Abstract
Background
Development of an HIV vaccine might be essential to ending the HIV/AIDS pandemic. However, vaccines can result in the emergence and spread of vaccine-resistant strains. Indeed, analyses of breakthrough infections in the HIV phase 3 vaccine trial RV144 identified HIV genotypes with differential rates of transmission in vaccine and placebo recipients. We hypothesized that, for HIV vaccination programs based on partially effective vaccines similar to RV144, HIV adaptation will rapidly diminish the expected vaccine impact.
Methods and findings
Using two HIV epidemic models, we simulated large-scale vaccination programs and, critically, included HIV strain diversity with respect to the vaccine response. We show here that rapid population-level viral adaptation can lead to decreased overall vaccine efficacy and substantially fewer infections averted by vaccination, when comparing scenarios with and without viral evolution (with outcomes depending on vaccination coverage, vaccine efficacy against the sensitive allele, and the initial resistant allele frequency). Translating this to the epidemic in South Africa, a scenario with 70% vaccination coverage may result in 250,000 infections (non-averted by vaccination) within 10 years of vaccine rollout that are due solely to HIV adaptation, all else being equal.
Conclusions
These findings suggest that approaches to HIV vaccine development, program implementation, and epidemic modeling may require attention to viral adaptation in response to vaccination.
Keywords: HIV vaccine, Mathematical model, South Africa, HIV prevention
1. Introduction
Despite concerted global effort and the existence of effective methods for prevention, HIV continues to be a public health crisis. The need for an HIV vaccine remains paramount. The phase 3 RV144 HIV vaccine trial is the only trial of an HIV vaccine to show modest success in preventing infection [1]. RV144 resulted in an estimated 31% vaccine efficacy (VE) at 3.5 years post-vaccination (p = .04, modified intent-to-treat analysis). The vaccine was partially protective but not therapeutic; i.e. vaccinated individuals had decreased rates of infection, but breakthrough infections were not associated with differences in early HIV plasma viral loads, post-infection CD4+ T cell counts, or HIV disease progression rates, when comparing vaccine and placebo recipients [2]. The RV144 results spurred the development of the recently initiated HVTN 702, a large phase 3 HIV vaccine trial in South Africa that aims to replicate the RV144 findings in a different study group, with regimen and schedule that follow from RV144 (with several modifications, including a vaccine insert specific to HIV subtype C, the most common subtype in South Africa).
Partially effective vaccines have been of enduring scientific, clinical, and theoretical interest. For HIV, two decades of mathematical modeling studies suggest that partially effective vaccines, whether protective or therapeutic, can have a substantial impact on the HIV pandemic [3–15]. More recently, HIV epidemic models were used to predict the impact of a partially effective (protective) vaccine similar to RV144 in terms of VE and duration. Models were used to assess the impact of vaccination programs with 30% and 60% population coverage of sexually active adults, with subsequent vaccine rollouts at 1- to 5-year intervals [16]. Results were consistent across several model and epidemic types, e.g., multiple vaccination rounds, at 60% coverage, were predicted to prevent 5–15% of new infections over 10 years [17–24]. The expected impact of vaccination programs depended on vaccination coverage, VE, and the duration of vaccine protection [25].
However, the potential for HIV adaptation at the populationlevel in response to vaccination was not considered in these modeling studies. The requirements for adaption (via natural selection) are few: there must be phenotypic variation in a population, this variation must be heritable (linked to genetic variation), and this variation must be related to fitness (differential reproduction) [26]. Evidence from RV144 follow-up studies suggest that, with respect to a partially effective protective vaccine, HIV meets these requirements. Namely, genetic sieve analyses of RV144 breakthrough infections showed that sequences from infected vaccine recipients differed from those isolated from infected placebo recipients. Two signatures were identified in the Env V2 region: in the vaccine recipients, K169X mutations were more frequent (34% vs. 17%) and 181I was more conserved (91% vs. 71%). VE against viruses matching the vaccine at position 169 was 48% (95% confidence interval (CI) 18% to 66%), whereas VE against viruses mismatching the vaccine at position 181 was 78% (CI 35% to 93%) [27,28]. Thus, heritable (genetic) variation in HIV can be associated with differential infection rates in a vaccinated population, making viral adaptation a potential outcome.
We hypothesized that HIV population-level adaptation after vaccine rollout will result from selection acting on a viral locus containing an allele that confers resistance to the vaccine response; i.e., viruses not blocked by a vaccine-elicited immune response will spread in the HIV-infected population. Our goal was to predict the public health impact of this viral evolution, under varying VE, population vaccination coverage, and initial frequency of vaccineresistant genotypes. We quantified this impact in terms of the resistant genotype frequency, the overall VE, and the cumulative HIV infections averted by vaccination.
2. Methods
We used two stochastic, individual-based HIV epidemic models, both of which were based on existing model frameworks (described in further detail in the Supplementary Materials, and in Tables S1 and S2). For each serodiscordant coital act, the probability of transmission from an HIV-infected person to an HIVnegative person was based primarily on viral load but also on disease stage, condom use, and antiretroviral use (see Supplementary Materials). The first model was roughly calibrated to the heterosexual epidemic of South Africa [29]. We ran the heterosexual epidemic simulation for 40 years, to mimic HIV epidemics from 1990 to 2030 in South Africa. The second model was parameterized using behavioral data from men who have sex with men (MSM) in the United States. The models differed in their underlying sexual contact networks; the heterosexual epidemic model contained three risk subgroups with distinct relationship durations, daily probability of sexual contact, and propensity for assortative mixing; the MSM model did not include subgroups with different risk profiles. Both models contained antiretroviral therapy (ART) to approximate the population-level effects of ART on background incidence and prevalence in our vaccine simulations, and we repeated all simulations under two ART population coverage levels: 30% and 70% for the heterosexual model, 40% and 70% for the MSM model (lower coverage levels reflect approximate current states; upper level is aspirational).
We designed the vaccine component of our models to reflect the empirical results from RV144 [1]. The vaccine in our models protected individuals from infection by decreasing the percontact probability of transmission; it did not affect HIV disease progression, viral load, or CD4 count in vaccinated individuals who became infected (consistent with RV144). The mean duration of VE was set to three years, with efficacy reduced immediately to 0% at the end of this period. For the MSM simulations, the vaccination program was initiated 10 years after equilibrium prevalence of ~25% was reached; this was generally around year 20 of the simulations. For the heterosexual simulations, the vaccination program was initiated at year 25 of the simulated epidemic. In both epidemic simulation types the vaccine rollout was continuous after program initiation. Target vaccination coverage was generally reached within three to four years. We modeled viral diversity with respect to vaccine-induced host response via one locus with two alleles: sensitive and resistant. Sensitive viruses had reduced per-contact probabilities of infecting vaccinated hosts. Resistant viruses experienced no change in transmission probability, regardless of the vaccination status of possible recipients (but see Supplementary Materials for cases in which resistance was incomplete). We used a single viral genetic locus as an approximation of the complete set of putative vaccine-resistant variants identified by sieve analyses [27,28]; in effect, we assumed that multiple independent vaccine-resistant alleles at low frequency will have a similar population-level impact as a single vaccine-resistant allele at high frequency. Our model did not allow de novo intra-host resistance development (mutation from vaccine sensitive to vaccine resistant after breakthrough infections of vaccinated individuals).
For each ART coverage level we evaluated two vaccine scenarios, at increasing vaccination coverage levels: (1) VE for the sensitive virus = 75% and initial resistant virus proportion = 0.25; and (2) VE for sensitive virus = 90% and initial resistant virus proportion = 0.50. We selected these scenarios because both correspond to an overall VE of roughly ~ 50% (56.25% and 45%, respectively, calculated as the weighted average of sensitive and resistant VE at first vaccine rollout), a VE that the HVTN 702 vaccine trial is powered to detect relative to the null hypothesis (VE ≤ 25%).
For the heterosexual model, we employed two separate approaches to vaccination: random and targeted. For random vaccination, all individuals had the same probability of being vaccinated, regardless of their risk group status. In this case, population vaccination coverage was a single target value and reflected the entire population of susceptible individuals. For targeted vaccination, the high-risk population subgroup was given a higher target vaccination coverage (50%, 70%, 90%), while the two lower-risk subgroups were both given 25% vaccination coverage targets.
3. Results
To confirm the validity of our models, we first examined the effects of introducing a partially effective vaccine into HIV epidemics with a single, vaccine-sensitive viral strain (all viruses were equally sensitive to the vaccine response). We found, in agreement with previous models [17–24], that a vaccine similar to RV144 can indeed have a modest impact. For example, an HIV vaccine with 45% VE and 50% coverage can prevent ~20% of cumulative infections within 10 years of vaccine rollout, in comparison to populations with no vaccine (Tables S3 and S4). As expected, these values increase with higher vaccination coverage and higher VE: a vaccine with 56% VE and 70% overall coverage can prevent ~ 40% of cumulative infections within 10 years (Tables S3 and S4).
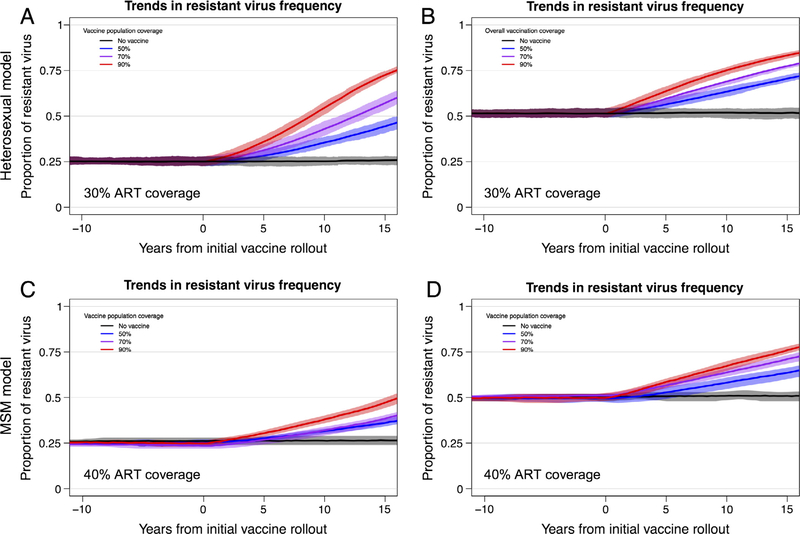
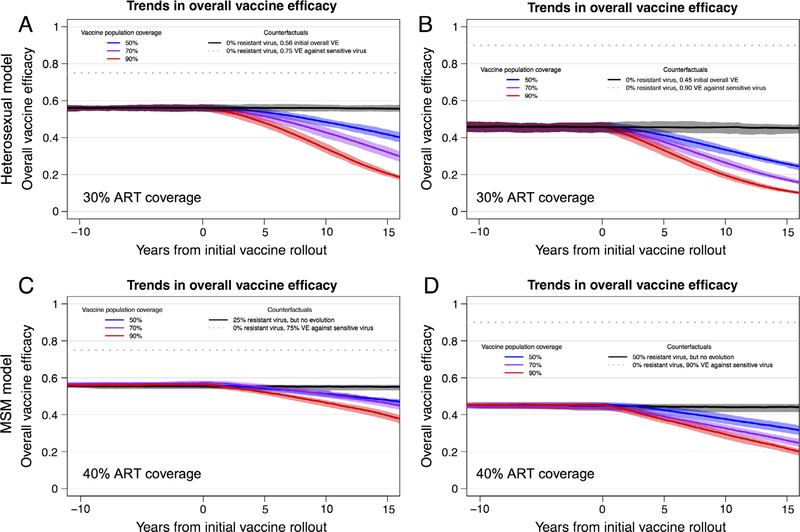
Next, we examined the impact of a partially effective vaccine in a population that contained resistant viruses (VE = 0 for the resistant viruses). In all simulations, for both heterosexual and MSM models, resistant virus increased in proportion after vaccine rollout (Fig. 1). In a representative scenario from the heterosexual model (Fig. 1A), with 30% ART coverage, 70% vaccination coverage, and 75% VE against the sensitive allele, the resistant allele population proportion increased from 0.25 to 0.38 within 10 years of vaccine rollout. With 70% ART coverage and 90% VE against the sensitive allele, the resistant allele increased from 0.51 to 0.69 within 10 years. Across all epidemic simulations, the rate at which the resistant allele frequency increased was higher with higher vaccination coverage and greater VE against the sensitive virus. As the resistant allele frequency increases, the overall VE correspondingly declines (Fig. 2); e.g., with 50% vaccination coverage, overall VE declines from 56% to 50%, or from 45% to 33% in 10 years (depending on sensitive virus VE and initial resistant frequency). Thus, we see that both programmatic and vaccine-related parameters can exert evolutionary pressure on HIV and impact vaccine effectiveness.
Fig. 1.
Trends in the proportion of HIV strains that are resistant to a vaccine-driven immune response. Trends in the proportion of resistant strains among all HIV strains (with interquartile ranges) for 20 replicate HIV epidemic simulations from heterosexual (panels A and B) and MSM (panels C and D) models. Panels A and C depict results from epidemic scenarios that included an initial resistant strain (VE = 0.0) at a proportion = 0.25 and a sensitive virus (VE = 0.75). Panels B and D depict results from scenarios with initial resistant strain proportion = 0.50 and a sensitive virus VE = 0.90. Background population ART coverage was at 30% for the heterosexual model and 40% for the MSM model. (See Fig. S1 for equivalent results from heterosexual model scenarios in which a high-risk subgroup is preferentially targeted for vaccination, which leads to substantially decreased overall vaccination coverage but results in similar effects of HIV adaptation on resistant virus frequency.)
Fig. 2.
Trends in overall vaccine efficacy (VE) for an HIV vaccine. Trends in overall vaccine efficacy (with interquartile ranges) for 20 replicate HIV epidemic simulations from heterosexual (panels A and B) and MSM (panels C and D) models. Overall VE was calculated as the weighted average of the VE for sensitive and resistant viruses at each time step (VE for resistant viruses = 0.0). Panels A and C depict results from scenarios with an initial resistant strain proportion = 0.25 and a sensitive virus VE = 0.75. Panels B and D depict results from scenarios with initial resistant strain proportion = 0.50 and a sensitive virus VE = 0.90. ART coverage was at 30% for the heterosexual model and 40% for the MSM model. (See Fig. S1 and Table S5 for equivalent results from heterosexual model scenarios in which a high-risk subgroup is preferentially targeted for vaccination, which leads to substantially decreased overall vaccination coverage but results in similar effects of HIV adaptation on overall vaccine efficacy
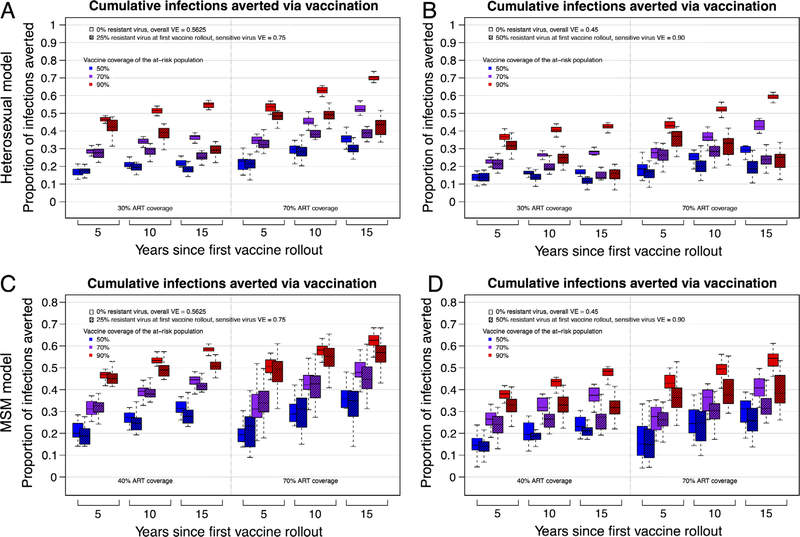
In terms of predicted public health impact, fewer infections were averted over time in scenarios with resistant strains, relative to counterfactual simulations with no vaccine-resistant HIV strains (Figs. 3 and 4). Since overall VEs at the time of first vaccine rollout were equivalent across runs, we can assign causality for the marginal differences (in the proportion of infections averted) to viral adaptation. The proportion of infections averted by vaccination decreased dramatically as resistant viruses increased in frequency and overall VE decreased. In the representative examples, above, with 70% vaccination coverage, 30% ART coverage, and either 75% or 90% VE against the sensitive allele and 25% or 50% resistant virus at vaccine rollout, respectively, infections averted decreased from 39% to 37%, or from 32% to 26%, within 10 years (Fig. 3A, Table S3).
Fig. 3.
The proportion of cumulative HIV infections averted by vaccination. Trends in cumulative infections averted by vaccination (with interquartile ranges) for 20 replicate epidemic simulations from heterosexual (panels A and B) and MSM (panels C and D) HIV epidemic models (comparing epidemic scenarios with and without vaccination programs). Panels A and C depict results from epidemic scenarios with an initial resistant strain (VE = 0) proportion = 0.25 and a sensitive virus VE = 0.75. Panels B and D depict results with initial resistant strain proportion = 0.50 and a sensitive virus VE = 0.90. All solid color boxplots represent epidemic scenarios with a vaccine but without HIV adaptation; the initial overall VE in these scenarios is equal to the overall VE at initial vaccine rollout in the scenarios with HIV adaptation (hashed boxplots, placed immediately to the right of the solid color boxplots). ART coverage was at 30% or 70% for the heterosexual model and 40% or 70% for the MSM model. (See Fig. S1 and Table S5 for equivalent results from heterosexual model scenarios in which a high-risk subgroup is preferentially targeted for vaccination, which leads to substantially decreased overall vaccination coverage but results in similar effects of HIV adaptation on infections averted.)
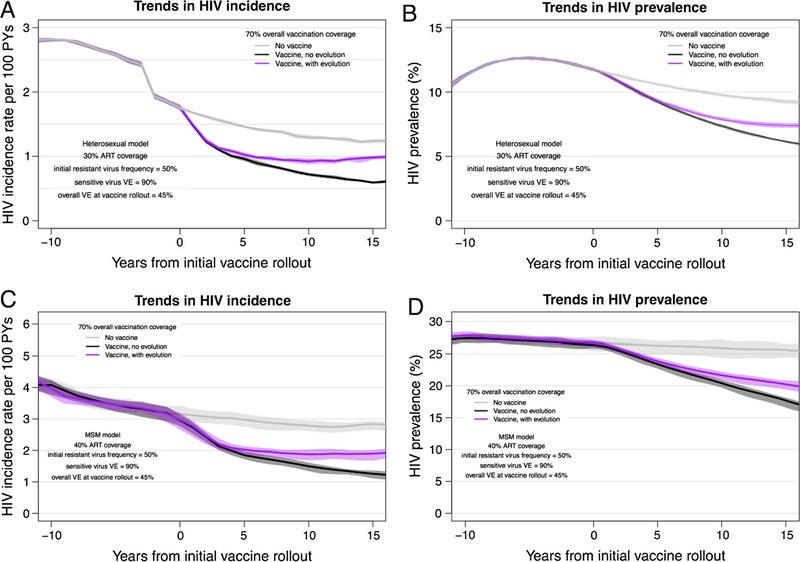
Fig. 4.
Trends in the incidence and prevalence for the HET and MSM HIV epidemic models, with and without HIV vaccination. Trends in HIV incidence (with interquartile ranges) for 20 replicate HIV epidemic simulations from heterosexual (panel A) and MSM (panel C) models. Trends in HIV prevalence from heterosexual (panel B) and MSM (panel D) models. All panels depict results from epidemic scenarios that included an initial resistant strain (VE = 0) proportion = 0.50 and a sensitive virus VE = 0.90. Background population ART coverage was at 30% for the heterosexual model and 40% for the MSM model. (See Fig. S1 and Table S5 for equivalent results from heterosexual model scenarios in which a high-risk subgroup is preferentially targeted for vaccination, which leads to substantially decreased overall vaccination coverage but results in similar effects of HIV adaptation on incidence and prevalence.)
In scenarios in which a high-risk subgroup in the heterosexual model is preferentially targeted for vaccination (Fig. S1, Table S5), we see equivalent declines in the proportion of infections averted, but with much lower overall vaccination coverage: up to 150,000 new infections in the first 10 years after vaccine rollout due to the emergence and spread of vaccine-resistant strains, in a scenario with 70% vaccination coverage of a high-risk subgroup but only 32% coverage of the total population. This increases to 350,000 new infections with 90% coverage of the high-risk subgroup and 35% total coverage.
4. Discussion
Our HIV-specific model predictions are consistent with findings from other pathogens. These include empirical evidence of vaccine-induced strain replacement in, e.g., Streptococcus pneumoniae, Haemophilus influenza, Neisseria meningitidis, Bordetella pertussis, Plasmodium falciparum, and hepatitis B virus (reviewed in [30,31]). Mathematical models have evaluated patterns and processes of strain replacement in, e.g., Mycobacterium tuberculosis [32], rotavirus [33], and S. pneumoniae [34,35], and generalized pathogens [30,36]. Yet, despite widespread acknowledgement of, and concern for, the evolutionary potential of HIV—with respect to resistance to ART and PrEP [37,38], vaccine design [39,40], the human immune response [41–44], and a potential vaccine-induced cellular immune response [45,46], HIV strain replacement in response to an imperfect vaccine had not been evaluated previously.
We observed population-level viral adaptation in response to an HIV vaccine in all model scenarios. Critically, our results predict that HIV adaptation in response to vaccination may have a considerable, and detrimental, public health impact. In the representative example discussed above (70% vaccination coverage), there can be a nearly 20% relative decrease in the proportion of infections averted by vaccination in the first 10 years alone, depending on VE and initial resistance allele frequency. This prediction can be placed in the context of the current HIV epidemic: in South Africa, with approximately 380,000 new HIV infections in 2015 [47], approximately 100,000–250,000 new infections may occur in the first decade after vaccine rollout that are due solely to the emergence and spread of vaccine-resistant strains (in scenarios with 70% vaccination coverage, 30% ART coverage, 25% resistant virus and 75% sensitive virus VE, and 50% resistant virus and 90% sensitive virus VE, respectively).
These results highlight the potential public health impact of HIV adaptation in response to vaccination. They also underscore the need to understand the underlying viral determinants of partially effective vaccines: despite their similar overall VE of ~50%, the distinct sensitive virus VE and resistant frequency scenarios (25% and 50% resistant virus; 75% and 90% sensitive virus VE) differed greatly in implications for public health.
We note that similar, but not identical, HIV population-level adaptation and diminished public health impacts were seen in the heterosexual and MSM models, despite differences between the models in population structure, transmission dynamics, HIV prevalence and incidence, and overall effect of vaccination. Additionally, the impacts of HIV adaptation were consistent across the two ART scenarios, suggesting that ART parameters will likely not substantially affect the rates or impact of viral adaptation. Furthermore, we note that vaccination efforts that use high-risk behavioral targeting result in similar patterns of viral adaptation and number of infections averted by vaccination, though at much lower overall population coverage levels. Such vaccination strategies must therefore also be prepared for significant impacts from the viral adaptation; further studies of the impact of transmission network structure on viral adaptation in response to vaccination, guided by empirical data, are warranted.
As noted in the Methods, our model did not include de novo intra-host development of vaccine resistance; further studies are needed to explore the question of whether, and to what extent, such de novo mutation and adaptation would further impact vaccine effectiveness over time. Another limitation was our description of viral diversity as simply two strains (resistant and sensitive), without consideration of the genomic location of the locus associated with vaccine resistance, the number of loci and alleles that may confer resistance, and the mechanism of resistance. Further model development may include the capacity for multiple vaccine-related loci, each with sensitive and resistant alleles, allele-specific VE, and potential for synergistic or antagonistic effects.
5. Conclusions
Previous epidemic models that have estimated the effects of partially effective HIV vaccines have likely overestimated the benefits conferred on a population by vaccination. Perhaps more pressing, strategies for HIV vaccine development and program implementation may benefit from careful attention to the potential evolutionary consequences of vaccination. This includes continued surveillance of viral genetic diversity, accompanied by vaccine design that limits the mutational pathways available for viral adaptation and subsequent emergence of vaccine-resistant viruses [40,48,49]. Our analysis is particularly relevant given the recent initiation of the HVTN 702 trial, which is the critical test for licensure in South Africa of the first vaccine to prevent HIV infection. If successful, such an HIV vaccination program may necessarily become a program similar to that in place for influenza, comprising an acceptable vaccine that requires periodic updating.
Supplementary Material
Acknowledgments
We thank Jonathan Carlson, Christian Selinger, members of the University of Washington International Clinical Research Center, and two anonymous reviewers for helpful comments and discussion.
Funding sources
This work was supported by grants from the U.S. National Institutes of Health (R01AI108490 to J.T.H., J.E.M., and S.M.G., R01GM125440 to J.T.H., and P30AI027757 to the University of Washington Center for AIDS Research) and by an Interagency Agreement with the US Army Medical Research and Material Command (Y1-AI-2642-12) and by a cooperative agreement between the Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc., and the U.S. Department of Defense (MR). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, the US Department of Defense or the Department of the Army. The authors declare no competing interests.
Footnotes
Conflict of interest
The authors declare no competing interests.
Appendix A. Supplementary material
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.vaccine.2017.12.004.
References
- [1].Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, Paris R, et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med 2009;361:2209–20. [DOI] [PubMed] [Google Scholar]
- [2].Rerks-Ngarm S, Paris RM, Chunsutthiwat S, Premsri N, Namwat C, Bowonwatanuwong C, et al. Extended evaluation of the virologic, immunologic, and clinical course of volunteers who acquired HIV-1 infection in a phase III vaccine trial of ALVAC-HIV and AIDSVAX B/E. J Infect Dis 2013;207:1195–205. [DOI] [PubMed] [Google Scholar]
- [3].Andersson KM, Owens DK, Vardas E, Gray GE, McIntyre JA, Paltiel AD. Predicting the impact of a partially effective HIV vaccine and subsequent risk behavior change on the heterosexual HIV epidemic in low- and middle-income countries: a South African example. J Acquir Immune Defic Syndr 2007;46:78–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Long EF, Brandeau ML, Owens DK. Potential population health outcomes and expenditures of HIV vaccination strategies in the United States. Vaccine 2009;27:5402–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Davenport MP, Ribeiro RM, Chao DL, Perelson AS. Predicting the impact of a nonsterilizing vaccine against human immunodeficiency virus. J Virol 2004;78:11340–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Owens DK, Edwards DM, Shachter RD. Population effects of preventive and therapeutic HIV vaccines in early- and late-stage epidemics. AIDS 1998;12:1057–66. [PubMed] [Google Scholar]
- [7].Abu-Raddad LJ, Boily MC, Self S, Longini IM Jr. Analytic insights into the population level impact of imperfect prophylactic HIV vaccines. J Acquir Immune Defic Syndr 2007;45:454–67. [DOI] [PubMed] [Google Scholar]
- [8].Smith RJ, Blower SM. Could disease-modifying HIV vaccines cause populationlevel perversity? Lancet Infect Dis 2004;4:636–9. [DOI] [PubMed] [Google Scholar]
- [9].Anderson R, Hanson M. Potential public health impact of imperfect HIV type 1 vaccines. J Infect Dis 2005;191(Suppl 1):S85–96. [DOI] [PubMed] [Google Scholar]
- [10].van Ballegooijen M, Bogaards JA, Weverling GJ, Boerlijst MC, Goudsmit J. AIDS vaccines that allow HIV-1 to infect and escape immunologic control: a mathematic analysis of mass vaccination. J Acquir Immune Defic Syndr 2003;34:214–20. [DOI] [PubMed] [Google Scholar]
- [11].Anderson RM, Swinton J, Garnett GP. Potential impact of low efficacy HIV-1 vaccines in populations with high rates of infection. Proc Biol Sci 1995;261:147–51. [DOI] [PubMed] [Google Scholar]
- [12].McLean AR, Blower SM. Imperfect vaccines and herd immunity to HIV. Proc Biol Sci 1993;253:9–13. [DOI] [PubMed] [Google Scholar]
- [13].Amirfar S, Hollenberg JP, Abdool Karim SS. Modeling the impact of a partially effective HIV vaccine on HIV infection and death among women and infants in South Africa. J Acquir Immune Defic Syndr 2006;43:219–25. [DOI] [PubMed] [Google Scholar]
- [14].Gray RH, Li X, Wawer MJ, Gange SJ, Serwadda D, Sewankambo NK, et al. Stochastic simulation of the impact of antiretroviral therapy and HIV vaccines on HIV transmission; Rakai, Uganda. AIDS 2003;17:1941–51. [DOI] [PubMed] [Google Scholar]
- [15].Blower SM, McLean AR. Prophylactic vaccines, risk behavior change, and the probability of eradicating HIV in San Francisco. Science 1994;265:1451–4. [DOI] [PubMed] [Google Scholar]
- [16].Hankins CA, Glasser JW, Chen RT. Modeling the impact of RV144-like vaccines on HIV transmission. Vaccine 2011;29:6069–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Andersson KM, Paltiel AD, Owens DK. The potential impact of an HIV vaccine with rapidly waning protection on the epidemic in Southern Africa: examining the RV144 trial results. Vaccine 2011;29:6107–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Andersson KM, Stover J. The potential impact of a moderately effective HIV vaccine with rapidly waning protection in South Africa and Thailand. Vaccine 2011;29:6092–9. [DOI] [PubMed] [Google Scholar]
- [19].Gray RT, Ghaus MH, Hoare A, Wilson DP. Expected epidemiological impact of the introduction of a partially effective HIV vaccine among men who have sex with men in Australia. Vaccine 2011;29:6125–9. [DOI] [PubMed] [Google Scholar]
- [20].Hontelez JA, Nagelkerke N, Barnighausen T, Bakker R, Tanser F, Newell ML, et al. The potential impact of RV144-like vaccines in rural South Africa: a study using the STDSIM microsimulation model. Vaccine 2011;29:6100–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Long EF, Owens DK. The cost-effectiveness of a modestly effective HIV vaccine in the United States. Vaccine 2011;29:6113–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Nagelkerke NJ, Hontelez JA, de Vlas SJ. The potential impact of an HIV vaccine with limited protection on HIV incidence in Thailand: a modeling study. Vaccine 2011;29:6079–85. [DOI] [PubMed] [Google Scholar]
- [23].Phillips AN, Cambiano V, Nakagawa F, Ford D, Lundgren JD, Roset-Bahmanyar E, et al. Potential future impact of a partially effective HIV vaccine in a southern African setting. PLoS One 2014;9:e107214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Schneider K, Kerr CC, Hoare A, Wilson DP. Expected epidemiological impacts of introducing an HIV vaccine in Thailand: a model-based analysis. Vaccine 2011;29:6086–91. [DOI] [PubMed] [Google Scholar]
- [25].Medlock J, Pandey A, Parpia AS, Tang A, Skrip LA, Galvani AP. Effectiveness of UNAIDS targets and HIV vaccination across 127 countries. Proc Natl Acad Sci USA 2017;114:4017–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Darwin C On the origin of species by means of natural selection, or the preservation of favoured races in the struggle for life. London: John Murray, Albemarle Street; 1859. [Google Scholar]
- [27].Edlefsen PT, Rolland M, Hertz T, Tovanabutra S, Gartland AJ, deCamp AC, et al. Comprehensive sieve analysis of breakthrough HIV-1 sequences in the RV144 vaccine efficacy trial. PLoS Comput Biol 2015;11:e1003973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Rolland M, Edlefsen PT, Larsen BB, Tovanabutra S, Sanders-Buell E, Hertz T, et al. Increased HIV-1 vaccine efficacy against viruses with genetic signatures in Env V2. Nature 2012;490:417–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Eaton JW, Johnson LF, Salomon JA, Barnighausen T, Bendavid E, Bershteyn A, et al. HIV treatment as prevention: systematic comparison of mathematical models of the potential impact of antiretroviral therapy on HIV incidence in South Africa. PLoS Med 2012;9:e1001245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Martcheva M, Bolker BM, Holt RD. Vaccine-induced pathogen strain replacement: what are the mechanisms? J R Soc Interface 2008;5:3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Read AF, Mackinnon MJ. Pathogen evolution in a vaccinated world In: Stearns SC, Koella J, editors. Evolution in health and disease. 2nd ed. Oxford University Press; 2008. p. 139–52. [Google Scholar]
- [32].Cohen T, Colijn C, Murray M. Modeling the effects of strain diversity and mechanisms of strain competition on the potential performance of new tuberculosis vaccines. Proc Natl Acad Sci USA 2008;105:16302–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Pitzer VE, Patel MM, Lopman BA, Viboud C, Parashar UD, Grenfell BT. Modeling rotavirus strain dynamics in developed countries to understand the potential impact of vaccination on genotype distributions. Proc Natl Acad Sci USA 2011;108:19353–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Lipsitch M Bacterial vaccines and serotype replacement: lessons from Haemophilus influenzae and prospects for Streptococcus pneumoniae. Emerg Infect Dis 1999;5:336–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Bottomley C, Roca A, Hill PC, Greenwood B, Isham V. A mathematical model of serotype replacement in pneumococcal carriage following vaccination. J R Soc Interface 2013;10:20130786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].McLean AR. Vaccination, evolution and changes in the efficacy of vaccines: a theoretical framework. Proc Biol Sci 1995;261:389–93. [DOI] [PubMed] [Google Scholar]
- [37].Lehman DA, Baeten JM, McCoy CO, Weis JF, Peterson D, Mbara G, et al. Risk of drug resistance among persons acquiring HIV within a randomized clinical trial of single- or dual-agent preexposure prophylaxis. J Infect Dis 2015;211:1211–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].TenoRes Study G Global epidemiology of drug resistance after failure of WHO recommended first-line regimens for adult HIV-1 infection: a multicentre retrospective cohort study. Lancet Infect Dis 2016;16:565–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Dimitrov D, Kublin JG, Ramsey S, Corey L. Are clade specific HIV vaccines a necessity? An analysis based on mathematical models. EBioMedicine 2015;2:2062–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Rolland M, Nickle DC, Mullins JI. HIV-1 group M conserved elements vaccine. PLoS Pathog 2007;3:e157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Rademeyer C, Korber B, Seaman MS, Giorgi EE, Thebus R, Robles A, et al. Features of recently transmitted HIV-1 çlade C viruses that impact antibody recognition: implications for active and passive immunization. PLoS Pathog 2016;12:e1005742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Bunnik EM, Euler Z, Welkers MR, Boeser-Nunnink BD, Grijsen ML, Prins JM, et al. Adaptation of HIV-1 envelope gp120 to humoral immunity at a population level. Nat Med 2010;16:995–7. [DOI] [PubMed] [Google Scholar]
- [43].Kawashima Y, Pfafferott K, Frater J, Matthews P, Payne R, Addo M, et al. Adaptation of HIV-1 to human leukocyte antigen class I. Nature 2009;458:641–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Carlson JM, Du VY, Pfeifer N, Bansal A, Tan VY, Power K, et al. Impact of preadapted HIV transmission. Nat Med 2016;22:606–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Fryer HR, McLean AR. Modelling the spread of HIV immune escape mutants in a vaccinated population. PLoS Comput Biol 2011;7:e1002289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Nowak MA, McLean AR. A mathematical model of vaccination against HIV to prevent the development of AIDS. Proc Biol Sci 1991;246:141–6. [DOI] [PubMed] [Google Scholar]
- [47].UNAIDS. Prevention gap report; 2016.
- [48].Mullins JI, Rolland M, Allen TM. Viral evolution and escape during primary human immunodeficiency virus-1 infection: implications for vaccine design. Curr Opin HIV AIDS 2008;3:60–6. [DOI] [PubMed] [Google Scholar]
- [49].Chen RT, Hu DJ, Dunne E, Shaw M, Mullins JI, Rerks-Ngarm S. Preparing for the availability of a partially effective HIV vaccine: some lessons from other licensed vaccines. Vaccine 2011;29:6072–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.