Abstract
Opioid abuse remains a public health crisis despite a tremendous outpouring of resources to address the problem. One factor that might complicate this issue is polydrug abuse. While cannabis is increasingly available due to legalization by states, phytocannabinoids do not appear to alter the abuse-related effects of opioids. Synthetic cannabinoids, which are not pharmacologically identical to phytocannabinoids, are also increasingly available, and differences among cannabinoids might affect their interactions with opioids. This study assessed the impact of one synthetic cannabinoid, JWH-018, on the effects of two μ opioid receptor agonists using two procedures that address different aspects of abuse. First, four monkeys could choose to self-administer the opioid remifentanil alone (0.32 μg/kg/infusion) or a mixture containing 0.32 μg/kg/infusion remifentanil and JWH-018 (1–10 μg/kg/infusion). On separate occasions, monkeys could choose between remifentanil available alone or combined with 100 μg/kg/infusion cocaine. While monkeys chose the remifentanil/cocaine mixture over remifentanil alone, they responded equally for remifentanil alone and the remifentanil/JWH-018 mixture. The ability of JWH-018 to reinstate extinguished responding previously maintained by heroin was examined in four other monkeys. When presented with drug-associated stimuli, heroin, but not JWH-018, reinstated responding, and when combined, JWH-018 did not increase the potency of heroin. While opioids and synthetic cannabinoids, including JWH-018, are abused, these results indicate that JWH-018 does not modify the behavioral effects of opioids in monkeys in a manner that would predict greater abuse liability of cannabinoid/opioid mixtures, a result that is consistent with a growing literature on mixtures of opioids and phytocannabinoids.
Keywords: Opioid, Cannabinoid, JWH-018, Self-Administration, Reinstatement, Rhesus Monkey, Heroin, Remifentanil
1. Introduction
The opioid abuse crisis in the US has resulted in a substantial number of opioid overdose deaths (Hedegaard et al., 2018). While changes in prescribing guidelines appear to have slowed the expansion of the opioid epidemic (e.g., Bohnert et al., 2018), the mortality rate from opioids remains very high. Among the many factors contributing to the opioid crisis is polydrug abuse, which predicts poorer treatment outcomes and increased likelihood of overdose (e.g., Darke et al., 2010; Kerr et al., 2007). With the increasing legalization of cannabis by states and increasing availability of synthetic cannabinoids, coabuse of opioids and cannabinoids is likely, prompting studies examining possible interactions between opioids and cannabinoids. When administered alone, opioids and cannabinoids can relieve pain; combining these drugs might improve treatment because cannabinoids can increase the potency of morphine to produce antinociception in monkeys (Maguire et al., 2013c; Maguire and France 2014). Moreover, cannabinoids do not enhance other effects of opioids, including potential adverse effects such as opioid self-administration (Li et al., 2012; Maguire et al., 2013c), respiratory depression (Weed et al., 2018), or physical dependence (Gerak and France, 2016). Thus, cannabinoids can enhance some, but not all effects of opioids; however, much less is known about interactions between opioids and synthetic cannabinoids.
Many effects of synthetic cannabinoids, including JWH-018, are similar to those of the predominant psychoactive component of cannabis, Δ9-tetrahydrocannabinol (THC), suggesting that interactions between THC and opioids might predict interactions between JWH-018 and opioids. For example, in monkeys discriminating THC, JWH-018 increases drug-lever responding, and these effects of THC and JWH-018 are similarly antagonized by rimonabant (Ginsburg et al., 2012), indicating that these effects of JWH-018 are mediated by agonist actions at CB1 receptors. Despite these pharmacological similarities, there is evidence to suggest that JWH-018 and THC are not identical. JWH-018 has higher affinity for and efficacy at CB2 receptors compared with THC (Rajasekaran et al., 2013). In addition, changes in other receptor systems have been observed during chronic treatment with JWH-018 (e.g., 5-HT1A; Elmore and Baumann, 2018). Such differences might account for some unexpected effects of JWH-018 in humans, including psychosis, convulsions and cardiovascular events. In addition, differences among cannabinoids could mean that the previous findings with phytocannabinoids such as THC will not accurately predict interactions between JWH-018 and opioids.
The current study determined whether JWH-018 enhances the abuse-related effects of opioids using two distinct procedures. A two-response self-administration procedure was used to model aspects of ongoing drug taking and determined whether monkeys prefer a mixture of the ultra-short acting opioid receptor agonist remifentanil and JWH-018 to remifentanil alone. In addition, a reinstatement procedure was used to model aspects of relapse. Responding that was maintained by infusions of heroin was extinguished and then reinstated by noncontingent drug administration and presentation of heroin-associated stimuli. JWH-018 was examined for its ability to reinstate extinguished responding when given alone and to enhance the potency of heroin to reinstate responding.
2. Methods
2.1. Subjects
Eight adult rhesus monkeys contributed to these studies. The four females (monkeys DAI, DAH, GA, JA) weighed between 7.1 and 10.7 kg, and the four males (monkeys CH, HU, MU, KI) weighed between 9.5 and 12.3 kg. Four males participated in the choice procedure, precluding assessment of sex differences in either assay. Monkeys received sufficient quantities of primate chow (High Protein Monkey Diet; Harlan Teklad, Madison, WI), fresh fruit, and peanuts daily to maintain healthy weights, had unlimited access to water, and were housed individually in a room that was maintained under a 14-/10-hour light/dark cycle. These monkeys previously responded for drug under procedures similar to those described here (e.g., Gerak et al., 2019; Weed et al., 2017). Monkeys were maintained in accordance with the Institutional Animal Care and Use Committee, The University of Texas Health Science Center at San Antonio, and the 2011 Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources on Life Sciences, National Research Council, National Academy of Sciences).
2.2. Surgery
Prior to surgery, monkeys were given 10 mg/kg ketamine (Henry Schein Animal Health, Dublin OH); thereafter, they were intubated and maintained on isoflurane anesthesia (Butler Animal Health Supply, Grand Prairie, TX) with oxygen delivered at a rate of 2 L/min. Silicone double-lumen catheters (monkeys HU, JA, KI, MU; Instech Solomon, San Antonio, TX) or polyurethane single-lumen catheters (monkeys CH, DAH, DAI, GA; SIMS Deltec Inc., St. Paul, MN, USA) were placed in a vein (e.g., femoral or jugular) and then tunneled s.c. to the back where each lumen was connected to a vascular access port (Access Technologies, Skokie, IL, USA). Monkeys received penicillin B and G (40,000 IU/kg) and meloxicam (0.1–0.2 mg/kg) postoperatively.
2.3. Apparatus
During experimental sessions, monkeys sat in commercially available chairs (Primate Products, Miami, FL), which were placed in ventilated and sound-attenuating chambers equipped with two response levers. Stimulus lights located above each lever could be illuminated either green or red. Syringes in syringe pumps (Med Associates, Inc.) were connected to each vascular access port with a 20-g Huber-point needle (Access Technologies) and 183-cm catheter extension sets (Baxter Healthcare, Deerfield, IL). The size of the syringe depended on the size of the monkey, and the infusion rate varied depending on the size of the syringe (2.3 ml/min for a 30-ml syringe; 3.4 ml/min for a 60-ml syringe). Experimental events were controlled and data recorded by a computer operating MedPC IV software (Med Associates, Inc., St. Albans, VT). White noise was present in chambers to mask extraneous sounds.
2.4. Procedures
2.4.1. Choice Procedure
Four monkeys (HU, JA, KI, and MU) responded under a fixed-ratio (FR) 30 schedule for i.v. infusions with two different solutions available for self-administration each day (Weed et al., 2017). Sessions were divided into two distinct types of trials; during the first two trials (forced trials), monkeys responded on one lever in the first trial and on the other lever in the second trial to receive a reinforcer, whereas during the rest of the trials (choice trials), monkeys could choose between the two alternatives. On all trials, availability of drug for self-administration was signaled by illumination of one (forced trials) or both (choice trials) green stimulus lights; when monkeys responded 30 consecutive times on a lever below an illuminated stimulus light, the green light above that lever changed to red for 5 sec before it was extinguished, and if the green light above the other lever was illuminated (i.e., choice trial), it was extinguished immediately. Completing the response requirement also activated the syringe pump which delivered the solution associated with responding on that lever for that session and initiated a 180-sec intertrial interval. The duration of the infusion ranged from 17–24 sec, depending on the weight of the monkey. Different unit doses were studied across sessions by changing the concentration of drug. During intertrial intervals, responses were recorded but had no programmed consequence. While the green light(s) were illuminated, a response on one lever reset the response requirement on the other lever. Monkeys were required to complete both forced trials, one on each lever, to advance to choice trials, and the two forced trials were presented in a random order. Sessions ended after completion of 24 choice trials or 100 minutes, whichever occurred first.
For all sessions, responding on one lever resulted in the delivery of 0.32 μg/kg/infusion remifentanil; this dose maintains high response rates and is selected over food (Maguire et al., 2013a) or saline (Maguire et al., 2013b; Weed et al., 2017) in choice procedures. The solution in the other syringe varied with each solution available for a minimum of 3 sessions. Experimental conditions changed when the following criterion was satisfied: for the last 3 sessions, the number of infusions of the variable solution did not differ by more than 20% from the mean of those sessions with no upward or downward trend in the number of infusions or response rates. If that criterion was not satisfied within 7 sessions, experimental conditions changed regardless of response choice. For the first change in experimental conditions, the solutions available for self-administration remained the same, and the lever designation was reversed so that responding on the lever that previously resulted in the delivery of the variable solution now resulted in the delivery of 0.32 μg/kg/infusion remifentanil with responding on the other lever resulting in the delivery of the variable solution. These conditions remained in place until the criterion was satisfied or for 7 sessions after which the variable solution changed, whichever occurred first.
While a fixed dose of 0.32 μg/kg/infusion remifentanil was always available on one lever, the solution available for self-administration on the other lever changed across conditions. For example, a dose-effect curve was obtained for remifentanil alone (0.1–1 μg/kg/infusion) by changing the unit dose available on one lever. To determine whether monkeys preferred remifentanil when it was combined with JWH-018 over remifentanil alone, a dose-effect curve was determined for JWH-018 mixed with 0.32 μg/kg/infusion remifentanil, beginning with 1 μg/kg/infusion and increasing in ½ log unit increments. On separate occasions, the variable solution was 100 μg/kg/infusion cocaine alone and in combination with 0.32 μg/kg/infusion remifentanil.
2.4.2. Reinstatement Procedure
Four monkeys (CH, DAI, DAH, and GA) participated in three different types of sessions (Gerak et al., 2019). All sessions lasted 90 min and began with delivery of a loading infusion to fill the catheter. For baseline self-administration sessions, the catheter was filled with heroin; one minute after activation of the syringe pump, the drug-associated stimulus, which was the red stimulus light located above the active lever, was illuminated for 5 sec, signaling the delivery of a noncontingent priming infusion of 10 μg/kg/infusion heroin. Once the priming infusion was delivered, the green stimulus light above the active lever was illuminated, and that unit dose of heroin was available under an FR30 schedule. Completion of the response requirement extinguished the green light, illuminated the red light for 5 sec, and initiated the drug infusion as well as the 180-sec timeout. The infusion duration depended on the weight of the monkey and ranged from 15 to 23 sec. At the end of the timeout, the green light was again illuminated, and monkeys could respond for another infusion of drug. The active lever was the left lever for three monkeys. Responses on the inactive lever and those on the active lever during timeouts were recorded but had no scheduled consequence. During baseline self-administration sessions, responding was considered stable when there was less than 20% difference in number of responses across the last three baseline sessions with no increasing or decreasing trend.
When the criterion was satisfied, extinction sessions were conducted, which began with a loading infusion of saline to fill the catheter. Thereafter, stimulus lights were not illuminated, and no infusion (contingent or noncontingent) was delivered, although responding was recorded throughout the 90-min session. Extinction sessions were conducted until the number of responses emitted during one session was less than 10% of the number of drug-reinforced responses that were emitted during the last self-administration session. Once that criterion was satisfied, a reinstatement session was conducted. Before the session, a solution was injected into the port, which was then connected to a syringe in the syringe pump containing saline; when the pump was activated at the start of the session, the solution in the port was delivered. Reinstatement sessions were identical to baseline sessions except that the syringe pump was not activated after the loading infusion was delivered, and in the presence of the green stimulus light, responding under the FR30 schedule resulted in a 5-sec presentation of the red light followed by a 180-sec timeout. After the reinstatement session, baseline self-administration conditions were again introduced.
Two different types of reinstatement tests were conducted. First, to determine whether JWH-018 increased reinstated responding, monkeys received one dose of JWH-018 (3.2–32 μg/kg) before a reinstatement session. The number of responses emitted after noncontingent administration of heroin (3.2–100 μg/kg) or cocaine (10–1000 μg/kg) were obtained for comparison. The ability of JWH-018 to enhance responding reinstated by heroin was determined by administering 3.2 μg/kg JWH-018 along with a dose of heroin (3.2–32 μg/kg) before the session; 100 μg/kg cocaine was also studied in combination with heroin.
2.5. Drugs
Remifentanil HCl, JWH-018, cocaine HCl and heroin HCl were generously provided by the National Institute on Drug Abuse Drug Supply Program (Bethesda, MD, USA). Remifentanil, cocaine and heroin were dissolved in saline. JWH-018 was dissolved in a vehicle containing 5% ethanol, 5% emulphor and 90% saline. A saline solution containing heparin (100 U/ml; Hospira Inc., Lake Forest, IL, USA) was placed in each lumen of the catheter after sessions.
2.6. Data Analyses
When monkeys chose between 0.32 μg/kg/infusion remifentanil and a solution that varied across sessions, the percentage choice of the variable solution, overall response rate during choice trials, and number of choice trials completed were plotted as a function of unit dose. Percentage choice was obtained by dividing the number of infusions of the variable solution by the total number of infusions during choice trials. Response rate was calculated by adding the number of responses emitted while the green lights were illuminated across levers and choice trials (i.e., responses during forced trials were excluded) and dividing that sum by the total time that the green lights were illuminated during choice trials. Each data point was determined twice, with the variable solution available on each lever. Data points shown in figures were obtained by averaging values across the last three sessions for a particular condition (variable solution and lever designation) and then averaging across the two determinations; those means (± 1 SEM) are shown in the figures. Separate one-factor repeated measures ANOVAs were conducted to detect statistical differences for each dependent variable with the factor being the variable solution; ANOVAs were followed by post-hoc Tukey’s multiple comparisons tests.
When monkeys responded during baseline self-administration or reinstatement sessions, the number of responses that occurred in the presence of the green lights was plotted as a function of dose. During extinction sessions when visual stimuli were not presented, the number of responses on the lever that was previously associated with heroin was plotted. Separate one-factor repeated measures ANOVAs were used to detect statistical differences for multiple determinations of each type of session (e.g., baseline self-administration sessions; points above BL, Figure 2). Responding across different types of sessions (e.g., baseline, extinction, reinstatement with heroin-associated stimuli alone and in combination with drug administered noncontingently) was compared using one-factor repeated measures ANOVA with the factor being type of session followed by post-hoc Tukey’s multiple comparisons tests. In addition, a two-factor repeated measures ANOVA was used to determine whether 100 μg/kg cocaine or 3.2 μg/kg JWH-018 altered heroin-induced reinstatement, with one factor being solution administered noncontingently with heroin and the other factor being heroin dose, followed by post-hoc Tukey’s multiple comparisons test. Data analyses were performed using GraphPad Prism (version 6.07 GraphPad, La Jolla, California, USA), and effects were considered significant when P<0.05.
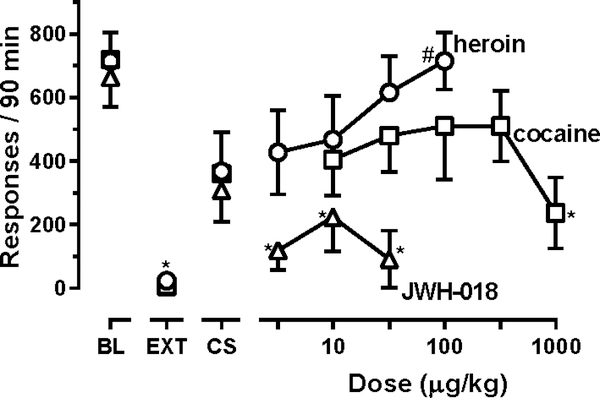
Figure 2.
Effects of heroin, cocaine and JWH-018 on reinstatement of responding previously maintained by heroin (n=4).
Leftmost points show the mean (± 1 SEM) number of responses during baseline sessions in which 10 μg/kg/infusion heroin was available for self-administration (points above BL) with the mean number of responses emitted during extinction also shown (points above EXT). The number of responses reinstated by presentation of drug-associated stimuli alone were obtained following noncontingent infusions of saline (points above CS). Noncontingent infusions of drug preceded the rest of the reinstatement sessions during which drug-associated stimuli were also presented. Separate one-factor repeated-measures ANOVAs were used to detect significant differences in responding across different types of sessions with a separate analysis used to compare across sessions for each of the three drugs tested during reinstatement sessions; * denotes conditions in which responding was significantly (P<0.05) lower than that maintained by 10 μg/kg/infusion heroin (BL), and # denotes conditions in which responding was significantly (P<0.05) greater than during extinction (EXT). Ordinate: number of responses in 90-min reinstatement sessions. Abscissa: dose (μg/kg; i.v.).
3. Results
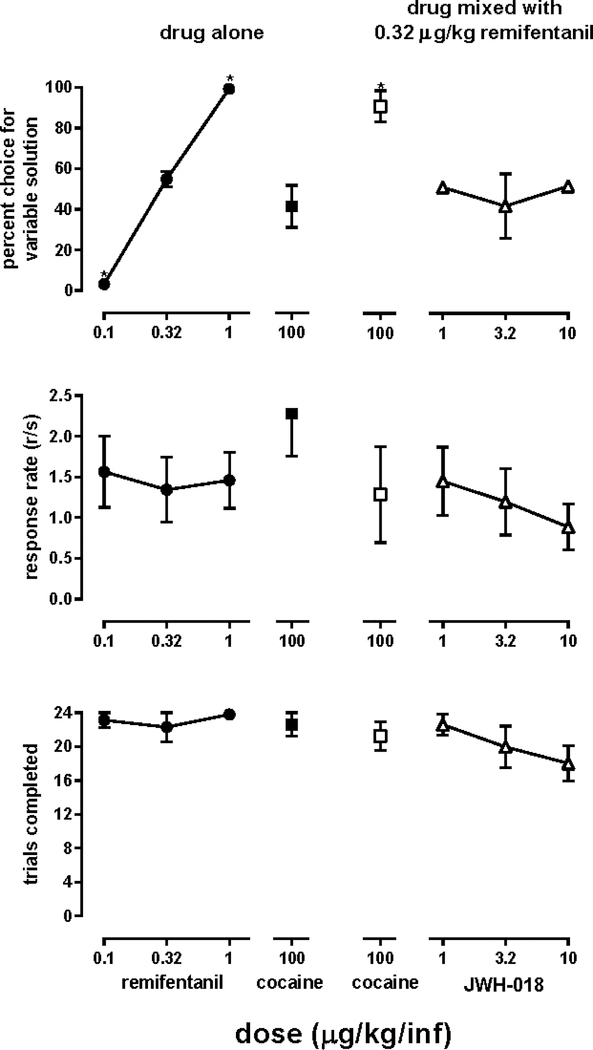
When 0.32 μg/kg/infusion remifentanil was available for self-administration on both levers, monkeys responded 54.9 ± 3.8% on one lever at a rate of 1.34 ± 0.40 responses/sec, and they completed 22.3 ± 1.7 choice trials. Most changes in experimental conditions (86%) occurred because monkeys satisfied the stability criterion within 7 sessions. When other doses of remifentanil were available on one lever, monkeys reliably chose the larger dose (filled circles, top panel, Figure 1); specifically, monkeys chose 0.32 over 0.1 μg/kg/infusion remifentanil and 1 over 0.32 μg/kg/infusion remifentanil. During other sessions, monkeys could choose between 0.32 μg/kg/infusion remifentanil and 100 μg/kg/infusion cocaine, and they responded for cocaine almost as frequently as they responded for remifentanil (filled square, top panel, Figure 1); however, combining those unit doses of cocaine and remifentanil resulted in monkeys choosing the cocaine/remifentanil mixture over that unit dose of remifentanil alone (open square, top panel, Figure 1). In contrast, when different unit doses of JWH-018 were combined with remifentanil, monkeys responded equally for remifentanil alone and for the JWH-018/remifentanil mixtures (triangles, top panel, Figure 1). One-factor ANOVA revealed that the solution available for self-administration significantly affected choice (F[1.28, 3.85]=15.6; P=0.017). Changing one solution did not significantly alter response rates (F[1.42, 4.24]=1.26; P=0.35; middle panels, Figure 1) or choice trials completed (F[1.91, 5.73]=2.63; P=0.16; bottom panels, Figure 1).
Figure 1.
Percentage choice of the variable solution (top panels), response rate during choice trials (middle panels), and choice trials completed (bottom panels) averaged across four monkeys responding on one lever for delivery of 0.32 μg/kg/infusion remifentanil and on a second lever for delivery of a solution that varied across sessions.
Each data point was determined twice (once with the variable solution available for self-administration on the left lever and once with the variable solution available on the right lever). Data points represent the mean (± 1 SEM) of the two determinations. Separate one-factor repeated-measures ANOVAs were used to detect significant differences in percentage choice of the variable solution, response rate, and number of trials completed with the variable solution as the factor; * (P<0.05) denotes conditions in which values were significantly different from those obtained when 0.32 μg/kg/infusion remifentanil was available on both levers. Ordinates: percentage choice of the variable solution (top panels), response rate in responses/sec (middle panels), and number of choice trials completed (bottom panels). Abscissae indicate the unit dose of remifentanil or cocaine available alone (left panels) or the unit dose of cocaine or JWH-018 combined with 0.32 μg/kg/infusion remifentanil (right panels). Missing error bars indicate the point encompasses the variance.
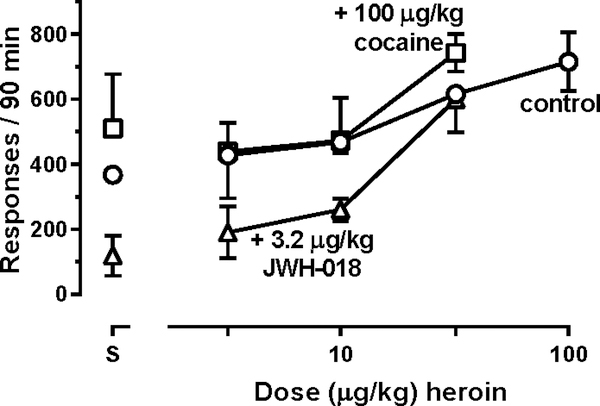
During baseline self-administration sessions when monkeys could respond to receive 10μg/kg/infusion heroin, they emitted, on average, 707 ± 71 responses; during extinction sessions, responding was significantly decreased to 24 ± 19 responses (circles above BL and EXT, Figure 2). When vehicle was administered noncontingently before sessions in which monkeys responded to receive heroin-associated stimuli, the average number of responses was 368 ± 153 (circles above CS, Figure 2). Responding did not vary significantly across multiple determinations of heroin self-administration sessions (F[1.26, 3.78]=1.52; P=0.30; points above BL, Figure 2), extinction sessions (F[1.02, 3.05]=1.17; P=0.36; points above EXT, Figure 2), or reinstatement sessions in which only vehicle was administered (F[1.21, 3.62]=0.17; P=0.74; points above CS, Figure 2). During reinstatement sessions in which vehicle was administered, responding was not significantly different from responding that occurred during self-administration or extinction sessions, increasing the noncontingent dose of heroin dose-dependently increased reinstated responding (F[2.04,6.10]=14.7; P=0.0046) with monkeys emitting significantly more responses after receiving 100 μg/kg heroin (715 ± 91 responses, on average) than those emitted during extinction sessions (circles, Figure 2). Responding was significantly decreased during extinction sessions and following noncontingent administration of 1000 μg/kg cocaine (F[1.90,5.69]=7.26; P=0.0046) and all doses of JWH-018 (F[1.70,5.09]=11.05; P=0.015); no dose of cocaine or JWH-018 significantly increased the number of responses above that emitted during extinction sessions (squares and triangles, respectively, Figure 2). Moreover, combining 100 μg/kg cocaine or 3.2 μg/kg JWH-018 with increasing doses of heroin did not alter the ability of heroin to reinstate responding (Figure 3) with two-factor ANOVA revealing a main effect of heroin dose (F[3,9]=29.45; P<0.0001) and no main effect of the solution given with heroin (F[2,6]=3.37; P=0.10) or an interaction between the two factors (F[6,18]=0.66; P=0.68).
Figure 3.
Effects of heroin administered noncontingently with saline (circles), cocaine (squares), or JWH-018 (triangles) on reinstatement of responding previously maintained by heroin (n=4).
Leftmost points represent the number of responses reinstated by each solution in the absence of heroin (points above S). Drug-associated stimuli were presented during each reinstatement session. A two-factor repeated-measures ANOVA was used to detect significant differences in responding when different solutions were combined with heroin. Ordinate: number of responses in 90-min reinstatement sessions. Abscissa: dose (μg/kg) heroin (i.v.).
4. Discussion
With increasing legalization of cannabis, the scientific and medical communities have been assessing its impact on opioid abusers. Some reports have speculated that cannabis serves as a substitute for opioids (Corroon et al., 2017) and that cannabis use increases the risk of opioid use disorder (Olfson et al., 2018a, 2018b). Others hypothesize that cannabis use in individuals receiving medication-assisted treatment might strengthen the relationship between pain and depression as well as pain and anxiety that are commonly comorbid in opioid use disorder (Wilson et al., 2018). While those findings imply that cannabinoid use by opioid abusers might be detrimental, other reports suggest that cannabinoids might be helpful in reducing opioid abuse. For example, opioid use decreases in abusers who use both opioids and cannabinoids compared with those who abuse opioids alone (Reinman et al., 2017). Moreover, with legalization of cannabis, rates of opioid-induced mortality (Bachhuber et al., 2014) as well as the number of opioid prescriptions (Bradford and Bradford 2016) has decreased; however, over the same time, policy changes were implemented to decrease opioid prescribing, use, and abuse. Thus, the impact of cannabis on opioid abuse remains unclear, and even fewer studies have examined a possible role of synthetic cannabinoids. The current study examined the impact of the synthetic cannabinoid JWH-018 on the abuse-related effects of opioids to determine whether it interacts with opioids in a manner that might exacerbate the opioid crisis.
The concurrent choice procedure modeled ongoing opioid abuse with monkeys choosing between remifentanil alone and a solution that varied in doses and constituents; this procedure was selected because it is sensitive to the reinforcing effects of drug combinations. For example, like cannabinoids, benzodiazepines are less likely than other drugs of abuse to be self-administered by monkeys (e.g., Griffiths and Weerts, 1997), and despite numerous anecdotal reports in humans of opioid/benzodiazepine coabuse (e.g., Gelkopf et al., 1999; Lavie et al., 2009; Peles et al., 2006; San et al., 1993), there was little evidence of increased self-administration of drug combinations. Recently, this procedure demonstrated that monkeys prefer opioid/benzodiazepine mixtures to larger doses of the opioid alone (Weed et al., 2017); consequently, this choice procedure was selected to study combinations of remifentanil and JWH-018. When different doses of remifentanil were available for self-administration, monkeys reliably chose the larger dose, indicating that they were sensitive to reinforcer magnitude. In contrast, monkeys did not prefer combinations of remifentanil and JWH-018 to the same dose of remifentanil alone.
Although several factors could impact preference, leading to this null result, some can be eliminated as likely explanations. For example, because it is important to know whether behaviorally active doses of JWH-018 were used, one factor involves the dose range of JWH-018 studied in combination with remifentanil. Although not statistically significant, response rate and the number of trials completed decreased for each monkey when remifentanil was studied in combination with the largest unit dose of JWH-018 (10 μg/kg/infusion) compared with responding when 0.32 μg/kg/infusion remifentanil alone was available on both levers; rates were reduced by 50% in one monkey, suggesting that this dose combination is the largest one that could be studied before monkeys began choosing remifentanil alone over the JWH-018/remifentanil mixture or, more likely, a marked decrease in response rate was observed. In addition, monkeys chose a combination of 10 μg/kg/infusion JWH-018 and 0.32 μg/kg/infusion remifentanil in 50% of trials (8–10 total infusions), resulting in total cumulative intake of 80–100μg/kg JWH-018, which is 2- to 3-fold larger than the dose that produces discriminative stimulus effects in monkeys (Ginsburg et al., 2012). These findings indicate that doses of JWH-018 used in the current study were behaviorally active.
A second possibility for the null result is insensitivity of the choice procedure to differential reinforcing effectiveness of drug mixtures. In addition to findings from a previous study with opioid/benzodiazepine mixtures (Weed et al., 2017), results from the current study suggest that insensitivity to mixtures does not account for the null result. When 0.32 μg/kg/infusion remifentanil was available on one lever and 100 μg/kg/infusion cocaine was available on the other lever, monkeys chose cocaine in 41% of trials; however, when combined, monkeys chose the remifentanil/cocaine mixture over the same dose of remifentanil alone. Thus, the concurrent choice procedure detected preference for opioid/cocaine and opioid/benzodiazepine mixtures and not for opioid/cannabinoid mixtures. These results are consistent with previous findings using other self-administration procedures (Li et al., 2012; Maguire and France, 2016, 2018; Maguire et al., 2013c) and suggest that JWH-018 does not increase preference for an opioid.
Another aspect of drug abuse that significantly impacts treatment outcome is relapse. This complex phenomenon cannot be easily studied in the preclinical laboratory; however, reinstatement procedures are used widely and thought to model some features of relapse. Presentation of heroin-associated stimuli increased extinguished responding, and that effect was further enhanced when 100 μg/kg heroin was given noncontingently before sessions (current study; Gerak et al., 2019). In contrast, neither cocaine nor JWH-018 increased reinstated responding beyond that produced by heroin-associated stimuli alone, up to doses of either drug that significantly decreased responding. In rats, the synthetic cannabinoid receptor agonists WIN-55,212, CP-55,940 and HU-210, but not THC, reinstated responding previously maintained by heroin (de Vries et al., 2003; Fattore et al., 2003, 2005, 2011). These differences among cannabinoids (and/or between species) demonstrate the need to study other synthetic cannabinoids like JWH-018; even less is known about responding that is reinstated by mixtures of opioids and cannabinoids. In the current study, neither JWH-018 nor cocaine increased the potency of heroin to reinstate responding. To the extent that this type of reinstatement procedure in monkeys is predictive of relapse in humans, these results suggest that JWH-018 is not likely to cause relapse to opioid use.
In summary, there appear to be differences in the effects of cannabis and synthetic cannabinoids, which might affect their potential impact on the opioid epidemic. The current study examined the effects of one synthetic cannabinoid, JWH-018, on opioid self-administration and reinstatement and showed that JWH-018 does not appear to increase abuse-related effects of opioids. Taken together, these results suggest that the synthetic cannabinoid JWH-018 is not likely to contribute directly to the ongoing opioid epidemic.
Highlights.
Phytocannabinoids do not appear to alter the abuse-related effects of opioids.
The study examines how the synthetic cannabinoid JWH-018 interacts with opioids.
JWH-018 with remifentanil was not preferred to remifentanil alone.
JWH-018 did not reinstate extinguished responding previously maintained by heroin.
This synthetic cannabinoid does not appear to increase abuse liability of opioids.
Acknowledgements
The authors would like to thank J. Juarez for her expert technical assistance.
Role of Funding Source
This work was supported by the National Institutes of Health, National Institute on Drug Abuse (Grants R01DA005018 and T32DA031115) and by the Welch Foundation (Grant AQ-0039). All funding sources had no involvement beyond financial support of this study.
Footnotes
Conflict of Interest
No conflict declared.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bachhuber MA, Saloner B, Cunningham CO, Barry CL, 2014. Medical cannabis laws and opioid analgesic overdose mortality in the United States, 1999–2010. JAMA Intern. Med 174, 1668–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohnert ASB, Guy GP Jr., Losby JL, 2018. Opioid prescribing in the United States before and after the Centers for Disease Control and Prevention’s 2016 opioid guideline. Ann. Intern. Med 169, 367–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford AC, Bradford WD, 2016. Medical marijuana laws reduce prescription medication use in Medicare Part D. Health Aff. (Millwood) 351, 1230–1236. [DOI] [PubMed] [Google Scholar]
- Corroon JM, Mischley LK, Sexton M, 2017. Cannabis as a substitute for prescription drugs–A cross sectional study. J. Pain Res 10, 989–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darke S, Ross J, Mills K, Teesson M, Williamson A, Havard A, 2010. Benzodiazepine use among heroin users: Baseline use, current use and clinical outcome. Drug Alcohol Rev. 29, 250–255. [DOI] [PubMed] [Google Scholar]
- de Vries TJ, Homberg JR, Binnekade R, Raasø H, Schoffelmeer AN, 2003. Cannabinoid modulation of the reinforcing and motivational properties of heroin and heroin-associated cues in rats. Psychopharmacology 168, 164–169. [DOI] [PubMed] [Google Scholar]
- Elmore JS, Baumann MH, 2018. Repeated exposure to the “spice” cannabinoid JWH-018 induces tolerance and enhances responsiveness to 5-HT1A receptor stimulation in male rats. Front. Psychiatry 9, 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fattore L, Spano S, Cossu G, Deiana S, Fadda P, Fratta W, 2005. Cannabinoid CB1 antagonist SR 141716A attenuates reinstatement of heroin self-administration in heroin-abstinent rats. Neuropharmacology 48, 1097–1104. [DOI] [PubMed] [Google Scholar]
- Fattore L, Spano MS, Cossu G, Deiana S, Fratta W, 2003. Cannabinoid mechanism inreinstatement of heroin- seeking after a long period of abstinence in rats. Eur. J. Neurosci 17, 1723–1726. [DOI] [PubMed] [Google Scholar]
- Fattore L, Spano MS, Melis V, Fadda P, Fratta W, 2011. Differential effect of opioid and cannabinoid receptor blockade on heroin-seeking reinstatement and cannabinoidsubstitution in heroin- abstinent rats. Br. J. Pharmacol 163, 1550–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelkopf M, Bleich A, Hayward R, Bodner G, Adelson M, 1999. Characteristics of benzodiazepine abuse in methadone maintenance treatment patients: A 1 year prospective study in an Israeli clinic. Drug Alcohol Depend. 55, 63–68. [DOI] [PubMed] [Google Scholar]
- Gerak LR, Collins GT, Maguire DR, France CP, 2019. Effects of lorcaserin on reinstatement of responding previously maintained by cocaine or remifentanil in rhesus monkeys. Exp. Clin. Psychopharmacol 27, 78–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerak LR, France CP, 2016. Combined treatment with morphine and Δ9-tetrahydrocannabinol in rhesus monkeys: antinociceptive tolerance and withdrawal. J. Pharmacol. Exp. Ther 357, 357–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsburg BC, Schulze DR, Hruba L, McMahon LR, 2012. JWH-018 and JWH-073: Δ9-tetrahydrocannabinol-like discriminative stimulus effects in monkeys. J. Pharmacol. Exp. Ther 340, 37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths RR, Weerts EM, 1997. Benzodiazepine self-administration in humans and laboratory animals— implications for problems of long-term use and abuse. Psychopharmacology 134, 1–37. [DOI] [PubMed] [Google Scholar]
- Hedegaard H, Miniño AM, Warner M, 2018. Drug overdose deaths in the United States, 1999–2017 NCHS Data Brief, No. 329. National Center for Health Statistics, Hyattsville, MD: Available from: https://www.cdc.gov/nchs/data/databriefs/db329-h.pdf. [Google Scholar]
- Kerr T, Fairbairn N, Tyndall M, Marsh D, Li K, Montaner J, Wood E, 2007. Predictors of non-fatal overdose among a cohort of polysubstance-using injection drug users. Drug Alcohol Depend. 87, 39–45. [DOI] [PubMed] [Google Scholar]
- Lavie E, Fatséas M, Denis C, Auriacombe M, 2009. Benzodiazepine use among opiate-dependent subjects in buprenorphine maintenance treatment: Correlates of use, abuse and dependence. Drug Alcohol Depend. 99, 338–344. [DOI] [PubMed] [Google Scholar]
- Li JX, Koek W, France CP, 2012. Interactions between Δ9-tetrahydrocannabinol and heroin: Self-administration in rhesus monkeys. Behav. Pharmacol 23, 754–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire DR, France CP, 2014. Impact of efficacy at the μ-opioid receptor on antinociceptive effects of combinations of μ-opioid receptor agonists and cannabinoid receptor agonists. J. Pharmacol. Exp. Ther 351, 383–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire DR, France CP, 2016. Effects of daily delta-9-tetrahydrocannabinol treatment on heroin self-administration in rhesus monkeys. Behav. Pharmacol 27, 249–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire DR, France CP, 2018. Reinforcing effects of opioid/cannabinoid mixtures in rhesus monkeys responding under a food/drug choice procedure. Psychopharmacology 235, 2357–2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire DR, Gerak LR, France CP, 2013a. Delay discounting of food and remifentanil in rhesus monkeys. Psychopharmacology 229, 323–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire DR, Gerak LR, France CP, 2013b. Effect of delay on self-administration of remifentanil under a drug versus drug choice procedure in rhesus monkeys. J. Pharmacol. Exp. Ther 347, 557–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire DR, Yang W, France CP, 2013c. Interactions between μ-opioid receptor agonists and cannabinoid receptor agonists in rhesus monkeys: antinociception, drug discrimination, and drug self-administration. J. Pharmacol. Exp. Ther 345, 354–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olfson M, Wall MM, Blanco C, 2018a. Medical marijuana and the opioid epidemic: Response to Theriault and Schlesinger. Am. J. Psychiatry 175, 285–285. [DOI] [PubMed] [Google Scholar]
- Olfson M, Wall MM, Liu SM, Blanco C, 2018b. Cannabis use and risk of prescription opioid use disorder in the United States. Am. J. Psychiatry 175, 47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peles E, Schreiber S, Adelson M, 2006. Factors predicting retention in treatment: 10-year experience of a methadone maintenance treatment (MMT) clinic in Israel. Drug Alcohol Depend. 82, 211–217. [DOI] [PubMed] [Google Scholar]
- Rajasekaran M, Brents LK, Franks LN, Moran JH, Prather PL, 2013. Human metabolites of synthetic cannabinoids JWH-018 and JWH-073 bind with high affinity and act as potent agonists at cannabinoid type-2 receptors. Toxicol. Appl. Pharmacol 269, 100–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinman A, Welty M, Solomon P, 2017. Cannabis as a substitute for opioid-based pain medication: Patient self-report. Cannabis Cannabinoid Res. 2, 160–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- San L, Tato J, Torrens M, Castillo C, Farré M, Camí J, 1993. Flunitrazepam consumption among heroin addicts admitted for in-patient detoxification. Drug Alcohol Depend. 32, 281–286. [DOI] [PubMed] [Google Scholar]
- Weed PF, France CP, Gerak LR, 2017. Preference for an opioid/benzodiazepine mixture over an opioid alone using a concurrent choice procedure in rhesus monkeys. J. Pharmacol. Exp. Ther 362, 59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weed PF, Gerak LR, France CP, 2018. Ventilatory-depressant effects of opioids alone and in combination with cannabinoids in rhesus monkeys. Eur. J. Pharmacol 833, 94–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson M, Gogulski HY, Cuttler C, Bigand TL, Oluwoye O, Barbosa-Leiker C, Roberts MA, 2018. Cannabis use moderates the relationship between pain and negative affect in adults with opioid use disorder. Addict. Behav 77, 225–231. [DOI] [PubMed] [Google Scholar]