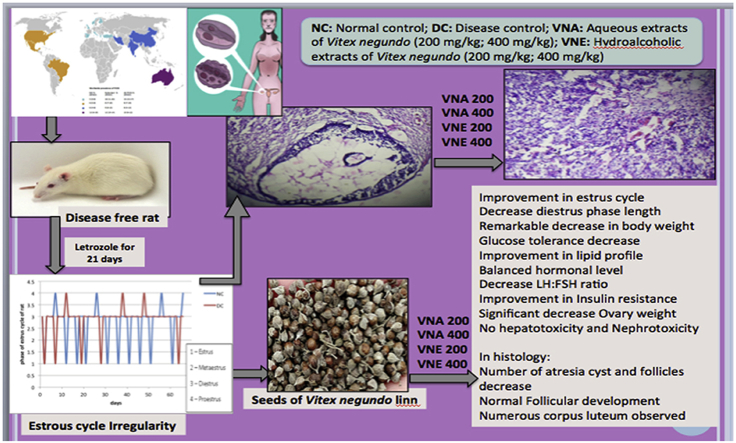
Abstract
The clinical management of PCOS is multifaceted but often unsatisfactory. The aim of the current study is to evaluate the effect of Vitex negundo L. in the letrozole-induced polycystic ovarian syndrome. Female Sprague-Dawley rats were divided into six groups, each containing 6 animals. Group I (Control) daily received 1% carboxymethylcellulose (CMC) suspension as a vehicle control. Letrozole (1 mg/kg) was administered per orally (p.o) for a period of 21 days for the induction of PCOS in Group II to VI. PCOS induced animals were treated with aqueous (Group III - 200 mg/kg and IV- 400 mg/kg) and hydroalcoholic extract (Group V- 200 mg/kg and VI- 400 mg/kg) of Vitex negundo up to 66 days using 0.5% w/v CMC as the vehicle. Body weight and estrous cycle phase were measured every day. Blood samples were collected on 0, 21 and 66 days for the measurement of fasting blood glucose, lipid profile, LH, FSH and hormonal level. Oral glucose tolerance test was performed to study insulin resistance effect. Toxicity markers; SGOT, SGPT, and creatinine also measured at the end of the study. The administration of Letrozole led to an abnormality in serum sex steroid profile, lipid profile, glucose and estrous cycle. It was able to successfully exert its protective effect by restoring parameters to the normal level and disappearance of cysts in ovaries. This can be attributed to phyto-components present in the extract. The aqueous and hydro-alcoholic extracts of seeds of Vitex negundo showed significant amelioration of Letrozole induced PCOS.
Keywords: Polycystic ovary syndrome, Letrozole, Vitex negundo, Insulin resistance, Estrogen
ABBREVIATIONS: PCOS, Polycystic ovary syndrome; KBIPER, K. B. Institute of Pharmaceutical Education and Research; LVG, Lallubhai Vrajlal Gandhi; CPCSEA, Committee for the Purpose of Control And Supervision of Experiments on Animals; PCG, Pharmacognosy; VN, Vitex negundo L.; VNA, Aqueous extract of Vitex negundo; VNE, Hydroalcoholic extract of Vitex negundo; NPD, Normal pellet diet; IAEC, Institutional Animal Ethics Committee; NC, Normal control; DC, Disease control; HCG, Human chorionic gonadotropin; CMC, Carboxymethylcellulose; DHEA, Dehydroepiandrosterone; NADH, Nicotinamide adenine dinucleotide
Graphical abstract
1. Introduction
Polycystic ovary syndrome (PCOS) is the most common endocrine disorder among women of fertile age, which causes anovulation in animal and in women. The prevalence of PCOS varies between 2.5 and 7.5%. Its hyperandrogenic manifestations include menstrual irregularity, acne, hirsutism and oligo-ovulation/anovulation. Metabolic abnormalities such as dyslipidemia, insulin resistance in PCOS responsible to make the patient more prone to diabetes, obesity, cancer, and infertility as well as coronary heart diseases.1
Clomiphene citrate, exogenous gonadotropins, Insulin sensitizers, such as metformin, are used to reduce insulin resistance which results in a reduction of ovarian androgen production and a consequent improvement in menstrual cyclicity.2 Although many drugs have been shown to be effective in the treatment of PCOS, alternatives are continuously being searched because of actual or possible side effects ranging from arthritis, joint or muscle pain,3 psychological disturbances,4 and lactic acidosis.5 In traditional herbal medicine, there are many exceptional herbal drugs that have the potential competence of preventing and curing PCOS 6, 7, 8, 9; however, many more herbs are not evaluated and much research has been not done on its mechanism of action.
Vitex negundo is a plant of (Linn) Family -Verbenaceae, Genus - Vitex and Species - negundo.10 It is commonly known as the five-leaved chaste tree or monk's pepper (Hindi- Sambhalu, Nirgundi, Gujarati-Nagod); is used as medicine fairly throughout the greater part of India and found mostly at warmer zones and ascending to an altitude of 1500 m in outer western himalayas.11
The seed is rounded drupe, 1–3 mm in diameter, 1/3 rd to 3/4 th of its size surrounded by a dull grey cup-like, persistent calyx along with pedicel; calyx cup may show one or two vertical splits; seeds color light brown to black. The seeds mainly contain 3β -acetoxyolean-12-en-27-oic acid; 2α, 3α-dihydroxyoleana-5,12-dien-28-oicacid; 2β,3α diacetoxyoleana-5,12-dien-28-oicacid; 2α,3β-diacetoxy-18 hydroxyoleana-5,12-dien-28-oic acid vitedoin-A; vitedoin-B; phenylnaphthalene-type lignan alkaloid, vitedoamine-A; five other lignan derivatives 6-hydroxy-4-(4-hydroxy-3- methoxy-phenyl)-3-hydroxymethyl-7-methoxy-3, 4-dihydro-2-naphthaldehyde β-sitosterol; p-hydroxybenzoic acid; 5-oxyisophthalic acid; n-tritriacontane, n-hentriacontane; n-pentatriacontane; n-nonacosane.10
Vitex negundo L. (VN) has been reported to possess a wide variety of biological effects like as a anti-inflammatory, analgesic,12 antioxidant,13 antifungal,14 insect repellant,15 antiviral, enzyme inhibitory action and also used in gynecological disorders10 Research studies indicate that VN has significantly improved insulin resistance and hyperglycemia condition16 which is often associated with PCOS. It also exhibits potent antiandrogenic17 and estrogenic (linoleic acid like estrogenic compounds)18 action, which may be beneficial to improve PCOS condition. On the basis of extensive literature review, we hypothesized that VN may be beneficial in the management of PCOS induced by letrozole.
2. Methodology
2.1. Collection and authentication of the plant material
The seeds of VN were purchased from LVG (Lallubhai Vrajlal Gandhi, Ambavadi, Ahmedabad, Gujarat 380006). The seeds were identified (Voucher specimen Number: KBIPER/2012/PCG-V/01) and authenticated by the expert, Department of Pharmacognosy, K. B. Institute of Pharmaceutical education and research, Gandhinagar.
2.2. Preparation of extracts of VN for pharmacological action
For Pharmacological activity, aqueous and hydroalcoholic extracts were selected because flavanoid and triterpenoids like phytoconstituents (A major active constituents) are mostly get extracted in water and alcohol.
2.2.1. Aqueous extract (VNA)
Seeds of VN were powdered and aqueous extract was prepared using hot maceration technique. 100 gm of powder was mixed with 1000 ml of distilled water and then it was heated on boiling water bath for six hours and allowed to stand overnight. The mixture was then filtered and the marc was extracted twice again in the same manner. The filtrates from each extraction step were pooled and concentrated to dryness (Yield: 22.56%). The extracts were kept in sterile bottles, under refrigerated conditions, until further use.
2.2.2. Hydroalcoholic extract (VNE)
Powdered seeds of VN. 100 g powder was extracted separately with 1000 ml of diluted alcohol (70:30 - Alcohol: Water) by heating under reflux on the water bath for 6 h at 55 °C. The mixture was then filtered and the marc was extracted twice again in the same manner. The filtrates from each extraction step were pooled and concentrated under vacuum using a rotary vacuum evaporator. The concentrate was evaporated to dryness at a temperature not exceeding 60 °C. (Yield: 29.48%) The extracts were kept in sterile bottles, under refrigerated conditions, until further use.
2.3. Phytochemical analysis of plant extracts
All prepared extracts were subjected to various qualitative tests to detect the presence of phytoconstituents like alkaloids, flavonoids, saponins, carbohydrates, sterols and terpenoids, anthraquinone glycosides and flavonoids.
Total flavonoid content was estimated using an AlCl3 method with little modification.19 0.5 ml of extract was mixed with 1.5 ml of 95% ethanol, 0.1 ml of 10% aluminum chloride, 0.1 ml of 1 M potassium acetate and 2.8 ml of distilled water. After incubation at room temperature for 30 min, the absorbance of the reaction mixture was measured at 415 nm with Shimadzu UV-160A spectrophotometer. The amount of 10% aluminum chloride was substituted by the same amount of distilled water in the blank. Percentage of total flavonoid was calculated from the calibration curve of quercetin (200–700 μg/ml), plotted by using the same procedure. Results were expressed in g/100 g of dry matter.
2.4. Selection of dose of the extracts
The standard dose for the adult human of the Vitex negundo L. in ayurvedic text reported is 1–3 gm per day.20 Using human to the animal dose conversion factor for rat (Conversion factor 0.018) according to body surface area,21 we calculate the dose for the rat and it is approximately between 100 mg/kg to 270 mg/kg. Other pharmacological studies on this plant have used extracts of this plant in the dose range of 100–500 mg/kg dose.22,23 On the basis of these reports, we selected two-dose levels 200 and 400 mg/kg for our research work.
2.5. Animals
Healthy female Sprague Dawley rats, 6–8 week old, were used for the study. The animals were procured from the animal house of K. B. Institute of pharmaceutical education and research, Gandhinagar, India and housed in standard polypropylene cages. They were maintained at controlled room temperature (20 °C - 22 °C) and relative humidity (30%–70%) with 12:12 h light and dark cycle. All the animals were fed with commercially available normal pellet diet (NPD) (Amrut feeds, Sangli, Maharashtra) and water ad libitum during the course of an experiment. The project protocol (Protocol No: KBIPER/2012/338) was approved by CPCSEA and the Institutional Animal Ethics Committee (IEAC) of K. B. Institute of Pharmaceutical Education & Research, Gandhinagar.
2.6. Treatment protocol
Animals were randomly divided into six groups containing (n = 6) animals. All the animals were checked for the estrous cycle regularity by vaginal smear test for 2 consecutive estrous cycles. Animals having irregular estrous cycle were eliminated from the study. All the animals except group I were treated with 1 mg/kg Letrozole p.o. dissolved in 1% CMC (2 ml/kg) once a daily for 21 days.24 Vaginal Smears were collected daily and evaluated microscopically using Giemsa stain to confirm the induction of PCOS. The disease was confirmed by an irregularity of estrous cycle.
From the 22nd day Group I (NC) received vehicle only, Group II (DC) served as disease control, Group III (VNA 200) was treated with 200 mg/kg aqueous extract of VN orally once a day for the remaining of the experimental duration i.e. up to the 66th day. Same way animals of group IV (VNA 400), Group V (VAE 200), Group VI (VAE 400) were treated with 400 mg/kg aqueous extract of VN, 200 mg/kg hydroalcoholic extract of VN, and 400 mg/kg hydroalcoholic extract of VN respectively.
The body weight of all animals was recorded at the beginning and at weekly intervals throughout the experiment. Vaginal smear test was performed daily to confirm the phase of an estrous cycle. Serum glucose, total cholesterol, HDL, LDL, triglyceride, LH, FSH were measured on day 0, 21 and 66. Oral Glucose Tolerance Test (OGTT) was carried out on day 15 and day 40. On the last day of experiment SGOT, SGPT and creatinine were measured to check the toxic effect on liver and kidney respectively. At the end of the experimental duration, a hormonal level was measured and ovary was collected for histopathology study.
2.7. Parameters to be assessed
2.7.1. Physical parameters
The body weight of all animals was recorded at the beginning and at weekly interval throughout the experiment.
2.7.2. Vaginal smear test
Pipette smear technique was used to collect a smear sample from the rat's vaginal lining to study estrous cycle. 0.2–0.3 ml of saline was flushed in a vagina of the rat using a small pipette and then that vaginal fluid was collected. A drop of this cell suspension was placed on a slide and covered with a coverslip. Staining was done using WBC dilution fluid and prepared slide was observed under the microscope using 10X and 45X lens.
2.7.3. Biochemical parameters
Serum glucose, triglyceride, total cholesterol, HDL, total protein levels were determined using diagnostic kits. (Span diagnostic Ltd, Gujarat, India) The serum LH and FSH were estimated by Immuno enzymometric assay using ERBA Fertikit LH.25 Serum Estradiol, progesterone, and testosterone were assayed by immunosorbent Sandwich ELISA colorimetric method in a 96 well plate ELISA microplate reader (Multiskan™ GO, Thermo Fischer scientific) using GenXbio kit.
2.7.4. Steroidogenic enzyme assay
The key steroidogenic enzymes - 3β hydroxysteroid dehydrogenase and 17β hydroxysteroid dehydrogenase were assayed to evaluate the enzyme activity of ovarian enzyme.26 In brief, the enzyme assay was carried out in 0.1 M Tris HCl buffer (pH 7.8) containing NAD (500 μM) and the substrate DHEA (100 μM) for 3β hydroxysteroid dehydrogenase or 17β estradiol (100 μM) for 17β hydroxysteroid dehydrogenase in a total volume of 3 ml. The reaction was started by adding the enzyme (100 μl) together with the color reagent, INT. The mixture was then incubated at 37 °C for 1 h. The reaction was terminated by the addition of 2.0 ml of phthalate buffer (pH 3.0) and read at 490- nm. The enzyme activity was calculated from the standard curve of NADH.
2.7.5. Oral glucose tolerance test (OGTT)
OGTT was performed on day 15 and 40 for all rats in the experiments.27 Next, glucose (2 g/kg body weight) was orally fed to the overnight fasted rats and blood samples were collected after time intervals of 0’ (Before glucose load) 30′, 60′, 90′ and 120′ minutes. The blood was subjected to 3000 rpm for 10 min and the serum was separated. Glucose was estimated using GOD-POD based kits.
2.7.6. Histopathology of the ovary
On the day 66, both the ovaries were collected from each animal. It was quickly removed, cleaned up and weighed. Afterward, ovary was fixed in 10% formalin solution and stored at 4 °C for HE (hematoxylin and eosin) staining and light microscopic examination. Partly wax-enveloped ovarian tissues were stained with HE and the growth of follicles observed by microscopy.28
2.8. Statistical evaluation
The results are expressed as mean ± SEM. The statistical significance of the data was determined by two-way analysis of variance (ANOVA) followed by Bonferroni post hoc test. The level of significance was set at p < 0.05. The statistical analysis of data was performed using Prism 5.0 software (Graphpad Software Inc., California, USA).
3. Result
3.1. Morphological study of Vitex negundo seeds
The seed was a rounded, 1–3 mm in diameter, 1/3 rd to 3/4th of its size surrounded by a dull grey cup-like, persistent calyx along with pedicel; calyx cup showed one or two vertical splits; seed color light brown to black; texture smooth, taste and odor not characteristic. These morphological characters were comparable with those mentioned for seeds of VN in literature.
3.2. Phytochemical screening
All the extracts were subjected to various chemical tests to detect the presence of compounds of different chemical groups. Aqueous extract of VN mainly contains flavonoids and carbohydrates wherever, Hydroalcoholic extract of VN mainly contain flavanoid, terpenoid, and glycosides. Total flavonoid content in VNA and VNE is 0.386 ± 0.120 and 0.416 ± 0.020 (%w/w) respectively. All these findings are consistent with those reported earlier by other investigators.29
3.3. Effect of Vitex negundo on different parameters in letrozole induced PCOS rat
3.3.1. Estrous cycle
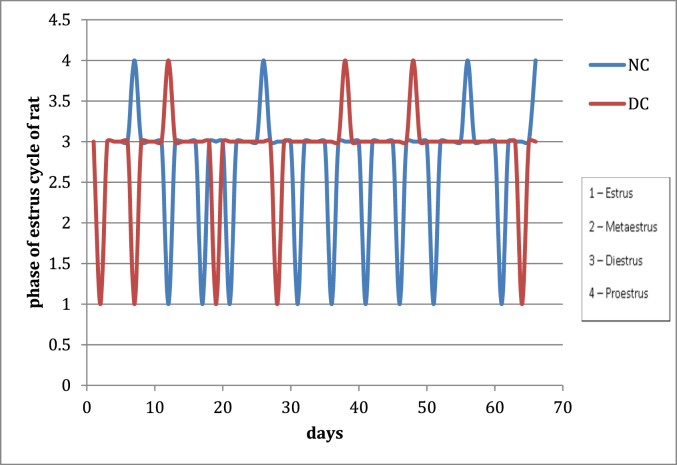
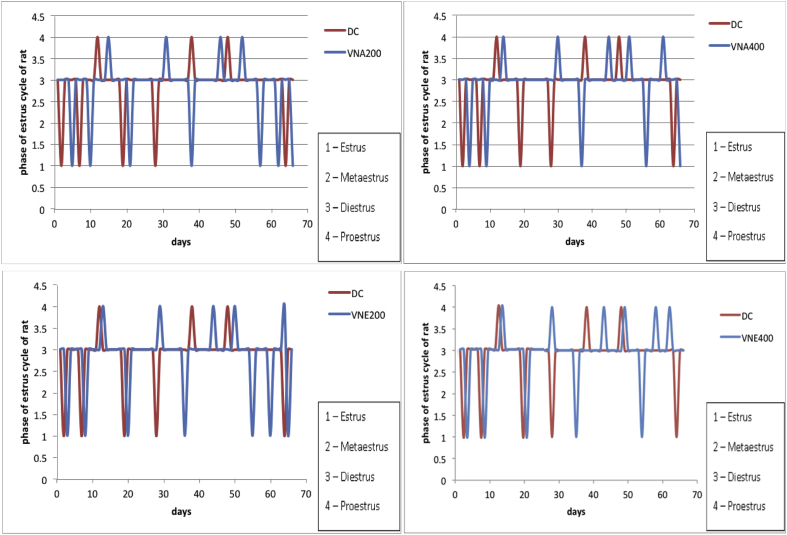
After 21 days treatment with letrozole, cause reproductive cycle has become irregular. During that time normal control group has a regular estrous cycle. (Fig. 1), It indicates the development of PCOS in animals. From 21 to 66 days, disease control group displayed irregular estrous cycles and exhibited constant diestrus phase. Treatment during 21–66 days with aqueous and hydroalcoholic extracts of VN cause improvement in the estrous cycle irregularity and decrease the length of diestrous phase as compared to disease control group (Fig. 2).
Fig. 1.
Effect of letrozole on menstrual cycle (NC: Normal control, DC: Disease control).
Fig. 2.
Effect of Vitex negundo extracts on estrous cycle in letrozole induced PCOS rat (NC: Normal control, DC: Disease control, VNA 200: Aqueous extract of Vitex negundo 200 mg/kg, VNA 400: Aqueous extract of Vitex negundo 400 mg/kg, VNE 200: Hydroalcoholic extract of Vitex negundo 200 mg/kg, VNE 400: Hydroalcoholic extract of Vitex negundo 400 mg/kg).
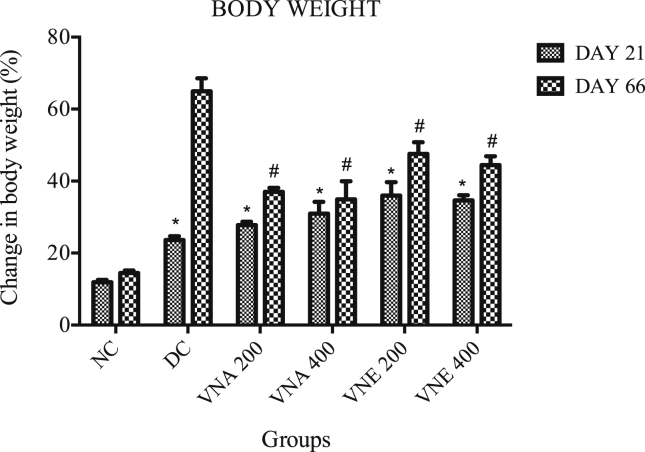
3.3.2. Bodyweight
Twenty-one day's treatment with letrozole causes the significant increase in the body weight in all groups as compared to normal control group. After the treatment with VN extracts a remarkable decrease in body weight was observed. VNA cause more decrease in body weight as compared to VNE (Fig. 3).
Fig. 3.
Effect of Vitex negundo extracts on body weight in letrozole induced PCOS rat.
(NC: Normal control, DC: Disease control, VNA 200: Aqueous extract of Vitex negundo 200 mg/kg, VNA 400: Aqueous extract of Vitex negundo 400 mg/kg, VNE 200: Hydroalcoholic extract of Vitex negundo 200 mg/kg, VNE 400: Hydroalcoholic extract of Vitex negundo 400 mg/kg).
Values are expressed as Mean ± SEM (n = 6).
* Indicates P < 0.05 vs NC on day 21.
# Indicates P < 0.05 vs DC on day 66.
As evaluated by ANOVA followed by Bonferroni tests.
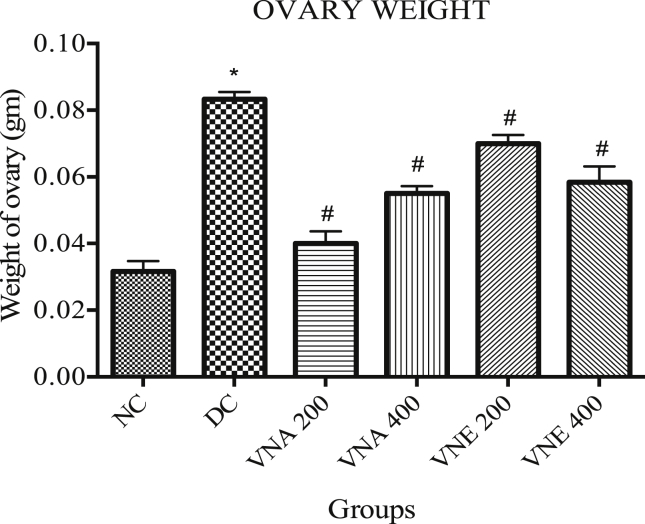
3.3.3. Ovary weight
Letrozole induced PCOS in the rat cause significantly increase ovary weight in the disease control group (0.083 ± 0.002) as compare to normal group (0.032 ± 0.003), and this condition was reversed back by treatment with VNA200 (0.04 ± 0.003), VNA400 (0.05 ± 0.002), VNE200 (0.070 ± 0.003), VNE400 (0.058 ± 0.005) (Fig. 4).
Fig. 4.
Effect of Vitex negundo on ovary weight in letrozole induced PCOS rat.
(NC: Normal control, DC: Disease control, VNA 200: Aqueous extract of Vitex negundo 200 mg/kg, VNA 400: Aqueous extract of Vitex negundo 400 mg/kg, VNE 200: Hydroalcoholic extract of Vitex negundo 200 mg/kg, VNE 400: Hydroalcoholic extract of Vitex negundo 400 mg/kg).
Values are expressed as Mean ± SEM (n = 6).
* Indicates P < 0.05 vs NC on day 66.
# Indicates P < 0.05 vs DC on day 66.
As evaluated by ANOVA.
3.3.4. Fasting blood glucose
There was no significant difference in fasting blood glucose between all groups on day 0. The significant increase in fasting blood glucose was observed in all groups as compared to normal control on day 21. After treatment with VN extracts glucose level is remarkably decrease indicate a positive effect on hyperglycemia which is a major condition present in PCOS patient (Table 1).
Table 2.
Effect of Vitex negundo extracts on serum hormones in letrozole induced PCOS rat.
| Parameter (mg/dl) | NC | DC | VNA 200 | VNA 400 | VNE 200 | VNE 400 |
|---|---|---|---|---|---|---|
| Estradiol | 22.06 ± 2.11 | 09.24 ± 1.62 | 18.06 ± 1.84# | 20.06 ± 1.46# | 16.82 ± 2.11 | 19.69 ± 2.61# |
| Progesterone | 30.68 ± 0.62 | 20.32 ± 1.02* | 24.44 ± 0.06# | 27.68 ± 1.99# | 23.68 ± 2.12 | 24.68 ± 1.99# |
| Testosterone | 32.66 ± 1.42 | 45.68 ± 0.38* | 37.68 ± 2.42# | 34.24 ± 1.44# | 42.66 ± 1.98 | 40.66 ± 1.42 |
Values are expressed as Mean ± SEM (n = 6).
As evaluated by two way ANOVA followed by Bonferroni tests.
(NC: Normal control, DC: Disease control, VNA 200: Aqueous extract of Vitex negundo 200 mg/kg, VNA 400: Aqueous extract of Vitex negundo 400 mg/kg, VNE 200: Hydroalcoholic extract of Vitex negundo 200 mg/kg, VNE 400: Hydroalcoholic extract of Vitex negundo 400 mg/kg).
Table 1.
Effect of Vitex negundo extracts on biochemical parameters in letrozole induced PCOS rat.
| NC | DC | VNA 200 | VNA 400 | VNE 200 | VNE 400 | |
|---|---|---|---|---|---|---|
| Glucose (mg/dl) | ||||||
| 0 day | 61.04 ± 6.86 | 59.94 ± 6.37 | 65.84 ± 6.06 | 60.21 ± 5.35 | 63.06 ± 4.10 | 56.55 ± 6.53 |
| 21 day | 61.77 ± 6.00 | 75.75 ± 7.14a | 78.38 ± 4.45a | 74.43 ± 4.39a | 78.33 ± 6.65a | 79.54 ± 7.01a |
| 66 day |
60.77 ± 7.21 |
76.10 ± 8.32 |
63.84 ± 8.12b |
62.71 ± 3.37b |
67.39 ± 2.43b |
66.55 ± 5.02b |
| Total Cholesterol (mg/dl) | ||||||
| 0 day | 37.41 ± 3.74 | 36.31 ± 3.20 | 36.27 ± 4.59 | 38.11 ± 6.82 | 37.15 ± 7.46 | 35.83 ± 8.25 |
| 21 day | 40.98 ± 3.63 | 40.04 ± 1.72 | 39.44 ± 4.60 | 41.28 ± 3.22 | 43.33 ± 1.90 | 40.63 ± 4.23 |
| 66 day | 35.07 ± 4.69 | 43.18 ± 3.43 | 37.02 ± 2.59 | 36.86 ± 3.21 | 41.85 ± 3.02 | 39.81 ± 5.20 |
| Triglyceride (mg/dl) | ||||||
| 0 day | 26.77 ± 3.95 | 23.74 ± 2.03 | 29.82 ± 1.33 | 28.27 ± 2.85 | 33.58 ± 2.71 | 35.05 ± 2.49 |
| 21 day | 33.39 ± 3.98 | 44.45a ± 1.403 | 38.32 ± 2.61a | 48.21 ± 3.23a | 45.25 ± 5.83a | 51.42 ± 2.62a |
| 66 day |
35.20 ± 1.76 |
45.24 ± 3.30 |
29.754 ± 2.3b |
37.17 ± 2.54b |
32.93 ± 2.86b |
43.825 ± 1.34b |
| HDL (mg/dl) | ||||||
| 0 day | 42.87 ± 2.44 | 40.61 ± 2.17 | 39.28 ± 3.07 | 41.83 ± 2.01 | 43.13 ± 1.65 | 41.33 ± 2.33 |
| 21 day | 40.91 ± 3.20 | 37.68 ± 1.69 | 39.97 ± 1.75 | 40.44 ± 0.81 | 39.98 ± 1.24 | 40.05 ± 1.12 |
| 66 day | 40.45 ± 2.07 | 36.71 ± 2.29 | 39.13 ± 1.67 | 37.77 ± 2.85 | 38.15 ± 2.29 | 39.38 ± 2.31 |
Values are expressed as Mean ± SEM (n = 6).
Indicates P < 0.05 vs NC on day 21.
Indicates P < 0.05 vs DC on day 66, As evaluated by two way ANOVA followed by Bonferroni tests. (NC: Normal control, DC: Disease control, VNA 200: Aqueous extract of Vitex negundo 200 mg/kg, VNA 400: Aqueous extract of Vitex negundo 400 mg/kg, VNE 200: Hydroalcoholic extract of Vitex negundo 200 mg/kg, VNE 400: Hydroalcoholic extract of Vitex negundo 400 mg/kg).
3.3.5. Lipid profile
Serum cholesterol (mg/dl), triglyceride and HDL level (mg/dl) were measured on day 0, 21 and 66. Study data shows that letrozole and VN extracts didn't show any effect on serum cholesterol and HDL level. Letrozole treatment cause triglyceride level significantly increase in all groups as compared to normal control group. These effect is significantly reverted back by both the extracts of VN (Table 1).
3.3.6. Serum hormonal assay
Serum Testosterone was markedly increased while progesterone and estradiol decreased significantly in PCOS group as compared to normal control group. Treatment with different extracts of VN (VNA 200 and VNA 400) cause a significant fall in testosterone levels, however, VNE 200 and 400 decrease the testosterone but not up to significant level. Estrogen and Progesterone levels were also improved in VNA 200, VNA 400 and VNE 400 groups.
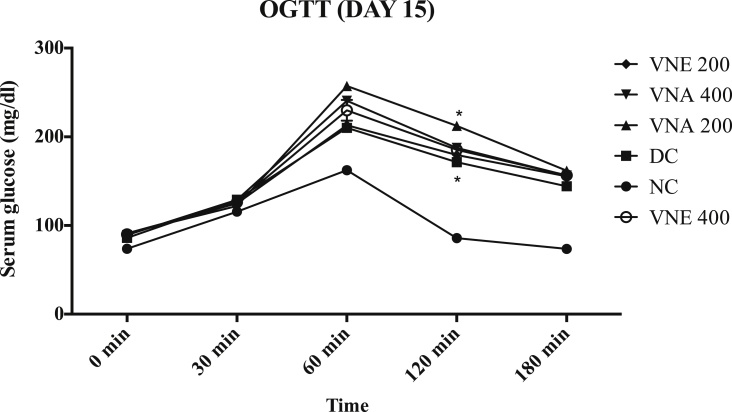
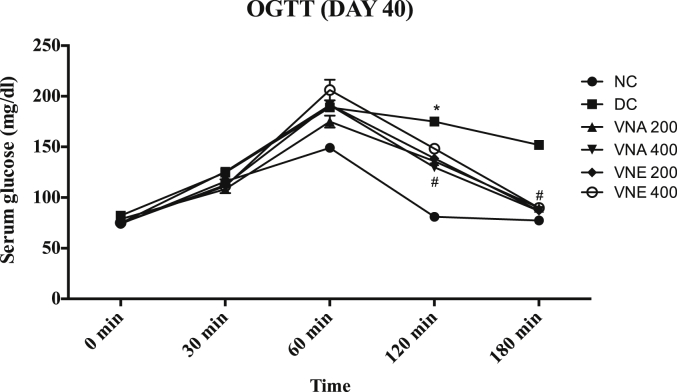
3.3.7. Oral glucose tolerance test
On day 15, the significantly increase glucose level at 60, 120 and 180 min after glucose loading in DC, VNA and VNE groups as compared to normal control (Fig. 5). this difference was not observed at 0 min glucose level. Generally, In normal condition after the glucose loading, serum glucose concentration is increased up to peak level and within two to three hours it comes into the normal range again but it was not observed in disease and treatment group on the day 21. It indicates the development of insulin resistance condition. OGTT test on the day 40 showed that due to treatment with different extracts of VN, developed resistance due to letrozole is significantly decrease as compared to disease control. Aqueous extract cause more improvement in insulin resistant as compared to hydroalcoholic extract (Fig. 6).
Fig. 5.
Effect of Vitex negundo extracts on Oral glucose tolerance test on day 15.
(NC: Normal control, DC: Disease control, VNA 200: Aqueous extract of Vitex negundo 200 mg/kg, VNA 400: Aqueous extract of Vitex negundo 400 mg/kg, VNE 200: Hydroalcoholic extract of Vitex negundo 200 mg/kg, VNE 400: Hydroalcoholic extract of Vitex negundo 400 mg/kg).
Values are expressed as Mean ± SEM (n = 6).
* Indicates P < 0.05 vs NC on 120 min.
As evaluated by two way ANOVA followed by Bonferroni tests.
Fig. 6.
Effect of Vitex negundo extracts on oral glucose tolerance test on day 40.
(NC: Normal control, DC: Disease control, VNA 200: Aqueous extract of Vitex negundo 200 mg/kg, VNA 400: Aqueous extract of Vitex negundo 400 mg/kg, VNE 200: Hydroalcoholic extract of Vitex negundo 200 mg/kg, VNE 400: Hydroalcoholic extract of Vitex negundo 400 mg/kg).
Values are expressed as Mean ± SEM (n = 6).
* Indicates P < 0.05 vs NC on 120 min.
# Indicates P < 0.05 vs DC on 120 min.
As evaluated by two way ANOVA followed by Bonferroni tests.
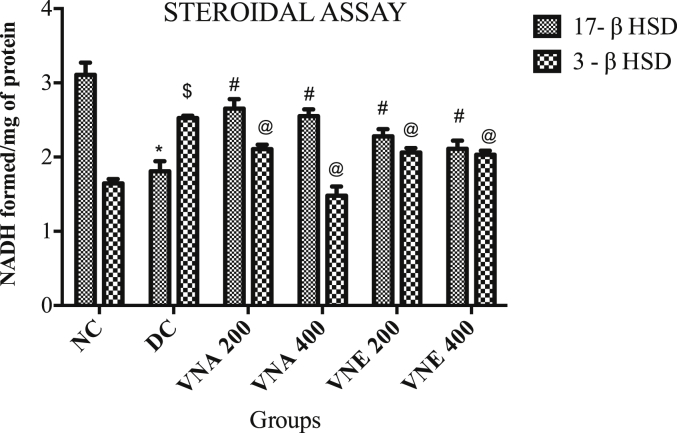
3.3.8. Steroidogenic bioassay
VN extract treatment in letrozole induced PCO animals caused an improvement in ovarian 3β hydroxysteroid dehydrogenase (3β HSD) and 17β hydroxysteroid dehydrogenase (17β HSD) activities, as compared to disease control group suggesting amelioration in steroid status (Fig. 7).
Fig. 7.
Effect of Vitex negundo extracts on steroidal assay in letrozole induced PCOS rat.
(NC: Normal control, DC: Disease control, VNA 200: Aqueous extract of Vitex negundo 200 mg/kg, VNA 400: Aqueous extract of Vitex negundo 400 mg/kg, VNE 200: Hydroalcoholic extract of Vitex negundo 200 mg/kg, VNE 400: Hydroalcoholic extract of Vitex negundo 400 mg/kg).
Values are expressed as Mean ± SEM (n = 6).
*Indicates P < 0.05 vs NC in 17- β HSD level.
# Indicates P < 0.05 vs DC in 17- β HSD level.
$ Indicates P < 0.05 vs NC in 3- β HSD level.
@ Indicates P < 0.05 vs DC in 3- β HSD level.
As evaluated by two way ANOVA followed by Bonferroni tests.
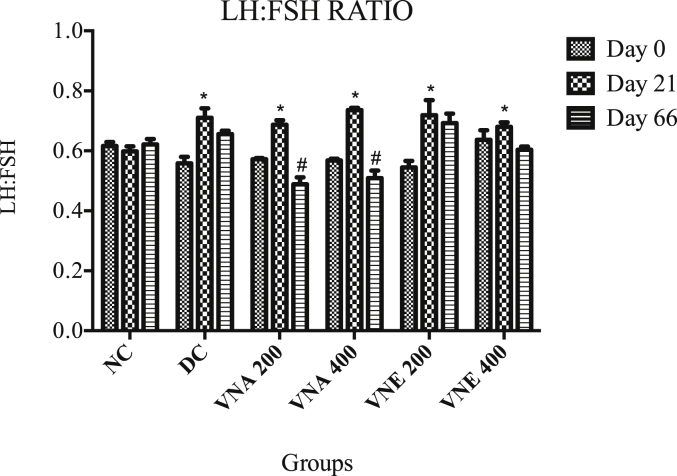
3.3.9. LH:FSH ratio
On the day zero, all the group had normal LH: FSH ratio. Treatment with the letrozole for 21 days cause greatly increase the LH: FSH ratio, which indicates PCOS development. Treatment with aqueous extracts of VN cause notably decrease the LH: FSH ratio. It recommends that VN balancing the hormone, which is disturbed in PCOS condition. Hydroalcoholic extracts didn't cause reclamation of LH and FSH hormones (Fig. 8).
Fig. 8.
Effect of Vitex negundo on LH:FSH ratio.
(NC: Normal control, DC: Disease control, VNA 200: Aqueous extract of Vitex negundo 200 mg/kg, VNA 400: Aqueous extract of Vitex negundo 400 mg/kg, VNE 200: Hydroalcoholic extract of Vitex negundo 200 mg/kg, VNE 400: Hydroalcoholic extract of Vitex negundo 400 mg/kg).
Values are expressed as Mean ± SEM (n = 6).
* Indicates P < 0.05 vs NC on day 21.
# Indicates P < 0.05 vs DC on day 66.
As evaluated by two way ANOVA followed by Bonferroni tests.
3.3.10. Ovarian follicular growth
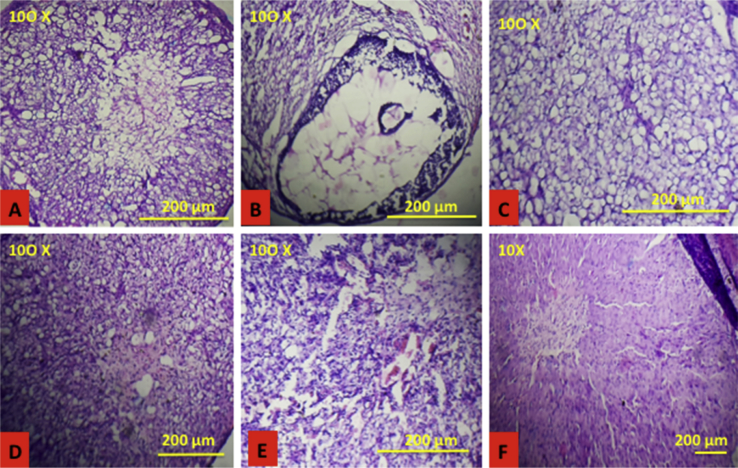
Many small and multiple ovarian follicles and atresic cyst was observed in letrozole-treated group along with less number of corpus luteum., whereas no histological abnormalities were observed in control rats. Histological studies of the treatment groups showed normal follicular development as compared to disease control group, which revealed decrease in the number of cyst formation (Fig. 9).
Fig. 9.
Effect of Vitex negundo extracts on ovarian follicular growth. A (NC): Normal follicular development in CMC treated group; B (DC): Letrozole treatment cause small cyst formation in the ovary; C (VNA 200), D (VNA 400), E (VNE 200), F (VNE 400): Treatment with Vitex negundo cause normal follicular development.
(NC: Normal control, DC: Disease control, VNA 200: Aqueous extract of Vitex negundo 200 mg/kg, VNA 400: Aqueous extract of Vitex negundo 400 mg/kg, VNE 200: Hydroalcoholic extract of Vitex negundo 200 mg/kg, VNE 400: Hydroalcoholic extract of Vitex negundo 400 mg/kg).
3.3.11. Biomarkers of toxicity
Even though a prolonged treatment (From 21 to 66 days) with aqueous and hydroalcoholic extracts of VN in Letrozole induced PCOS rat, no toxic effects were noticed on kidney and liver functions (Table 3).
Table 3.
Effect of Vitex negundo extracts on biotoxicity parameters in letrozole induced PCOS rat.
| Parameter (mg/dl) | NC | DC | VNA 200 | VNA 400 | VNE 200 | VNE 400 |
|---|---|---|---|---|---|---|
| Serum SGPT | 48.32 ± 1.62 | 49.61 ± 1.03 | 46.81 ± 2.28 | 51.44 ± 2.42 | 46.46 ± 1.80 | 47.65 ± 1.24 |
| Serum SGOT | 51.23 ± 2.42 | 49.87 ± 1.42 | 48.92 ± 1.36 | 54.61 ± 1.66 | 52.34 ± 2.12 | 50.22 ± 2.48 |
| Serum Creatinine | 00.59 ± 0.21 | 00.57 ± 0.14 | 00.61 ± 0.16 | 00.53 ± 0.24 | 00.55 ± 0.26 | 00.58 ± 0.11 |
Values are expressed as Mean ± SEM (n = 6).
As evaluated by two way ANOVA followed by Bonferroni tests.
(NC: Normal control, DC: Disease control, VNA 200: Aqueous extract of Vitex negundo 200 mg/kg, VNA 400: Aqueous extract of Vitex negundo 400 mg/kg, VNE 200: Hydroalcoholic extract of Vitex negundo 200 mg/kg, VNE 400: Hydroalcoholic extract of Vitex negundo 400 mg/kg).
4. Discussion
Polycystic Ovarian Syndrome (PCOS) is defined as one of the most common hormonal disorder affecting women. It has a reproductive, metabolic and cardiovascular health complication across the lifespan. It is also defined as a presence of 12 or more follicles on ultrasound sonography.30 Insulin resistance, with compensatory hyperinsulinemia, plays a major role in the metabolic abnormalities associated with PCOS. Although, not all women with PCOS are insulin-resistant or develop compensatory hyperinsulinemia, implying that these features are not essential to develop PCOS. However, androgen excess and higher LH level are the principal biochemical abnormality in women with PCOS, and the clinical manifestations of hyperandrogenemia usually appear around puberty.31
Numerous experimental animal models for PCOS include neonatal androgenization, human chorionic gonadotropin (HCG) administration to hypothyroid rats, injection of estradiol valerate and maintenance of animals in constant light have been developed in rats.32,33 None of these animal models are fully convincing and mimic with the conditions of human PCOS completely. Letrozole, a non-steroidal aromatase inhibitor produces a PCOS model which in numerous ways depicts human PCOS. It blocks the conversion of testosterone and androstenedione to estradiol and estrone respectively and simulates PCOS like condition24 by causing hormonal imbalance, circulating hyperandrogenism and intraovarian androgen excess leading to the appearance of a polycystic ovary, hyperglycemic condition, and metabolic disturbances. Follicular atresia and abnormal follicular development are observed due to the induced elevation of androgen levels inside the ovary.4 Due to striking resemblance of the letrozole induced PCOS rats to humans with respect to hormonal imbalance and insulin resistance make it more useful model for the preclinical efficacy study of PCOS.
Obesity and abdominal obesity worsen the clinical features of menstrual irregularity and infertility34 and are correlated with increased serum androgens and luteinizing hormone.35 An increase in body weight is associated with increased androgen levels in both women with PCOS and in normal controls.36 A complex interrelationship thus exists between obesity, abdominal obesity, insulin resistance, androgen level and LH level in the etiology and pathogenesis of PCOS. In our study, the increase in body weight and presence of estrous cycle irregularities after oral administration of letrozole; suggest that development of PCOS in rats due to increased androgen and LH hormone. In the present study, a significant decrease in body weight was observed in VNA200, VNA400, VNE200, and VNE400 as compared to disease control group. Aqueous extract shows more decrease in body weight as compared to hydroalcoholic extract.
Letrozole produces estrous irregularity due to hormonal imbalance, circulating hyperandrogenism and excess intraovarian androgen. It leads to an appearance of the polycystic ovary. In our study, 21-day treatment with the letrozole cause diestrus phase continues for the longer time in disease control group and other treatment groups. All the extracts of VN cause decrease diestrus phase length and estrus cycle irregularity. Moreover, both the dose shows the same effect. It indicates, VN might have the capacity to normalize the irregular cycle in the clinical setting and it will be a better treatment for menstrual irregularities.
Although women have lower basal levels of androgen compared with men, several studies suggest that an increase in androgen levels can also affect metabolism and food intake in women, resulting in metabolic imbalances and weight gain.37,38 Elevated androgen (testosterone) levels are also associated with bulimia nervosa in women, an eating disorder characterized by frequent binge-eating episodes. Bulimic women have higher levels of testosterone but lower meal-related satiety peptide secretion than those without the disorder.39 It is a reason for the increase a significant weight in PCOS patient. We also noted a significant decrease in testosterone level due to VNA 200 and 400. However, VNE does not improve the abnormal level in both the dose. A decrease in testosterone level may be one cause to decrease food intake in rats during the study period.
Polycystic ovary syndrome is frequently associated with various patterns of dyslipidemia including low high-density lipoprotein cholesterol (HDL-C), high levels of triglycerides, total cholesterol, and low-density lipoprotein cholesterol (LDL-C).40 Although the data from large series suggest that the mean values for circulating lipids in women with PCOS are in normal limits, up to 70% of patients have at least one abnormal lipid level according to NCEPATP III criteria.41 In our study, there are not any notable changes occur in HDL level and total cholesterol level in disease and treatment groups. But there is a significant increase the triglyceride level in disease control group and it is also proficiently decreased by the VN extracts. VNA has a more decrease as compared to VNE and effect is more significant at a lower dose as compared to the higher dose. Finally, it indicates that extracts also have an impact on the metabolic complication (dyslipidemia) of the PCOS.
Beyond representing the most frequent cause of hyperandrogenism and female infertility, PCOS puts young women at increased risks for diabetes and cardiovascular diseases. Moreover, conversion from normal (NGT) to impaired (IGT) glucose tolerance42 and from IGT to type 2 diabetes mellitus (T2DM) is increased two-to fivefold in the PCOS population.42,43 Because of these increased metabolic risks, many organizations recommend screening for T2DM in PCOS women. Moreover, the Androgen Excess Society recommends screening these women with an OGTT instead of fasting glucose (FG).44 Similar results found with our study that glucose tolerance is significantly increased after 21-day oral letrozole dosing. We found that VNA has more decrease the fasting blood glucose as compare to VNE and both the dose has equal effect. These extracts show similar pattern effect on glucose tolerance also. So, Vitex negundo may act as an insulin sensitizer and it will influence the choice of PCOS treatment because it causes the conversion from abnormal glucose tolerance (AGT) to normal glucose tolerance.
In PCOS, follicular development arrests at the stage of selection of the dominant follicle when aromatase activity in the granulosa cells (GC) and production of androgen normally increase.45 The amount of estrogen (E) produced by the dominant follicle indicates the ‘‘vitality’’ of the follicle and successful ovulation.46 In PCOS the follicular fluid (FF) concentrations of androgens are higher and Es lower than in women without PCOS.47 In our study, as estrogen synthesis is inhibited by the use of aromatase inhibitor in PCOS model, the 3-β HSD activity is higher as compared to 17-β HSD and androgen production is also to be higher than estrogen production. VN extracts treatment bought both the level in normal range again. This effect is more prominent in the aqueous extract as compare to hydroalcoholic extracts. Improvement is also observed in estrogen and progesterone level with the treatment of VNA 200, VNA 400 and VNE 400. In lower dose (VNE 200), the improvement was not observed in estrogen and progesterone level. At the end of study both estrogen and progesterone level significantly improve, which may have the correlation with the decrease in diestrus phase and regularization of the estrus cycle.
From a neuroendocrine perspective, a number of studies using frequent sampling over extended periods of time have documented a marked increase in mean serum LH concentrations related to augmented pulse amplitude and frequency in PCOS women, providing evidence for accelerated hypothalamic gonadotrophin-releasing hormone (GnRH) pulse generator output in this disorder 48,49. Concomitantly, several studies have also documented increased pituitary sensitivity to GnRH in PCOS women 50 with both neuroendocrine abnormalities leading eventually to increased LH secretion as a key factor that contributes to overproduction of ovarian androgens. Similar results also found with our study, that LH level is significantly increased after the letrozole treatment. Due to that LH: FSH ratio is also increased, which significantly decreases in VNA 200 and VNA 400 groups. VNE does not cause the notable decrease in LH: FSH ratio.
Reversion of estrus cyclicity and normal follicular growth to normal following the VN extract treatment could be attributed to phytochemical components present in the extracts of it, that maintain the steroidal status, enabling fertility status to be regained. It is reported that Vitex negundo has antiandrogenic activity due to flavonoids present in it, so it may responsible chemical constituent for this anti-PCOS action. Primary phytochemical evaluation indicates that the Aqueous and hydroalcoholic extracts of VN mainly contain flavonoids and carbohydrate. We estimated total flavonoid content in both the extracts because flavonoids from the plant sources have versatile health benefits and reported numerous pharmacological activities. The higher content of the flavonoid may be a responsible factor for the pharmacological activity observed in this study. Toxicity parameters do not deviate from the normal range even after the long treatment with VN. It indicates that long-time continuous treatment with VN is advisable to the patient which is a major drawback of currently available therapy.
5. Conclusion
The results of the present study reveal that Vitex negundo L. contributes significantly to the treatment of the PCOS induced by letrozole. It is clear that drug has positive effects on the ovary and also displaying effects on the glucose tolerance, estrous cycle irregularities, LH: FSH ratio, steroidogenic enzymes and cardiovascular parameters, an important factor in the treatment of PCOS. Furthermore, Vitex negundo L. available widely in most of the area and it can be used easily and conveniently by the community persons. It definitely a cost-effective and safest drug for further development as an effective anti-PCOS drug. It may be used either alone or in conjunction with the metformin and other allopathic treatment and surely beneficial for PCOS women.
Funding acknowledgment
We acknowledged K. B. Institute of Pharmaceutical Education and Research, Gandhinagar for funding this project.
Authors' contribution
-
•
NK contributed to study design, manuscript writing, data analysis and supervised the laboratory work.
-
•
PP contributed to laboratory work, data analysis, the collection of plant samples and histopathological studies.
-
•
GBS and SSD designed the study, data analysis and helped in manuscript writing.
Conflicts of interest
The authors declare no conflicts of interest.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
References
- 1.Maharjan R., Nagar P.S., Nampoothiri L. Effect of Aloe barbadensis Mill. formulation on Letrozole induced polycystic ovarian syndrome rat model. J Ayurveda Integr Med. 2010 doi: 10.4103/0975-9476.74090. 1(4)(4):273-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Poretsky L., Clemons J., Bogovich K. Hyperinsulinemia and human chorionic gonadotropin synergistically promote the growth of ovarian follicular cysts in rats. Metabolism. 1992;41(8):903–910. doi: 10.1016/0026-0495(92)90175-a. [DOI] [PubMed] [Google Scholar]
- 3.Badawy A., Elnashar A. Treatment options for polycystic ovary syndrome. Int J Womens Health. 2011;3(1):25–35. doi: 10.2147/IJWH.S11304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choi S.-H., Shapiro H., Robinson G.E. Psychological side-effects of clomiphene citrate and human menopausal gonadotrophin. J Psychosom Obstet Gynecol. 2005;26(2):93–100. doi: 10.1080/01443610400022983. [DOI] [PubMed] [Google Scholar]
- 5.Dunaif A. Drug insight: insulin-sensitizing drugs in the treatment of polycystic ovary syndrome—a reappraisal. Nat Rev Endocrinol. 2008;4(5):272–283. doi: 10.1038/ncpendmet0787. [DOI] [PubMed] [Google Scholar]
- 6.Arentz S., Abbott J.A., Smith C.A., Bensoussan A. Herbal medicine for the management of polycystic ovary syndrome (PCOS) and associated oligo/amenorrhoea and hyperandrogenism; a review of the laboratory evidence for effects with corroborative clinical findings. BMC Compl Alternative Med. 2014;14(1):1. doi: 10.1186/1472-6882-14-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ried K. Chinese herbal medicine for female infertility: an updated meta-analysis. Compl Ther Med. Feb 2015;23(1):116–128. doi: 10.1016/j.ctim.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 8.Hosseinkhani A., Asadi N., Pasalar M., Zarshenas M.M. Traditional Persian Medicine and management of metabolic dysfunction in polycystic ovary syndrome. J Tradit Complementary Med. Jan 2018;8(1):17–23. doi: 10.1016/j.jtcme.2017.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Borzoei A., Rafraf M., Niromanesh S., Farzadi L., Narimani F., Doostan F. Effects of cinnamon supplementation on antioxidant status and serum lipids in women with polycystic ovary syndrome. J Tradit Complementary Med. Jan 2018;8(1):128–133. doi: 10.1016/j.jtcme.2017.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vishwanathan A., Basavaraju R. A review on Vitex negundo L–a medicinally important plant. Eur J Biol Sci. 2010;3(1):30–42. [Google Scholar]
- 11.Tandon V.R. Medicinal uses and biological activities of Vitex negundo. Nat Prod Radiance. 2005;4(3):162–165. [Google Scholar]
- 12.Dharmasiri M., Jayakody J., Galhena G., Liyanage S., Ratnasooriya W. Anti-inflammatory and analgesic activities of mature fresh leaves of Vitex negundo. J Ethnopharmacol. 2003;87(2):199–206. doi: 10.1016/s0378-8741(03)00159-4. [DOI] [PubMed] [Google Scholar]
- 13.Kulkarni R.R., Virkar A.D., D'Mello P. Antioxidant and antiinflammatory activity of Vitex negundo. Indian J Pharmaceut Sci. Nov 2008;70(6):838–840. doi: 10.4103/0250-474X.49140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sathiamoorthy B., Gupta P., Kumar M., Chaturvedi A.K., Shukla P.K., Maurya R. New antifungal flavonoid glycoside from Vitex negundo. Bioorg Med Chem Lett. Jan 1 2007;17(1):239–242. doi: 10.1016/j.bmcl.2006.09.051. [DOI] [PubMed] [Google Scholar]
- 15.Karunamoorthi K., Ramanujam S., Rathinasamy R. Evaluation of leaf extracts of Vitex negundo L. (Family: verbenaceae) against larvae of Culex tritaeniorhynchus and repellent activity on adult vector mosquitoes. Parasitol Res. Aug 2008;103(3):545–550. doi: 10.1007/s00436-008-1005-5. [DOI] [PubMed] [Google Scholar]
- 16.Sundaram R., Naresh R., Shanthi P., Sachdanandam P. Antihyperglycemic effect of iridoid glucoside, isolated from the leaves of Vitex negundo in streptozotocin-induced diabetic rats with special reference to glycoprotein components. Phytomedicine. Feb 15 2012;19(3-4):211–216. doi: 10.1016/j.phymed.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 17.Bhargava S. Antiandrogenic effects of a flavonoid-rich fraction of Vitex negundo seeds: a histological and biochemical study in dogs. J Ethnopharmacol. 1989;27(3):327–339. doi: 10.1016/0378-8741(89)90007-x. [DOI] [PubMed] [Google Scholar]
- 18.Hu Y., Zhang Q.-Y., Hou T.-T. Estrogen-like activities in Vitex species from China determined by a cell based proliferation assay. Pharmazie. 2007;62(11):872–875. [PubMed] [Google Scholar]
- 19.Chang C.-C., Yang M.-H., Wen H.-M., Chern J.-C. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J Food Drug Anal. 2002;10(3) [Google Scholar]
- 20.Ayurvedic Pharmacopiea of India. In: welfare MohaF, ed. Vol. vol. 5. India:183.
- 21.Medhi B., Prakash A. Jaypee Brothers, Medical Publishers Pvt. Limited; 2010. Practical Manual of Experimental and Clinical Pharmacology. [Google Scholar]
- 22.Petchi R.R., Vijaya C., Parasuraman S. Antidiabetic activity of polyherbal formulation in streptozotocin - nicotinamide induced diabetic wistar rats. J Tradit Complementary Med. Apr 2014;4(2):108–117. doi: 10.4103/2225-4110.126174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sarvankumar G., Lalitha V., Sengottuvelu S., Sharif S.H., Sivakumar T. Nephroprotective activity of Vitex negundo Linn bark against chemical induced toxicity in experimenal Rats. Int J Adv Pharmaceut Sci. 2011;2:462–470. [Google Scholar]
- 24.Kafali H., Iriadam M., Ozardalı I., Demir N. Letrozole-induced polycystic ovaries in the rat: a new model for cystic ovarian disease. Arch Med Res. 2004;35(2):103–108. doi: 10.1016/j.arcmed.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 25.Robertson D.M., Foulds L.M., Fry R.C., Cummins J.T., Clarke I. Circulating half-lives of follicle-stimulating hormone and luteinizing hormone in pituitary extracts and isoform fractions of ovariectomized and intact ewes. Endocrine. Oct 1991;129(4):1805–1813. doi: 10.1210/endo-129-4-1805. [DOI] [PubMed] [Google Scholar]
- 26.Shivanandappa T., Venkatesh S. A colorimetric assay method for 3β-hydroxy-Δ 5-steroid dehydrogenase. Anal Chem. 1997;254(1):57–61. doi: 10.1006/abio.1997.2406. [DOI] [PubMed] [Google Scholar]
- 27.Du Vigneaud V., Karr W.G. Carbohydrate utilization I. Rate of disappearance of d-glucose from the blood. J Biol Chem. 1925;66(1):281–300. [Google Scholar]
- 28.Wang F., Yu B., Yang W., Liu J., Lu J., Xia X. Polycystic ovary syndrome resembling histopathological alterations in ovaries from prenatal androgenized female rats. J Ovarian Res. 2012;5(1):1. doi: 10.1186/1757-2215-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meena A.K., Niranjan U., Rao M., Padhi M., Babu R. A review of the important chemical constituents and medicinal uses of Vitex genus. Asian J Tradit Med. 2011;6(2):54–60. [Google Scholar]
- 30.Roy M., Yasmin S. Changing concepts in polycystic ovarian syndrome. Apollo Med. 2009;6(3):222–226. [Google Scholar]
- 31.Franks S. Adult polycystic ovary syndrome begins in childhood. Best Pract Res Clin Endocrinol Metabol. 2002;16(2):263–272. doi: 10.1053/beem.2002.0203. [DOI] [PubMed] [Google Scholar]
- 32.Mahajan D.K. Polycystic ovarian disease: animal models. Endocrinol Metab Clin N Am. Dec 1988;17(4):705–732. [PubMed] [Google Scholar]
- 33.Mahesh V.B., Mills T.M., Bagnell C.A., Conway B.A. Animal models for study of polycystic ovaries and ovarian atresia. Adv Exp Med Biol. 1987;219:237–257. doi: 10.1007/978-1-4684-5395-9_12. [DOI] [PubMed] [Google Scholar]
- 34.Clark A., Ledger W., Galletly C. Weight loss results in significant improvement in pregnancy and ovulation rates in anovulatory obese women. Hum Reprod. 1995;10(10):2705–2712. doi: 10.1093/oxfordjournals.humrep.a135772. [DOI] [PubMed] [Google Scholar]
- 35.Holte J., Bergh T., Berne C., Berglund L., Lithell H. Enhanced early insulin response to glucose in relation to insulin resistance in women with polycystic ovary syndrome and normal glucose tolerance. J Clin Endocrinol Metab. 1994;78(5):1052–1058. doi: 10.1210/jcem.78.5.8175959. [DOI] [PubMed] [Google Scholar]
- 36.Gambineri A., Pelusi C., Vicennati V., Pagotto U., Pasquali R. Obesity and the polycystic ovary syndrome. Int J Obes Relat Metab Disord. 2002;26(7) doi: 10.1038/sj.ijo.0801994. [DOI] [PubMed] [Google Scholar]
- 37.Barber T., McCarthy M., Wass J., Franks S. Obesity and polycystic ovary syndrome. Clin Endocrinol. 2006;65(2):137–145. doi: 10.1111/j.1365-2265.2006.02587.x. [DOI] [PubMed] [Google Scholar]
- 38.Sundblad C., Bergman L., Eriksson E. High levels of free testosterone in women with bulimia nervosa. Acta Psychiatr Scand. 1994;90(5):397–398. doi: 10.1111/j.1600-0447.1994.tb01613.x. [DOI] [PubMed] [Google Scholar]
- 39.Naessen S., Carlstrom K., Bystrom B., Pierre Y., Hirschberg A.L. Effects of an antiandrogenic oral contraceptive on appetite and eating behavior in bulimic women. Psychoneuroendocrinology. 2007;32(5):548–554. doi: 10.1016/j.psyneuen.2007.03.008. Epub 2007 May 2002. [DOI] [PubMed] [Google Scholar]
- 40.Xiang S.-K., Hua F., Tang Y., Jiang X.-H., Zhuang Q., Qian F.-J. Relationship between serum lipoprotein ratios and insulin resistance in polycystic ovary syndrome. Internet J Endocrinol. 2012;2012 doi: 10.1155/2012/173281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Legro R.S., Kunselman A.R., Dunaif A. Prevalence and predictors of dyslipidemia in women with polycystic ovary syndrome. Am J Med. 2001;111(8):607–613. doi: 10.1016/s0002-9343(01)00948-2. [DOI] [PubMed] [Google Scholar]
- 42.Ehrmann D.A. Polycystic ovary syndrome. N Engl J Med. 2005;352(12):1223–1236. doi: 10.1056/NEJMra041536. [DOI] [PubMed] [Google Scholar]
- 43.Legro R.S., Gnatuk C.L., Kunselman A.R., Dunaif A. Changes in glucose tolerance over time in women with polycystic ovary syndrome: a controlled study. J Clin Endocrinol Metab. 2005;90(6):3236–3242. doi: 10.1210/jc.2004-1843. [DOI] [PubMed] [Google Scholar]
- 44.Gagnon C., Baillargeon J.-P. Suitability of recommended limits for fasting glucose tests in women with polycystic ovary syndrome. Can Med Assoc J. 2007;176(7):933–938. doi: 10.1503/cmaj.060607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gougeon A. Regulation of ovarian follicular development in primates: facts and hypotheses. Endocr Rev. 1996;17(2):121–155. doi: 10.1210/edrv-17-2-121. [DOI] [PubMed] [Google Scholar]
- 46.Hillier S.G., Reichert L.E., Jr., Van hall E.V. Control of preovulatory follicular estrogen biosynthesis in the human ovary. J Clin Endocrinol Metab. 1981;52(5):847–856. doi: 10.1210/jcem-52-5-847. [DOI] [PubMed] [Google Scholar]
- 47.Eden J., Jones J., Carter G., Alaghband-Zadeh J. Follicular fluid concentrations of insulin-like growth factor 1, epidermal growth factor, transforming growth factor-alpha and sex-steroids in volume matched normal and polycystic human follicles. Clin Endocrinol. 1990;32(4):395–405. doi: 10.1111/j.1365-2265.1990.tb00879.x. [DOI] [PubMed] [Google Scholar]
- 48.Arroyo A., Laughlin G., Morales A., Yen S. Inappropriate gonadotropin secretion in polycystic ovary syndrome: influence of adiposity 1. J Clin Endocrinol Metab. 1997;82(11):3728–3733. doi: 10.1210/jcem.82.11.4377. [DOI] [PubMed] [Google Scholar]
- 49.Taylor A.E., McCourt B., Martin K.A. Determinants of abnormal gonadotropin secretion in clinically defined women with polycystic ovary syndrome 1. J Clin Endocrinol Metab. 1997;82(7):2248–2256. doi: 10.1210/jcem.82.7.4105. [DOI] [PubMed] [Google Scholar]
- 50.Cheung A.P., Chang R.J. Endocrinology: pituitary responsiveness to gonadotrophin-releasing hormone agonist stimulation: a dose—response comparison of luteinizing hormone/follicle-stimulating hormone secretion in women with polycystic ovary syndrome and normal women. Hum Reprod. 1995;10(5):1054–1059. doi: 10.1093/oxfordjournals.humrep.a136093. [DOI] [PubMed] [Google Scholar]