Abstract
Background
Staphylococcus aureus is a common nasal colonizer in 20–30% of the general population. When mucosal and cutaneous barriers are disrupted, S. aureus can cause severe infections. While MRSA nasal carriers have an increased risk of infections when compared to non-carriers, prolonged exposure to the hospital environment may cause an increase in carriage of MRSA.
Materials and methods
A survey questionnaire was filled for analyzing risk factors of colonization. Swab isolates were identified as S. aureus by traditional microbiological assays. Antibiotic susceptibility profiles were performed following the CLSI standard guidelines. Multiplex PCR was conducted to determine the presence of genes mecA and lukS-PV/lukF-PV. Chi-squared, univariate, and multivariate logistic regressions were applied to find statistically significant associations between risk factors and the presence of S. aureus and MRSA.
Results
One hundred and eighty-six isolates were identified as S. aureus. The strains showed high resistance to penicillin, oxacillin, azithromycin, erythromycin, clindamycin (inducible), and tetracycline. The overall prevalence of MRSA in medical students was 45.9% [40.4–51.6] 95% CI. PCR showed a prevalence of mecA gene in MRSA isolates of 6.1% while lukS-PV/lukF-PV gene was present in 3.2% [1.2–6.9] 95% CI of the S. aureus samples. The risk factors frequency of antibiotic intake and repeated visits to hospitals demonstrated statistical significance.
Conclusion
S. aureus and MRSA isolates have a high prevalence of colonization, and antibiotic resistance in the population studied. MRSA resistance was not related to the presence of the mecA gene. The prevalence of PVL genes was low, but it could represent a risk because they are circulating in the community.
Keywords: mecA, medical students, MRSA, Panton-Valentine leukocidin, Staphylococcus aureus
Introduction
Staphylococcus aureus is part of the human skin and mucosal microbiota and a common opportunistic pathogen that causes significant infections.1 Staphylococcus aureus is carried in the nasal mucosa in 20–30% of the general population.2 However, when mucosal and cutaneous barriers are disrupted, S. aureus can produce bloodstream, heart, skin and soft tissues, bones, joints, and device-related infections.1–3 While there are still some strains of S. aureus sensitive to more commonly prescribed antibiotics, emerging S. aureus isolates have developed antimicrobial resistance. Those resistant to the antibiotic methicillin are termed methicillin-resistant Staphylococcus aureus (MRSA)3,4 because they have acquired a mecA gene, a coding gene of an essential protein to bacterial cell wall synthesis called PBP2ʹ, which is found in a mobile genetic element called staphylococcal chromosome cassette mec (SSCmec).4
Prevalence of hospital-associated MRSA (HA-MRSA) in Latin America ranges from 6% to 80%, where Cuba has the lowest percentage and Peru the highest.5 Differences in prevalence data between countries may be due to differences between the population studied (from primary, secondary, and tertiary health care facilities origin), microbiology laboratory protocols, and available technologies. Guzman-Blanco et al showed that more than 50% of Latin American countries have more than 50% prevalence of HA-MRSA.5 Zurita et al in a study conducted in Ecuador demonstrated that the frequency of MRSA is 46.97%.6
MRSA nasal carriers have an increased risk of infections when compared to non-carriers.7 However, when the bacterium has virulence factors, such as cytolytic toxin Panton-Valentine leukocidin (PVL) (a cytotoxin associated with tissue necrosis and leukocyte destruction), the chance of complicated infections increments. Depends on the geographical area or the type of patients, the presence of PVL in S. aureus isolates can vary from 3% to 75%,8–10 but in Latin America, the presence of these genes in circulating HA-MRSA or community-associated (CA) MRSA has not been fully elucidated.11
Evidence suggests that prolonged exposure to the hospital environment may cause an increase in carriage of MRSA.12 Work-related factors for MRSA carriage among health care workers include work experience and area of service, employment in areas of high patient MRSA prevalence and close contact with them, high workload, and poor hand hygiene.13 Although medical students are not technically health care workers, they have intermittent exposure to hospital environments. Little is known regarding S. aureus and MRSA colonization among medical students.14–19 Additionally, MRSA surveillance studies carried out in community settings are necessary to better understand the molecular and clinical epidemiology of emerging MRSA isolates.20 Therefore, the purpose of the present study was to determine the antibiotic susceptibility profiles and S. aureus and MRSA colonization prevalence in nose and throat, and its association with risk factors in healthy medical students at the Universidad de Las Américas.
Materials and methods
Population, study design, ethical considerations, and sample collection
Our work was an exploratory and cross-sectional study in a small cohort of medical students from the Universidad de Las Americas (UDLA) in Quito, Ecuador. The study was performed at the Research Laboratories at the UDLA from March to November of 2018. The Ethics Committee for Investigation on Human Beings at Universidad San Francisco de Quito approved the research with the approval code 2018-026E in accordance with the Declaration of Helsinki. All participants signed the previously approved informed consent.
The sample size was calculated using the epidemiological program Epi Info 7.2.2.1, StatCalc based on the number of enrolled students in Medical School in the second period of 2017. The total amount was 1349 students. We used an expected frequency of 46.97% according to the reports of Zurita et al6 and an error range of 5%. The final sample size calculated was 298 students, with a reliability level of 95%. The sample was taken from students that signed the informed consent and filled up a survey questionnaire that was designed according to Halablab et al to determine the risk factors of S. aureus colonization in healthy carriers.21 Nasal and pharyngeal swabs were taken from every participant, cultured in blood agar (Medibac INC S.A, Ecuador), and incubated for 16–18 hrs at 36°C.22
Phenotypic identification
Isolates that grown with beta-hemolysis in blood agar (Medibac INC S.A, Ecuador) were cultured in mannitol salt agar (BD DifcoTM, USA) and incubated for 16–18 hrs at 36°C.22 Isolates that were positive for mannitol salt agar were tested by Gram stain, coagulase, and catalase assays. Strains that were mannitol positive, Staphylococcus Gram +, catalase positive and coagulase positive were identified as Staphylococcus aureus and isolated for future experiments.
Antibiotic susceptibility
Antibiotic susceptibility was performed by the disk diffusion method following the Clinical & Laboratory Standards Institute (CLSI) guidelines.22 Briefly, we took one or two colonies, previously grown on nutrient agar (BD DifcoTM, USA), and diluted them in Muller Hinton broth (BD DifcoTM, USA) until reaching 0.5 McFarland scale. After that, we followed the disc diffusion method according to Kirby-Bauer.23 The antibiotics used were as follow: penicillin (10U), oxacillin (30 µg), vancomycin (E- Test), gentamicin (10 µg), erythromycin (15 µg), linezolid (30 µg), tetracycline (30 µg), ciprofloxacin (5 µg), trimethoprim-sulfamethoxazole (1.25/23.75 μg), chloramphenicol (30 µg), rifampicin (5 µg), clindamycin (2 µg), azithromycin (15 µg), mupirocin (100 µg). Clindamycin and erythromycin were used in a D-test to assess inducible clindamycin resistance. The strain Staphylococcus aureus ATCC 25923 was used as a control.
DNA extraction and molecular identification of genes mecA and LukS-PV/LukF-PV
DNA extraction was performed following the Chelex-100 methodology with some modifications.24 Briefly, 6–10 colonies of S. aureus were resuspended on 200 µL of Chelex 10% (Sigma-Aldrich, USA) and 5 µL of Proteinase K (Invitrogen, USA). Samples were incubated at 56°C for 60 mins. After that, the samples were vortexed and centrifuged at 8000 g for 2 mins. Another incubation was made at 96°C for 20 mins. Finally, samples were centrifuged at 8000 g for 5 mins, and the supernatant was transferred to a clean tube and stored at −20°C.
Polymerase chain reaction (PCR) was performed with modifications of the protocol reported by Zhang et al.25 The reaction mix for the multiplex PCR contained 1.3 U/µL Platinum Taq DNA polymerase (Invitrogen, USA), 1X PCR buffer (Invitrogen, USA), 2.3 mM MgCl2 (Invitrogen, USA), 0.4 mM dNTPs (Invitrogen, USA), 10 ng/µL of DNA and 0.6 µM primers Staph 756F and Staph 750 R,26 0.3 µM of primers Nuc1 and Nuc2,26 0.6 µM of primers MecA147-F and MecA 147-R27 and 0.5 µM of primers Luk-PV-1 and Luk-PV-2.28 The PCR amplification was performed in a thermocycler cycler Eppendorf, Mastercycler® Gradient (Eppendorf, Germany) with the initial denaturation step at 94°C for 7 min, followed by two cycling steps of 30 cycles of denaturation at 94°C for 30 s, annealing at 56°C for 30 s, and extension at 72°C for 45 s and 20 cycles of denaturation at 94°C for 30s, annealing at 63°C for 30 s, and extension at 72°C for 45 s. A final extension was performed at 72°C for 7 mins. PCR products were analyzed by 2% (w/v) agarose gel containing DNA stain SyBr Safe (Invitrogen, USA) in 1X TBE buffer and electrophoresis was performed at 100 V for 35 min in a Labnet Enduro Gel XL Electrophoresis System (Labnet International, Inc, USA).
The agarose gel was visualized under UV light using a gel documentation system ChemiDoc™ Imaging Systems (BioRad, USA). The length of amplicons was determined by comparing with a 100 bp DNA ladder (Invitrogen, USA). The lengths of amplification of expected products were 756 bp for 16S rRNA (Stapy756-F and Staph750-R), 279 bp for thermostable nuclease (nuc) (Nuc-1 and Nuc-2), 147 bp for mecA (mecA147-F and mecA147-R), and 433 pb for PVL genes lukS-PV/lukF-PV (Luk-PV-1 and Luk-PV-2). The strain PV-STAPH (kindly donated by the Hospital del Mar de Barcelona, Spain) was used as a positive control for all the four genes. The strain S. aureus ATCC 25923 was used as a control for genes 16S and nuc.
Statistical analysis
All results were grouped depending on the outcome of interest: 1) S. aureus carriers, 2) S. aureus MRSA, 3) Presence of mecA gene and 4) Presence of PVL.
Chi-squared, uni, and multivariate logistic regressions were applied to find statistically significant associations between risk factors and the presence of 1) S. aureus carriers, 2) S. aureus MRSA, 3) Presence of mecA gene and 4) Presence of Panton- Valentine.
For all statistical analyses, p-values <0.05 were considered significant. All studies were carried out in R version 3.5.3. and JASP (2018).
The frequency of isolates confirmed as S. aureus was determined as sensitive, intermediate resistant, and resistant.
Results
A total of 322 medical students from the first to the eleventh semester participated in the survey. Most of the participants were in the fourth and fifth semesters in the medical curriculum with 36.6% (n=118/322) and 16.1% (n=52/322), respectively. The mean age of the participants was 20.8 years (minimum: 17 years, maximum: 34 years), 46.3% (n=149/322) were males, and 53.7% (n=173/322) were females.
All 322 samples were analyzed using traditional microbiological assays. 17.1% (n=55/322) were isolated from nose, 25.1% (n=81/322) from throat, and 15.5% (n=50/322) from both localizations. S. aureus was present in 57.8% [52.2–63.2]95% CI of the samples (n=186/322).
S. aureus antibiotic susceptibility profiles
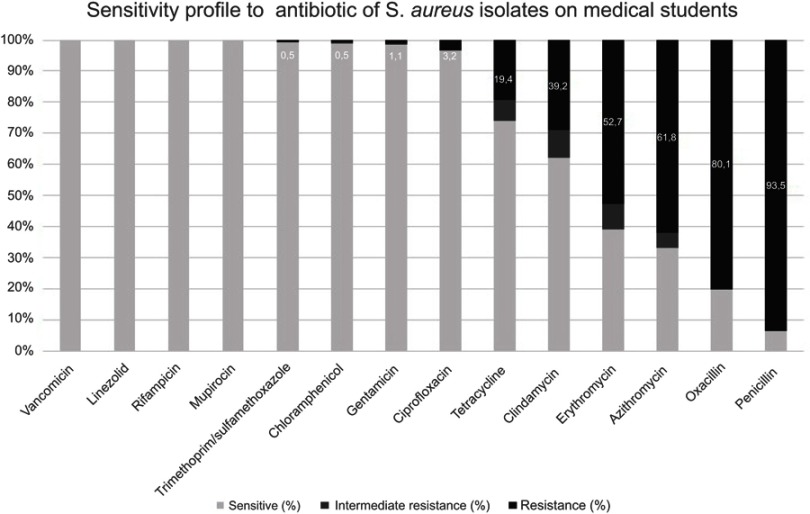
Using a disc diffusion method, the resistance profiles of the 186 S. aureus isolates were determined as follows. The highest percentage of resistance was against penicillin 93.5% (n=174/186), followed by oxacillin with 79.6% (n=148/186), azithromycin with 61.8% (n=115/186), erythromycin with 52.7% (n=98/186), clindamycin (using D-test) with 39.2% (73/186), tetracycline with 19.4% (36/186), ciprofloxacin with 3.2% (n=6/186), gentamicin with 1.1% (n=2/186), and the lowest percentage of resistance was for both trimethoprim/sulfamethoxazole and chloramphenicol with 0.5% (n=1/186). All the 186 isolates were sensitive to vancomycin (using Etest), linezolid (>21 mm), rifampicin (>20 mm), and mupirocin (>1 mm). According to the oxacillin resistance, the overall prevalence of MRSA in medical students was 45.9% [40.4–51.6] 95% CI (n=148/322) (Figure 1).
Figure 1.
Antibiotic susceptibility profiles of Staphylococcus aureus isolated from nasal and pharyngeal swabs from medical students.
Note: Numbers inside the bars indicate resistance.
Molecular analysis of MRSA
Using a multiplex PCR assay, we determined a prevalence of mecA gene in MRSA isolates of 6.1% [2.8–11.2]95% CI (n=9/148). The frequency of lukS-PV/lukF-PV genes in S. aureus was 3.2% [1.2–6.9]95% CI (n=6/186), from these, 2.7% [0.8–6.2]95% CI (n=5/186) were MRSA. No isolates were found as lukS-PV/lukF-PV and mecA gene positive at the same time.
Risk factors analysis
Non-statistical association was found for the standard demographic indicators such as age and sex for neither S. aureus nor MRSA colonization.
S. aureus colonization
Logistic regression found that S. aureus colonization was negatively associated if the students took just one cycle of antibiotic during the 12 months before the sampling (p=0.039). S. aureus colonization was negatively associated as well with students of the third semester (p=0.046).
MRSA colonization
The chi-squared test revealed that MRSA colonization was associated with the intake/not intake of antibiotics due to skin infections (p=0.038) or due to another medical condition (p=0.025), during the 12 months before taking the sample.
Logistic regression showed a significant negative association of MRSA colonization in students that took antibiotics during skin infections (p=0.042), or when they took antibiotics due to other medical conditions (p=0.026), or when they took only one cycle of antibiotics (p=0.029) during the twelve months before to the sampling.
Related to the level in the medical curriculum and visits to clinics or hospitals during their training, we identified that students who were taking the third semester (p=0.022) or those who visited a health unit twice a week (p=0.035) were less likely to be colonized by MRSA.
Discussion
To our knowledge, this is the first report that analyses antibiotic susceptibility profiles in S. aureus isolates from nose and throat swabs, and its association with risk factors for colonization in healthy medical students in Ecuador.
The overall prevalence of S. aureus in our study was 57.8%. Individually, the rate of the nasal carriage was 17.1%, and pharyngeal carriage was 25.4%. Previous studies, based on nasal swabs, have reported similar prevalences in medical students between 15% to 22.1%.14,15,17–19 Additionally, other reports have shown high variability in the nasal and pharyngeal carriage of S. aureus between populations.29–32 When Senn et al combined three sites (nose, groin, and throat), the sensitivity of S. aureus detection by culture increased to 96%.33 In our report, combining two anatomical sites for screening, the overall prevalence reached 57.8%. Although S. aureus is most often found in the nose,34 our study has shown that this bacteria colonizes throat (25.4%) more frequently than the nose (17.1%) in medical students at the UDLA. Therefore, it is crucial to carry out investigations for screening samples from different anatomical niches to improve the rate of S. aureus detection and understand the real burden of colonization.
Antibiotic susceptibility pattern of S. aureus isolates in our study is comparable to other similar studies when were included the most commonly used drugs like penicillin, oxacillin (methicillin), azithromycin, erythromycin, clindamycin, and tetracycline.16,19 When we used the D-test, the resistance of S. aureus to clindamycin was 39.2% (73/186) instead of 3.2% (n=6/186) (data not shown) when a disc of clindamycin was used alone. It evidences the importance of including in routine susceptibility test a D-test in all S. aureus isolates.35 In contrast with a study conducted in Nepal that found resistances between 20–33.3%, our work reported low rates of resistance against ciprofloxacin, gentamicin, and trimethoprim/sulfamethoxazole17 maybe because these antibiotics have a low frequency of use in the population studied. In agreement with other studies, all the isolates in our report were sensitive to vancomycin,16–18 linezolid,18 and rifampicin.18 Similar studies with medical students have not included mupirocin in their analyses.
Interestingly, the frequency of MRSA (using phenotypic methods) reached 79.5% (n=148/186), but only 6.1% (n=9/148) of the MRSA isolates presented the mecA gene. Our results contrast sharply with data published in Sudan, which found that 90.2% (111/123) of the isolates were mecA positive.36 They suggest that finding of mecA gene is the principal evidence for the detection of MRSA isolate. However, we found only 6.1% (n=9/148) isolates mecA positives. These data suggest that resistance to oxacillin is due to other genetic alternatives. Patterson et al have identified the emergence of a variant of the mecA gene known as the mecC gene with a genetic similarity of 70%,37 which could explain the low frequency of the mecA gene in this study. Elhassan et al suggest searching for other intrinsic factors that may compete with the mecA gene in producing resistance events in regions with a high prevalence of MRSA.36
Additionally, our study identified lukS-PV/lukF-PV gene in 3.2% of the samples. The prevalence of PVL-positive MRSA varies considerably from country to country. While in France the prevalence is as lower as 3%,9 in the United States reach 98%.8 These findings alert to health authorities about the high rate of MRSA circulating in a community and a non-hospital population as would be expected. Based on the chance of person-to-person transmission, we support the implementation of surveillance systems both in hospital and community settings sampling different patient body sites to identify the emergence of new strains, the antibiotic resistance profile, the risk factors associated with infection,38 and the potential contributions of undetected carriers to MRSA transmission.39 Today, the standard MRSA surveillance method is a culture-based strategy, but Ábrók et al developed an approach to minimize time-consuming culturing-based MRSA screening combining MALDI-TOF MS optimized and PBP2′ latex agglutination assay.40 Therefore, to prevent the MRSA dissemination, rapid and accurate detection of MRSA strains are appropriate measures to understand the situation of MRSA distribution in the community and health care facilities.
The present report failed to identify a statistical association between age and sex and S. aureus and MRSA colonization. We sought to analyze whether the level in the medical curriculum could help prone the students to S. aureus and MRSA colonization taking in count that students in higher semesters have longer stays in clinical practice than students in lower semesters. They did not find any significant association between the semester and the acquisition of S. aureus among medical students. On the contrary, who was taking the third semester or those who visited a clinic or hospital twice a week were less likely to be colonized by MRSA. In contrast, research conducted in Ethiopia found that more than half of their study participants who were exposed to a university medical center for more than two years were colonized with MRSA.19 Unexpectedly, our report found that S. aureus and MRSA colonization was negatively associated with the use of antibiotics. Fanelly et al recognized that long-term use of antibiotics decreased the prevalence of S. aureus colonization by nearly 70%, and a reduced frequency of S. aureus colonization was identified with the use of oral and topical antibiotics.41
The limitations of our study were that we could not distinguish between persistent and intermittent carriers because this was a cross-sectional study. Persistent carriers are individuals that almost always carry one type of S. aureus in 20% of the population while 60% of them harbor this bacterium intermittently.42 Another factor was that we analyzed only one strain from each participant, so we cannot be able to ensure if participants colonized with S. aureus at nose and throat are carriers of the same or different strains. Additionally, only 10% of medical students agreed to participate when they were chosen at random and notified via email. As a result, we invited all 1300 medical students in the same university to increase and achieve the appropriate sample size, and it could have introduced selection bias. Furthermore, information concerning to antibiotics used in the last 12 months, names, doses, and other data could have introduced recall bias in our study.
Acknowledgments
We acknowledge the support of the Facultad de Medicina, the Facultad de Ingenierías y Ciencias Aplicadas, and the Dirección General de Investigación at UDLA, who made this work possible. The funders had no part in study design, data collection, analysis, or preparation of the manuscript. We also thank Maira Rojas for collecting some samples, and all of the students who agreed to participate in this research.
Author Contributions
All authors contributed towards data analysis, drafting and critically revising the paper, gave final approval of the version to be published, and agreed to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Tong SYC, Davis JS, Eichenberger E, Holland TL, Fowler VG. Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clin Microbiol Rev. 2015;28(3):603–661. doi: 10.1128/CMR.00134-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee AS, de Lencastre H, Garau J, et al. Methicillin-resistant Staphylococcus aureus. Nat Rev Dis Primers. 2018;4:1–23. doi: 10.1038/nrdp.2018.33 [DOI] [PubMed] [Google Scholar]
- 3.Peacock SJ, Paterson GK. Mechanisms of methicillin resistance in Staphylococcus aureus. Annu Rev Biochem. 2015;84(1):577–601. doi: 10.1146/annurev-biochem-060614-034516 [DOI] [PubMed] [Google Scholar]
- 4.Gajdács M. The continuing threat of methicillin-resistant Staphylococcus aureus. Antibiotics. 2019;8(2):52. doi: 10.3390/antibiotics8020052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guzmán-Blanco M, Mejía C, Isturiz R, et al. Epidemiology of methicillin-resistant Staphylococcus aureus (MRSA) in Latin America. Int J Antimicrob Agents. 2009;34(4):304–308. doi: 10.1016/j.ijantimicag.2009.06.005 [DOI] [PubMed] [Google Scholar]
- 6.Zurita J, Barba P, Ortega-Paredes D, Mora M, Rivadeneira S. Local circulating clones of Staphylococcus aureus in Ecuador. Br J Infect Dis. 2016;1–9. doi: 10.1016/j.bjid.2016.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stenehjem E, Rimland D. MRSA nasal colonization burden and risk of MRSA infection. Am J Infect Control. 2013;41(5):405–410. doi: 10.1016/j.ajic.2012.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moran GJ, Krishnadasan A, Gorwitz RJ, et al. Methicillin-resistant S. aureus infections among patients in the emergency department. N Engl J Med. 2006;355:666–674. doi: 10.1056/NEJMoa055356 [DOI] [PubMed] [Google Scholar]
- 9.Del Giudice P, Blanc V, Durupt F, et al. Emergence of two populations of methicillin-resistant Staphylococcus aureus with distinct epidemiological, clinical and biological features, isolated from patients with community-acquired skin infections. Br J Dermatol. 2006;154(1):118–124. doi: 10.1111/j.1365-2133.2005.06910.x [DOI] [PubMed] [Google Scholar]
- 10.Tristan A, Bes M, Meugnier H, et al. Global distribution of panton- valentine leukocidin–positive methicillin-resistant. Emerg Infect Dis. 2007;13(4):594–600. doi: 10.3201/eid1304.061316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reyes J, Rincon S, Diaz L, et al. Dissemination of methicillin-resistant Staphylococcus aureus USA300 sequence type 8 lineage in Latin America. Clin Infect Dis. 2009;49(12):1861–1867. doi: 10.1086/648426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Al-Anazi AR. Prevalence of Methicillin-Resistant Staphylococcus aureus in a teaching hospital in Riyadh, Saudi Arabia. Biomed Res. 2009;20(1):7–14. [Google Scholar]
- 13.Albrich WC, Harbarth S. Health-care workers: source, vector, or victim of MRSA? Lancet Infect Dis. 2008;8(5):289–301. doi: 10.1016/S1473-3099(08)70097-5 [DOI] [PubMed] [Google Scholar]
- 14.Chen C-S, Chen C-Y, Huang Y-C. Nasal carriage rate and molecular epidemiology of methicillin-resistant Staphylococcus aureus among medical students at a Taiwanese university. Int J Infect Dis. 2012;16:e799–e803. doi: 10.1016/j.ijid.2012.07.004 [DOI] [PubMed] [Google Scholar]
- 15.Zakai S. Prevalence of methicillin-resistant Staphylococcus aureus nasal colonization among medical students in Jeddah, Saudi Arabia. SMJ. 2015;36(7):807–812. doi: 10.15537/smj.2015.7.11609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Okamo B, Moremi N, Seni J, Mirambo MM, Kidenya BR, Mshana SE. Prevalence and antimicrobial susceptibility profiles of Staphylococcus aureus nasal carriage among pre-clinical and clinical medical students in a Tanzanian University. BMC Res Notes. 2016;1–6. doi: 10.1186/s13104-016-1858-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ansari S, Gautam R, Shrestha S, Ansari SR, Subedi SN, Chhetri MR. Risk factors assessment for nasal colonization of Staphylococcus aureus and its methicillin resistant strains among pre-clinical medical students of Nepal. BMC Res Notes. 2016;1–8. doi: 10.1186/s13104-016-2021-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Al-Tamimi M, Himsawi N, Abu-Raideh J, et al. Nasal colonization by methicillin-sensitive and methicillin-resistant Staphylococcus aureus among medical students. J Infect Dev Ctries. 2018;12(05):326–335. doi: 10.3855/jidc.9908 [DOI] [PubMed] [Google Scholar]
- 19.Efa F, Alemu Y, Beyene G, Gudina EK, Kebede W. Methicillin-resistant Staphylococcus aureus carriage among medical students of Jimma University, Southwest Ethiopia. Heliyon. 2019;e01191. doi: 10.1016/j.heliyon.2019.e01191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sharma Y, Jain S, Singh H, Govil V. Research article Staphylococcus aureus: screening for nasal carriers in a community setting with special reference to MRSA. Scientifica. 2014;1–5. doi: 10.1155/2014/479048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Halablab MA, Hijazi SM, Fawzi MA, Araj GF. Staphylococcus aureus nasal carriage rate and associated risk factors in individuals in the community. Epidemiol Infect. 2009;138(05):702–705. doi: 10.1017/S0950268809991233 [DOI] [PubMed] [Google Scholar]
- 22.CLSI. Performance Standards for Antimicrobial Susceptibility Testing. 28th ed. CLSI supplement M100. Wayne, PA: Clinical and Laboratory Standards Institute. 2018: 1–282. [Google Scholar]
- 23.Bauer AW, Kirby W, Sherris JC, Turck M. Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol. 1966;45(4):493–496. [PubMed] [Google Scholar]
- 24.Suenaga E, Nakamura H. Evaluation of three methods for effective extraction of DNA from human hair. J Chromatogr B. 2005;820(1):137–141. doi: 10.1016/j.jchromb.2004.11.028 [DOI] [PubMed] [Google Scholar]
- 25.Zhang K, McClure J-A, Elsayed S, Louie T, Conly JM. Novel multiplex PCR assay for simultaneous identification of community-associated methicillin-resistant Staphylococcus aureus strains USA300 and USA400 and detection of mecA and Panton-Valentine leukocidin genes, with discrimination of Staphylococcus aureus from coagulase-negative staphylococci. J Clin Microbiol. 2008;46(3):1118–1122. doi: 10.1128/JCM.01309-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang K, Sparling J, Chow BL, et al. New quadriplex PCR assay for detection of methicillin and mupirocin resistance and simultaneous discrimination of Staphylococcus aureus from coagulase-negative staphylococci. J Clin Microbiol. 2004;42(11):4947–4955. doi: 10.1128/JCM.42.11.4947-4955.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang K, McClure JA, Elsayed S, Louie T, Conly JM. Novel multiplex PCR assay for characterization and concomitant subtyping of staphylococcal cassette chromosome mec types I to V in methicillin-resistant Staphylococcus aureus. J Clin Microbiol. 2005;43(10):5026–5033. doi: 10.1128/JCM.43.10.5026-5033.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lina G, Piémont Y, Godail-Gamot F, et al. Involvement of panton-valentine leukocidin–producing Staphylococcus aureus in primary skin infections and pneumonia. Clin Infect Dis. 1999;29:1128–1132. doi: 10.1086/313461 [DOI] [PubMed] [Google Scholar]
- 29.Lestari ES, Severin JA, Filius PMG, et al. Antimicrobial resistance among commensal isolates of Escherichia coli and Staphylococcus aureus in the Indonesian population inside and outside hospitals. Eur J Clin Microbiol Infect Dis. 2007;27(1):45–51. doi: 10.1007/s10096-007-0396-z [DOI] [PubMed] [Google Scholar]
- 30.Ruimy R, Angebault C, Djossou F, et al. Are host genetics the predominant determinant of persistent nasal Staphylococcus aureus carriage in humans? J Infect Dis. 2010;202(6):924–934. doi: 10.1086/655901 [DOI] [PubMed] [Google Scholar]
- 31.Sollid JUE, Furberg AS, Hanssen AM, Johannessen M. Staphylococcus aureus: determinants of human carriage. Infect Genet Evol. 2014;21(C):531–541. doi: 10.1016/j.meegid.2013.03.020 [DOI] [PubMed] [Google Scholar]
- 32.Ouedraogo A-S, Dunyach-Remy C, Kissou A, et al. High nasal carriage rate of Staphylococcus aureus containing panton-valentine leukocidin- and EDIN-encoding genes in community and hospital settings in Burkina Faso. Front Microbiol. 2016;7(348):4131–4137. doi: 10.3389/fmicb.2016.01406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Senn L, Basset P, Nahimana I, Zanetti G, Blanc DS. Which anatomical sites should be sampled for screening of methicillin-resistant Staphylococcus aureus carriage by culture or by rapid PCR test? Eur Soc Clin Infect Dis. 2012;18(2):E31–E33. doi: 10.1111/j.1469-0691.2011.03724.x [DOI] [PubMed] [Google Scholar]
- 34.Kaspar U, Kriegeskorte A, Schubert T, et al. The culturome of the human nose habitats reveals individual bacterial fingerprint patterns. Environ Microbiol. 2016;18(7):2130–2142. doi: 10.1111/1462-2920.12891 [DOI] [PubMed] [Google Scholar]
- 35.Shrestha B, Rana SS. D test: a simple test with big implication for Staphylococcus aureus macrolide-lincosamide-streptograminB resistance pattern. Nepal Med Coll J. 2014;16(1):88–94. [PubMed] [Google Scholar]
- 36.Elhassan MM, Ozbak HA, Hemeg HA, Elmekki MA, Ahmed LM. Research article absence of the mecA gene in methicillin resistant Staphylococcus aureus Isolated from different clinical specimens in Shendi City, Sudan. Biomed Res Int. 2015;1–5. doi: 10.1155/2015/895860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paterson GK, Harrison EM, Holmes MA. The emergence of mecC methicillin-resistant Staphylococcus aureus. Trends Microbiol. 2014;22(1):42–47. doi: 10.1016/j.tim.2013.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mejía C, Zurita J, Guzmán-Blanco M. Epidemiology and surveillance of methicillin-resistant Staphylococcus aureus in Latin America. Br J Infect Dis. 2010;14(2):S79–S86. [DOI] [PubMed] [Google Scholar]
- 39.Currie A, Davis L, Odrobina E, et al. Sensitivities of nasal and rectal swabs for detection of methicillin-resistant Staphylococcus aureus colonization in an active surveillance program. J Clin Microbiol. 2008;46(9):3101–3103. doi: 10.1128/JCM.00848-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ábrók M, Lázár A, Szécsényi M, Deák J, Urbán E. Combination of MALDI-TOF MS and PBP2′ latex agglutination assay for rapid MRSA detection. J Microbiol Methods. 2018;144:122–124. doi: 10.1016/j.mimet.2017.11.021 [DOI] [PubMed] [Google Scholar]
- 41.Fanelli M. Antibiotics, acne, and Staphylococcus aureus colonization. Arch Dermatol. 2011;147(8):917–921. doi: 10.1001/archdermatol.2011.67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kluytmans J, van Belkum A, Verbrugh H. Nasal carriage of Staphylococcus aureus: epidemiology, underlying mechanisms, and associated risks. Clin Microbiol Rev. 1997;10(3):505–520. [DOI] [PMC free article] [PubMed] [Google Scholar]