Abstract
This article serves as a brief primer on planaria for behavior scientists. In the 1950s and 1960s, McConnell’s planarian laboratory posited that conditioned behavior could transfer after regeneration, and through cannibalization of trained planaria. These studies, the responses, and replications have been collectively referred to as the “planarian controversy.” Successful behavioral assays still require refinement with this organism, but they could add valuable insight into our conceptualization of memory and learning. We discuss how the planarian’s distinctive biology enables an examination of biobehavioral interaction models, and what behavior scientists must consider if they are to advance behavioral research with this organism. Suggestions for academics interested in building planaria learning laboratories are offered.
Electronic supplementary material
The online version of this article (10.1007/s40614-018-00176-w) contains supplementary material, which is available to authorized users.
Keywords: Planaria, Invertebrate learning, Behavior analysis, Conditioning, Memory
Nonhuman animal research contributes substantially to understanding human behavior (see Lattal, 2001), and continues to inform theory and clinical treatment (American Psychological Association [APA], 2017). Planaria are organisms with a unique research history, and provide a model for examining complex learning within their simple behavioral repertoire. However, species used in research appear to be selected based on convention, or ease of access. At first, pigeons were selected to emphasize that mammalian brains and associated cognitive processes were not required to demonstrate complex behavior (e.g., Epstein, 1981), but they have remained a conventional research model. The American Psychological Association (APA) noted that 90% of nonhuman psychology research uses rodents and avians (APA, n.d.). Focusing on the “white rat” to the exclusion of other species minimizes the breadth of scientific discovery (Breland & Breland, 1961).
Traditional behavior science uses a limited variety of species in research, potentially leaving undiscovered biological mechanisms and phylogenic contingencies particular to each species (Breland & Breland, 1961; Skinner, 1966). Zimmermann, Watkins, and Poling (2015) reviewed animal subjects in studies published in the Journal of the Experimental Analysis of Behavior (JEAB) between 1958 and 2013. The most common subjects were avians (47.9% pigeons), rats (24.2%), humans (17%), nonhuman primates (7.5%), and other (5.2%).1 The majority in the “other” category comprise vertebrates like fish and reptiles. Invertebrate species accounted for 0.13% of research studies that appeared in JEAB between 1958 and 2007 (Sokolowski, Disma, & Abramson, 2010). Despite invertebrates dominating a disproportionate amount of the animal kingdom (almost 95%), they are on the fringe of research efforts in psychology (Bitterman, 1965; Corning & Lahue, 1972; McConnell, 1966). Contrary to anthropocentric opinion, a spine is not a prerequisite for neural complexity, nor is one required to confer insight into human behavior.
Invertebrate research is worthwhile for many reasons. Such research is inexpensive relative to vertebrate models, and the care and maintenance of the animals is less labor intensive. In addition, institutions utilizing an institutional animal care and use committee (IACUC) typically waive regulatory oversight for invertebrate use in research and teaching activities (regardless, all activities should be ethically conducted). Numerous species like aplysia (Downey & Jahan-Parwar, 1972), blow flies (Sokolowski et al., 2010), cockroaches (Dixon, Daar, Gunnarsson, Johnson, & Shayter, 2016), green crabs (Abramson & Feinman, 1990), honeybees (Grossmann, 1973), and planaria (Shomrat & Levin, 2013) have been used in operant research, and far more have been used in the respondent literature. Skinner, known for work with pigeons, also conducted research with ants (Barnes & Skinner, 1930).
The purpose of this article is to be a primer on planaria behavior. We start by introducing the organism, then cover classic behavior science research on planaria and its implications, then finally we discuss what can be learned by integrating this organism in modern behavior science. There are several reasons to seriously consider the experimental analysis of planarian behavior. First, although the evolutionary lineages of planaria and humans diverged long ago, the neurobiology of a planarian is surprisingly similar to that of vertebrates, and it is considered one of the first organisms to have a “true brain” (Pagán, 2014). Second, due to their ability to regenerate after being dissected there are many interesting research questions for experimental analysis. Consider that it may be possible to train a planarian to engage in a target behavior, then dissect the planarian and determine if the head or tail segment retained prior training. Lastly, Katz (1978) proposed many years ago that planaria would be useful in university laboratories, and they continue to be recommended for that purpose despite being rarely used (Chicas-Mosier & Abramson, 2015).
Introducing the Planarian
Planaria belong to the phylum Platy-helminthes, which translates to “flat-worm.” Figure 1 depicts the basic anatomical layout of the planarian. Sizes vary by species, where some are as short as 1 millimeter in length, others as long as 90 millimeters (Pagán, 2014). Most planaria species are found in freshwater (Reddien & Alvarado, 2004), but marine and terrestrial species exist (Pagán, 2014). Aquatic species use cilia and tail motion to glide in water. Planaria are sensitive to many stimuli including: chemical gradients, texture, vibration, electric fields, weak gamma radiation, magnetic fields, light (Nicolas, Abramson, & Levin, 2008), and gravity (Adell, Saló, Van Loon, & Auletta, 2014), so it is essential to minimize background interference.
Fig. 1.
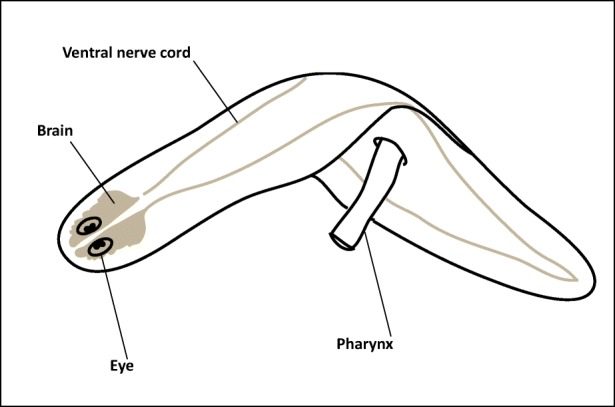
Simplified anatomical depiction of the planarian CNS. The anterior contains the head, brain and eyes; the middle comprises the abdominal trunk and pharynx. Ingested nutrients diffuse to the rest of the body
Feeding in the Planaria
Evasion and hunting patterns, potentially mediated by activity or ability to adhere to surfaces, vary by species (Best, 1960). Although most flat worms are parasitic, planaria are opportunistic predators and scavengers (Pagán, 2014), preying predominantly upon small insects, larvae, and other invertebrates (Reddien & Alvarado, 2004). Freshwater planaria commence the feeding process with a light investigatory head touch to the food, then the head is retracted. Finally, the planarian quickly wraps around and latches on the food (Best, 1960). Chemical signals in the water play a major role in localizing food. Planaria are also attracted to live prey that produce disturbances in the water (Reynoldson & Young, 1963). They leave a mucus trail on surfaces they contact, which assists in surface adherence and capturing prey (Bocchinfuso et al., 2012). In research, planaria have displayed a preference for struggling prey (trapped with petroleum jelly) compared to inert controls (Reynoldson & Young, 1963). After ingesting food, locomotion and responsivity to stimuli is much reduced. If stressed sufficiently after eating, planaria will expel previously ingested food enabling more efficient evasion. Latency to feed increases in the presence of novel stimuli, often requiring a habituation period prior to eating (Best & Rubinstein, 1962). Planaria can be attracted to water movement without the presence of prey (Allen, 1915). This is possibly a discrimination issue, that is to say planaria may “confuse” the movement with presence of prey.
In laboratory conditions planaria can thrive on raw liver or brine shrimp, but they will eat almost any animal tissue readily (Jenkins, 1967). Muscle tissue is reportedly not as effective for maintaining planarian growth (McConnell, 1967). Freezing is standard for laboratory feeding, but there is a slight reduction in the growth factor compared to fresh samples (McConnell, 1965).
Locomotion in the Planaria
In general, planaria avoid open spaces (Talbot & Schötz, 2011) and demonstrate a preference for container walls (Akiyama, Agata, & Inoue, 2015). Two-dimensional tracking is usually sufficient to characterize locomotion of planaria. In laboratory environments the upward lifting of the head is minimized due to the shallow depth (1 cm) of the water in petri dishes, and because this action is usually made in response to food or obstacles encountered (Talbot & Schötz, 2011). S. mediterranea average speeds up to 2mms-1, which is about 10 times faster when compared to other invertebrates like C. elegans (Talbot & Schötz, 2011). Speed has been reported to be stable within size differences of 1–2.6 cm between planaraia (Raffa, Holland, & Schulingkamp, 2001). There are several dimensions of movement one could measure, such as velocity, time immobile, the number and direction of turns, contractions not resulting in direction changes, and worm paths depicting density of locations frequented. The head region, containing the brain, is important in movement and orientating toward or away from stimuli. If the head is separated it will move away from the less mobile headless body (Reddien & Alvarado, 2004).
When a planarian is placed in a novel environment, there is an initial exploratory phase. After 5–30 minutes alone in a petri dish, a planarian will become immobile and “scrunch” their head into their body. This action can also be elicited by physically touching the planarian’s head and possibly confers some protection to the brain region. While scrunched up, a planarian may be less sensitive to stimulation that would previous elicit or evoke a response. Researchers have opted to poke, shock, or pipette planaria to locomotion. To purposefully immobilize planaria anesthetizing agents, chilled water, or thicker mediums to slow movement can be used (Dexter, Tamme, Lind, & Collins, 2014). Researchers developed a chip that renders a planarian immobile without side-effects (Dexter et al., 2014). After dissection, both head and tail segments will typically stay close to concave curves of outer walls of containers and from this position they are less likely to engage in head turning unless out in the open (Akiyama et al., 2015).
Drug Effects in Relation to Movement
The planaria central nervous system (CNS) contains nearly every neurotransmitter found in mammalian vertebrates, and for this reason they have been used extensively pharmacological research (Nicolas et al., 2008). Planaria are administered pharmacological agents by bathing the worm in a drug solution. Ethanol mixed in a 3% in water solution has been shown to suppress locomotor activity in Schmidtea mediterranea (3–6 mm in size) with no permanent decline in their regenerative ability, even after repeated exposure (Stevenson & Beane, 2010). Acute exposure to stimulants does not always result in increased locomotion, possibly because planaria move at their top speeds. Stimulant withdrawal can result in decreases in movement (Ramoz et al., 2012). Mephedrone, cocaine, and nicotine have been shown to induce stereotypic contractions, referred to as screw-like or “C-like” hyperkinesias (Pagán et al., 2013; Raffa et al., 2001; Ramoz et al., 2012). Cocaine requires an intact brain to induce contractions, because decapitated segments do not contract, whereas nicotine induces this behavior in the head or headless segments (Pagán et al., 2013). It should be noted that planarian size should be reported because this could affect the pharmacokinetics of drug metallization.
Sexual Behavior
Planaria can reproduce asexually by fission, where a worm divides into two or more sections, with each piece regenerating to form genetic clones. Dissection imitates asexual fission, which occurs naturally when a planarian gets larger or is exposed to stressors. Some describe this process as potentially resulting in an “immortal life-history” for planaria (Sahu, Dattani, & Aboobaker, 2017). Certain species engage in sexual reproduction as cross-fertilizing hermaphrodites (Reddien & Alvarado, 2004), laying cocoons that give birth to hatchlings (Sahu et al., 2017). Sexuality reportedly can be induced in some planaria by exposing them to colder temperatures then returning them to room temperatures (Jenkins, 1967). Sex allocation in some flatworms can be determined by “penis fencing,” where partners stab one another until injecting enough sperm to assign one as female (Ramm, 2016).
Group and Species Behavior
After acclimatizing to an environment, planaria of different species cluster together (Reynierse, 1967; Reynierse & Ellis, 1967; Reynierse, Gleason, & Ottemann, 1969). Planaria engage in mutually protective behaviors such as crowding together under ultraviolet stimulation, which confers some protection from the harmful effects of the radiation (Allee & Wilder, 1939). Certain species are more likely to engage in cannibalization of other species. For example, D. tigrina will attack C. foremani at night and when starved, but attacks do not occur in the other direction even though C. foremani predates on larger more active prey like the mosquito wriggler (Best, 1960). Housing species separately could prevent cannibalization and transference of diseases between colonies.
Species Used in Research
Biologists interested in behavior have studied several species of planaria. Dugesia japonica, Schmidtea mediterranea, and Girardia tigrina are often preferred models (Auletta, Adell, Colagè, D’Ambrosio, & Salò, 2012). S. mediterranea (see Fig. 2) have been genomically sequenced, making them an attractive option for molecular biologists (Nicolas et al., 2008), and D. japonica are sometimes favored for behavioral experiments because they are highly active and appear to adapt well to training paradigms (Shomrat & Levin, 2013).
Fig. 2.
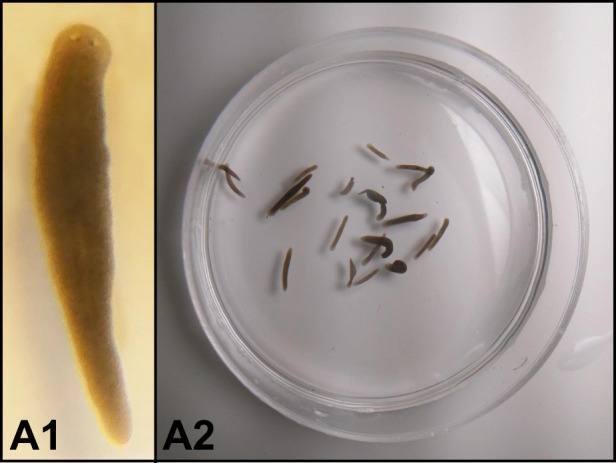
A1 depicts a single 10 mm planarian (S. mediterranea) at 12x magnification. A2 depicts a group of S. mediterranea on a chilled plate to slow movement for image capturing
Classic Behavior Science Research
The planarian brain is actively involved in avoidance paradigms, satiation reflexes, and mating behavior (Egger, Gschwentner, & Rieger, 2007). The planaria can perfectly regenerate all elements of its morphology, including its head and brain. Indeed, these regenerative abilities exceed many other animal regeneration capabilities including the axetlotl, spiny mouse, and zebrafish (Newmark & Alvarado, 2002). Researchers have been curious what learning, if any, from a trained planaria transfers to the head or tail portions after dissection. Behavior science has developed numerous learning models, and the planarian could be the key to unlocking what physiological changes relate to different psychological learning processes. Unfortunately, the planarian behavioral training literature contains many controversial findings and misinterpretations. Behavior science as a field is positioned to offer guidance where there has been a failure to understand crucial elements in the conditioning literature.
Respondent Conditioning
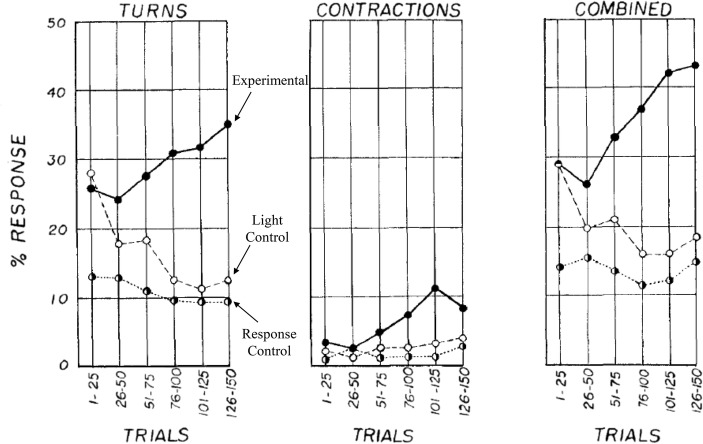
The “planarian controversy” stems from early classical conditioning experiments using light and shock pairings with turns and contractions (Corning & Riccio, 1970). Thompson and McConnell (1955) attempted to condition turns and contractions in G. dorotocephala using a group design. The conditioned stimulus (CS) was light, and the unconditioned stimulus (US) was shock. In the experimental group, light was presented for 3 s, with shock overlapping the final 1 s. To determine if conditioning occurred number of turns and contractions were examined by comparing three control groups, one where light was presented in the same manner but without shock (light control [LC]), one with no experimental stimuli (response control [RC]) and one in which shock was presented the same number of times as the experimental group (shock control [SC]). Figure 3 depicts turns and contractions LC or RC groups’ decreased in frequency or were stable, but the experimental group showed response increases, supporting the premise that conditioning occurred. The SC group was used to ascertain if shock sensitized responding to light. Thompson and McConnell (1955) noted minimal differences between the first and last 15 trials. McConnell, Jacobson, and Kimble (1959) extended this earlier work by dissecting the worms post training, and examining learning in regenerated segments. A response control group received no directed training, but they were also cut in half to eliminate the possibility that the act of dissection sensitized their responsiveness to shock or light. Their results offered some support that conditioning survived the regeneration process, surprisingly for both the head and tail sections (McConnell et al., 1959). However, critiques included that the study was not well-controlled (Halas, James, & Knutson, 1962).
Fig. 3.
Percentage of turns and contractions of control and conditioned worms. Adapted from Thompson and McConnell (1955)
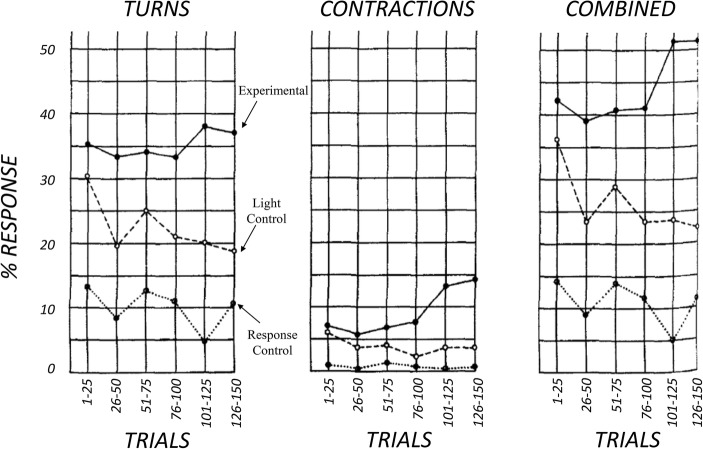
Halas et al. (1962) replicated Thompson and McConnell’s study. Halas et al. found smaller differences between experimental and LC groups than in Thompson and McConnell’s original study, but otherwise noted that their data were similar. Halas et al. suggested that light actually served as a weak US for turns or contractions, and that the results of the experimental group could be better explained through sensitization. Halas et al. (1962) were correct in identifying light as a weak US, however, they were incorrect in their interpretation of sensitization. Visual analysis of their results (see Fig. 4) support the notion that conditioning occurred, specifically the experimental group data are steady or upward trending, whereas control data are clearly trending down. An examination of both Halas et al. and Thompson and McConnell’s data show that there was no case for sensitization, because in the SC groups there were decreasing turns with continued trials, and little to no differences in contractions. Halas et al. (1962) had used null-hypothesis significance testing (NHST) to conclude differences between the experimental group and controls were not significant, despite the clear visual analysis of data (It should be note that NHST in psychology is deeply problematic [Branch, 2014]). The social context of the science at the time is fascinating, and several records are available indicating that McConnell was a controversial figure in science, and potentially this could have motivated others to discredit his findings (e.g., Duhaime-Ross, 2015).
Fig. 4.
Percentage of turns and contractions of control and conditioned worms. Adapted from Halas et al. (1962)
Halas et al. (1962) surmised responding was a product of some process other than conditioning. Arguments against conditioning were presented two ways. Either the shock sensitized responding to light by altering the planarian’s physiological response to any stimuli (as noted above, data sets from SC groups across the studies do not support this interpretation). Pseudo-conditioning was presented as the alternative argument, because potentially random presentations of light and shock stimuli could result in similar results to the experimental groups in the classic studies (Travis, 1981). Baxter and Kimmel (1963) added an unpaired control group to Thompson and McConnell’s (1955) procedure, which received the same light or shock stimuli as the experimental group except they were separated by 15 s. Conditioned contractions increased over time for the experimental group and decreased for the unpaired group, but differences between the groups disappeared quickly during extinction. Jacobson, Horowitz, and Fried (1967) established that the same light-shock forward pairing resulted in higher responses compared to backward or simultaneous presentations. Both studies substantially weakened pseudo-conditioning and sensitization as potential explanations for Thompson and McConnell’s (1955) results (Rilling, 1996).
The planarian controversy culminated when McConnell (1962) posited that respondent learning could transfer from trained to untrained planaria through cannibalization. The study included a lack of contemporaneous controls related to the biology of the organism (i.e., no cannibalized shock alone or cannibalized light alone controls were used) leading critics to quickly attribute results to again rely on pseudo-conditioning or sensitization to refute the results, rather than “memory transfer” (Walker, 1966; Walker & Milton, 1966). Much of this research was denigrated as folklore within psychology, and largely forgotten (Duhaime-Ross, 2015). Since then, molecular experiments examining RNA interference have offered support for McConnell’s results and interpretation (Smalheiser, Manev, & Costa, 2001).
Operant Responding
A majority of behavioral research with planaria has used classical conditioning procedures (Shomrat & Levin, 2013), but there have been operant conditioning experiments (Crawford & Skeen, 1967; Chicas-Mosier & Abramson, 2015; Krantz, 1964; Lee, 1963; Wells, Jennings, & Davis, 1966). Lee (1963) attempted to condition a free operant response. When a planarian passed through a photoelectric cell beam, this response was recorded and reinforced by the termination of the aversive stimulus of light (a negative reinforcement procedure). In an extinction phase, the rate of passing through the beam decreased to near zero levels. Krantz (1964) and Crawford and Skeen (1967) successfully replicated Lee’s operant responding study, also with yoked controls. Although results were clear, Halas (1963) critiqued that passing through the beam may not have been an operant response, suggesting it was an artifact of turning the light on. This would have resulted in increased locomotor activity that altered the probability that the planarian would accidentally touch the beam, and subsequently turn off the light source. After a period of activity planaria usually reduce movement particularly in the dark, thus resulting in the planaria already being closer to the photobeam, and more likely to turn it off. Halas (1963) aptly debated whether this was in fact an instrumental response, but with Skinnerian conceptualizations of operant behavior this distinction becomes more difficult. We will return to the necessity and validity of the operant–respondent distinction below.
Chicas-Mosier and Abramson (2015) reported a procedure for shaping planaria to move longer distances along the edge of a half petri dish to seek water reinforcement. This was based on early maze work where water was used as reinforcement (Best, 1965) combined with Skinnerian shaping. This procedure was robust in design demonstrating that controls do not travel as fast without the training procedure.
The slime trails left by planaria can serve as cues in maze learning based on a preference for “slimed areas” (McConnell, 1966). A simple preparation reducing planaria handling and the need for hand shaping is the modified Van Oye Maze, which will be discussed in more detail in the section “Opportunities for University Learning Labs,” below (Wells et al., 1966).
Operant–Respondent Distinction
Within behavior science, even with traditional animal models, there are challenges to distinguish between operant and respondent processes. The overlapping interactive effects in processes such as autoshaping, differential outcomes effect, unsignaled avoidance, adjunctive schedule induced behavior, and conditioned suppression lead to these challenges (Pear & Eldridge, 1984). Theorists using biobehavioral models have concluded that operant and respondent conditioning are one process with different procedures (e.g., Donahoe & Palmer, 2004). The procedural definitions have been based on structural ordering of events related to behavior, but these purely behavioral definitions can be (and have been) greatly improved with biological data (Fox, 2018). In a classic example, biobehavioral data have shown that respondent processes in food aversion are enduring and do not require immediate pairing (Garcia, Kimeldorf, & Koelling, 1955). Biological sources of information can inform the discussion as to whether theoretically it is worthwhile to consider conditioning separate or interlocking processes (Stein, 1997). Maintaining the operant–respondent distinction may largely be for the sake of established procedures in applied behavior analysis and for instructional ease when introducing these concepts.
Integrating Planaria with Modern Behavior Science
Inheritance of Behavioral Traits
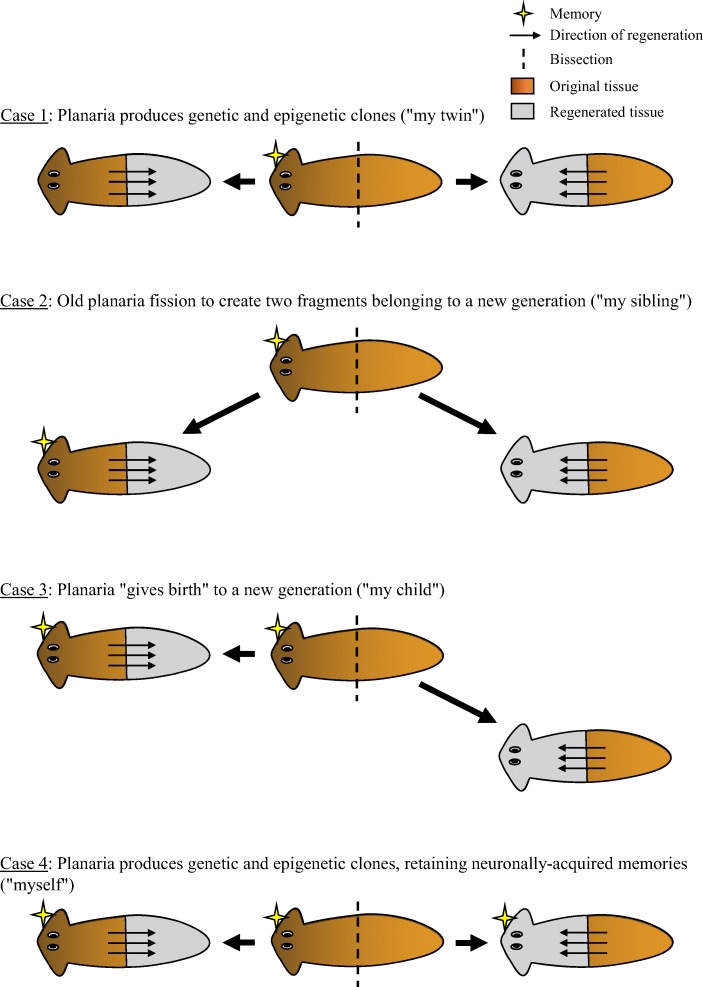
The planarian’s regenerative powers are being investigated to defy the aging process and unlock regeneration for humans (Sahu et al., 2017). Planarian regeneration is also of interest as to what facilitates memory and learning. Learning to approach and eat food under aversive lighting retains after dissection to both tail and head segments (Shomrat & Levin, 2013). Retention of eating behavior could reflect, like taste aversion, that there is a biobehavioral interplay related to survival (Garcia et al., 1955). Retention of training of an arbitrary operant behavior has yet to be demonstrated. It is interesting that biologists have also noted that conditioned avoidance retains from a larva to moth, a process that is characterized by substantial morphological reorganization (Blackiston, Silva Casey, & Weiss, 2008). The implication is, from this and planaria research, that there are biological mechanisms for conditioning that do not require repeated experience even after such substantial morphological changes. If this is the case, it could defy many of the widely held assumptions regarding learning and inheritance (Neuhof, Levin, & Rechavi, 2016). In essence, it is unknown whether all neuronally encoded learning from the host is regenerated in the asexual cloning process. Neuhof et al. (2016) describe four cloning case scenarios in a hypothesis paper presented in Fig. 5 where clones resemble (1) twins with an erasure of the host memory; (2) siblings where the head section retains memories of the host but their brains were the same at some point in the planaria’s life span; (3) birth of a child where the brain is made of naïve brain tissue constituting a new generation; and a (4) clone of self and memories.
Fig. 5.
Adapted from Neuhof et al. (2016)
Although, planaria naturally engage in asexual cloning in the wild, in laboratories it is possible to transplant the head of one planarian onto another planarian, where the new head will take control of a headless body (Reddien & Alvarado, 2004). There are only a few species that can survive a surgical procedure like a brain transplant. Successful brain transplants (defined by regaining close to normal behavior after 48 hours) have been accomplished with marine polyclad Notoplana acticola (Pagán, 2014). Axolotls are one of the few vertebrates that can survive such a transplantation (De Both, 1968), and there is evidence that avoidance responses maintain after a “trained brain” is transferred to a naïve host axolotl (Hershkowitz, Segal, & Samuel, 1972).
Opportunities for University Learning Labs
Conditioned place preference occurs with planaria in many scenarios. In a typical scenario, one half of a petri dish is illuminated and the other is in darkness, and the planarian is placed in the mid-section (Ramoz et al., 2012). Time spent and number of entrances to both sides are measured in baseline (Ramoz et al., 2012; Raffa, Shah, Tallarida, & Rawls, 2013). In general, naïve worms spend their time on the darkened side. Later, however, after the planarian is exposed to a drug while exposed to light, preference will shift to spending more time in the illuminated side of the dish (Ramoz et al., 2012). This demonstrates drugs can perturb the effectiveness of a stimulus as a reinforcer or punisher, and are important factors to consider within avoidance assays.
The Van Oye Maze also serves as housing, eliminating the need to handle the worms. Groups of worms can be placed in a 250 ml beaker where a baited hook is suspended in the water and gradually lowered (8 mm, 16 mm, 24 mm, and 32 mm). The percentage of worms reaching the goal are compared to control groups at the same 32 mm lowered bait level, who did not receive the gradual lowering (Wells, 1967). A testing condition without food, just the hook, allows differentiating between chemical gradient detection and conditioned place preference (Wells et al., 1966). Researchers note that this preparation still constitutes one of the most convincing evidence of operant learning due to the number of successful replications (Nicolas et al., 2008), and because its methodological rigor makes it challenging to reach other conclusions. The above Van Oye Maze and conditioned place preference procedure do not require expert handling or hand shaping for convincing demonstrations of training.
Many older experiments involving planaria lacked appropriate controls (Block & McConnell, 1967; Halas et al., 1962; Thompson & McConnell, 1955). Planaria laboratories traditionally used numerous and varied training procedures, and often relied on hand-training methods to prompt a planarian to motion, and as a result there have been numerous replication failures (Nicolas et al., 2008). Automated devices track behavior and eliminate experimenter bias while minimizing handling or differentials in researcher training expertise (Blackiston, Shomrat, Nicolas, Granata, & Levin, 2010). These devices are costly, and it is still crucial to understand functional behavioral processes when designing an experiment. In some cases, hand shaping has been successfully applied, and is cost effective compared to automated devices (Chicas-Mosier & Abramson, 2015). Hand shaping might even be warranted for complex behaviors that cannot be integrated into algorithms and automated. An extension of Chicas-Mosier and Abramson’s hand-shaping procedure using water (described in “Operant Responding” section) could be to yoke a control worm in an adjoined half petri dish, side by side with the experimental worm, so that all movements of the dish are experienced by both worms. In our estimation, the shaping procedure for the experimental worm requires that the dish be moved substantially. Although water access is likely the functional reinforcer, the contribution of movement to the results has not been examined.
Light is one of the most common stimuli used in the behavior training literature with planaria, and has been a preferred training stimulus by these authors. Light is considered a weaker unconditioned stimulus compared to vibration and shock (Vandeventer & Ratner, 1964). Researchers have examined the specific avoidance responses elicited and or evoked by differing light stimuli (Boring, 1912; Marriott, 1958; Pirenne & Marriott, 1955; Paskin, Jellies, Bacher, & Beane, 2014). In general, light will stimulate a planarian into locomotion, and they will come to rest in the dark (Boring, 1912), we refer to this pattern of behavior as photonegative responding.
Responsivity to light can be suppressed after an injury, eating, or if the dish contains slime trails (Riccio & Corning, 1969). Besides the photonegative moving away from light, planaria engage in distinct responses to light, the first being stereotypic head turns (“wig-waggling”), and the second being a longitudinal body turn, which has previously been referred to as contractions (Halas, James, & Stone, 1961). Eye-gouged samples engage in a similar cephalic “wig-waggling” response to light, albeit sluggishly (Taliaferro, 1920), and only planaria with intact eyes move away from a lateral light sources (Boring, 1912). Therefore, the eyes are integral to directional moving away from light but not “wig-waggling,” which potentially indicates there are photo-receptors on the body of the planaria mediating small head turns. Note planaria may have directional turn preferences, and reversing these preferences back and forth across trials is required to demonstrate effective stimulus control (Abbott & Wong, 2008).
Planaria are more sensitive to light on the ultraviolet side of the spectrum than infrared (Pirenne & Marriott, 1955). Overdoses of ultraviolet light can cause the death of planaria (Allee & Wilder, 1939; McConnell, 1965). Paskin et al. (2014) replicated some of this previous research by collecting data on photonegative responses measured by the distance of worms in four quadrants after 2 minutes of light exposure. They then also recording the angle of head turns away from lasers of differing wavelengths. The actual angle of the bend was greater when using ultraviolet light was than red light (Paskin et al., 2014). Planaria appear to be least sensitive to light in the red spectrum (Shomrat & Levin, 2013).
From our investigations, the use of red ambient light as illumination, with contingent omnidirectional light delivery (from the sides and vertically from the bottom), where any type of turn immediately switches off white light, has been useful for training planaria. After an initial turn training in either direction, the researcher can select a specific turn to reinforce with the removal of aversive lighting to initiate directional training. In a video included as supplementary material, the use of this device is demonstrated. In the four main panels, one single worm receives baseline (red background light), left turn training (light termination for turning left), right turn training (light termination for turning right), and omission training (light termination for not turning). The controls consist of two separate worms receiving either red light, or an automated delivery of light on for three seconds and off for seven seconds. The reason why ultraviolet light was not selected as the training stimulus is because a weak aversive stimulus should be selected with hand shaping, as otherwise accidental overexposure to a strong aversive stimulus will result in punishing the desired response beyond easy reacquisition (Hoffman & Fleshler, 1959).
Conclusion
Experimental analysis of behavior (EAB), applied behavior analysis (ABA), and clinical service delivery have been described as three interlocking domains (Moore & Cooper, 2003; Morris, 1992), and a threat to one affects the others in the long term. Although ABA and service delivery appear to be thriving (Deochand & Fuqua, 2016), EAB has been encountering new hurdles (Fox, 2018). Funding and jobs for basic research in psychology have dwindled, as have undergraduate opportunities to gain formative animal lab learning experiences (see Abramson, 2015). If EAB as a field is to thrive, effective strategies to conduct nonhuman research must be in place (Critchfield, 2011). Perhaps conducting research with new species, which can be cost-effectively maintained in a laboratory, is a solution; diversification of EAB within the higher education environment could be the “ultimate key to survival” (Poling, 2010). Invertebrate research may provide both professional research programs and student research laboratories with the flexibility they need to survive in the current academic climate.
Unraveling the diverse tapestry linking species to their evolutionary origins will require integrating behavioral assays among surviving members of each species. Planaria have unique morphology allowing for reconceptualizing the nature of memory from the bottom up in a biobehavioral model, and could inform what mode of conditioning may require higher brain function. Researchers within behavior science are continuing to develop innovative techniques for training planaria, which could become standard practice (Chicas-Mosier & Abramson, 2015). The boundaries of learning have yet to be delineated in planaria. Planaria have been known to go to the top of an electrode to avoid shock (McConnell, 1965), which is as functional a response as the rat that opted for breakfast in bed (Azrin & Holz, 1966). Conditioning has been suggested to be one reinforcement process, shown in two procedures (Donahoe & Palmer, 2004; Calvin & McDowell, 2016), which explains why it was challenging to distinguish between the two in the murky behavioral history of the planaria. If various behavioral assays demonstrate training can be preserved in the planaria in different segments, then these results implicate different biological processes regarding memory transference and retention.
Regeneration is dependent upon neuronal firing to amputated sections (Singer, 1952) and is likely essential for regaining the functionality of limbs post regeneration, but mammals may experience phantom pain after similar amputation. Phantom limb pain remains something of an enigma in psychology (Weeks, Anderson-Barnes, & Tsao, 2010), but mammal models only allow one amputation whereas regenerative models offer an infinite number of attempts and potential insight into such phenomena. The reliance of methodologically flawed functional magnetic resonance imaging studies may do little to advance the science and investigation into psychological phenomena even with human subjects (Fiedler, 2011), perhaps more will be learned from organisms like the planaria as it relates to the chemistry and physiology of learning.
In summary, many traditional nonhuman subjects are no longer as convenient to use, leading to dwindling opportunities and resources for students to engage in animal research, subverting the formative educational experiences for psychology majors (Abramson, 2015). Planaria are suitable for use for both training and research paradigms. Extending our reach to incorporating other animal models serves as strong evidence regarding the interspecies generality of our behavioral technology (Sidman, 1960). Selecting an organism based on tradition ignores Skinner’s advice to drop everything to study interesting behavioral phenomena (Skinner, 1956). Behavioral research with planaria is ongoing and will happen with or without our input, therefore ensuring behavioral science contributes is a “no brainer”.
Electronic Supplementary Material
(MP4 22402 kb)
Acknowledgments
The authors thank Rachel L. Burroughs for helping with editing an earlier draft of this manuscript.
Compliance with Ethical Standards
Disclaimer
All material contained herein is not plagiarized.
Conflict of Interest
The authors declare that they have no conflict of interest.
Footnotes
Two or more species were used in some studies, therefore the summed total of all the listed species is over 100%.
References
- Abbott SM, Wong GK. The conditioning and memory retention of planaria (Dugesia tigrina) for directional preferences. Bios. 2008;79(4):160–170. [Google Scholar]
- Abramson CI. A crisis in comparative psychology: Where have all the undergraduates gone? Frontiers in Psychology. 2015;6:1589. doi: 10.3389/fpsyg.2015.01500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abramson CI, Feinman RD. Lever-press conditioning in the crab. Physiology & Behavior. 1990;48(2):267–272. doi: 10.1016/0031-9384(90)90311-q. [DOI] [PubMed] [Google Scholar]
- Adell, T., Saló, E., van Loon, J. J., & Auletta, G. (2014). Planarians sense simulated microgravity and hypergravity. BioMed Research International, 1–10. 10.1155/2014/679672. [DOI] [PMC free article] [PubMed]
- Akiyama Y, Agata K, Inoue T. Spontaneous behaviors and wall-curvature lead to apparent wall preference in planarian. PLoS One. 2015;10:1–17. doi: 10.1371/journal.pone.0142214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allee WC, Wilder J. Group protection for Euplanaria dorotocephala from ultra-violet radiation. Physiological Zoology. 1939;12(2):110–135. [Google Scholar]
- Allen GD. Reversibility of the reactions of Planaria dorotocephala to a current of water. Biological Bulletin. 1915;29:111–128. [Google Scholar]
- American Psychological Association (APA). (2017). Resolution reaffirming support for research and teaching with nonhuman animals. Retrieved from http://www.apa.org/about/policy/animal-research-resolution.pdf.
- American Psychological Association (APA). (n.d.). Research animals in psychology. Retrieved from https://www.apa.org/research/responsible/research-animals.pdf.
- Auletta G, Adell T, Colagè I, D’Ambrosio P, Salò E. Space research program on Planarian Schmidtea Mediterranea’s establishment of the anterior-posterior axis in altered gravity conditions. Microgravity Science and Technology. 2012;24:419–425. [Google Scholar]
- Azrin NH, Holz WC. Punishment. In: Honig WK, editor. Operant behavior: areas of research and application. East Norwalk, CT: Appleton-Century-Crofts; 1966. pp. 380–447. [Google Scholar]
- Barnes TC, Skinner BF. The progressive increase in the geotropic response of the ant Aphaenogaster. The Journal of General Psychology. 1930;4:102–112. [Google Scholar]
- Baxter R, Kimmel HD. Conditioning and extinction in the planarian. American Journal of Psychology. 1963;76:665–669. [PubMed] [Google Scholar]
- Best JB. Diurnal cycles and cannibalism in planaria. Science. 1960;131:1884–1885. doi: 10.1126/science.131.3417.1884. [DOI] [PubMed] [Google Scholar]
- Best JB. Behavior of planaria in instrumental learning paradigms. Animal Behaviour. 1965;13(Suppl. 1):69–75. [Google Scholar]
- Best JB, Rubinstein I. Environmental familiarity and feeding in a planarian. Science. 1962;135(3507):916–918. doi: 10.1126/science.135.3507.916-a. [DOI] [PubMed] [Google Scholar]
- Bitterman EM. Phyletic differences in learning. American Psychologist. 1965;20:396–410. doi: 10.1037/h0022328. [DOI] [PubMed] [Google Scholar]
- Blackiston D, Shomrat T, Nicolas CL, Granata C, Levin M. A second-generation device for automated training and quantitative behavior analyses of molecularly-tractable model organisms. PLoS One. 2010;5:1–20. doi: 10.1371/journal.pone.0014370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackiston DJ, Silva Casey E, Weiss MR. Retention of memory through metamorphosis: can a moth remember what it learned as a caterpillar? PLoS One. 2008;3:e1736. doi: 10.1371/journal.pone.0001736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block RA, McConnell JV. Classically conditioned discrimination in the Planarian, Dugesia dorotocephala. Nature. 1967;215:1465–1466. doi: 10.1038/2151465a0. [DOI] [PubMed] [Google Scholar]
- Bocchinfuso DG, Taylor P, Ross E, Ignatchenko A, Ignatchenko V, Kislinger T, et al. Proteomic profiling of the planarian Schmidtea mediterranea and its mucous reveals similarities with human secretions and those predicted for parasitic flatworms. Molecular & Cellular Proteomics. 2012;11:681–691. doi: 10.1074/mcp.M112.019026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boring EG. Notes on the negative reaction under light-adaptation in the planarian. Journal of Animal Behavior. 1912;2(4):229. [Google Scholar]
- Branch M. Malignant side effects of null-hypothesis significance testing. Theory & Psychology. 2014;24:256–277. [Google Scholar]
- Breland K, Breland M. The misbehavior of organisms. American Psychologist. 1961;16:681. [Google Scholar]
- Calvin OL, McDowell JJ. Extending unified-theory-of-reinforcement neural networks to steady-state operant behavior. Behavioral Processes. 2016;127:52–61. doi: 10.1016/j.beproc.2016.03.016. [DOI] [PubMed] [Google Scholar]
- Chicas-Mosier AM, Abramson CI. A new instrumental/operant conditioning technique suitable for inquiry-based activities in courses on experimental psychology, learning, and comparative psychology using planaria (Dugesia dorotocephala and Dugesia tigrina) Comprehensive Psychology. 2015;4:1–6. [Google Scholar]
- Corning WC, Lahue R. Invertebrate strategies in comparative learning studies. American Zoologist. 1972;12:455–469. [Google Scholar]
- Corning WC, Riccio D. The planarian controversy. In: Bryne W, editor. Molecular approaches to learning and memory. New York, NY: Academic Press; 1970. pp. 107–150. [Google Scholar]
- Crawford FT, Skeen SLC. Operant responding in the planarian: a replication study. Psychological Reports. 1967;20:1023–1027. [Google Scholar]
- Critchfield TS. To a young basic scientist, about to embark on a program of translational research. The Behavior Analyst. 2011;34(2):137–148. doi: 10.1007/BF03392245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Both NJ. Transplantation of axolotl heads. Science. 1968;162:460–461. doi: 10.1126/science.162.3852.460. [DOI] [PubMed] [Google Scholar]
- Deochand N, Fuqua RW. BACB certification trends: state of the states (1999 to 2014) Behavior Analysis in Practice. 2016;9:243–252. doi: 10.1007/s40617-016-0118-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dexter JP, Tamme MB, Lind CH, Collins EMS. On-chip immobilization of planarians for in vivo imaging. Scientific Reports. 2014;4:1–9. doi: 10.1038/srep06388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon MR, Daar JH, Gunnarsson K, Johnson ML, Shayter AM. Stimulus preference and reinforcement effects of the Madagascar hissing cockroach (Gromphordahina portentosa): a case of reverse translational research. The Psychological Record. 2016;66:41–51. [Google Scholar]
- Donahoe JW, Palmer DC. Learning and complex behavior. Richmond, MA: Ledgetop Publishing; 2004. [Google Scholar]
- Downey P, Jahan-Parwar B. Cooling as reinforcing stimulus in Aplysia. American Zoologist. 1972;12:507–512. [Google Scholar]
- Duhaime-Ross, A. (2015, March 18). Memory in the flesh: a radical 1950’s scientist suggested memories could survive outside the brain—and he may have been right. The Verge. Retrieved August 24, 2018, from https://www.theverge.com/2015/3/18/8225321/memory-research-flatworm-cannibalism-james-mcconnell-michael-levin.
- Egger B, Gschwentner R, Rieger R. Free-living flatworms under the knife: past and present. Development Genes and Evolution. 2007;217:89. doi: 10.1007/s00427-006-0120-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein R. On pigeons and people: a preliminary look at the Columban Simulation Project. The Behavior Analyst. 1981;4:43–55. doi: 10.1007/BF03391851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiedler K. Voodoo correlations are everywhere—not only in neuroscience. Perspectives on Psychological Science. 2011;6:163–171. doi: 10.1177/1745691611400237. [DOI] [PubMed] [Google Scholar]
- Fox AE. The future is upon us. Behavior Analysis: Research & Practice. 2018;18:144–150. [Google Scholar]
- Garcia J, Kimeldorf DJ, Koelling RA. Conditioned aversion to saccharin resulting from exposure to gamma radiation. Science. 1955;122:157–158. [PubMed] [Google Scholar]
- Grossmann KE. Continuous, fixed-ratio, and fixed-interval reinforcement in honey bees. Journal of the Experimental Analysis of Behavior. 1973;20:105–109. doi: 10.1901/jeab.1973.20-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halas ES. Operant conditioning in planaria: a criticism. Science. 1963;141:390–392. doi: 10.1126/science.141.3579.390-a. [DOI] [PubMed] [Google Scholar]
- Halas ES, James RL, Knutson CS. An attempt at classical conditioning in the planarian. Journal of Comparative & Physiological Psychology. 1962;55(6):969–971. doi: 10.1037/h0040092. [DOI] [PubMed] [Google Scholar]
- Halas ES, James RL, Stone LA. Types of responses elicited in planaria by light. Journal of Comparative & Physiological Psychology. 1961;54:302. doi: 10.1037/h0045739. [DOI] [PubMed] [Google Scholar]
- Hershkowitz M, Segal M, Samuel D. The acquisition of dark avoidance by transplantation of the forebrain of trained newts (Pleurodeles waltl) Brain Research. 1972;48:366–369. doi: 10.1016/0006-8993(72)90191-6. [DOI] [PubMed] [Google Scholar]
- Hoffman HS, Fleshler M. Aversive control with the pigeon. Journal of the Experimental Analysis of Behavior. 1959;2:213–218. doi: 10.1901/jeab.1959.2-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson AL, Horowitz SD, Fried C. Classical conditioning, pseudoconditioning, or sensitization in the planarian. Journal of Comparative & Physiological Psychology. 1967;64(1):73. doi: 10.1037/h0024808. [DOI] [PubMed] [Google Scholar]
- Jenkins MM. Aspects of planarian biology and behavior. In: Corning WC, Ratner SC, editors. Chemistry of learning. New York, NY: Plenum Press; 1967. pp. 116–143. [Google Scholar]
- Katz AN. Inexpensive animal learning exercises for huge introductory laboratory classes. Teaching of Psychology. 1978;5(2):91–93. [Google Scholar]
- Krantz ML. Operant conditioning of planarians. Beta Beta Beta Biological Society. 1964;35(3):139–141. [Google Scholar]
- Lattal KA. The human side of animal behavior. The Behavior Analyst. 2001;24:147–161. doi: 10.1007/BF03392026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RM. Conditioning of a free operant response in planaria. Science. 1963;139:1048–1049. doi: 10.1126/science.139.3559.1048. [DOI] [PubMed] [Google Scholar]
- Marriott FHC. The absolute light-sensitivity and spectral threshold curve of the aquatic flatworm Dendrocoelum lacteum. Journal of Physiology. 1958;143:369–379. doi: 10.1113/jphysiol.1958.sp006065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell JV. Memory transfer through cannibalism in planarians. Journal of Neuropsychiatry. 1962;3(1):542–548. [Google Scholar]
- McConnell JV. Comparative physiology: learning in invertebrates. Annual Review of Physiology. 1966;28:107–136. doi: 10.1146/annurev.ph.28.030166.000543. [DOI] [PubMed] [Google Scholar]
- McConnell JV, editor. A manual of psychological experimentation on planarians. 2. Ann Arbor, MI: Worm Runner's Digest; 1967. [Google Scholar]
- McConnell JV, Jacobson AL, Kimble DP. The effects of regeneration upon retention of a conditioned response in the planarian. Journal of Comparative & Physiological Psychology. 1959;52:1–5. doi: 10.1037/h0048028. [DOI] [PubMed] [Google Scholar]
- Moore J, Cooper JO. Some proposed relations among the domains of behavior analysis. The Behavior Analyst. 2003;26:69–84. doi: 10.1007/BF03392068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris EK. The aim, progress, and evolution of behavior analysis. The Behavior Analyst. 1992;15:3–29. doi: 10.1007/BF03392582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhof M, Levin M, Rechavi O. Vertically- and horizontally-transmitted memories: the fading boundaries between regeneration and inheritance in planaria. Biology Open. 2016;5:1177–1188. doi: 10.1242/bio.020149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newmark PA, Alvarado AS. Not your father's planarian: a classic model enters the era of functional genomics. Nature Reviews Genetics. 2002;3:210. doi: 10.1038/nrg759. [DOI] [PubMed] [Google Scholar]
- Nicolas CL, Abramson CI, Levin M. Analysis of behavior in the planarian model. In: Raffa RB, Rawls SM, editors. Planaria: a model for drug action and abuse. Austin, TX: Landes Bioscience; 2008. pp. 83–94. [Google Scholar]
- Pagán OR. The first brain: the neuroscience of planarians. New York, NY: Oxford University Press; 2014. [Google Scholar]
- Pagán OR, Deats S, Baker D, Montgomery E, Wilk G, Tenaglia M, Semon J. Planarians require an intact brain to behaviorally react to cocaine, but not to react to nicotine. Neuroscience. 2013;246:265–270. doi: 10.1016/j.neuroscience.2013.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paskin TR, Jellies J, Bacher J, Beane WS. Planarian phototactic assay reveals differential behavioral responses based on wavelength. PLoS One. 2014;9:e114708. doi: 10.1371/journal.pone.0114708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pear JJ, Eldridge GD. The operant-respondent distinction: future directions. Journal of the Experimental Analysis of Behavior. 1984;42:453–467. doi: 10.1901/jeab.1984.42-453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirenne MH, Marriott FHC. Light sensitivity of the aquatic flatworm Dendrocoelum lacteum. Nature. 1955;175:642. [Google Scholar]
- Poling A. Looking to the future: will behavior analysis survive and prosper? The Behavior Analyst. 2010;33:7–17. doi: 10.1007/BF03392200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffa RB, Holland LJ, Schulingkamp RJ. Quantitative assessment of dopamine D2 antagonist activity using invertebrate (Planaria) locomotion as a functional endpoint. Journal of Pharmacological and Toxicological Methods. 2001;45(3):223–226. doi: 10.1016/s1056-8719(01)00152-6. [DOI] [PubMed] [Google Scholar]
- Raffa RB, Shah S, Tallarida CS, Rawls SM. Amphetamine conditioned place preference in planarians. Journal of Behavioral & Brain Science. 2013;3:131–136. [Google Scholar]
- Ramm SA. Exploring the sexual diversity of flatworms: ecology, evolution, and the molecular biology of reproduction. Molecular Reproduction & Development. 2016;84(2):120–131. doi: 10.1002/mrd.22669. [DOI] [PubMed] [Google Scholar]
- Ramoz L, Lodi S, Bhatt P, Reitz AB, Tallarida C, Tallarida RJ, et al. Mephedrone (“bath salt”) pharmacology: insights from invertebrates. Neuroscience. 2012;208:79–84. doi: 10.1016/j.neuroscience.2012.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddien PW, Alvarado AS. Fundamentals of planarian regeneration. Annual Review of Cell and Developmental Biology. 2004;20:725–757. doi: 10.1146/annurev.cellbio.20.010403.095114. [DOI] [PubMed] [Google Scholar]
- Reynierse JH. Aggregation formation in planaria, Phagocata gracilis and Cura foremani: species differentiation. Animal Behaviour. 1967;15:270–272. doi: 10.1016/0003-3472(67)90011-5. [DOI] [PubMed] [Google Scholar]
- Reynierse JH, Ellis RR. Aggregation formation in three species of planaria: distance to nearest neighbour. Nature. 1967;214:895–896. doi: 10.1038/214895a0. [DOI] [PubMed] [Google Scholar]
- Reynierse JH, Gleason KK, Ottemann R. Mechanisms producing aggregations in planaria. Animal Behaviour. 1969;17:47–63. [Google Scholar]
- Reynoldson TB, Young JO. The food of four species of lake-dwelling triclads. Journal of Animal Ecology. 1963;32:175–191. [Google Scholar]
- Riccio D, Corning WC. Slime and planarian behavior. The Psychological Record. 1969;19:507–513. [Google Scholar]
- Rilling M. The mystery of the vanished citations: James McConnell’s forgotten 1960s quest for planarian learning, a biochemical engram, and celebrity. American Psychologist. 1996;51:589–598. [Google Scholar]
- Sahu S, Dattani A, Aboobaker AA. Secrets from immortal worms: what can we learn about biological ageing from the planarian model system? Seminars in Cell & Developmental Biology. 2017;70:108–121. doi: 10.1016/j.semcdb.2017.08.028. [DOI] [PubMed] [Google Scholar]
- Shomrat T, Levin M. An automated training paradigm reveals long-term memory in planarians and its persistence through head regeneration. Journal of Experimental Biology. 2013;216:3799–3810. doi: 10.1242/jeb.087809. [DOI] [PubMed] [Google Scholar]
- Sidman M. Tactics of scientific research. New York, NY: Basic Books; 1960. [Google Scholar]
- Singer M. The influence of the nerve in regeneration of the amphibian extremity. Quarterly Review of Biology. 1952;27:169–200. doi: 10.1086/398873. [DOI] [PubMed] [Google Scholar]
- Skinner BF. A case history in scientific method. American Psychologist. 1956;11(5):221. [Google Scholar]
- Skinner BF. The phylogeny and ontogeny of behavior. Science. 1966;153:1205–1213. doi: 10.1126/science.153.3741.1205. [DOI] [PubMed] [Google Scholar]
- Smalheiser NR, Manev H, Costa E. RNAi and brain function: was McConnell on the right track? Trends in Neurosciences. 2001;24:216–218. doi: 10.1016/s0166-2236(00)01739-2. [DOI] [PubMed] [Google Scholar]
- Sokolowski MBC, Disma G, Abramson CI. A paradigm for operant conditioning of blow flies (Phorma terrae novae robineau-desvoidy, 1830) Journal of the Experimental Analysis of Behavior. 2010;93:81–89. doi: 10.1901/jeab.2010.93-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein L. Biological substrates of operant conditioning and the operant-respondent distinction. Journal of the Experimental Analysis of Behavior. 1997;67:246–253. doi: 10.1901/jeab.1997.67-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson CG, Beane WS. A low percent ethanol method for immobilizing planarians. PLoS One. 2010;5:1–12. doi: 10.1371/journal.pone.0015310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbot J, Schötz EM. Quantitative characterization of planarian wild-type behavior as a platform for screening locomotion phenotypes. Journal of Experimental Biology. 2011;214:1063–1067. doi: 10.1242/jeb.052290. [DOI] [PubMed] [Google Scholar]
- Taliaferro WH. Reactions to light in Planaria maculata, with special reference to the function and structure of the eyes. Journal of Experimental Zoology. 1920;31:58–116. [Google Scholar]
- Thompson R, McConnell JV. Classical conditioning in planarian, Dugesia dorotocephala. Journal of Comparative and Physiological Psychology. 1955;48:65–68. doi: 10.1037/h0041147. [DOI] [PubMed] [Google Scholar]
- Travis GD. Replicating replication? Aspects of the social construction of learning in planarian worms. Social Studies of Science. 1981;11:11–32. [Google Scholar]
- Vandeventer JM, Ratner SC. Variables affecting the frequency of response of planaria to light. Journal of Comparative & Physiological Psychology. 1964;57:407–411. doi: 10.1037/h0042776. [DOI] [PubMed] [Google Scholar]
- Walker DR. Memory transfer in planarians: an artifact of the experimental variables. Psychonomic Science. 1966;5:357–358. [Google Scholar]
- Walker DR, Milton GA. Memory transfer vs. sensitization in cannibal planarians. Psychonomic Science. 1966;5:293–294. [Google Scholar]
- Weeks SR, Anderson-Barnes VC, Tsao JW. Phantom limb pain: theories and therapies. The Neurologist. 2010;16:277–286. doi: 10.1097/NRL.0b013e3181edf128. [DOI] [PubMed] [Google Scholar]
- Wells PH. Training flatworms in a Van Oye maze. In: Corning WC, Ratner SC, editors. Chemistry of learning. New York, NY: Plenum Press; 1967. pp. 251–254. [Google Scholar]
- Wells PH, Jennings LB, Davis M. Conditioning planarian worms in a van Oye type maze. American Zoologist. 1966;6(3):295. [Google Scholar]
- Zimmermann Z, Watkins E, Poling A. JEAB research over time: species used, experimental designs, statistical analyses, and sex of subjects. The Behavior Analyst. 2015;38:203–218. doi: 10.1007/s40614-015-0034-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(MP4 22402 kb)