Abstract
Infection can negatively impact brain functions. Here, Duan et al., (2018) show that specific PDGFRβ-expressing cell subtypes of the neurovascular unit release the chemokine CCL2 rapidly after systemic infection, leading to increased neural excitability.
When infection spreads in the body, organs suffer. In some circumstances, brain inflammation may develop from infection and affect neuronal functions (Klein et al., 2017). Understanding of the precise sequence of cellular and molecular events that occur in the brain after infection could guide the development of treatments to limit brain damage. Glial cells, mainly microglia and astrocytes, are key players in neuroinflammation. The resident immune cells of the nervous system, microglia are considered as first responders. Microglia have motile processes that constantly survey the parenchyma and physically respond to local injury within minutes (Davalos et al., 2005; Nimmerjahn et al., 2005). Both microglia and astrocytes contribute to acute and chronic inflammation by proliferating and releasing immune factors, such as cytokines and chemokines. For example, in response to systemic lipopolysaccharide (LPS) administration to model bacterial infection, microglia become activated with delayed directed process motility and repress hippocampal neurogenesis (Gyoneva et al., 2014; Monje et al., 2003). Systemic infection may compromise the integrity of the blood-brain barrier (BBB), which is regulated by endothelial cell tight junctions, astrocytic endfeet, microglia, and pericytes that together restrict the passage of plasma proteins and cells into the brain. In most studies, the effects of infection on cells that control BBB integrity are assessed at time points 24 hr and longer after initial infection.
In this issue of Neuron, Duan et al. (2018) shed new light on the very early triggers of neuroinflammation by investigating the molecular events that occur in the brain 2 hr following systemic bacterial or viral infection. Using a multi-pronged experimental approach including infection models in mice, single-cell RNA sequencing (scRNA-seq), histology, slice electrophysiology, behavior, and in vitro cell culture studies, the authors show that two subtypes of perivascular cells expressing platelet-derived growth factor receptor beta (PDGFRβ) were the predominant early source of the CC chemokine ligand 2 (CCL2, also referred to as monocyte chemotactic protein-1, MCP1) (Duan et al., 2018). The authors find that PDGFRβ+ cell – secreted CCL2 increased excitatory synaptic transmission by acting on neurons (see Figure 1). This work provides in vivo demonstration of PDGFRβ+ cells as early neuroinflammatory mediators and reveals a mechanism by which systemic infection can impact neuronal activity.
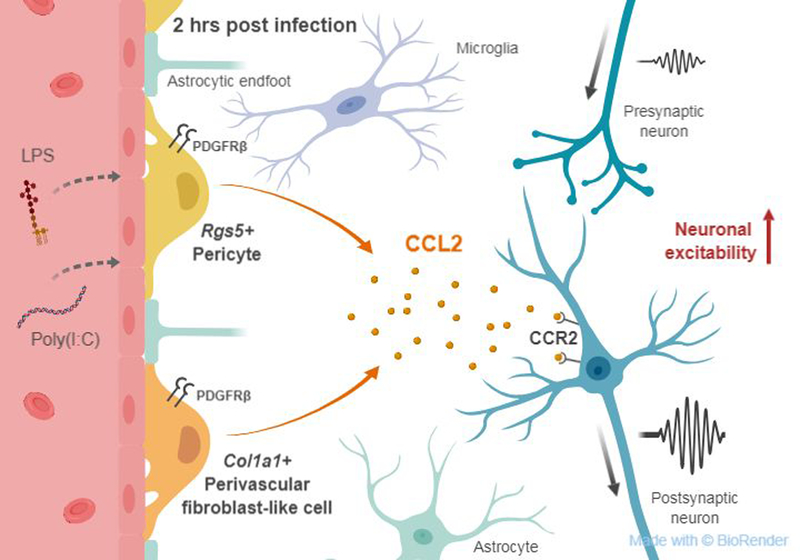
Figure 1. PDGFRβ+ cells as neuroinflammatory mediators after infection.
Within 2 hr of systemic bacterial or viral infection, two subtypes of PDGFRβ+ cells expressing either Col1a1 or Rgs5 are the major producers of the chemokine CCL2. CCL2 increases excitatory synaptic neurotransmission via the CCR2 receptor in neurons.
Duan et al. (2018) first assessed the hippocampal transcript and protein levels of cytokines and chemokines considered the “usual suspects” in neuroinflammation at 2 hr post intraperitoneal injection of bacterial or viral mimetics into 2 week old mice. The authors determined that CCL2 was the most highly induced chemokine in the hippocampus 2 hr after infection, and made the unanticipated discovery that Ccl2 mRNA was mainly upregulated in PDGFRβ+ cells, but not in endothelial cells, microglia, or astrocytes. The authors performed scRNA-seq to further dissect the transcriptional profile and identity of Ccl2-expressing cells after LPS injection. The cell populations with the highest Ccl2 expression were Collagen type I, alpha 1 (Col1a1)-expressing cells, Regulator of G Protein Signaling 5 (Rgs5)-expressing cells, microglia, and astrocytes. Microglia expressed Ccl2 at similar levels in both control and LPS treatment, and astrocytes expressed Ccl2 but at much lower levels than Pdgfrb-expressing cells. By cross-referencing previous profiling studies with their own scRNA-seq and histological verification, Duan et al. (2018) determined that CCL2 was mainly expressed by Col1a1+Pdgfrb+ cells, a perivascular fibroblast-like cell that lies at the meninges and large blood vessels and Rgs5+Pdgfrb+ cells, a population of cells lining small vessels that include pericytes.
Further evidence that PDGFRβ+ cells were the major contributors of CCL2 at 2 hr post-infection was obtained from conditional knockout mouse studies. Specific deletion of CCL2 in Pdgfrβ+ cells, but not in Tie2+ endothelial cells, reduced total hippocampal Ccl2 gene expression and CCL2 protein levels after infection. Cell-specific deletion of Ccl2 in PDGFRβ+ cells also rescued LPS-induced prolonged immobility during the mouse tail suspension test, a proposed model for sickness behavior. What is striking about these findings is that PDGFRβ+ cells, which include pericytes and other perivascular fibroblast-like cells, are generally not considered as first responders in neuroinflammation. Indeed, the described functions of pericytes include primarily the establishment and regulation of the BBB and other cerebrovascular functions (Sweeney et al., 2016). While pericytes express pathogen-associated molecular patterns (PAMP) receptors, release cytokines, and present antigens (Rustenhoven et al., 2017), data supporting a potential role for pericytes in neuroinflammation comes mostly from in vitro studies. Now, Duan et al. (2018) provide in vivo functional evidence that two subpopulations of PDGFRβ+ cells, namely the Rgs5+ Pdgfrβ+ pericytes along with a Col1a1+ Pdgfrβ+ perivascular fibroblast-like cell type, rapidly respond to systemic infection by producing the bulk of CCL2.
Although previous studies have demonstrated that CCL2 signaling can influence neuronal excitability (Cerri et al., 2017), Duan et al. (2018) use a robust set of in vitro and ex vivo experimental approaches, including whole cell recordings in acute postnatal brain slices, to determine the effect of PDGFRβ+ cell-derived CCL2 on neuronal activity. They find that CCL2 intensified AMPA receptor-mediated evoked excitatory postsynaptic potentials (EPSCs) accompanied by an increase in total synaptic charge. Notably, the paired-pulse ratio as a measure of presynaptic release probability and GABAergic inhibitory neurotransmission were not affected by CCL2, suggesting that CCL2 specifically increased postsynaptic neuronal excitability. Using mice deficient for the major CCL2 receptor C-C chemokine receptor type 2 (CCR2) or decreasing CCR2 in neurons by RNAi showed that neuronal CCR2 is required for CCL2 to increase excitatory neurotransmission. Importantly, CCL2 derived from culture human brain vascular pericytes stimulated with LPS or the viral mimetic Poly(I:C) was sufficient to increase total neuronal excitability when applied to slices. In acute brain slice recordings, LPS-injected Ccl2 knockout mice or mice lacking Ccl2 specifically in PDGFRβ+cells had lower mEPSC frequencies than LPS-injected littermate controls. Given that these effects were observed in pyramidal cells of the primary somatosensory cortex, hippocampal CA1 and CA3 regions, and in granule cells of the dentate gyrus, PDGFRβ+ cells may mediate inflammation-induced aberrant neuronal activity via CCl2 on a brain-wide scale. Given the importance of AMPA receptor signaling in memory and learning, could CCL2 released by PDGFRβ+cells affect cognitive functions? Furthermore, are any of these mechanisms altered with age? The authors show that their major findings can be recapitulated in adult mice around 2 and 7 months of age, but whether these effects occur in aged mice remains to be tested. Future studies are warranted to reveal whether a boost in excitatory neurotransmission by PDGFRβ+ cell-derived CCL2 drives changes to neuronal circuits in vivo.
Another important consideration raised by Duan et al. (2018) concerns the relative contributions of microglia and PDGFRβ+ cells to neuroinflammation during infection. While Duan et al. (2018) show that PDGFRβ+ cell subtypes were the largest early source of CCL2, microglia also produce CCL2 and other immune signaling molecules although perhaps at a time scale longer than 2 hr post infection. Is early CCL2 release by PDGFRβ+ cells a transient response or is it also required for chronic inflammatory responses by microglia and astrocytes? Are the effects of early CCL2 release additive or synergistic to microglial CCL2 or release of other immunomodulators during chronic inflammation? The authors provide transcriptional and protein evidence that PDGFRβ+ cells were perhaps the quickest responders to infection. At 2 and 6 hr post infection, Duan et al. (2018) do not observe microgliosis and astrogliosis in the hippocampus. However, a previous in vivo two-photon imaging study demonstrated that microglial process motility dynamics are altered 48 hr after systemic infection (Gyoneva et al., 2014). Is it possible that not only microglial process motility is affected, but also that pericytes and other perivascular cells modify their physical dynamics around blood vessels in response to infection? Finally, the authors suggest that the BBB is not open at 2 hr post LPS injection because of negligible leakage of the serum albumin-binding dye Evans blue in the brain at this time point. To unequivocally demonstrate that the BBB is not altered, more detailed examination, including assessment of tight junctions and leakage of other plasma proteins, is needed (Petersen et al., 2018). Indeed, microglia and pericytes are cell targets of the plasma protein fibrinogen (Petersen et al., 2018). Fibrinogen induces Ccl2 expression in microglia and is a trigger for rapid and sustained microglial responses (Petersen et al., 2018). A provocative study has shown an interaction between microglia and pericytes in disease by identifying that RGS5+ pericytes can assume a microglial-like phenotype after ischemic stroke (Rustenhoven et al., 2017). These studies together with Duan et al., (2018) provide a rationale for future investigation of the cross-talk between microglia, pericytes, neurons, and the BBB after systemic infection, cerebrovascular damage, and chronic inflammation.
In summary, the study by Duan et al. (2018) describes a new role for PDGFRβ+ cells as early neuroinflammatory mediators that can influence neural excitability after infection. Transcriptional profiling of subpopulations of cells at the neurovascular unit by single-cell analysis has the potential to change the way we think about the cellular mechanisms that regulate the communication between the brain and the periphery. Determining the types of resident brain cells that are causally involved in neuroinflammation will expand our understanding of how systemic infection alters the bidirectional communication between cells of the neurovascular unit to modulate brain functions. Future research should examine whether similar brain-immune communication pathways control neuronal activity in non-infectious inflammatory conditions, such as in autoimmune or neurodegenerative diseases with chronic brain inflammation.
Footnotes
DECLARATION OF INTERESTS
The authors declare financial interests potentially related to this work. K.A. is a co-founder and scientific advisor of MedaRed, Inc. Her interests are managed by the Gladstone Institutes in accordance with its conflict of interest policy.
REFERENCES
- Cerri C, Caleo M, and Bozzi Y (2017). Chemokines as new inflammatory players in the pathogenesis of epilepsy. Epilepsy Res 136, 77–83. [DOI] [PubMed] [Google Scholar]
- Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, Littman DR, Dustin ML, and Gan WB (2005). ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci 8, 752–758. [DOI] [PubMed] [Google Scholar]
- Duan L, Zhang X, Miao W, Sun Y, Xiong G, Wu Q, Li G, Yang P, Yu H, Li H, et al. (2018). PDGFRβ cells rapidly relay inflammatory signal from the circulatory system to neurons via chemokine CCL2. Neuron In press. [DOI] [PubMed] [Google Scholar]
- Gyoneva S, Davalos D, Biswas D, Swanger SA, Garnier-Amblard E, Loth F, Akassoglou K, and Traynelis SF (2014). Systemic inflammation regulates microglial responses to tissue damage in vivo. Glia 62, 1345–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein RS, Garber C, and Howard N (2017). Infectious immunity in the central nervous system and brain function. Nat Immunol 18, 132–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monje ML, Toda H, and Palmer TD (2003). Inflammatory blockade restores adult hippocampal neurogenesis. Science 302, 1760–1765. [DOI] [PubMed] [Google Scholar]
- Nimmerjahn A, Kirchhoff F, and Helmchen F (2005). Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 308, 1314–1318. [DOI] [PubMed] [Google Scholar]
- Petersen MA, Ryu JK, and Akassoglou K (2018). Fibrinogen in neurological diseases: mechanisms, imaging and therapeutics. Nat Rev Neurosci 19, 283–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rustenhoven J, Jansson D, Smyth LC, and Dragunow M (2017). Brain Pericytes As Mediators of Neuroinflammation. Trends Pharmacol Sci 38, 291–304. [DOI] [PubMed] [Google Scholar]
- Sweeney MD, Ayyadurai S, and Zlokovic BV (2016). Pericytes of the neurovascular unit: key functions and signaling pathways. Nat Neurosci 19, 771–783. [DOI] [PMC free article] [PubMed] [Google Scholar]