Abstract
In general, neurons belonging to the central nervous system (CNS), such as retinal ganglion cells (RGCs), do not regenerate. Due to this, strategies have emerged aimed at protecting and regenerating these cells. Neurotrophic factor (NTF) supplementation has been a promising approach but is limited by length of delivery and delivery vehicle. For this study, we tested a polymeric delivery system (sulfonated reverse thermal gel or SRTG) engineered to deliver cilliary neurotrophic factor (CNTF), while also being injectable. A rat optic nerve crush (ONC) model was used to determine the neuroprotective and regenerative capacity of our system. The results demonstrate that one single intravitreal injection of SRTG-CNTF following ONC showed significant protection of RGC survival at both 1 and 2 week time points, when compared to the control groups. Furthermore, there was no significant difference in the RGC count between the eyes that received the SRTG-CNTF following ONC and a healthy control eye. Intravitreal injection of the polymer system also induced noticeable axon regeneration 500 μm downstream from the lesion site compared to all other control groups. There was a significant increase in Müller cell response in groups that received the SRTG-CNTF injection following optic nerve crush also indicative of a regenerative response. Finally, higher concentrations of CNTF released from SRTG-CNTF showed a protective effect on RGCs and Müller cell response at a longer time point (4 weeks). In conclusion, we were able to show a neuroprotective and regenerative effect of this polymer SRTG-CNTF delivery system and the viability for treatment of neurodegenerations.
Keywords: biomimetic, retinal ganglion cells, reverse thermal gel, regeneration, neurotrophic factors
Graphical Abstract
INTRODUCTION
The first question to ask when devising a treatment for optic neuropathies is: how do retinal ganglion cells (RGCs) die as a result of optic nerve damage? The answer to this question is unfortunately extremely complex, with several molecular pathways likely contributing to RGC death.1 However, recent progress in this field has increased our understanding of what leads to RGC degeneration following optic nerve (ON) injury. The answer to this question is now believed to lie with neurotrophic factors (NTFs) and their role in a healthy retina.2 NTFs are a family of small diffusible molecules that are strongly implicated in the control of adult neurogenesis, axon extension, proliferation, and cellular survival.3 In a healthy eye, axon terminals are responsible for the uptake and transport of these NTFs to the RGC cell body.4 However, when RGC axons become injured, they are unable to transport NTFs, leaving the RGCs susceptible to apoptotic signals and subsequent cell death.1,5
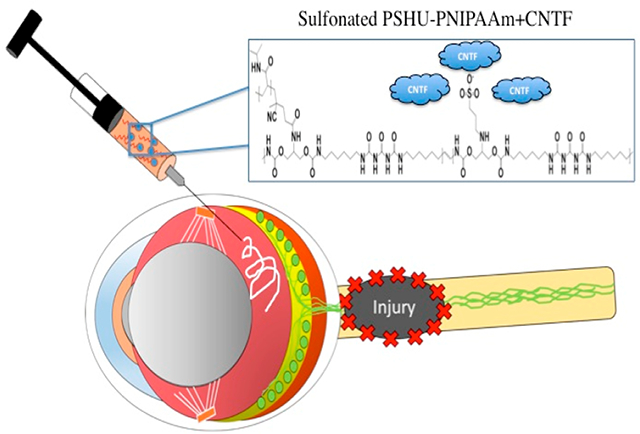
These recent discoveries in the mechanisms behind RGC death following optic nerve damage have led to the formation of new potential treatments. One such example are treatments aimed at neuroprotecting RGCs from cell death and loss of function.6 NTF supplementation strategies have been one area of research extensively studied to deliver the necessary NTFs directly to RGCs and provide them with a neuroprotective effect.7–9 However, preliminary studies using NTF supplementation typically involve delivering free NTF unaccompanied through a simple bolus injection.10 Although somewhat promising, initial studies show that free NTFs administered through bolus injection presents physiochemical instability, rapid diffusion, and a short half-life.8 Due to this, multiple high concentration injections would be required to realize the full effects of the NTFs, making the use of NTFs as it stands impractical and potentially dangerous in the clinical setting.11 To achieve effective and controlled therapeutic NTF levels, a delivery system sustaining NTF expression in the specific zone of interest while maintaining NTF bioactivity must be designed. For these reasons, research surrounding polymeric delivery systems has become of particular interest for making the use of NTF therapeutics a viable treatment option for various optic neuropathies, including glaucoma.12,13 An ideal polymeric delivery system would allow (1) simple injectable delivery of the NTF, (2) NTF localization, (3) functional properties to improve NTF stability, and (4) long-term stable release of the NTF into the surrounding tissue. To answer these requirements, we propose the use of a multicomponent injectable reverse thermal gel (RTG) polymer system, poly(serinol hexamethylene urea) backbone (PSHU) conjugated to poly(N-isopropylacrylamide) PNIPAAm (PSHU–PNIPAAm), functionalized with sulfonate groups (Sul–PSHU–PNIPAAm or sulfonated reverse thermal gel, SRTG) (Figure S1). The first component, PNIPAAm, will provide the RTG properties and allow injectability of this polymer as well as entrapment of the NTF within the polymer matrix. The next component of this polymer system is a functionalizable PSHU backbone capable of attaching a large quantity of functional groups (18 potential linkages per molecule). In this case, the polymer backbone will be modified with PNIPAAm as well as negatively charged sulfonate groups to make the SRTG. Modeled off of interactions between native extracellular matrix (ECM) and NTFs (e.g., heparin interacting with NTFs), this electrostatic interaction between the sulfonate groups and the positively charged NTF can protect the proteins from proteolytic degradation, preserve their bioactivity, and increase their half-life.14 Thus, we conjugated the negatively charged sulfonate groups to the polymer backbone with the goal of prolonging the potency of the delivered growth factor while also increasing the duration of release from the polymer network. This SRTG system can be administered intravitreally and provide RGCs with localized and sustained release of NTF, promoting substantial neuroprotection of RGCs following optic nerve crush (ONC) damage. To analyze this delivery system, we will utilize cilliary neurotrophic factor (CNTF) as a model NTF. Early studies first identified increased CNTF as a response to disease conditions or injury of retinal neurons, after axotomy, ischemia, and experimental glaucoma.15,16 It was further discovered that CNTF works to provide neuroprotection to the RGCs as well as induce axon regeneration following injury.17
The first aim of this work is to design and characterize an injectable polymer system that is capable of sustaining bioactive NTF release to the retinal ganglion cell layer. Lower critical solution temperature (LCST) and energy-dispersive X-ray spectroscopy (EDS) will be used to analyze gelling properties and quantify the functionalization process, respectively. Next, a release test with IR-labeled CNTF and subsequent fluorescent analysis will be performed to determine the release profile of CNTF as released from the SRTG and RTG. Furthermore, the effects of the SRTG system on RGC neuroprotection and axon regeneration following ONC will be examined (with the appropriate controls) after 7, 14, and 28 days post-ONC. Brn3a, growth associated protein 43 (GAP43), and glial fibrillary acidic protein (GFAP) staining will be used to study the neurprotective effects, axon regeneration, and Müller cell activation, respectively.
MATERIALS AND METHODS
Detailed experimental methods are provided in the Supporting Information Materials and Methods.
PSHU–PNIPAAm Fabrication
To synthesize the RTG, we began by synthesizing a functionalizable biomimetic polymer backbone (PSHU, Mw: 10 500) using urea, N-BOC-serinol, and hexamethylene diisocyanate as described previously.18,19 PNIPAAm (Mw: 11 000) was synthesized as previously described19 and conjugated to PSHU to form the PSHU–PNIPAAm copolymer. To do this, PNIPAAm–COOH (0.75 g, 1.21 mmol), EDC (5× molar excess), and NHS (5× molar excess) were dissolved in 5 mL of anhydrous DMF and reacted for 24 h under a nitrogen atmosphere. Purified PSHU (0.125 g/mL) was then added to the reactant mixture and allowed to react for 48 h at room temperature. The product mixture was purified by precipitation in anhydrous diethyl ether twice followed by dialysis (MWCO: 12 000–14 000 Da) against ultrapure water for 5 days. The final product was lyophilized at −45 °C for 24 h and stored at room temperature.
Sul–PSHU–PNIPAAM Fabrication
To synthesize the SRTG, sulfonation of the RTG was performed as previously described.20 In short, PS (0.034 g, 5 mmol) and t-BuOK (0.032 g, 5 mmol) were dissolved in 3 mL of anhydrous DMF. RTG (0.1 g/mL) dissolved in anhydrous DMF was slowly added to the flask and allowed to react for 3 days at 60 °C under a nitrogen atmosphere. The product mixture was then precipitated in anhydrous diethyl ether twice and dialyzed (MWCO: 12 000–14 000 Da) against ultrapure water for 48 h at room temperature. The final product was lyophilized at −45 °C for 24 h and stored at room temperature.
Animals
All animal experiments were performed under a protocol approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Colorado Anschutz Medical Campus. Male adult wistar rats (250–300 g) were allowed to acclimate for 1 week prior to surgical procedures. Rats were maintained on a 14/10 h light/dark cycle with a continuous supply of fresh air and access to food and water ad libitum. Rats were anesthetized with 5% isoflurane in oxygen and maintained on 0.5–1% isoflurane in oxygen for the remainder of the surgery. To maintain body temperature, rats were placed on a warm recirculating water blanket. Rats were divided into four separate groups according to the experiment. The time course of the ONC and subsequent treatment included three different time points (1, 2, and 4 weeks). To determine the percentage of RGCs surviving post-ONC and axon regrowth, 3–5 rats were used per time point.
Optic Nerve Crush Model
ONC and subsequent treatment was performed on the right eye with the left eyes serving as intact contralateral controls with no crush and no treatment applied. Detailed methods are provided in Supporting Information Materials and Methods.
Intravitreal Injection
Following the nerve crush procedure, animals received intravitreal injections depending on the group (saline, SRTG, free CNTF (0.5 μg), RTG-CNTF (1 wt % polymer) (0.5 μg CNTF), SRTG-CNTF (1 wt % polymer) (0.5 μg CNTF), and SRTG-CNTF (1 wt % polymer) (2.5 μg CNTF)). The CNTF dosage was determined from previously reported studies.21–23 For each intravitreal injection, 5 μL of saline or 5 μL of the formulations was injected into the vitreous chamber using a 32 gauge, 4 mm long needle and a Hamilton glass syringe. When injected, the needle was inserted into the superior hemisphere of the eye at a 45° angle taking care to avoid any injury to the lens or retina. Following injection, the needle was held in the injection site for 30 s to prevent leakage of the treatment and then removed slowly.
Retina and Optic Nerve Immunohistochemistry (IHC)
IHC was performed to analyze RGC survival, axon regrowth, and Müller cell activation. Detailed methods may be found in Supporting Information Materials and Methods.
Quantification of Neuronal Survival and Axonal Growth
After fixation, sectioning, and staining of the tissue, the number of Brn3a positive cells was analyzed to determine the neuroprotective capabilities of the treatment. GAP-43 staining was used to determine the affects of each treatment on RGC axon growth in vivo (for details, see Supporting Information Materials and Methods).
Statistical Analysis
All results are expressed as means ± standard error of the mean. Analysis of variance (ANOVA) was used to determine significant differences between groups. Statistical significance was considered when p < 0.05.
RESULTS
Characterization of Negatively Charged SRTG
We began by characterizing PSHU. Both 1H nuclear magnetic resonance spectroscopy (NMR) and Fourier transform infrared spectroscopy FT-IR (data previously reported)18,20 were used to confirm the overall polymer structure and ensure the presence of free functionalizable amines on the backbone. Elemental analysis, through EDS, was used to confirm the sulfonation process and synthesis of SRTG.24 The approximate mass percent of sulfonate groups chemically conjugated to the SRTG was 0.09% as compared to 0.00% seen in the RTG (Figure S2). Next, we examined the gelling properties, through LCST, of the polymer system before and after sulfonation. The LCST describes the temperature that the polymer system transitions from solution to solid form and is therefore an extremely important characteristic for this application, allowing for facile injection into the intravitreal space. It is possible that the introduction of sulfonate groups on the polymer backbone could alter the gelling properties of the polymer system. Figure S3 displays the LCST of both SRTG and RTG. We can see that the sulfonated polymer system still displayed very similar gelling properties to the RTG precursor, including a similar temperature of gelation and relative rate of gelation. This is likely because the addition of the small sulfonate groups to the polymer backbone is not sufficient to alter the large hydrophobic/hydrophilic properties of the PNIPAAm polymer that dictates the liquid-to-solid phase transition. Finally, we analyzed the release profile of CNTF from each of the polymer types (SRTG and RTG). As discussed earlier, negatively charged sulfonate groups were conjugated to the polymer backbone to form the SRTG. The motivation behind this step was to encourage polymer–protein interaction between the negatively charged sulfonate groups and the large positively charged receptor binding site on CNTF.25 These interactions would not only preserve the integrity of the protein but also prolong the duration of release by dampening the burst release of CNTF from the polymer system. To compare the release profiles of CNTF released from the RTG to the release profile of CNTF released from the SRTG, we took samples of release buffer and analyzed these samples for fluorescently labeled CNTF using a fluorescent imaging system (Figure S4). We can see from Figure S4 that the 1% SRTG polymer showed a decreased burst release of CNTF compared to the initial burst release from the 1% RTG polymer samples. This indicates that the increased charge diminished the initial expulsion of the CNTF from the polymer potentially leaving more CNTF entrapped within the polymer system. The decreased burst release could prolong the duration of CNTF release from the SRTG lending to the superior results during in vivo studies.
Delivery of CNTF from the SRTG Promotes Survival of RGCs Post-ONC
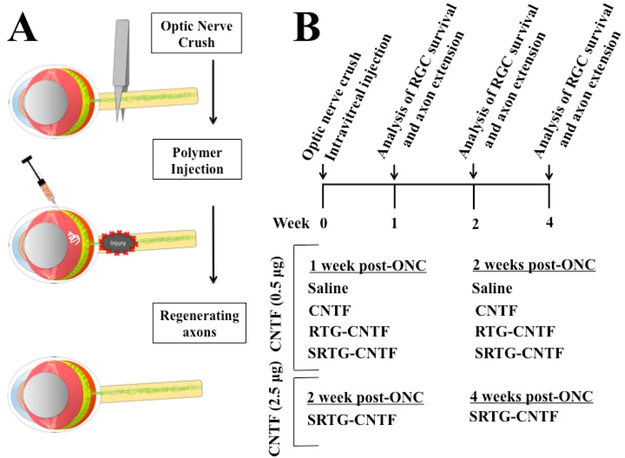
We first investigated the neuroprotective effects of the NTF delivery system. To do this, the polymer system loaded with CNTF or a control treatment was applied as an intravitreal injection following ONC and the remaining RGCs were analyzed using immunostaining procedures. Experimental groups included animals with ONC that received: (i) a single intravitreal injection of saline (5 μL) (n = 5, 1 week; n = 5, 2 weeks); (ii) a single intravitreal injection of SRTG (5 μL) (n = 5, 2 weeks), (iii) a single intravitreal injection of free CNTF (0.5 μg in 5 μL of saline) (n = 3, 1 week; n = 5, 2 weeks); (iv) a single intravitreal injection of RTG-CNTF (5 μL, 1 wt % polymer solution loaded with 0.5 μg CNTF) (n = 5, 1 week; n = 5, 2 weeks); (v) a single intravitreal injection of SRTG-CNTF (5 μL, 1 wt % polymer solution loaded with 0.5 μg CNTF) (n = 5, 1 week; n = 5, 2 weeks; n = 5, 4 weeks), and (vi) a single intravitreal injection of SRTG-CNTF (5 μL, 1 wt % polymer solution loaded with 2.5 μg CNTF) (n = 3, 2 weeks; n = 3, 4 weeks) (Figure 1).
Figure 1.
Experimental protocol and animal groups. ONC was performed followed by the appropriate intravitreal injection. At 1, 2, or 4 weeks post-ONC, rats from each group were euthanized and the tissue was analyzed (A). Both 1 and 2 week time points included the groups: saline, CNTF (0.5 μg), RTG-CNTF (0.5 μg), and SRTG-CNTF (0.5 μg) (n = 3–5). The 2 and 4 week time points also included the group: SRTG-CNTF (2.5 μg) (B).
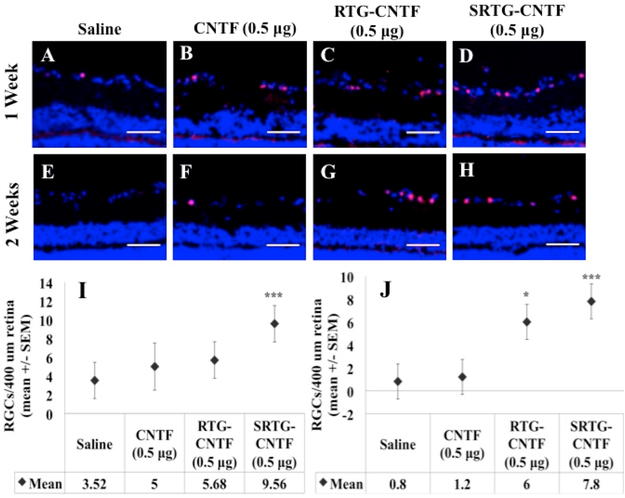
At the correct time points, animals from each group were analyzed for RGC survival using Brn3a immunostaining followed by confocal imaging of the ganglion cell layer. At 1 week post-ONC, the group that received a single intravitreal injection of saline (Figure 2A) and the group that received a single intravitreal injection of free CNTF (0.5 μg) (Figure 2B) showed a decrease in the number of RGCs. In addition, quantitative analysis showed that a comparable number of RGCs survived post-ONC in the saline group (3.52 ± 1.945 RGCs/400 μm retina, mean ± SEM) compared to the free CNTF group (5 ± 2.511 RGCs/400 μm retina) (Figure 2I). These numbers are in fact consistent with previous studies showing survival rates of RGCs 1 week post-ONC with no treatment applied being around 37%–40%,26,27 indicating that both the saline and free CNTF injections provided no significant neuroprotective effect for RGCs 1 week post-ONC. We then examined if a single intravitreal injection of polymer systems (RTG-CNTF and SRTG-CNTF) loaded with 0.5 μg of CNTF, injected at the time of nerve injury, could facilitate RGC survival 1 week post-ONC. Despite a slight increase, there was no statistically significant difference between the RTG-CNTF group (5.68 ± 1.945 RGCs/400 μm retina) when compared to both the saline treatment group and free CNTF (Figure 2I). However, there was a dramatic increase in the survival of RGCs in the SRTG-CNTF treatment group (9.56 ± 1.945 RGCs/400 μm retina) (Figure 2I). In addition, there was a statistically significant difference between the saline treatment group and the SRTG-CNTF (p-value: <0.0001) as well as between the free CNTF group and the SRTG-CNTF groups (p-value: 0.0082) (Figure 2I), indicating that the prolonged release of CNTF from the SRTG polymer can enhance the survival of RGCs 1 week post-ONC.
Figure 2.
Expression of Brn3a in the retina following ONC and the appropriate intravitreal injection. One week (A–D) and 2 week (E–H) retina sections from the four treatment groups (SRTG-CNTF (0.5 μg), RTG-CNTF (0.5 μg), CNTF (0.5 μg), and saline) are shown in the top two rows. Blue staining indicates DAPI. Red staining indicates Brn3a expression. Scale bar = 50 μm. *p < 0.05 and ***p < 0.001.
Two weeks post-ONC, both groups that received a single intravitreal injection of saline (0.8 ± 1.526 RGCs/400 μm retina) or a single intravitreal injection of free CNTF (0.5 μg) (1.2 ± 1.526 RGCs/400 μm retina) showed similar numbers of surviving RGCs (Figure 2E,F). In addition, both of these groups showed a number of surviving RGCs consistent with previous reports on the number of surviving RGCs 2 weeks post-ONC with no applied treatment. These expected results indicate that both the saline and free CNTF injections had no significant neuroprotective effect 2 weeks post-ONC but provided good controls for comparison to the polymer treatment groups. In contrast, both polymer groups showed a dramatic increase in the number of surviving RGCs post-ONC (Figure 2G,H). One single intravitreal injection of RTGCNTF (0.5 μg) (6 ± 1.526 RGCs/400 μm retina) or one single intravitreal injection of SRTG-CNTF (0.5 μg) (7.8 ± 1.526 RGCs/400 μm retina) both displayed a significant difference in surviving RGCs when compared to both the saline and free CNTF groups; however, the SRTG-CNTF group displayed the highest mean value of surviving RGCs (Figure 2J). To determine if the SRTG itself was having any effect on RGC survival, we included an additional group that received a single injection of SRTG alone following the ONC. Results from this study show no significant difference between an injection of SRTG and saline at the 2 week time point (Figure S5), indicating that the previously described neuroprotective effects of the SRTG-CNTF system are likely not due to the polymer itself.
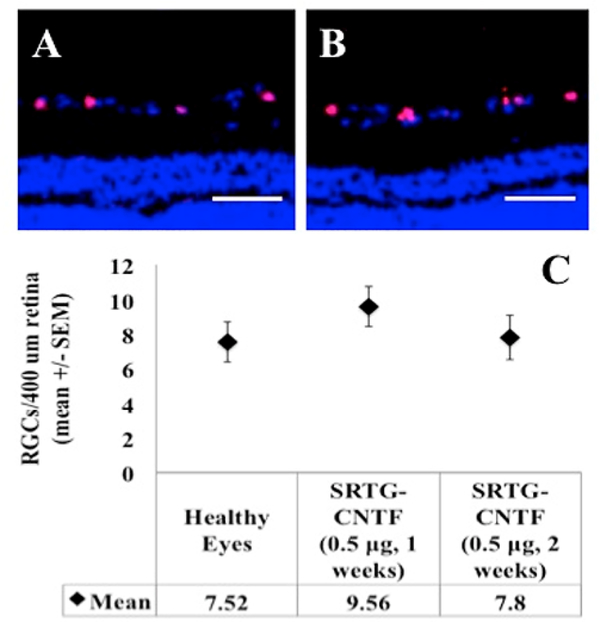
Treatment with SRTG-CNTF Following ONC Shows Preservation of RGCs Comparable to That of a Healthy Eye
Following the evaluation of each treatment group as shown above, we took the analysis one step further and compared eyes treated with SRTG-CNTF (0.5 μg) following ONC with eyes that have had no ONC and no intravitreal injection (i.e., healthy eyes). This analysis was done using a Brn3a immunostain and the same quantification process as described above. Figure 3 shows immunostained representative zoomed images of retinal cross sections from each group. Rats that had received the ONC followed by an intravitreal injection of SRTG-CNTF (0.5 μg) showed significant Brn3a expressing RGCs within the ganglion cell layer of the retina (Figure 3A). Interestingly, healthy eyes (Figure 3B) showed a comparable number of Brn3a expressing cells in the ganglion cell layer. Further quantification comparing the Brn3a cell count between these two groups shows no significant difference at both the 1 and 2 week time points (Figure 3C).
Figure 3.
Expression of Brn3a in retinal cross sections following ONC and Sul–PSHU–PNIPAAm intravitreal treatment after 2 weeks (A) when compared to a healthy eye receiving no ONC and no treatment (B). There was no significant difference observed between these two groups (C). Blue staining indicates DAPI. Red staining indicates Brn3a expression. Scale bar = 50 μm.
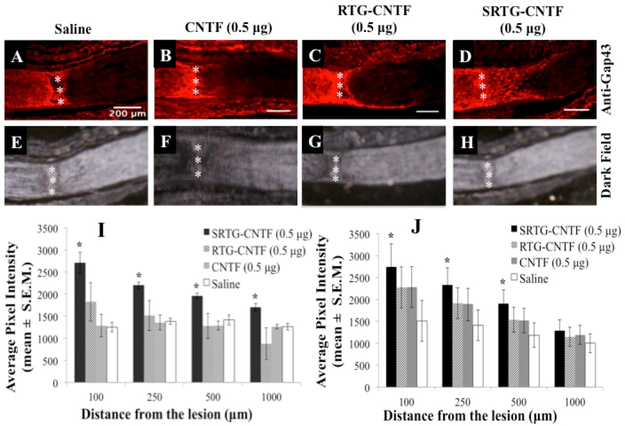
Delivery of CNTF from SRTG Promotes Growth of Injured RGC Axons
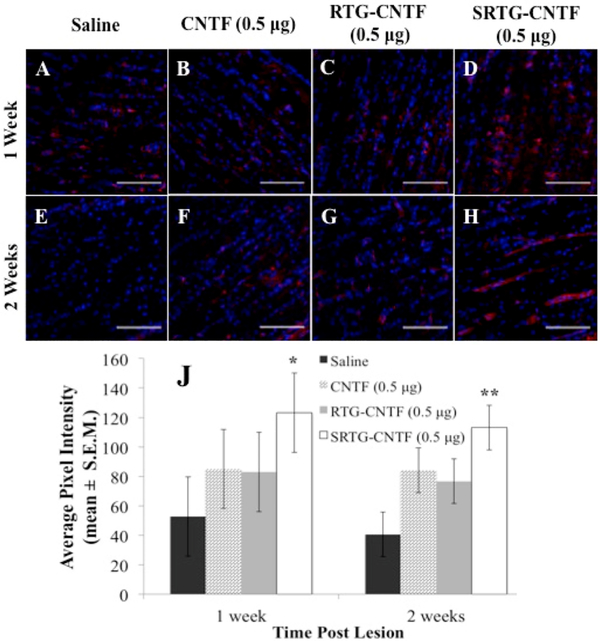
To investigate whether the increased RGC survival seen in the polymer treatment groups correlated to greater axon regeneration, we analyzed for damaged and regenerating RGC axons within ON cross sections using a GAP-43 immunostain.28 This stain was used not only to help deduce the crush site but also to elucidate any regenerating axons downstream from the injury. After 2 weeks, controls injected with saline or free CNTF (0.5 xg) following ONC showed minimal amounts of axons crossing over the glial scar (Figure 4A,B). Additionally, animals that received an intravitreal injection of RTG-CNTF (0.5 μg) showed negligible RGC growth downstream from the lesion 2 weeks post-ONC (Figure 4C). In contrast, the group that received an intravitreal injection of SRTG-CNTF (0.5 μg) post-ONC showed extensive upregulation of GAP-43 well beyond the injury site (Figure 4D). Dark field imaging was used to elucidate the lesion site in the same slide that received the GAP-43 stain (Figure 4E–H). Quantification analysis of axon growth showed that the expression of GAP-43 was upregulated for the SRTG-CNTF group at 100, 250, and 500 μm past the lesion site, when compared to control groups 1 week post-ONC (Figure 4I). For example, the average pixel intensity of the GAP-43 stain seen within the SRTG-CNTF group at 100, 250, and 500 μm was 2709.06 ± 409.2 (mean ± S.E.M), 2200.0 ± 125.3, and 1962.1 ± 108.1, respectively compared to the saline group values of 1251.7 ± 184.1, 1383.9 ± 124.1, and 1418.5 ± 192.9 respectively. These groups showed a statistically significant difference for distances 100, 250, and 500 μm with p-values of 0.0182, 0.0445, and 0.0371, respectively (Figure 4I). Quantification of 2 week data also showed an upregulation of GAP-43 expression for the SRTGCNTF group at 100, 250, and 500 μm past the lesion site, when compared to control groups. More specifically, the average pixel intensity of the GAP-43 stain seen within the SRTG-CNTF group at 100, 250, and 500 μm was 2741.3 ± 523.1 (mean ± S.E.M), 2333.2 ± 384.2, and 1903.6 ± 314.7, respectively, compared to the saline group value of 1510.1 ± 467.9, 1409.2 ± 350.7, and 1183.4 ± 281.5, respectively. These groups showed a statistically significant difference for distances 100, 250, and 500 μm with p-values of 0.0135, 0.0135, and 0.0204, respectively (Figure 4J). Furthermore, within the SRTG-CNTF group, there was no significant difference between the 1 and 2 week time points at all distances from the lesion (100, 250, 500, and 1000 μm).
Figure 4.
Intravitreal treatment of SRTG-CNTF (0.5 μg) induced RGC axon growth following ONC. Saline, CNTF (0.5 μg), and RTG-CNTF (0.5 μg) groups showed minimal axon regrowth 2 weeks post-ONC (A–C). SRTG-CNTF (0.5 μg) showed robust RGC axon growth 2 weeks post-lesion (D). The injury site was distinguished using dark field microscopy (E–H). Triple (vertical) asterisks represent the ONC site. Average pixel intensity (mean ± S.E.M.) of GAP-43 stain present past the lesion site following intravitreal injection of saline, CNTF (0.5 μg), RTG-CNTF (0.5 μg), and SRTG-CNTF (0.5 μg) at 1 week post-ONC (I) and 2 weeks post-ONC (J). Scale bar = 200 μm. *p < 0.05.
Although there was no statistically significant difference between the groups at 1000 μm downstream from the lesion site for both 1 and 2 weeks post-ONC, we decided to image cross sections of the nerve at 40× to detect any regeneration (Figure 5I). After staining with GAP-43, nerve sections from each group were imaged at approximately 1000 μm downstream from the lesion site (as identified through dark field microscopy). We can see that there is minimal GAP-43 staining present in groups, saline, CNTF, and RTG-CNTF at both 1 and 2 week time points (Figure 5A–C,E–G). However, the rats that received an intravitreal treatment of SRTG-CNTF showed a visible increase in GAP-43 expression 1000 μm down stream from the crush site at both time points (Figure 5D,H). Quantification was done through measuring the average pixel intensity of each section. At the 1 and 2 week time points, there was a statistical difference between the saline and SRTGCNTF group (p-values: 0.0181 and 0.0004, respectively) (Figure 5J).
Figure 5.
Longitudinal images of GAP-43 immunostained ON approximately 1000 μm downstream from the crush site. At both the 1 and 2 week time points, groups saline, CNTF, and RTG-CNTF showed minimal GAP-43 expression (A–C, E–G). At both 1 and 2 week time points, SRTG-CNTF showed increased GAP-43 expression at these downstream points (D, H). Quantification of the average pixel intensity showed a significant difference between SRTG-CNTF and other groups at both 1 and 2 week time points (J). Red staining indicates GAP-43 expression. Photomicrographs were captured at 40× magnification. Scale bar = 100 μm. *p < 0.05 and **p < 0.01.
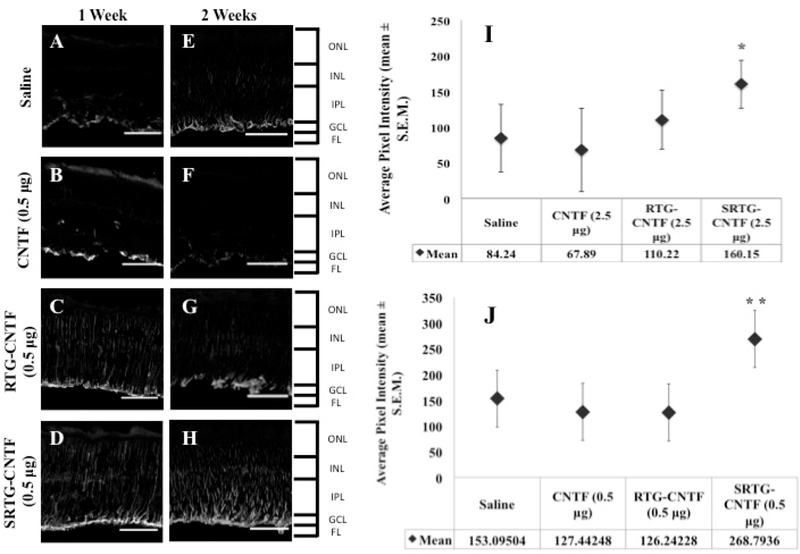
Intravitreal Injection of SRTG-CNTF Alters the Müller Cell Response 1 and 2 Weeks Post-ONC
Next, we wanted to investigate the effects of each intravitreal treatment on Müller cell activation following ONC. Müller cells are the principal glial cells residing in the retina. It is thought that Müller cells, like other types of glial cells, play important roles in the support and protection of retinal neurons following injury.29,30 For example, it has been previously reported that injury to the ON caused by ONC can trigger morphological and cellular changes in Müller cells, suggesting that Müller cells play a key role in aiding the regeneration of RGC axons.31 Some of these changes seen from activated Müller cells can aid in the neuroprotection and regeneration of a damaged retina.32,33 However, it is important to note that the complete function of Müller cells may not be limited to a regenerative effect. Müller cell activation is also linked to the inflammatory response, and while inflammation is part of the regenerative process, prolonged inflammation can lead to additional tissue damage.34,35 To see resulting Müller cell activation caused by ONC and the subsequent intravitreal treatment, we performed retinal immunostaining using an antibody against GFAP (Figure 6A–H). At 1 and 2 weeks post-ONC, the treatment groups that received saline and free CNTF (0.5 μg) injections showed GFAP staining that was limited to the astrocytes and Müller cell end-feet present within the nerve fiber layer (Figure 6A,B,E,F). The treatment group that received an intravitreal injection of RTG-CTNF (0.5 μg) showed an increase in Müller cell staining with expression moving down through the retina at both the 1 and 2 week time points (Figure 6C,G). However, the treatment group that received an intravitreal injection of SRTG-CNTF (0.5 μg) following ONC displayed robust GFAP labeling with a large number of Müller cell processes observed spanning the entire retina (Figure 6D,H). We further quantified these results by analyzing the fluorescence of the stained and imaged section. Figure 6I shows a significant difference between the SRTG-CNTF group and the other three groups at the 1 week time point (p-value 0.025). Similarly, Figure 6J shows a significant difference at the 2 week time point between the SRTG-CNTF and the remaining three groups (p-value 0.0037). These results indicate that the release of CNTF from the SRTG system was able to induce a significant Müller cell response that was likely contributing to the neuroprotective and neuroregenerative effect we were seeing in this group.
Figure 6.
Response of Müller glial cells to ONC and each subsequent intravitreal injection examined using a GFAP antibody. After 1 week post-ONC and appropriate intravitreal injection, GFAP staining in Müller cells is shown in saline, CNTF, RTG-CNTF, and SRTG-CNTF groups (A–D). Quantification shows an increase in Müller cell expression seen in the SRTG-CNTF group compared to the saline control at the 1 week time point (I). At 2 weeks post-ONC and appropriate intravitreal injection, there was an increased amount of Müller cell expression in all groups (E–H). There was a significant increase in the SRTG-CNTF group compared to all other groups (J). ONL: outer nuclear layer; INL: inner nuclear layer; IPL: inner plexiform layer; GCL: ganglion cell layer; FL: fiber layer. Staining indicates GFAP expression. Photomicrographs were captured at 20× magnification. Scale bar = 100 μm. *p < 0.05 and **p < 0.01.
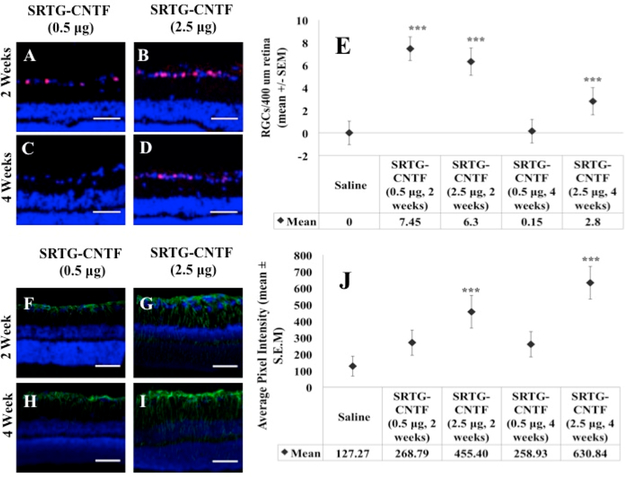
Increasing the Amount of CNTF Can Prolong the Neuroprotective Effect of the Polymer System
As mentioned earlier, we were not able to see a significant difference between the SRTG group and the remaining three groups 4 weeks post-ONC when using a low CNTF amount (0.5 μg). To determine if the low amount of CNTF was the reason for the limited effect length, we increased the CNTF amount to a higher amount (2.5 μg) and analyzed the effects of SRTG-CNTF (2.5 xg) at 2 and 4 weeks and compared these results to corresponding SRTG-CNTF (0.5 μg) and saline control groups. Figure 7A shows the quantification following Brn3a staining to elucidate RGC survival post-ONC. We can see from this figure that the groups SRTG-CNTF (0.5 μg, 2 weeks), SRTG-CNTF (2.5 μg, 2 weeks), and SRTG-CNTF (2.5 μg, 4 weeks) all showed a significant difference in the number of RGCs that survived post-ONC (all p-values: <0.0001). In addition, we analyzed the Müller cell response with this higher dose at extended time points. Figure 7B shows the quantification of fluorescence from the GFAP stained slides. We can see from these results that there was a significant difference between the SRTG-CNTF (2.5 μg, 2 weeks) and SRTG-CNTF (2.5 μg, 4 weeks) compared to the controls (p-values: <0.0001), demonstrating that this increase in CNTF concentration caused a Müller cell response well into the 4-week time point indicating longer term neuroprotection and neuroregeneration
Figure 7.
Larger amounts of CNTF loaded into the SRTG system increased the length of survival of RGCs and expression of GFAP to the 4 week time point. After 2 weeks post-ONC, both low and high CNTF groups showed a significant difference in the number of remaining Brn3a positive cells to the saline control (A, B, E). After 4 weeks post-ONC, only the high CTNF groups showed a significant difference in the number of Brn3a expressing cells compared to the control (C, D, E). For GFAP expression analysis, after 2 weeks post-ONC, both low and high CNTF groups showed a significant difference between the saline control (F, G, J). After 4 weeks post-ONC, there was no significant difference between the low CNTF group and saline (H, J). After 4 weeks, there was a significant difference between the high CNTF group and saline group (I, J). Red staining in A–D indicates Brn3a expression. Green staining in F–I indicates GFAP expression. Photomicrographs were captured at 20× magnification. Scale bar = 50 μm. ***p < 0.001.
DISCUSSION
It has been proposed that RGC death in glaucoma may be due to axonal transport failure of NTFs from axon tip to the RGC cell body. As these NTFs are responsible for many crucial cell functions, deprivation of NTFs can lead cells into subsequent apoptotic degeneration.36,37 In addition, recent developments indicate that damage to central nervous system (CNS) neurons, such as RGCs, can cause them to re-express NTF markers commonly found during development in an attempt to bolster NTF production and begin to regenerate.38–40 These discoveries turned the focus to new treatment strategies aimed at neuroprotecting and regenerating RGCs by delivering NTFs to injured cells. NTF supplementation therapies have been tested using many different and complicated methods including viral gene transfers to retinal cells causing them to overexpress NTFs,8,41 intraocular transplantation of genetically modified cells,42 etc. Perhaps the simplest and most straightforward method of delivering NTFs to RGCs is through a facile intravitreal bolus injection. However, single intravitreal injections of NTFs result in limited neuroprotection and axon regeneration due to rapid clearance, degradation, and short half-life of the NTF.43 Polymeric delivery systems could potentially mitigate the pitfalls of repeated NTF injections by providing continuous delivery of NTF to the appropriate areas. Currently, there is a diverse range of polymeric materials that could aid in the delivery of drugs/NTFs to the eye.44–46
In the present study, we investigated the use of an injectable polymer system to deliver CNTF to RGCs following ONC. CNTF was chosen for this analysis because it has been shown to have a neuroprotective effect on injured RGCs in various pathological conditions.47,48,23 In addition, unlike brain-derived neurotrophic factor (BDNF) or fibroblast growth factor (FGF), CNTF was also shown to stimulate RGC axon regeneration following injury.49,50 CNTF was intermixed within the injectable polymeric delivery system or unconfined in free bolus and delivered intravitreally following ONC. The ONC model has been used extensively to not only study the mechanisms of RGC death but also to devise new neuroprotective and repair strategies for the injured ON.51,52 This model generates direct trauma to RGC axons leading to a severe injury and almost absolute depletion in RGCs after 2 weeks.53 Although this model typically represents a more severe pathogenesis than seen in glaucoma, it provided us with a highly reliable and “extreme-case” model to study the effects of the NTF delivery system. In other words, because this model is so severe, any neuroprotection or regeneration seen would be due to a robust treatment effect and thus will be more easily detected with a smaller standard deviation.
We found that a single intravitreal injection of SRTG-CNTF resulted in significant preservation of RGCs in the ganglion cell layer when compared to the control groups (saline or free CNTF) at both 1 and 2 week time points. We consider it remarkable that this CNTF delivery system was able to rescue such a large amount of RGCs from apoptosis following a severe injury in which control groups experienced almost complete depletion of RGCs after 2 weeks. Additionally, rats that received an intravitreal injection of SRTG-CNTF following severe ON injury retained a similar number of RGCs at the 2 week time point when compared to a healthy eye that had no manipulations or ONC performed on it. However, it is important to note that the number of RGCs did begin to decrease following this 2 week time point, likely due to the insufficient amount of CNTF being released at these longer time points. Considering the robust effect of this polymer system following an ONC which induces severe and rapid depletion of RGCs, it would be interesting to evaluate the neuroprotective capacity of the polymer system using an animal model that more closely resembles the slow and variable loss of RGCs seen with glaucoma.54–56
Along with a neuroprotective effect, CNTF has been shown to induce axon regeneration following ON injury.48,57,50 We observed this regenerative effect of CNTF in eyes that received an intravitreal injection of the polymer system following ONC. Specifically, at both the 1 and 2 week time points post-ONC, we observed robust and significant axon regeneration in mature rat RGCs that received an intravitreal injection of SRTG-CNTF, whereas eyes that received intravitreal injections of saline, free CNTF, or RTG-CNTF showed minimal axon regrowth past the lesion. The separation seen with the SRTGCNTF group is likely due to the addition of sulfonate groups, as this difference was not seen with the unsulfonated polymer (RTG-CNTF). This lends to the idea that the negatively charged sulfonate groups sequestered or stabilized the CNTF before releasing it into the surrounding retina.
Recently, it has been shown that RGC regeneration is closely correlated with activation of retinal astrocytes and Müller cells.58 This is likely because activated Müller cells express CNTF as a response to nerve injury,59,16,60 aiding in protecting RGCs from apoptosis and promoting regeneration of damaged axons.47,61,59,57 Even more interesting, new developments show that CNTF also leads to activation of Müller cells,62 meaning that CNTF contributes to a positive feedback loop leading to RGC protection and axon regeneration following injury. In this study, we saw the effects of prolonged exposure of CNTF on Müller cell activation. At both 1 and 2 week time points, there was a large increase in GFAP staining for Müller cells in the groups that received intravitreal injections of SRTG-CNTF, extending processes spanning the entire width of the retina. In contrast, there was minimal GFAP staining present in the three other groups (saline, free CNTF, and RTG-CNTF) at both time points. This result indicates that prolonged exposure of CNTF led to activation of Müller cells, which could have bolstered neuroprotection of RGCs and axon regeneration by releasing additional CNTF produced by Müller cells. Furthermore, we were able to induce a longer effect simply through increasing the amount of CNTF initially loaded into the polymer system. Using this higher amount of CNTF, we were able to see a significant increase in RGC survival and Müller cell activation at the 4 week time point.
CONCLUSION
With this work, we aimed to show the viability of this polymer as a NTF delivery system for the treatment of retinal degenerations. However, in reality, supplementation of NTFs to the injury site only resolves part of the disease state. The other aspect of glaucoma and other retinal degenerations is the depletion and permanent loss of RGCs. Since these cells do not regenerate, replenishing this population must involve some form of cell transplantation therapy. Due to the number of functional groups available on the polymer backbone (PSHU), this polymer is highly modifiable and may be conjugated with more than one functional unit. For example, in a previous work, we have chemically conjugated biomolecules aimed at promoting cell survival and attachment of neurons (specifically RGCs), making this polymer system a viable scaffold for cell transplantation therapies.63,18,19 Through this work and previous works, we have shown that this polymer system may be used simultaneously for NTF delivery and as a scaffold for cell transplantations giving a 2-fold approach to the treatment of retinal degenerations. In summary, we believe that this polymer system may be utilized as a multifaceted approach to prevent RGC degeneration (through NTF release) as well as provide a new RGC population (through cellular scaffolding).
Supplementary Material
ACKNOWLEDGMENTS
Dr. Mark Petrash provided the equipment needed for cryosectioning and imaging. Pat Lenhart aided in histology training and techniques.
Funding
This work was supported by NIH EY025333–01 and EY023711–01A1.
Footnotes
The authors declare no competing financial interest.
ASSOCIATED CONTENT
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsbiomaterials.8b00803.
Materials; equipment; elemental analysis; solution to gel phase transition; IR-dye conjugation to CNTF; in vitro CNTF release test; optic nerve crush model; retina and optic nerve immunohistochemistry; analysis of neuronal survival; quantification of axonal growth; Figure S1, schematic of the synthesis process for Sul–PSHU–PNIPAAm; Figure S2, elemental analysis of SRTG and RTG; Figure S3, temperature-dependent phase transition of SRTG and RTG; Figure S4, in vitro release profile of CNTF; Figure S5, expression of Brn3a in retinal cross sections (PDF)
REFERENCES
- (1).Almasieh M; Wilson AM; Morquette B; Cueva Vargas JL; Di Polo A The molecular basis of retinal ganglion cell death in glaucoma. Prog. Retinal Eye Res 2012, 31, 152–81. [DOI] [PubMed] [Google Scholar]
- (2).Kuehn MH; Fingert JH; Kwon YH Retinal ganglion cell death in glaucoma: mechanisms and neuroprotective strategies. Ophthalmol. Clin. North Am 2005, 18, 383–395. [DOI] [PubMed] [Google Scholar]
- (3).Huang EJ; Reichardt LF Annu. Rev. Neurosci 2001, 24, 677–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Raff MC; et al. Programmed Cell Death and the Control of Cell Survival: Lessons from the Nervous System. Science 1993, 262, 695–700. [DOI] [PubMed] [Google Scholar]
- (5).Calkins DJ Critical Pathogenic Events Underlying Progresison of Neurodegeneration in Glaucoma. Prog. Retinal Eye Res 2012, 31, 702–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Levin L a. Retinal Ganglion Cells and Neuroprotection for Glaucoma. Surv. Ophthalmol 2003, 48, S21–S24. [DOI] [PubMed] [Google Scholar]
- (7).Pease ME; et al. Effect of CNTF on retinal ganglion cell survival in experimental glaucoma. Invest. Ophthalmol. Visual Sci 2009, 50, 2194–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Flachsbarth K; et al. Neural stem cell-based intraocular administration of ciliary neurotrophic factor attenuates the loss of axotomized ganglion cells in adult mice. Invest. Ophthalmol. Visual Sci 2014, 55, 7029–39. [DOI] [PubMed] [Google Scholar]
- (9).Parrilla-Reverter G; et al. Effects of different neurotrophic factors on the survival of retinal ganglion cells after a complete intraorbital nerve crush injury: a quantitative in vivo study. Exp. Eye Res 2009, 89, 32–41. [DOI] [PubMed] [Google Scholar]
- (10).Tang Y; Singh J Biodegradable and biocompatible thermosensitive polymer based injectable implant for controlled release of protein. Int. J. Pharm 2009, 365, 34–43. [DOI] [PubMed] [Google Scholar]
- (11).Deveza L; Choi J; Yang F Therapeutic angiogenesis for treating cardiovascular diseases. Theranostics 2012, 2, 801–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Bertram JP; Rauch MF; Chang K; Lavik EB Using polymer chemistry to modulate the delivery of neurotrophic factors from degradable microspheres: Delivery of BDNF. Pharm. Res 2010, 27, 82–91. [DOI] [PubMed] [Google Scholar]
- (13).Checa-Casalengua P; et al. Retinal ganglion cells survival in a glaucoma model by GDNF/Vit e PLGA microspheres prepared according to a novel microencapsulation procedure. J. Controlled Release 2011, 156, 92–100. [DOI] [PubMed] [Google Scholar]
- (14).Sangaj N; et al. Heparin mimicking polymer promotes myogenic differentiation of muscle progenitor cells. Biomacromolecules 2010, 11, 3294–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Arakawa Y; Sendtner M; Thoenen H Survival Effect of Ciliary Neurotrophic Factor (CNTF) on Chick Embryonic Motoneurons in Culture: Comparison with Other Neurotrophic Factors and Cytokines. J. Neurosci 1990, 10, 3507–3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Chun M; Ju W; Kim K; Lee M; et al. Upregulation of ciliary neurotrophic factor in reactive Muller cells in the rat retina following optic nerve transection. Brain Res. 2000, 868, 358–362. [DOI] [PubMed] [Google Scholar]
- (17).Cui Q; Yip HK; Zhao RC; So K-F; Harvey AR Intraocular elevation of cyclic AMP potentiates ciliary neurotrophic factor-induced regeneration of adult rat retinal ganglion cell axons. Mol. Cell. Neurosci 2003, 22, 49–61. [DOI] [PubMed] [Google Scholar]
- (18).Yun D; Lee YM; Laughter MR; Freed CR; Park D Substantial Differentiation of Human Neural Stem Cells Into Motor Neurons on a Biomimetic Polyurea. Macromol. Biosci 2015, 15, 1206–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Yun D; Laughter MR; Park D A Biomimetic Reverse Thermal Gel for 3-Dimensional Neural Tissue Engineering. Austin J. Biomed. Eng 2014, 1 (4), 1019. [Google Scholar]
- (20).Peña B; Shandas R; Park D A heparin-mimicking reverse thermal gel for controlled delivery of positively charged proteins. J. Biomed. Mater. Res., Part A 2015, 103, 2102–2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Unoki K; LaVail MM Protection of the rat retina from ischemic injury by brain- derived neurotrophic factor, ciliary neurotrophic factor, and basic fibroblast growth factor. Invest. Ophthalmol. Visual Sci 1994, 35, 907–915. [PubMed] [Google Scholar]
- (22).Zhang C-W; et al. CNTF and BDNF Have Similar Effects on Retinal Ganglion Cell Survival but Differential Effects on Nitric Oxide Synthase Expression Soon after Optic Nerve Injury. Invest. Ophthalmol. Visual Sci 2005, 46, 1497. [DOI] [PubMed] [Google Scholar]
- (23).Ji J CNTF promotes survival of retinal ganglion cells after induction of ocular hypertension in rats: the possible involvement of STAT3 pathway. Eur. J. Neurosci 2004, 19, 265–272. [DOI] [PubMed] [Google Scholar]
- (24).Park D; Wu W; Wang Y A functionalizable reverse thermal gel based on a polyurethane/PEG block copolymer. Biomaterials 2011, 32, 777–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Ibáñez CF Emerging themes in structural biology of neurotrophic factors. Trends Neurosci. 1998, 21, 438–444. [DOI] [PubMed] [Google Scholar]
- (26).Kwong JMK; Quan A; Kyung H; Piri N; Caprioli J Quantitative analysis of retinal ganglion cell survival with Rbpms immunolabeling in animal models of optic neuropathies. Invest. Ophthalmol. Visual Sci 2011, 52, 9694–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Nadal-Nicolas FM; et al. Brn3a as a marker of retinaĺ ganglion cells: qualitative and quantitative time course studies in naive and optic nerve-injured retinas. Invest. Ophthalmol. Visual Sci 2009, 50, 3860–8. [DOI] [PubMed] [Google Scholar]
- (28).Xu G; et al. Optic nerve regeneration in polyglycolic acidchitosan conduits coated with recombinant L1-Fc. Neuroreport 2004, 15, 2167–2172. [DOI] [PubMed] [Google Scholar]
- (29).Newman E; Reichenbach A The Muller cell: a functional element of the retina. Trends Neurosci. 1996, 19, 307–312. [DOI] [PubMed] [Google Scholar]
- (30).García M; Vecino E Role of Müller glia in neuroprotection and regeneration in the retina. Histol Histopathol. 2003, 1205–1218. [DOI] [PubMed] [Google Scholar]
- (31).Caminos E; Becker E; Martin-Zanca D; et al. Neurotrophins and Their Receptors in the Tench Retina During Optic Nerve Regeneration. J. Comp. Neurol 1999, 404, 321–331. [DOI] [PubMed] [Google Scholar]
- (32).Bringmann A; et al. Cellular signaling and factors involved in Müller cell gliosis: neuroprotective and detrimental effects. Prog. Retinal Eye Res 2009, 28, 423–51. [DOI] [PubMed] [Google Scholar]
- (33).Cao W; Wen R; Li F; Cheng T; Steinberg RH Induction of Basic Fibroblast Growth Factor mRNA by Basic Fibroblast Growth Factor in Muller Cells. Invest. Ophthalmol. Visual Sci 1997, 38, 1358–1366. [PubMed] [Google Scholar]
- (34).Müller A; Hauk TG; Fischer D Astrocyte-derived CNTF switches mature RGCs to a regenerative state following inflammatory stimulation. Brain 2007, 130, 3308–3320. [DOI] [PubMed] [Google Scholar]
- (35).Leon S; Yin Y; Nguyen J; Irwin N; Benowitz LI Lens injury stimulates axon regeneration in the mature rat optic nerve. J. Neurosci 2000, 20, 4615–4626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Pease ME; Mckinnon SJ; Quigley HA; Baumrind LAK; Zack DJ Obstructed axonal transport of BDNF and its receptor TrkB in experimental glaucoma. Invest. Ophthalmol. Visual Sci. 2000, 41, 764–774. [PubMed] [Google Scholar]
- (37).Quigley HA; et al. Retrograde Axonal Transport of BDNF in Retinal Ganglion Cells Is Blocked by Acute IOP Elevation in Rats. Invest. Ophthalmol. Visual Sci 2000, 41, 3460–3466. [PubMed] [Google Scholar]
- (38).Gao H; Qiao X; Hefti F; Hollyfield JG; Knusel B Elevated mRNA Expression of Brain-Derived Neurotrophic Factor in Retinal Ganglion Cell Layer After Optic Nerve Injury. Invest. Ophthalmol. Visual Sci 1997, 38, 1840–1847. [PubMed] [Google Scholar]
- (39).Eckenstein P; Shipley D; Nishi R Acidic and Basic Fibroblast Growth Factors in the Nervous System Distribution and Differential Alteration of Levels after Injury of Central versus Peripheral Nerve. J. Neurosci 1991, 11, 412–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Fujino H; et al. Axonal protection by brain-derived neurotrophic factor associated with CREB phosphorylation in tumor necrosis factor-alpha-induced optic nerve degeneration. Acta Neuropathol. 2009, 117, 75–84. [DOI] [PubMed] [Google Scholar]
- (41).Wilson a M.; Di Polo a. Gene therapy for retinal ganglion cell neuroprotection in glaucoma. Gene Ther. 2012, 19, 127–36. [DOI] [PubMed] [Google Scholar]
- (42).Zhang R; et al. Neuroprotective effect of intravitreal cell-based glucagon-like peptide-1 production in the optic nerve crush model. Acta Ophthalmol. 2011, 89, e320–6. [DOI] [PubMed] [Google Scholar]
- (43).Dittrich F; Theonen H; Sendtner M Ciliary Neurotrophic Factor: Pharmacokinetics and Acute-Phase Response in Rat. Ann. Neurol 1994, 35, 151–163. [DOI] [PubMed] [Google Scholar]
- (44).Yasukawa TMD; Kimura H; Tabata Y; Ogura Y Adv. Drug Delivery Rev 2001, 52, 25–36. [DOI] [PubMed] [Google Scholar]
- (45).Yasukawa T; Ogura Y; Sakurai E; Tabata Y; Kimura H Intraocular sustained drug delivery using implantable polymeric devices. Adv. Drug Delivery Rev 2005, 57, 2033–46. [DOI] [PubMed] [Google Scholar]
- (46).Achouri D; Alhanout K; Piccerelle P; Andrieu V Recent advances in ocular drug delivery. Drug Dev. Ind. Pharm 2013, 39, 1599–617. [DOI] [PubMed] [Google Scholar]
- (47).Mey J; Thanos S Intravitreal injections of neurotrophic factors support the survival of axotomized retinal ganglion cells in adult rats in vivo. Brain Res. 1993, 602, 304–317. [DOI] [PubMed] [Google Scholar]
- (48).Hellström M; Pollett M. a; Harvey AR Post-injury delivery of rAAV2-CNTF combined with short-term pharmacotherapy is neuroprotective and promotes extensive axonal regeneration after optic nerve trauma. J. Neurotrauma 2011, 28, 2475–83. [DOI] [PubMed] [Google Scholar]
- (49).Lingor P; et al. ROCK inhibition and CNTF interact on intrinsic signalling pathways and differentially regulate survival and regeneration in retinal ganglion cells. Brain 2008, 131, 250–263. [DOI] [PubMed] [Google Scholar]
- (50).Müller A; Hauk TG; Leibinger M; Marienfeld R; Fischer D Exogenous CNTF stimulates axon regeneration of retinal ganglion cells partially via endogenous CNTF. Mol. Cell. Neurosci. 2009, 41, 233–246. [DOI] [PubMed] [Google Scholar]
- (51).Benowitz LI; Yin Y Combinatorial Treatments for Promoting Axon Regeneration in the CNS: Strategies for Overcoming Inhibitory Signals and Activating Neurons’ Intrinsic Growth State. Dev. Neurobiol 2007, 67, 1148–1165. [DOI] [PubMed] [Google Scholar]
- (52).Harvey AR; et al. Gene therapy and transplantation in CNS repair: The visual system. Prog. Retinal Eye Res 2006, 25, 449–489. [DOI] [PubMed] [Google Scholar]
- (53).Berkelaar M; Clarke DB; Wang Y; Bray GM; Aguayo AJ Axotomy Ganglion Results in Delayed Death and Apoptosis Cells in Adult Rats of Retinal Ganglion Cells in Adult Rats. J. Neurosci 1994, 14, 4368–4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Johnson TV; Tomarev SI Rodent models of glaucoma. Brain Res. Bull 2010, 81, 349–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).McKinnon SJ; Schlamp CL; Nickells RW Mouse models of retinal ganglion cell death and glaucoma. Exp. Eye Res 2009, 88, 816–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (56).Pang IH; Clark AF Nonprimate models for glaucoma retinopathy and optic neuropathy. Neuromethods 2010, 46, 139–164. [DOI] [PubMed] [Google Scholar]
- (57).Leaver SG; et al. AAV-mediated expression of CNTF promotes long-term survival and regeneration of adult rat retinal ganglion cells. Gene Ther. 2006, 13, 1328–1341. [DOI] [PubMed] [Google Scholar]
- (58).Hauk TG; Müller A; Lee J; Schwendener R; Fischer D Neuroprotective and axon growth promoting effects of intraocular inflammation do not depend on oncomodulin or the presence of large numbers of activated macrophages. Exp. Neurol 2008, 209, 469–482. [DOI] [PubMed] [Google Scholar]
- (59).Lee MY; Deller T; Kirsch M; Frotscher M; Hofmann HD Differential regulation of ciliary neurotrophic factor (CNTF) and CNTF receptor alpha expression in astrocytes and neurons of the fascia dentata after entorhinal cortex lesion. J. Neurosci 1997, 17, 1137–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (60).Sarup V; Patil K; Sharma SC Ciliary neurotrophic factor and its receptors are differentially expressed in the optic nerve transected adult rat retina. Brain Res. 2004, 1013, 152–158. [DOI] [PubMed] [Google Scholar]
- (61).Meyer-Franke A; Kaplan MR; Pfrieger FW; Barres BA Characterization of the Signaling Interactions That Promote the Survival and Growth of Developing Retinal Ganglion Cells in Culture. Neuron 1995, 15, 805–819. [DOI] [PubMed] [Google Scholar]
- (62).Wahlin KJ; Campochiaro PA; Zack DJ; Adler R Neurotrophic Factors Cause Activation of Intracellular Signaling Pathways in Muller Cells and Other Cells of the Inner Retina, but Not Photoreceptors. Invest. Ophthalmol. Visual Sci 2000, 41, 927–936. [PubMed] [Google Scholar]
- (63).Laughter MR; et al. A Self-Assembling Injectable Biomimetic Microenvironment Encourages Retinal Ganglion Cell Axon Extension in Vitro. ACS Appl. Mater. Interfaces 2016, 8, 20540–20548. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.