Abstract
Introduction
Non-invasive ventilation (NIV) is recommended as first-line therapy in respiratory failure of critically ill immunocompromised patients as it can decrease intubation and mortality rates as compared with standard oxygen. However, its recommendation is only conditional. Indeed, the use of NIV in this setting has been challenged recently based on results of trials finding similar outcomes with or without NIV or even deleterious effects of NIV. To date, NIV has been compared with standard oxygen but not to high-flow nasal oxygen therapy (HFOT) in immunocompromised patients. Several studies have found lower mortality rates using HFOT alone than when using HFOT with NIV sessions in patients with de novo respiratory failure, and even in immunocompromised patients. We are hypothesising that HFOT alone is more effective than HFOT with NIV sessions and reduces mortality of immunocompromised patients with acute hypoxemic respiratory failure.
Methods and analysis
This study is an investigator-initiated, multicentre randomised controlled trial comparing HFOT alone or with NIV in immunocompromised patients admitted to intensive care unit (ICU) for severe acute hypoxemic respiratory failure. Around 280 patients will be randomised with a 1:1 ratio in two groups. The primary outcome is the mortality rate at day 28 after inclusion. Secondary outcomes include the rate of intubation in each group, length of ICU and hospital stay and mortality up to day 180.
Ethics and dissemination
The study has been approved by the ethics committee and patients will be included after informed consent. The results will be submitted for publication in peer-reviewed journals.
Trial registration number
Keywords: immunosuppression, acute respiratory failure, non-invasive ventilation, high-flow nasal cannula oxygen therapy, clinical trial, mortality
Strengths and limitations of this study.
This trial is the first to compare high-flow nasal oxygen therapy (HFOT) alone versus HFOT with non-invasive ventilation (NIV) sessions for treatment of acute hypoxemic respiratory failure in immunocompromised patients admitted to intensive care unit.
The settings of the oxygenation techniques compared have been protocolised based on physiological studies in order to optimise their efficiency (improvement in oxygenation, decrease in work of breathing, limitation of patient self-inflicted lung injury).
The sample size of this trial has been designed to have the power to detect a difference in mortality rates of patients with severe acute hypoxemic respiratory failure.
The individual study assignments of the patients will not be masked. Given the nature of the two strategies under evaluation, a double-blind trial is not possible.
Introduction
Background and rationale
Acute hypoxemic respiratory failure is the leading cause of admission to intensive care units (ICUs) in immunocompromised patients.1 Intubation and subsequent invasive mechanical ventilation are needed in about two-thirds of cases and are associated with particularly high mortality reaching 50%–70% of cases.1–3 Therefore, it is crucial to assess the best strategy of oxygenation with the aim of avoiding the need for intubation in immunocompromised patients.
According to a large international cohort study, non-invasive ventilation (NIV) is used in up to 21% of cases in this setting.4 It is worth noting that recent European/American clinical practice guidelines have recommended NIV as first-line therapy for management of acute hypoxemic respiratory failure in immunocompromised patients.5 Indeed, by pooling all randomised controlled trials, NIV has been associated with decreased intubation and mortality rates as compared with standard oxygen (table 1).6–9 However, the largest randomised controlled trial comparing NIV vs standard oxygen found no difference in intubation or mortality rates and application of NIV was consequently only a conditional recommendation.6
Table 1.
Characteristics and outcomes of previous trials comparing non-invasive ventilation to oxygen therapy in immunocompromised patients
| Authors | Year | Setting | N= | Arms | Intubation rate (%) | In-ICU mortality rate (%) |
| Antonelli et al | 2000 | ICU, monocentre | 20 | O2 | 70 | 50 |
| 20 | NIV | 20 | 20 | |||
| Hilbert et al | 2001 | ICU, monocentre | 26 | O2 | 77 | 69 |
| 26 | NIV | 46 | 38 | |||
| Lemiale et al | 2015 | ICU, multicentre | 183 | O2 | 45 | 25 |
| 191 | NIV | 38 | 21 | |||
| Squadrone et al | 2010 | Ward, monocentre | 20 | O2 | 40 | 75* |
| 20 | CPAP | 10 | 15* | |||
| Frat et al† | 2016 | ICU, multicentre | 30 | O2 | 43 | 20 |
| 26 | NIV | 65 | 42 | |||
| 26 | HFOT | 31 | 15 |
Outcomes of patients in the control arm are displayed in italics.
*Hospital mortality (ICU mortality was not indicated in the article).
†Post hoc analysis of a randomised trial.
CPAP, continuous positive airway pressure; HFOT, high-flow nasal oxygen therapy;ICU, intensive care unit; NIV, non-invasive ventilation; O2, oxygen therapy.
All previous studies have compared NIV to standard oxygen and not versus high-flow nasal oxygen therapy (HFOT).5 Recently, better outcomes have been reported with HFOT than with standard oxygen, and even as compared with HFOT with NIV in patients with acute hypoxemic respiratory failure.10–12 However, the design of these studies (retrospective monocentre or post-hoc) excludes any definite conclusion on the best treatment option for immunocompromised critically ill.10 11 13 Therefore, there is an urgent need for a dedicated trial designed to compare NIV to HFOT in immunocompromised critically ill patients taking into account the suggested deleterious effects of NIV.10 11 Indeed, NIV may be associated with harmful effects in de novo respiratory failure,14 especially in patients generating strong inspiratory efforts and subsequent large tidal volumes due to high transpulmonary pressures.15 16 It could be argued that NIV protocol had not been protective enough, that is, by applying low levels of pressure-support (PS) to avoid large tidal volumes that may worsen underlying lung injury,17 by applying high levels of positive end-expiratory pressure (PEEP) to promote alveolar recruitment as is the case in patients invasively ventilated for acute respiratory distress syndrome (ARDS),18 19 and by applying prolonged sessions of NIV to avoid derecruitment during NIV breakoffs.20 21 Indeed, while most of these patients meet the clinical criteria for ARDS,22 optimisation of ventilator settings during NIV could lead to better outcomes, as is the case in patients under invasive mechanical ventilation.
Objectives
We are aiming to conduct a prospective multicentre randomised controlled trial comparing HFOT alone or with optimised NIV sessions in immunocompromised patients admitted to ICU for acute hypoxemic respiratory failure. Our hypothesis is that HFOT alone may reduce mortality rate at day 28 as compared with HFOT with NIV, despite application of NIV with protective ventilator settings.
Primary objective
To compare the mortality rate at day 28 after inclusion between HFOT alone and HFOT with NIV in immunocompromised patients admitted to ICU for acute hypoxemic respiratory failure.
Secondary objectives
To compare the rates of intubation, and of mortality in ICU, in hospital, at day 90 and at day 180 after inclusion between the two strategies.
To compare length of stay in ICU, in hospital and number of ventilator-free days (invasive or noninvasive mechanical ventilation) within the 28 days following inclusion.
To compare tolerance between the two strategies.
Trial design
The FLORALI-IM study is an investigator-initiated, prospective, multicentre, randomised, controlled, open trial comparing two strategies of oxygenation using HFOT alone or with NIV in immunocompromised patients admitted to ICU for acute hypoxemic respiratory failure. Patients will be randomly assigned to the HFOT alone group or the HFOT with NIV group with a 1:1 ratio.
Methods
Participants, interventions and outcomes.
Study setting
The FLORALI-IM study is taking place in 29 ICUs in France and 1 ICU in Italy.
Eligibility criteria
Inclusion criteria
Adult immunocompromised patients admitted to ICU for acute hypoxemic respiratory failure are considered eligible.
Acute hypoxemic respiratory failure is defined by respiratory rate ≥25 breaths/min, and PaO2/FiO2 ≤300 mm Hg while spontaneously breathing under standard oxygen with oxygen flow rate of at least 10 L/min, under HFOT, or under NIV. For patients under standard oxygen, FiO2 is calculated according to the following formula: FiO2=0.21 + 0.03 per supplemental litre of oxygen.14
Immunosuppression is defined by one of the following criteria: haematological malignancy (active or remitting for <5 years), allogenic stem cell transplantation within the last 5 years, active solid cancer, leucopenia <1 G/L or neutropenia ≤0.5 G/L induced by chemotherapy, solid organ transplantation, AIDS, systemic steroids ≥0.5 mg/kg/day of prednisone equivalent for at least 3 weeks, immunosuppressive or immunomodulatory drugs.23
Exclusion criteria
Patients fulfilling one of the following criteria will not be included: PaCO2 above 50 mm Hg, patients who could strongly benefit from NIV, that is, with underlying chronic lung disease, cardiogenic pulmonary oedema or postoperative patients; severe shock defined as vasopressor dose >0.3 µg/kg/min of norepinephrine-equivalent to maintain systolic blood pressure >90 mm Hg or with impaired consciousness with a Glasgow coma score ≤12; patients with urgent need for intubation, that is, respiratory or cardiac arrest, respiratory pauses with loss of consciousness or gasping for air, severe hypoxemia defined as SpO2 lower than 90% despite maximal oxygen support; patients with do-not-intubate order at time of inclusion; or patients with contraindication to NIV according to the French consensus conference,24i.e. patient refusal, cardiorespiratory arrest, coma, non-drained pneumothorax, unresolved vomiting, upper airway obstruction, haematemesis or severe facial trauma.
Intervention
Patients eligible for inclusion will be informed, asked for consent, then randomised within the first 6 hours after they meet inclusion criteria, and assigned to one of the two following groups: (1) the patients assigned to control group will receive HFOT with NIV sessions and (2) the patients assigned to interventional group will receive HFOT alone.
The purpose of this 6-hour time frame is to avoid the possibly harmful delayed initiation of oxygenation strategies. As NIV may be more effective in haematological or neutropenic patients,7 randomisation will be stratified according to the existence of underlying haematological malignancy, leucopenia <1 G/L or neutropenia ≤0.5 G/L induced by chemotherapy.
Interventional group: HFOT alone
Immediately after randomisation, patients assigned to the interventional group will be continuously treated by HFOT (Optiflow or AIRVO2, Fisher & Paykel, Auckland, New Zealand) with a flow of 60 L/min and FiO2 adjusted to obtain adequate oxygenation (SpO2 ≥92%) through a heated humidifier (MR 850, Fisher & Paykel, Auckland, New Zealand) set to the ‘intubation’ position. For patients experiencing HFOT intolerance due to high flow levels despite reinsurance, flow will be decreased to the maximal tolerated level.
Control group: HFOT with NIV
Immediately after randomisation, NIV will be initiated with a first session of at least 4 hours until clinical improvement (assessed by the attending physician) and then applied by sessions of at least 1 hour for a minimal duration of at least 12 hours a day. NIV will be carried out with a ventilator dedicated for NIV (ICU ventilator after activation of NIV mode or NIV bi-level ventilator)25 in PS ventilatory mode with a minimal PS level of 5 cm H2O targeting a tidal volume of 6 mL/kg of predicted body weight and avoiding tidal volumes exceeding 8 mL/kg, a PEEP level of at least 8 cm H2O, and FiO2 adjusted to obtain adequate oxygenation (SpO2 ≥92%). Between NIV sessions, HFOT will be delivered as in the interventional group. For patients experiencing NIV intolerance despite reinsurance, physicians will be encouraged to modify NIV settings (level of PS and PEEP, minimising leaks, adjustment of inspiratory trigger and cycling, interface switch) to improve NIV tolerance.
Duration of treatment
In the two groups, strategies of oxygenation will be applied for a minimal duration of 48 hours. After that, continuation of the treatment will be decided according to patient respiratory status (figure 1).
Figure 1.
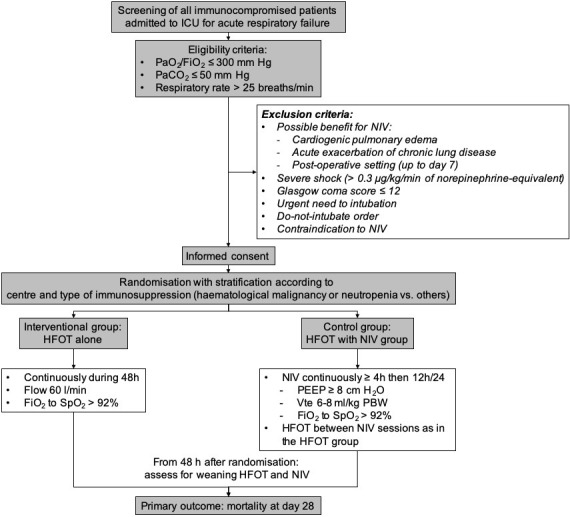
Flowchart of the patients and study design. HFOT, high-flow nasal oxygen therapy; ICU, intensive care unit; NIV, non-invasive ventilation; PBW, predicted body weight; PEEP, positiveend-expiratory pressure; Vte, expired tidal volume
Criteria for weaning oxygenation strategies
As there is no consensual method of weaning from HFOT or NIV, we propose a standardised weaning protocol to mitigate differences between centres. From 48 hours after inclusion, weaning from both oxygenation strategies will be assessed twice a day during the investigator’s round.
In the HFOT alone group, HFOT will be stopped and switched to standard oxygen when respiratory rate is <25 breaths/min and SpO2 ≥92% with FiO2 ≤50% and flow ≤50 L/min.
In the NIV group, NIV will be stopped first when respiratory rate is <25 breaths/min and SpO2 ≥92% with FiO2 ≤50%, and then HFOT will be stopped and switched to standard oxygen as in the HFOT alone group.
At any time after weaning of oxygenation techniques, if respiratory rate is ≥25 breaths/min or SpO2 <92% HFOT or HFOT with NIV sessions will be resumed according to randomisation group.
Prespecified intubation criteria
In order to avoid harmful effects of delayed intubation in patients treated with NIV26 27 and HFOT,28 intubation will be performed if at least one of the following criteria is fulfilled: neurological failure defined as agitation or altered consciousness defined as a Glasgow coma scale below 12 points, haemodynamic failure defined as the need for a dose of norepinephrine >0.3 µg/kg/min of norepinephrine-equivalent to maintain systolic blood pressure >90 mm Hg, persisting or worsening respiratory failure defined by the presence of at least two criteria among the following: respiratory rate >40/min, lack of improvement of high respiratory muscle workload, severe hypoxemia defined as a need for FiO2 of 100% to maintain SpO2 ≥92% or PaO2/FiO2 ≤100 mm Hg, and acidosis defined as pH <7.35 units.
Outcomes
Primary outcome
The primary outcome is mortality at day 28 after randomisation.
Secondary outcomes
Secondary outcome variables include the following:
Mortality in ICU, in hospital, at day 90 and at day 180.
Intubation at day 28 from randomisation.
Length of stay in ICU and in hospital.
Number of ventilator-free days, and number of oxygenation techniques-free days within the 28 days following randomisation.
Tolerance of oxygenation techniques.
Sample size
We determined that inclusion of 280 analysable patients would provide a power of 80% to highlight an absolute difference of 15% in rate of mortality at day 28 after randomisation between the control group using HFOT with NIV (mortality rate estimated of 35%) and the intervention group using HFOT alone (mortality rate estimated of 20%). As NIV may be more effective according to type of immunosuppression, stratification will be performed in order to have the same number of patients with haematological malignancy, leucopenia or neutropenia induced by chemotherapy in each group.
Estimated rates of mortality in the two groups
The estimated mortality rates in the two groups are based on the recent literature. Mortality rates at day 28 reported in patients treated with HFOT and NIV are particularly homogeneous: 37% in a retrospective monocentre study,13 38% in a post hoc analysis of a randomised trial10 and 36% in our preliminary study.11 A lower mortality rate (24%) has been reported in patients treated with NIV in a randomised trial.6 However, this difference could be explained by the lower severity of respiratory failure at admission. According to our previous studies, we can estimate a mortality rate of 20% in the interventional group.10 11 A recent trial reported a mortality rate of 36% in patients treated with HFOT alone.29 However, a high proportion of patients died without prior intubation in the HFOT alone group (55 patients, 40%), that is, with a do-not-intubate order, and the actual mortality rate was closer to 25% after exclusion of these patients.
Recruitment
Initial expected duration of patient inclusion is 2 years, starting in January 2017.
End of 2015: grant award;
2016: approval by an independent ethics committee.
2017: inclusion of patients.
2019: end of inclusions, monitoring of participating centres and queries to investigators; overseeing by the steering committee at the REVA Network meetings; blind review to determine protocol violation, to define intention-to-treat and per-protocol analysis populations; new queries to investigators, cleaning and closure of the database.
2020–2021: data analysis, writing of the manuscript and submission for publication.
Methods: assignement of intervention, data collection, management and analysis
Allocation and sequence intervention
A computer-generated randomisation is performed with stratification according to centre and the type of immunosuppression (haematological malignancy or leucopenia <1 G/L or neutropenia ≤0.5 G/L vs the other types of immunosuppression) in a 1:1 ratio and by blocks, using a centralised web-based management system (Clinfile). After randomisation, the strategy assigned to the patient (HFOT alone or with NIV) will be initiated immediately.
Data collection and management
Data will be collected on an electronic-Case Report Form (e-CRF) by a trained investigator or research assistant at each centre (figure 2). At time of inclusion, the following data will be collected: informed consent, demographic characteristics, Charlson score,28 vital signs, current oxygenation settings (oxygen flow under standard oxygen, FiO2 and gas flow under HFOT, and FiO2, PS levels and PEEP under NIV), tolerance to oxygenation devices using a visual analogue scale, arterial blood gases and analysis of chest X-ray. Similar data and an evaluation of dyspnoea using a 5-point Likert scale will be recorded at H1, between H6 and H12, at H24 ±6 hours, H48 ±6 hours and H72 ±6 hours after randomisation. Duration of the first NIV session and total duration of NIV within the first 24 hours, between H24 and H48 and between H48 and H72 will be collected to ensure adherence to the protocol. The type of ventilator used for NIV and the NIV interface will be noted. For intubated patients, time and reason for intubation will be documented according to the above-mentioned criteria. Invasive ventilatory settings, arterial blood gases and chest X-ray will be recorded during the first 3 days following intubation. At day 28, vital status, need for intubation, total duration of invasive ventilation and of each oxygenation technique studied will be recorded. At ICU and hospital discharge, vital status and length of stay will be noted. At day 90 and day 180, vital status and Eastern Cooperative Oncology Group score will be recorded.30
Figure 2.
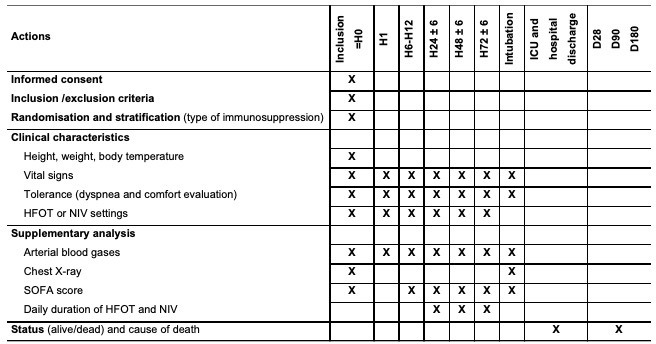
Flowchart of timing in collection of different variables. FiO2, fraction of inspired oxygen; HFOT, high-flow nasal oxygen therapy; ICU, intensive care unit; NIV, non-invasive ventilation; PaO2, PaO2 of arterial oxygen; PaCO2, PaO2 of arterial carbon dioxide; SpO2, peripheral capillary oxygen saturation.
As the absence of aetiology of acute respiratory failure could influence mortality,31 investigators are strongly encouraged to have an active diagnostic strategy. Results of the non-invasive diagnostic tests, bronchoalveolar lavage and chest CT-scan will be collected.32
Statistical methods
All the analyses will be performed by the study statistician according to a predefined statistical analysis plan and using statistical software (SAS V.9.4). A two-tailed p value of <0.05 will be considered as indicating statistical significance.
Descriptive analysis of patient groups at baseline
Continuous variables will be summarised with the classic parameters of descriptive analysis (median and interquartile ranges or mean and standard deviation), while indicating the number of missing data. Category variables will be presented in the form of absolute frequency and percentage in each modality. The analysis will be performed on an intention-to-treat basis, including all patients having undergone randomisation. Deviations from the protocol will be described and analysed on a case-by-case basis after validation by a blind review committee.
No imputation for missing values will be carried out.
Analysis pertaining to the main criteria of evaluation
Mortality rates at day 28 after randomisation will be compared between the two groups by means of a χ2 test. Analysis of this primary outcome will subsequently be completed by multivariate logistic regression after testing for interactions between treatment effect and strata. Survival time will be described by means of Kaplan-Meier method and compared with a log-rank test at day 28. A Cox proportional-hazards model will be used to calculate hazard ratio with 95% confidence interval.
Logistic and Cox regression maximal models will include all the variables associated with mortality at day 28 with a p value <0.20 in the univariate analysis. The final model will be obtained by a backward-selection procedure and will include variables significantly associated with mortality at day 28 with a p value of <0.05.
Analysis pertaining to the secondary criteria of evaluation
Length of stay, number of ventilator-free days and number of oxygenation technique-free days will be compared between the two treatment groups using the Student’s t-test (or Mann-Whitney test if necessary). Time to ICU death, time to hospital death or time to intubation will be described by means of the Kaplan-Meier method and compared between the two treatment groups with a log-rank test. Efficacy and tolerance of oxygenation techniques will be compared between the two groups using Student’s t-test (or Mann-Whitney test) for quantitative variables and χ2 test for qualitative variables. Ventilator-free days at day 28 will be calculated as one point for each day between inclusion to day 28 that patients are both alive and free of mechanical ventilation.
Per-protocol analysis
The proportion of patients treated according to the prespecified intervention goals will be calculated for each randomisation group. According to their sample size, their outcomes will be compared using the same methods as in the intention-to-treat analysis.
Predetermined subgroup analysis
Randomisation is stratified according to type of immunosuppression in order to have the same number of patients with haematological malignancy, leucopenia or neutropenia induced by chemotherapy in each group. A subgroup analysis will consequently be performed for the main and secondary criteria of evaluation in this subgroup of patients and in patients with another type of immunosuppression. Prior to adjustment, an interaction test will be carried out to detect heterogeneity of treatment effect according to type of immunosuppression.
As benefits of HFOT may be influenced by baseline PaO2/FiO2, a subgroup analysis will be performed for the main and secondary criteria of evaluation in patients with PaO2/FiO2≤200 mm Hg at inclusion.
Subgroup analysis will be performed according to:
Ancillary study
Data on nutrition practice in patients with acute respiratory failure is scarce.34 In voluntary participating centres, we have planned to collect nutrition practice. Therefore, in an ancillary study, we will describe daily nutritional intake from inclusion to day 28 or intubation or ICU discharge or death, type of nutrition, amount of calories intake, existence of complications related to nutrition and the reason for maintaining patient fasting.
Data monitoring
An investigator at each centre will be responsible for daily patient screening, enrolling patients in the study, ensuring adherence to the protocol and completing the e-CRF. Research assistants will regularly monitor all the centres on site to check adherence to the protocol and accuracy of the data recorded.
Patient and public involvement
Patients and public were not involved in the study
Study status
Current status: the last patient was included on 4 March 2019.
Expected date of complete data collection: mid-September 2019 (6-month follow-up of the last patient included).
Expected date of the end of monitoring of participating centres: December 2019.
Expected starting date of data analysis: first trimester 2020.
Ethics and dissemination
Consent or assent
Patients will be included after verification of the eligibility criteria and having provided an informed consent to the investigator according to the decision of the central ethics committee. For patients not able to provide informed consent, their next-of-kin will be contacted according to the same procedure. Patients will be informed as soon as possible by the investigator of their participation in the study and their consent to continue to participate in the study will be retrieved.
Confidentiality
Data will be handled according to French law. All original records will be archived at trial sites for 25 years. The clean database file will be deidentified and kept for 25 years.
Declaration of interest
The FLORALI-IM study is an investigator-initiated trial supported by the French Ministry of Health with funds obtained in 2015 from an inter-regional hospital clinical research programme (‘Programme Hospitalier de Recherche Clinique Inter-Régional 2015’). The European research network REVA has endorsed the study project. The study is promoted by the University Hospital of Poitiers. The study promoter has received a grant from AADAIRC and Le Nouveau Souffle. Fisher & Paykel Healthcare provides high-flow oxygen therapy equipment and face masks for NIV to all the participating centres but has no other involvement in the study.
Access to data
All investigators will have access to the final data set. Participant-level data sets will be made accessible on a controlled access basis.
Dissemination policy
Findings will be published in peer-reviewed journals and presented at local, national and international meetings and conferences to publicise and explain the research to clinicians, commissioners and service users.
Discussion
In immunocompromised patients, invasive ventilation is associated with particularly high mortality rates and application of NIV is currently recommended as a means of avoiding intubation.5 Almost 20 years ago, two randomised controlled trials including a small sample of patients reported decreased intubation and mortality rates with NIV as compared with standard oxygen therapy.7 8 By contrast, more recent studies including larger samples of patients have found either similar outcomes or even an increased risk of mortality in patients treated with NIV compared with oxygen alone.6 10 In a large controlled trial including 376 immunocompromised patients, outcomes were similar between patients treated with NIV and those treated with oxygen therapy.6 However, a high proportion of patients had mild respiratory failure, more than one-third of the patients in the control group received HFOT while those in the interventional group received short sessions of NIV, and all these factors together may have mitigated the difference between the two groups.6 In a post hoc analysis of a randomised controlled trial including 82 immunocompromised patients with severe acute hypoxemic respiratory failure, patients treated with HFOT alone had lower mortality than those treated with HFOT with NIV sessions.10
To explain the lack of effect or harmful effects of NIV, it could be argued that NIV was not carried out with optimal ventilator settings for patients of whom the majority met the clinical criteria for ARDS. Indeed, they had particularly large tidal volumes under NIV, which could be associated with increased risk of mortality by potential worsening of pre-existing lung injury.15–17 35 36 PEEP levels remained relatively low whereas the treatment represents a major adjustment in ARDS patients, and NIV was applied for a duration of only 8 hours in mean within the first 24 hours.6 10 Another study has found that NIV performed with helmet may be more efficient than with face mask.33 Interestingly, patients treated with helmet also received lower PS levels and higher PEEP levels than those treated with facemask, thereby highlighting the potential impact of ventilatory settings on outcomes.33 Consequently, we decided to apply a protective NIV protocol aiming at avoiding large tidal volumes, and applying prolonged sessions of NIV with high PEEP levels.
In a recent large randomised controlled trial including 776 immunocompromised patients, mortality rates at day 28 did not differ between patients treated with HFOT and those treated with standard oxygen.29 However, 40% of the deceased patients in the HFOT group died without prior intubation and the high proportion of patients with do-not-intubate order may have mitigated the beneficial effects of HFOT.29 By contrast, several studies have reported promising results of HFOT alone versus standard oxygen or NIV in patients with de novo respiratory failure, even in immunocompromised patients.10–12 14
The FLORALI-IM trial has several strengths. First, it will be the first study comparing HFOT alone versus HFOT with NIV sessions in immunocompromised patients. Second, the study will include only patients with severe acute hypoxemic respiratory failure. Third, NIV will be optimised using low levels of PS targeting a tidal volume between 6 and 8 mL/kg, PEEP levels of at least 8 cm H2O and duration of NIV >12 hours a day during the first 48 hours.
In conclusion, the FLORALI-IM trial is an investigator-initiated randomised controlled trial empowered to test the hypothesis that HFOT alone may in comparison with HFOT and NIV decrease mortality rate at day 28 of immunocompromised patients admitted to ICU for acute respiratory failure. Innovative aspects include the two groups of treatment in this clinical setting and the optimised protocol to carry out NIV and HFOT.
Supplementary Material
Footnotes
Contributors: RC, J-PF and AWT, in collaboration with SE, FP, NT, MD, GP, CG, DC, JB, AG, CG, JD, DM, GL, J-PQ, AH, SJ, JD, DB, EV, SN, GC, DT, GG, MA, CG, DB, TL, AK, SR and the REVA Network designed the study and wrote the manuscript together. SR provided substantial contributions to the conception and design of the study, and wrote the statistical analysis plan and estimated the sample size with RC. RC, J-PF, SE, FP, NT, MD, GP, CG, DC, JB, AG, CG, JD, DM, GL, J-PQ, AH, SJ, JD, DB, EV, SN, GC, DT, GG, MA, CG, DB, TL, AK, SR, and AWT contributed in drafting the work, revising it critically for important intellectual content and approved the final version of the manuscript. RC, J-PF, SE, FP, NT, MD, GP, CG, DC, JB, AG, CG, JD, DM, GL, J-PQ, AH, SJ, JD, DB, EV, SN, GC, DT, GG, MA, CG, DB, TL, AK, SR, and AWT give their agreement to be accountable for all aspects of the work, and ensure the accuracy and integrity of any part of the work.
Funding: The study was funded by the ‘Programme Hospitalier de Recherche Clinique InterRégional 2015’ of the French Ministry of Health. The study promoter is the University Hospital of Poitiers, Poitiers, France.
Disclaimer: The firm Fisher & Paykel provided the high-flow oxygen therapy equipment and masks for non-invasive ventilation to all the participating centres but has no other involvement in the study.
Competing interests: RC reports travel expense coverage to attend scientific meetings from Fisher & Paykel and MSD. JPF reports travel expense coverage to attend scientific meetings and personal fees from Fisher & Paykel and SOS Oxygène. SE reports consulting fees from Aerogen, La diffusion technique française, Baxter, Bayer, lecture fees from Aerogen, Fisher & Paykel, unrestricted research grants / research support from from Fisher & Paykel, Hamilton medical, Aerogen, La diffusion technique française. Chr G reports travel expense coverage to attend scientific meetings, personal fees and logistic support from Fisher & Paykel, Resmed and Lowenstein Medical. AWT reports travel expense coverage to attend scientific meetings and payment for lectures from Fisher & Paykel, Covidien, Maquet-Getinge, General Electric Healthcare. SJ reports personal fees for lectures from Hamilton Medical and Nihon Kohden. GG reports payment for lectures from Getinge, Draeger Medical, Pfizer, Fisher&Paykel, and travel / accommodation/congress registration support from Biotest and Getinge.
Patient consent for publication: Not required.
Ethics approval: The first version of the study protocol has been approved by the central ethics committee (Ethics Committee Ouest III, Poitiers, France) with the registration number 2016-A00834-47 (23 March 2016).
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Azoulay E, Mokart D, Pène F, et al. Outcomes of critically ill patients with hematologic malignancies: prospective multicenter data from France and Belgium—A Groupe de Recherche Respiratoire en Réanimation Onco-Hématologique study. J Clin Oncol 2013;31:2810–8. 10.1200/JCO.2012.47.2365 [DOI] [PubMed] [Google Scholar]
- 2. Azoulay E, Pickkers P, Soares M, et al. Acute hypoxemic respiratory failure in immunocompromised patients: the Efraim multinational prospective cohort study. Intensive Care Med 2017;43:1808–19. 10.1007/s00134-017-4947-1 [DOI] [PubMed] [Google Scholar]
- 3. Gristina GR, Antonelli M, Conti G, et al. Italian group for the evaluation of interventions in intensive care medicine). noninvasive versus invasive ventilation for acute respiratory failure in patients with hematologic malignancies: a 5-year multicenter observational survey. Crit Care Med 2011;39:2232–9. [DOI] [PubMed] [Google Scholar]
- 4. Cortegiani A, Madotto F, Gregoretti C, et al. Immunocompromised patients with acute respiratory distress syndrome: secondary analysis of the lung safe database. Critical Care 2018;22 10.1186/s13054-018-2079-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rochwerg B, Brochard L, Elliott MW, et al. Official ERS/ATS clinical practice guidelines: noninvasive ventilation for acute respiratory failure. Eur Respir J 2017;50 10.1183/13993003.02426-2016 [DOI] [PubMed] [Google Scholar]
- 6. Lemiale V, Mokart D, Resche-Rigon M, et al. Groupe de Recherche en Réanimation Respiratoire du patient d’Onco-Hématologie (GRRR-OH). Effect of Noninvasive Ventilation vs Oxygen Therapy on Mortality Among Immunocompromised Patients With Acute Respiratory Failure: A Randomized Clinical Trial. JAMA 2015;314:1711–9. [DOI] [PubMed] [Google Scholar]
- 7. Hilbert G, Gruson D, Vargas F, et al. Noninvasive ventilation in immunosuppressed patients with pulmonary infiltrates, fever, and acute respiratory failure. N Engl J Med 2001;344:481–7. 10.1056/NEJM200102153440703 [DOI] [PubMed] [Google Scholar]
- 8. Antonelli M, Conti G, Bufi M, et al. Noninvasive ventilation for treatment of acute respiratory failure in patients undergoing solid organ transplantation. JAMA 2000;283:235–41. 10.1001/jama.283.2.235 [DOI] [PubMed] [Google Scholar]
- 9. Squadrone V, Massaia M, Bruno B, et al. Early CPAP prevents evolution of acute lung injury in patients with hematologic malignancy. Intensive Care Med 2010;36:1666–74. 10.1007/s00134-010-1934-1 [DOI] [PubMed] [Google Scholar]
- 10. Frat J-P, Ragot S, Girault C, et al. Effect of non-invasive oxygenation strategies in immunocompromised patients with severe acute respiratory failure: a post-hoc analysis of a randomised trial. Lancet Respir Med 2016;4:646–52. 10.1016/S2213-2600(16)30093-5 [DOI] [PubMed] [Google Scholar]
- 11. Coudroy R, Jamet A, Petua P, et al. High-Flow oxygen therapy through a nasal cannula versus noninvasive ventilation versus in immunocompromised patients with acute respiratory failure. Ann Intensive Care 2016;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sklar MC, Mohammed A, Orchanian-Cheff A, et al. The impact of high-flow nasal oxygen in the immunocompromised critically ill: a systematic review and meta-analysis. Respir Care 2018;63:1555–66. 10.4187/respcare.05962 [DOI] [PubMed] [Google Scholar]
- 13. Mokart D, Geay C, Chow-Chine L, et al. High-Flow oxygen therapy in cancer patients with acute respiratory failure. Intensive Care Med 2015;41:2008–10. 10.1007/s00134-015-3994-8 [DOI] [PubMed] [Google Scholar]
- 14. Frat J-P, Thille AW, Mercat A, et al. High-Flow oxygen through nasal cannula in acute hypoxemic respiratory failure. N Engl J Med 2015;372:2185–96. 10.1056/NEJMoa1503326 [DOI] [PubMed] [Google Scholar]
- 15. Carteaux G, Millán-Guilarte T, De Prost N, et al. Failure of noninvasive ventilation for de novo acute hypoxemic respiratory failure. Crit Care Med 2016;44:282–90. 10.1097/CCM.0000000000001379 [DOI] [PubMed] [Google Scholar]
- 16. Frat J-P, Ragot S, Coudroy R, et al. Predictors of intubation in patients with acute hypoxemic respiratory failure treated with a noninvasive oxygenation Strategy*. Crit Care Med 2018;46:208–15. 10.1097/CCM.0000000000002818 [DOI] [PubMed] [Google Scholar]
- 17. Brochard L, Slutsky A, Pesenti A. Mechanical ventilation to minimize progression of lung injury in acute respiratory failure. Am J Respir Crit Care Med 2017;195:438–42. 10.1164/rccm.201605-1081CP [DOI] [PubMed] [Google Scholar]
- 18. Briel M, Meade M, Mercat A, et al. Higher vs lower positive end-expiratory pressure in patients with acute lung injury and acute respiratory distress syndrome. JAMA 2010;303:865–73. 10.1001/jama.2010.218 [DOI] [PubMed] [Google Scholar]
- 19. Morais CCA, Koyama Y, Yoshida T, et al. High positive end-expiratory pressure renders spontaneous effort Noninjurious. Am J Respir Crit Care Med 2018;197:1285–96. 10.1164/rccm.201706-1244OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sehgal IS, Dhooria S, Agarwal R. High-Flow nasal cannula oxygen in respiratory failure. N Engl J Med 2015;373. [DOI] [PubMed] [Google Scholar]
- 21. Nava S, Navalesi P, Conti G. Time of non-invasive ventilation. Intensive Care Med 2006;32:361–70. 10.1007/s00134-005-0050-0 [DOI] [PubMed] [Google Scholar]
- 22. Ards definition Task force, Ranieri VM, Rubenfeld Gd, thompson Bt, Ferguson Nd, Caldwell E, FAN E, Camporota L, Slutsky as. acute respiratory distress syndrome: the Berlin definition. JAMA 2012;307:2526–33. [DOI] [PubMed] [Google Scholar]
- 23. Rubin LG, Levin MJ, Ljungman P, et al. 2013 IDSA clinical practice guideline for vaccination of the immunocompromised host. Clinical Infectious Diseases 2014;58:e44–100. 10.1093/cid/cit684 [DOI] [PubMed] [Google Scholar]
- 24. SPLF Conference de consensus commune 2006 VNI. Available: https://www.srlf.org/wp-content/uploads/2015/12/2006_10_12_conference_de_consensus_commune_ventilation_non_invasive_resume.pdf [Accessed 11 Oct 2018].
- 25. Coudroy R, Hoppe MA, Robert R, et al. Influence of the noninvasive ventilation protocol on Intubations rates in patients with de novo acute respiratory failure: a systematic review of randomized trials. Ann Intensive Care 2019;9(Suppl 1). [Google Scholar]
- 26. Esteban A, Frutos-Vivar F, Ferguson ND, et al. Noninvasive positive-pressure ventilation for respiratory failure after extubation. N Engl J Med 2004;350:2452–60. 10.1056/NEJMoa032736 [DOI] [PubMed] [Google Scholar]
- 27. Carrillo A, Gonzalez-Diaz G, Ferrer M, et al. Non-Invasive ventilation in community-acquired pneumonia and severe acute respiratory failure. Intensive Care Med 2012;38:458–66. 10.1007/s00134-012-2475-6 [DOI] [PubMed] [Google Scholar]
- 28. Kang BJ, Koh Y, Lim C-M, et al. Failure of high-flow nasal cannula therapy may delay intubation and increase mortality. Intensive Care Med 2015;41:623–32. 10.1007/s00134-015-3693-5 [DOI] [PubMed] [Google Scholar]
- 29. Azoulay E, Lemiale V, Mokart D, et al. Effect of high-flow nasal oxygen vs standard oxygen on 28-day mortality in immunocompromised patients with acute respiratory failure. JAMA 2018;320 10.1001/jama.2018.14282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the eastern cooperative Oncology Group. Am J Clin Oncol 1982;5:649–56. 10.1097/00000421-198212000-00014 [DOI] [PubMed] [Google Scholar]
- 31. Contejean A, Lemiale V, Resche-Rigon M, et al. Increased mortality in hematological malignancy patients with acute respiratory failure from undetermined etiology: a Groupe de Recherche en Réanimation Respiratoire en Onco-Hématologie (Grrr-OH) study. Ann Intensive Care 2016;6:102 10.1186/s13613-016-0202-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Azoulay E, Mokart D, Lambert J, et al. Diagnostic strategy for hematology and oncology patients with acute respiratory failure: randomized controlled trial. Am J Respir Crit Care Med 2010;182:1038–46. 10.1164/rccm.201001-0018OC [DOI] [PubMed] [Google Scholar]
- 33. Patel BK, Wolfe KS, Pohlman AS, et al. Effect of noninvasive ventilation delivered by helmet vs face mask on the rate of endotracheal intubation in patients with acute respiratory distress syndrome. JAMA 2016;315:2435–41. 10.1001/jama.2016.6338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Terzi N, Darmon M, Reignier J, et al. Initial nutritional management during noninvasive ventilation and outcomes: a retrospective cohort study. Crit Care 2017;21 10.1186/s13054-017-1867-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bellani G, Laffey JG, Pham T, et al. Noninvasive ventilation of patients with acute respiratory distress syndrome. insights from the lung safe study. Am J Respir Crit Care Med 2017;195:67–77. 10.1164/rccm.201606-1306OC [DOI] [PubMed] [Google Scholar]
- 36. Slutsky AS, Ranieri VM, injury V-inducedlung. Ventilator-Induced lung injury. N Engl J Med 2013;369:2126–36. 10.1056/NEJMra1208707 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


