Abstract
Purpose
To examine outcomes of 23-gauge (23G) pars plana vitrectomy (PPV) for complex diabetic tractional retinal detachment (TRD) in Chicago’s Cook County Health and Hospitals System (CCHHS).
Materials and methods
This is a retrospective noncomparative study of diabetic TRD cases that underwent PPV at CCHHS. Primary retinal reattachment rate, visual function, and postoperative complications were analyzed.
Results
Sixty nine consecutive cases were included. Primary reattachment and final attachment were achieved in 68/69 eyes (98.6%). Secondary retinal detachment was noted in 1 eye (1.4%). Vitreous hemorrhage requiring repeat PPV developed in 5 eyes (7.2%) and reoperation due to other complications was required in 4/69 eyes (5.8%). Perfluoropropane (C3F8) gas tamponade was used in 91.3% of eyes and silicone oil in 8.7% of eyes. Mean LogMAR visual acuity significantly improved from 1.84 ± 0.61 to 0.93 ± 0.66, (P<0.0001). Vision was stabilized or improved in 66 eyes (95.7%). Visual acuity of 20/200 or better was achieved in 49/69 eyes (71.0%) and 20/50 or better in 16/69 eyes (23.2%).
Conclusions
Even in patients with severe and advanced diabetic TRD pathology and unique demographics as seen in CCHHS, modern vitrectomy techniques can provide excellent anatomical and visual outcomes.
Introduction
Tractional retinal detachment (TRD) represents one of the most severe complications and a major cause of vision loss in patients with proliferative diabetic retinopathy (PDR)[1]. Hyperglycemia and molecular events related to diabetes lead to retinal vessel endothelial cell damage, increased vascular permeability, bleeding, retinal vessel occlusion, and subsequently retinal ischemia. An ischemic retina secretes locally active cytokines, such as vascular endothelial growth factor and connective tissue growth factor, which lead to neovascularization and connective tissue formation in the proliferative stage of diabetic retinopathy. This fibrovascular proliferation grows into the vitreoretinal interface and may contract, potentially resulting in TRD[2].
Diabetic TRD is repaired with pars plana vitrectomy (PPV), which allows for simultaneous visualization and manipulation of the retina[3]. In 1981, Michels described posterior membrane segmentation as a surgical technique for the management of TRD by relieving anterior and tangential traction caused by fibrovascular membranes, allowing for reattachment of the retina[4]. The surgical repair of diabetic TRD is among the most challenging surgeries for a vitreoretinal specialist due to the friable nature of the ischemic retina and the presence of extensive fibrovascular membranes[5]. Despite the difficulty of repair, recent advancements in vireoretinal surgery, including smaller gauge instruments, wide angle viewing systems, use of tamponade, self-retaining endoillumination, and the development of medications targeting vascular endothelial growth factor, have greatly improved outcomes in complex TRD cases[6–9].
Cook County Health and Hospitals System (CCHHS) is one of the largest public county hospital systems in the United States and is an important safety net hospital for the city of Chicago. CCHHS serves a primarily underserved population with 43.8% of outpatients using Medicaid as a payor source and 22.4% of outpatients requiring charity care[10]. Furthermore, the patient population of CCHHS is primarily from minority groups, with 52.4% of patients identifying their race as Black and 27.6% of patients identifying their ethnicity as Hispanic or Latino[10].
CCHHS serves patients with severe diabetes and limited access to preventative medical and retinal screening. These patients commonly present with advanced PDR and severe longstanding diabetic TRD with broadly adherent plaques of fibrovascular proliferation. Surgery for diabetic TRD has a high rate of complications postoperatively, especially for eyes with complex pathology. With just a handful of previous studies investigating PPV for diabetic TRD in a county hospital, limited data are available for outcomes of surgical repair in patient cohorts with such demographics and severity of pathology[5,8,11]. The purpose of this study is to examine the visual and anatomical outcomes and complication rates of 23-gauge (23G) PPV for diabetic TRD at CCHHS.
Material and methods
We conducted a retrospective review of the medical records of all consecutive surgical cases of diabetic TRD performed by a single vitreoretinal surgeon (DS) at CCHHS, between November 2013 and March 2016. Eyes that underwent 23G PPV for primary repair of diabetic TRD and had at least 3 months of follow-up were eligible for inclusion; 8 patients with less than 3 months of postoperative follow-up, or patients with other proliferative vitreoretinal disease, such as proliferative sickle cell retinopathy, were excluded from this study. This study was conducted in accordance with the Declaration of Helsinki. The collection and evaluation of all protected health information was performed in a Health Insurance Portability and Accountability Act (HIPAA)-compliant manner. Ethical approval for this study was obtained from Chicago’s Cook County Health and Hospitals System Institutional Review Board (IRB). Informed consent was not obtained because it was waived by the IRB that approved the study.
23-gauge vitrectomy was performed using the Alcon Constellation vitrectomy machine (Alcon Laboratories, Inc., Fort Worth, TX, USA) using a wide-angle viewing system and scleral depression during the vitreous base dissection. 25 and 27-gauge vitrectomy was not used as it was not available at the hospital where the surgeries were performed. Fibrovascular membrane dissection was performed using a combination of one or more of the following without bimanual technique: the vitrector handpiece, internal limiting membrane forceps, curved scissors, vertical scissors, and/or blunt delaminating spatula. Triamcinolone staining and Perfluoro-n-octane (PFO) liquid was used as needed. Endolaser photocoagulation was applied around retinal breaks and in panretinal photocoagulation (PRP) fashion using a flexible curved illuminated laser. Fluid-air exchange was followed by injection of long-acting tamponade of perfluoropropane gas (C3F8) (14–16% concentration) or silicone oil. The choice of tamponade was determined by the extent of detachment, the presence and location and size of retinotomy or retinectomy, and the amount of residual traction/fibrovascular membranes. C3F8 gas tamponade was favored in the vast majority of cases except in eyes requiring large inferior retinectomies and with significant residual traction/fibrovascular membranes. All sclerotomies were sutured. Scleral buckle (SB) was used in cases with significant peripheral vitreoretinal traction or with significant scar tissue that required inferior retinectomy. In patients with a preoperative visually significant cataract, cataract extraction with intraocular lens replacement (CE/IOL) was performed at the time of vitrectomy by a cataract surgeon. To our knowledge, all lens calculations were made using A-Scan ultrasound biometry and measurements of the fellow eye.
During the postoperative period, patients were instructed to maintain face-down positioning for up to 3 weeks at least 80% of the time. Patients were seen weekly for the first 3 weeks after surgery to monitor the postoperative course and reinforce and ensure compliance with instructions. Primary outcome measures included postoperative best-corrected Snellen visual acuity (BCVA), rate of primary reattachment with a single surgery, and rates of postoperative complications including secondary retinal detachment, vitreous hemorrhage needing repeat PPV, anterior hyaloid fibrovascular proliferation (AHFVP), fibrinoid syndrome, neovascular glaucoma, and epiretinal membrane formation (ERM) needing PPV.
BCVA was converted to the logarithm of the minimum angle of resolution (LogMAR) for quantitative comparison. A LogMAR value of 1.98 was used for vision limited to counting fingers (CF) and 2.28 for hand motions (HM)[12]. Vision limited to light perception (LP) or no light perception (NLP) could not be converted to LogMAR, as these do not represent visual acuities, and were thus excluded from this analysis. Preoperative and postoperative LogMAR values were analyzed using a paired, two-tailed t-test, and a P-value <0.05 was considered statistically significant.
Results
69 consecutive eyes from 61 patients that underwent 23G PPV for primary repair of diabetic TRD were included in this study. Patient characteristics are described in Table 1. The average age of all patients was 47 years (range, 21–65 years) and average follow-up was 11.4 months (range, 3–28 months). 36 patients were male and 25 were female. 36 patients were Hispanic, 21 were African American, 3 were Caucasian, and 1 patient was Asian.
Table 1. Patient characteristics (n = 61).
| Patient Characteristics | Number of Patients (%) |
|---|---|
| Age (years) | |
| Average | 47 |
| Range | 21–65 |
| Sex | |
| Male | 36 (59.0) |
| Female | 25 (41.0) |
| Ethnicity | |
| Hispanic | 36 (59.0) |
| African American | 21 (34.4) |
| Caucasian | 3 (4. |
| Asian | 1 (1.6) |
| Follow-up Time (months) | |
| Average | 11.4 |
| Range | 3–28 |
| HgbA1c (average) | 9% |
69.6% of eyes were characterized by detachments involving the macula, 44.9% by combined tractional and rhegmatogenous retinal detachments (TRD/RRD), and 85.5% by high complexity detachments or severe fibrovascular proliferation (Table 2 along with pre-operative findings, complications, and other anatomic/functional outcomes). TRD/RRD cases included those with preexisting breaks only, a combination of iatrogenic and preexisting breaks, or iatrogenic breaks only. No specific associations were apparent between the presence of retinal breaks and patient age or retinal ischemia. Combined SB was performed in 8/69 eyes (11.6%), combined CE/IOL in 6/69 eyes (8.7%), and combined pars plana lensectomy (PPL) in 3/69 eyes (4.3%), which is summarized in Table 3. Long-acting tamponade was used in all cases, with C3F8 in 63/69 eyes (91.3%) and silicone oil in 6/69 (8.7%). There were 56 eyes that were phakic at the time of operation, of which 9 underwent combined cataract extraction or lensectomy. Of the 47 eyes that were phakic immediately after vitrectomy, 17 (36.2%) required postoperative CE/IOL in the follow-up period. General anesthesia was used in 96% of cases, monitored anesthesia care (MAC) anesthesia was used in 4% of cases, and mean surgical time was 181 minutes (SD 65.4). Mean post-operative day 1 intraocular pressure (IOP) was 20.0 mmHg (SD 9.3), post-operative week 1 IOP was 15.7 mmHg (SD 4.1), and post-operative month 1 IOP was 15.0 mmHg (SD 4.2). Patients with elevated IOP responed to topical therapy, which was tapered as IOP improved. No patients required surgical intervention for elevated IOP. Fig 1 highlights three cases from the cohort, which effectively represent the typical complexity encountered in the patients analyzed in this study.
Table 2. TRD characteristics, anatomical and functional outcomes, and complications (n = 69).
| Number of Eyes (%) | |
|---|---|
| TRD characteristics | |
| TRD only | 38 (55.1) |
| Combined TRD/RRD | 31 (44.9) |
| Macula Involvement | 48 (69.6) |
| High Complexity Detachment or Severe Fibrovascular Proliferation | 59 (85.5) |
| Phakic | 56 (81.2) |
| Anatomic Outcomes | |
| Primary Reattachment with Single Surgery | 68 (98.6) |
| Final Attachment | 68 (98.6) |
| Visual Outcomes | |
| Postoperative BCVA | |
| > = 20/50 | 16/69 (23.2) |
| > = 20/100 | 38/69 (55.1) |
| > = 20/200 | 49/69 (71.0) |
| > = 5/200 | 54/69 (78.3) |
| Better | 61/69 (88.4) |
| Stable | 5/69 (7.2) |
| Worse | 3/69 (4.3) |
| No Light Perception | 0 (0) |
| Complications | |
| Vitreous Hemorrhage | 41 (59.4) |
| Vitreous Hemorrhage Requiring PPV | 5 (7.2) |
| Secondary Retinal Detachment | 1 (1.4) |
| Neovascular Glaucoma | 1 (1.4) |
| Neovascular Glaucoma Requiring Filtering Surgery | 0 (0) |
| Anterior Hyaloid Fibrovascular Proliferation | 1 (1.4) |
| Fibrinoid Syndrome | 1 (1.4) |
| Epiretinal Membrane Requiring PPV | 1 (1.4) |
TRD, tractional retinal detachment; RRD, rhegmatogenous retinal detachment; BCVA, best-corrected Snellen visual acuity; PPV, pars plana vitrectomy.
Table 3. Intraoperative procedures (n = 69).
| Surgery Characteristics | Number of Eyes (%) |
|---|---|
| Combined Scleral Buckle | 8 (11.6) |
| Primary Combined PPL | 3 (4.3) |
| Combined CE/IOL | 6 (8.7) |
| Long-Acting Tamponade | |
| C3F8 gas | 63 (91.3) |
| Silicone Oil | 6 (8.7) |
PPL, pars plana lensectomy; CE/IOL, cataract extraction with intraocular lens implantation.
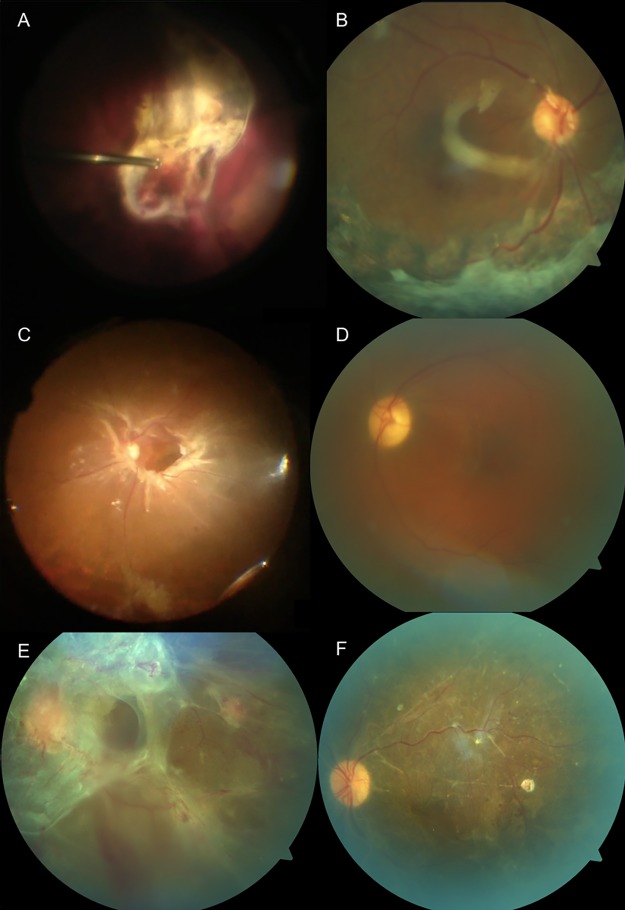
Fig 1. Fundus photographs of advanced diabetic tractional retinal detachments repaired with surgery.
(A-B) Intraoperative and postoperative fundus photos in a 50 y/o male with 20-year history of type 2 diabetes mellitus who presented with combined tractional and rhegmatogenous retinal detachments (TRD/RRD) with extensive fibrovascular plaques covering the posterior pole and inferior retina. (C-D) Intraoperative and postoperative fundus photos in a 50 y/o male with 5-year history of diabetes mellitus with combined TRD/RRD and ring of fibrovascular membrane along arcades. (E-F) Preoperative and postoperative fundus photos in a 30 y/o female patient with history of type I diabetes mellitus that presented with chronic macula off TRD with extensive preretinal fibrovascular proliferation. Vision improved from count fingers to 20/80 postoperatively.
Retinal reattachment with a single surgery was achieved in 68 of 69 eyes (98.6%) (Table 2). One patient had persistent shallow chronic subretinal fluid in the macula after the primary repair and repeat vitrectomy was performed for draining of persistent submacular fluid. Secondary retinal detachment was observed in 1 eye (1.4%) at 4.5 months postoperatively and was reattached successfully with one additional surgery with combined vitrectomy and lensectomy with scleral buckle and silicone oil tamponade. Final reattachment was achieved in 68 eyes (98.6%). Postoperative vitreous hemorrhage requiring repeat PPV was noted in 5 eyes (7.2%). Although not systematically quanitified, in combined CE/IOL and SB cases inflammation appeared increased. There was one case of neovascular glaucoma that was managed medically and did not require filtering surgery. No eyes developed endophthalmitis, phthisis, corneal decompensation, or secondary angle closure. No patients were noted to have NLP vision and no patients had complications (e.g. IOL positioning) due to prolonged face-down positioning.
Overall, the mean preoperative visual acuity improved from LogMAR 1.84 (SD 0.61, 95% confidence interval (CI) 1.69–1.98) to 0.93 (0.66, 0.77–1.09) after vitrectomy, P<0.0001. Significant improvements in postoperative visual acuity were observed in eyes that received C3F8 but not silicone oil, which is summarized in Table 4. Both eyes with TRD and eyes with TRD/RRD had significant improvement of visual acuity postoperatively (Table 5). Eyes with TRD (55.1%) improved from LogMAR 1.87 (0.58, 1.68–2.05) to 0.78 (0.59, 0.56–0.96), P<0.0001, and eyes with combined TRD/RRD (44.9%) improved from LogMAR 1.80 (0.65, 1.57–2.03) to 1.12 (0.70, 0.88–1.37), P = 0.00007. Of note, two eyes had preoperative vision that were LP and one eye had postoperative vision that was LP, and therefore could not be converted to LogMAR[12].
Table 4. Visual outcomes based on tamponade agent.
| Form of Tamponade | Number of Eyes (%) | Mean Preoperative LogMAR (BCVA) | Mean Postoperative LogMAR (BCVA) | P-value |
|---|---|---|---|---|
| C3F8 Gas | 63 (91.3) | 1.81 ± 0.62 (~20/1300) | 0.87 ± 0.64 (~20/150) | < 0.0001 |
| Silicone Oil | 6 (8.7) | 2.10 ± 0.45 (~20/2500) | 1.52 ± 0.52 (~20/670) | 0.11 |
| Total | 69 (100) | 1.84 ± 0.61 (~20/1400) | 0.93 ± 0.66 (~20/170) | < 0.0001 |
LogMAR, logarithm of the minimum angle of resolution; BCVA, best-corrected Snellen visual acuity
Table 5. Visual outcomes based on type of retinal detachment.
| Type of Detachment | Number of Eyes (%) | Mean Preoperative LogMAR (BCVA) | Mean Postoperative LogMAR (BCVA) | P-value |
|---|---|---|---|---|
| TRD | 38 (55.1) | 1.87 ± 0.58 (~20/1500) | 0.78 ± 0.59 (~20/120) | < 0.0001 |
| TRD/RRD | 31 (44.9) | 1.80 ± 0.65 (~20/1250) | 1.12 ± 0.70 (~20/270) | 0.00007 |
TRD, traction retinal detachment; RRD, rhegmatogenous retinal detachment; LogMAR, logarithm of the minimum angle of resolution; BCVA, best-corrected Snellen visual acuity.
Improved visual acuity was observed in 61/69 eyes (88.4%). Visual acuity was stabilized in 5/69 eyes (7.2%) and was worse in 3/69 eyes (4.3%). Differences between preoperative and postoperative BCVA are summarized in Tables 2 and 6. Regarding the eyes with stable visual acuity, visual outcomes were limited by macular ischemia/photoreceptor loss due to macula off RD despite retinal reattachment. Of the eyes with worse postoperative acuity, one surgery was complicated by severe hemorrhagic choroidals at the end of surgery as a result of severe bucking due to airway irritation. This patient developed anterior hyaloid proliferation with hypotony and cyclitic membrane 3 months postoperatively and underwent repeat vitrectomy. One patient developed fibrinoid syndrome noted on postoperative day 1, which was managed with systemic and topical steroids without significant response. This patient declined further intervention due to serious comorbidities and eventually developed a white cataract and hypotony. In the third patient, vision was limited by macular ischemia with photoreceptor loss and subsequent dense posterior capsular opacification, ERM, and cystoid macular edema formation under silicone oil for which the patient declined further surgical intervention.
Table 6. Visual function comparing preoperative and postoperative visual acuity (n = 69).
| BCVA | Number of Eyes (%), Preoperative | Number of Eyes (%), Postoperative |
|---|---|---|
| > = 20/50 | 1/69 (1.4) | 16/69 (23.2) |
| > = 20/200 | 12/69 (17.4) | 49/69 (71.0) |
| > = 5/200 | 18/69 (26.1) | 54/69 (78.3) |
BCVA, best-corrected Snellen visual acuity.
Discussion
In this study, we report a high rate of anatomic success and low incidence of complications in patients undergoing diabetic TRD repair. The rate of primary reattachment with single surgery and persistent attachment was 98.6% in our series despite the severe pathology seen at CCHHS. Additional vitrectomy was required for postoperative vitreous hemorrhage in 7.2% of cases. Only 5.8% (4/69) of eyes required repeat operation due to other causes including secondary retinal detachment, AHFVP, and ERM formation. Neovascular glaucoma developed in 1 eye (1.4%) but was managed medically without the need for surgery.
Previous studies that have analyzed outcomes of PPV for diabetic TRD have reported a wide range of results. Table 7 provides a detailed review of all relevant literature identified regarding small gauge (23G, 25G, and 27G) vitrectomy outcomes (n ≥ 20) in patients with diabetic TRD and TRD/RRD. For increased clarity in presenting previous outcomes data, we decided to group studies before or after 2007, as this appears to be the year when Eckardt’s transconjunctival sutureless 23G vitrectomy technique began to gain popularity over traditional 20G PPV,[13–18]. Before 2007, the overall rate for final retinal reattachment in TRD repair ranged from 53–88%[19–22] and the rate of reoperation was between 16–43% for TRD[19,20,22]. After 2007, the overall rate for final reattachment in TRD repair improved to 67%-100% [5,8,9,23–34] and the rate of reoperation decreased slightly to 6–31% [24,25,28,30,35]. The high reoperation rate both before and after 2007 is likely due to complications that can develop after diabetic vitrectomy. The most frequent complication is postoperative vitreous hemorrhage, which historically had an incidence of up to 75%[36,37], but more recently has been reported in 16–55% of cases [25,26,28,30,35,38]. Despite the large prevalence of this complication, vitreous hemorrhage typically only requires repeat vitrectomy in 5–10% of total cases[5,32,39]. Other common complications include secondary retinal detachment in up to 36% of cases, AHFVP in up to 13% of cases, and fibrinoid syndrome in up to 5% of cases, which represent serious conditions that require repeat vitrectomy and can significantly affect final vision[8],[5,32,40,41],[42]. Neovascular glaucoma occurred in 0–29% of cases before 2007[19–22,43–45] and 0–8% after 2007[5,8,9,18,25–28], with increased incidence in cases with lens removal during vitrectomy[32,46–48].
Table 7. Summary of previous literature on small gauge (23G, 25G, and 27G) vitrectomy outcomes (n ≥ 20) in patients with diabetic TRD and TRD/RRD.
| Author | Year | # Eyes DTRDa | Vitrectomy Gauge and Type | DTRD Pre-op VA (LogMAR unless otherwise noted) | DTRD Post-op VA (LogMAR unless otherwise noted) | Significant DTRD VA improvement (P value) | Tamponade | DTRD Complications | Primary Anatomic Success | Final Anatomic Success |
|---|---|---|---|---|---|---|---|---|---|---|
| Rahimy et. al[5] | 2015 | 62 (14.5% TRD/RRD) | 23G and 20G | 2 +/- 0.5 | 1.4 +/- 0.8 | Yes (0.0007) | None/air (37.1%), SO (25.8%), SF6 (19.4%), C3F8 (17.7%) | Secondary RRD (17.7%), NVG (8%) | — | 90.3% |
| Dikopf et. al[32] | 2015 | 70 (70% TRD/RRD) | 25G | 1.59 +/- 0.88 | 0.68 +/- 0.77 | Yes (<0.001) | SF6 (31.4%), C3F8 (31.4%), BSS (30%), SO (7.1%) | ERM (24%), recurrent vitreous hemorrhage (33%), OHTN (36%), hypotony (3%), NLP (5.7%) | 90% | 99% |
| Wang et. al[33] | 2016 | 66 (33.3% TRD/RRD in 4-port group and 26.7% TRD/RRD in 23G group) | 4-port bimanual 23G (45%) and 23G (55%) | 2.04 +/- 0.76 (4-port), 2.21 +/- 0.67 (23G) | 1.16 +/- 0.6 (4-port), 1.31 +/- 0.75 (23G) | Yes (<0.001 in both groups) | C3F8 (58.3% in 4-port and 50% in 23G), SO (41.7% in 4-port and 50% in 23G) | Secondary RD (5.6% 4-port and 10% in 23G), Hypotony (16.7% in 4-port and 3.3% in 23G), NVG (2.8% in 4-port and 6.7% in 23G), reoperation (11.1% in 4-port and 20% in 23G) | 94.4% (4-port group), 93.3% (23G group) | 100% |
| Mikhail et. al[34] | 2017 | 109 (29.3% TRD/RRD) | 25G | 1.17 | 0.812 | Yes (<0.05) | Air (38%), C3F8 (32%), SF6 (23%), SO (7%) | Hypotony (4.6%), OHTN post-op day 1 (10%), NVG (1.8%), recurrent VH requiring vitrectomy (10%) | 91% | 98% |
| Storey et. al[8] | 2018 | 403 (21.3% TRD/RRD) | 20G, 23G, and 25G | 1.73 | 1.23 | Yes (<0.0001) | None (37%), air (7.4%), C3F8 (23%), SF6 (8.4%), SO (24.3%) | Secondary RRD (5.2%), NVG (4.7%), VH post-op day 1 (28%), VH post-op week 1 (33%), VH post-op month 1 (20.9%), | 87.60% | 92.60% |
| Shroff et. al[9] | 2018 | 315 (33.9% macular TRD, 45.4% extramacular TRD, 15.2% TRD/RRD, 5.4% FVP with minimal detachment) | 23G and 25G | 1.67 +/- 0.63 | 0.78 +/- 0.63 | Yes (<0.001) | SF6 and C3F8 (52.7%), SO (25.7%), none (21.6%) | Secondary RD (1.6%), iatrogenic retinal break (52.1%), recurrent VH (18.4%), ERM (7.9%), sclerotomy site break (4.1%), intractable glaucoma (2.5%) endophthalmitis (0.6%) | 98.40% | 99% |
| Choovuthayakorn et. al[58] | 2019 | 455 (890–36.6% TRD and 13.5% TRD/RRD of entire cohort) | 20G and 23G (46% of TRD and TRD/RRD cases 23G) | 44% of eyes improved 2 or more Snellen lines | — | SO (40.4%) | RD (9%) | No DTRD subgroup analysis | ||
DTRD, diabetic tractional retinal detachment; TRD, tractional retinal detachment; RRD, rhegmatogenous retinal detachment; VA, visual acuity; LogMAR, logarithm of the minimum angle of resolution; SO, silicon oil,; RD, retinal detachment; AHFVP, anterior hyaloidal fibrovascular proliferation; NVG, neovascular glaucoma; VH, vitreous hemmorage; C3F8, perfluoropropane; SF6, sulfer hexafluoride; ERM, epiretinal membrane; OHTN, ocular hypertension; FVP, fibrovascular proliferation.
a all averages and percentages labeled DTRD represent both TRD and TRD/RRD unless otherwise noted.
Studies assessing visual outcomes for TRD repair typically report reaching postoperative visual acuity > = 20/100 in 7%-43% of cases, > = 20/200 in 36.2–62.5% of cases, > = 5/200 in 67–77% of cases, and visual improvement in up to 90% of cases [9,19–23,26,39,43,45,46,49–53]. In our series, 69.6% of eyes were characterized by preoperative macular involvement and 44.9% by combined TRD/RRD, with 55.1% of eyes reaching postoperative acuity > = 20/100, 71.0% of eyes reaching > = 20/200, 78.3% of eyes reaching > = 5/200, and visual improvement in 88.4% of eyes. Despite using tamponade in all cases, only 36.2% of phakic eyes required cataract surgery in the short-term follow-up period in this series. This rate is consistent with other studies describing the need for cataract extraction in 33%-87.1% of vitrectomized eyes for diabetic indications in one to four years postoperatively irrespective of tamponade status[30,54–56]. Visual outcomes in this study seem to be favorable compared to other studies with lower rates of complications, such as secondary retinal detachment and neovascular glaucoma.
It is reasonable to consider that the results of this study could partially be attributed to differences in disease severity or intraoperative technique. However, the patient population in our series represents an underserved community at a large urban county hospital, and is comprised of mostly Hispanic and African-American patients (93%) who frequently present with more advanced PDR[57]. As expected, these demographic and socioeconomic trends are reflected in the high anatomic complexity of these cases. Preoperatively and intraoperatively, many eyes were characterized by combined TRD/RRD (44.9%) and/or macular involvement (69.6%), and over 80% represented severe cases characterized by extensive broad adherent fibrovascular plaques. Thus, the patients in this series likely represent a more complex population than observed in previous studies.
Our overall surgical approach was consistent with many recent studies, but special attention was focused on meticulous removal of fibrovascular membranes and residual hyaloid with triamcinolone staining, which may contribute to both our favorable outcomes and our longer surgical durations [5,7–9]. Full PRP including the anterior retina with scleral depression could contribute to the lower rates of neovascular glaucoma and postoperative vitreous hemorrhage requiring PPV. It is unlikely that the results can be exclusively attributed to minor surgical differences alone since most studies for diabetic TRDs mention thorough removal of membranes and hyaloid and full PRP. Moreover, a distinction in our study was the use of long-acting tamponade in all cases, in particular gas tamponade with C3F8 14–16% in more than 90%% of cases, and prolonged face-down positioning. In most diabetic TRD studies in the literature, the tamponade is variable, including cases without any tamponade, air tamponade, variable concentrations of sulfur hexafloride (SF6) and C3F8, or silicon oil and information regarding patient positioning is rarely addressed.
Currently there are no strict guidelines for the use and the type of tamponade (short versus long-acting and gas versus silicon oil) or face-down positioning after vitrectomy for diabetic TRD repair. In this series, we favored the use of long-acting C3F8 rather than silicone oil, limiting the use of silicone oil mostly to cases involving large inferior retinectomies. In this study, we observed a significant improvement in BCVA in eyes that underwent C3F8 tamponade but not silicone oil. However, the six eyes that received silicone oil had worse preoperative BCVA and were selected to receive silicone oil tamponade due to their severe pathology needing large inferior retinectomies. This study is not randomized so definitive conclusions for the use of silicone oil versus gas cannot be drawn. Since C3F8 was used in more than 90% of the cases, our data indicates that excellent outcomes can be achieved by using long-acting C3F8 in complex TRD cases, even those with severe FVP and combined TRD/RRD.
Strengths of this study include the large population of eyes with complex diabetic TRD operated on by a single surgeon with consistent techniques at a single institution with unique demographics. Limitations of this study include the retrospective design, lack of grading of vitreous hemorrhage, lack of retinal break descriptions, potential for loss to follow-up bias due to the wide range of follow-up times (3–28 months), and the inability to make definite conclusions about tamponade agent and the need for prolonged face down positioning. Although our data suggests that long-acting C3F8 with prolonged face-down positioning may contribute to improved outcomes, the lack of randomization for the type of tamponade and duration of positioning and the absence of a control group do not allow us to make a definite conclusion.
In conclusion, our findings show that 23G vitrectomy can provide improved outcomes in patients with complex TRD pathology as described in our study representing Chicago’s urban population in a large public hospital. Despite our efforts to prevent late-stage diabetic retinopathy and TRD, many patients are unfortunately faced with these debilitating conditions. Although medical and surgical advancements have greatly improved treatment modalities, further research is warranted to better elucidate which techniques lead to the best outcomes for our patients.
Supporting information
All data used to calculate means, standard deviations, confidence intervals, and P values in manuscript.
(XLSX)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Fong DS, Ferris FL, Davis MD, Chew EY. Causes of severe visual loss in the early treatment diabetic retinopathy study: ETDRS report no. 24. Early Treatment Diabetic Retinopathy Study Research Group. Am J Ophthalmol. 1999;127: 137–141. 10.1016/s0002-9394(98)00309-2 [DOI] [PubMed] [Google Scholar]
- 2.Ryan SJ, Schachat AP, Wilkinson CP, Hinton DR, Sadda SR, Wiedemann P. Retina. Elsevier Health Sciences; 2012. [Google Scholar]
- 3.Machemer R, Buettner H, Norton EW, Parel JM. Vitrectomy: a pars plana approach. Trans Am Acad Ophthalmol Otolaryngol. 1971;75: 813–820. [PubMed] [Google Scholar]
- 4.Michels RG. Proliferative diabetic retinopathy: pathophysiology of extraretinal complications and principles of vitreous surgery. Retina. 1981;1: 1–17. [PubMed] [Google Scholar]
- 5.Rahimy E, Pitcher JD, Gee CJ, Kreiger AE, Schwartz SD, Hubschman J-P. Diabetic tractional retinal detachment repair by vitreoretinal fellows in a county health system. Retina. 2015;35: 303–309. 10.1097/IAE.0000000000000310 [DOI] [PubMed] [Google Scholar]
- 6.Khan MA, Kuley A, Riemann CD, Berrocal MH, Lakhanpal RR, Hsu J, et al. Long-Term Visual Outcomes and Safety Profile of 27-Gauge Pars Plana Vitrectomy for Posterior Segment Disease. Ophthalmology. 2018;125: 423–431. 10.1016/j.ophtha.2017.09.013 [DOI] [PubMed] [Google Scholar]
- 7.Khan MA, Samara WA, Hsu J, Garg S. Short-term outcomes of hybrid 23-, 25-, and 27-gauge vitrectomy for complex diabetic tractional retinal detachment repair. Retin Cases Brief Rep. 2017; 10.1097/ICB.0000000000000571 [DOI] [PubMed] [Google Scholar]
- 8.Storey PP, Ter-Zakarian A, Philander SA, Olmos de Koo L, George M, Humayun MS, et al. Visual and anatomical outcomes after diabetic traction and traction-rhegmatogenous retinal detachment repair. Retina. 2017; 10.1097/IAE.0000000000001793 [DOI] [PubMed] [Google Scholar]
- 9.Shroff CM, Gupta C, Shroff D, Atri N, Gupta P, Dutta R. Bimanual microincision vitreous surgery for severe proliferative diabetic retinopathy: outcome in more than 300 eyes. Retina. 2018;38 Suppl 1: S134–S145. 10.1097/IAE.0000000000002093 [DOI] [PubMed] [Google Scholar]
- 10.2014 Hospital Profile rev 11-19-15.pdf [Internet]. http://www.idph.state.il.us/about/hfpb/pdf/2014%20Hospital%20Profile%20rev%2011-19-15.pdf
- 11.Flaxel CJ, Dustin L, Kim J, Bekendam P, Row P. Outcome of diabetic vitrectomy in Latino population. Retina. 2007;27: 1274–1278. 10.1097/IAE.0b013e31805d0bfb [DOI] [PubMed] [Google Scholar]
- 12.Lange C, Feltgen N, Junker B, Schulze-Bonsel K, Bach M. Resolving the clinical acuity categories “hand motion” and “counting fingers” using the Freiburg Visual Acuity Test (FrACT). Graefes Arch Clin Exp Ophthalmol. 2009;247: 137–142. 10.1007/s00417-008-0926-0 [DOI] [PubMed] [Google Scholar]
- 13.Eckardt C. Transconjunctival sutureless 23-gauge vitrectomy. Retina. 2005;25: 208–211. [DOI] [PubMed] [Google Scholar]
- 14.Wimpissinger B, Kellner L, Brannath W, Krepler K, Stolba U, Mihalics C, et al. 23-Gauge versus 20-gauge system for pars plana vitrectomy: a prospective randomised clinical trial. Br J Ophthalmol. 2008;92: 1483–1487. 10.1136/bjo.2008.140509 [DOI] [PubMed] [Google Scholar]
- 15.Tsang C-W, Cheung BT-O, Lam RF, Lee GK-Y, Yuen CY-F, Lai TY-Y, et al. Primary 23-gauge transconjunctival sutureless vitrectomy for rhegmatogenous retinal detachment. Retina. 2008;28: 1075–1081. 10.1097/IAE.0b013e31817b98ba [DOI] [PubMed] [Google Scholar]
- 16.Oliveira LB, Reis PAC. Silicone oil tamponade in 23-gauge transconjunctival sutureless vitrectomy. Retina. 2007;27: 1054–1058. 10.1097/IAE.0b013e318113235e [DOI] [PubMed] [Google Scholar]
- 17.El-Batarny AM. Transconjunctival Sutureless 23-gauge Vitrectomy for Vitreoretinal Diseases: Outcome of 30 Consecutive Cases. Middle East Afr J Ophthalmol. 2008;15: 99–105. 10.4103/0974-9233.51983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fine HF, Iranmanesh R, Iturralde D, Spaide RF. Outcomes of 77 consecutive cases of 23-gauge transconjunctival vitrectomy surgery for posterior segment disease. Ophthalmology. 2007;114: 1197–1200. 10.1016/j.ophtha.2007.02.020 [DOI] [PubMed] [Google Scholar]
- 19.Thompson JT, de Bustros S, Michels RG, Rice TA. Results and prognostic factors in vitrectomy for diabetic traction retinal detachment of the macula. Arch Ophthalmol. 1987;105: 497–502. 10.1001/archopht.1987.01060040067035 [DOI] [PubMed] [Google Scholar]
- 20.Williams DF, Williams GA, Hartz A, Mieler WF, Abrams GW, Aaberg TM. Results of vitrectomy for diabetic traction retinal detachments using the en bloc excision technique. Ophthalmology. 1989;96: 752–758. 10.1016/s0161-6420(89)32813-2 [DOI] [PubMed] [Google Scholar]
- 21.Meier P, Wiedemann P. Vitrectomy for traction macular detachment in diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 1997;235: 569–574. [DOI] [PubMed] [Google Scholar]
- 22.Mason JO, Colagross CT, Haleman T, Fuller JJ, White MF, Feist RM, et al. Visual outcome and risk factors for light perception and no light perception vision after vitrectomy for diabetic retinopathy. Am J Ophthalmol. 2005;140: 231–235. 10.1016/j.ajo.2005.02.052 [DOI] [PubMed] [Google Scholar]
- 23.Altan T, Acar N, Kapran Z, Unver YB, Ozdogan S. Transconjunctival 25-gauge sutureless vitrectomy and silicone oil injection in diabetic tractional retinal detachment. Retina. 2008;28: 1201–1206. 10.1097/IAE.0b013e3181853d3c [DOI] [PubMed] [Google Scholar]
- 24.Arevalo JF. En bloc perfluorodissection for tractional retinal detachment in proliferative diabetic retinopathy. Ophthalmology. 2008;115: e21–25. 10.1016/j.ophtha.2008.02.008 [DOI] [PubMed] [Google Scholar]
- 25.Oshima Y, Shima C, Wakabayashi T, Kusaka S, Shiraga F, Ohji M, et al. Microincision vitrectomy surgery and intravitreal bevacizumab as a surgical adjunct to treat diabetic traction retinal detachment. Ophthalmology. 2009;116: 927–938. 10.1016/j.ophtha.2008.11.005 [DOI] [PubMed] [Google Scholar]
- 26.Yorston D, Wickham L, Benson S, Bunce C, Sheard R, Charteris D. Predictive clinical features and outcomes of vitrectomy for proliferative diabetic retinopathy. Br J Ophthalmol. 2008;92: 365–368. 10.1136/bjo.2007.124495 [DOI] [PubMed] [Google Scholar]
- 27.Tao Y, Jiang Y-R, Li X-X, Gao L, Jonas JB. Long-term results of vitrectomy without endotamponade in proliferative diabetic retinopathy with tractional retinal detachment. Retina. 2010;30: 447–451. 10.1097/IAE.0b013e3181d374a5 [DOI] [PubMed] [Google Scholar]
- 28.Farouk MM, Naito T, Sayed KM, Nagasawa T, Katome T, Radwan G, et al. Outcomes of 25-gauge vitrectomy for proliferative diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 2011;249: 369–376. 10.1007/s00417-010-1506-7 [DOI] [PubMed] [Google Scholar]
- 29.Fortun JA, Hubbard GB. New viscodissection instrument for use with microincisional vitrectomy in the treatment of diabetic tractional retinal detachments. Arch Ophthalmol. 2011;129: 352–355. 10.1001/archophthalmol.2011.15 [DOI] [PubMed] [Google Scholar]
- 30.Gupta B, Wong R, Sivaprasad S, Williamson TH. Surgical and visual outcome following 20-gauge vitrectomy in proliferative diabetic retinopathy over a 10-year period, evidence for change in practice. Eye. 2012;26: 576–582. 10.1038/eye.2011.348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Velez-Montoya R, Guerrero-Naranjo JL, Garcia-Aguirre G, Morales-Cantón V, Fromow-Guerra J, Quiroz-Mercado H. Perfluorocarbon-perfused 23 gauge three-dimensional vitrectomy for complicated diabetic tractional retinal detachment. Clin Ophthalmol. 2011;5: 1709–1715. 10.2147/OPTH.S26838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dikopf MS, Patel KH, Setlur VJ, Lim JI. Surgical outcomes of 25-gauge pars plana vitrectomy for diabetic tractional retinal detachment. Eye. 2015;29: 1213–1219. 10.1038/eye.2015.126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Z-Y, Zhao K-K, Li J-K, Rossmiller B, Zhao P-Q. Four-port bimanual 23-gauge vitrectomy for diabetic tractional retinal detachment. Acta Ophthalmol. 2016;94: 365–372. 10.1111/aos.12951 [DOI] [PubMed] [Google Scholar]
- 34.Mikhail M, Ali-Ridha A, Chorfi S, Kapusta MA. Long-term outcomes of sutureless 25-G+ pars-plana vitrectomy for the management of diabetic tractional retinal detachment. Graefes Arch Clin Exp Ophthalmol. 2017;255: 255–261. 10.1007/s00417-016-3442-7 [DOI] [PubMed] [Google Scholar]
- 35.Ozone D, Hirano Y, Ueda J, Yasukawa T, Yoshida M, Ogura Y. Outcomes and complications of 25-gauge transconjunctival sutureless vitrectomy for proliferative diabetic retinopathy. Ophthalmologica. 2011;226: 76–80. 10.1159/000328407 [DOI] [PubMed] [Google Scholar]
- 36.Novak MA, Rice TA, Michels RG, Auer C. Vitreous hemorrhage after vitrectomy for diabetic retinopathy. Ophthalmology. 1984;91: 1485–1489. 10.1016/s0161-6420(84)34099-4 [DOI] [PubMed] [Google Scholar]
- 37.Schachat AP, Oyakawa RT, Michels RG, Rice TA. Complications of vitreous surgery for diabetic retinopathy. II. Postoperative complications. Ophthalmology. 1983;90: 522–530. 10.1016/s0161-6420(83)34540-1 [DOI] [PubMed] [Google Scholar]
- 38.Schoenberger SD, Miller DM, Riemann CD, Foster RE, Sisk RA, Hutchins RK, et al. Outcomes of 25-gauge pars plana vitrectomy in the surgical management of proliferative diabetic retinopathy. Ophthalmic Surg Lasers Imaging. 2011;42: 474–480. 10.3928/15428877-20110901-02 [DOI] [PubMed] [Google Scholar]
- 39.Steinmetz RL, Grizzard WS, Hammer ME. Vitrectomy for diabetic traction retinal detachment using the multiport illumination system. Ophthalmology. 2002;109: 2303–2307. 10.1016/s0161-6420(02)01291-5 [DOI] [PubMed] [Google Scholar]
- 40.Lewis H, Abrams GW, Williams GA. Anterior hyaloidal fibrovascular proliferation after diabetic vitrectomy. Am J Ophthalmol. 1987;104: 607–613. 10.1016/0002-9394(87)90173-5 [DOI] [PubMed] [Google Scholar]
- 41.Sebestyen JG. Fibrinoid syndrome: a severe complication of vitrectomy surgery in diabetics. Ann Ophthalmol. 1982;14: 853–856. [PubMed] [Google Scholar]
- 42.Meleth AD, Carvounis PE. Outcomes of vitrectomy for tractional retinal detachment in diabetic retinopathy. Int Ophthalmol Clin. 2014;54: 127–139. 10.1097/IIO.0000000000000021 [DOI] [PubMed] [Google Scholar]
- 43.Tolentino FI, Freeman HM, Tolentino FL. Closed vitrectomy in the management of diabetic traction retinal detachment. Ophthalmology. 1980;87: 1078–1089. 10.1016/s0161-6420(80)35115-4 [DOI] [PubMed] [Google Scholar]
- 44.Canny CL, O’Hanley GP, Wells GA. Pars plana vitrectomy for the complications of diabetic retinopathy: a report on 131 cases. Can J Ophthalmol. 1985;20: 11–15. [PubMed] [Google Scholar]
- 45.Flynn HW, Chew EY, Simons BD, Barton FB, Remaley NA, Ferris FL. Pars plana vitrectomy in the Early Treatment Diabetic Retinopathy Study. ETDRS report number 17. The Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology. 1992;99: 1351–1357. 10.1016/s0161-6420(92)31779-8 [DOI] [PubMed] [Google Scholar]
- 46.La Heij EC, Tecim S, Kessels AGH, Liem ATA, Japing WJ, Hendrikse F. Clinical variables and their relation to visual outcome after vitrectomy in eyes with diabetic retinal traction detachment. Graefes Arch Clin Exp Ophthalmol. 2004;242: 210–217. 10.1007/s00417-003-0815-5 [DOI] [PubMed] [Google Scholar]
- 47.Rice TA, Michels RG, Maguire MG, Rice EF. The effect of lensectomy on the incidence of iris neovascularization and neovascular glaucoma after vitrectomy for diabetic retinopathy. Am J Ophthalmol. 1983;95: 1–11. 10.1016/0002-9394(83)90328-8 [DOI] [PubMed] [Google Scholar]
- 48.Kwon J, Jee D, La TY. Neovascular glaucoma after vitrectomy in patients with proliferative diabetic retinopathy. Medicine (Baltimore). 2017;96 10.1097/MD.0000000000006263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smiddy WE, Flynn HW. Vitrectomy in the management of diabetic retinopathy. Surv Ophthalmol. 1999;43: 491–507. [DOI] [PubMed] [Google Scholar]
- 50.Wu WC, Lin JC. The experience to use a modified en bloc excision technique in vitrectomy for diabetic traction retinal detachment. Kaohsiung J Med Sci. 1999;15: 461–467. [PubMed] [Google Scholar]
- 51.Rice TA, Michels RG, Rice EF. Vitrectomy for diabetic traction retinal detachment involving the macula. Am J Ophthalmol. 1983;95: 22–33. 10.1016/0002-9394(83)90330-6 [DOI] [PubMed] [Google Scholar]
- 52.Berrocal MH. All-probe vitrectomy dissection techniques for diabetic tractional retinal detachments: Lift and shave. Retina. 2018;38 Suppl 1: S2–S4. 10.1097/IAE.0000000000001884 [DOI] [PubMed] [Google Scholar]
- 53.Sternfeld A, Axer-Siegel R, Stiebel-Kalish H, Weinberger D, Ehrlich R. Advantages of diabetic tractional retinal detachment repair. Clin Ophthalmol. 2015;9: 1989–1994. 10.2147/OPTH.S90577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Silva PS, Diala PA, Hamam RN, Arrigg PG, Shah ST, Murtha TL, et al. Visual outcomes from pars plana vitrectomy versus combined pars plana vitrectomy, phacoemulsification, and intraocular lens implantation in patients with diabetes. Retina. 2014;34: 1960 10.1097/IAE.0000000000000171 [DOI] [PubMed] [Google Scholar]
- 55.Ostri C, Lux A, Lund-Andersen H, la Cour M. Long-term results, prognostic factors and cataract surgery after diabetic vitrectomy: a 10-year follow-up study. Acta Ophthalmol. 2014;92: 571–576. 10.1111/aos.12325 [DOI] [PubMed] [Google Scholar]
- 56.Kumar A, Duraipandi K, Gogia V, Sehra SV, Gupta S, Midha N. Comparative evaluation of 23- and 25-gauge microincision vitrectomy surgery in management of diabetic macular traction retinal detachment. Eur J Ophthalmol. 2014;24: 107–113. 10.5301/ejo.5000305 [DOI] [PubMed] [Google Scholar]
- 57.Emanuele N, Sacks J, Klein R, Reda D, Anderson R, Duckworth W, et al. Ethnicity, Race, and Baseline Retinopathy Correlates in the Veterans Affairs Diabetes Trial. Diabetes Care. 2005;28: 1954–1958. 10.2337/diacare.28.8.1954 [DOI] [PubMed] [Google Scholar]
- 58.Choovuthayakorn J, Khunsongkiet P, Patikulsila D, Watanachai N, Kunavisarut P, Chaikitmongkol V, et al. Characteristics and Outcomes of Pars Plana Vitrectomy for Proliferative Diabetic Retinopathy Patients in a Limited Resource Tertiary Center over an Eight-Year Period. J Ophthalmol. 2019;2019: 9481902 10.1155/2019/9481902 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
All data used to calculate means, standard deviations, confidence intervals, and P values in manuscript.
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.