Abstract
Aims
Emerging evidence suggests that maternal vitamin D status may be associated with gestational diabetes (GDM). However, the temporal relation remains unclear due to the lack of longitudinal data on vitamin D over pregnancy. We aimed to prospectively and longitudinally investigate vitamin D status during early to mid-pregnancy in relation to GDM risk.
Methods
In a nested case-control study of 107 GDM cases and 214 controls within the Fetal Growth Studies-Singleton Cohort, plasma levels of 25-hydroxyvitamin D2 and D3 (25(OH)D) and vitamin D binding protein were measured at gestational weeks 10–14, 15–26, 23–31, and 33–39; we further calculated total, free, and bioavailable 25(OH)D. Conditional logistic regression models and linear mixed-effects models were used.
Results
We observed a threshold effect for the relation of vitamin D biomarkers with GDM risk. Vitamin D deficiency (<50 nmol/L) at 10–14 gestational weeks was associated with a 2.82-fold increased risk for GDM [odds ratio (OR) =2.82, 95% confidence interval (CI): 1.15–6.93]. Women with persistent vitamin D deficiency at 10–14 and 15–26 weeks of gestation had a 4.46-fold elevated risk for GDM compared to women persistently non-deficient (OR=4.46, 95% CI: 1.15–17.3).
Conclusions
Maternal vitamin D deficiency as early as the first trimester of pregnancy was associated with an elevated risk of GDM. The association was stronger for women who were persistently deficient through the 2nd trimester. Assessment of vitamin D status in early pregnancy may be clinically important and valuable for improving risk stratification and developing effective interventions for the primary prevention of GDM.
INTRODUCTION
Gestational diabetes mellitus (GDM) is one of the most common metabolic complications of pregnancy, affecting up to 9.2% of pregnant women in the United States (U.S.).1 GDM is also a global epidemic and is thought to affect up to 12.9% of all pregnancies worldwide.2 Women with GDM have an increased risk of developing type 2 diabetes after delivery; and their offspring may be predisposed to childhood obesity and type 2 diabetes later in life.3 Therefore, identifying potentially modifiable factors that may inform the prevention of GDM may not only improve pregnant women’s health, but also their children’s.
Although the precise mechanisms underlying the pathophysiology of GDM remain unclear, both β-cell dysfunction and pregnancy-induced insulin resistance are thought to be key components.4 Accumulating data indicates that vitamin D may modulate pancreatic β-cell function, improve insulin sensitivity, and alter glucose metabolism.5,6 Vitamin D deficiency is recognized as a common health concern during pregnancy at a prevalence up to 84% worldwide, depending on the country of residence and other related factors.7,8 Emerging evidence suggests that vitamin D deficiency may contribute to the development of GDM and human studies have been summarized in two recent meta-analyses of observational studies.9,10 However, previous studies focused on a single measurement of 25-hydroxyvitamin D [25(OH)D] levels and did not include serial measurements to reliably reflect a time-integrated measure of vitamin D status during pregnancy.11–13 Limited and inconsistent findings from clinical trials to examine the effect of vitamin D supplementation on GDM have been reported.14–17 Thus, the temporal association between maternal vitamin D status and GDM risk remains unclear. Longitudinal data on maternal vitamin D status are needed to elucidate the variation of vitamin D levels and requirements over pregnancy and better understand the role of vitamin D metabolism and function on GDM development.
In addition, accumulating evidence indicates that free and bioavailable 25(OH)D may better reflect biological activity of vitamin D than total 25(OH)D.18,19 The bioavailability of vitamin D and its metabolites is largely regulated by vitamin D binding protein (VDBP); however, the validity of monoclonal immunoassays has been criticized for lack of sensitivity to VDBP isoforms determined by genetic polymorphisms.20–23 Further, there are no standardized assays for VDBP and free or bioavailable 25(OH)D to be well-validated in ethnically diverse populations. Thus, there remains a controversy regarding the optimal markers for determining vitamin D status and action.
In a prospective, multiracial cohort of U.S. pregnant women, we focused primarily on total 25(OH)D levels (including 25(OH)D2 and 25(OH)D3) assessed by the presumed gold standard liquid chromatography-tandem mass spectrometry (LC-MS/MS), which is the most widely used and clinically accepted biomarker for vitamin D status. We aimed to investigate 1) the longitudinal trajectories of vitamin D biomarkers over pregnancy; 2) the prospective associations between levels of vitamin D biomarkers during early to mid-pregnancy and subsequent risk of GDM; and 3) whether the levels of vitamin D biomarker from early to mid-pregnancy and their prospective associations with GDM risk are modified by race/ethnicity, pre-pregnancy BMI, physical activity, parity, or family history of diabetes.
METHODS
Study design and population
We performed a nested case-control study using the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Fetal Growth Studies-Singleton cohort (2009–2013), consisting of 2,802 generally healthy multiracial women (2,334 non-obese and 468 obese women) with singleton pregnancies and aged 18–40 years at enrollment. All women were enrolled between 8 weeks 0 days and 13 weeks 6 days of gestation at 12 clinical centers throughout the U.S. and were followed up throughout their pregnancies.24 For participants to be eligible, ultrasound estimates of gestational age at enrollment were required to be consistent (±5–7 days) with gestational dating, calculated by last menstrual period. Sampling and eligibility criteria are described in detail elsewhere.24 The study was approved by all participating institutions including NICHD. All study participants gave their written informed consent prior to enrollment.
In this prospective cohort study, maternal blood samples were longitudinally collected from each participant at four targeted study visits: gestational weeks 8–13 (enrollment visit), 16–22, 24–29, and 34–37. However, the actual time ranges for blood collection were gestational weeks 10–14, 15–26, 23–31, and 33–39, respectively. All biospecimens were processed immediately and stored at −80°C before assay. All women were instructed to fast overnight for 8–14 h before their blood samples were drawn at the second visit (weeks 15–26). The screening or diagnosis of GDM was conducted according to standard clinical care, with an average gestational age of 27 weeks. A total of 107 women with incident GDM were identified as cases and matched randomly at a ratio of 1:2 to non-GDM controls on age (±2 years), race/ethnicity (non-Hispanic white, non-Hispanic black, Hispanic, or Asian/Pacific Islander), and gestational age at blood collection (±2 weeks). Overall, our case-control study consisted of 107 women with GDM and 214 women without GDM for a total of 321 women.
Outcome ascertainment
Gestational diabetes was ascertained by review of medical records. Of 107 cases, the vast majority (n=95) had a confirmed diagnosis of GDM based on 100-g, 3-h oral glucose tolerance test (OGTT) results. The Carpenter and Coustan diagnostic criteria were used for GDM diagnosis.25 For those without available OGTT results, hospital discharge diagnosis after delivery was reviewed and women who received medication for GDM were considered to have GDM (n=12). Among the 214 matched controls, 195 women underwent GDM screening by a 50-g, 1-h glucose challenge test (GCT). Among the remaining women (n=19) without available GCT results, either an OGTT with the Carpenter and Coustan criteria thresholds (n=12) or review of hospital discharge diagnoses (n=7) was used to confirm non-GDM status.
Laboratory assessment
Biomarkers were measured at all four-time points of biospecimen collection among all cases (n=107) and one of the matched controls (n=107). In the remaining control subjects (n=107), assays were performed only for the two specimens collected prior to GDM screening (i.e., 10–14 and 15–26 gestational weeks) which are most informative for prospectively investigating biomarkers of GDM. All biospecimen samples of matched cases and controls were assayed in random order in the same analytic run, without knowledge of GDM status. Plasma levels of ergocalciferol (D2) and cholecalciferol (D3) were measured in ng/mL using liquid chromatography-tandem mass spectrometry (LC-MS/MS). Plasma VDBP was measured in ng/mL using a quantitative sandwich enzyme immunoassay (R&D Systems, Inc., Minneapolis, MN). Plasma albumin was measured using the bromcresol purple method (Roche Diagnostics, Indianapolis, IN). Total 25(OH)D was reflected by the summation of 25(OH)D2 and 25(OH)D3. Based on the lab data, free 25(OH)D and bioavailable 25(OH)D were derived using equations adapted from that of Vermeulen et al..26 Plasma glucose and insulin were measured using hexokinase and immunosorbent assays (Roche Diagnostics, Indianapolis, IN), respectively. HOMA-IR, as a surrogate measure of insulin sensitivity, was computed by multiplying fasting plasma insulin (FPI) mU/L by fasting plasma glucose (FPG) mmol/L, then dividing by the constant 22.5, i.e. HOMA-IR = (FPI × FPG)/22.5.27 Plasma levels of total cholesterol, HDL cholesterol, and triglycerides were measured using enzymatic assays (Roche Diagnostics, Indianapolis, IN). Plasma LDL cholesterol was calculated by the Friedewald’s formula:28
LDL cholesterol = total cholesterol – HDL cholesterol – triglycerides/5 The inter-assay coefficients of variation for all above-mentioned analytes were in the range of 1.3–12.9%. We reported plasma 25(OH)D levels in nmol/L, multiply by 2.50 to convert from ng/mL to nmol/L (for 25(OH)D2, 1 ng/mL=2.42 nmol/L).
Covariates
Information on participant demographics, lifestyle factors, and past medical history was collected through self-reported questionnaire. A priori selection of conventional GDM risk factors, including nulliparity (yes/no), prepregnancy BMI (kg/m2), and family history of diabetes (yes/no), was assessed at study enrollment. Based on the clinical centers where participants were enrolled, we categorized geographical regions by latitude as Southern (≤37°N), Middle (>37°N to 40°N), and Northern (>40°N).29 Season of blood draw (February to April, May to July, August to October, and November to January) and physical activity (quartiles) at each study visit prior to GDM screening were also considered in our analysis. Physical activity was assessed using the Pregnancy Physical Activity Questionnaire.30 Given that cases were matched with controls within a certain range of maternal age (years) and gestational age at biospecimen collection (weeks), we also included these two matching variables as covariates to derive conservative GDM risk estimates.
Statistical analysis
Participant characteristics were compared according to GDM status using generalized linear mixed-effect models for continuous variables and binomial/multinomial logistic regression with generalized estimating equations for categorical variables, accounting for matched case-control pairs. To illustrate the longitudinal trends of vitamin D biomarkers throughout pregnancy in both cases and controls, median levels of each biomarker were displayed graphically by study visit; generalized linear mixed-effects regression models, accounting for matched case-control pairs, were implemented for case-control comparisons at each study visit. Spearman’s partial correlation coefficients adjusting for maternal age were calculated to examine the associations of vitamin D biomarkers at either 10–24 or 15–26 gestational weeks with fasting plasma glucose during weeks 15–26, respectively. We performed the complete data analysis by excluding participants with missing measurements of vitamin D biomarkers. Additionally, one case at weeks 10–14 and five cases at weeks 15–26 were excluded from the final analysis, as their blood samples were collected after GDM diagnosis.
To evaluate the associations of maternal vitamin D levels with subsequent risk of GDM and identify the optimal timing of vitamin D assessment in relation to GDM risk, separate multivariable conditional logistic regression models were performed for each study visit prior to GDM diagnosis, i.e., gestational weeks 10–14 and 15–26. The levels of each vitamin D biomarker were parameterized as quartiles with the lowest quartile as the reference. To account for seasonal variation in vitamin D levels, season-specific quartile cutoffs of 25(OH)D were determined according to blood draw dates among the controls and applied for all the cases and controls. We also categorized total 25(OH)D levels into deficiency (<50 nmol/L) and non-deficiency (≥50 nmol/L) according to previously published criteria for vitamin D status.31 The main multivariable logistic models were adjusted for race/ethnicity, maternal age, gestational age at blood collection, geographic latitude, prepregnancy BMI, parity, and family history of diabetes. We further adjusted for physical activity in separate models for sensitivity analysis considering its potential confounding and modifying effects. Tests for linear and nonlinear trends were performed by modeling the median levels of vitamin D within each category as a continuous explanatory variable and using restricted cubic splines, respectively.
We also investigated longitudinal vitamin D profiles in relation to GDM. Between the two visits that included blood collection prior to GDM screening (i.e., the first to second trimester), the following longitudinal patterns of changes in 25(OH)D levels were identified and conditional logistic regression models was used to assess the associations between changing patterns of vitamin D status and the subsequent risk for GDM, with persistent non-deficiency being the reference group: 1) persistent non-deficiency (≥50 nmol/L); 2) persistent deficiency (<50 nmol/L); 3) non-deficiency to deficiency; or 4) deficiency to non-deficiency. We also fitted linear mixed-effects models accounting for matched case-control pairs to compare the longitudinal trajectories of vitamin D levels during early and mid-pregnancy before the diagnosis of GDM in individuals with GDM and normal glucose levels, with adjustment for the above-listed confounders at baseline. The log-transformed levels of 25(OH)D were parameterized as a continuous variable in the models. The least-squares means were back transformed to the original scale for result presentation.
To explore possible effect modification in the relationship between vitamin D deficiency and GDM, we first performed subgroup analyses stratified by race/ethnicity (non-Hispanic White, non-Hispanic Black, Hispanic, or Asian/Pacific Islander) and other factors, such as pre-pregnancy weight status (normal weight or overweight/obese), maternal age (<30 or ≥30 years), physical activity (quartiles), parity (nulliparous or parous), and family history of diabetes (yes/no). We performed interaction analyses with multiplicative interaction terms to formally test their potential modifying effects on the association between vitamin D deficiency and GDM. All statistical analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC).
RESULTS
Compared with non-GDM controls, GDM cases had a higher proportion of a family history of diabetes, lower HDL cholesterol, and higher prepregnancy BMI, triglycerides, fasting glucose, fasting insulin, and HOMA-IR (Table 1).
Table 1.
Participant characteristics among women with GDM and their matched control subjects, the NICHD Fetal Growth Studies-Singleton cohort
| Variables | Case subjects with GDM (n = 107) |
Control subjects (n = 214) |
P-value† | |
|---|---|---|---|---|
| Age (years) | mean ± SD | 30.5 ± 5.7 | 30.4 ± 5.4 | — |
| Race/ethnicity | n (%) | — | ||
| Non-Hispanic white | 25 (23.4) | 50 (23.4) | ||
| Non-Hispanic black | 15 (14.0) | 30 (14.0) | ||
| Hispanic | 41 (38.3) | 82 (38.3) | ||
| Asian/Pacific Islander | 26 (24.3) | 52 (24.3) | ||
| Education | n (%) | 0.18 | ||
| Less than high school | 17 (15.9) | 26 (12.1) | ||
| High school graduate or equivalent | 15 (14.0) | 23 (10.7) | ||
| More than high school | 75 (70.1) | 165 (77.1) | ||
| Prepregnancy BMI (kg/m2) | n (%) | <0.001 | ||
| <25.0 | 37 (34.6) | 123 (57.5) | ||
| 25.0–29.9 | 35 (32.7) | 56 (26.2) | ||
| 30.0–34.9 | 20 (18.7) | 17 (7.9) | ||
| 35.0–44.9 | 15 (14.0) | 16 (7.5) | ||
| Unknown/missing | 0 | 2 (0.9) | ||
| Nulliparity | n (%) | 48 (44.9) | 96 (44.9) | 1.00 |
| Smoking 6 months before pregnancy | n (%) | 4 (3.7) | 1 (0.5) | 0.06 |
| Alcohol consumption 3 months before pregnancy | n (%) | 61 (57.0) | 137 (64.0) | 0.22 |
| Family history of diabetes | n (%) | 40 (37.4) | 48 (22.4) | 0.003 |
| Geographical latitude (Clinical center) | n (%) | 0.38 | ||
| Southern (≤37°N) | 40 (37.4) | 77 (36.0) | ||
| Middle (>37°N to 40°N) | 22 (20.6) | 37 (17.3) | ||
| Northern (>40°N) | 42 (39.3) | 100 (46.7) | ||
| Season of blood draw (weeks 10–14) | n (%) | 0.01 | ||
| February-April | 28 (26.9) | 65 (30.4) | ||
| May-July | 26 (25.0) | 39 (18.2) | ||
| August-October | 32 (30.8) | 50 (23.4) | ||
| November-January | 18 (17.3) | 60 (28.0) | ||
| Season of blood draw (weeks 15–26) | n (%) | 0.02 | ||
| February-April | 24 (25.5) | 65 (30.4) | ||
| May-July | 22 (23.4) | 39 (18.2) | ||
| August-October | 30 (31.9) | 50 (23.4) | ||
| November-January | 18 (19.2) | 60 (28.0) | ||
| Physical activity at weeks 10–14 (MET-minutes per week) | mean ± SD | 419 (214, 1112) | 489 (185, 1059) | 0.77 |
| Physical activity at weeks 15–26 (MET-minutes per week) | mean ± SD | 288 (69, 558) | 299 (117, 647) | 0.34 |
| Gestational age at blood collection (weeks 10–14) |
mean ± SD | 12.8 ± 0.9 | 12.9 ± 0.8 | 0.55 |
| Gestational age at blood collection (weeks 15–26) |
mean ± SD | 19.2 ± 2.4 | 19.4 ± 2.2 | 0.27 |
| Metabolic biomarkers at gestational weeks 10–14 | ||||
| Triglycerides (mmol/L) | median (IQR) | 1.7 (1.2, 2.2) | 1.3 (1.1, 1.7) | <0.001 |
| Total cholesterol (mmol/L) | median (IQR) | 4.8 (4.2, 5.0) | 4.6 (4.1, 5.2) | 0.73 |
| HDL-cholesterol (mmol/L) | median (IQR) | 1.5 (1.3, 1.7) | 1.6 (1.9, 3.2) | 0.001 |
| LDL-cholesterol (mmol/L) | median (IQR) | 2.4 (1.9, 2.7) | 2.3 (1.8, 2.7) | 0.83 |
| Metabolic biomarkers at gestational weeks 15–26 | ||||
| Triglycerides (mmol/L) | median (IQR) | 1.8 (1.5, 2.2) | 1.5 (1.2, 1.9) | <0.001 |
| Total cholesterol (mmol/L) | median (IQR) | 5.1 (4.6, 5.8) | 5.4 (4.6, 5.9) | 0.17 |
| HDL-cholesterol (mmol/L) | median (IQR) | 1.7 (1.4, 1.9) | 1.8 (1.5, 2.1) | 0.005 |
| LDL-cholesterol (mmol/L) | median (IQR) | 2.5 (2.1, 3.1) | 2.7 (2.1, 3.2) | 0.22 |
| Fasting glucose (mmol/L) | median (IQR) | 4.9 (4.6, 5.4) | 4.6 (4.3, 4.8) | <0.001 |
| Fasting insulin (pmol/L) | median (IQR) | 10.6 (6.9, 18.4) | 6.7 (4.2, 10.6) | <0.001 |
| HOMA-IR | median (IQR) | 2.7 (1.8, 5.0) | 1.6 (0.9, 2.6) | <0.001 |
P-value for differences between case and control subjects were obtained by generalized linear mixed-effect models for continuous variables and binomial/multinomial logistic regression with generalized estimating equations for binary/multilevel categorical variables, accounting for matched case-control pairs.
IQR, interquartile range.
Over the entire gestational period, median levels of 25(OH)D2 and 25(OH)D3 as well as total and free 25(OH)D increased whereas median bioavailable 25(OH)D levels decreased with gestational week among both cases and controls (Figure 1 and Supplementary Figure 1). Median levels of VDBP increased from gestational weeks 10–14 up to weeks 15–26, with a subsequent decline until the end of pregnancy. There was no statistically significant difference in any vitamin D biomarkers between GDM cases and non-GDM controls at any study visit. After adjustment for maternal age, total, free, and bioavailable 25(OH)D were significantly and inversely correlated with fasting glucose at 15–26 gestational weeks (Supplementary Table 1).
Figure 1.
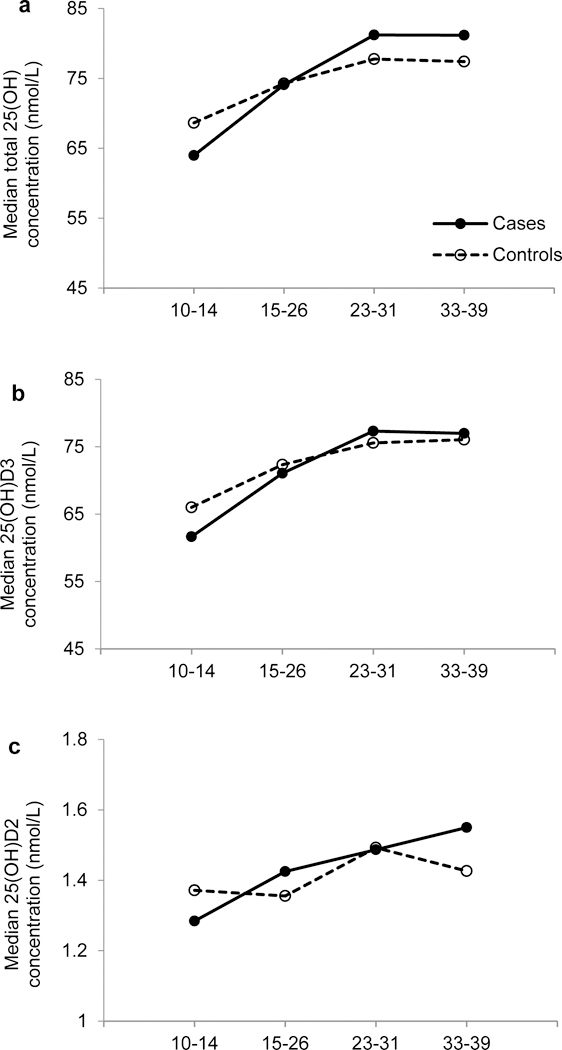
Median levels of total 25(OH)D (a), 25(OH)D3 (b), and 25(OH)D2 (c) according to gestational age at blood collection among women with GDM (solid line) and their matched control subjects (dashed line).
At 10–14 gestational weeks, women with higher season-specific levels of total 25(OH)D biomarkers appeared to have a 50–60% lower subsequent risk of GDM compared with women in the lowest season-specific quartile (Supplementary Table 2). The association was similar with 25(OH)D3. Although the significant linear trends between levels of total 25(OH)D (P=0.04) and 25(OH)D3 (P=0.03) and GDM risk were robust to adjustment for race/ethnicity, maternal age, geographical latitude, and gestational age at blood collection, we did not observe linear associations after additional adjustment for prepregnancy BMI, parity, and family history of diabetes. However, significant nonlinear associations of total 25(OH)D (P=0.025) and 25(OH)D3 (P=0.016) with GDM were observed. While increasing levels of free and bioavailable 25(OH)D were suggestive of a lower risk for GDM, none of the quartile associations or linear trends were significant. VDBP was not associated with GDM risk. No significant results were observed at 15–26 gestational weeks.
First trimester vitamin D deficiency (10–14 gestational weeks) was significantly associated with an increased risk of developing GDM after adjusting for potential confounding factors (OR=2.82, 95% CI: 1.15–6.93) (Figure 2). This association remained significant after further adjustment for physical activity (OR=3.02, 95% CI: 1.20–7.57). Furthermore, women with persistent vitamin D deficiency (10 GDM cases and 13 controls) in both the first and second trimester (10–14 and 15–26 weeks) had over a 4-fold significantly higher risk for GDM than those with persistently non-deficient vitamin D levels (68 GDM cases and 166 controls; OR=4.46, 95% CI: 1.15–17.3). However, this significant association was not observed in women with a change from non-deficiency to deficiency (1 GDM cases and 11 controls; OR=0.29, 95% CI: 0.03–2.59) or vice versa (11 GDM cases and 19 controls; OR=2.59, 95% CI: 0.82–8.19).
Figure 2.
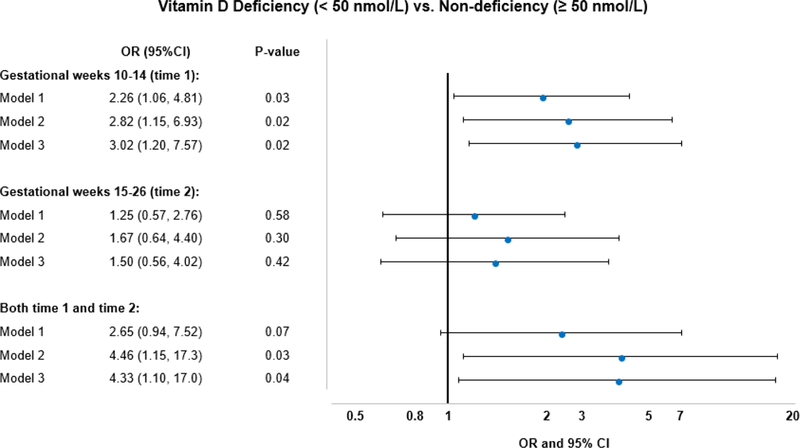
ORs for GDM by vitamin D deficiency status at gestational weeks 10–14 and 15–26. ORs for GDM in women with persistent vitamin D deficiency compared with those with persistent non-deficiency at both gestational weeks 10–14 and 15–26 were also shown. Model 1 adjusted for race/ethnicity, maternal age, gestational age at blood collection, and geographical latitude (clinical center); Model 2 (main model) further adjusted for pre-pregnancy BMI, parity, season of blood draw, and family history of diabetes; Model 3 additionally adjusted for physical activity.
In the adjusted mixed-effects models, longitudinal trajectories of log-transformed total 25(OH)D and 25(OH)D3 levels during early to mid-pregnancy differed significantly between women with GDM and non-GDM controls (Table 2 and Supplementary Table 3). Compared to women without GDM, those who developed GDM appeared to have lower levels of total 25(OH)D at 10–14 gestational weeks (β=−0.07, 95% CI: −0.15–0.02) and had a 6% greater incremental rate of total 25(OH)D levels on a logarithmic scale from 10–14 weeks to 15–26 weeks (β=0.06, 95% CI: 0.001–0.11). Least-squares means of total 25(OH)D further illustrated this significant difference in the longitudinal increase of total 25(OH)D levels from the first to second trimester between GDM cases and non-GDM controls: least-squares means appeared to be different at 10–14 weeks between cases and controls (63.0 vs. 67.4 nmol/L; p=0.10), but were similar at 15–26 weeks (69.5 vs. 70.3 nmol/L; p=0.76) (Figure 3 and Supplementary Figure 2). Longitudinal change in least-squares means from 10–14 weeks to 15–26 weeks among cases was significantly greater than that among controls (p=0.046). We obtained very similar results for 25(OH)D3.
Table 2.
Change of total 25(OH)D, 25(OH)D3, and 25(OH)D2 from the 1st to 2nd trimester for individuals with GDM and normal glucose levels in multivariate mixed-effects models
| Model 2† | Model 3‡ | |||||
|---|---|---|---|---|---|---|
| β | 95% CI | P-value | β | 95% CI | P-value | |
| Total 25(OH)D | ||||||
| GDM | −0.07 | (−0.15, 0.02) | 0.11 | −0.06 | (−0.14, 0.02) | 0.15 |
| Visit | 0.09 | (0.06, 0.13) | <0.001 | 0.09 | (0.06, 0.13) | <0.001 |
| GDM*Visit | 0.06 | (0.001, 0.11) | 0.046 | 0.06 | (0.001, 0.11) | 0.046 |
| 25(OH)D3 | ||||||
| GDM | −0.06 | (−0.15, 0.02) | 0.14 | −0.06 | (−0.14, 0.03) | 0.17 |
| Visit | 0.09 | (0.06, 0.13) | <0.001 | 0.09 | (0.06, 0.13) | <0.001 |
| GDM*Visit | 0.05 | (0.00001, 0.11) | 0.049 | 0.05 | (−0.00003, 0.11) | 0.05 |
| 25(OH)D2 | ||||||
| GDM | −0.05 | (−0.23, 0.13) | 0.57 | −0.04 | (−0.21, 0.14) | 0.68 |
| Visit | 0.02 | (−0.05, 0.09) | 0.65 | 0.02 | (−0.05, 0.09) | 0.64 |
| GDM*Visit | 0.08 | (−0.05, 0.20) | 0.22 | 0.08 | (−0.05, 0.20) | 0.22 |
Model 2 (main model) adjusted for race/ethnicity, maternal age, gestational age at blood collection, geographical latitude (clinical center), pre-pregnancy BMI, parity, season of blood draw, and family history of diabetes.
Model 3 further adjusted for physical activity.
β coefficient for “GDM” represents the difference of vitamin D biomarker levels between GDM cases and non-GDM controls when their gestational age was 10–14 weeks;
β coefficient for “Visit” represents the longitudinal change of vitamin D levels from 10–14 to 15–26 weeks of gestation among non-GDM controls;
β coefficient for “GDM*Visit” represents the difference in longitudinal change of vitamin D biomarker levels from 10–14 to 15–26 weeks of gestation between GDM cases and non-GDM controls.
Figure 3.
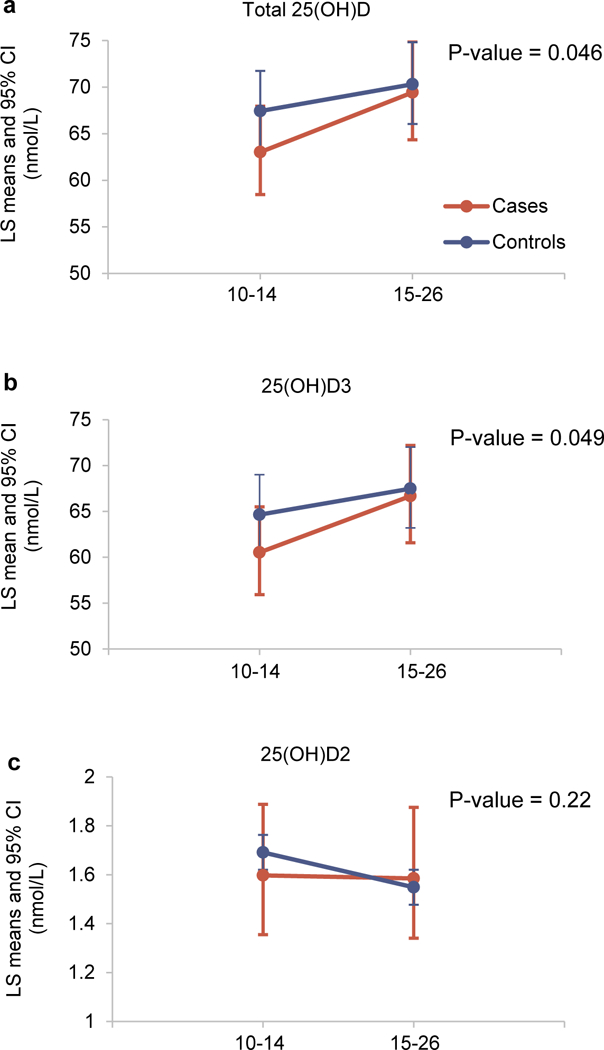
Longitudinal change of vitamin D biomarkers, including total 25(OH)D (a), 25(OH)D3 (b), and 25(OH)D2 (c), for individuals with GDM (case, orange line) and normal glucose levels (control, blue line). Back-transformed LS means and the corresponding 95% CIs of vitamin D biomarkers at gestational weeks of 10–14 and 15–26 from linear mixed-effects models, adjusting for race/ethnicity, maternal age, gestational age at blood collection, geographical latitude (clinical center), pre-pregnancy BMI, parity, season of blood draw, and family history of diabetes (Model 2).
Lastly, we did not observe significant effect modification by race/ethnicity, pre-pregnancy BMI, maternal age, physical activity, parity, or family history of diabetes.
DISCUSSION
In this ethnically diverse longitudinal study, our results showed evidence of a significant longitudinal change in the vitamin D biomarkers during pregnancy with no differences between GDM cases and controls at each visit. We found a nonlinear threshold effect of total 25(OH)D and 25(OH)D3 levels on GDM risk, and vitamin D deficiency as defined by total 25(OH)D <50nmol/L during early and mid-pregnancy was significantly associated with risk of developing GDM among initially healthy pregnant women. The association appeared to be independent of conventional risk factors for GDM. We observed no significant effect modification by race/ethnicity, pre-pregnancy BMI, maternal age, physical activity, parity, or family history of diabetes. Our findings indicate that assessment of vitamin D status during early pregnancy may be clinically valuable for developing risk stratification and intervention strategies for GDM prevention.
To our knowledge, only one study has investigated the longitudinal profiles of serum 25(OH)D during pregnancy (at 12–14, 20–22, and 32–34 weeks) and prospectively examined the associations between trimester-specific vitamin D status and GDM in a cohort of 523 Korean women.32 Similar to our study, there was no statistically significant difference in 25(OH)D between GDM cases and controls at any time points. However, they did not examine the associations between GDM risk and longitudinal changes of 25(OH)D levels, as well as other vitamin D biomarkers. In addition, they found no association between vitamin D status in either the first or second trimester and GDM. The small case number (23 GDM cases) may limit their statistical power to identify the significant association between first-trimester vitamin D deficiency and GDM. Whereas, the present study prospectively and longitudinally examined and demonstrated, for the first time, the significant and inverse association between maternal vitamin D levels based on repeated measurements and subsequent risk of GDM. Our main findings not only are in line with our prior work and a large body of the existing literature,11,12,33 showing an inverse association between vitamin D in early gestation and subsequent risk of GDM, but also further extend these findings by showing an association between longitudinal vitamin D status during early pregnancy to mid-gestation and subsequent risk for GDM which may be more clinically relevant for prevention of GDM. We previously observed that vitamin D deficiency before 20 weeks of gestation was associated with an increased risk of developing GDM in the Omega Study.11 Another prospective study indicated that the risk of incident GDM increased by 40% with 1 SD decrease in 25(OH)D levels during gestational weeks 6–13.12 Evidence from a recent case-control study among a cohort of Saudi pregnant women consisting of 116 GDM cases and 303 control subjects suggested that vitamin D deficiency in the first trimester was associated with a 2.87-fold greater risk of subsequent GDM.33 In the present study, we further observed that longitudinal trajectories of 25(OH)D levels during early and mid-pregnancy differed significantly between GDM cases and women with normal glucose levels. Compared to controls, GDM cases had lower 25(OH)D levels in early pregnancy, but increased to very similar levels at weeks 15–26. This might reflect the fact that pregnant women were more likely to take prenatal vitamin supplements due to counselling at their routine prenatal visits. Our findings also suggest the importance of vitamin D levels during early pregnancy but not the mid-pregnancy in the development of GDM. Compared to women with non-deficient levels of vitamin D (≥50 nmol/L) from the first to second trimester of gestation, those with persistently deficient vitamin D levels (<50 nmol/L) had significantly increased risk for developing GDM. These results indicate that prenatal vitamin supplements may not be enough for GDM prevention, especially for women with deficient levels of vitamin D in early pregnancy. It is also possible that these participants with persistently deficient vitamin D levels may have not taken any vitamin D supplements or had poor adherence or response to vitamin D supplements or dietary counselling. Overall, the trajectories of total 25(OH)D levels between GDM cases and controls were different from the first to second trimester of pregnancy and the associations of vitamin D deficiency with GDM were significant either during the first trimester or persistently through the second trimester. These findings indicate the importance of assessing trajectories of vitamin D status across gestation in relation to GDM risk.
In contrast, inconsistent findings were also reported.34–36 A cross-sectional study of a Turkish population found no association between first-trimester vitamin D deficiency and GDM risk, potentially due to small sample size (50 GDM cases and 50 controls).34 Similar findings were reported by another nested case-control study of 180 pregnant women in North Carolina (60 GDM cases and 120 controls).35 With more than 50% white women, the prevalence of vitamin D deficiency in this study was much lower (7.2%) compared to our study (22.4%) as well as another study with a nationally representative sample of U.S. pregnant women (33%);37 some important confounding effects, such as geographical latitude and family history of diabetes, were also not accounted for. Therefore, their inference may be lack of generalizability and reliability. Our finding of no association between the second-trimester vitamin D deficiency and GDM was also less consistent with that from a birth cohort of 1,314 U.S. pregnant women.36 They found an inverse association between second-trimester severe vitamin D deficiency (<25 nmol/L) and GDM, although the association was attenuated to non-significance after adjusting for maternal BMI in addition to other risk factors.
A recent review pointed out that controversial findings from observational studies on vitamin D and GDM may be affected by heterogeneity in study design and insufficient considerations of confounding factors.38 However, the results from clinical trials have also been inconsistent.14–17 Two randomized clinical trials (n=500 and n=90) in Iran found that vitamin D supplementation intake (50,000 IU every 2 weeks or 5,000 IU weekly) started in the first trimester of gestation decreased incidence of GDM,14,15 which are consistent with our findings. In contrast, the results from two randomized clinical trials conducted in Sydney (n=179) and Iran (n=210) showed that vitamin D supplementation as doses from 400 to 5,000 IU daily had no effects on incidence of GDM or maternal glucose levels.16,17 Their negative findings may be due to: 1) relatively small sample size in each treatment arm; 2) late initiation of vitamin D supplementation (gestational age of <20 weeks for Sydney’s trial and 14–16 weeks for Iran’s trial); 3) the control group containing low dose of vitamin D supplements; 4) extreme cutoffs used for definition of vitamin D deficiency (80 nmol/L in Sydney’s trial and 10 nmol/L in Iran’s trial); or 5) potentially low compliance since adherence rate was not reported in either study.
Although the exact biological mechanisms underlying vitamin D and glucose metabolism in pregnancy remain unclear, the observed associations may be explained by the influence of vitamin D on glucose homeostasis. The biological effects of vitamin D on regulation of pancreatic β-cell function and insulin secretion involve its biologically active metabolite, 1,25(OH)2D, binding to the vitamin D receptor in β-cells of the pancreas.39 Vitamin D deficiency may affect normal release of insulin by regulating the calcium pool of β-cells intracellularly and extracellularly. Since the secretion of insulin is mediated by a calcium-dependent mechanism,40 vitamin D deficiency may decrease the insulin response to glucose. Vitamin D may also influence insulin sensitivity through vitamin D receptors in adipose tissue and skeletal muscle through its role in activating the peroxisome proliferator activator receptor-δ, which is involved in the metabolism of fatty acids in adipose tissue and skeletal muscle.41 Thus, vitamin D deficiency may affect peripheral target tissues of insulin and thus lead to insulin resistance. Another possible indirect pathway by which vitamin D could affect glucose homeostasis is through systemic inflammation. Chronic inflammation can trigger β-cell dysfunction or death and directly induce insulin resistance.42 Defects in insulin secretion and insulin sensitivity (insulin resistance) can contribute to GDM development. Through inhibiting production and action of inflammatory cytokines, 1,25(OH)2D can lower systemic inflammation and promote β-cell survival.42,43
A major strength of our study is the use of longitudinal measurements of plasma levels of 25(OH)D, VDBP, and albumin before GDM diagnosis and throughout pregnancy, which provided the opportunity to examine the temporal relationship between longitudinal vitamin D status during pregnancy and risk of GDM. In particular, we were able to examine a panel of vitamin D biomarkers and compare them for the strength of their associations with GDM risk. The multiethnic diversity of the study cohort increased the generalizability of our results. Furthermore, findings from our observational research will complement and extend findings from existing and future clinical research of the effects of vitamin D supplements on GDM. Clinical trials are ideal to define a causal relationship between vitamin D and GDM; however, some of logistical limitations may restrict their ability to address some unanswered questions about vitamin D as follows: (1) only certain fixed dose levels of vitamin D can be tested; (2) a relatively narrow range of vitamin D levels in the trial participants cannot allow for assessment of the full spectrum of vitamin D levels; (3) inability to assess a panel of novel vitamin D biomarkers relative to physiological levels of total 25(OH)D, including VDBP, free or bioavailable 25(OH)D, may hinder our further understandings of the physiological role of vitamin D in relation to GDM.
However, some limitations need to be acknowledged. First, the relatively small sample size limited our ability to fully address ethnic disparities in the relationship between vitamin D and GDM risk, and we were only able to explore possible effect modification of these associations by race/ethnicity. Second, a monoclonal ELISA assay used for VDBP measurements has been criticized for lack of sensitivity to genetically determined isoforms, yielding an underestimation of VDBP levels in Blacks due to a high frequency of Gc-1F alleles in this population, compared with polyclonal or LC-MS/MS-based VDBP measurements.20–23,44,45 Thus, our main findings have focused primarily on total 25(OH)D levels assessed by the LC-MS/MS, which is the most widely used and clinically accepted biomarker of vitamin D status, and its associations with GDM risk across race/ethnicity. It is worth noting that a recent study of 368 healthy white pregnant women found that directly measured free 25(OH)D had stronger correlations with gestational age and markers of bone metabolism, lipid metabolism, and kidney function than total 25(OH)D.46 Their findings implicate the importance of free 25(OH)D in monitoring of maternal vitamin D status, although further research is needed to clarify the validity and utility of free 25(OH)D in reflecting tissue-specific activities or overall status of vitamin D. Third, parathyroid hormone (PTH), known for its synergistic role with vitamin D endocrine system,31 was not measured. Emerging evidence has also suggested the potential effects of an interaction between vitamin D and PTH on glucose metabolism.47,48 Lastly, due to lack of information on determinants of vitamin D levels such as sun exposure or outdoor activities, residual confounding from them cannot be completely ruled out.
In conclusion, our results suggest that early-pregnancy vitamin D deficiency may increase the risk of developing GDM in pregnant women. These findings suggest that the assessment of vitamin D in the first trimester of gestation may contribute to the identification of women at risk for developing GDM. For those identified as high-risk, clinical vitamin D supplementation and dietary recommendations might be considered in clinical-care strategies to aid in the prevention of GDM associated with vitamin D deficiency. Further longitudinal studies with larger sample size and accurate assessment of vitamin D-related biomarkers measured by well-validated and standardized assays are required to confirm our findings. If confirmed, future randomized controlled trials are warranted to clarify the preventive dosage and therapeutic time windows of vitamin D supplementation to prevent GDM and address potential racial/ethnic disparities.
Supplementary Material
Acknowledgements
We would like to acknowledge the research teams at our participating clinical centers, including Christiana Care Health Systems, Wilmington, DE, USA; University of California, Irvine, CA, USA; Long Beach Memorial Medical Center, CA, USA; Northwestern University, Evanston, IL, USA; Medical University of South Carolina, Charleston, SC, USA; Columbia University, New York, NY, USA; New York Hospital Queens, NY, USA; St Peters’ University Hospital, New Brunswick, NJ, USA; University of Alabama at Birmingham, AL, USA; Women and Infants Hospital of Rhode Island, Providence, RI, USA; Fountain Valley Regional Hospital and Medical Center, CA, USA; and Tufts University, Medford, MA, USA. We would also like to thank the C-TASC Corporation, Owings Mill, MD, USA for providing data coordination, as well as the Department of Laboratory Medicine and Pathology, University of Minnesota, Minneapolis, MN, USA, for providing the laboratory support and resources for the analysis of blood samples and biomarkers.
Funding
This work was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development intramural funding as well as the American Recovery and Reinvestment Act funding [grant numbers HHSN275200800013C, HHSN275200800002I, HHSN27500006, HHSN275200800003IC, HHSN275200800014C, HHSN275200800012C, HHSN275200800028C, HHSN275201000009C, and HHSN275201000001Z]. YS was supported by a grant [R01-HL113056] from the National Institutes of Health (NIH)/National Heart, Lung, and Blood Institute (NHLBI), Bethesda, MD; JX and YS were supported by the Indiana University Health-Indiana University School of Medicine Strategic Research Initiative Grant, Indianapolis, IN.
Footnotes
Conflict of interest
None.
References
- 1.DeSisto CL, Kim SY, Sharma AJ. Prevalence estimates of gestational diabetes mellitus in the United States, Pregnancy Risk Assessment Monitoring System (PRAMS), 2007–2010. Prev Chronic Dis 2014;11:E104 doi: 10.5888/pcd11.130415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu Y, Zhang C. Prevalence of gestational diabetes and risk of progression to type 2 diabetes: a global perspective. Current Diabetes Reports 2016;16(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reece EA, Leguizamon G, Wiznitzer A. Gestational Diabetes: The need for a common ground. Lancet 2009;373:1789–1797. [DOI] [PubMed] [Google Scholar]
- 4.Buchanan TA, Xiang AH. Gestational diabetes mellitus. J Clin Invest 2005;115:485–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ken Chiu C, Audrey C. Hypovitaminosis D is associated with insulin resistance and Bcell dysfunction. Am J Clin Nutr 2004;79(5):820–825. [DOI] [PubMed] [Google Scholar]
- 6.Forouhi NG, Luan J, Cooper A, Boucher BJ, Wareham NJ. Baseline serum 25-hydroxyvitamin D is predictive of future glycemic status and insulin resistance: the Medical Research Council Ely Prospective Study 1990–2000. Diabetes 2008; 10.2337/db08-0593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van der Meer IM, Karamali NS, Boeke AJ, Lips P, Middlekoop BJ, Verhoeven I et al. High prevalence of vitamin D deficiency in pregnant non-Western women in The Hague, Netherlands. Am J Clin Nutr 2006;84(2):350–3. [DOI] [PubMed] [Google Scholar]
- 8.Bodnar LM, Simhan HN, Powers RW, Frank MP, Cooperstein E, Roberts JM. High prevalence of vitamin D insufficiency in black and white pregnant women residing in the northern United States and their neonates. J Nutr 2007;137(2):447–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang MX, Pan GT, Guo JF, Li BY, Qin LQ, Zhang ZL. Vitamin D deficiency increases the risk of gestational diabetes mellitus: A meta-analysis of observational studies. Nutrients 2015;7(10): 8366–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aghajafari F, Nagulesapillai T, Ronksley PE, Tough SC, O’Beirne M, Rabi DM. Association between maternal serum 25-hydroxyvitamin D level and pregnancy and neonatal outcomes: systematic review and meta-analysis of observational studies. BMJ 2013;346: f1169. [DOI] [PubMed] [Google Scholar]
- 11.Zhang C, Qiu C, Hu FB, David RM, van Dam RM, Bralley A et al. Maternal plasma 25-Hydroxyvitamin D concentrations and the risk for gestational diabetes mellitus. PLoS One 2008;3(11): e3753 doi: 10.1371/journal.pone.0003753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lacroix M, Battista MC, Doyon M, Houde G, Menard J, Ardilouze JL et al. Lower vitamin D levels at first trimester are associated with higher risk of developing gestational diabetes mellitus. Acta Diabetol 2014;51(4):609–16. [DOI] [PubMed] [Google Scholar]
- 13.Parlea L, Bromberg IL, Feig DS, Vieth R, Merman E, Lipscombe LL. Association between serum 25-hydroxyvitamin D in early pregnancy and risk of gestational diabetes mellitus. Diabet Med 2012;29, e25–e32. [DOI] [PubMed] [Google Scholar]
- 14.Shahgheibi S, Farhadifar F, Pouya B. The effect of vitamin D supplementation on gestational diabetes in high-risk women: Results from a randomized placebo-controlled trial. J Res Med Sci 2016;21:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mojibian M, Soheilykhah S, Fallah Zadeh MA, Jannati Moghadam M. The effects of vitamin D supplementation on maternal and neonatal outcome: A randomized clinical trial. Iran J Reprod Med 2015;13(11):687–96. [PMC free article] [PubMed] [Google Scholar]
- 16.Yap C, Cheung NW, Gunton JE, Athayde N, Munns CF, Duke A, McLean M. Vitamin D supplementation and the effects on glucose metabolism during pregnancy: a randomized controlled trial. Diabetes Care 2014;37(7):1837–44. [DOI] [PubMed] [Google Scholar]
- 17.Tehrani HG, Mostajeran F, Banihashemi B. Effect of vitamin D supplementation on the incidence of gestational diabetes. Adv Biomed Res 2017;6:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Powe CE, Ricciardi C, Berg AH, Erdenesanaa D, Collerone G, Ankers E et al. Vitamin d-binding protein modifies the vitamin d-bone mineral density relationship. J Bone Miner Res 2011;26:1609–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhan I, Powe CE, Berg AH, Ankers E, Wenger JB, Karumanchi SA et al. Bioavailable vitamin D is more tightly linked to mineral metabolism than total vitamin D in incident hemodialysis patients. Kidney Int 2012;82(1):84–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nielson CM, Jones KS, Bouillon R, Osteoporotic Fractures in Men (MrOS) Research Group, Chun RF, Jacobs J, Wang Y, Hewison M, Adams JS, Swanson CM, Lee CG, Vanderschueren D, Pauwels S, Prentice A, Smith RD, Shi T, Gao Y, Zmuda JM, Lapidus J, Cauley JA, Schoenmakers I, Oroll ES. Role of assay type in determining free 25-hydorxyvitamin D levels in diverse populations. N Engl J Med 2016;374(17):1695–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Neilson CM, Jones KS, Chun RF, Jacobs JM, Wang Y, Hewison M, Adams JS, Swanson CM, Lee CG, Vanderschueren D, Pauwels S, Prentice A, Smith RD, Shi T, Gao Y, Schepmoes AA, Zmuda JM, Lapidus J, Cauley JA, Bouillon R, Schoenmakers I, Orwoll ES; Osteoporotic Fractures in Mean (MrOS) Research Group. Free 25-hydroxyvitamin D: impact of vitamin D binding protein assays on racial-genotypic associations. J Clin Endocrinol Metab 2016;101(5):2226–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Genetic Bouillon R. and racial differences in the vitamin D endocrine system. Endocrinol Metab Clin North Am 2017;46(4):1119–1135. [DOI] [PubMed] [Google Scholar]
- 23.Bikle DD, Malmstroem S, Schwartz J. Current controversies: Are free vitamin metabolite levels a more accurate assessment of vitamin D status than total levels? Endocrinol Metab Clin North Am 2017;46(4):901–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grewal J, Grantz KL, Zhang C, Sciscione A, Wing DA, Grobman WA et al. Cohort Profile: NICHD Fetal Growth Studies-Singletons and Twins. Int J Epidemiol 2018;47(1):25–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Committee on Practice B-O. Practice Vulletin No. 137: gestational diabetes mellitus. Obstet Gynecol 2013;122(2 Pt 1):406–416. [DOI] [PubMed] [Google Scholar]
- 26.Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab 1999;84:3666–3672. [DOI] [PubMed] [Google Scholar]
- 27.Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care 2004;27:1487–1495. [DOI] [PubMed] [Google Scholar]
- 28.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the conentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972;18:499–502. [PubMed] [Google Scholar]
- 29.Millen AE, Pettinger M, Freudenheim JL, Langer RD, Rosenberg CA, Mossavar-Rahmani Y. Incident invasive breast cancer, geographic location of residence, and reported average time spent outside. Cancer Epidemiol Biomarkers Prev 2009;18(2):495–507. [DOI] [PubMed] [Google Scholar]
- 30.Chasan-Taber L, Schmidt MD, Roberts DE, Hosmer D, Markenson G, Freedson PS. Development and validation of a Pregnancy Physical Activity Questionnaire. Med Sci Sports Exerc 2004;36(10):1750–60. [DOI] [PubMed] [Google Scholar]
- 31.Holick MF. Vitamin D deficiency. N Engl J Med 2007;357:266–281. [DOI] [PubMed] [Google Scholar]
- 32.Park S, Yoon HK, Ryu HM, Han YJ, Lee SW, Park BK, Park SY, Yim CH, Kim SH. Maternal vitamin D deficiency in early pregnancy is not associated with gestational diabetes mellitus development or pregnancy outcomes in Korean pregnant women in a prospective study. J Nutr Sci Vitaminol (Tokyo) 2014;60(4):269–75. [DOI] [PubMed] [Google Scholar]
- 33.AI-Ajlan A, S AI-Musharaf, Fouda MA, Krishnaswamy S, Wani K, Aljohani NJ, AI-Serehi A, Sheshah E, Alshingetti NM, Turkistani IZ, Afrah Alharbi A, Alraqebah BA, Ali AM, AI-Saeed G, AI-Daghri NM. Lower vitamin D levels in Saudi pregnant women are associated with higher risk of developing GDM. BMC Pregnancy Childbirth 2018;18(1):86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bal M, Şahin Ersoy G, Demirtas Ö, Kurt S, Taşyurt A. Vitamin D deficiency in pregnancy is not associated with diabetes mellitus development in pregnant women at low risk for gestational diabetes. Turk J Obstet Gynecol 2016;13(1):23–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baker AM, Haeri S, Camargo CA Jr, Stuebe AM, Boggess KA. First-trimester naternal vitamin D status and risk for gestational diabetes (GDM) a nested case-control study. Diabetes Metab Res Rev 2012;28(2):164–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Burris HH, Rifas-Shiman SL, Kleinman K, Litonjua AA, Huh SY, Rich-Edwards JW, Camargo CA Jr, Gillman MW. Vitamin D deficiency in pregnancy and gestational diabetes mellitus. Am J Obstet Gynecol 2012;207(3): 182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ginde AA, Sullivan AF, Mansbach JM, Camargo CA Jr. Vitamin D insufficiency in pregnant and nonpregnant women of childbearing age in the United States. Am J Obstet Gynecol 2010;202(5):436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Von Websky K, Hasan AA, Reichetzeder C, Tsuprykov O, Hocher B. Impact of vitamin D on pregnancy-related disorders and on offspring outcome. J Steroid Biochem Mol Biol 2018;180:51–64. [DOI] [PubMed] [Google Scholar]
- 39.Johnson JA, Grande JP, Roche PC, Kumar R. Immunohistochemical localization of the 1,25(OH)2D3 receptor and calbindin D28k in human and rat pancreas. Am J Physiol 1994;267(3 Pt 1):E356–360. [DOI] [PubMed] [Google Scholar]
- 40.Charles MA, Lawecki J, Pictet R, Grodsky GM. Insulin secretion: Interrelationships of glucose, cyclic adenosine 3’:5’-monophosphate, and calcium. J Biol Chem 1975;250(15):6134–6140. [PubMed] [Google Scholar]
- 41.Pittas AG, Dawson-Hughes B. Vitamin D and diabetes. J Steroid Biochem Mol Biol 2010;121(1–2):425–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pittas AG, Lau J, Hu FB, Dawson-Hughes B. The role of vitamin D and calcium in type 2 diabetes: A systematic review and meta-analysis. J Clin Endocrinol Metab 2007;92(6):2017–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shab-Bidar S, Neyestani T, Djazayery A, Eshraghian M, Houshiarrad A, Kalayi A. Improvement of vitamin D status resulted in amelioration of biomarkers of systemic inflammation in the subjects with type 2 diabetes. Diabetes Metab Res Rev 2012;28:423–430. [DOI] [PubMed] [Google Scholar]
- 44.Hoofnagle AN, Eckfeldt JH, Lutsey PL. Vitamin D-binding protein concentrations quantified by mass spectrometry. N Engl J Med 2015;373:1480–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Denburg MR, Hoofnagle AN, Sayed S, Gupta J, de Boer IH, Appel LJ, Durazo-Arvizu R, Whitehead K, Feldman HI, Leonard MB Chronic Renal Insufficiency Cohort study i. Comparison of two elisa methods and mass spectrometry for measurement of vitamin D-binding protein: Implications for the assessment of bioavailable vitamin D concentrations across genotypes. J Bone Miner Res 2016;31(6):1128–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tsuprykov O, Buse C, Skoblo R, Hocher B. Comparison of free and total 25-hydroxyvitamin D in normal human pregnancy. J Steroid Biochem Mol Biol 2019;190:29–36. [DOI] [PubMed] [Google Scholar]
- 47.Stanley T, Bredella MA, Pierce L, Misra M. The ratio of parathyroid hormone to vitamin D is a determinant of cardiovascular risk and insulin sensitivity in adolescent girls. Metab Syndr Relat Disord 2013;11:56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kramer CK, Swaminathan B, Hanley AJ, Connelly PW, Sermer M, Zinman B. Prospective associations of vitamin D status with β-cell function, insulin sensitivity, and glycemia: the impact of parathyroid hormone status. Diabetes 2014; 10.2337/db14-0489. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


