Abstract
Background:
Extended-release naltrexone (XR-NTX, Vivitrol®) and daily oral naltrexone tablets (O-NTX) are FDA-approved mu opioid receptor antagonist medications for alcohol dependence treatment. Despite the efficacy of O-NTX, non-adherence and poor treatment retention have limited its adoption into primary care. XR-NTX is a once-a-month injectable formulation that offers a potentially more effective treatment option in reducing alcohol consumption and heavy drinking episodes among persons with alcohol use disorders.
Methods:
This pragmatic, open-label, randomized controlled trial examines the effectiveness of XR-NTX vs. ONTX in producing a Good Clinical Outcome, defined as abstinence or moderate drinking (< 2 drinks/day, men; < 1 drink/day, women; and < 2 heavy drinking occasions/month) during the final 20 of 24 weeks of primary care-based Medical Management treatment for alcohol dependence. Secondary aims will estimate the cost effectiveness of XR-NTX vs. O-NTX, in conjunction with primary-care based Medical Management for both groups, and patient-level characteristics associated with effectiveness in both arms. Alcohol dependent persons are recruited from the community into treatment in a New York City public hospital primary care setting (Bellevue Hospital Center) for 24 weeks of either XR-NTX (n = 117) or O-NTX (n = 120).
Results:
We describe the rationale, specific aims, design, and recruitment results to date. Alternative design considerations and secondary aims and outcomes are reported.
Conclusions:
XR-NTX treatment in a primary care setting is potentially more efficacious, feasible, and cost-effective than oral naltrexone when treating community-dwelling persons with alcohol use disorders. This study will estimate XR-NTX’s treatment and cost effectiveness relative to oral naltrexone.
Keywords: Naltrexone, Extended-release naltrexone, Alcohol dependence, Alcohol use disorder, Medical management, Primary care treatment
1. Introduction and background
Alcohol use disorders (AUDs) are common, debilitating, and costly. An estimated 64.2 million (about 25%) U.S. adults ≥18 reported current binge drinking (≥5 drinks for males and ≥ 4 drinks for females on any one occasion, past 30 days); 16.1 million (7%) adults reportedly engaged in heavy drinking (≥5 drinks for males and ≥ 4 drinks for females on ≥5 occasions, past 30 days) in the past month, and approximately 14.6 million adults (6%) met DSM-IV criteria for alcohol abuse or dependence in the past year in the 2016 National Survey on Drug Use and Health (NSDUH) [1]. This widespread excessive alcohol use is a leading cause of disability exacting a tremendous toll in mortality, morbidity, and cost [2–6]. Strikingly, less than one third of persons with AUDs are enrolled in specialty treatment, and many fewer (< 10%) are prescribed medications to reduce or moderate their drinking [7].
Systematic reviews of daily oral medications used to treat alcohol dependence (including acamprosate, disulfiram, and oral naltrexone) have shown promise in reducing alcohol consumption and achieving or maintaining sobriety [8,9]. Recent recommendations from the U.S. Preventive Services Task Force to screen adults for AUDs [10] as well as NIAAA prioritization of the expansion of effective alcohol pharmacotherapies [11] may potentially increase treatment in primary care settings. However, unlike with other chronic conditions (e.g. asthma, diabetes), barriers to integrating alcohol pharmacotherapies in primary care settings persist. Health care providers’ unfamiliarity with these medications and their effectiveness, poor patient adherence to medication regimens, concerns about potential side effects, and medication costs are all plausible factors contributing to alcohol pharmacotherapies’ underutilization [12–14]. Furthermore, these settings may lack formal protocols, healthcare personnel, and infrastructure to expand psychosocial interventions or patient education.
Efforts to improve adherence to alcohol treatment led to the development of an extended-release naltrexone formulation (XR-NTX), which gained FDA approval for the treatment of alcohol dependence in 2006 [15]. Despite the potential benefits of injectable naltrexone in eliminating daily adherence obstacles and yielding a more predictable plasma concentration compared to oral naltrexone (O-NTX) [16], few studies have demonstrated the efficacy of XR-NTX among adults with AUDs [17], and none have compared its effectiveness versus O-NTX. In an earlier pilot study conducted at this same site, a single-arm evaluation of XR-NTX in monthly primary care medical management (MM) of alcohol dependence, we demonstrated good acceptability/feasibility, high rates of retention, and significant decreases in daily and heavy drinking [18].
To inform the expansion of these pharmacotherapies, this trial evaluates the relative clinical effectiveness of XR-NTX versus O-NTX in combination with Medical Management in a “real-world” primary care setting. Patient-level characteristics associated with effectiveness in both arms are identified to inform treatment choice. In 2010, alcohol use disorders cost the U.S. economy approximately $249 billion [19], which presents a staggering, but likely avoidable, economic burden. This study is the first to rigorously analyze the cost benefit associated with each form of naltrexone.
2. Research design and study population
2.1. Study design
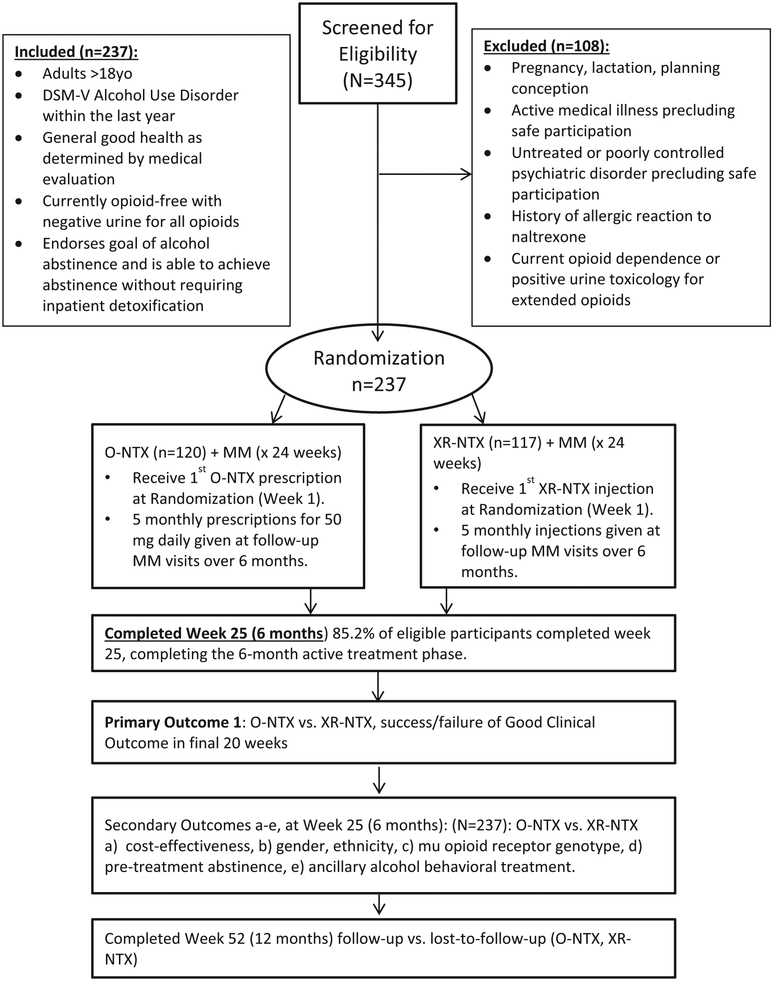
XON is an N = 237 open-label, 24 week randomized clinical trial assessing the effectiveness of XR-NTX (n = 117) vs. O-NTX (n = 120) among adults diagnosed with AUD in primary care (Fig. 1). The primary study aim is a binary measure of success (yes/no) in achieving a Good Clinical Outcome defined by abstinence or moderate drinking (≤2 drinks/day, men; ≤1 drink/day, women; and ≤ 2 heavy drinking occasions/month) during the final 20 of 24 weeks of study treatment. Secondary aims measure: the incremental cost-effectiveness of XR-NTX vs. O-NTX, drinking measures, medication treatment adherence, patient-level predictors of effectiveness, and other study-related measures and events (i.e. HIV sexual risk scores, depression ratings, and safety events).
Fig. 1.
Study flow.
2.2. Research questions and hypotheses
This study is primarily interested in whether an AUD adult population assigned to XR-NTX will exhibit higher rates of abstinence or moderate drinking compared to those assigned O-NTX. We hypothesize that individuals receiving XR-NTX will demonstrate less drinking overall and achieve a Good Clinical Outcome at twice the rate of O-NTX individuals. Of additional interest is the cost effectiveness of XR-NTX relative to O-NTX, and we hypothesize that XR-NTX will be more cost-effective than O-NTX. Additionally, in a more exploratory fashion, drinking measures, medication treatment adherence, patient-level predictors of effectiveness such as gender, ethnicity, mu opioid receptor genotype, pre-treatment abstinence, ancillary alcohol behavioral treatment involvement that may be associated with treatment effectiveness, and other study-related measures and events will be examined. These analyses will be exploratory, as the study is not powered to adequately test these multiple and secondary hypotheses.
2.3. Study setting
Eligible participants are recruited from in-clinic referrals and community advertisements. All study visits take place at Bellevue Hospital Center in New York City. Study Medical Management visits are structured as ambulatory care outpatient new and existing patient encounters and take place in the Adult Primary Care clinic, or at the NYU-H + H Clinical Translational Science Institute if additional clinical space is required. Study clinicians are all Internal Medicine physicians; some are credentialed in Addiction Medicine.
2.4. Study population and inclusion/exclusion criteria
To maximize a ‘real-world’ comparative effectiveness design, this pragmatic trial has few exclusion criteria and attempts to recruit a comprehensive population of adults with AUDs. Eligible participants for both randomized arms are: 1) adults 18 years of age or older, 2) DSM-V diagnosis of alcohol use disorder as determined by the study physician and a standard DSM-V checklist; 3) able to provide informed consent in English or Spanish; and 4) endorse a goal of alcohol abstinence without requiring inpatient detoxification. Exclusion criteria for both arms are: 1) current opioid dependence or opioid-positive urine toxicology; 2) pregnancy or female and planning conception; 3) allergy to naltrexone or its diluent; 4) severe liver disease, liver failure, or liver function test (LFT) levels greater than three times the upper limit of normal; and 5) other severe or untreated medical or psychiatric illness potentially exacerbated by participation in treatment.
2.5. Recruitment procedures by study visits
Recruitment is participant or provider-initiated and takes place through in-clinic referrals of existing primary care patients or through community recruitment of new participants who are not currently active in the site’s primary care practice. Employing similar methods as our prior pilot study [18], our recruitment success relies on a blend of in-network physicians and local community outreach advertisements. Individuals interested in the study call an intake number and are pre-screened by a research coordinator to determine prospective eligibility, after which an initial in-person screening visit is scheduled if eligibility seems likely based on phone-screen. Current participants can refer friends or acquaintances through word-of mouth aided by IRB-approved study business cards and flyers.
2.6. Informed consent
The in-person, two-part screening visit includes obtaining informed consent and completing diagnostic study procedures. Screening visits take place at Bellevue Hospital Center’s Adult Primary Care clinic or Clinical Translational Science Institute. Per standard Good Clinical Practice guidelines, study staff facilitates a discussion of potential risks, benefits and voluntary, confidential study participation. Interested participants must sign the informed consent form and complete a consent quiz to proceed. The consent quiz is implemented in order to ensure the participants’ understanding of study rationale, risks, procedures, and voluntary participation and is increasingly standard practice in addiction clinical research [20]. Any incorrect answers on the consent quiz prompt additional discussion and re-testing until all answers on quiz are correct.
2.7. Screening, randomization, and follow-up procedures
After informed consent is completed, study staff conducts a diagnostic interview that includes assessment forms, urine toxicology testing, blood alcohol content breathalyzer testing, physical exam, medical and psychiatric history review, and liver function testing. After eligibility and lab results are confirmed, a randomization visit is scheduled as soon as possible, typically within 8 weeks of screening. Randomization is stratified by gender and recruitment status (internal primary care vs. community referrals), 1:1 XR-NTX: O-NTX, in randomly permuted blocks of sizes 2 and 4. Sealed envelopes were prepared by a statistician independent of the study and are opened sequentially by study staff at the randomization visit.
The randomization visit signifies official enrollment in the study for both arms (day 1, week 1) and includes both a Medical Management (MM) medication induction visit and further baseline assessments. Ambulatory care follow-up visits at weeks 3–25 consist of MM sessions, medication refills, and brief research assessments. These visits occur biweekly for the first 9 weeks, then monthly thereafter. Longer research-only assessment visits occur at weeks 13, 25, and 48 and may be conducted by telephone or independent of the same week’s MM visit. In alignment with an aim to replicate “real-world” primary care, treatment and medication are free, but this study is not otherwise heavily incentivized. Participants are paid at 5 of the total 13 study visits for a maximum of $340 during the 52-week study. All participants are made aware of this incentive schedule at the initial screening visit. The 24-week treatment phase constitutes the active trial phase, and marks the endpoint for the primary Good Clinical Outcome. Thereafter, participants are followed for 24 weeks to gather data on treatment outcomes and safety information.
3. Data management
Written and electronic data entry and data management uses the REDCap (Research Electronic Data Capture) [21] platform on a secure New York University School of Medicine server. Data management and study staff review reports for data integrity and address data cleaning tasks in real-time. A final data cleaning will be completed following the last subject visit, followed by data lock and analysis.
4. Regulatory affairs and data and safety monitoring
4.1. Approvals and certifications
NYU School of Medicine’s Institutional Review Board (IRB) approved the study protocol. Public clinical trial registration was completed on ClinicalTrials.gov (). A Federal Certificate of Confidentiality was obtained to prevent disclosure of individual’s study data.
4.2. Data safety monitoring board
The Data Safety Monitoring Board (DSMB) ensures that all data, protocol compliance, and safety standards are compliant with Good Clinical Practices. The DSMB convenes annually, and includes closed sessions with or without the research team and an executive closed DSMB session. The study’s principal investigator and site IRB will monitor local recruitment, retention, and safety outcomes, and ongoing DSMB meetings include reviews of these topics. Site and independent outside monitors annually review procedures and data quality. Adverse events (AEs) and serious adverse events (SAEs) are logged in sequential, open-ended fashion during monthly assessments using AE and SAE logs. AEs and SAEs are determined to be medication-related by the study clinician and/or site PI. The study team reports medication-related SAEs to the local IRB, the DSMB, study sponsor (NIAAA), and FDA.
5. Study interventions
5.1. Medical management treatment visits
Medical Management (MM) counseling provides support and assessment for common, anticipated side effects (including nausea, headache, and fatigue following the initial dose of O-NTX or X-NTX), community treatment and recovery participation, and risk reduction for relapse that mimics a typical ambulatory care office visit. Study physicians are all adult primary care staff board-certified in internal medicine. The content of the MM component is the same in both arms, and is based on the initial and follow-up MM visits outlined in the COMBINE MM manual and adapted by the NIAAA Clinician’s Guide [11]. MM emphasizes: a) education surrounding the alcohol dependence diagnosis, b) a recommendation and emphasis on drinking abstinence, c) support for 12-step involvement (referrals to specialty outpatient treatment will not be part of the MM strategy – patients interested in such will not be prohibited from self-referral, and specialty referrals will be made in cases of relapse/treatment failure), d) self-efficacy counseling surrounding medication adherence, e) education and trouble-shooting of medication side effects, f) feedback on the success of drinking reductions, and g) non-specific support and motivational enhancement to make further changes toward abstinence. After the 24-week active trial phase, all participants are advised to continue appropriate aftercare and are encouraged to continue as patients in the primary care clinic.
5.2. O-NTX medical management treatment visits
Participants randomized to O-NTX will receive a one-month prescription of oral naltrexone (50 mg daily dose) following each monthly MM visit provided by the facility’s pharmacy. Alternatively, participants may obtain a written or electronic prescription provided by the study physician to an outside pharmacy of their choice.
5.2.1. XR-NTX medical management treatment visits
Participants randomized to XR-NTX receive a single, 380 mg intramuscular injection to the upper outer gluteal (buttock) quadrant at each monthly MM visit provided by the facility’s pharmacy. The study physician administers subsequent injections to alternating upper outer gluteal quadrants.
6. Assessments
The schedule of visits and assessment instruments is summarized in Table 1. Medication are dispensed (XR-NTX injections or O-NTX prescriptions) at each monthly MM visit following randomization (weeks 5, 9, 13, 17, and 21). Research-only assessments are conducted at weeks 13 and 25 during the treatment phase. The week 25 visit concludes the treatment phase for all participants and a final study visit occurs at week 48 for research-only purposes for all participants.
Table 1.
Schedule of assessments and procedures.
| Assessments | Baseline | Treatment | Research-only visits | Safety visit | ||
|---|---|---|---|---|---|---|
| Bi-weekly | Monthly | Month 3 & 6 | Month 3, 6, & 12 | |||
| (Weeks) | (0/1a) | (3,7) | (5,9,17,21)a | (13a, 25) | (13,25,48) | (26) |
| DSM-V AUD | X | |||||
| Informed consent | X | |||||
| Inclusion/exclusion | X | |||||
| Consent quiz | X | |||||
| Urine toxicology | X | X | X | |||
| Pregnancy test | X | X | X | |||
| Blood alcohol Breathalyzer Test | X | X | X | |||
| Serum (AST, ALT, GGT, %CDT) | X | X | ||||
| Serum (“G” mu opioid allele) | X | |||||
| Vitals | X | X | X | X | ||
| Demographics | X | |||||
| Timeline follow back | X | X | X | X | X | X |
| Med/psych history checklist | X | |||||
| RAB (modified) | X | X | ||||
| Drinking severity (AUDIT and OCDS) | X | X | ||||
| Charlson comorbidity index | X | |||||
| Economic form 90 | X | X | ||||
| ASSIST | X | |||||
| PHQ-9 | X | X | X | |||
| WHOQOL | X | X | ||||
| AE/SAE | X | X | X | X | X | |
| MMAS-8 O-NTX adherenceb | X | X | X | |||
| O-NTX dispensation and tracking formb | X | X | X | |||
| XR-NTX administrationc | X | X | X | |||
| Injection site abnormalityc | X | X | X | |||
| Alcohol good clinical outcome | X | X | X | X | ||
Medication dispensation visits.
O-NTX arm only.
XR-NTX arm only.
6.1. Primary outcome
This study’s primary outcome is the success or failure of a dichotomous Good Clinical Outcome (GCO) defined as two or fewer heavy drinking days during each 4-week block spanning weeks 5–24 of the 24-week treatment phase. Heavy drinking is defined as 5 or more drinks per occasion (men) and 4 or more (women). Each of these 5 months (months 2–6 of study treatment) is noted as GCO+/− based on self-report on the timeline follow-back (TLFB) [22] drinking calendar source document. A participant needs to report drinking below this level for each successive month 2–6 to qualify for the overall ‘GCO-success’ primary outcome. One or more ‘GCO-negative’ months during this month 2–6 period would disqualify the participant as ‘GCO-success’ (the primary outcome would be designated as negative or ‘failure’).
6.2. Secondary outcome
Secondary outcomes of interest include: a) the incremental cost effectiveness of O-NTX vs. XR-NTX; b) continuous measures of alcohol drinking rates (including: percentage of days abstinent, percentage of heavy drinking days, mean drinks per day, time to first drink, time to first heavy drinking day, and biomarkers); and c) exploratory analysis of factors possibly associated with effectiveness, such as gender, pre-treatment abstinence, baseline drinking severity, and mu opioid receptor (OPRM1) genotypes. The study also evaluates group differences in medication treatment adherence, study retention, treatment services utilization, HIV sexual risk, depression ratings, and safety events.
The Economic Form 90 [23] and World Health Organization Quality of Life Survey (WHOQOL) [24] are used to assess economic data for cost-effectiveness outcome measures. The timeline follow-back (TLFB), Alcohol Use Disorders Identification Test (AUDIT) [25] and Obsessive-Compulsive Drinking Scale (OCDS) [26] are all validated measures used to identify alcohol and drug use along with regular urine toxicology and breathalyzer tests. The Alcohol, Smoking and Substance Involvement Screening Test (ASSIST) [27] is used to assess for substance abuse or dependence. The Seek, Test, Treat and Retain (STTR) measures collect demographics. The Patient Health Questionnaire (PHQ-9) [28] is used to assess for patient’s depression severity. The Morisky Medication Adherence Scale (MMAS-8) [29,30] is administered to the O-NTX arm only, providing information on adherence to self-administered medications at monthly visits. Liver function tests (aspartate transaminase [AST], alanine transaminase [ALT], gamma-glutamyl transferase [GGT] and carbohydrate-deficient transferrin [CDT]) is collected every three months (weeks 0/1, 13, and 25), with AST, ALT, and GGT results available in real-time to study physicians to inform clinical care and counseling.
7. Statistical analysis
7.1. Primary aim: a good clinical outcome
An intention-to-treat (ITT) comparison of all randomized participants will compare the probabilities of a Good Clinical Outcome for the XR-NTX and O-NTX treatment groups. The analysis will utilize a logistic regression with indicator variables for treatment and randomization strata (gender and referral source). We will fit an initial model that includes interaction terms to test if effect differs across strata. If the tests for interaction are not significant, we will fit a simpler model of that assumes an overall, homogenous treatment effect. Odds ratios will be calculated to quantify the risk to treatment failure of significant predictors. In the event of a missed visit or dropout, missing TLFB days will be assumed to have resumed baseline heavy drinking rates and therefore will be determined as Good Clinical Outcome failures and will contribute to the relapse primary outcome. An instrumental variable analysis will be implemented to assess the effect of adhering to study drug. Continuous measures of alcohol consumption will also be analyzed. In addition, we plan multiple secondary per protocol, or as-treated, analyses, focusing on participants successfully exposed to both study medication and those completing the recommended 24-week treatment phase. We will use instrumental variable or principal stratification methods to make unbiased estimates.
7.2. Secondary aim 1: cost-effectiveness
We will estimate the incremental cost-effectiveness of XR-NTX compared to O-NTX. The incremental cost-effectiveness is the ratio of incremental costs to incremental benefits. More specifically, it is equal to (Cost of XR-NTX minus Cost of O-NTX) divided by (Effectiveness of XR-NTX minus Effectiveness of O-NTX), and requires estimates of costs and effectiveness. Consequently, primary data analyses from this study (Specific Aim 1 in the proposal) will inform costs and effectiveness estimates in the short-term, and will be synthesized with published reports that allow us to estimate the longer-term, downstream costs and effects of the changes in alcohol consumption observed in Specific Aim 1.
Cost assessment will be conceptually similar to recently published analyses of the COMBINE interventions, and will include medical and non-medical costs as well as payer and societal costs. Effectiveness assessments will include alcohol consumption measures as described earlier, as well as quality of life using the World Health Organization Quality of Life Survey (WHO-QOL) [24]. We will calculate mean intervention-specific and overall costs of care by intervention arm from the societal and healthcare system perspectives separately [31]. We will estimate mean costs per subject for the intervention and 1 year of follow-up for each trial arm (H2a) using simple means and estimated mean costs using multivariable generalized linear models with a log link and gamma distribution [32].
7.3. Longer-term estimates of costs and effectiveness
To estimate the comparative effectiveness of XR-NTX relative to ONTX over longer time horizons than are reflected by our data, we will incorporate these short-term results within a state-transition (Markov) computer simulation of outcomes that enables a cohort of hypothetical alcohol-dependent patients to be followed over time until death, and to be exposed to alternative alcohol interventions for specified time durations beyond the week 24 assessment. The simulation will represent alcohol-related events that have a particularly important influence on health and/or resource utilization, including chronic liver disease, alcohol-related cancers (eg, cervical, colorectal, breast, lung, prostate), and traumatic injuries (eg, unintentional injuries, suicide, homicide). The simulation will be able to aggregate the lifetime benefit of remitting alcohol dependence for designated periods of time using trial endpoints (e.g. frequency of heavy drinking days), and also can quantify benefits of the levels of improvement short of full remission (e.g. if alcohol dependence remits but the patient still is an at-risk drinker). The simulation will estimate lifetime costs and benefits over a lifetime horizon, measured in both life-years and quality-adjusted life-years. (Quality-adjusted life years are a preference-based, quality of life metric that considers quality of life simultaneously with quantity of life, and instantiates the notion that a typical person would trade away some quantity of life to get a greater quality of life.) In sensitivity analyses we will consider time horizons shorter than lifetime (10 year and 20 year) because these horizons are sometimes preferred by decision makers, even though the longer, lifetime horizon is advocated by the Panel on Cost-Effectiveness in Health and Medicine.
7.4. Secondary aim 2: patient-level predictors of effectiveness
To differentiate time to a Good Clinical Outcome failure, we will use a “cure model.” The form of a cure model is H(t) = 1 − p + pS(t), where H(t) is the probability of failure at a time greater than t, p represents the probability of failure, and S(t) the distribution of time to failure, conditional on failure occurring. The parameters will be estimated using both non-parametric Kaplan Meier methods and alternative parametric methods. The equality of the values of p for the two treatment arms will be tested using a nonparametric likelihood ratio test. For the parametric test, logistic regression will be used to model p and a Weibull survival distribution will be used to model time to failure, S(t).
Both models allow the use of covariates. We will expand the primary outcome regression models to include additional predictors and interaction terms to identify specific patient characteristics that might be associated with naltrexone’s effectiveness in either the oral or XR formulation, including gender, ethnicity, pre-treatment abstinence, voluntary specialty alcohol treatment and Alcoholics Anonymous involvement (the study does not require ancillary psychosocial treatment or 12-step involvement), and Asp40 OPRM1 SNP status.
7.5. Missing data
Maximum efforts in terms of outreach, participant contact, and, if optimal, telephone research visit and remote data collection are used to minimize missing data. For the GCO primary outcome, which relies on self-reported drinking Timeline Followback, a negative GCO is assumed only if TLFB data is entirely missing through the week 48 research visit window. Participants do not need to be present at MM or research visits, or active on study treatment, so long as TLFB self-reported drinking recall is eventually completed by the week 48 window. While most TLFB data is expected to be collected in-person on a monthly basis, the study allows for less frequent recall over longer intervals. For example, a participant could randomize to either medication, miss all scheduled in-person study visits, complete a week 48 research assessment visit by telephone, report minimal or no drinking during the 48 weeks, and accrue a GCO for all months 1–6. For secondary outcomes, including self-reported drinking rates, self-reported adherence, laboratory values, and adverse events, missing data will be analyzed as both missing-as-missing and missing-at-random.
8. Sample size, power, and effect size
A projected randomized clinical trial sample targeted 234 participants randomized 1:1 to receive XR-NTX (n = 117) vs. O-NTX (n = 117). Our recent feasibility study of XR-NTX in primary care [18], Roozen’s single review comparing the two medications [33], and the ONTX treatment retention and adherence literature [34–36], is the basis for powering our current study on the assumption that XR-NTX is approximately twice as effective as O-NTX at achieving drinking reductions. Results from our pilot data indicated that 36 of 40 persons completing 12 weeks of XR-NTX achieved the moderate-drinking-only Good Clinical Outcome, and findings from the COMBINE study showed adherent oral naltrexone/MM patients with high rates of the same moderate-drinking-only Good Clinical Outcome during the 8 of 16 study weeks [37]. From our pilot study data, the current literature, and commercial refill information, we predict that 40–50% of patients will have a Good Clinical Outcome after 24 weeks of treatment on XR-NTX, compared to < 20% on O-NTX, roughly the same as adherence rates [18]. With 100 subjects per group, we anticipate a power of 0.84 to detect a 20% absolute difference in the primary outcome based on the Fisher’s exact test. These projected rates, however, are not well-established, nor are they based on large datasets of heterogeneous alcohol populations. Therefore we have increased the target screening to n = 468, with a very conservative assumption of 2 subjects screened for every one enrolled, for a target sample of N = 234 and N = 117 per arm, which increases the projected power to 0.90.
9. Results
Recruitment began in July of 2014 and ended in August of 2017. Our final accruals are N = 237, XR-NTX (n = 117) and O-NTX (n = 120). Our site has obtained consent from 345 participants and successfully randomized 237 eligible participants: 117 to XR-NTX and 120 to O-NTX. Of the 345 consented participants, 108 were excluded from randomization after failing to meet inclusion criteria.
The study recruited a primarily male, ethnic minority population. At baseline, mean AUDIT and OCDS scores indicated the overall study population had hazardous and harmful patterns of alcohol use. Mean drinks per day over the last 28 days was 10.3 for XR-NTX and 9.1 for ONTX (Table 2), demonstrating daily heavy drinking for participants in both groups.
Table 2.
Demographics.
| XR-NTX | O-NTX | |
|---|---|---|
| (n = 117) | (n = 120) | |
| # (%) | # (%) | |
| Male | 82 (70%) | 84 (70%) |
| Age, mean (SD) | 48 (10.6) | 49 (10.7) |
| Hispanic | 24 (21%) | 27 (23%) |
| Non-hispanic black | 67 (57%) | 62 (52%) |
| Non-hispanic white | 30 (26%) | 44 (37%) |
| High school graduate or higher | 100 (85%) | 99 (83%) |
| Employed (full or part-time) | 64 (55%) | 72 (60%) |
| AUDIT scores, mean (SD) | 24.6 (8.3) | 23.6 (7.8) |
| Obsessive compulsive drinking scores, mean (SD) | 17.7 (8.5) | 16.4 (7.5) |
| Current daily drinking, average drinks per day in the last 28 days (SD) | 10.3 (12.5) | 9.1 (10.7) |
Retention in study visits across the study has been high: visit 2 (64%), visit 3 (66%), visit 4 (38%), visit 5 (55%), visit 6 (80%), research-only visit 1 (89%), visit 7 (55%), visit 8 (56%), visit 9 (75%), research-only visit 2 (85%), safety visit (51%), and research-only visit 3 (81%). Thus far, 171 participants have completed the study, 24 are in long-term follow-up, and 42 participants have been lost to follow-up, XR-NTX (n = 17) and O-NTX (n = 25).
10. Discussion
XON, a pragmatic, open-label, randomized controlled trial, is to our knowledge the first large-scale RCT to compare the effectiveness of XRNTX to O-NTX among adults with alcohol use disorders in relatively simple, low threshold, low intensity primary care Medical Management paradigm. Results to date have been characterized by robust rates of recruitment and accruals, with the overall randomized sample on target and enrolled on schedule. In addition, we have noted high rates of follow-up and data completeness throughout the trial. The randomized sample appears representative of community-dwelling adults with alcohol use disorders in New York City, and is characteristic of under-served populations who are more likely to access care at a public hospital primary care setting. As reported by the New York City Department of Health and Mental Hygiene in 2014, New York City binge drinkers were primarily male (59%), ethnic minorities (55%), living in neighborhoods with medium to high poverty levels (61%) [38].
Hazardous and heavy drinking adults and those with moderate-to-severe alcohol use disorders are highly likely to be seen in general health care settings, versus specialty drug-alcohol treatment programs, yet alcohol medication treatments such as naltrexone have been underutilized in primary care and general behavioral health settings [8,39]. Health insurance expansion, parity reforms, and the growth in medical home and accountable care organizational approaches remain on track to potentially re-define primary care as a first-line alcohol treatment setting. Findings from comparative effectiveness studies such as XON are uniquely positioned to guide treatment protocols and resource allocation.
Our current trial is innovative both as a ‘head-to-head’ evaluation of XR-NTX vs. O-NTX in primary care, and as a study that does not preclude participation of primarily Medicaid-covered or uninsured participants based on their medical and psychiatric comorbidities, which is often the case in efficacy trials. We hypothesize that a slow-release, injectable preparation of naltrexone would overcome barriers to oral naltrexone adherence. Alternatively, some patients may over time resist repeated monthly IM injections, versus the ease of at home pill taking. We prioritize a binary Good Clinical Outcome of moderate drinking or abstinence only to anchor the study on a clinically meaningful outcome with face validity to both patients and providers. The Good Clinical Outcome is based on reducing heavy drinking days and does not require complete abstinence. Epidemiologic data consistently associate increased drinking levels with increased health problems, including cancers, cognitive impairment, liver disease, stroke, and depression [4,9]. Total abstinence is likely not required to achieve meaningful health gains and alcohol-related risk reduction during naltrexone treatment.
Past research studies have called for a rigorous cost-effectiveness analysis of XR-NTX and primary care MM compared to oral naltrexone, since XR-NTX is substantially more expensive than O-NTX (~$1100 vs. ~$100 per month) [40]. A study commissioned by Alkermes, the manufacturer of XR-NTX, showed that XR-NTX could be more economically effective compared to O-NTX, based off of reduced usage of expensive emergency and detox services [41]. We are attempting to independently and more rigorously examine these cost issues using a prospective randomized design, which includes long-term outcomes one year after beginning treatment.
This study has several strengths, including assessment of multiple domains of patient-level characteristics, and a large patient/provider-initiated sample with few exclusion criteria, which lends to its credibility as a relatively representative sample of individuals with alcohol use disorders seeking community-based treatment across the US. It also has several limitations. This open-label effectiveness trial lacks a placebo control group and blinding to both treatment assignment and primary/secondary outcomes, which makes recall and assessment biases more likely than in a placebo-controlled trial. Further, our recruited sample has a substantial proportion of African American adults, which is generalizable to the general population of underserved New Yorkers with alcohol use disorders, but among whom it has been speculated naltrexone would have diminished or no effects due primarily to lower rates of the mu receptor ‘G’ allele [42]. We note however that during the course of this study, results from another RCT evaluating the impact of mu receptor polymorphism resulted in no effects – which suggests that predicting which patients will respond best to naltrexone in any formulation based on allele status is ineffective [43].
Disseminating an expensive medication for free and financially incentivizing participants are traditional clinical trial designs and may bias findings from our optimal pragmatic trial strategy. In an attempt to ‘de-link’ cost, utilization, and longitudinal drinking data from a naturalistic observation of usual care patterns, we conduct three Research-only Assessment visits separately from the scheduled MM treatment visits. These visits do not hinge on a participant’s treatment retention status, are heavily incentivized to encourage participation ($100 for time and travel), and are conducted whenever possible in-person but also by phone if a participant is otherwise unwilling or unable to appear in clinic. To minimize the influence of extra assessments, attention, and the monetary research participation incentive on a participant’s willingness to continue with treatment, the MM treatment visits are not incentivized beyond the provision of no-cost care and free study medication.
In summary, the delivery of XR-NTX versus O-NTX using a Medical Management primary care treatment model offers an innovative and potentially cost-effective approach to reducing the burden of AUDs in primary care settings. This trial will describe the clinical impact of XRNTX versus O-NTX, defined as a dichotomous Good Clinical Outcome (i.e., abstinence or moderate drinking). Secondary outcomes will compare cost effectiveness measures and will explore patient characteristics that may be associated with treatment effectiveness (e.g., gender, pre-treatment abstinence, and mu opioid receptor (OPRM1) genotypes). Findings from this study will have important implications for health systems that seek to leverage primary care settings and effective alcohol pharmacotherapies for populations with alcohol use disorders.
Funding source
This work was supported by the National Institute on Alcohol Abuse and Alcoholism [R01AA020836].
Footnotes
Conflicts of interest
We wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
References
- [1].Substance Abuse and Mental Health Services Administration, Key substance use and mental health indicators in the United States: Results from the 2016 National Survey on Drug Use and Health, HHS Publication No. SMA 17–05==5044, NSDUH Series H-52, 2017. https://www.samhsa.gov/data/, Accessed date: 4 June 2018. [Google Scholar]
- [2].Mann K, Schafer DR, Langle G, Ackermann K, Croissant B, The long-term course of alcoholism, 5, 10 and 16 years after treatment, Addict. 100 (6) (2005) 797–805, 10.1111/j.1360-0443.2005.01065.x. [DOI] [PubMed] [Google Scholar]
- [3].Rivara FP, Garrison MM, Ebel B, McCarty CA, Christakis DA, Mortality attributable to harmful drinking in the United States, 2000, J. Stud. Alcohol 65 (4) (2004) 530–536, 10.15288/jsa.2004.65.530. [DOI] [PubMed] [Google Scholar]
- [4].Hasin DS, Dasin FS, Ogburn E, Grant BF, Prevalence, correlates, disability, and comorbidity of DSM-IV alcohol abuse and dependence in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions, Arch. Gen. Psychiatry 64 (7) (2007) 830–842, 10.1001/archpsyc.64.7.830. [DOI] [PubMed] [Google Scholar]
- [5].Mertens JR, Weisner C, Ray GT, Fireman B, Walsh K, Hazardous drinkers and drug users in HMO primary care: prevalence, medical conditions, and costs, Alcohol. Clin. Exp. Res 29 (6) (2005) 989–998, 10.1097/01.ALC.0000167958.68586.3D. [DOI] [PubMed] [Google Scholar]
- [6].Teesson M, Baillie A, Lynskey M, Manor B, Degenhardt L, Substance use, dependence and treatment seeking in the United States and Australia: a cross-national comparison, Drug Alcohol Depend. 81 (2) (2006) 149–155, 10.1016/j.drugalcdep.2005.06.007. [DOI] [PubMed] [Google Scholar]
- [7].Jonas DE, Amick HR, Feltner C, Bobashev G, Thomas K, Wines R, et al. , Pharmacotherapy for adults with alcohol use disorders in outpatient settings a systematic review and meta-analysis, JAMA 311 (18) (2014) 1889–1900, 10.1001/jama.2014.3628. [DOI] [PubMed] [Google Scholar]
- [8].Berglund M, Thelander S, Salaspuro M, Franck J, Andreasson S, Ojehagen A, Treatment of alcohol abuse: an evidence-based review, Alcohol Clin. Exp. Res 27(10) (2003) 1645–1656, 10.1097/01.ALC.0000090144.99832.19. [DOI] [PubMed] [Google Scholar]
- [9].Pettinati HM, Weiss RD, Miller WR, Donovan D, Ernst DB, Rounsaville BJ, Medical Management Treatment Manual: A Clinical Research Guide for Medically Trained Clinicians Providing Pharmacotherapy as Part of the Treatment for Alcohol Dependence, DHHS Publication No. (NIH) 04–5289, COMBINE Monograph Series Volume 2 NIAAA, Bethesda, MD, 2004. [Google Scholar]
- [10].Moyer VA, And preventive services task force, screening and behavioral counseling interventions in primary care to reduce alcohol misuse: U.S. preventive services task force recommendation statement, Ann. Intern. Med 159 (3) (2013) 210–218, 10.7326/0003-4819-159-3-201308060-00652. [DOI] [PubMed] [Google Scholar]
- [11].N.I.A.A.A, 5 Year Strategic Plan FY17–21, https://www.niaaa.nih.gov/sites/default/files/StrategicPlan_NIAAA_optimized_2017-2020.pdf, (2017) (accessed 4 June 2018). [Google Scholar]
- [12].Oliva EM, Maisel NC, Gordon AJ, Harris AHS, Barriers to use of pharmacotherapy for addiction disorders and how to overcome them, Curr. Psychiatry Rep 13 (5) (2011) 374–381, 10.1007/s11920-011-0222-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Roman PM, Abraham AJ, Knudsen HK, Using medication-assisted treatment for substance use disorders: evidence of barriers and facilitators of implementation, Addict. Behav 36 (6) (2011) 584–589, 10.1016/j.addbeh.2011.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Weiss RD, Adherence to pharmacotherapy in patients with alcohol and opioid dependence, Addiction 99 (11) (2004) 1382–1392. [DOI] [PubMed] [Google Scholar]
- [15].Naccari C, FDA approves naltrexone for extended-release injectable suspension for the treatment of alcohol dependence, Cns Spectrums 11 (5) (2006) 345–345. [Google Scholar]
- [16].Dunbar JL, Turncliff RZ, Hayes SC, Farrell CB, Population pharmacokinetics of extended-release injectable naltrexone (XR-NTX) in patients with alcohol dependence, J. Stud. Alcohol Drugs 68 (6) (2007) 862–870, 10.15288/jsad.2007.68.862. [DOI] [PubMed] [Google Scholar]
- [17].Kranzler HR, Wesson DR, Billot L, DrugAbuse sciences naltrexone depot study group, naltrexone depot for treatment of alcohol dependence: a multicenter, randomized, placebo-controlled clinical trial, Alcohol. Clin. Exp. Res 28 (7) (2004) 1051–1059, 10.1097/01.ALC.0000130804.08397.29. [DOI] [PubMed] [Google Scholar]
- [18].Lee JD, Grossman E, DiRocco D, Truncali A, Hanley K, Stevens D, et al. , Extended release naltrexone for treatment of alcohol dependence in primary care, J. Subst. Abus. Treat 39 (1) (2010) 14–21, 10.1016/j.jsat.2010.03.005. [DOI] [PubMed] [Google Scholar]
- [19].Bouchery EE, Hardwood HJ, Sacks JJ, Simon CJ, Brewer RD, Economic costs of excessive alcohol consumption in the U.S., 2006, Am. J. Prev. Med 41 (5) (2011) 516–524, 10.1016/j.amepre.2011.06.045. [DOI] [PubMed] [Google Scholar]
- [20].Kiluk BD, Nich C, Carroll KM, Neurocognitive indicators predict results of an informed-consent quiz among substance-dependent treatment seekers entering a randomized clinical trial, J. Stud. Alcohol Drugs 71 (5) (2010) 704–712, 10.15288/jsad.2010.71.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG, Research electronic data capture (REDCap) - a metadata-driven methodology and workflow process for providing translational research informatics support, J. Biomed. Inform 42 (2) (2009) 377–381, 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Hoeppner BB, Stout RL, Jackson KM, Barnett NP, How good is fine-grained timeline follow-back data? Comparing 30-day TLFB and repeated 7-day TLFB alcohol consumption reports on the person and daily level, Addict. Behav 35 (12) (2012) 113843, , 10.1016/j.addbeh.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Bray JW, Zarkin GA, Miller WR, Mitra D, Kivlahan DR, Martin DJ, et al. , Measuring economic outcomes of alcohol treatment using the economic form 90, J. Stud. Alcohol Drug 68 (2) (2015), 10.15288/jsad.2007.68.248. [DOI] [PubMed] [Google Scholar]
- [24].The WHOQOL Group, Development of the World Health Organization WHOQOLBREF quality of life assessment, Psychol. Med 28 (1998) 551–558, 10.1017/S0033291798006667. [DOI] [PubMed] [Google Scholar]
- [25].Bohn MJ, Babor TF, Kranzler HR, The alcohol use disorders identification test (AUDIT): validation of a screening instrument for use in medical settings, J. Stud. Alcohol Drug 56 (4) (2005) 423–432, 10.15288/jsa.1995.56.423. [DOI] [PubMed] [Google Scholar]
- [26].Anton RF, Moak DH, Latham P, The obsessive compulsive drinking scale: a self-rated instrument for the quantification of thoughts about alcohol and drinking behavior, Alcoholism: Clin. Exp. Res 19 (1) (1995) 92–99, 10.1111/j.1530-0277.1995.tb01475.x. [DOI] [PubMed] [Google Scholar]
- [27].WHO ASSIST Working Group, The alcohol, smoking and substance involvement screening test (ASSIST): development, reliability and feasibility, AddictionAddict 97 (2002) 1183–1194, 10.1046/j.1360-0443.2002.00185.x. [DOI] [PubMed] [Google Scholar]
- [28].Lowe B, Kroenke K, Herzog W, Grafe K, Measuring depression outcome with a brief self-report instrument: sensitivity to change of the patient health questionnaire (PHQ-9), J. Affect. Disord 81 (1) (2004) 61–66, 10.1016/S0165-0327(03)00198-8. [DOI] [PubMed] [Google Scholar]
- [29].Tan X, Patel I, Chang J, Review of the four item morisky medication adherence scale (MMAS-4) and eight item Morisky medication adherence scale (MMAS-8), Innov. Pharm 5 (3) (2014) 165, , 10.24926/iip.v5i3.347. [DOI] [Google Scholar]
- [30].Morisky DE, et al. , Predictive validity of a medication adherence measure in an outpatient setting, J. Clin. Hypertens. (Greenwich) 10 (5) (2008) 348–354, 10.1111/j.1751-7176.2008.07572.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [31].Neumann PJ, Sanders GD, Russell LB, Siegel JE, Ganiats TG, Cost-Effectiveness in Health and Medicine, 2nd edition, Oxford University Press, New York, NY, 2017. [Google Scholar]
- [32].Basu A, Manning WG, Mullahy J, Comparing alternative models: log vs Cox proportional hazard? Health Econ. 13 (8) (2003) 749–765, 10.1002/hec.852. [DOI] [PubMed] [Google Scholar]
- [33].Roozen HG, de Waart R, van den Brink W, Efficacy and tolerability of naltrexone in the treatment of alcohol dependence: oral versus injectable delivery, Eur. Addict. Res 13 (4) (2007) 201–206, 10.1159/000104882. [DOI] [PubMed] [Google Scholar]
- [34].Kranzler HR, Stephenson JJ, Montejano S, Gastfriend Wang DR, Persistence with oral naltrexone for alcohol treatment: implications for health-care utilization, Addiction 103 (11) (2008) 1801–1808, 10.1111/j.1360-0443.2008.02345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Hermos JA, Young MM, Gagnon DR, Fiore LD, Patterns of dispensed disulfiram and naltrexone for alcoholism treatment in a veteran patient population, Alcohol. Clin. Exp. Res 28 (8) (2004) 1229–1235, 10.1097/01.ALC.0000134234.39303.17. [DOI] [PubMed] [Google Scholar]
- [36].Harris KM, DeVries A, Dimidjian K, Datapoints: trends in naltrexone use among members of a large private health plan, Psychiatric Serv 55 (3) (2014) 221, 10.1176/appi.ps.55.3.221. [DOI] [PubMed] [Google Scholar]
- [37].Anton RF, O’Malley SS, Ciraulo DA, Cisler RA, Couper D, Donovan DM, et al. , Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial, JAMA 295 (17) (2006) 2003–2017, 10.1001/jama.295.17.2003. [DOI] [PubMed] [Google Scholar]
- [38].Capua J, Tuazon E, Paone D Binge, Drinking and Associated Health-Related Behaviors among Adults in New York City, 2014, New York City Department of Health and Mental Hygiene: Epi Data Brief (77), October 2016. [Google Scholar]
- [39].Ducharme LJ, Knudsen HK, Roman PM, Trends in the adoption of medications for alcohol dependence, J. Clin. Psychopharmacol 26 (Supply 1) (2006) S13–S19, 10.1097/01.jcp.0000246209.18777.14. [DOI] [PubMed] [Google Scholar]
- [40].Lee JD, Grossman E, Huben L, Manseau M, McNeely J, Rotrosen J, et al. , Extended-release naltrexone plus medical management alcohol treatment in primary care: findings at 15 months, J. Subst. Abus. Treat 43 (4) (2012) 458–462, 10.1016/j.jsat.2012.08.012. [DOI] [PubMed] [Google Scholar]
- [41].Mark TL, Gastfriend DR, Kranzler HR, Chalk M, Montejano L, Characteristics and cost outcomes of insured patients treated with extended-release naltrexone (XR-NTX) or oral alcohol dependence medications, Value Health 13 (3) (2010), 10.1016/S1098-3015(10)72509-2A106-A106. [DOI] [Google Scholar]
- [42].Ray LA, Oslin DW, Naltrexone for the treatment of alcohol dependence among African Americans: results from the COMBINE study, Drug Alcohol Depend 105 (3) (2009. December 1) 256–258, 10.1016/j.drugalcdep.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Oslin DW, Leong SH, Lynch KG, Berrettini W, O’Brien CP, Gordon AJ,Rukstalis M, Naltrexone vs placebo for the treatment of alcohol dependence: a randomized clinical trial, JAMA Psychiatr. 72 (5) (2015) 430–437, 10.1001/jamapsychiatry.2014.3053. [DOI] [PubMed] [Google Scholar]