Abstract
Emerging findings suggest that Parkinson’s disease (PD) pathology (α-synuclein accumulation) and neuronal dysfunction may occur first in peripheral neurons of the autonomic nervous system including the enteric branches of the vagus nerve. The risk of PD increases greatly in people over the age of 65, a period of life in which chronic inflammation is common in many organ systems including the gut. Here we report that chronic mild focal intestinal inflammation accelerates the age of disease onset in α-synuclein mutant PD mice. Wild-type and PD mice treated with 0.5% dextran sodium sulfate (DSS) in their drinking water for 12 weeks beginning at 3 months of age exhibited histological and biochemical features of mild gut inflammation. The age of onset of motor dysfunction, evaluated using a rotarod test, gait analysis, and grip strength measurements, was significantly earlier in DSS-treated PD mice compared to control PD mice. Levels of the dopaminergic neuron marker tyrosine hydroxylase in the striatum and numbers of dopaminergic neurons in the substantia nigra were reduced in PD mice with gut inflammation. Levels of total and phosphorylated α-synuclein were elevated in enteric and brain neurons in DSS-treated PD mice, suggesting that mild gut inflammation accelerates α-synuclein pathology. Markers of inflammation in the colon and brain, but not in the blood, were elevated in DSS-treated PD mice, consistent with retrograde transneuronal propagation of α-synuclein pathology and neuroinflammation from the gut to the brain. Our findings suggest that interventions that reduce gut inflammation may prove beneficial in the prevention and treatment of PD.
Keywords: Enteric neurons, Alpha-synuclein, Neuroinflammation, Dopaminergic neurons, Parkinson’s disease
Introduction
Parkinson’s disease (PD) is the second most common age-related neurodegenerative disorder and the fourteenth cause of death; over 1 million Americans currently suffer from PD (Murphy et al. 2012). The diagnosis of PD is based on abnormalities in the control of body movements (tremor, bradykinesia, and rigidity) which are known to result from the degeneration of dopaminergic neurons in the substantia nigra (Gazewood et al. 2013). Data suggest that vulnerable neuronal populations in PD experience mitochondrial impairment, oxidative damage, and accumulation of the self-aggregating protein α-synuclein (Dias et al. 2013). In addition, microglial cell activation and production of pro-inflammatory cytokines occur in association with neuronal degeneration in PD (Tansey et al. 2008). Progress in the genetics of PD and the development of transgenic mouse models have established major roles for the intraneuronal accumulation of aggregating α-synuclein and mitochondrial dysfunction in the neurodegenerative process (Dawson et al. 2010; Houlden and Singleton 2012; Pickrell and Youle 2015).
Emerging evidence from studies of human subjects and animal models suggests that α-synuclein pathology and associated neuronal dysfunction may occur first in peripheral neurons, including the enteric nervous system and branches of the vagus nerve that innervate the gut, heart, and other organs (Del Tredici and Braak 2016). The pathology may then spread retrogradely to the brainstem, midbrain, and cerebral cortex. Evidence that enteric and autonomic nervous system dysfunction occurs prior to motor impairment comes from studies of PD patients and animal models. PD patients often have a history of chronic constipation and dysregulation of heart rate, consistent with a deficit in vagal cholinergic tone (Awerbuch and Sandyk 1994; Savica et al. 2010; Klingelhoefer and Reichmann 2015; Adams-Carr et al. 2016). α-synuclein pathology is present in gut tissues and enteric neurons of PD patients, and has been observed in gut surgical samples from subjects up to 20 years prior to their being diagnosed with PD (Stokholm et al. 2016). Transgenic mice overexpressing mutant α-synuclein exhibit an age-related reduction in gastrointestinal motility associated with vagal α-synuclein pathology (Noorian et al. 2012). Transgenic mice overexpressing wild-type human α-synuclein exhibit an early decrease in fecal water content and increased gut transit time, which are associated with α-synuclein accumulation in axons of the autonomic nervous system (Hallett et al. 2012). Colon tissue from PD patients exhibits an inflammatory phenotype compared to age-matched neurologically normal subjects (Devos et al. 2013). A pro-inflammatory diet (high fat, glucose, and fructose) exacerbates autonomic (vagal parasympathetic) dysfunction, whereas an anti-inflammatory intermittent fasting diet ameliorates dysfunction of brainstem cardiovagal neurons in transgenic mice overexpressing mutant human α-synuclein (Griffioen et al. 2013). However, it is not known whether local gut inflammation is sufficient to accelerate brain PD neuropathology and associated motor dysfunction.
In the present study, we tested the hypothesis that chronic mild gut inflammation can accelerate the age of disease onset, and worsen α-synuclein pathology and neuroinflammation in A53T α-synuclein mutant transgenic mice (PD mice). Gut inflammation was induced by treatment with 0.5% (w/v) dextran sodium sulfate (DSS) in the drinking water. We found that the age of onset of motor dysfunction was significantly earlier, and α-synuclein pathology and dopamine neuron degeneration were exacerbated, in DSS-treated PD mice compared to control PD mice. Markers of inflammation were increased in the brain, but not in the blood, of PD mice with gut inflammation compared to control PD mice. Our findings suggest the possibility that chronic mild gut inflammation can accelerate the onset of PD in predisposed individuals.
Methods
Mice, DSS Treatment, and Tissue Harvesting
Female transgenic mice overexpressing human mutant (A53T) α-synuclein (PD mice) and age-matched female littermate wild-type (WT) control mice were used for all experiments. The generation and initial phenotypic characterization of this line of PD mice is described in Chandra et al. (2005), and findings from a study showing impaired regulation of brainstem cardiovagal control of heart rate in this PD mouse line have been reported (Griffioen et al. 2013). Mice had ad libitum access to food and water and were maintained on a 12-h light/12-h dark cycle. We employed only female mice in the present study because preliminary observations indicated less inter-animal variability in the age of onset of motor dysfunction in female a-synuclein mutant mice compared to males. WT and PD mice were treated with or without 0.5% (w/v) DSS with a molecular weight of 40,000 (Alfa Aesar, Ward Hill, MA) in their drinking water for 12 weeks beginning at 3 months of age. Body weights were recorded every 2 weeks beginning immediately before initiation of DSS treatment. At the end of the study, mice were euthanized using isoflurane anesthetic, and the serum was removed from the inferior vena cava and the vasculature then perfused with cold saline. The small intestine, colon, and brain were rapidly removed on ice. After measuring their lengths, the small intestine and colon were cut transversely into several pieces. Some of the pieces were flash-frozen and the other pieces were fixed in a solution of 4% paraformaldehyde in PBS. The brain was cut in the sagittal plane at the midline; half of the brain was fixed in a solution of 4% paraformaldehyde in PBS, and the striatum was removed from the other half-brain and flash-frozen. Frozen tissue was stored at − 80 °C. All procedures were approved by the Animal Care and Use Committee of the National Institute on Aging Intramural Research Program.
Motor Performance Tests
Three different tests were used to evaluate motor performance, namely, rotarod, gate analysis, and grip strength. Mice were placed on a rotarod apparatus (3 cm diameter rod; Med Associates; St. Albans, VT) with an initially slow rotation speed of four revolutions per minute (RPM). The rod was then slowly accelerated to 40 RPM over a 300-s period, and the latency until the mouse fell from the rotating rod was recorded. Each mouse was tested three times. Mice were tested on the rotarod every 2 weeks. Gate analysis and grip strength measurements were made at the 12-week intervention time point. For the gate analysis, mice were acclimated to the testing apparatus for 3 days prior to testing; the apparatus consisted of a runway (4.5 cm wide and 42 cm long, 12 cm high) leading to a dark box (20 × 15 × 12 cm). All four paws of the mouse were wetted with commercially available ink and the mouse was let to walk freely down the runway towards the goal box. From the ink foot tracks, the stride lengths of the front and hind legs were quantified. Grip strength of the forepaws was quantified using a grip strength meter (Bioseb, Chaville, France) as described previously (Sconce et al. 2015).
Evaluation of Gut Pathology
Feces were collected every 2 weeks and evaluated for the presence of blood (guaiac paper test; ColoScreen; Helena Laboratories, Beaumont, Texas). Fixed tissue sections of intestine were stained with hematoxylin and eosin (Richard Allen Scientific, Kalamazoo, MI) and evaluated for histo-pathology by an investigator blinded as to mouse genotype and treatment group. Chronic inflammation of the mucosal layer (infiltration of lymphoid cells and macrophages, crypt loss, or crypt distortion) was scored on the following scale: 0, none; 1, mild (patchy, limited to one or two foci); two, moderate (≧ 3 foci, but area limited to < 1/3 of colon); and three, severe (more than 1/3 area of the colon is afflicted). Active inflammation (neutrophil infiltration in the mucosal layer, surface epithelial cells with reactive atypia) was scored on the following scale: 0, absent; 1, present (neutrophils and regenerating epithelium); 2, present (erosion and/or cryptitis, in addition to neutrophils and regenerating epithelium). Submucosal inflammation (infiltration of lymphoid cells into the submucosal layer) was scored on the following scale: 0, none; 1, observed in 1 or 2 foci; and 2, observed in ≧ 3 foci.
Immunoblots
For immunoblot analysis, brain tissues were homogenized with sonication in 10 volumes (w/v) of TBS containing 1 mM DTT, 1 mM PMSF, 0.2 mM Na3VO4, 0.02% NaN3, and a proteinase inhibitor cocktail (Roche), and centrifuged at 21000 × g 30 min at 4 °C. The supernatants were used for analysis. Protein concentration was measured by BCA assay kit (Pierce, Rockford, IL) and sample mixed with NuPAGE LDL sample buffer (Novex) were separated (12 μg protein/lane) in a 4–12% gradient Bis–Tris gel (Novex). The proteins were then transferred from the gel to a 0.45-μm-thick nitro-cellulose membrane (Novex). After Blocking with 5% skim milk in TBS-T (20 mM Tris HCl, pH 7.5, 0.05% Tween-20) for 1 h, membranes were incubated overnight at 4 °C in TBS-T containing a primary antibody which included a rabbit polyclonal α-synuclein antibody (AB15530, Abcam) which reacts with human but not mouse α-synuclein; a mouse monoclonal tyrosine hydroxylase (TH) antibody (MAB318, Millipore); and a rabbit polyclonal β-actin antibody (A2066, Sigma Aldrich) for protein loading control. All primary antibodies were used at a 1:1000 dilution in 5% skim milk/TBS-T. Secondary antibodies included anti-mouse and anti-rabbit IgG conjugated with HRP and were used at 1:2000 dilution in 5% skim milk/TBS-T (1 h incubation at room temperature). The immunoreactive proteins were detected using an enhanced chemiluminescence kit (Thermo Fisher Scientific). The signal intensities of immunoreactive protein bands were quantified using ImageJ software (National Institutes of Health, Bethesda, MD). The optical density of the α-synuclein and TH immunoreactive bands was normalized to the β-actin band in the same lanes.
Immunohistochemistry
Tissue samples were fixed in 4% paraformaldehyde in PBS, embedded in paraffin, sectioned at 10 μm thickness, and mounted on Superfrost Plus microscope slides (Fisher Scientific, Pittsburgh, PA). Sections were de-paraffinized, rehydrated, incubated in boiling citrate buffer (10 mM citric acid, 0.05% Tween-20, pH 6.0) for 10 min in a microwave, and then incubated in 0.3% hydrogen peroxide in methanol for 30 min to block endogenous peroxidases. For immunofluorescence staining, tissue sections were incubated in blocking buffer (5% normal horse serum and 0.2% Triton X-100 in PBS) for 1 h, and then incubated with a primary antibody in blocking buffer overnight at 4 °C. The primary antibodies included a rabbit polyclonal α-synuclein antibody (AB15530, Abcam; 1:200 dilution), a rabbit polyclonal antibody against a pathogenic phosphorylated form of α-synuclein (pS129 αSyn (PPS091, R&D Systems; 1:200 dilution), a mouse monoclonal TH antibody (MAB318, Millipore; 1:200 dilution), and a rabbit polyclonal antibody against the microglial marker protein Iba1 (019–19741, Wako; 1:200 dilution). After thorough washes in PBS, sections were then incubated with a biotinylated secondary antibody (Vectastain Universal–Elite ABC Kit, Vector Laboratories) for 1 h. After washing with PBS, the sections were incubated in the avidin/biotin complex (Vectastain Universal–Elite ABC Kit, Vector laboratories) for 30 min. The antibody complex was detected using the peroxidase ABC system with diaminobenzidine (DAB) as the substrate following the manufacturer’s instructions (Vector Laboratories). The sections were examined and images were acquired using a Nikon bright-field microscope (Nikon, Ellipse E600) with 20X and 40X objectives. TH immunoreactive neurons were counted in every sixth section through the entire volume of the substantia nigra. Counts were performed in a blinded manner using ImageJ software (National Institutes of Health, Bethesda, MD).
Quantification of Cytokine and α-Synuclein Levels
Brain, colon tissues, and serum levels of cytokines (TNF-α, IL-6, IL-1β, and IL-10) were measured using a multiplex immunoassay kit (K15048D) and α-synuclein levels in colon and brain tissues were quantified using an immunoassay kit (K151TGD) from Meso Scale Discovery (Rockville, MD). The assays were performed using a QuickPlex SQ120 analyzer with MSD Discovery Workbench 4.0 software (Meso Scale Discovery, Rockville, MD) according to the manufacturer’s instructions with samples run in duplicate.
Statistical Analyses
Data were analyzed by two-way ANOVA with Bonferroni’s post hoc test, and Student’s t test for individual comparisons between groups, using GraphPad Prism (GraphPad software, Inc., San Diego, CA). Data are expressed as mean ± standard error of the mean (SEM), and p values of 0.05 or less were considered significant.
Results
Chronic Mild Gut Inflammation Hastens the Onset of Motor Dysfunction in PD Mice
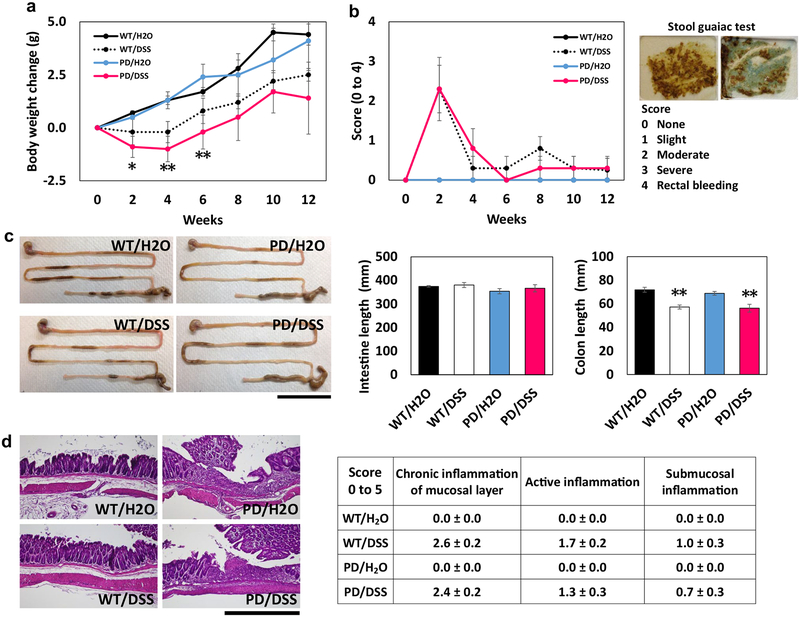
The line of α-synuclein mutant (A53T) transgenic mice used in the present study develop discernable motor dysfunction beginning at approximately 8 months of age, which becomes progressively worse over a period of several weeks until the mice are no longer able to ambulate (Chandra et al. 2005; Griffioen et al. 2013). We randomly assigned 3-month-old α-synuclein mutant transgenic mice (PD mice) and wild-type mice (WT) to DSS treatment or water control groups. Whereas control WT and PD mice gained weight progressively during the 12-week period, DSS-treated WT and PD mice did not gain weight during the first 4 weeks and thereafter gained weight at rates similar to mice not treated with DSS (Fig. 1a). At 2 and 4 weeks of DSS treatment, the body weights of PD mice were significantly lower than those of PD control mice. Analysis of fecal samples using a guaiac test revealed a transient appearance of blood at 2 weeks with recovery to normality by 4–6 weeks (Fig. 1b). At the 12-week time point, mice were euthanized and gut and brain tissues were collected. While the length of the small intestine was not different among the four groups of mice, the colons of DSS-treated WT and PD mice were significantly shorter than WT and PD mice not treated with DSS (Fig. 1c). Tissue sections of the colons of each mouse were stained with hematoxylin and eosin and scored in a blinded manner by an expert on cellular manifestations of gut inflammation (W. H.). There was no evidence of inflammation in WT or PD mice not treated with DSS, whereas all DSS-treated mice exhibited moderate levels of inflammation in the epithelial layer but not in the submucosal layer, with no significant differences between WT and PD mice (Fig. 1d). The intestinal wall did not exhibit pathological features in mice in any of the four groups.
Fig. 1.
Chronic DSS ingestion results in transient gut leakiness and chronic mild intestinal inflammatory changes in wild-type and α-synuclein mutant transgenic mice. Beginning at 3 months of age (time 0), mice were provided water alone (H2O) or water containing DSS. a Body weights of wild-type mice (WT) and α-synuclein mutant transgenic PD mice (PD) treated with water (H2O) or water containing DSS. *p < 0.05, **p < 0.01 compared to the PD/H2O group. b Fecal blood scores (guaiac test) in mice in the four groups. c Lengths of the small intestine and colon in mice in the four groups. The images at the left show representative images and the graphs show the quantitative data. Scale bar, 5 cm. **p < 0.01 compared to mice in the WT/H2O and PD/H2O groups. d Histological analyses of cellular indicators of gut inflammation. The images at the left show examples of hematoxylin and eosin-stained sections of the intestinal wall, and the table shows the results of the scoring of the tissue sections from the mice. Scale bar, 200 μm. For all panels, n = 7 for WT/H2O, WT/DSS, and PD/DSS groups, and n = 8 for the PD/H2O group
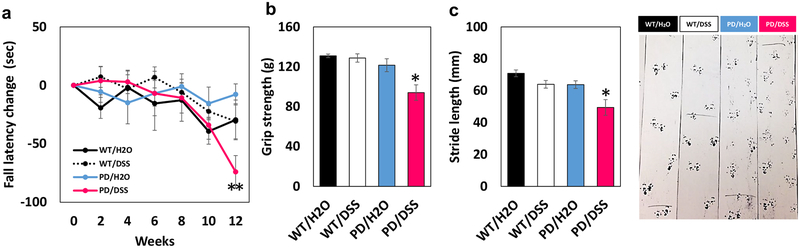
Motor performance on a rotarod was evaluated every 2 weeks after initiation of DSS treatment, and tests of grip strength and stride length were performed at 12 weeks. Rotarod performance was similar in all four groups through week eight of treatment (Fig. 2a). However, whereas rotarod performance was maintained in WT mice and control PD mice through 12 weeks, DSS-treated mice exhibited a highly significant decline in performance by 12 weeks (Fig. 2a). We therefore performed two additional tests of motor performance, grip strength and stride length, at that time point. DSS-treated PD mice exhibited significantly reduced grip strength compared to control PD mice and WT mice (Fig. 2b). Stride analysis revealed that stride length during walking was significantly reduced in PD mice in the DSS group compared to PD mice in the control group and compared to wild-type mice treated with DSS (Fig. 2c). Because there was a clear decline in motor function at 12 weeks after initiation of DSS treatment (6 months of age), we chose to euthanize all mice at this time point with the expectation that neuropathological differences between control and DSS-treated PD mice would be discernable.
Fig. 2.
Chronic mild gut inflammation hastens the onset of motor dys-function in α-synuclein mutant transgenic mice. a Mice in each of the four groups were tested on the rotarod at baseline (time 0) and every 2 weeks after the initiation of DSS treatment. *p < 0.05 compared to mice in the PD/H2O group, +p < 0.05, ++p < 0.01 compared to mice in the WT/DSS group. b Results of the grip strength test. *p < 0.05 compared to mice in the PD/DSS group. c Results of the stride length analysis test. *p < 0.05 compared to mice in the PD/DSS group, +p < 0.05 compared to each of the WT groups. The images at the right show examples of ink foot prints of one mouse in each group. For all panels, n = 7 for WT/H2O, WT/DSS, and PD/DSS groups, and n = 8 for the PD/H2O group
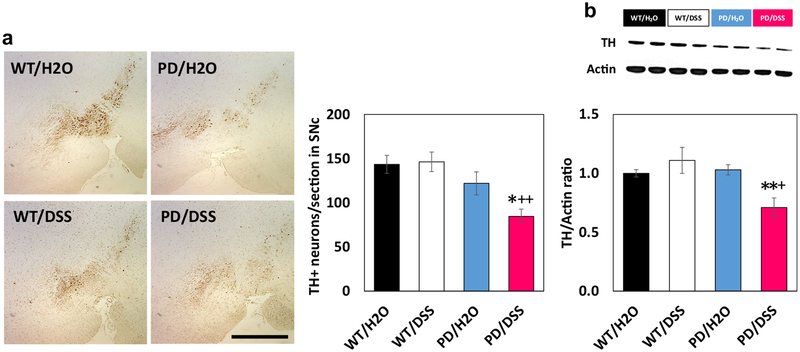
Chronic Gut Inflammation Triggers Degeneration of Dopaminergic Neurons, and Exacerbates α‑Synuclein Pathology in the Gut and Brain of PD Mice
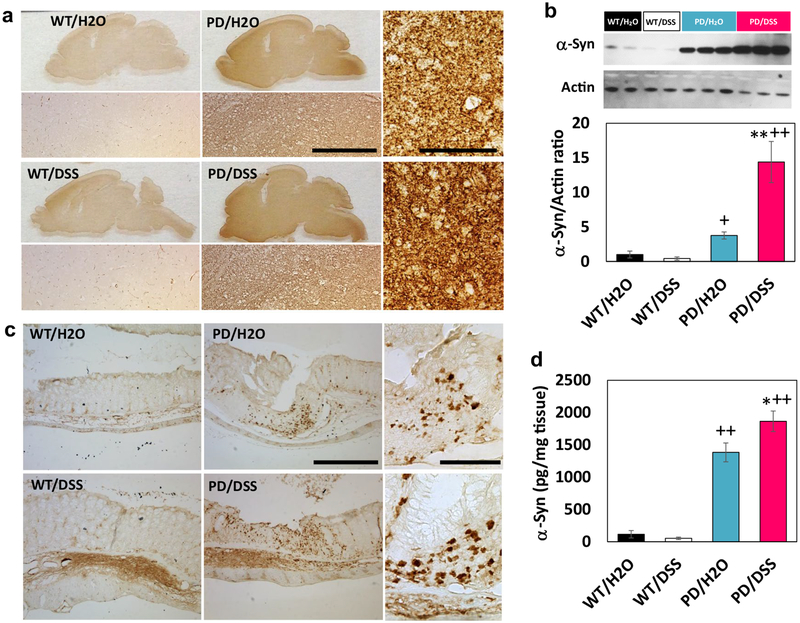
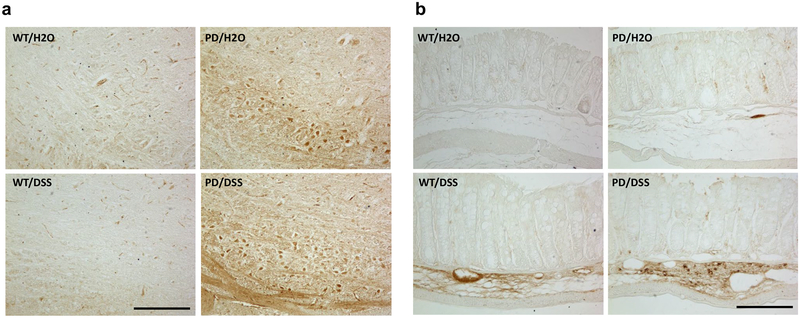
One hemisphere of the brain was fixed and processed for immunohistochemical analyses, and the striatum was removed and used for immunoblot and cytokine assays. Whereas numbers of tyrosine hydroxylase (TH) immunoreactive dopaminergic neurons in the substantia nigra were similar in WT control mice, DSS-treated WT mice, and control PD mice, there was a significant decrease in dopaminergic neuron numbers in DSS-treated PD mice compared to control PD mice (Fig. 3a). Immunoblot analysis demonstrated a significant reduction in TH protein levels in the striatum of DSS-treated PD mice compared to each of the other three groups of mice (Fig. 3b). As expected, α-synuclein immunoreactivity was overtly greater in brain tissue sections from PD mice compared to WT mice (Fig. 4a). A visual comparison suggested that levels of α-synuclein immunoreactivity were greater in brains of DSS-treated PD mice compared to control PD mice, and quantification of immunoblots demonstrated that α-synuclein protein levels were 3.8-fold greater in the striatum of DSS-treated PD mice compared to control PD mice (Fig. 4a, b). The α-synuclein immunoreactivity in both colon submucosal and myenteric plexus layers of DSS-treated PD mice was also higher than control PD mice (Fig. 4c). ELISA analysis of soluble α-synuclein levels also demonstrated a significant elevation of α-synuclein concentrations in the colon of DSS-treated PD mice compared to PD mice not treated with DSS (Fig. 4d). There was no detectable α-synuclein in the serum of mice in any of the four groups.
Fig. 3.
Chronic mild gut inflammation triggers loss of substantia nigra dopaminergic neurons in α-synuclein mutant transgenic mice. a The images show TH immunoreactivity in neurons in the substantia nigra of mice in the indicated groups. Scale bar, 500 μm. The graph shows the results of counts of TH positive neurons. b The upper panel is an image of an immunoblot showing relative levels of TH and β-actin in striatal tissue samples from two mice in each of the indicated groups. The lower panel shows the results of densitometric analyses of TH protein levels (normalized to actin levels) in all mice. *p < 0.05, **p < 0.01 compared to mice in the PD/H2O group and +p < 0.05, ++p < 0.01 compared to mice in the WT/DSS group. For all panels, n = 7 for WT/H2O, WT/DSS, and PD/DSS groups, and n = 8 for the PD/H2O group
Fig. 4.
Chronic mild gut inflammation increases the accumulation of α-synuclein in neurons in the enteric nervous system and brain in α-synuclein mutant transgenic mice. a Images showing α-synuclein immunoreactivity in sagittal brain sections from mice in the indicated groups. Immediately below each low-magnification micrograph is a high-magnification image of the midbrain. Scale bar, 200 μm. The two high-magnification images at the right are from the substantia nigra of mice in the PD/H2O (upper) and PD/DSS (lower) groups. Scale bar, 100 μm. b The upper panel is an image of an immunoblot showing relative levels of α-synuclein and β-actin in striatal tissue samples from two mice in each of the WT groups and three mice in each of PD groups. The middle panel shows the results of densitometric analyses of α-synuclein protein levels (normalized to actin levels) in all mice. **p < 0.01 compared to mice in the PD/H2O group and +p < 0.05, ++p < 0.01 compared to each of the WT groups. c Images showing α-synuclein immunoreactivity in colon tissue sections from mice in the indicated groups. Scale bar, 500 μm. The two high-magnification images at the right show α-synuclein immunoreactive enteric neurons in the PD/H2O (upper) and PD/DSS (lower) groups. Scale bar, 100 μm. d Results of ELISA analysis of α-synuclein protein levels in colon tissue homogenates from mice in the indicated groups. *p < 0.05 compared to the PD/H2O group and ++p < 0.01 compared to each of the WT groups. For all panels, n = 7 for WT/H2O, WT/DSS, and PD/DSS groups, and n = 8 for the PD/H2O group
We next immunostained sections of brain and colon with an antibody that selectively binds to α-synuclein phosphorylated on amino acid (serine) 129. Previous findings suggest that this phosphorylated form of α-synuclein is particularly protein to accumulation within neurons affected in PD (Oueslati 2016). We focused on neurons in the brainstem nucleus tractus solitarius (NTS) which regulate vagal parasympathetic outflow to the gut (Travagli and Anselmi 2016) and are also neurons vulnerable to degeneration in PD (Del Tredici et al. 2002). We found that p-S129 phosphorylated α-synuclein immunoreactivity was abundant in neuron in the brainstem nucleus tractus solitarius of PD mice in the DSS group, less abundant in PD mice in the water control group, and absent in wildtype mice in either water or DSS groups (Fig. 5a). In the colon, p-S129 phosphorylated α-synuclein immunoreactivity was prominent in presumptive enteric neurons of DSS-treated PD mice, and sparse or absent in mice in the other three groups (Fig. 5b).
Fig. 5.
Chronic mild gut inflammation increases the accumulation of phosphorylated (p-S129) α-synuclein in neurons in the brainstem and enteric nervous system in α-synuclein mutant transgenic mice. a Images showing p-S129 α-synuclein immunoreactivity in neurons of the nucleus tractus solitarius in the brainstem of mice in the indicated groups. Scale bar, 200 μm. b Images showing p-S129 α-synuclein immunoreactivity in the colon of mice in the indicated groups. Scale bar, 200 μm
DSS Treatment Induces Inflammation in the Gut and Brain, but not in the Blood
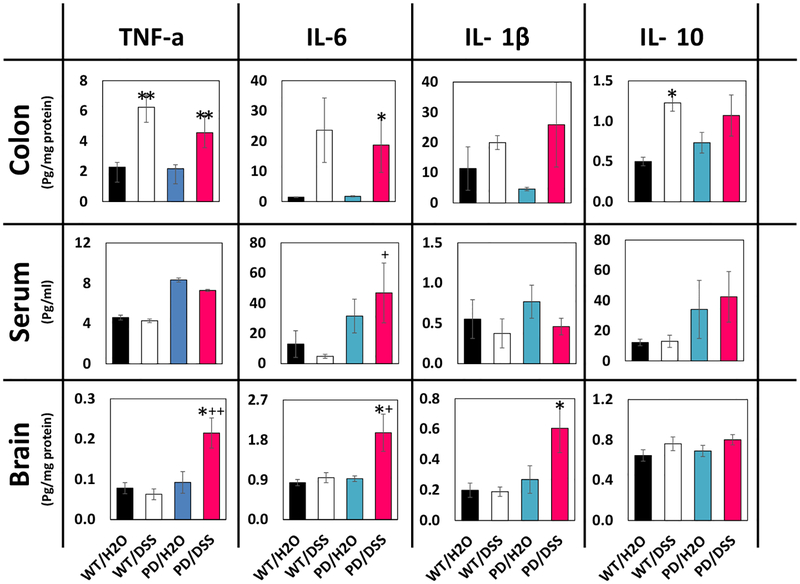
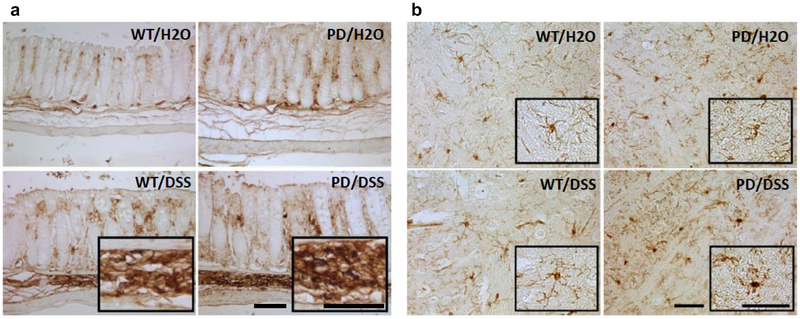
To confirm that DSS treatment did in fact increase inflammation in the gut, we measured levels of cytokines that are typically associated with tissue inflammation in colon tissue samples from mice in all four groups. Levels of tumor necrosis factor a (TNF-α) were significantly elevated 2.7- and 2.1-fold in DSS-treated WT and PD mice, respectively, compared to untreated WT and PD mice (Fig. 6). Interleukin 6 (IL-6) levels were significantly elevated 9.4-fold in colon tissue from DSS-treated PD mice, compared to untreated PD mice. There were non-significant trends towards elevation of interleukin 1β (IL-1β) and interleukin 10 (IL-10) concentrations in DSS-treated PD mice compared to untreated PD mice (5.7- and 1.5-fold, p = 0.08 and 0.18, respectively). On the other hands, there were no significant effects of DSS treatment in both WT and PD mice on levels of any of the four cytokines in the serum (Fig. 6). In contrast to the lack of effect of DSS treatment on serum cytokine levels, DSS-treated PD mice exhibited elevated levels of TNF-α, IL-6, and IL-1β in striatal tissue samples (2.3-, 1.9-, and 2.3-fold, respectively) (Fig. 6). As another indicator of local tissue inflammation, we evaluated colon and brain tissue sections that had been immunostained with an antibody specific for microglia/macrophages. Macrophage infiltration was observed in intestinal tissue of DSS-treated WT and PD mice with no obvious differences between WT and PD mice (Fig. 7a). Microglia with an activated morphology (unramified and intensely stained with Iba-1) were evident in striatum of DSS-treated PD mice, to a lesser extent in control PD mice, and rarely or not at all in striatal sections from WT mice which instead contained microglia with a quiescent-ramified morphology (Fig. 7b).
Fig. 6.
Chronic mild gut inflammation increases the levels of pro-inflammatory cytokines in the gut and brain, but not in the blood. Concentrations of the indicated cytokines were quantified in brain (striatum) tissue, serum, and colon tissue from mice in the indicated groups. For all panels, n = 7 for WT/H2O, WT/DSS, and PD/DSS groups, and n = 8 for the PD/H2O group. *p < 0.05, **p < 0.01 compared to the value for mice in the H2O group of the same genotype and +p <0.05, ++p < 0.01 compared to each of the WT groups
Fig. 7.
Chronic mild gut inflammation induces activation of macrophages/microglia in the gut and brain. Sections of gut and brain tissue were immunostained with antibody Iba-1 which reacts with an antigen specific for macrophages and microglia. a Images of colon tissue sections from mice in the indicated groups. Scale bar, 100 μm. b Images of striatal tissue sections from mice in the indicated groups. Scale bar, 50 μm
Discussion
Previous clinical findings indicated that PD patients often exhibit impaired regulation of gut motility which manifests as chronic constipation, and inflammatory bowel diseases or irritable bowel syndrome-like symptoms, occurring prior to the onset of motor symptoms (Lai et al. 2014; Villum-sen et al. 2018; Adams-Carr et al. 2016). Such disorders of gut motility typically involve local inflammation in the gut tissue (Reichardt et al. 2017). However, it is not known whether chronic gut inflammation affects the pathogenic processes involved in the dysfunction and degeneration of dopaminergic neurons in the brain. Our findings demonstrate that chronic mild gut inflammation induced by a low concentration of DSS in the drinking water (0.5%) is sufficient to accelerate the onset of motor dysfunction in an α-synuclein mutant mouse model of PD. Compared to control PD mice, those subjected to experimental chronic low-grade gut inflammation exhibited exacerbations of α-synuclein pathology and neuroinflammation, and loss of nigrostriatal dopaminergic neurons. Acute (1–7 days) severe gut inflammation results in either a reduction or no change in α-synuclein protein levels in gut tissue of wild-type mice (Prigent et al. 2018), but the relevance of the latter findings to PD pathogenesis is unclear.
Postmortem analyses of brain tissue samples have documented increased levels of pro-inflammatory cytokines and microglial hyperactivation in vulnerable brain regions in PD (Hirsch et al. 2012). Evidence from studies of cell culture and animal models suggests that abnormal accumulation of α-synuclein in neurons can trigger microglial activation and pro-inflammatory cytokine production (Chung et al. 2009; Allen Reish and Standaert 2015). Conversely, an inflammatory cellular milieu exacerbates α-synuclein neuropathology (Choi et al. 2009; Gao et al. 2011). Such reciprocal interactions between α-synuclein pathology and inflammation are therefore likely involved in the adverse effects of local low-grade gut inflammation on dopaminergic neuron function and viability documented in the present study.
Markers of tissue inflammation were elevated in the gut and brain tissues, but not in the blood of DSS-treated mice suggesting a non-systemic mode of disease propagation from the gut to the brain. We found that chronic gut inflammation exacerbates the accumulation of α-synuclein in neurons in the gut, which are known to receive innervation from the vagus nerve. Emerging evidence from studies of PD patients and animal models suggests that α-synuclein pathology occurs in enteric neurons and the vagus nerve axons that innervate the gut before α-synuclein neuropathology is evident in the brain (Del Tredici and Braak 2016). Pathogenic accumulation of α-synuclein may then propagate retrogradely to the brainstem via vagal parasympathetic axons, and thence from brainstem neurons to midbrain dopaminergic neurons. α-synuclein is normally expressed in relatively high amounts in enteric neurons and associated vagal preganglionic axon terminals (Phillips et al. 2008) and accumulates excessively in enteric neurons in α-synuclein mutant transgenic mice (Bencsik et al. 2014). When PD patient brain cell lysates and recombinant α-synuclein into the intestinal wall in rats, α-synuclein pathology is propagated via the vagus nerve to brainstem cholinergic neurons (Holmqvist et al. 2014). Similarly, when a viral vector expressing human α-synuclein was injected directly into the vagus nerve in the neck, α-synuclein pathology developed in brainstem neurons and spread to the midbrain and cerebral cortex (Ulusoy et al. 2013). A causal role for retrograde propagation of α-synuclein pathology and neuroinflammation from gut to brain in PD pathogenesis was suggested from a study that found patients who had undergone vagotomy as a treatment for duodenal ulcers had a reduced risk for PD (Svensson et al. 2015). However, a subsequent study of a different cohort found no significant effect of vagotomy on PD risk (Liu et al. 2017). Exposure of mice to the pesticide and mitochondrial toxin rotenone causes accumulation of α-synuclein in enteric and brainstem vagal neurons, and this was prevented by hemivagotomy (Pan-Montojo et al. 2012). Our findings are therefore consistent with the possibility that local gut inflammation can trigger or exacerbate brain α-synuclein pathology and dopaminergic neuron degeneration by a mechanism involving retrograde vagus nerve-mediated propagation of the PD disease process from the gut to the brain.
The ability of focal chronic low-grade gut inflammation to accelerate α-synuclein pathology and associated dopaminergic neuron degeneration and neuroinflammation in the brain in a mouse model has implications for diagnosis and treatment of PD. For example, one could envision evaluation of gut α-synuclein pathology in tissue biopsies or by imaging methods similar to those now being used for analyses of amyloid β-peptide plaque and Tau tangle pathologies in AD (Villemagne 2016; Hall et al. 2017). Western diets high in saturated fats and sugars promote gut inflammation (Reichardt et al. 2017) and can exacerbate brain neuropathological and associated behavioral deficits in animal models of AD and PD (Rotermund et al. 2014). It was also reported that such pro-inflammatory diets can exacerbate impairment of brainstem cardiovagal control of heart rate in α-synuclein mutant transgenic mice (Griffioen et al. 2013). Interventions that enhance parasympathetic tone and/or protect the vagal neurons against α-synuclein pathology and inflammation may therefore be useful in the prevention and treatment of PD. Consistent with this possibility, exercise and intermittent fasting enhance parasympathetic tone and are neuroprotective in animal models of PD (Mattson and Wan 2005; Mattson 2012 Griffioen et al. 2013). Moreover, it was recently reported that vagal nerve stimulation improves locomotion and protects substantia nigra dopaminergic neurons in a rat PD model (Farrand et al. 2017). Based upon our findings, another approach would be to reduce gut inflammation. In this regard, it was reported that manipulations of the gut microbiota that suppress gut inflammation reduce motor deficits in α-synuclein overexpressing transgenic mice, whereas fecal transplants from PD patients exacerbated the motor deficits (Sampson et al. 2016). Future clinical trials of interventions that suppress gut inflammation in human PD patients would therefore seem warranted.
Significance Statement.
Parkinson’s disease (PD) patients exhibit gut dysfunction associated with local inflammation and enteric neuron α-synuclein pathology that often precede clinical diagnosis based on motor symptoms. However, it is not known whether gut inflammation is causally involved in the development of brain neuropathology and motor dysfunction in PD. Here we show that chronic mild gut inflammation is sufficient to accelerate the onset of motor dysfunction and dopaminergic neuron degeneration in α-synuclein mutant transgenic PD mice. The gut inflammation exacerbates brain α-synuclein pathology and inflammation without causing systemic inflammation. These findings support a scenario in which gut inflammation accelerates the propagation of α-synuclein from the gut to the brain via a transneuronal, vagus nerve-mediated mechanism. Our findings suggest that interventions that reduce gut inflammation may prove beneficial in the prevention and treatment of PD.
Acknowledgements
This research was supported by the Intramural Research Program of the National Institute on Aging, National Institutes of Health.
Footnotes
Conflict of interest The authors have no conflicts to declare.
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Adams-Carr KL, Bestwick JP, Shribman S, Lees A, Schrag A, & Noyce AJ (2016). Constipation preceding Parkinson’s disease: A systematic review and meta-analysis. Journal of Neurology, Neurosurgery and Psychiatry, 87, 710–716. [DOI] [PubMed] [Google Scholar]
- Allen Reish HE, & Standaert DG (2015). Role of α-synuclein in inducing innate and adaptive immunity in Parkinson disease. Journal of Parkinson’s Disease, 5, 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awerbuch GI, & Sandyk R (1994). Autonomic functions in the early stages of Parkinson’s disease. International Journal of Neuroscience, 74, 9–16. [DOI] [PubMed] [Google Scholar]
- Bencsik A, Muselli L, Leboidre M, Lakhdar L, & Baron T (2014). Early and persistent expression of phosphorylated α-synuclein in the enteric nervous system of A53T mutant human α-synuclein transgenic mice. Journal of Neuropathology and Experimental Neurology, 73, 1144–1151. [DOI] [PubMed] [Google Scholar]
- Chandra S, Gallardo G, Fernández-Chacón R, Schlüter OM, & Südhof TC (2005). Alpha-synuclein cooperates with CSPalpha in preventing neurodegeneration. Cell, 123, 383–396. [DOI] [PubMed] [Google Scholar]
- Choi DY, Liu M, Hunter RL, Cass WA, Pandya JD, Sullivan PG, et al. (2009). Striatal neuroinflammation promotes Parkinsonism in rats. PLoS ONE, 4(5), e5482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung CY, Koprich JB, Siddiqi H, & Isacson O (2009). Dynamic changes in presynaptic and axonal transport proteins combined with striatal neuroinflammation precede dopaminergic neuronal loss in a rat model of AAV alpha-synucleinopathy. Journal of Neuroscience, 29, 3365–3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson TM, Ko HS, & Dawson VL (2010). Genetic animal models of Parkinson’s disease. Neuron, 66, 646–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Tredici K, & Braak H (2016). Review: Sporadic Parkinson’s disease: Development and distribution of α-synuclein pathology. Neuropathology and Applied Neurobiology, 42, 33–50. [DOI] [PubMed] [Google Scholar]
- Del Tredici K, Rüb U, De Vos RA, Bohl JR, & Braak H (2002). Where does parkinson disease pathology begin in the brain? Journal of Neuropathology and Experimental Neurology, 61, 413–426. [DOI] [PubMed] [Google Scholar]
- Devos D, Lebouvier T, Lardeux B, Biraud M, Rouaud T, Pouclet H, et al. (2013). Colonic inflammation in Parkinson’s disease. Neurobiology of Diseases, 50, 42–48. [DOI] [PubMed] [Google Scholar]
- Dias V, Junn E, & Mouradian MM (2013). The role of oxidative stress in Parkinson’s disease. Journal of Parkinson’s Disease, 3, 461–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrand AQ, Helke KL, Gregory RA, Gooz M, Hinson VK, & Boger HA (2017). Vagus nerve stimulation improves locomotion and neuronal populations in a model of Parkinson’s disease. Brain Stimulation, 10, 1045–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao HM, Zhang F, Zhou H, Kam W, Wilson B, & Hong JS (2011). Neuroinflammation and α-synuclein dysfunction potentiate each other, driving chronic progression of neurodegeneration in a mouse model of Parkinson’s disease. Environmental Health Perspectives, 119, 807–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazewood JD, Richards DR, & Clebak K (2013). Parkinson disease: An update. American Family Physician, 87, 267–273. [PubMed] [Google Scholar]
- Griffioen KJ, Rothman SM, Ladenheim B, Wan R, Vranis N, Hutchison E, et al. (2013). Dietary energy intake modifies brainstem autonomic dysfunction caused by mutant α-synuclein. Neurobiology of Aging, 34, 928–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall B, Mak E, Cervenka S, Aigbirhio FI, Rowe JB, & O’Brien JT (2017). In vivo tau PET imaging in dementia: Pathophysiology, radiotracer quantification, and a systematic review of clinical findings. Ageing Research Reviews, 36, 50–63. [DOI] [PubMed] [Google Scholar]
- Hallett PJ, McLean JR, Kartunen A, Langston JW, & Isacson O (2012). α-Synuclein overexpressing transgenic mice show internal organ pathology and autonomic deficits. Neurobiology of Diseases, 47, 258–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch EC, Vyas S, & Hunot S (2012). Neuroinflammation in Parkinson’s disease. Parkinsonism and Related Disorders, 18(Suppl 1), S210–S212. [DOI] [PubMed] [Google Scholar]
- Holmqvist S, Chutna O, Bousset L, Aldrin-Kirk P, Li W, Björklund T, et al. (2014). Direct evidence of Parkinson pathology spread from the gastrointestinal tract to the brain in rats. Acta Neuropathologica, 128, 805–820. [DOI] [PubMed] [Google Scholar]
- Houlden H, & Singleton AB (2012). The genetics and neuropathology of Parkinson’s disease. Acta Neuropathologica, 124, 325–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingelhoefer L, & Reichmann H (2015). Pathogenesis of Parkinson disease–the gut-brain axis and environmental factors. Nature Reviews Neurology, 11, 625–636. [DOI] [PubMed] [Google Scholar]
- Lai SW, Liao KF, Lin CL, & Sung FC (2014). Irritable bowel syndrome correlates with increased risk of Parkinson’sdisease in Taiwan. European Journal of Epidemiology, 29, 57–62. [DOI] [PubMed] [Google Scholar]
- Liu B, Fang F, Pedersen NL, Tillander A, Ludvigsson JF, Ekbom A, et al. (2017). Vagotomy and Parkinson disease: A Swedish register-based matched-cohort study. Neurology, 88, 1996–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP (2012). Energy intake and exercise as determinants of brain health and vulnerability to injury and disease. Cell Metabolism, 16, 706–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, & Wan R (2005). Beneficial effects of intermittent fasting and caloric restriction on the cardiovascular and cerebrovascular systems. Journal of Nutritional Biochemistry, 16, 129–137. [DOI] [PubMed] [Google Scholar]
- Murphy SL, Xu J, & Kochanek KD (2012). Deaths: Preliminary data for 2010. CDC National Vital Statistics Reports, 60, 1–52. [PubMed] [Google Scholar]
- Noorian AR, Rha J, Annerino DM, Bernhard D, Taylor GM, & Greene JG (2012). Alpha-synuclein transgenic mice display age-related slowing of gastrointestinal motility associated with transgene expression in the vagal system. Neurobiology of Diseases, 48, 9–19. [DOI] [PubMed] [Google Scholar]
- Oueslati A (2016). Implication of alpha-synuclein phosphorylation at S129 in synucleinopathies: What have we learned in the last decade? Journal of Parkinson’s Disease, 6, 39–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan-Montojo F, Schwarz M, Winkler C, Arnhold M, O’Sullivan GA, Pal A, et al. (2012). Environmental toxins trigger PD-like progression via increased alpha-synuclein release from enteric neurons in mice. Scientific Reports, 2, 898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips RJ, Walter GC, Wilder SL, Baronowsky EA, & Powley TL (2008). Alpha-synuclein-immunopositive myenteric neurons and vagal preganglionic terminals: Autonomic pathway implicated in Parkinson’sdisease? Neuroscience, 153, 733–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickrell AM, & Youle RJ (2015). The roles of PINK1, parkin, and mitochondrial fidelity in Parkinson’s disease. Neuron, 85, 257–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prigent A, Gonzales J, Durand T, Le Berre-Scoul C, Rolli-Derkinderen M, Neunlist M, et al. (2018). Acute inflammation down-regulates alpha-synuclein expression in enteric neurons. Journal of Neurochemistry, 148(6), 746–760. 10.1111/jnc.14656. [DOI] [PubMed] [Google Scholar]
- Reichardt F, Chassaing B, Nezami BG, Li G, Tabatabavakili S, Mwangi S, et al. (2017). Western diet induces colonic nitrergic myenteric neuropathy and dysmotility in mice via saturated fatty acid- and lipopolysaccharide-induced TLR4 signalling. Journal of Physiology, 595, 1831–1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotermund C, Truckenmüller FM, Schell H, & Kahle PJ (2014). Diet-induced obesity accelerates the onset of terminal phenotypes in α-synuclein transgenic mice. Journal of Neurochemistry, 131, 848–858. [DOI] [PubMed] [Google Scholar]
- Sampson TR, Debelius JW, Thron T, Janssen S, Shastri GG, Ilhan ZE, et al. (2016). Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson’s disease. Cell, 167, 1469–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savica R, Rocca WA, & Ahlskog JE (2010). When does Parkinson disease start? Archives of Neurology, 67, 798–801. [DOI] [PubMed] [Google Scholar]
- Sconce MD, Churchill MJ, Greene RE, & Meshul CK (2015). Intervention with exercise restores motor deficits but not nigrostriatal loss in a progressive MPTP mouse model of Parkinson’s disease. Neuroscience, 299, 156–174. [DOI] [PubMed] [Google Scholar]
- Stokholm MG, Danielsen EH, Hamilton-Dutoit SJ, & Borghammer P (2016). Pathological α-synuclein in gastrointestinal tissues from prodromal Parkinson disease patients. Annals of Neurology, 79, 940–949. [DOI] [PubMed] [Google Scholar]
- Svensson E, Horváth-Puhó E, Thomsen RW, Djurhuus JC, Pedersen L, Borghammer P, et al. (2015). Vagotomy and sub-sequent risk of Parkinson’s disease. Annals of Neurology, 78, 522–529. [DOI] [PubMed] [Google Scholar]
- Tansey MG, Frank-Cannon TC, McCoy MK, Lee JK, Martinez TN, McAlpine FE, et al. (2008). Neuroinflammation in Parkinson’s disease: Is there sufficient evidence for mechanism-based interventional therapy? Front Biosci, 13, 709–717. [DOI] [PubMed] [Google Scholar]
- Travagli RA, & Anselmi L (2016). Vagal neurocircuitry and its influence on gastric motility. Nature Reviews Gastroenterology and Hepatology, 13, 389–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulusoy A, Rusconi R, Pérez-Revuelta BI, Musgrove RE, Helwig M, Winzen-Reichert B, et al. (2013). Caudo-rostral brain spreading of α-synuclein through vagal connections. EMBO Molecular Medicine, 5, 1119–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villemagne VL (2016). Amyloid imaging: Past, present and future perspectives. Ageing Research Reviews, 30, 95–106. [DOI] [PubMed] [Google Scholar]
- Villumsen M, Aznar S, Pakkenberg B, Jess T, & Brudek T (2018). Inflammatory bowel disease increases the risk of Parkinson’s disease: A Danish nationwide cohort study 1977–2014. Gut, 68(1), 18–24. [DOI] [PubMed] [Google Scholar]