Growing up, Sarah spent nearly every weekend playing soccer, yet one of her most vivid childhood memories of practicing with her team has nothing to do with sports. The practices lasted several hours and were made manageable by a much-cherished snack break. One week in spring, she grabbed her water bottle after leaving the field and ran over to the snack table, as she had countless times before. She spotted a box of doughnuts on the table, and without thinking, picked up a chocolate doughnut and took a bite. Within seconds, she recoiled in horror and embarrassment at what she had done and ran to the nearest trash can to spit it out. The Jewish holiday of Passover had started several days earlier, and she was definitely not supposed to be eating doughnuts–or, for that matter, any of the other snacks. How could she have made such a mistake? It was not that she had forgotten about the holiday or was especially hungry: the problem was that the context had changed but her brain was stuck in the same weekly routine.
Moments like this happen to nearly everyone, but what makes them so shocking and memorable is that they highlight a fundamental paradox in the human experience: despite our sense of control and purpose in our lives, a significant proportion of our daily behavior is actually driven by habit. Although the degree to which habit drives human behavior is difficult to estimate, one study asked participants to record their actions every hour and found that nearly half of their actions were performed almost daily and in the same context (1). Habits serve a critical purpose in making our behavior more efficient, reducing the decision burden we face each day and freeing up mental energy for more demanding tasks. But in order to keep established habits from interfering with current needs and plans, the brain has to be able to use and switch between two different strategies: one based on habits and one based on goals.
The modern concept of habit was first clearly laid out by the psychologist William James in the late 19th century (2). He described habit as a routine, behavior, or even cognitive process that starts spontaneously but is repeated automatically as a result of prior experience. While Sarah’s instinctive reach for the doughnut was clearly based on previous experience getting snacks during breaks, what exactly led her to do it that day? Was it simply the sight of the snack table or the familiar logo on the doughnut box? Habits are context dependent; they strengthen through repetition and associations with cues from the surrounding environment such that their expression becomes dependent on the relevant cues. Once habits form, the perception of the cue (in Sarah’s case, the visual cue of the snack table) is sufficient to automatically trigger the response (1). Habits do not require much cognitive input because they are performed quickly and automatically; they also tend to be relatively inflexible. On the other hand, goal-directed behavior is performed based on predicted or expected outcomes, which allows for adaptation to changes in context.
To understand how habits are represented in the brain, one has to first be able to distinguish habitual and goal-directed strategies in a laboratory setting. Dickinson et al. (3) first achieved this by establishing two experimental paradigms in which researchers manipulated the relationship of a trained behavior to its outcome. The first paradigm is called reward devaluation and involves simply reducing the reward that follows a behavior. For example, a rat trained to press a lever in order to obtain a reward is said to have developed a habitual response using this paradigm if it continues to press the lever at the same rate even if the reward has been switched to become aversive. The second paradigm is called contingency degradation. This involves disrupting an established connection between an action and a rewarded outcome. For example, using this approach, the rat would be said to have a habitual response if it continues to press the lever at the same rate even if the reward is then provided randomly, without any correlation to the timing of the lever press. Researchers have since developed other behavioral paradigms for separating habitual and goal-directed behavior that are more effective at capturing the nuance of habitual behavior in humans (4). For example, slips-of-action tasks test whether participants can suppress previously learned responses that no longer yield valuable outcomes–essentially the same situation Sarah faced when she accidentally reached for the doughnut. Other approaches, such as sequential decision tasks, require participants to develop an internal representation of how the task is structured and respond based on this cognitive model rather than responding habitually based on the most recent response.
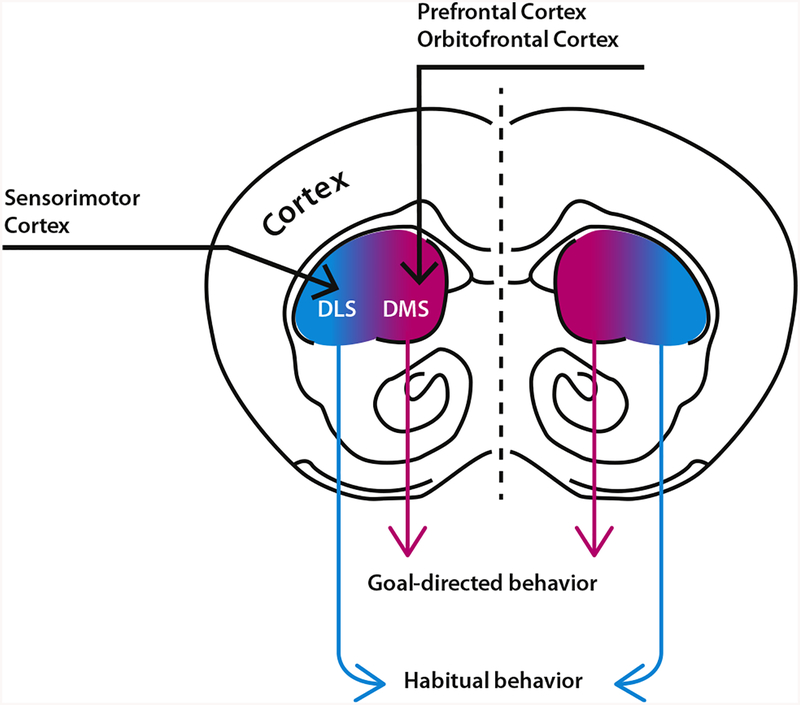
With behavioral models that can disentangle habitual and goal-directed behaviors, neuroscientists have now been able to address the crucial question of how these systems are represented in the brain. Decades of research using a range of techniques, from lesion studies to functional imaging and interventional approaches, have shown that there are indeed separate but interacting systems in the brain for each of these behavioral strategies. While both habitual and goal-directed behavior involve connections between the cortex and striatum, they are represented by distinct pathways (Figure 1) (5,6). Goal-directed behavior has been linked to the corticostriatal associative loop, which connects the prefrontal cortex and orbitofrontal cortex with the dorsomedial striatum. Habitual behavior, on the other hand, has been linked to the corticostriatal sensorimotor loop, which connects the sensorimotor cortex to the dorsolateral striatum.
Figure 1.
Corticostriatal circuits that contribute to habitual and goal-directed behavior. The associative corticostriatal loop, associated with goal-directed behavior, links the prefrontal cortex and orbitofrontal cortex (shown here in coronal section) with the dorsomedial striatum (DMS). The sensorimotor corticostriatal loop, associated with habitual behavior, links the sensorimotor cortex to the dorsolateral striatum (DLS).
As learned behaviors become increasingly stereotyped and automatic, the sensorimotor loop takes a more active role in encoding the features of the behavior. Remarkably, lesioning components of the goal-directed loop is actually sufficient to drive an animal to start behaving more habitually.
As Sarah discovered during the doughnut incident, the real challenge in our daily lives is being able to dynamically shift between habitual and goal-directed strategies. This balance is challenging to study experimentally but is thought to depend on the modulation of local striatal circuits by the cortex. One elegant study explored this in mice using a task in which different reward interval schedules allowed the animals to alternate in real time between goal-directed and habitual strategies (6). By simultaneously recording the activity of neurons in different components of the corticostriatal circuits, the investigators found that activity in the orbitofrontal cortex is necessary for switching from habitual to goal-directed strategies. This shifting is dependent on the plasticity of corticostriatal connections, a process mediated by multiple neurotransmitter systems, including dopaminergic, glutamatergic, and endocannabinoid signaling (7).
The implications of a dual system for habitual and goal-directed behavior extends far beyond the scope of daily rituals and mishaps. Just as our habits can disrupt our aspirations and goals if they become too entrenched, imbalances in habitual and goal-directed behavior may underlie certain forms of psychopathology. In fact, increasing evidence has emerged for biases towards habitual behavior in a range of compulsive behavioral disorders, including obsessive-compulsive disorder, Tourette syndrome, and substance abuse (8).
Habitual tendencies in compulsive behavioral disorders could have two possible biological explanations: an over-reliance on habitual processes or inadequate regulation of goal-directed control. The degree to which these potential underlying mechanisms contribute to compulsivity remains an active area of inquiry. One recent study conducted neuroimaging in subjects with obsessive-compulsive disorder as they completed a sequential decision task. The results suggested that the subjects’ deficits in goal-directed planning were associated with decreased functional connectivity between components of the goal-directed associative loop (9). These results are consistent with an emerging consensus that habitual biases in obsessive-compulsive disorder may be related to disruptions in circuits underlying goal-directed control (4).
A potential confounder in the link between habitual behavior and psychopathology is that habitual behavior is also highly regulated by stress. Just as your nervousness before a major examination or job interview might lead you to rely more on your morning routine, acute and chronic stress has been shown to increase subjects’ reliance on habitual strategies in both animal and human studies (10). Although relying on habit when stressed may increase the risk of errors in failing to adapt to contextual changes, it likely represents an adaptive reallocation of cognitive resources to reduce the likelihood of unreliable performance overall.
Despite our advances in understanding how habit is represented in the brain and the role it might play in compulsive behavioral disorders, we still have a long way to go to understand how these circuits contribute to the development of major psychiatric illnesses and how we might leverage this knowledge to develop more effective treatments. At the same time, beyond its implications for illness, studying how the brain balances habits and goals may provide insight into one of the most challenging questions about being human: to what degree do we actually have control over our behavior? Or in other words, are we just creatures of habit?
Acknowledgments and Disclosures
Clinical Commentaries are produced in collaboration with the National Neuroscience Curriculum Initiative (NNCI). David A. Ross, in his dual roles as co-chair of the NNCI and as Education Editor of Biological Psychiatry, manages the development of these commentaries but plays no role in the decision to publish each commentary. The NNCI is supported by the National Institutes of Health Grant Nos. R25 MH08646607S1 and R44 MH115546–01.
I thank Melissa Arbuckle for her assistance developing the manuscript.
Footnotes
The author reports no biomedical financial interests or potential conflicts of interest.
References
- 1.Wood W, Rünger D (2016): Psychology of habit. Annu Rev Psychol 67:289–314. [DOI] [PubMed] [Google Scholar]
- 2.James W (1890): Habit. New York: Henry Holt and Company. [Google Scholar]
- 3.Dickinson A (1985): Actions and habits: The development of behavioural autonomy. Philos Trans R Soc Lond B Biol Sci 308:67–78. [Google Scholar]
- 4.de Wit S, Kindt M, Knot SL, Verhoeven AAC, Robbins TW, Gasull-Camos J, et al. (2018): Shifting the balance between goals and habits: Five failures in experimental habit induction. J Exp Psychol 147:1043–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O’Hare J, Calakos N, Yin HH (2018): Recent insights into corticostriatal circuit mechanisms underlying habits. Curr Opin Behav Sci 20:40–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gremel CM, Costa RM (2013): Orbitofrontal and striatal circuits dynamically encode the shift between goal-directed and habitual actions. Nat Commun 4:2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lovinger DM (2010): Neurotransmitter roles in synaptic modulation, plasticity and learning in the dorsal striatum. Neuropharmacology 58:951–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Wit S (2018): The balance between goal-directed and habitual action control in disorders of compulsivity In: Morris R, Bornstein A, Shenhab A, editors. Goal-Directed Decision Making: Computations and Neural Circuits. San Diego, CA: Academic Press, 331–365. [Google Scholar]
- 9.Vaghi MM, Vértes PE, Kitzbichler MG, Apergis-Schoute AM, van der Flier FE, Fineberg NA, et al. (2017): Specific frontostriatal circuits for impaired cognitive flexibility and goal-directed planning in obsessive-compulsive disorder: Evidence from resting-state functional connectivity. Biol Psychiatry 81:708–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schwabe L, Wolf OT (2013): Stress and multiple memory systems: From ‘thinking’ to ‘doing.’ Trends Cogn Sci 17:60–68. [DOI] [PubMed] [Google Scholar]