Abstract
Gallarhois (GR) is a traditional oriental herbal medicine with various pharmacological effects; however, its effect on gastric ulcer has not been previously explored. We firstly investigated the component and antioxidant activity of GR extract (EtGR) by HPLC analysis and 1,1-diphenyl-2-picrylhydrazyl (DPPH) assay. The results showed that EtGR consisted of gallotannin (68.7%), gallic acid (27.2%) and methyl gallate (4.1%) and that it had a high antioxidant value (IC50 value; 1.93 μg/mL). To evaluate the possible anti-gastric ulcer potential of EtGR, we investigated the effects of EtGR in the model of ethanol/hydrochloric acid (EtOH/HCl)-induced gastric ulcer. Gross and histological gastric lesions, biochemical and gene expression parameters were taken into consideration. The results showed that EtOH/HCl treatment produced mucosal injuries with morphological and histological damage, whereas EtGR co-treatment reduced the gastric injuries. EtGR treatment also decreased the contents of malonaldehyde (MDA) activity relative to the vehicle group. Moreover, EtGR decreased the levels of interleukin-1β (IL-1β), interleukin-6 (IL-6) and cyclo-oxygenase-2 (COX-2) expression. Finally, EtGR did not induce any specific toxicity in the livers or kidneys of the EtOH/HCl-induced gastric ulcer model. These results suggest that EtGR had stronger antioxidant activity and could be a new useful natural drug for gastroprotection against gastric ulcer. Moreover, these findings provide a scientific basis for the development of drugs from traditional oriental herbal medicines.
Keywords: Gallarhois, Anti-gastric ulcer, Antioxidant, Toxicity, Animal model
Abbreviations: GR, Gallarhois; EtGR, Gallarhois extract; EtOH/HCl, ethanol/hydrochloric acid; MDA, malonaldehyde; IL-1β, Interleukin-1β; IL-6, Interleukin-6; COX-2, cyclo-oxygenase-2
Graphical abstract
1. Introduction
Gastric ulcers are a serious disease that affects about 5–10% of the world's population.1 Gastric ulcers are caused by injury to the gastrointestinal mucosa by smoking, stress, alcohol consumption, prolonged ingestion of nonsteroidal anti-inflammatory drugs (NSAIDs), gastric acid hypersecretion, pepsin activity, gastric contractions, gastric mucosa ischemia and particularly infection by the bacteria, Helicobacter pylori.2, 3 It is well known orally consumed ethanol is rapidly absorption into the blood stream from the stomach and intestine.4, 5 In areas of acute gastric ulcer, high-concentration of ethanol directly destroyed gastric mucosa, infiltration of neutrophil, the over-expression of Nuclear factor-kB (NF-kB), and the release of pro-inflammatory cytokines.6, 7 This process induces the production of reactive oxygen species (ROS), which are responsible for cell damage and death.8 Furthermore, there is increasing evidence that oxidative stress plays a pivotal role in the pathological processes of gastric ulcer.9 Therefore, many efforts have been made to treat gastritis using antioxidants that regulate oxidative stress.10, 11, 12
Although they are not representative of the exact mechanism of all gastric ulcers, various gastric ulcer animal models are known, including ceramide-induced ulcer, indomethacin induced ulcer, Hcl-induced ulcer, cysteamine induced ulcer and ethanol induced ulcer.13, 14, 15 Among these, the ethanol (EtOH)-induced ulcer model is most preferential used animal model because it enables rapid induction and can be widely employed to test the efficacy of potential drugs independent of gastric acid secretion. Moreover, the ethanol-induced gastric ulcer model resembles acute gastric ulcers in human.16, 17 In recent years, there has been an increased effort to identify herbal derived therapeutic agents with reduced side effects for the treatment of gastric ulcers caused in animal models.18, 19 In Asian countries, including Korea, Japan and China, traditional medicines such as herbal extract have long been used to treat infectious and inflammatory diseases.20, 21, 22 GallaRhois (GR) has long been used in traditional herbal medicine to treat diarrhea, persistent coughing and spontaneous perspiration. This material is found in various parts of Korea, Japan and China. The main component of GR is tannin (50–60%), which affects branching and hemostasis, and inhibits the glandular secretions. In addition, GR has antimicrobial, antioxidant, and antithrombotic effects.23, 24 Interestingly, Zaidi et al. have reported that GR has an inhibitory effect on Helicobacter pylori infection.25 However, the anti-ulcer activities of EtGR toward gastric ulcer are unknown. Therefore, we observed the components and antioxidant activity of EtGR and investigated its activity against EtOH/HCl -induced gastric ulcer experimental models in mice.
2. Materials and methods
2.1. Preparation of EtGR
The GR, which was obtained from HongcheonNonghyup (http://www.hcari.co.kr), was harvested in the Hongcheon area during October or 2013. The specimens were dried at 60 °C in a hot air dryer (JSR, Seoul, Korea) and then stored. The aqueous extract of GR was obtained from the powder of the dried sample at 90 °C for 9 h using a circulation extractor (IKA Labortechnik, Staufen, Germany) at a fixed liquid ratio (solid GR powder/water ratio, 1:10). These solutions were then passed through a 0.4 μm filter and concentrated by vacuum evaporation and lyophilization using a circulating extractor (IKA Labortechnik, Staufen, Germany).
2.2. Analysis of functional compounds
A standard curve was generated using standard components such as, Gallic acid monohydrate (IUPAC name; 3,4,5-trihydroxybenzoic acid, MW: 170.12 g/mol, Sigma-Aldrich Co., St. Louis, MO, USA), methyl gallate (IUPAC name; methyl 3,4,5-trihydroxybenzoate, MW: 184.15 g/mol, Sigma-Aldrich Co.) and gallotanin (IUPAC name; 3,5-dihydroxy-2-(3,4,5-trihydroxybenzoyl)oxy-6-[(3,4,5-trihydroxybenzoyl)oxymethyl]oxan-4-yl] 3,4,5-trihydroxybenzoate, MW: 1701.20 g/mol, Sigma-Aldrich Co.). The maximum absorption wavelengths of pure gallic acid, pure methyl gallate, commercial gallotannin and gallnut extract were 212/257, 214/268, 213/278 and 212/275 nm, respectively. The UV-VIS spectra of pure methyl gallate, pure gallotannin and gallnut extracts were detected at 212–214 nm and 257–278 nm. The UV-Vis spectra were then analyzed by the curve analysis technique using a linear least-squares fit for a combination of Lorentzian and Gaussian curve shapes.
The HPLC analysis of EtGR was performed using a Summit Dual-Gradient HPLC System (Dionex, Sunnyvale, CA, USA) with a PDA UV-vis detector at the Korea Bio-IT Foundry Busan Center. The separation was performed on a YMC-Triart C18 column (S-5 mm/12 nm, 150 mm × 4.6 mm I.D.) maintained at 40 °C. The mobile phase consisted of solvent A (0.4% formic acid in water) and solvent B (acetonitrile). The gradient condition of the mobile phase was as follows: 0–5 min, 10% B; 5–6 min, 10%–15% B; 6–40 min, 15% B; 40–41 min, 15%–30% B; 41–50 min, 30% B; 50–55 min, 30%–10% B; 55–60 min, 10% B. The injection volume was 5 mL in full loop injection, while the flow rate was 0.8 mL/min and detection was performed at 280 nm.26
2.3. DPPH radical scavenging assay
DPPH (2,2-diphenyl-1-picryl-hydrazyl-hydrate) was purchased from Sigma Chemical Co. (St. Louis, MO). The DPPH free radical method is an antioxidant assay based on electron-transfer that produces a violet solution in ethanol. A 200 μl test sample was mixed with 4 mL of 99.5% ethanol containing DPPH (0.07 mM), after which the absorbance of the mixture at 517 nm was measured from 0 min to 60 min using a Versa-max plate reader (Molecular Devices, Sunnyvale, CA, USA). The half maximal inhibitory concentration (IC)50 value is defined as the concentration of substrate that causes a 50% loss in DPPH activity. Each sample (containing 13 different concentrations of GEGRs (0.122–125 μg/ml)) was analyzed in triplicate.26, 27
2.4. Animal experiment
Six-week-old male Korl:ICR mice were obtained from the Department of Laboratory Animal Resources in the National Institute of Food and Drug Safety Evaluation (NIFDS, Cheongju, Korea). The mice were provided with commercial food (Samtako Inc., Osan, Korea) and water. During the experiment, mice were maintained in a specific pathogen free condition under a strict light cycle (lights on at 08:00 h and off at 20:00 h) at 23 ± 2 °C and 50 ± 10% relative humidity. The mice were housed in the Pusan National University-Laboratory Animal Resources Center accredited by the Korea Ministry of Food and Drug Safety (MFDS) in accordance with the Laboratory Animal Act (Accredited Unit Number-000231) and AAALAC International according to the National Institutes of Health guidelines (Accredited Unit Number; 001525). Prior to the animal experiment, the protocols were approved by the Pusan National University-Institutional Animal Care and Use Committee (PNU-IACUC; Approval Number PNU-2015-0916). Moreover, alterations in body weight were measured using an electronic balance (Mettler Toledo, Greifensee, Switzerland) once a week according to the MFDS guideline.
2.5. Induction of gastric ulcer using EtOH/HCl treatment
To evaluate the anti-gastric ulcer activity of the EtGR extract, gastric ulcer was induced by modified methods. After 24 h of food starvation, mice (six-week old, n = 8) were randomly divided into five groups. The first (NO group, n = 6) received a constant volume of dH2O, while the second group (vehicle + Ulc group, n = 10) were administered 0.2 mL of 70% EtOH/150 Mm HCl. The remaining groups were pre-treated with EtGR (100, 200 and 400 mg/kg; EtGR1, 2, 3 + Ulc groups) for 7 days, then treated with 0.2 mL of 70% EtOH/150 Mm HCl. One hour after treatment, all animals were euthanized using a chamber filled with CO2 gas and experimental samples were collected.
2.6. Gastric lesions analysis
To harvest the sample of gastric lesions, the stomachs were removed from the ICR mice of each experimental group and opened along the greater curvature. After repeatedly washing with 1× PBS, the inside of the stomach was photographed using a Canon® digital camera (Canon Inc., Japan). The area of visible erosive lesions was measured using the Leica Application Suite (Leica Microsystems, Wetzlar, Germany) and the images were analyzed using the Image J software (National Institutes of Health, Maryland, USA). The ulcer lesion index was calculated as follows28: Lesions index (%) = (gastric injury area/gastric total area of mice)×100%.
2.7. Histological analysis
For histological analysis, the stomach tissues were fixed in 10% formalin for 24 h, after which they were embedded in paraffin and then sectioned into 4 μm thick slices. The stomach sections were subsequently stained with hematoxylin and eosin (H&E) and examined by light microscopy for alterations in histological structure. To accurately assess the lesions, specimens were analyzed according to previously defined criteria.29 Briefly, a 1 cm segment of each histological section was assessed for mucosal injury including epithelial cell loss (score: 0–3), edema (score: 0–3), and the presence of inflammatory cells (score: 0–3).
2.8. Reverse transcription-polymerase chain reaction (RT-PCR) analysis
For RT-PCR analysis, stomach specimens were homogenized in RNAzol B solution (Tet-Test Inc., Texas, USA), and preparation of cDNA was synthesized by reverse transcriptase. Subsequently, primers were added, and the reaction mixture was subjected to 32 cycles of amplification in a Perkin-Elmer Thermal Cycler as follows: 1 min at 94 °C, 1 min at 62 °C, and 1 min at 72 °C. RT-PCR was also conducted using β-actin specific primers to ensure RNA integrity. The primers sequences for IL-Iβ sense and antisense primers were as follows: 5′-GCA CAT CAA CAA GAG CTT CAG-3′ and 5′-GGT ACA TCA GCA CCT CAC AAG CAGAG-3'. The primer sequences for IL-6 sense and antisense primers were 5′-TTG GGA CTG ATG TTG TTG ACA-3′ and 5′-TCA TCG CTG TTC ATA CAA TCA GA-3'. The primer sequences for Cox-2 sense and antisense primers were 5′-CAG GTC ATT GGT GGA GAG GTG TAT C-3′ and 5′-CCA GGA GGA TGG AGT TAT TAT AGA G-3'. The sequences of the β-actin sense and antisense primers were 5′-TGG AAT CCT GTG GCA TCC ATGAAA C-3′ and 5′-TAA AAC GCA GCT CAG TAACAG TCC G-3′, respectively. All samples were analyzed in triplicate, and the final PCR products were separated by 1% agarose gel electrophoresis and visualized by ethidium bromide staining.
2.9. Determination of MDA level
The MDA concentration in gastric mucosa ware evaluated using a Lipid Peroxidation (MDA) Assay Kit (Cat. No. MAK085, Sigma-Aldrich Co.) The level of MDA was measured absorbance at 523 nm by Vmax ELISA reader (Molecular Devices). The final results were reported as nmol/mg protein.
2.10. Serum biochemical analysis
To harvest the serum sample, whole blood of the target mice in the individual groups was collected from their abdominal veins and incubated for 1 h at room temperature. Serum was then obtained by centrifugation and analyzed for alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), creatinine (Crea) and blood urea nitrogen (BUN) using an automatic serum analyzer (HITACHI 747, Tokyo, Japan). All assays were conducted in triplicate using fresh serum.
2.11. Statistical analysis
One-way ANOVA was used to identify significant differences between EtOH/HCl plus vehicle and EtOH/HCl plus EtGR treated groups (SPSS for Windows, Release 10.10, Standard Version, Chicago, Illinois, USA). All values are reported as the means ± standard deviation (SD) and a P value of<0.05 was considered significant.
3. Result
3.1. Identification of EtGR components and evaluation of antioxidant activity of EtGR
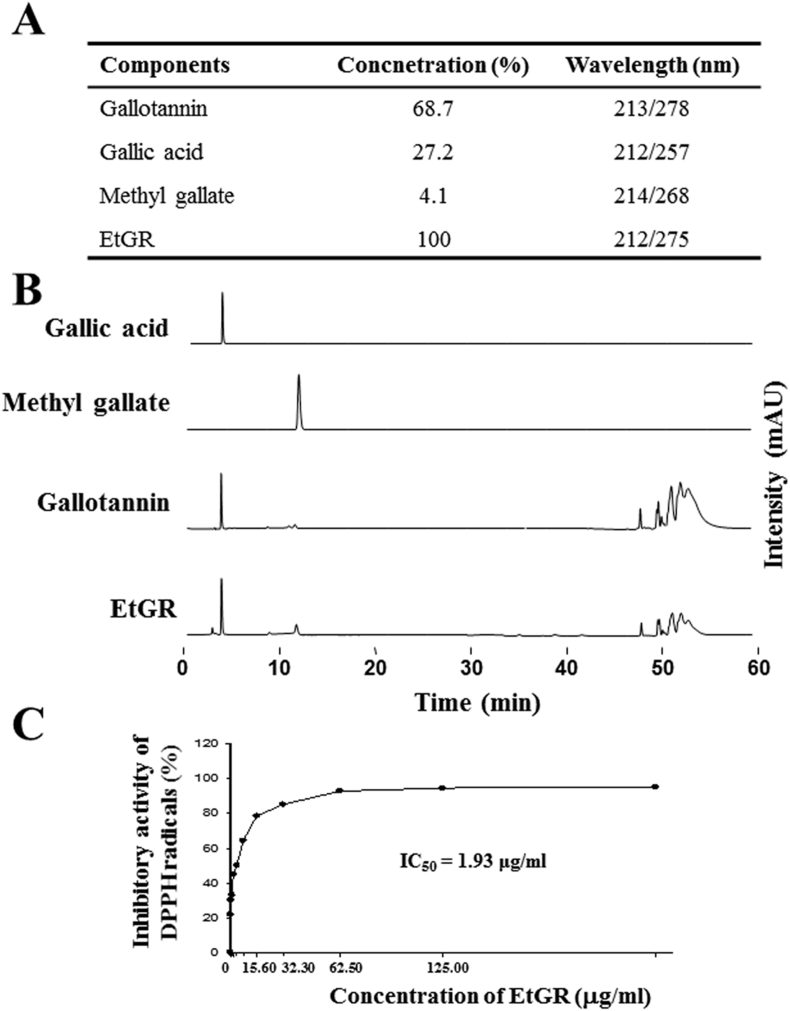
HPLC analysis was used to identify the tannins in EtGR (Fig. 1A and B). The tannins of EtGR produced three prominent peaks corresponding to gallotannin (68.7%), gallic acid (27.2%) and methyl gallate (4.1%). The antioxidant activity of EtGR for scavenging of free radicals in vitro was evaluated by DPPHA assay. As shown in Fig. 1C, EtGR demonstrated radical scavenging activity and an IC50 value of EtGR of 1.93 μg/mL. The results are similar to those of the previous experiments. However, the antioxidant activity of EtGR was slightly decreased.26
Fig. 1.
Effects of EtGR on the anti-oxidant activity and determination of components by HPLC analysis. (A) The distribution of three major components of EtGR, gallotannin, gallic acid and methylgallate, was analyzed based on their UV-Vis spectra. (B) HPLC chromatograms of pure gallic acid (commercial chemical), pure methylgallate (commercial chemical), pure gallotannin (commercial chemical) and EtGR. (C) DPPH radical scavenging activity of EtGR. The data shown represent the means ± SD of three replicates.
3.2. Evaluation of oral administration toxicity to EtOH/HCl and EtGR in mice
It is well known that changes in body weight can be used to determine the toxicity of a chemical compound toward an animal. After 24 h of food starvation, the No group mice received a constant volume of dH2O, while other groups (Vehicle + Ulc and EtGR + Ulc treated group) were administered 0.2 mL 70%EtOH in 150 mM HCl. There were no significant changes in body weight during treatment with EtOH/HCl and EtGR (Table 1), indicating that EtOH/HCl administration to induce gastric ulcer and EtGR treatment did not affect changes in body weight in mice.
Table 1.
Changes in body weight in ICR mice.
| Groups | Con | Ve | G100 | G200 | G400 |
|---|---|---|---|---|---|
| Before Ad. | 33.50 ± 2.59 | 34.80 ± 2.25 | 30.60 ± 2.84 | 34.30 ± 1.77 | 32.10 ± 2.73 |
| After Ad. | 32.54 ± 2.64 | 33.98 ± 3.79 | 33.67 ± 2.63 | 33.67 ± 2.11 | 32.29 ± 2.63 |
The data shown represent the means ± SD of three replicates. Abbreviation: Ad, administration.
3.3. Effect of EtGR on gastric lesions in EtOH/HCl-induced gastric ulcer model
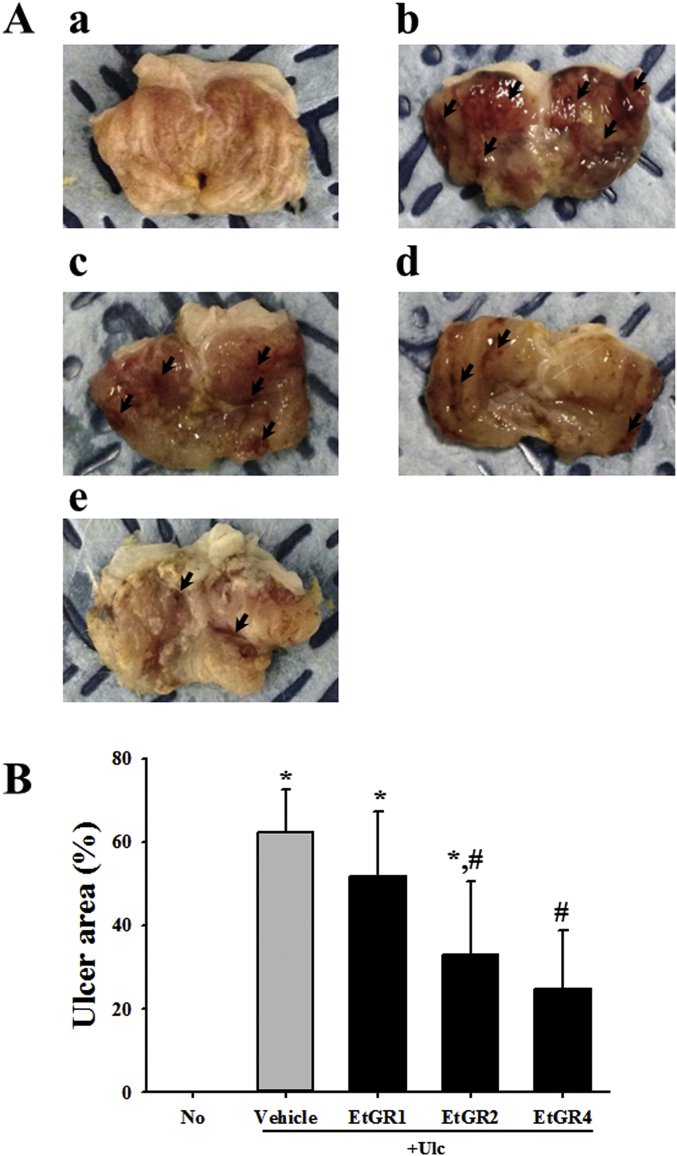
To analyze the effects of EtGR on EtOH/HCl-induced gastric ulcers, we harvested stomach specimen following administration of three different concentrations of EtGR to EtOH/HCl treated mice. As shown in Fig. 2, the Vehicle + Ulc group displayed typical gastric lesion index of the stomach when compared with the No group. However, the lesion index of EtGR + Ulc treated group was markedly decreased in a dose dependent manner when compared with the vehicle + Ulc treated group.
Fig. 2.
Effects of EtGR on lesions in the stomachs of ICR mice with EtOH/HCl -induced gastritis. (A) After opening along the greater curvature, morphological features were observed in the lesion of stomachs of EtOH/HCl treated ICR mice. Arrows indicate the area of hemorrhagic lesions in the inner surface of the stomach. (B) After measurement of the area of visible erosive lesions, the lesion index was calculated using the formula provided in the materials and methods. The data shown represent the means ± standard deviation (SD) of three replicates. *, P < 0.05 relative to the No group. #, P < 0.05 relative to the Vehicle + Ulc group.
3.4. Histopathological evaluation of EtOH/HCl-induced gastric ulcer model
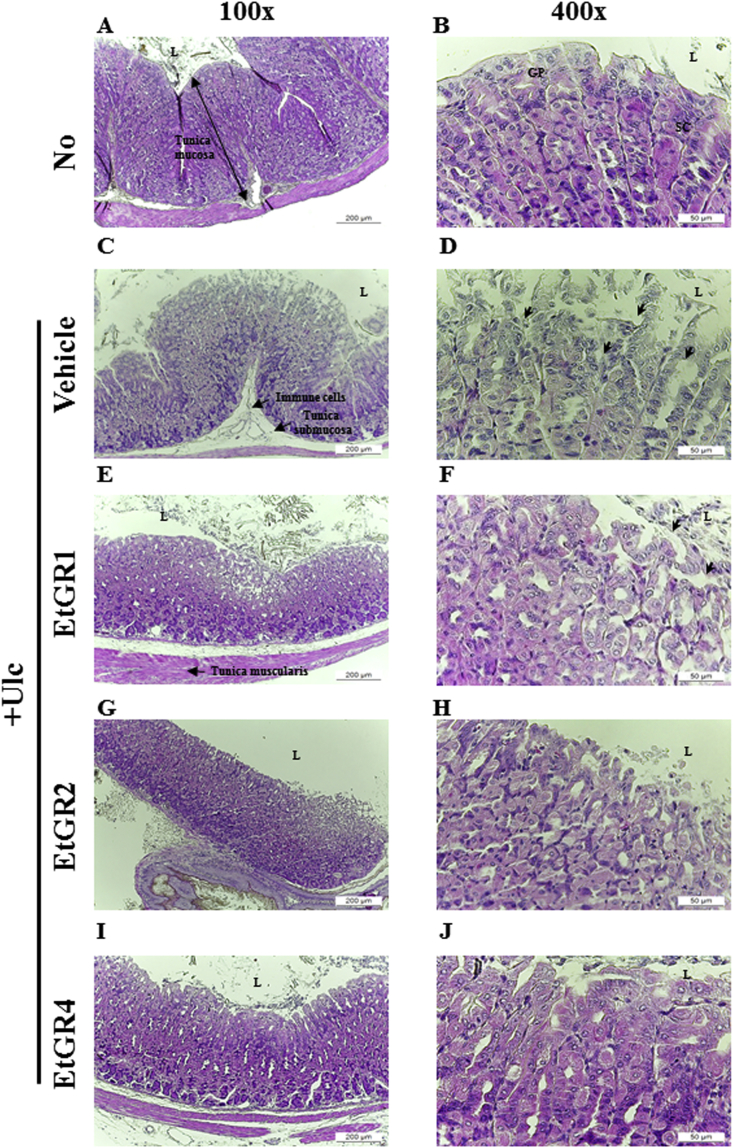
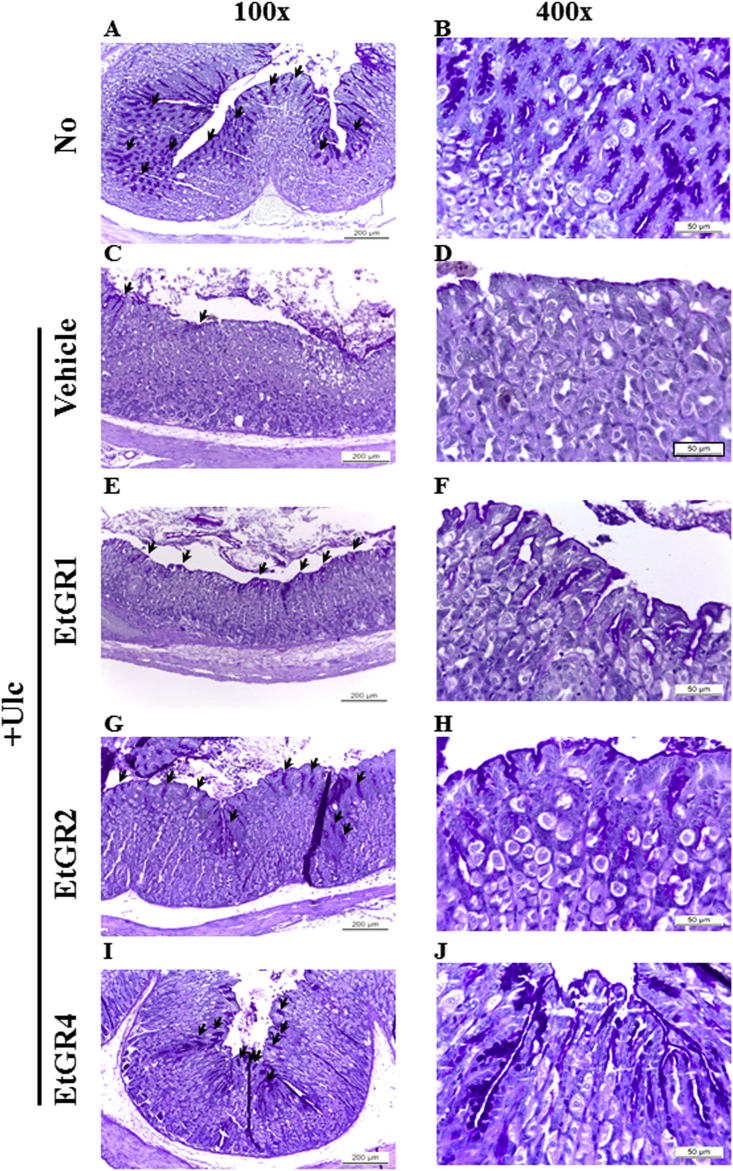
Histopathological alterations in stomach specimens of the EtGR treated group are shown in Fig. 3. Stomach specimens in the Vehicle + Ulc group showed dramatic increases in loss of the mucous membrane, necrosis and congestion and inflammatory cell number relative to the No group. In contrast, treatment with EtGR improved these alternations in a dose-dependent manner, as indicated by less mucosal damage, milder edema and loss of inflammatory cell infiltration when compared to the vehicle+ Ulc group. Moreover, histological observation of mucus production in the vehicle + Ulc group revealed significant decreases in the pathological score relative to the No group. In contrast, treatment with EtGR caused increased mucus production when compared with vehicle group (Fig. 4). In addition, the histomorphometrical analysis of gastric mucosa in stomach specimens showed similar patterns as hostopathological analysis (Table 2). Taken together, these results suggest that treatment of mice with EtOH/HCl induced gastric ulcer, while EtGR prevented EtOH/HCl induced gastric injury in a dose-dependent manner.
Fig. 3.
Effects of EtGR on histopathological changes of the stomachs of ICR mice with EtOH/HCl-induced gastritis. Histopathological analysis of stomach tissue. After collection of stomach specimens from each group, the histopathological changes in the slide sections of stomach tissue were identified by staining with H&E followed by observation at 100× and 400× magnification. Abbreviations: L, lumen; GP, gastric pits; SC, surface mucous cells.
Fig. 4.
Effects of EtGR on the production of mucus in the stomachs of ICR mice with EtOH/HCl -induced gastritis. Alcian blue (pH 2.5) stained sections of stomach specimen in mice from the No treated group, vehicle + Ulc group and EtGR + Ulc group were observed at 100× (upper corner in left column) and 400× (left column) using a light microscope. Mucin secreted from crypt layer cells are indicated by arrows.
Table 2.
Histomorphometrical analysis of gastric mucosa in EtOH/HCl treated ICR mice.
| Semiquantative scores | No | +Ulc |
|||
|---|---|---|---|---|---|
| Vehicle | EtGR1 | EtGR2 | EtGR4 | ||
| Mucosal injury (Max = 3) | 0 ± 0 | 2.4 ± 0.55∗ | 1.8 ± 0.45∗ | 0.8 ± 0.45# | 0.6 ± 0.55# |
| Edema (Max = 3) | 0 ± 0 | 2.8 ± 0.45∗ | 2.4 ± 0.55∗ | 1.6 ± 0.55∗,# | 0.6 ± 0.55# |
| Inflammatory cells (Max = 3) | 0 ± 0 | 2.8 ± 0.45∗ | 1.0 ± 0.71∗,# | 0.6 ± 0.55# | 0.6 ± 0.55# |
The data shown represent the means ± SD of three replicates. *, P < 0.05 relative to the No group. #, P < 0.05 relative to the Vehicle + Ulc group.
3.5. Effects of EtGR on expression of inflammatory cytokines in EtOH/HCl-induced gastric ulcer model
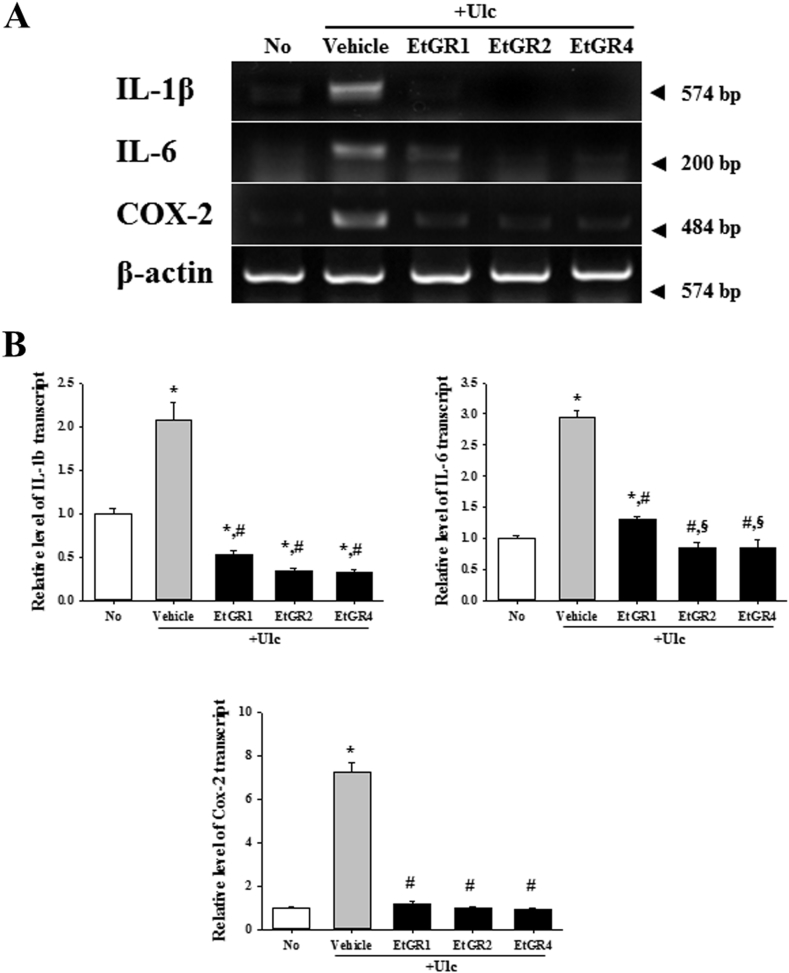
To confirm the anti-inflammatory activity of EtGR, we conducted RT-PCR of IL-1β, IL-6 and COX-2 in stomach specimens using a commercial RT-PCR kit (Fig. 5). As expected, the mRNA level of inflammatory cytokines and COX-2 was significantly increased in stomach specimens of vehicle + Ulc mice when compared with the No group mice. However, higher doses of EtGR (400 mg/ml; EtGR4 + Ulc) showed marked reductions in inflammatory cytokines and COX-2 when compared to the vehicle group and low dose EtGR group (100 mg/ml; EtGR1+Ulc). These results revealed that EtGR inhibited the production of inflammatory cytokines and COX-2 in gastric ulcer.
Fig. 5.
Effects of EtGR on the cytokine expression in the stomach of ICR mice with EtOH/HCl-induced gastritis. (A) The mRNA levels of IL-1β, IL-6 and COX-2 in stoma specimen were measured by RT-PCR using specific primers. (B) After the intensity of each band was analyzed using an imaging densitometer, the relative mRNA levels of the IL-1β, IL-6 and COX-2 were calculated based on the mRNA level of β-actin internal control. Data represent the means ± SD of three replicates. *, p < 0.05 compared to the No treated group. #, p < 0.05 compared to the vehicle + Ulc group.
3.6. Effect of EtGR on lipid peroxidation in the stomach of EtOH/HCl-treated mice
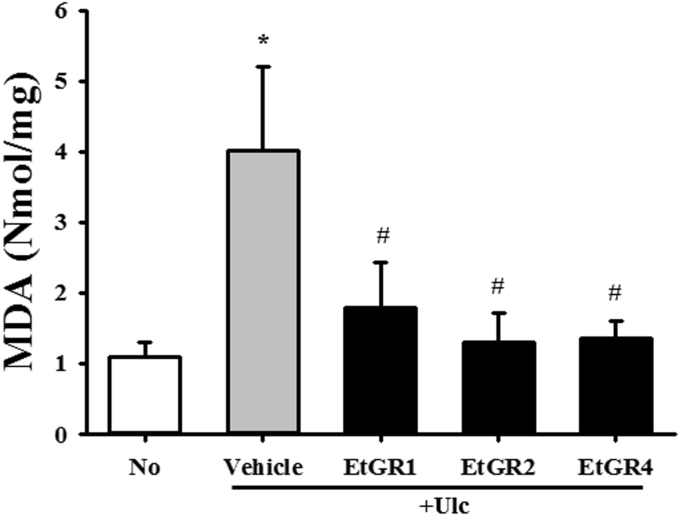
To investigate the correlation between therapeutic effects of GR and the suppression of oxidative stress, the levels of MDA representing lipid peroxidation were determined in the stomachs of a gastric ulcer model using commercial kits. As shown in Fig. 6, EtOH/HCl treatment significantly increased MDA activity in stomach tissues compared with the No group. However, their level was dramatically lower in all EtGR + Ulc groups, although EtGR concentration had no effect on the MDA level. Therefore, these results indicate that therapeutic effects of GR against gastric ulcer are tightly correlated with anti-oxidative activity.
Fig. 6.
Effects of EtGR on lipid peroxidation in the stomachs of ICR mice with EtOH/HCl-induced gastritis. MDA concentration in the stomach specimens of ICR mice treated with EtGR. The level of MDA was determined in tissue samples of ICR mice using a lipid peroxidation assay kit that could detect MDA at 0.1 nmole/mg to 20 nmole/mg. Three samples were assayed in triplicate by MDA assay. Data are reported as the means ± SD.*, P < 0.05 relative to the No group. #, P < 0.05 relative to the Vehicle + Ulc group.
3.7. Effect of EtGR on organ damage in EtOH/HCl-treated mice
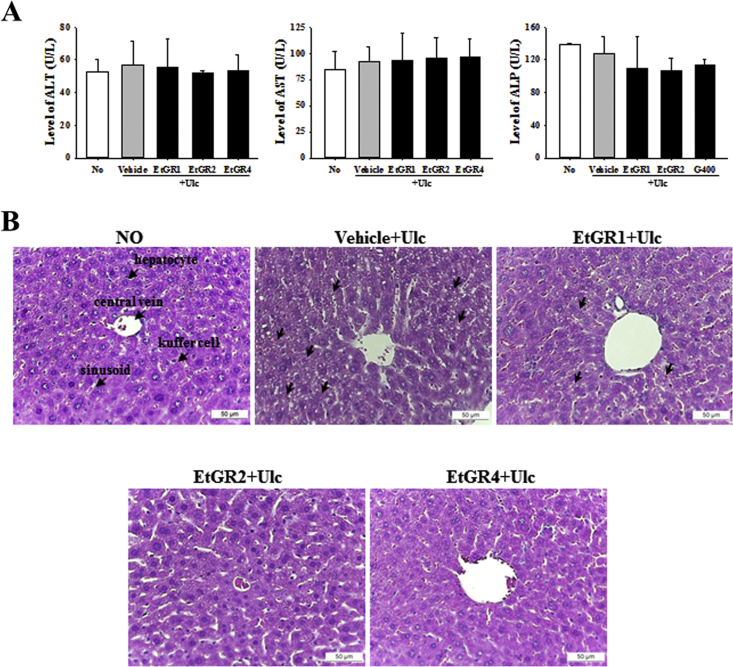
Finally, we analyzed the toxicity of EtGR to evaluate the possibility for human application. As shown in Fig. 7A, exposure to EtOH/HCl did not lead to differences in liver damage serum markers such as ALT, AST and ALP relative to the No group. In addition, there were no changes in liver damage serum markers in the group treated with EtGR. In contrast, exposure of mice to EtOH/HCl revealed production of lipid pores in liver specimens along with lipid peroxidation induced by liver damage (Fig. 7B). Treatment with EtGR improved these alternations compared to the vehicle + Ulc group.
Fig. 7.
Effects of EtGR on lipid peroxidation in serum parameters and histological features of the livers of ICR mice with EtOH/HCl-induced gastritis. (A) Serum was collected from the abdominal veins of ICR mice and the serum concentrations of ALP, AST and ALT were then analyzed as described in the Materials and Methods. (B) Liver tissues were stained with H&E and cellular morphology was viewed at 400× magnification. The data shown represent the means ± SD of three replicates. Arrow indicates hepatocytes or kuffer cells or sinusoid.
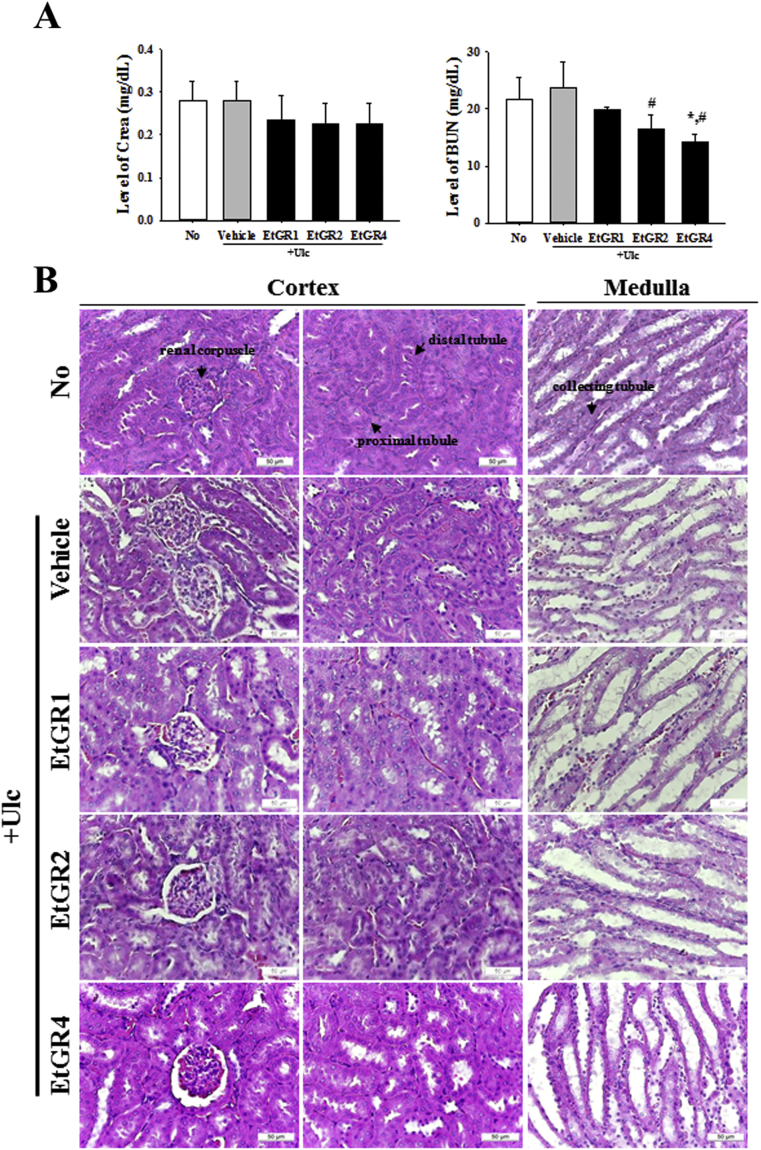
We next evaluated the levels of Crea and BUN in serum using commercial kits to confirm the effect of EtGR on EtOH/HCl induced kidney damage. No significant differences in Crea and BUN were observed among groups (Fig. 8A). In addition, exposure to EtOH/HCl did not induce changes in the histological structures of kidney tissue, and the effects of EtGR demonstrated similar patterns (Fig. 8B). These results clearly indicated that EtGR does not induce any specific toxicity in the livers and kidneys of ICR mice at doses of 400 mg/kg body weight/day, indicating that this is the no observed adverse effect level (NOAEL).
Fig. 8.
Effects of EtGR on lipid peroxidation in serum parameters and histological features of the kidneys of ICR mice with EtOH/HCl-induced gastritis. (A) Serum was collected from the abdominal veins of ICR mice and serum concentrations of BUN and CRE were then analyzed as described in the Materials and Methods. (B) Kidney tissues were stained with H&E, and cellular morphology was viewed at 400× magnification. The data shown represent the means ± SD of three replicates. Arrow, distal convoluted tubules; arrow head, proximal convoluted tubules; RC, renal corpuscle.
4. Discussion
Gallarhois (GR) is a traditional oriental drug used to treat diarrhea, persistent coughing and spontaneous perspiration, although there is little scientific evidence supporting these pharmacological effects in humans. Recently, several studies revealed that tannin-derived components of GR effectively inhibit bacteria, fungi and viruses.30, 31 In addition, methyl gallate and ethyl gallate isolated from GR also showed significant anti-inflammatory activity in lipopolysaccharide (LPS)-stimulated RAW264.7 macrophages via induction of hemoxic enzyme 1 and inhibition of iNOS/COX-2.22, 32 Therefore, this study was conducted using a gastric ulcer animal model to evaluate the efficacy of GR as a good candidate for treatment of gastric ulcers and determine its possible mechanism. The results revealed that treatment with EtGR notably inhibited EtOH/HCl-induced gastric ulcer by inhibiting inflammatory reactions, improving oxidative stress and protecting against organ damage.
Various animal models have been developed and used to develop therapeutic agents for gastric ulcers, as well as to elucidate precise mechanisms of disease. Because multiple factors, such as inactive lifestyle, alcohol consumption, spicy foods, drugs and bacterial infections, are responsible for gastric ulcers, various gastric ulcer models are also developed and used in accordance with the mechanisms that are attracting attention. For example, indomethacin, a non-steroidal anti-inflammatory agent, has the capacity to induce gastric ulcer. These models have applied to study the role of prostaglandins (PG) in gastric cytoprotection because of Indomethacin decreased COX (cyclooxygenase) activity, and endogenous level of PG in the gastric mucosa.33 Finally, decreasing PG leads to gastric and vascular disturbances through increasing levels of reactive oxygen species (ROS) and infiltration of neutrophils. The indomethacin-induced gastric ulcer model is useful for investigation of PG-mediated gastric ulceration.34 This Because ethanol easily penetrates into gastric mucosa, leading to gastritis of mucosal edema, hemorrhages, and inflammatory cell infiltration, while HCl increases oxidative stress and corrosive damage to gastric mucus, ethanol and ethanol/HCl are the most commonly utilized experimental models for evaluation of antiulcer activity in mice.35 It is also well known that excessive alcohol consumption causes health problems and social problems. In the present study, we used an EtOH/HCl induced gastric ulcer model to investigate the anti-ulceration activity of GR. The EtGR was effective in this model, which may have been due to inhibition of EtOH/HCl induced damage events, such as mucosal injury, edema and infiltration of inflammatory cells, as well as decreased secretion of the mucus that protects gastric mucosa from corrosive effects of EtOH/HCl. Moreover, EtGR has antiulcer properties that significantly reduce the expression of inflammatory cytokines and COX-2 induced by EtOH/HCl, suggesting that its anti-gastritis effects involve decreased production of PG and infiltration of inflammatory cells in gastric lesions.
Although alcohol consumption leads to harmful effects in the gastrointestinal tract, the exact mechanism still remains unknown. There is growing evidence that ethanol-induced gastric injury is related to increases in the reactive oxygen species (ROS) levels.36, 37 In gastric ulcers, oxidative stress is also increased by penetration of activated neutrophils, which produce reactive oxygen species. Neutrophil infiltration has been shown to play a decisive role in the development of gastric mucosal inflammation and injury.38 Infiltration of neutrophils into gastric mucosal tissues is assessed by the activity of MDA, which is one of the common markers of oxidative stress and a valuable index of oxidative stress intensity. Lipid peroxidation is caused by a process in which free radicals interact with cell membranes to produce lipid peroxides such as MDA.39 In this study, MDA activity in stomach specimens was significantly increased after EtOH/HCl administration, and neutrophil infiltration was observed in the lesion area. However, the treatment of EtOH/HCl-induced gastric ulcer with EtGR decreased neutrophil infiltration and MDA level in stomach specimens, suggesting the ability of EtGR to prevent neutrophil infiltration and oxidative stress in ulcer-causing gastric tissues. Moreover, other oxidative stress markers measure the level of antioxidants in tissues. It is well known to that glutathione (GSH) and superoxide dismutase (SOD) are important antioxidants that protect the gastric mucosa from oxidative damage caused by reactive oxygen species.40 Although we did not measure SOD and GSH in this study, we could investigate oxidative stress by measuring MDA levels. Further studies of the levels of antioxidants may verify the effects of GR based on more accurate evaluations of its effects on oxidative stress.
5. Conclusion
In conclusion, this study demonstrated that GR exerted a protective effect on EtOH/HCl-induced gastric ulcer, which was demonstrated by biochemical, histopathological and RT-PCR analysis data. The anti-ulcer effects of EtGR were primarily attributed to its modulation of oxidative stress, the inhibitory effects on inflammatory cell infiltration and inhibition of IL-1β, IL-6 and COX-2. In addition, GR did not cause hepatotoxicity and renal toxicity. Further and more comprehensive studies are needed to elucidate the gastrointestinal protective mechanism of GR.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Acknowledgments
We thank JinHyang Hwang, the animal technician, for directing the animal care and use at the Laboratory Animal Resources Center. This project was supported by a grant from BIOREIN (Laboratory Animal Bio Resources Initiative) from the Ministry of Food and Drug Safety in 2015.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
Contributor Information
HyunKeun Song, Email: kali71@hanmail.net.
DaeYoun Hwang, Email: dyhwang@pusan.ac.kr.
References
- 1.Sumbul S., Ahmad M.A., Mohd A. Role of phenolic compounds in peptic ulcer: an overview. J Pharm Bioallied Sci. 2011;3(3):361–367. doi: 10.4103/0975-7406.84437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amaral G.P., de Carvalho N.R., Barcelos R.P. Protective action of ethanolic extract of Rosmarinus officinalis L. in gastric ulcer prevention induced by ethanol in rats. Food Chem Toxicol. 2013;55:48–55. doi: 10.1016/j.fct.2012.12.038. [DOI] [PubMed] [Google Scholar]
- 3.Chen H.Y., Lin C.L., Chen W.C., Kao C.H. Does Helicobacter pylori eradication reduce the risk of open angle glaucoma in patients with peptic ulcer disease? Med Baltim. 2015;94(39):e1578. doi: 10.1097/MD.0000000000001578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boltin D., Niv Y. Pharmacological and alimentary alteration of the gastric barrier. Best Pract Res Clin Gastroenterol. 2014;28(6):981–994. doi: 10.1016/j.bpg.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 5.Matsuda H., Shimoda H., Ninomiya K., Yoshikawa M. Inhibitory mechanism of costunolide, a sesquiterpene lactone isolated from Laurus nobilis, on blood-ethanol elevation in rats: involvement of inhibition of gastric emptying and increase in gastric juice secretion. Alcohol Alcohol. 2002;37(2):121–127. doi: 10.1093/alcalc/37.2.121. [DOI] [PubMed] [Google Scholar]
- 6.Amirshahrokhi K., Khalili A.R. The effect of thalidomide on ethanol-induced gastric mucosal damage in mice: involvement of inflammatory cytokines and nitric oxide. Chem Biol Interact. 2015;225:63–69. doi: 10.1016/j.cbi.2014.11.019. [DOI] [PubMed] [Google Scholar]
- 7.Dockray G.J., Varro A., Dimaline R., Wang T. The gastrins: their production and biological activities. Annu Rev Physiol. 2001;63:119–139. doi: 10.1146/annurev.physiol.63.1.119. [DOI] [PubMed] [Google Scholar]
- 8.El-Maraghy S.A., Rizk S.M., Shahin N.N. Gastroprotective effect of crocin in ethanol-induced gastric injury in rats. Chem Biol Interact. 2015;229:26–35. doi: 10.1016/j.cbi.2015.01.015. [DOI] [PubMed] [Google Scholar]
- 9.Bhattacharyya A., Chattopadhyay R., Mitra S., Crowe S.E. Oxidative stress: an essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol Rev. 2014;94(2):329–354. doi: 10.1152/physrev.00040.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arda-Pirincci P., Bolkent S., Yanardag R. The role of zinc sulfate and metallothionein in protection against ethanol-induced gastric damage in rats. Dig Dis Sci. 2006;51(12):2353–2360. doi: 10.1007/s10620-006-9301-3. [DOI] [PubMed] [Google Scholar]
- 11.Wang F.Y., Liu J.M., Luo H.H., Liu A.H., Jiang Y. Potential protective effects of Clostridium butyricum on experimental gastric ulcers in mice. World J Gastroenterol. 2015;21(27):8340–8351. doi: 10.3748/wjg.v21.i27.8340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang X., Jiang A., Qi B. Resveratrol protects against Helicobacter pylori-Associated gastritis by combating oxidative stress. Int J Mol Sci. 2015;16(11):27757–27769. doi: 10.3390/ijms161126061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chakraborty S., Yadav S.K., Subramanian M., Iwaoka M., Chattopadhyay S. dl-trans-3,4-Dihydroxy-1-selenolane (DHSred) heals indomethacin-mediated gastric ulcer in mice by modulating arginine metabolism. Biochim Biophys Acta. 2014;1840(12):3385–3392. doi: 10.1016/j.bbagen.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 14.Gadekar R., Singour P.K., Chaurasiya P.K., Pawar R.S., Patil U.K. A potential of some medicinal plants as an antiulcer agents. Pharmacogn Rev. 2010;4(8):136–146. doi: 10.4103/0973-7847.70906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakashita M., Suzuki H., Miura S. Attenuation of acetic acid-induced gastric ulcer formation in rats by glucosylceramide synthase inhibitors. Dig Dis Sci. 2013;58(2):354–362. doi: 10.1007/s10620-012-2350-x. [DOI] [PubMed] [Google Scholar]
- 16.Abdelwahab S.I., Taha M.M., Abdulla M.A. Gastroprotective mechanism of Bauhinia thonningii Schum. J Ethnopharmacol. 2013;148(1):277–286. doi: 10.1016/j.jep.2013.04.027. [DOI] [PubMed] [Google Scholar]
- 17.Brzozowski T., Konturek P.C., Konturek S.J. Involvement of endogenous cholecystokinin and somatostatin in gastroprotection induced by intraduodenal fat. J Clin Gastroenterol. 1998;27(suppl 1):S125–S137. doi: 10.1097/00004836-199800001-00020. [DOI] [PubMed] [Google Scholar]
- 18.Klein L.C., Jr., Gandolfi R.B., Santin J.R., Lemos M. Cechinel Filho V, de Andrade SF. Antiulcerogenic activity of extract, fractions, and some compounds obtained from Polygala cyparissias St. Hillaire & Moquin (Polygalaceae) Naunyn Schmiedeb Arch Pharmacol. 2010;381(2):121–126. doi: 10.1007/s00210-009-0485-x. [DOI] [PubMed] [Google Scholar]
- 19.Wittschier N., Faller G., Hensel A. Aqueous extracts and polysaccharides from liquorice roots (Glycyrrhiza glabra L.) inhibit adhesion of Helicobacter pylori to human gastric mucosa. J Ethnopharmacol. 2009;125(2):218–223. doi: 10.1016/j.jep.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 20.Choi J.G., Kang O.H., Lee Y.S. Antibacterial activity of methyl gallate isolated from Galla Rhois or carvacrol combined with nalidixic acid against nalidixic acid resistant bacteria. Molecules. 2009;14(5):1773–1780. doi: 10.3390/molecules14051773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choi J.G., Mun S.H., Chahar H.S. Methyl gallate from Galla rhois successfully controls clinical isolates of Salmonella infection in both in vitro and in vivo systems. PLoS One. 2014;9(7):e102697. doi: 10.1371/journal.pone.0102697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chae H.S., Kang O.H., Choi J.G. Methyl gallate inhibits the production of interleukin-6 and nitric oxide via down-regulation of extracellular-signal regulated protein kinase in RAW 264.7 cells. Am J Chin Med. 2010;38(5):973–983. doi: 10.1142/S0192415X10008391. [DOI] [PubMed] [Google Scholar]
- 23.Djakpo O., Yao W. Rhus chinensis and Galla Chinensis–folklore to modern evidence: review. Phytother Res. 2010;24(12):1739–1747. doi: 10.1002/ptr.3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pettinari A., Amici M., Cuccioloni M., Angeletti M., Fioretti E., Eleuteri A.M. Effect of polyphenolic compounds on the proteolytic activities of constitutive and immuno-proteasomes. Antioxid Redox Signal. 2006;8(1–2):121–129. doi: 10.1089/ars.2006.8.121. [DOI] [PubMed] [Google Scholar]
- 25.Zaidi S.F., Muhammad J.S., Usmanghani K., Sugiyama T. Review: pharmacological ins and outs of medicinal plants against Helicobacter pylori: a review. Pak J Pharm Sci. 2015;28(3 suppl l):1171–1176. [PubMed] [Google Scholar]
- 26.Kim J.E., Go J., Koh E.K. Gallotannin-enriched extract isolated from galla rhois may Be a functional candidate with laxative effects for treatment of loperamide-induced constipation of SD rats. PLoS One. 2016;11(9):e0161144. doi: 10.1371/journal.pone.0161144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim J.E., Lee Y.J., Kwak M.H., Ko J., Hong J.T., Hwang D.Y. Aqueous extracts of Liriope platyphylla induced significant laxative effects on loperamide-induced constipation of SD rats. BMC Complement Altern Med. 2013;13:333. doi: 10.1186/1472-6882-13-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ku S.K., Seo B.I., Park J.H. Effect of Lonicerae Flos extracts on reflux esophagitis with antioxidant activity. World J Gastroenterol. 2009;15(38):4799–4805. doi: 10.3748/wjg.15.4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laine L., Weinstein W.M. Histology of alcoholic hemorrhagic "gastritis": a prospective evaluation. Gastroenterology. 1988;94(6):1254–1262. doi: 10.1016/0016-5085(88)90661-0. [DOI] [PubMed] [Google Scholar]
- 30.Lee H.A., Hong S., Chung Y.H., Song K.D., Kim O. Anticoccidial effects of Galla rhois extract on Eimeria tenella-infected chicken. Lab Anim Res. 2012;28(3):193–197. doi: 10.5625/lar.2012.28.3.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee J.J., Cho W.K., Kwon H., Gu M., Ma J.Y. Galla rhois exerts its antiplatelet effect by suppressing ERK1/2 and PLCbeta phosphorylation. Food Chem Toxicol. 2014;69:94–101. doi: 10.1016/j.fct.2014.03.032. [DOI] [PubMed] [Google Scholar]
- 32.Park P.H., Hur J., Kim Y.C., An R.B., Sohn D.H. Involvement of heme oxygenase-1 induction in inhibitory effect of ethyl gallate isolated from Galla Rhois on nitric oxide production in RAW 264.7 macrophages. Arch Pharm Res. 2011;34(9):1545–1552. doi: 10.1007/s12272-011-0917-2. [DOI] [PubMed] [Google Scholar]
- 33.Halter F., Tarnawski A.S., Schmassmann A., Peskar B.M. Cyclooxygenase 2-implications on maintenance of gastric mucosal integrity and ulcer healing: controversial issues and perspectives. Gut. 2001;49(3):443–453. doi: 10.1136/gut.49.3.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Laine L., Takeuchi K., Tarnawski A. Gastric mucosal defense and cytoprotection: bench to bedside. Gastroenterology. 2008;135(1):41–60. doi: 10.1053/j.gastro.2008.05.030. [DOI] [PubMed] [Google Scholar]
- 35.Li C.Y., Xu H.D., Zhao B.T., Chang H.I., Rhee H.I. Gastroprotective effect of cyanidin 3-glucoside on ethanol-induced gastric lesions in rats. Alcohol. 2008;42(8):683–687. doi: 10.1016/j.alcohol.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 36.Pan J.S., He S.Z., Xu H.Z. Oxidative stress disturbs energy metabolism of mitochondria in ethanol-induced gastric mucosa injury. World J Gastroenterol. 2008;14(38):5857–5867. doi: 10.3748/wjg.14.5857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sidahmed H.M., Hashim N.M., Amir J. Pyranocycloartobiloxanthone A, a novel gastroprotective compound from Artocarpus obtusus Jarret, against ethanol-induced acute gastric ulcer in vivo. Phytomedicine. 2013;20(10):834–843. doi: 10.1016/j.phymed.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 38.Elliott S.N., Wallace J.L. Neutrophil-mediated gastrointestinal injury. Can J Gastroenterol. 1998;12(8):559–568. doi: 10.1155/1998/398384. [DOI] [PubMed] [Google Scholar]
- 39.Kwiecien S., Brzozowski T., Konturek S.J. Effects of reactive oxygen species action on gastric mucosa in various models of mucosal injury. J Physiol Pharmacol. 2002;53(1):39–50. [PubMed] [Google Scholar]
- 40.Danese S., Mantovani A. Inflammatory bowel disease and intestinal cancer: a paradigm of the Yin-Yang interplay between inflammation and cancer. Oncogene. 2010;29(23):3313–3323. doi: 10.1038/onc.2010.109. [DOI] [PubMed] [Google Scholar]