Abstract
Adequate skeletal muscle plasticity is an essential element for our well-being. Inversely, compromised muscle function can drastically affect quality of life, morbidity and mortality. Surprisingly however, skeletal muscle remains one of the most under-medicated organs. Accordingly, interventions in muscle diseases are scarce, not only in neuromuscular dystrophies, but also in highly prevalent secondary wasting pathologies such as sarcopenia and cachexia. Even in other diseases that exhibit a well-established risk correlation with a sedentary life-style and exercise, e.g. type 2 diabetes or cardiovascular pathologies, current treatments are mostly targeted on non-muscle tissues. In recent years, a renewed focus on skeletal muscle has led to the discovery of various novel drug targets and the design of new pharmacological approaches. This review will provide an overview of the current knowledge of the key mechanisms involved in muscle wasting conditions, and novel pharmacological avenues that could ameliorate muscle diseases.
Keywords: muscle wasting, atrophy, proteostasis, muscular dystrophy, gene therapy, exon-skipping
Introduction
Muscle wasting is observed in a variety of pathologies such as cancer, chronic kidney disease, heart failure, chronic obstructive pulmonary disease (COPD) as well as after prolonged inactivity or during aging. Reduced muscle mass and function is associated with a higher morbidity and mortality as well as reduced quality of life (1; 2). For example, loss of muscle mass is associated with a poorer prognosis and reduced response to therapy in cancer patients. Inversely, exercise exerts powerful effects on the prevention and treatment of many diseases (1; 2). Besides muscle wasting secondary to a pathology, there are several inherited muscular dystrophies characterized by muscle weakness. The etiology and mechanisms of these diseases are diverse and different to secondary muscle wasting. Even though the prevalence of people suffering from any kind of muscle weakness is high, there is no available treatment for muscle wasting and muscle remains an under-medicated organ. This review will provide an overview of the mechanistic aspects of muscle wasting, and summarize novel experimental and clinical pharmacological avenues aimed at skeletal muscle.
Muscle plasticity in different pathological conditions: key players and mechanisms
Skeletal muscle is a plastic tissue that constantly adapts its size and function according to external and internal stimuli. For example, mechanical loading, hormones, cytokines and nutrients shift the balance between protein synthesis and degradation and thereby determine muscle fiber size and contractile function (3). An imbalance in proteostasis is observed in several catabolic conditions such as sarcopenia, cachexia and disuse leading to muscle atrophy. Understanding the molecular mechanisms that regulate protein synthesis or degradation is therefore essential for the identification of potential targets for pharmacological stimulation or inhibition to counteract muscle wasting (Fig. 1). In this section, we will provide an overview of the important mechanisms regulating skeletal muscle proteostasis.
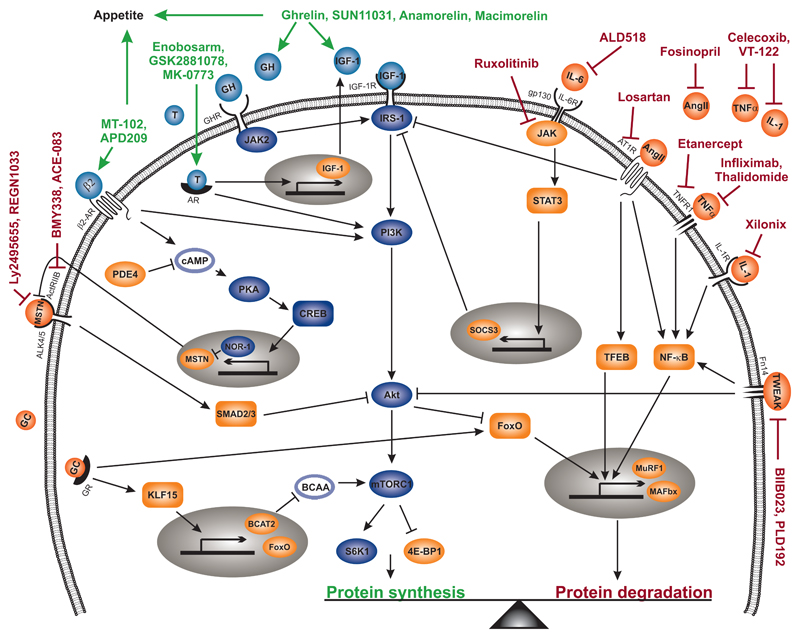
Figure 1. Signaling pathways involved in secondary muscle wasting and potential drugs to reduce loss of muscle mass.
Several classes of drugs are tested in clinical trials aimed at either inhibiting catabolic signaling (drug name red) and thereby reducing protein degradation (orange), or stimulating anabolic pathways (drug name green) and thereby increasing protein synthesis (blue). Activation via IGF-1, but also other upstream signals such as GH, androgens or β2-agonist, stimulate the PI3K – Akt – mTOR pathway and, as a consequence, protein synthesis is increased. In addition, Akt suppresses protein degradation by phosphorylating FoxO and thereby reducing its transcriptional activity. Several catabolic pathways do not only contribute to muscle atrophy by increasing the expression of E3 ligases MAFbx and MuRF1 via the transcription factors FoxO, TFEB or NF-κB, which all stimulate protein degradation, but also by inhibiting PI3K – Akt signaling and thereby reduce protein synthesis. MSTN is a major negative regulator of muscle mass by inhibiting Akt signaling. The multi-factorial nature of secondary muscle wasting and the different signaling pathways involved in muscle atrophy aggravates the development of effective drugs. Abbreviations not mentioned in the text: T = testosterone; GC = glucocorticoid
The mammalian target of rapamycin (mTOR), found in two distinct complexes in almost every cell, is a key regulatory nexus of protein synthesis and degradation. Binding of insulin or insulin-like growth factor 1 (IGF-1) to its receptor IGF-1R recruits insulin receptor substrate 1 (IRS-1), which subsequently leads to the activation of the phosphoinositide 3-kinase (PI3K) – Akt/protein kinase B (PKB) – mTOR pathway (3). In addition to IGF-1, growth hormone (GH) also induces PI3K-Akt signaling through the JAK2-mediated activation of IRS-1 (4). mTOR complex 1 (mTORC1), molecularly characterized by the protein raptor, controls protein synthesis mainly by phosphorylating the two downstream effectors S6 kinase 1 (S6K1) and eukaryotic translation initiation factor 4E-binding protein 1 (4E-BP1) (3). In addition to the activation of mTOR and the ensuing stimulation of protein synthesis, Akt also negatively affects muscle atrophy (5). Specifically, Akt-induced phosphorylation of forkhead box O (FoxO) results in its nuclear exclusion and thereby prevents the induction of the E3 ubiquitin ligases muscle atrophy F-box protein (MAFbx) and muscle RING finger-containing protein 1 (MuRF1) (5). Besides the insulin and GH signaling pathways, mTORC1 is also activated by amino acids, in particular branched-chain amino acids (BCAA) such as leucine or its metabolite β-hydroxy-β-methylbutyrate (HMB) in an Akt-independent manner (6), as well as other anabolic stimuli including activation of β2-adrenoceptors (β2-AR). Stimulation of these guanine nucleotide-binding G protein-coupled receptors (GPCRs) activates the PI3K – Akt signaling pathway via the Gβƴ dimer (7). In addition, β2-agonists might also promote muscle hypertrophy via the Gα- cAMP – PKA signaling pathway (7; 8). The PKA-mediated phosphorylation of the cAMP response element-binding protein (CREB) induces the transcription of the orphan nuclear receptor NOR-1 which inhibits myostatin (MSTN) expression and thereby contributing to muscle growth (9–11). Accordingly, phosphodiesterases (PDEs), the enzymes that hydrolyze and thereby degrade cAMP, can be a target to interfere with cAMP-dependent modulation of muscle hypertrophy (7).
In contrast to mTOR with its potent positive effect on protein synthesis, MSTN, also known as growth and differentiation factor 8 (GDF-8), is an important negative regulator of muscle mass (12). Upon binding to the receptor complex consisting of activin receptor type-IIB (ActRIIB) and activin receptor-like kinase 4 (ALK4) or ALK5, phosphorylation of the transcription factors SMAD2 and SMAD3 is stimulated and downstream signaling activated (13; 14). A reduction in Akt signaling and activation of FoxO are major targets of MSTN signaling, which thereby negatively regulates protein synthesis and stimulates protein degradation (15). Moreover, recruitment of activated SMAD3 to the SMAD-binding element in the promoter region of MuRF1 and MAFbx synergistically increases transcriptional activity of FoxO (16). In addition to MSTN, GDF11, GDF15 and activin likewise signal through the same receptor complex and are involved in the negative regulation of muscle mass (17; 18). Interestingly, GDF15 also promotes muscle wasting by negatively affecting food intake (19; 20). Thus, elevated GDF15 serum levels in patients with cancer, chronic kidney disease or COPD could contribute to the development of cachexia by reducing appetite as well as inducing muscle-inherent catabolic pathways (19; 21).
In various catabolic conditions associated with muscle wasting such as cancer, aging, denervation and glucocorticoid treatment, the expression of the effector proteins MAFbx and MuRF1 are highly elevated (22). MuRF1 and MAFbx have a distinct role in inducing muscle atrophy: MuRF1 is primarily involved in the proteolysis of sarcomeric proteins and enzymes regulating metabolic processes by ubiquitination of myosin heavy chains (MyHC) and enzymes involved in the generation of ATP, respectively (3; 22). MAFbx on the other hand downregulates protein synthesis and muscle fiber differentiation by mainly targeting the eukaryotic initiation factor 3 subunit f (elF3-f) and the myogenic regulatory transcription factor myogenic differentiation 1 (MyoD) (3; 22). Accordingly, MAFbx and MuRF1 are potential targets to blunt fiber atrophy, as evidenced by the amelioration of denervation-induced muscle mass loss by skeletal muscle-specific deletion of either MAFbx or MuRF1 (23). Numerous upstream signals induce the transcription of these E3 ubiquitin ligases and thereby promote muscle atrophy. For example, in conditions with reduced Akt signaling, dephosphorylated FoxO translocates into the nucleus and promotes transcription of MAFbx and MuRF1 (5; 24). Furthermore, in patients receiving glucocorticoid therapy, but also in sepsis or cancer, chronically elevated glucocorticoid levels promote the translocation of glucocorticoid receptor (GR) into the nucleus leading to increased transcription of various target genes including FoxO1/3, kruppel-like factor-15 (KLF15), MAFbx, and MuRF1 (25). Once induced by the GR, KLF15 can promote gene expression of MAFbx and MuRF1 directly as well as by upregulating FoxO (25). Moreover, KLF15-mediated regulation of branched-chain aminotransferase 2 (BCAT2) reduces mTOR activity and thereby shifts proteostasis towards degradation in a multi-pronged manner (25). Accordingly, GR-induced alterations in protein synthesis and degradation can be restored by BCAA-mediated stimulation of mTOR activity, which in turn inhibits GR signaling (25).
The nuclear factor κB (NF-κB) is another important transcription factor regulating the expression of the E3 ligases besides FoxO (26). In the context of elevated systemic inflammation (i.e. cancer), muscle atrophy is promoted by the canonical or non-canonical NF-κB signaling pathways (26). In response to binding of tumor necrosis factor α (TNFα) or interleukin 1 (IL-1) to their receptors TNFR1 and IL-1R, respectively, a series of events lead to the phosphorylation and subsequent proteosomal degradation of inhibitor of κB (IκB) by the IκB kinases (IKKα and IKKβ)/NF-κB essential modulator (NEMO/IKKƴ) complex (27). This allows the translocation of NF-κB into the nucleus and the induction of gene transcription, including that of MuRF1 (27). TNF-like weak inducer of apoptosis (TWEAK) binding to the receptor fibroblast growth factor inducible 14 (Fn14) likewise increases NF-κB signaling (28). TWEAK/Fn14-induced muscle atrophy however is context-dependent, as TWEAK-signaling is for example increased in response to denervation, but not in response to glucocorticoids (29). Denervation massively increases Fn14 levels rather than TWEAK expression itself (29). TWEAK also regulates muscle mass by inhibiting Akt, which results in reduced protein synthesis as well as indirect promotion of the transcription of E3 ligases (28). In contrast to the activation of NF-κB in response to TWEAK, TNFα or IL-1, IL-6-induced muscle atrophy is primarily mediated by signal transducer and activators of transcription 3 (STAT3) signaling. The binding of IL-6 to the IL-6R/glycoprotein 130 (gp130) complex activates Janus kinases (JAKs), which in turn phosphorylate STAT3, resulting in its nuclear translocation and transcription of target genes such as the suppressor of cytokine signaling 3 (SOCS3) (30). SOCS3 disrupts IGF-1 signaling by inhibiting IRS-1 and thereby impairs protein synthesis (31). IL-6 and JAK signaling are therefore also promising targets to reduce inflammation-induced muscle wasting (32).
In heart failure, chronic kidney disease and other conditions leading to increased antiotensin II (AngII) levels, MuRF1 expression is induced by NF-κB signaling as well as the transcription factor EF (TFEB) (31; 33). Moreover, AngII also disrupts Akt – mTOR signaling by phosphorylating IRS-1 and thereby reducing PI3K activity (34). Besides the prominent effect of AngII on muscle protein turnover, AngII reduces food intake, which furthermore contributes to the development of cachexia (31).
To summarize, a complex network of numerous signals stimulating different pathways lead to muscle atrophy. In many muscle pathologies, e.g. in cancer cachexia, the origin of muscle wasting is multi-factorial, including systemic inflammation, reduced food intake and diminished physical activity, which hamper the treatment of these diseases and accordingly, no approaches are currently available to counteract this type of muscle wasting (35). However, several drugs are being tested that interfere with one or more of the described pathways involved in muscle proteostasis (Fig. 1). In the following section, the different classes of drugs and the current state of knowledge are summarized.
Novel pharmacological interventions to improve muscle function
Anti-inflammatory drugs
Several pro-inflammatory cytokines are often elevated in plasma of patients with muscle wasting conditions and hence, anti-inflammatory drugs are attractive candidates to treat muscle wasting, albeit with mixed success. For example, direct inhibition of NF-κB signaling was effective to prevent denervation- or tumor-induced muscle mass loss in mice (26). Likewise, treatment of tumor-bearing mice with monoclonal antibody against Fn14 successfully prolonged survival and prevented loss of body weight, muscle and fat mass (36). Somewhat less pronounced, treatment with soluble TNFα receptor or IL-6 receptor antibodies ameliorated the cachectic phenotype, but could not completely reverse muscle wasting in animals (37; 38). Similarly, even though an early study showed beneficial effects of TNFα inhibitor (Thalidomide) on weight and muscle loss in cancer patients (39), several other clinical trials testing the effects of TNFα inhibitors or soluble TNFα receptors failed to show an improvement in appetite, body weight or muscle volume (see Table 1) (40; 41). Inhibition of IL-1α signaling by an IL1α-specific humanized monoclonal antibody (Xilonix, MABp1) increased lean body mass (LBM), however no control group was included in the study (42). Further studies are now ongoing and recruiting colorectal and pancreatic cancer patients (NCT02138422, NCT03207724). The humanized IL-6 monoclonal antibody ALD518 had no effect on LBM, but improved fatigue, which was most likely caused by the beneficial effect on anemia (43; 44). Whether a block in downstream signaling of IL-6 by the JAK1/2 inhibitor Ruxolitinib ameliorates muscle wasting in cancer patients remains to be investigated (NCT02072057). Likewise, pharmacological targeting of TWEAK action has so far only been tested in healthy volunteers regarding muscle plasticity. Due to the key role in the synthesis of prostanoids and prostaglandins, inhibition of cyclooxygenases (COXs) can potently affect systemic inflammation (45). Accordingly, plasma levels of IL-6 and IL-1 were significantly reduced and muscle mass increased upon treatment of aged rats with COX inhibitors (45). Furthermore, the COX2 blocker Meloxicam reduced rheumatoid arthritis-induced muscle mass loss in rats, together with a marked reduction in MAFbx and MuRF1 expression (46). This drug has so far not been tested in human patients in the context of muscle wasting. The COX2 inhibitor Celebrex elicited mixed results, with no effect on LBM in one study and a positive outcome on LBM and grip strength, as well as reduced serum TNFα in cancer patients in a second trial (47; 48).
Table 1. Table summarizing clinical trials performed to ameliorate secondary muscle wasting or muscular dystrophies.
| Drug name | Type of drug | Company | Completed (NCT-ID) | Phase | Patients | Results | Ongoing (NCT-ID) | Phase | Patients |
|---|---|---|---|---|---|---|---|---|---|
| Secondary muscle wasting | |||||||||
| Anti-inflammatory | |||||||||
| Etanercept | Soluble TNFα receptor | Mayo Clinic Cancer Center | NCT00046904 | Phase III | Cancer patients | ↔ BW, appetite | |||
| Etanercept (Enbrel) | Soluble TNFα receptor | Amgen | NCT00802815 | Phase I | Body myositis | No results | |||
| Infliximab | Anti-TNFα | Various | NCT00060502 | Phase II | Cancer patients | ↔ BW, appetite | |||
| NCT00040885 | Phase III | ||||||||
| Lenalidomide (Revlimid) | Anti-TNFα | NCT01127386 | Phase I/II | Cancer patients | No results found | ||||
| Thalidomide | Anti-TNFα | Celgene Corporation | NCT00379353 | Phase II | Cancer patients | ↔ BW, appetite | |||
| Xilonix (MABp1) | Anti-IL-1α | XBiotech, Inc. | NCT01021072 | Phase I | Cancer patients | NCG | NCT03207724 | Phase I | Cancer patients |
| ↑ LBM | NCT02138422 | Phase III | |||||||
| ALD518 | Anti-IL-6 | Alder Biopharmaceuticals, Inc. | NCT00866970 | Phase II | Cancer patients | ↔ LBM ↓ fatigue |
|||
| Ruxolitinib | JAK 1/2 inhibitor | Novartis | NCT02072057 | Phase II | Cancer patients | ||||
| BIIB023 | Anti-TWEAK | Biogen | NCT01407406 | Phase I | Healthy volunteers | Pharmacokinetics No results found on muscle atrophy | |||
| NCT01943513 | Phase I | ||||||||
| PDL192 | Anti-TWEAK receptor | Abbott Biotherapeutics Corp. | NCT00738764 | Phase II | Cancer patients | Tolerability | |||
| OHR/AVR 118 | Peptide nucleic acid immune-modulator | OHR Pharma | NCT01206335 | Phase II | Cancer patients | NCG ↔ BW, GS |
|||
| Celecoxib (Celebrex) | Cox2 inhibitor | Pfizer | Phase II | Cancer patients | Study with NCG: ↑ LBM, GS ↓ serum TNFα Study with CG: ↔ LBM |
||||
| VT-122 | β-blocker + COX inhibitor | Vicus Therapeutics | NCT00527319 | Phase II | Cancer patients | No statistics found | NCT01265576 | Phase II | Cancer patients |
| Anti-MSTN/anti-ActRII | |||||||||
| ACE-083 | Soluble ActRIIB | Acceleron Pharma, Inc. | NCT02257489 | Phase I | Healthy volunteers | ↑ MV | NCT02927080 | Phase II | Facioscapulo humeral Muscular Dystrophy |
| BMY338 (Bimagrumab) | Anti-ActRII | Novartis Pharmaceuticals | NCT01433263 | Phase II | Cancer patients, COPD, body myositis |
↑ LBM, MV ↔ FP |
NCT02152761 | Phase II | Elderly |
| NCT01669174 | Phase II | NCT02333331 | Phase II | ||||||
| NCT01925209 | Phase II/III | NCT02468674 | Phase II | ||||||
| NCT02573467 | Phase III | ||||||||
| LY2495655 | Anti-MSTN | Eli Lilly and Company | NCT01604408 | Phase II | Elderly, cancer patients | ↑ LBM, MV mixed results on FP | |||
| NCT01369511 | Phase II | ||||||||
| NCT01505530 | Phase II | ||||||||
| REGN1033 (SAR391786) | Anti-MSTN | Regeneron Pharmaceuticals | NCT01963598 | Phase II | Elderly | ↑ LBM ↔ FP |
|||
| Anti-catabolic | |||||||||
| Fosinopril | ACE inhibitor | Imperial College London, Medical Research Council | NCT01014338 | Phase IV | COPD | ↔ MV, MAFbx and MuRF1 mRNA | |||
| Losartan | Ang-receptor blocker | Johns Hopkins University | NCT01989793 | Phase II | Elderly | No results yet | |||
| Anabolic | |||||||||
| Enobosarm (GTx-024) | SARM | GTx | NCT00467844 | Phase II | Cancer patients | ↑ LBM ↑ stair climbing ↔ GS, 6MWT |
NCT02463032 | Phase II | Cancer patients |
| NCT01355484 | Phase III | ||||||||
| NCT01355497 | Phase III | ||||||||
| GSK2881078 | SARM | GSK Clinical Trials, GlaxoSmithKline | NCT02045940 | Phase I | Healthy volunteers, elderly women | Pharmacokinetics and tolerability | NCT03359473 | Phase II | COPD |
| NCT02567773 | Phase I | ||||||||
| Ly2452473 | SARM | Dana-Farber Cancer Institute | NCT02499497 | Phase II | Cancer patients | ||||
| MK-0773 | SARM | Merck Sharp & Dohme Corp. | NCT00529659 | Phase II | Elderly women | ↑ LBM ↔ FP | |||
| Anamorelin HCl | Ghrelin receptor agonist | Helsinn Therapeutics (U.S.), Inc | NCT01387282 | Phase III | Cancer patients | ↑ BW, LBM ↔ GS |
NCT03035409 | Phase II | Cancer patients |
| NCT01395914 | Phase III | ||||||||
| Ghrelin | Natural ghrelin | Cantonal Hospital St. Gallen KSSG | NCT00933361 | Phase I/II | Cancer patients | Phamacokinetics | |||
| Macimorelin (AEZS-130) | Ghrelin receptor agonist | AEterna Zentaris | NCT01614990 | Phase II | Cancer patients | ||||
| SUN11031 | Synthetic analog of human ghrelin | Daiichi Sankyo, Inc. | NCT00698828 | Phase II | COPD | ↑ BW, LBM ↔ FP |
|||
| APD209 | Combination of megestrol and formoterol (β2- agonist) | Acacia Pharma Ltd | NCT00895726 | Phase II | Cancer patients | only 7/14 finished; ↑ MV ↔ GS |
|||
| MT-102 (Espindolol) | Non-selective β- blocker with central seretonin 1A receptor and partial β2 receptor agonist effects | PsiOxus Therapeutics Ltd | NCT01238107 | Phase II | Cancer patients | ↑ LBM, GS ↔ FP | |||
| NGM120 | Anti-GFRAL | NGM Biopharmaceuticals, Inc | NCT03392116 | Phase I | Healthy volunteers | ||||
| Muscular dystrophies | |||||||||
| Gene therapy | |||||||||
| AAV-GALGT2 | rAAVrh74.MCK.GrAAVrh74.MCK.GALGT2 (β-1,4 Nacetylgalactosaminyltransferase 2(GALGT2 or B4GALNT2)) (via a major lower limbartery) | The Research Institute at Nationwide Children's Hospital | NCT03333590 | Phase I/II | DMD | ||||
| AAV-microdystrophin | rAAVrh74.MCK.M icro-Dystrophin (i.m.) | The Research Institute at Nationwide Children's Hospital | NCT02376816 | Phase I | DMD | No results available yet | |||
| AAV-microdystrophin | SGT-001 (AAV9 vector containing muscle-specific promoter and microdystrophin construct); (i.v.) | Solid Biosciences, LLC | NCT03368742 | Phase I/II | DMD | ||||
| AAV-microdystrophin | rAAVrh74.MHCK 7.micro-dystrophin (i.v.) | The Research Institute at Nationwide Children's Hospital | NCT03375164 | Phase I/II | DMD | ||||
| AAV-minidystrophin | rAAV2.5-CMV-minidystrophin (d3990) (i.m.) | Asklepios Biopharmaceutical,Inc. and the Research Institute at Nationwide Children's Hospital | NCT00428935 | Phase I | DMD | Detection of vector DNA;Mini-dystrophin protein detected in 2/4 patients | |||
| AAV-minidystrophin | PF-06939926 (rAAV9 carrying a truncated mini-dystrophin); (i.v.) | Pfizer | NCT03362502 | Phase I | DMD | ||||
| AAV-dysferlin | rAAVrh74.MHCK 7.DYSF.DV (i.m.) | The Research Institute at Nationwide Children's Hospital | NCT02710500 | Phase I | Dysferlinopathy | ||||
| AAV-SGCA | rAAV1.tMCK.human-alpha-sarcoglycan (i.m. and via a major lower limb artery) | The Research Institute at Nationwide Children's Hospital | NCT00494195 | Phase I | LGMD2D | No adverse effects SGCA mRNA and protein detectable in 2/3 | NCT01976091 | Phase I/II | LGMD2D |
| AAV-SGCG | AAV1-gamma-sarcoglycan vector injection (i.m.) | Genethon | NCT01344798 | Phase I | LGMD2C | No adverse effects SGCG mRNA level detectable in 4/9 and protein in 1/9 | |||
| PTC read-through | |||||||||
| Ataluren (Translarna,PTC124) | PTC read-through | PTC Therapeutics | NCT00264888 | Phase II | DMD, BMD |
Well tolerated ↓ CK levels ↔ 6MWT overall ↑ 6MWT in subgroup |
NCT0281955 | Phase II | DMD, BMD |
| NCT00592553 | Phase II | NCT01557400 | Phase III | ||||||
| NCT01826487 | Phase III | NCT02090959 | Phase III | ||||||
| NCT01247207 | Phase III | ||||||||
| NCT02369731 | PASS | ||||||||
| NCT03179631 | Phase III | ||||||||
| Gentamicin | PTC read-through | The Research Institute at Nationwide Children's Hospital | NCT00005574 | Phase I | DMD, BMD |
↓ CK levels Oto-nephrotoxicity | |||
| NCT00451074 | Phase I | ||||||||
| NPC-14 (Arbekacin) | PTC read-through | Kobe University | NCT01918384 | Phase II | DMD | ||||
| Exon-skipping | |||||||||
| DS-5141b | Skipping exon 45 (s.c.); ENA | Daiichi Sankyo Co., Ltd. | NCT02667483 | Phase I/II | DMD | ||||
| SRP-4045 | Skipping exon 45 (i.v.); PMO | Sarepta Therapeutics | NCT02530905 | Phase I | DMD | ||||
| NCT02500381 | Phase III | ||||||||
| Eteplirsen (EXODYS 51, AVI-4658) | Skipping exon 51 (i.v. and i.m NCT00159250); PMO | Sarepta Therapeutics | NCT00159250 | Phase I/II | Well tolerated ↑ 6MWT ↓ incidence of loss of ambulation |
NCT03218995 | Phase II | DMD | |
| NCT00844597 | Phase I/II | NCT02286947 | Phase II | ||||||
| NCT01396239 | Phase II | NCT02420379 | Phase II | ||||||
| NCT01540409 | Phase II | NCT02255552 | Phase III | ||||||
| SRP-5051 | Skipping exon 51 (i.v.); PPMO | Sarepta Therapeutics | NCT03375255 | Phase I | DMD | ||||
| SRP-4053 | Skipping exon 53 (i.v.); PMO | Sarepta Therapeutics | NCT02310906 | Phase I/II | DMD | ||||
| NCT02500381 | Phase III | ||||||||
| NS-065/NCNP-01 | Skipping exon 53 (i.v.); PMO | NS Pharma, Inc. | NCT02740972 | Phase II | DMD | ||||
| NCT03167255 | Phase II | ||||||||
Abbreviations: BW = body weight; LBM = lean body mass; MV = muscle volume; GS = grip strength; 6MWT = 6-minute walk test; FP = functional parameters; NCG = no control group, CG = control group; i.m. = intramuscular; i.v. = intravenous; SGCA = α-sarcoglycan; SGCG = ƴ-sarcoglycan; PMO = phosphorodiamidate morpholino oligonucleotides; PPMO = next-generation peptide PMO; ENA = 2'-O,4'-C-ethylene-bridged nucleic acid; PASS = post-approval safety study.
Clinical trials performing intervention studies to counteract secondary muscle wasting (i.e. cachexia, sarcopenia) by targeting muscle tissue were searched on January 2018 using www.clinicaltrial.gov; Phase I and II trials were only selected if finished within the last 5 or 10 years, respectively or if followed-up with additional trials; no combined interventions were included (e.g. drug and exercise).
Clinical trials performing intervention studies to ameliorate muscular dystrophies were searched on February 2018 using www.clinicaltrial.gov; only gene therapy, PTC read-through and exon-skipping were included in the search.
Interfering with MSTN/ActRII signaling
MSTN powerfully reduces muscle mass and therefore, MSTN and the related signaling pathways mediated by the ActRII are promising avenues to counteract muscle wasting. Earlier trials aimed at exclusively blocking MSTN action have shown little clinical efficacy (49). In contrast, novel strategies that target the MSTN receptor and therefore additionally reduce the activity of other ligands seem more promising. For example, blocking MSTN and activin A signaling by administration of soluble ActRIIB preserved body weight and muscle mass and dramatically extended survival in tumor-bearing mice (50). This striking effect on cachexia and survival was achieved without affecting tumor mass or systemic inflammation (50). Several drugs inhibiting ActRIIB signaling are currently being tested in clinical trials (Table 1). At this point, there are two main strategies to target MSTN signaling: first, by directly neutralizing MSTN with a humanized MSTN antibody (LY2495655 or REGN1033) and second by blocking ActRII with a soluble ActRIIB (ACE-031 or ACE-083) or ActRII antibody (Bimagrumab/BMY338). LY2495655 treatment resulted in a mixed outcome by slightly increasing appendicular LBM and gait speed in elderly subjects (51). However, despite the gain in muscle mass, grip strength was not affected (51). In contrast, in pancreatic cancer patients, LY2495655 improved muscle mass as well a grip strength (52). Similarly, REGN1033 increased LBM with no effect on muscle strength and function in patients with sarcopenia (53). Even though blocking ActRII using BMY338 massively increased muscle mass in mice and prevented dexamethasone-induced muscle atrophy (54), the results in humans were less pronounced (55). LBM, muscle mass and 6-minutes-walk test seem to improve after 8 weeks of treatment in patients with body myositis, however no significant differences were observed after 24 weeks of treatment (55). Moreover, no beneficial effects on these parameters were reported in cancer patients, whereas in patients with COPD, muscle volume increased with no effect on functional measures, which is similar to the effects of BMY338 in patients with sarcopenia (56). Thus, so far, mainly muscle mass could be improved with these treatments with less consistent effects on muscle strength and other functional parameters. Nevertheless, several additional clinical trials are ongoing (NCT02152761, NCT02333331, NCT02468674).
Similar to the positive outcome of anti-MSTN treatment in animal models, GDF15 blockage in tumor-bearing mice prevented cachexia and massively prolonged survival (20). Besides its effect on skeletal muscle, GDF15 also signals through the glial cell-derived neurotrophic factor (GDNF) receptor alpha-like (GFRAL) in the brainstem to control food intake (57). To leverage these effects, a trial is starting in 2018 testing a monoclonal antibody against the GDF15 receptor GFRAL (NGM120) for the treatment of anorexia and cachexia in cancer patients (NTC03392116). To our knowledge, a direct targeting of GDF15 has so far not been tested in humans.
Other anti-catabolic drugs
Application of an angiotensin converting enzyme (ACE) inhibitor resulting in reduced AngII signaling preserved grip strength in tumor-bearing mice, but was not able to prevent loss of muscle mass (58). In patients with COPD, the ACE inhibitor Fosinopril did not affect muscle volume, strength or expression levels of E3 ligases in quadriceps muscle (59). In old mice, the AngII inhibitor Losartan prevented immobilization-induced muscle loss (60). However, no results have so far been published on the trial with Losartan in elderly subjects (NCT01989793).
Stimulation of protein synthesis
Instead of inhibition of protein degradation, stimulation of protein synthesis is another strategy to beneficially affect proteostasis and thereby counteract muscle wasting. For example, increasing muscle mass by testosterone treatment is well documented although its exact mechanism of action is still not fully elucidated (61; 62). Although the effects of testosterone on protein synthesis by binding to the androgen receptor (AR) may be muscle specific and more pronounced in perineal muscles, it is likely that AR signaling also induces IGF-1 transcription as well as activates the Akt – mTOR pathway by interacting with PI3K in limb muscles (63–65). However, the potential therapeutic anabolic application of testosterone on muscle tissue is marred by a wide number of unwanted and adverse effects. Therefore, tissue-specific non-steroid selective androgen receptor modulators (SARM), which retain anabolic, but not androgenic and other unwanted properties, are of great interest in treating muscle wasting (66). The treatment of healthy men, sarcopenic elderly or cancer patients with SARMs (LGD-4033, MK-0773 or Enobosarm) increased LBM, albeit without enhancing strength (67–70). Various SARMs are currently being tested in clinical trials (NCT03359473, NCT02463032, NCT02499497).
Although β2-agonists such as Clenbuterol or Formoterol can reduce cancer- or denervation-induced muscle atrophy in rats (71; 72), clinical trials using β2-agonists to treat muscle wasting are scarce. In cancer patients, APD209 (a combination of Megestrol, an orexigenic progestin with anti-androgen activity, and Formoterol) slightly increased muscle mass with no effect in strength (73). However, only 7 (out of 14) patients finished the study and no control group was included. Espindolol (a non-selective β-blocker with central serotonin 1A receptor and partial β2 receptor agonist effects) on the other hand increased LBM and grip strength in cancer patients, but could not improve in functional parameters (74).
Ghrelin exerts its effect on muscle wasting by several means such as stimulating appetite as well as the release of GH which in turn increases liver IGF-1 production (75). Ghrelin also has an anti-atrophic effect by stimulating Akt signaling independent of the GH/IGF-1-axis (76). Although ghrelin receptor agonists or synthetic ghrelin analogs such as Anamorelin and SUN11031 increased body weight and LBM, grip strength and other functional parameters were not increased in cachectic cancer patients (77–80). To complement the multimodal approach in cancer cachexia, numerous clinical trials aimed at orexigenic central effects have been executed, e.g. with Megestrol or cannabinoids, exhibiting only minor effects on muscle mass or functional parameters (81), even though several trials are still ongoing (NCT02359123, NCT02802540, NCT03245658). Similarly, nutritional interventions are being considered, for example the use of BCAAs, HMB or L-carnitine to mitigate muscle wasting (81). Even though some studies indicated that these nutrients provide some relief in cancer cachexia (81), more clinical studies are needed to quantify efficacy.
Inherited and sporadic muscular dystrophies
Besides secondary pathologies such as cachexia or sarcopenia, muscular dystrophies are a diverse group of diseases associated with often lethal muscle wasting. Even though some of these diseases are sporadic, many muscular dystrophies are caused by mutations of a particular gene leading to a truncated or dysfunctional protein. There is a large variability in disease etiology, onset, primarily affected muscles, severity as well as the involvement of respiratory and cardiac muscles (82). Accordingly, muscular dystrophies are divided into different subtypes such as congenital muscular dystrophy (CMD), Duchenne or Becker muscular dystrophy (DMD or BMD, respectively) or limb-girdle muscular dystrophy (LGMD) based on gene mutation and/or phenotype (83). The different subtypes of muscular dystrophies sharing a similar phenotype can be caused by mutations in distinct proteins with a similar cellular function (82). Even though almost every organelle and cellular structures, including metabolic pathways, mitochondrial function or the contractile apparatus can be affected and lead to the development of a dystrophic phenotype (84), the majority of mutated proteins belong to the dystrophin-associated glycoprotein complex composed of dystrophin, sarcoglycans and dystroglycans, (82). This complex links the cytoskeleton to the extracellular matrix and thereby provides stability to the muscle fiber against contraction-induced mechanical forces (85). Therefore, a dysfunctional protein leading to impaired stability of the dystrophin-associated glycoprotein complex renders the muscle fiber more vulnerable to contraction- and stretch-induced muscle damage. Small tears in the plasma membrane in response to mechanical stress cause calcium influx into the cytoplasm activating a series of events normally leading to repair and regeneration of the muscle fiber (85). In dystrophic muscles, an impaired regenerative capacity, exacerbated muscle damage and the ensuing dysregulation of signaling pathways however results in degeneration and necrosis of the muscle (85). Histologically, muscle fibers of dystrophic patients are thus often characterized by centrally located nuclei, infiltration of macrophages, fiber necrosis and replacement of muscle tissue by fat and fibrotic tissue (83). Additionally, due to increased muscle damage, serum creatine kinase (CK) levels are elevated and used as a diagnostic marker (83).
Among the inherited muscular dystrophies, DMD is the most common childhood muscle disease affecting approximately 1 in 6000 newborn boys worldwide (86). DMD is caused by a mutation of the dystrophin gene, which is located on the X chromosome (82). A nonsense mutation or in-frame deletion in the dystrophin gene leading to a truncated protein induces a milder form of the disease called BMD (85). Dystrophin plays an important role in linking the cytoskeleton to the dystroglycan complex by N-terminal binding to F-actin and C-terminal binding to β-dystroglycan (85). As the most functional domains of dystrophin are in the N- and C-terminus, deletion of exons located in the central rod-domain of the gene by exon-skipping would not affect the main functionality and could thereby ameliorate DMD (87).
Mutations of sarcolemma-associated proteins such as the sarcoglycans or dysferlin are generally the underlying cause of LGMDs (82). LGMDs can be inherited in a dominant or recessive manner and are named LGMD1 or LGMD2, respectively (82). Defects in extracellular matrix proteins including laminin α2 or collagen VI predominantly result in CMD (82). Interestingly, mutations in genes encoding enzymes involved in the glycosylation of α-dystroglycan can cause LGMDs as well as CMD, highlighting the complexity and diversity of the etiology of muscular dystrophies (82). Even mutations within the same gene can lead to different phenotypes as seen in patients with mutations in dysferlin causing either LGMD2B affecting predominantly proximal muscles or Miyoshi muscular dystrophy affecting distal muscles (88) or the glycosyltransferase fukutin associated with either LGMD2M or CMD (82). Even though a great number of genes of which mutations lead to muscular dystrophies are known, the complete spectrum of the function of these proteins is not fully elucidated. Therefore, the diversity of genes responsible for muscular dystrophies as well as the different manifestation of the disease aggravates the development of therapies for patients. Based on the etiology of these dystrophies, specific genes or even particular mutations have to be targeted in a personalized manner. In the following section, we will provide an overview of the different novel approaches to treat muscular dystrophies.
Therapeutic strategies to treat muscular dystrophies
Up to date, there is no effective treatment available for the vast majority of muscular dystrophies. For example, standard care for DMD patients includes glucocorticoid treatment, which decelerates the decline in muscle strength and function and thereby prolongs ambulation (89). These benefits, which might be based on the anti-inflammatory effect of these compounds (90), are somewhat paradoxical in light of the pro-atrophic action of the GR in muscle, and caveats about potential adverse effects in long-term treatment have been raised (89). In DMD and other muscular dystrophies with cardio-respiratory impairment, therapies also address cardiological as well as respiratory symptoms, the latter by mechanical ventilation (83). Furthermore, various efforts are made to treat the skeletal muscle wasting symptoms of dystrophies in a similar manner as in secondary muscle wasting, including the use of anti-inflammatory drugs, MSTN blockers (PF-06252616/Domagrozumab, BMS-986089, follistatin by rAAV1.CMV.huFollistin344), anti-oxidants (Raxone/Idebenone), histone deacetylase (HDAC) inhibitors (Givinostat), ACE or PDE5 inhibitors (Tadalafil), or by stimulation of muscle regeneration (91; 92). Other approaches are targeted at the specific genes affected in muscular dystrophies, e.g. replacement of the defective protein by pharmacological upregulation of a protein that compensates for the lost function. In DMD, the induction of utrophin, a functional homolog of dystrophin that normally is only expressed in the subsynaptic region of adult muscle, by small molecules such as Ezutromid/SMTC1100 or the second-generation compound SMT022357, aims at ameliorating disease progression (93; 94). Of these compounds, Ezutromid currently is in phase II trials in DMD patients (NCT02858362). Intriguingly, several compounds boost utrophin gene expression by inducing an oxidative muscle phenotype in pre-clinical models, including the AMP-dependent kinase (AMPK) activator AICAR, resveratrol, metformin and L-Arginine. Of note, the latter two have now entered phase I clinical trials (92).
In muscular dystrophies with known gene mutations, strategies aimed at targeting the primary defect by replacing the mutated gene, premature termination codon (PTC) read-through and exon-skipping have made tremendous progress recently. So far, adeno-associated viral (AAV)-based vectors are the most efficient system to deliver DNA into nuclei of non-dividing cells with a low immunogenicity (95). Although in most cases, AAV is a suitable vehicle for human use and to induce long-term expression of the payload gene, the packaging capacity of the virus is limited to ~4.7 kb (95). However, even though full-length genes might exceed this capacity (i.e. the full-length dystrophin cDNA is 14 kb in size), a smaller, truncated version of the target gene containing the most functional parts of the protein could still exert therapeutic effects by reducing the disease phenotype. In mice or golden retrievers with DMD, transduction of two different truncated forms of dystrophin, mini-dystrophin and micro-dystrophin, ameliorated disease progression and extended lifespan (96–99). Based on these promising pre-clinical data, several phase I/II clinical trials are currently being planned or conducted aiming at treating DMD patients with mini- or micro-dystrophin (see Table 1) (100). Phase I clinical trials of LGMD2C and LGMD2D patients treated with AAV vectors containing either ƴ-sarcoglycan or α-sarcoglycan, respectively, have been completed or are ongoing. Even though no adverse effects have been reported, detection of the transcript and protein is variable (101–103). Clinical trials are currently ongoing to treat LGMD2B patients with AAV vectors containing dysferlin. So far, the clinical trials have only used intramuscular injections as proof-of-concept. In a next step, efficient systemic delivery via intravenous injections, which enables gene transfer in all muscles, is being established and optimized. The validity of this approach is still gaining traction and several pre-clinical trials showed encouraging results. For example, a combination of elevation of a mini-agrin together with specifically designed linker proteins to enhance the polymerization of laminins enhanced muscle function and life expectancy of a mouse model of merosin-deficient CMD (104).
In 10% of DMD patients, nonsense mutations lead to a PTC, resulting in a truncated dystrophin and, depending on the size of the remaining protein, in a milder phenotype of the disease (105). Premature termination can be suppressed by the binding of a read-through agent such as the aminoglycoside antibiotic gentamicin to the ribosome and thereby preventing the recognition of the stop codon, thus allowing full-length translation of the protein (106). However, due to serious oto-nephrotoxic side effects of gentamicin, Ataluren (also known as PTC124/Translarna) with little off-target effects and a better safety profile is currently being pursued as a PTC agent in patients (106; 107). In Phase II and III trials, Ataluren successfully increased dystrophin levels in the majority of treated DMD patients, lead to a reduction in CK levels, but could not robustly improve walking ability (108–110). Thus, while Ataluren is approved by the European Medicine Agency (EMA) under the trade name Translarna, approval by the FDA has so far been declined (111). Several clinical trials with Ataluren are still ongoing and currently recruiting DMD patients (see Table 1). Although other muscular dystrophies could likewise benefit from PTC suppression by Ataluren, for example as suggested in experiments in myotubes from patients with a nonsense mutation in the dysferlin gene, this drug has only been tested in clinical trials in DMD so far (112).
The majority of mutations in DMD patients are large deletions (68%) of which 80% are located between exon 45-55 (105). These patients accordingly will not benefit from PTC-based strategies. However, exon-skipping would help in individuals with frame-shift-inducing and other non-PTC mutations by restoring the expression of a truncated dystrophin protein similar to that of BMD patients with an in-frame deletion, hence resulting in a milder phenotype. To induce exon skipping, antisense oligonucleotides (AONs) are delivered to muscle tissue as 2´-O-methyl-phosphorothionate RNA olionucleotides (2’OMe) or phosphorodiamidate morpholino oligonucleotides (PMOs), which then bind to the targeted exon of the pre-mRNA (113–115). By masking the recognition site, spliceosome binding is hindered, leading to the splicing of the intron as well as the targeted exon (91; 115). In DMD, skipping of exon 51 is applicable for 14% of patients representing the largest group, followed by exon 53 and 45 with 10.1% and 9% eligible patients, respectively (105). Of all therapies for DMD, exon skipping is the most advanced and promising so far, with the AON Eteplirsen (also known as AVI-4658/Exondys 51) as the first accelerated FDA-approved representative of this class of drugs (116). Further clinical trials are being performed by Sarepta Therapeutics to further substantiate the clinical benefits in DMD (see Table 1). Treating DMD patients with Eteplirsen partially restores dystrophin expression and, importantly, significantly improves walking ability (114; 117–119). Sarepta Therapeutics has designed other AONs targeting exon 45 (SRP-4045/Casimersen) and 53 (SRP-4053/Golodirsen), currently in phase III, and a second-generation exon 51 AON (SRP-5051) in phase I (120). Two other AONs, DS-5141b and NS-065/NCNP-01, targeting exon 45 and 53, respectively, are also in clinical trials. The development of the AONs Drisapersen/Kyndrisa/PRO051/GSK2402968, BMN044/PRO044, BMN045/PRO045 and BMN053/PRO053 by BioMerin Pharmaceutical have been terminated because the phase III trial of Drisapersen failed to improve walking ability (NCT01254019) (121) and FDA approval was accordingly refused (122).
Conclusion and perspectives
To summarize, despite the massive efforts to develop therapies to treat muscle wasting, there are still very few drugs available in a small subset of these pathologies. Even though some aspects of muscle wasting could be ameliorated with some of the tested drugs (i.e. muscle mass), most trials fail to show significant improvement in the defined clinical endpoints, which usually are functional parameters. Therefore, drug discovery will have to go hand-in-hand with the development of sensitive tests to measure clinically relevant functional improvements, which then could help clinical trials to reach their primary goals. In addition, due to the multi-factorial nature of secondary muscle wasting and the large variations between patients, a multipronged approach that includes pharmacological, nutritional and exercise interventions might be necessary, aimed at skeletal muscle and other tissues, e.g. orexigenic effects in the brain. Furthermore, as more mechanistic aspects of muscle wasting are being revealed, novel potential targets are emerging, most of which exhibited promising results in experimental and pre-clinical experiments. For example, elevated parathyroid-hormone-related peptide (PTHrP) levels in models of cancer or kidney failure induces muscle atrophy as well as browning of white adipose tissue and thereby exacerbates energy expenditure (123; 124). Neutralizing PTHrP accordingly prevented cancer cachexia (123; 124). Similarly, the peroxisome proliferator-activated receptor α (PPARα) emerged from a metabolic analysis of liver function in cancer cachexia and pharmacological activation of PPARα by Fenofibrate also blunted muscle wasting in cancer cachexia by systemic remodeling of metabolism (125). Finally, the PPARγ coactivator 1α (PGC-1α), a regulatory nexus of endurance adaptation in skeletal muscle, reduced fiber damage and atrophy, as well as increased contractile function in different animal models of muscle wasting, including DMD (126; 127). Nutritional and pharmacological interventions aimed at these and similar factors could constitute potential therapeutic avenues to treat muscle wasting, e.g. by leveraging exercise-associated mechanisms (see Sidebar) (128–130). However, these factors and the related pharmacological activators will have to stand the test of time in a clinical setting.
Sidebar.
“Exercise mimetics”: a new principle for the pan-treatment of muscle wasting symptoms?
In recent years, the idea of designing and applying so-called “exercise mimetics”, pharmacological agents that regulate the effects of endurance and/or resistance exercise, has gained tremendous traction. Partial proof-of-concept has been provided for a range of compounds, mostly aimed at boosting an oxidative, high endurance muscle phenotype in experimental models. Currently, most of these agents ultimately affect the expression and/or activity of PGC-1α (reviewed in ref. 128). In several animal models of muscle wasting disorders, AICAR, resveratrol and other members of this type of compounds resulted in an amelioration of the disease phenotype. However, none of these “exercise mimetics” has been translated into clinical application to date. Moreover, the concept of “exercise mimetics” is highly controversial and compelling arguments have been put forward that a pharmacological agent is unable to elicit the pleiotropic and complex exercise-induced plastic changes in skeletal muscle and other organs (reviewed in refs. 128; 130).
Exon-skipping and gene editing are areas were currently more concrete progress is observed in regard to optimization of existing and the development of new treatment strategies. In many cases, exon skipping by AONs is hampered by poor cellular uptake after systemic administration and the tissue-specific skipping efficiency, which is better in skeletal muscle as compared to cardiac muscles (91). In animals, alternative strategies to improve the delivery efficiency of AONs have been developed (131). For instance, linking the antisense sequence to a small nuclear RNA (U7 snRNA) enhances the engagement in RNA processing (92). By using an AAV vector for delivery, exon-skipping was efficiently induced and dystrophin levels restored in this study (92). In addition, delivery efficiency of AONs to skeletal as well as cardiac muscle could be significantly enhanced by peptide-conjugated PMOs, providing another alternative method to rescue dystrophin levels in both tissues (132). Systemic delivery of PMOs could also be enhanced by natural carriers such as Saponin (133). Furthermore, the systemic delivery of a tricycle-DNA-AON was more efficient in improving muscle function compared to 2’OMe or PMOs (134). Importantly, dystrophin levels were not only restored in limb skeletal muscles, but also in diaphragm and the heart, showing the potential of this approach to enhance cardio-respiratory function (134; 135). The disadvantage of AONs to target individual exons can be overcome by using multi-exon-skipping targeting the mutation hot spot (exon 45-55), which would allow a common therapy of approximately 63% of DMD patients (136; 137). Finally, CRISPR/Cas9 as a tool for genome editing has emerged as a novel approach to treat muscular dystrophies (138). Experimentally, delivery of the CRISPR/Cas9 components Cas9 nuclease and specific single-guide RNAs to the muscle by AAV vectors lead to the rescue of dystrophin expression, either by inducing exon-skipping, generation of a full-length protein or inducing an in-frame deletion (139; 140). The use of Cpf1, an alternative nuclease to Cas9, exhibited similar results in regard to the restoration of dystrophin in mice (141). The ability to directly editing DNA and thereby induce permanent genomic changes is a major advantage of CRISPR/Cas9 technology (138). However, various hurdles, including delivery systems and potential off-target effects will have to be addressed before clinical application will be possible. Along the same lines, stem cell treatment of muscle dystrophies, either in a heterologous manner from healthy donors, or by engineering of autologous stem cells or inducible pluripotent stem cells (iPS), has been successfully used in pre-clinical experiments and clinical proof-of-concept studies, but systemic application in patients remains to be established (138; 142–144). Therefore, even though few therapies have arrived in the clinic so far, a wide variety of novel avenues is on the horizon to fix the current drought in drugs for muscle wasting pathologies.
Summary points.
-
-
Despite a high prevalence of secondary muscle wasting, i.e. in sarcopenia and cachexia, mechanistic insights into disease etiology and therapeutic options remain rare.
-
-
Muscular dystrophies, many of which are fatal, suffer from a lack of treatment options.
-
-
In recent years, new targets and approaches have been identified and are currently being tested in clinical trials.
-
-
For secondary muscle wasting pathologies, multipronged approaches aimed at pharmaceutical, nutritional and exercise interventions will have to be considered.
-
-
In muscular dystrophies, gene replacement and editing strategies might lead to future clinical breakthroughs.
Acknowledgments
The work in our laboratory is supported by the Swiss National Science Foundation, the European Research Council (ERC) Consolidator grant 616830-MUSCLE_NET, Swiss Cancer Research grant KFS-3733-08-2015, the Swiss Society for Research on Muscle Diseases (SSEM), SystemsX.ch, the “Novartis Stiftung für Medizinisch-Biologische Forschung” and the University of Basel.
Terms and Definitions
- AAV
adeno-associated virus
- ActRIIB
activin receptor type-IIB
- AON
antisense oligonucleotides
- BMD
Becker muscular dystrophy
- CMD
congenital muscular dystrophy
- DMD
Duchenne muscular dystrophy
- GH
growth hormone
- GR
glucocorticoid receptor
- LBM
lean body mass
- LGMD
limb-girdle muscular dystrophy
- MAFbx
muscle atrophy F-box protein
- MuRF1
muscle RING finger-containing protein 1
- MSTN
myostatin
- mTOR
mammalian target of rapamycin
- NF-κB
nuclear factor κB
- PGC-1α
peroxisome proliferator-activated receptor γ coactivator 1α
- PTC
premature termination codon
- SARM
selective androgen receptor modulator
- TNFα
tumor necrosis factor α
- TWEAK
TNF-like weak inducer of apoptosis
Footnotes
Reprint request
Unfortunately, due to copyright-relatedt issues, we are not able to post the post-print pdf version of our manuscript - in some cases, not even any version of our manuscript. Thus, if you would like to request a post-production, publisher pdf reprint, please click send an email with the request to christoph-dot-handschin_at_unibas-dot-ch (see http://www.biozentrum.unibas.ch/handschin).
Information about the Open Access policy of different publishers/journals can be found on the SHERPA/ROMEO webpage: http://www.sherpa.ac.uk/romeo/
Reprint Anfragen
Aufgrund fehlender Copyright-Rechte ist es leider nicht möglich, dieses Manuskript in der finalen Version, z.T. sogar in irgendeiner Form frei zugänglich zu machen. Anfragen für Reprints per Email an christoph-dot-handschin_at_unibas-dot-ch (s. http://www.biozentrum.unibas.ch/handschin).
Informationen zur Open Access Handhabung verschiedener Verlage/Journals sind auf der SHERPA/ROMEO Webpage verfügbar: http://www.sherpa.ac.uk/romeo/
Conflict of interest statement
The authors have no conflict of interest related to this manuscript.
Contributor Information
Regula Furrer, Email: regular.furrer@unibas.ch.
Christoph Handschin, Email: christoph.handschin@unibas.ch.
References
- 1.Handschin C, Spiegelman BM. The role of exercise and PGC1alpha in inflammation and chronic disease. Nature. 2008;454:463–9. doi: 10.1038/nature07206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pedersen BK, Saltin B. Exercise as medicine - evidence for prescribing exercise as therapy in 26 different chronic diseases. Scandinavian journal of medicine & science in sports. 2015;25(Suppl 3):1–72. doi: 10.1111/sms.12581. [DOI] [PubMed] [Google Scholar]
- 3.Egerman MA, Glass DJ. Signaling pathways controlling skeletal muscle mass. Crit Rev Biochem Mol Biol. 2014;49:59–68. doi: 10.3109/10409238.2013.857291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perrini S, Laviola L, Carreira MC, Cignarelli A, Natalicchio A, Giorgino F. The GH/IGF1 axis and signaling pathways in the muscle and bone: mechanisms underlying age-related skeletal muscle wasting and osteoporosis. J Endocrinol. 2010;205:201–10. doi: 10.1677/JOE-09-0431. [DOI] [PubMed] [Google Scholar]
- 5.Stitt TN, Drujan D, Clarke BA, Panaro F, Timofeyva Y, et al. The IGF-1/PI3K/Akt pathway prevents expression of muscle atrophy-induced ubiquitin ligases by inhibiting FOXO transcription factors. Mol Cell. 2004;14:395–403. doi: 10.1016/s1097-2765(04)00211-4. [DOI] [PubMed] [Google Scholar]
- 6.Laplante M, Sabatini DM. mTOR signaling at a glance. J Cell Sci. 2009;122:3589–94. doi: 10.1242/jcs.051011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lynch GS, Ryall JG. Role of beta-adrenoceptor signaling in skeletal muscle: implications for muscle wasting and disease. Physiol Rev. 2008;88:729–67. doi: 10.1152/physrev.00028.2007. [DOI] [PubMed] [Google Scholar]
- 8.Joassard OR, Durieux AC, Freyssenet DG. beta2-Adrenergic agonists and the treatment of skeletal muscle wasting disorders. Int J Biochem Cell Biol. 2013;45:2309–21. doi: 10.1016/j.biocel.2013.06.025. [DOI] [PubMed] [Google Scholar]
- 9.Pearen MA, Ryall JG, Lynch GS, Muscat GE. Expression profiling of skeletal muscle following acute and chronic beta2-adrenergic stimulation: implications for hypertrophy, metabolism and circadian rhythm. BMC Genomics. 2009;10:448. doi: 10.1186/1471-2164-10-448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pearen MA, Ryall JG, Maxwell MA, Ohkura N, Lynch GS, Muscat GE. The orphan nuclear receptor, NOR-1, is a target of beta-adrenergic signaling in skeletal muscle. Endocrinology. 2006;147:5217–27. doi: 10.1210/en.2006-0447. [DOI] [PubMed] [Google Scholar]
- 11.Fass DM, Butler JE, Goodman RH. Deacetylase activity is required for cAMP activation of a subset of CREB target genes. J Biol Chem. 2003;278:43014–9. doi: 10.1074/jbc.M305905200. [DOI] [PubMed] [Google Scholar]
- 12.McPherron AC, Lawler AM, Lee SJ. Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature. 1997;387:83–90. doi: 10.1038/387083a0. [DOI] [PubMed] [Google Scholar]
- 13.Rebbapragada A, Benchabane H, Wrana JL, Celeste AJ, Attisano L. Myostatin signals through a transforming growth factor beta-like signaling pathway to block adipogenesis. Mol Cell Biol. 2003;23:7230–42. doi: 10.1128/MCB.23.20.7230-7242.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sartori R, Milan G, Patron M, Mammucari C, Blaauw B, et al. Smad2 and 3 transcription factors control muscle mass in adulthood. Am J Physiol Cell Physiol. 2009;296:C1248–57. doi: 10.1152/ajpcell.00104.2009. [DOI] [PubMed] [Google Scholar]
- 15.Trendelenburg AU, Meyer A, Rohner D, Boyle J, Hatakeyama S, Glass DJ. Myostatin reduces Akt/TORC1/p70S6K signaling, inhibiting myoblast differentiation and myotube size. Am J Physiol Cell Physiol. 2009;296:C1258–70. doi: 10.1152/ajpcell.00105.2009. [DOI] [PubMed] [Google Scholar]
- 16.Bollinger LM, Witczak CA, Houmard JA, Brault JJ. SMAD3 augments FoxO3 induced MuRF-1 promoter activity in a DNA-binding-dependent manner. Am J Physiol Cell Physiol. 2014;307:C278–87. doi: 10.1152/ajpcell.00391.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loumaye A, Thissen JP. Biomarkers of cancer cachexia. Clin Biochem. 2017;50:1281–8. doi: 10.1016/j.clinbiochem.2017.07.011. [DOI] [PubMed] [Google Scholar]
- 18.Jones JE, Cadena SM, Gong C, Wang X, Chen Z, et al. Supraphysiologic Administration of GDF11 Induces Cachexia in Part by Upregulating GDF15. Cell Rep. 2018;22:1522–30. doi: 10.1016/j.celrep.2018.01.044. [DOI] [PubMed] [Google Scholar]
- 19.Johnen H, Lin S, Kuffner T, Brown DA, Tsai VW, et al. Tumor-induced anorexia and weight loss are mediated by the TGF-beta superfamily cytokine MIC-1. Nat Med. 2007;13:1333–40. doi: 10.1038/nm1677. [DOI] [PubMed] [Google Scholar]
- 20.Lerner L, Tao J, Liu Q, Nicoletti R, Feng B, et al. MAP3K11/GDF15 axis is a critical driver of cancer cachexia. J Cachexia Sarcopenia Muscle. 2016;7:467–82. doi: 10.1002/jcsm.12077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patel MS, Lee J, Baz M, Wells CE, Bloch S, et al. Growth differentiation factor-15 is associated with muscle mass in chronic obstructive pulmonary disease and promotes muscle wasting in vivo. J Cachexia Sarcopenia Muscle. 2016;7:436–48. doi: 10.1002/jcsm.12096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bodine SC, Baehr LM. Skeletal muscle atrophy and the E3 ubiquitin ligases MuRF1 and MAFbx/atrogin-1. Am J Physiol Endocrinol Metab. 2014;307:E469–84. doi: 10.1152/ajpendo.00204.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bodine SC, Latres E, Baumhueter S, Lai VK, Nunez L, et al. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science. 2001;294:1704–8. doi: 10.1126/science.1065874. [DOI] [PubMed] [Google Scholar]
- 24.Sandri M, Sandri C, Gilbert A, Skurk C, Calabria E, et al. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell. 2004;117:399–412. doi: 10.1016/s0092-8674(04)00400-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shimizu N, Yoshikawa N, Ito N, Maruyama T, Suzuki Y, et al. Crosstalk between glucocorticoid receptor and nutritional sensor mTOR in skeletal muscle. Cell Metab. 2011;13:170–82. doi: 10.1016/j.cmet.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 26.Cai D, Frantz JD, Tawa NE, Jr, Melendez PA, Oh BC, et al. IKKbeta/NF-kappaB activation causes severe muscle wasting in mice. Cell. 2004;119:285–98. doi: 10.1016/j.cell.2004.09.027. [DOI] [PubMed] [Google Scholar]
- 27.Hayden MS, Ghosh S. NF-kappaB, the first quarter-century: remarkable progress and outstanding questions. Genes Dev. 2012;26:203–34. doi: 10.1101/gad.183434.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dogra C, Changotra H, Wedhas N, Qin X, Wergedal JE, Kumar A. TNF-related weak inducer of apoptosis (TWEAK) is a potent skeletal muscle-wasting cytokine. FASEB J. 2007;21:1857–69. doi: 10.1096/fj.06-7537com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mittal A, Bhatnagar S, Kumar A, Lach-Trifilieff E, Wauters S, et al. The TWEAK-Fn14 system is a critical regulator of denervation-induced skeletal muscle atrophy in mice. J Cell Biol. 2010;188:833–49. doi: 10.1083/jcb.200909117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Belizario JE, Fontes-Oliveira CC, Borges JP, Kashiabara JA, Vannier E. Skeletal muscle wasting and renewal: a pivotal role of myokine IL-6. Springerplus. 2016;5:619. doi: 10.1186/s40064-016-2197-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yoshida T, Tabony AM, Galvez S, Mitch WE, Higashi Y, et al. Molecular mechanisms and signaling pathways of angiotensin II-induced muscle wasting: potential therapeutic targets for cardiac cachexia. Int J Biochem Cell Biol. 2013;45:2322–32. doi: 10.1016/j.biocel.2013.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bonetto A, Aydogdu T, Jin X, Zhang Z, Zhan R, et al. JAK/STAT3 pathway inhibition blocks skeletal muscle wasting downstream of IL-6 and in experimental cancer cachexia. Am J Physiol Endocrinol Metab. 2012;303:E410–21. doi: 10.1152/ajpendo.00039.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Du Bois P, Pablo Tortola C, Lodka D, Kny M, Schmidt F, et al. Angiotensin II Induces Skeletal Muscle Atrophy by Activating TFEB-Mediated MuRF1 Expression. Circ Res. 2015;117:424–36. doi: 10.1161/CIRCRESAHA.114.305393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Song YH, Li Y, Du J, Mitch WE, Rosenthal N, Delafontaine P. Muscle-specific expression of IGF-1 blocks angiotensin II-induced skeletal muscle wasting. J Clin Invest. 2005;115:451–8. doi: 10.1172/JCI22324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baracos VE, Martin L, Korc M, Guttridge DC, Fearon KCH. Cancer-associated cachexia. Nat Rev Dis Primers. 2018;4:17105. doi: 10.1038/nrdp.2017.105. [DOI] [PubMed] [Google Scholar]
- 36.Johnston AJ, Murphy KT, Jenkinson L, Laine D, Emmrich K, et al. Targeting of Fn14 Prevents Cancer-Induced Cachexia and Prolongs Survival. Cell. 2015;162:1365–78. doi: 10.1016/j.cell.2015.08.031. [DOI] [PubMed] [Google Scholar]
- 37.White JP, Baynes JW, Welle SL, Kostek MC, Matesic LE, et al. The regulation of skeletal muscle protein turnover during the progression of cancer cachexia in the Apc(Min/+) mouse. PLoS One. 2011;6:e24650. doi: 10.1371/journal.pone.0024650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Steffen BT, Lees SJ, Booth FW. Anti-TNF treatment reduces rat skeletal muscle wasting in monocrotaline-induced cardiac cachexia. J Appl Physiol (1985) 2008;105:1950–8. doi: 10.1152/japplphysiol.90884.2008. [DOI] [PubMed] [Google Scholar]
- 39.Gordon JN, Trebble TM, Ellis RD, Duncan HD, Johns T, Goggin PM. Thalidomide in the treatment of cancer cachexia: a randomised placebo controlled trial. Gut. 2005;54:540–5. doi: 10.1136/gut.2004.047563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jatoi A, Dakhil SR, Nguyen PL, Sloan JA, Kugler JW, et al. A placebo-controlled double blind trial of etanercept for the cancer anorexia/weight loss syndrome: results from N00C1 from the North Central Cancer Treatment Group. Cancer. 2007;110:1396–403. doi: 10.1002/cncr.22944. [DOI] [PubMed] [Google Scholar]
- 41.Jatoi A, Ritter HL, Dueck A, Nguyen PL, Nikcevich DA, et al. A placebo-controlled, double-blind trial of infliximab for cancer-associated weight loss in elderly and/or poor performance non-small cell lung cancer patients (N01C9) Lung Cancer. 2010;68:234–9. doi: 10.1016/j.lungcan.2009.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hong DS, Hui D, Bruera E, Janku F, Naing A, et al. MABp1, a first-in-class true human antibody targeting interleukin-1alpha in refractory cancers: an open-label, phase 1 dose-escalation and expansion study. Lancet Oncol. 2014;15:656–66. doi: 10.1016/S1470-2045(14)70155-X. [DOI] [PubMed] [Google Scholar]
- 43.Rigas JR, Schuster M, Orlov SV, Milovanovic B, Prabhash K, et al. Efect of ALD518, a humanized anti-IL-6 antibody, on lean body mass loss and symptoms in patients with advanced non-small cell lung cancer (NSCLC): Results of a phase II randomized, double-blind safety and efficacy trial. Journal of Clinical Oncology. 2010;28:7622. [Google Scholar]
- 44.Schuster M, Rigas JR, Orlov SV, Milovanovic B, Prabhash K, et al. ALD518, a humanized anti-IL-6 antibody, treats anemia in patients with advanced non-small cell lung cancer (NSCLC): Results of a phase II randomized, double-blind, placebo-controlled trial. Journal of Clinical Oncology. 2010;28:7631. [Google Scholar]
- 45.Rieu I, Magne H, Savary-Auzeloux I, Averous J, Bos C, et al. Reduction of low grade inflammation restores blunting of postprandial muscle anabolism and limits sarcopenia in old rats. J Physiol. 2009;587:5483–92. doi: 10.1113/jphysiol.2009.178319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Granado M, Martin AI, Villanua MA, Lopez-Calderon A. Experimental arthritis inhibits the insulin-like growth factor-I axis and induces muscle wasting through cyclooxygenase-2 activation. Am J Physiol Endocrinol Metab. 2007;292:E1656–65. doi: 10.1152/ajpendo.00502.2006. [DOI] [PubMed] [Google Scholar]
- 47.Mantovani G, Maccio A, Madeddu C, Serpe R, Antoni G, et al. Phase II nonrandomized study of the efficacy and safety of COX-2 inhibitor celecoxib on patients with cancer cachexia. J Mol Med (Berl) 2010;88:85–92. doi: 10.1007/s00109-009-0547-z. [DOI] [PubMed] [Google Scholar]
- 48.Lai V, George J, Richey L, Kim HJ, Cannon T, et al. Results of a pilot study of the effects of celecoxib on cancer cachexia in patients with cancer of the head, neck, and gastrointestinal tract. Head Neck. 2008;30:67–74. doi: 10.1002/hed.20662. [DOI] [PubMed] [Google Scholar]
- 49.Rodino-Klapac LR, Haidet AM, Kota J, Handy C, Kaspar BK, Mendell JR. Inhibition of myostatin with emphasis on follistatin as a therapy for muscle disease. Muscle Nerve. 2009;39:283–96. doi: 10.1002/mus.21244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhou X, Wang JL, Lu J, Song Y, Kwak KS, et al. Reversal of cancer cachexia and muscle wasting by ActRIIB antagonism leads to prolonged survival. Cell. 2010;142:531–43. doi: 10.1016/j.cell.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 51.Becker C, Lord SR, Studenski SA, Warden SJ, Fielding RA, et al. Myostatin antibody (LY2495655) in older weak fallers: a proof-of-concept, randomised, phase 2 trial. Lancet Diabetes Endocrinol. 2015;3:948–57. doi: 10.1016/S2213-8587(15)00298-3. [DOI] [PubMed] [Google Scholar]
- 52.Jameson GS, Hoff DDV, Weiss GJ, Richards DA, Smith DA, et al. Safety of the antimyostatin monoclonal antibody LY2495655 in healthy subjects and patients with advanced cancer. Journal of Clinical Oncology. 2012;30:2516. [Google Scholar]
- 53.Ebner N, von Haehling S. Unlocking the wasting enigma: Highlights from the 8th Cachexia Conference. J Cachexia Sarcopenia Muscle. 2016;7:90–4. doi: 10.1002/jcsm.12106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lach-Trifilieff E, Minetti GC, Sheppard K, Ibebunjo C, Feige JN, et al. An antibody blocking activin type II receptors induces strong skeletal muscle hypertrophy and protects from atrophy. Mol Cell Biol. 2014;34:606–18. doi: 10.1128/MCB.01307-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Amato AA, Sivakumar K, Goyal N, David WS, Salajegheh M, et al. Treatment of sporadic inclusion body myositis with bimagrumab. Neurology. 2014;83:2239–46. doi: 10.1212/WNL.0000000000001070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rooks D, Praestgaard J, Hariry S, Laurent D, Petricoul O, et al. Treatment of Sarcopenia with Bimagrumab: Results from a Phase II, Randomized, Controlled, Proof-of-Concept Study. J Am Geriatr Soc. 2017;65:1988–95. doi: 10.1111/jgs.14927. [DOI] [PubMed] [Google Scholar]
- 57.Hsu JY, Crawley S, Chen M, Ayupova DA, Lindhout DA, et al. Non-homeostatic body weight regulation through a brainstem-restricted receptor for GDF15. Nature. 2017;550:255–9. doi: 10.1038/nature24042. [DOI] [PubMed] [Google Scholar]
- 58.Murphy KT, Chee A, Trieu J, Naim T, Lynch GS. Inhibition of the renin-angiotensin system improves physiological outcomes in mice with mild or severe cancer cachexia. Int J Cancer. 2013;133:1234–46. doi: 10.1002/ijc.28128. [DOI] [PubMed] [Google Scholar]
- 59.Shrikrishna D, Tanner RJ, Lee JY, Natanek A, Lewis A, et al. A randomized controlled trial of angiotensin-converting enzyme inhibition for skeletal muscle dysfunction in COPD. Chest. 2014;146:932–40. doi: 10.1378/chest.13-2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Burks TN, Andres-Mateos E, Marx R, Mejias R, Van Erp C, et al. Losartan restores skeletal muscle remodeling and protects against disuse atrophy in sarcopenia. Sci Transl Med. 2011;3:82ra37. doi: 10.1126/scitranslmed.3002227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dubois V, Laurent M, Boonen S, Vanderschueren D, Claessens F. Androgens and skeletal muscle: cellular and molecular action mechanisms underlying the anabolic actions. Cell Mol Life Sci. 2012;69:1651–67. doi: 10.1007/s00018-011-0883-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rossetti ML, Steiner JL, Gordon BS. Androgen-mediated regulation of skeletal muscle protein balance. Mol Cell Endocrinol. 2017;447:35–44. doi: 10.1016/j.mce.2017.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.White JP, Gao S, Puppa MJ, Sato S, Welle SL, Carson JA. Testosterone regulation of Akt/mTORC1/FoxO3a signaling in skeletal muscle. Mol Cell Endocrinol. 2013;365:174–86. doi: 10.1016/j.mce.2012.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Basualto-Alarcon C, Jorquera G, Altamirano F, Jaimovich E, Estrada M. Testosterone signals through mTOR and androgen receptor to induce muscle hypertrophy. Med Sci Sports Exerc. 2013;45:1712–20. doi: 10.1249/MSS.0b013e31828cf5f3. [DOI] [PubMed] [Google Scholar]
- 65.Chambon C, Duteil D, Vignaud A, Ferry A, Messaddeq N, et al. Myocytic androgen receptor controls the strength but not the mass of limb muscles. Proc Natl Acad Sci U S A. 2010;107:14327–32. doi: 10.1073/pnas.1009536107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schmidt A, Harada S, Kimmel DB, Bai C, Chen F, et al. Identification of anabolic selective androgen receptor modulators with reduced activities in reproductive tissues and sebaceous glands. J Biol Chem. 2009;284:36367–76. doi: 10.1074/jbc.M109.049734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dobs AS, Boccia RV, Croot CC, Gabrail NY, Dalton JT, et al. Effects of enobosarm on muscle wasting and physical function in patients with cancer: a double-blind, randomised controlled phase 2 trial. Lancet Oncol. 2013;14:335–45. doi: 10.1016/S1470-2045(13)70055-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Papanicolaou DA, Ather SN, Zhu H, Zhou Y, Lutkiewicz J, et al. A phase IIA randomized, placebo-controlled clinical trial to study the efficacy and safety of the selective androgen receptor modulator (SARM), MK-0773 in female participants with sarcopenia. J Nutr Health Aging. 2013;17:533–43. doi: 10.1007/s12603-013-0335-x. [DOI] [PubMed] [Google Scholar]
- 69.Basaria S, Collins L, Dillon EL, Orwoll K, Storer TW, et al. The safety, pharmacokinetics, and effects of LGD-4033, a novel nonsteroidal oral, selective androgen receptor modulator, in healthy young men. J Gerontol A Biol Sci Med Sci. 2013;68:87–95. doi: 10.1093/gerona/gls078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Crawford J, Prado CM, Johnston MA, Gralla RJ, Taylor RP, et al. Study Design and Rationale for the Phase 3 Clinical Development Program of Enobosarm, a Selective Androgen Receptor Modulator, for the Prevention and Treatment of Muscle Wasting in Cancer Patients (POWER Trials) Curr Oncol Rep. 2016;18:37. doi: 10.1007/s11912-016-0522-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Busquets S, Toledo M, Marmonti E, Orpi M, Capdevila E, et al. Formoterol treatment downregulates the myostatin system in skeletal muscle of cachectic tumour-bearing rats. Oncol Lett. 2012;3:185–9. doi: 10.3892/ol.2011.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Goncalves DA, Silveira WA, Lira EC, Graca FA, Paula-Gomes S, et al. Clenbuterol suppresses proteasomal and lysosomal proteolysis and atrophy-related genes in denervated rat soleus muscles independently of Akt. Am J Physiol Endocrinol Metab. 2012;302:E123–33. doi: 10.1152/ajpendo.00188.2011. [DOI] [PubMed] [Google Scholar]
- 73.Greig CA, Johns N, Gray C, MacDonald A, Stephens NA, et al. Phase I/II trial of formoterol fumarate combined with megestrol acetate in cachectic patients with advanced malignancy. Support Care Cancer. 2014;22:1269–75. doi: 10.1007/s00520-013-2081-3. [DOI] [PubMed] [Google Scholar]
- 74.Stewart Coats AJ, Ho GF, Prabhash K, von Haehling S, Tilson J, et al. Espindolol for the treatment and prevention of cachexia in patients with stage III/IV non-small cell lung cancer or colorectal cancer: a randomized, double-blind, placebo-controlled, international multicentre phase II study (the ACT-ONE trial) J Cachexia Sarcopenia Muscle. 2016;7:355–65. doi: 10.1002/jcsm.12126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Collden G, Tschop MH, Muller TD. Therapeutic Potential of Targeting the Ghrelin Pathway. Int J Mol Sci. 2017;18 doi: 10.3390/ijms18040798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Porporato PE, Filigheddu N, Reano S, Ferrara M, Angelino E, et al. Acylated and unacylated ghrelin impair skeletal muscle atrophy in mice. J Clin Invest. 2013;123:611–22. doi: 10.1172/JCI39920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Currow D, Temel JS, Abernethy A, Milanowski J, Friend J, Fearon KC. ROMANA 3 a phase 3: safety extension study of anamorelin in advanced non-small-cell lung cancer (NSCLC) patients with cachexia. Ann Oncol. 2017;28:1949–56. doi: 10.1093/annonc/mdx192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Levinson B, Gertner J. Randomized study of the efficacy and safety of SUN11031 (synthetic human ghrelin) in cachexia associated with chronic obstructive pulmonary disease. e-Spen J. 2012;7:e171–e5. [Google Scholar]
- 79.Katakami N, Uchino J, Yokoyama T, Naito T, Kondo M, et al. Anamorelin (ONO-7643) for the treatment of patients with non-small cell lung cancer and cachexia: Results from a randomized, double-blind, placebo-controlled, multicenter study of Japanese patients (ONO-7643-04) Cancer. 2018;124:606–16. doi: 10.1002/cncr.31128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Temel JS, Abernethy AP, Currow DC, Friend J, Duus EM, et al. Anamorelin in patients with non-small-cell lung cancer and cachexia (ROMANA 1 and ROMANA 2): results from two randomised, double-blind, phase 3 trials. Lancet Oncol. 2016;17:519–31. doi: 10.1016/S1470-2045(15)00558-6. [DOI] [PubMed] [Google Scholar]
- 81.Aversa Z, Costelli P, Muscaritoli M. Cancer-induced muscle wasting: latest findings in prevention and treatment. Therapeutic advances in medical oncology. 2017;9:369–82. doi: 10.1177/1758834017698643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mercuri E, Muntoni F. Muscular dystrophies. Lancet. 2013;381:845–60. doi: 10.1016/S0140-6736(12)61897-2. [DOI] [PubMed] [Google Scholar]
- 83.Emery AE. The muscular dystrophies. Lancet. 2002;359:687–95. doi: 10.1016/S0140-6736(02)07815-7. [DOI] [PubMed] [Google Scholar]
- 84.Muscular Dystrophy Association. List of neuromuscular diseases. [Accessed on February 28, 2018]; Available from https://www.mda.org/disease/list.
- 85.Ruegg MA, Glass DJ. Molecular mechanisms and treatment options for muscle wasting diseases. Annu Rev Pharmacol Toxicol. 2011;51:373–95. doi: 10.1146/annurev-pharmtox-010510-100537. [DOI] [PubMed] [Google Scholar]
- 86.Mah JK, Korngut L, Dykeman J, Day L, Pringsheim T, Jette N. A systematic review and meta-analysis on the epidemiology of Duchenne and Becker muscular dystrophy. Neuromuscul Disord. 2014;24:482–91. doi: 10.1016/j.nmd.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 87.Rinaldi C, Wood MJA. Antisense oligonucleotides: the next frontier for treatment of neurological disorders. Nat Rev Neurol. 2018;14:9–21. doi: 10.1038/nrneurol.2017.148. [DOI] [PubMed] [Google Scholar]
- 88.Gayathri N, Alefia R, Nalini A, Yasha TC, Anita M, et al. Dysferlinopathy: spectrum of pathological changes in skeletal muscle tissue. Indian J Pathol Microbiol. 2011;54:350–4. doi: 10.4103/0377-4929.81636. [DOI] [PubMed] [Google Scholar]
- 89.Matthews E, Brassington R, Kuntzer T, Jichi F, Manzur AY. Corticosteroids for the treatment of Duchenne muscular dystrophy. Cochrane Database Syst Rev. 2016 doi: 10.1002/14651858.CD003725.pub4. CD003725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Angelini C. The role of corticosteroids in muscular dystrophy: a critical appraisal. Muscle Nerve. 2007;36:424–35. doi: 10.1002/mus.20812. [DOI] [PubMed] [Google Scholar]
- 91.Nakamura A. Moving towards successful exon-skipping therapy for Duchenne muscular dystrophy. J Hum Genet. 2017;62:871–6. doi: 10.1038/jhg.2017.57. [DOI] [PubMed] [Google Scholar]
- 92.Guiraud S, Davies KE. Pharmacological advances for treatment in Duchenne muscular dystrophy. Curr Opin Pharmacol. 2017;34:36–48. doi: 10.1016/j.coph.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 93.Guiraud S, Squire SE, Edwards B, Chen H, Burns DT, et al. Second-generation compound for the modulation of utrophin in the therapy of DMD. Hum Mol Genet. 2015;24:4212–24. doi: 10.1093/hmg/ddv154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tinsley JM, Fairclough RJ, Storer R, Wilkes FJ, Potter AC, et al. Daily treatment with SMTC1100, a novel small molecule utrophin upregulator, dramatically reduces the dystrophic symptoms in the mdx mouse. PLoS One. 2011;6:e19189. doi: 10.1371/journal.pone.0019189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Okada T, Takeda S. Current Challenges and Future Directions in Recombinant AAV-Mediated Gene Therapy of Duchenne Muscular Dystrophy. Pharmaceuticals (Basel) 2013;6:813–36. doi: 10.3390/ph6070813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gregorevic P, Allen JM, Minami E, Blankinship MJ, Haraguchi M, et al. rAAV6-microdystrophin preserves muscle function and extends lifespan in severely dystrophic mice. Nat Med. 2006;12:787–9. doi: 10.1038/nm1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ragot T, Vincent N, Chafey P, Vigne E, Gilgenkrantz H, et al. Efficient adenovirus-mediated transfer of a human minidystrophin gene to skeletal muscle of mdx mice. Nature. 1993;361:647–50. doi: 10.1038/361647a0. [DOI] [PubMed] [Google Scholar]
- 98.Wang B, Li J, Xiao X. Adeno-associated virus vector carrying human minidystrophin genes effectively ameliorates muscular dystrophy in mdx mouse model. Proc Natl Acad Sci U S A. 2000;97:13714–9. doi: 10.1073/pnas.240335297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Le Guiner C, Servais L, Montus M, Larcher T, Fraysse B, et al. Long-term microdystrophin gene therapy is effective in a canine model of Duchenne muscular dystrophy. Nat Commun. 2017;8 doi: 10.1038/ncomms16105. 16105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mendell JR, Campbell K, Rodino-Klapac L, Sahenk Z, Shilling C, et al. Dystrophin immunity in Duchenne's muscular dystrophy. N Engl J Med. 2010;363:1429–37. doi: 10.1056/NEJMoa1000228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mendell JR, Rodino-Klapac LR, Rosales-Quintero X, Kota J, Coley BD, et al. Limb-girdle muscular dystrophy type 2D gene therapy restores alpha-sarcoglycan and associated proteins. Ann Neurol. 2009;66:290–7. doi: 10.1002/ana.21732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Herson S, Hentati F, Rigolet A, Behin A, Romero NB, et al. A phase I trial of adeno-associated virus serotype 1-gamma-sarcoglycan gene therapy for limb girdle muscular dystrophy type 2C. Brain. 2012;135:483–92. doi: 10.1093/brain/awr342. [DOI] [PubMed] [Google Scholar]
- 103.Mendell JR, Rodino-Klapac LR, Rosales XQ, Coley BD, Galloway G, et al. Sustained alpha-sarcoglycan gene expression after gene transfer in limb-girdle muscular dystrophy, type 2D. Ann Neurol. 2010;68:629–38. doi: 10.1002/ana.22251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Reinhard JR, Lin S, McKee KK, Meinen S, Crosson SC, et al. Linker proteins restore basement membrane and correct LAMA2-related muscular dystrophy in mice. Sci Transl Med. 2017;9 doi: 10.1126/scitranslmed.aal4649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bladen CL, Salgado D, Monges S, Foncuberta ME, Kekou K, et al. The TREATNMD DMD Global Database: analysis of more than 7,000 Duchenne muscular dystrophy mutations. Hum Mutat. 2015;36:395–402. doi: 10.1002/humu.22758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Keeling KM, Xue X, Gunn G, Bedwell DM. Therapeutics based on stop codon readthrough. Annu Rev Genomics Hum Genet. 2014;15:371–94. doi: 10.1146/annurev-genom-091212-153527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Welch EM, Barton ER, Zhuo J, Tomizawa Y, Friesen WJ, et al. PTC124 targets genetic disorders caused by nonsense mutations. Nature. 2007;447:87–91. doi: 10.1038/nature05756. [DOI] [PubMed] [Google Scholar]
- 108.Bushby K, Finkel R, Wong B, Barohn R, Campbell C, et al. Ataluren treatment of patients with nonsense mutation dystrophinopathy. Muscle Nerve. 2014;50:477–87. doi: 10.1002/mus.24332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Finkel RS, Flanigan KM, Wong B, Bonnemann C, Sampson J, et al. Phase 2a study of ataluren-mediated dystrophin production in patients with nonsense mutation Duchenne muscular dystrophy. PLoS One. 2013;8:e81302. doi: 10.1371/journal.pone.0081302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.McDonald CM, Campbell C, Torricelli RE, Finkel RS, Flanigan KM, et al. Ataluren in patients with nonsense mutation Duchenne muscular dystrophy (ACT DMD): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390:1489–98. doi: 10.1016/S0140-6736(17)31611-2. [DOI] [PubMed] [Google Scholar]
- 111.PTC Therapeutics. Approved medicines. [Accessed on February 28, 2018]; Available from https://www.ptcbio.com/en/pipeline/our-approved-medicines/
- 112.Wang B, Yang Z, Brisson BK, Feng H, Zhang Z, et al. Membrane blebbing as an assessment of functional rescue of dysferlin-deficient human myotubes via nonsense suppression. J Appl Physiol (1985) 2010;109:901–5. doi: 10.1152/japplphysiol.01366.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.van Deutekom JC, Janson AA, Ginjaar IB, Frankhuizen WS, Aartsma-Rus A, et al. Local dystrophin restoration with antisense oligonucleotide PRO051. N Engl J Med. 2007;357:2677–86. doi: 10.1056/NEJMoa073108. [DOI] [PubMed] [Google Scholar]
- 114.Kinali M, Arechavala-Gomeza V, Feng L, Cirak S, Hunt D, et al. Local restoration of dystrophin expression with the morpholino oligomer AVI-4658 in Duchenne muscular dystrophy: a single-blind, placebo-controlled, dose-escalation, proof-of-concept study. Lancet Neurol. 2009;8:918–28. doi: 10.1016/S1474-4422(09)70211-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bennett CF, Baker BF, Pham N, Swayze E, Geary RS. Pharmacology of Antisense Drugs. Annu Rev Pharmacol Toxicol. 2017;57:81–105. doi: 10.1146/annurev-pharmtox-010716-104846. [DOI] [PubMed] [Google Scholar]
- 116.U.S. Food and Drug Administration. FDA grants accelerated approval to first drug for Duchenne muscular dystrophy. [Accessed on February 28, 2018]; Available from https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm521263.htm.
- 117.Cirak S, Arechavala-Gomeza V, Guglieri M, Feng L, Torelli S, et al. Exon skipping and dystrophin restoration in patients with Duchenne muscular dystrophy after systemic phosphorodiamidate morpholino oligomer treatment: an open-label, phase 2, dose-escalation study. Lancet. 2011;378:595–605. doi: 10.1016/S0140-6736(11)60756-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mendell JR, Goemans N, Lowes LP, Alfano LN, Berry K, et al. Longitudinal effect of eteplirsen versus historical control on ambulation in Duchenne muscular dystrophy. Ann Neurol. 2016;79:257–71. doi: 10.1002/ana.24555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mendell JR, Rodino-Klapac LR, Sahenk Z, Roush K, Bird L, et al. Eteplirsen for the treatment of Duchenne muscular dystrophy. Ann Neurol. 2013;74:637–47. doi: 10.1002/ana.23982. [DOI] [PubMed] [Google Scholar]
- 120.SAREPTA Therapeutics. Exon-skipping for duchenne - our pipeline. [Accessed on February 28, 2018]; Available from https://www.sarepta.com/pipeline/exon-skipping-duchenne.
- 121.Goemans N, Mercuri E, Belousova E, Komaki H, Dubrovsky A, et al. A randomized placebo-controlled phase 3 trial of an antisense oligonucleotide, drisapersen, in Duchenne muscular dystrophy. Neuromuscul Disord. 2018;28:4–15. doi: 10.1016/j.nmd.2017.10.004. [DOI] [PubMed] [Google Scholar]
- 122.Xconomy. BioMarin shelves duchenne drug. [Accessed on February 28, 2018]; Available from https://www.xconomy.com/san-francisco/2016/05/31/two-years-after-paying-680m-biomarin-shelves-duchenne-drug/
- 123.Kir S, White JP, Kleiner S, Kazak L, Cohen P, et al. Tumour-derived PTH-related protein triggers adipose tissue browning and cancer cachexia. Nature. 2014;513:100–4. doi: 10.1038/nature13528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kir S, Komaba H, Garcia AP, Economopoulos KP, Liu W, et al. PTH/PTHrP Receptor Mediates Cachexia in Models of Kidney Failure and Cancer. Cell Metab. 2016;23:315–23. doi: 10.1016/j.cmet.2015.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Goncalves MD, Hwang SK, Pauli C, Murphy CJ, Cheng Z, et al. Fenofibrate prevents skeletal muscle loss in mice with lung cancer. Proc Natl Acad Sci U S A. 2018;115:E743–E52. doi: 10.1073/pnas.1714703115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Svensson K, Handschin C. Modulation of PGC-1alpha activity as a treatment for metabolic and muscle-related diseases. Drug discovery today. 2014;19:1024–9. doi: 10.1016/j.drudis.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 127.Handschin C, Kobayashi YM, Chin S, Seale P, Campbell KP, Spiegelman BM. PGC-1alpha regulates the neuromuscular junction program and ameliorates Duchenne muscular dystrophy. Genes Dev. 2007;21:770–83. doi: 10.1101/gad.1525107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Handschin C. Caloric restriction and exercise "mimetics": Ready for prime time? Pharmacological research. 2016;103:158–66. doi: 10.1016/j.phrs.2015.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Weihrauch M, Handschin C. Pharmacological targeting of exercise adaptations in skeletal muscle: Benefits and pitfalls. Biochemical pharmacology. 2018;147:211–20. doi: 10.1016/j.bcp.2017.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Booth FW, Laye MJ. Lack of adequate appreciation of physical exercise's complexities can pre-empt appropriate design and interpretation in scientific discovery. J Physiol. 2009;587:5527–39. doi: 10.1113/jphysiol.2009.179507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Godfrey C, Desviat LR, Smedsrod B, Pietri-Rouxel F, Denti MA, et al. Delivery is key: lessons learnt from developing splice-switching antisense therapies. EMBO Mol Med. 2017;9:545–57. doi: 10.15252/emmm.201607199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yin H, Saleh AF, Betts C, Camelliti P, Seow Y, et al. Pip5 transduction peptides direct high efficiency oligonucleotide-mediated dystrophin exon skipping in heart and phenotypic correction in mdx mice. Mol Ther. 2011;19:1295–303. doi: 10.1038/mt.2011.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wang M, Wu B, Shah SN, Lu P, Lu Q. Saponins as Natural Adjuvant for Antisense Morpholino Oligonucleotides Delivery in vitro and in mdx Mice. Mol Ther Nucleic Acids. 2018 doi: 10.1016/j.omtn.2018.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Goyenvalle A, Griffith G, Babbs A, El Andaloussi S, Ezzat K, et al. Functional correction in mouse models of muscular dystrophy using exon-skipping tricyclo-DNA oligomers. Nat Med. 2015;21:270–5. doi: 10.1038/nm.3765. [DOI] [PubMed] [Google Scholar]
- 135.Relizani K, Griffith G, Echevarria L, Zarrouki F, Facchinetti P, et al. Efficacy and Safety Profile of Tricyclo-DNA Antisense Oligonucleotides in Duchenne Muscular Dystrophy Mouse Model. Mol Ther Nucleic Acids. 2017;8:144–57. doi: 10.1016/j.omtn.2017.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Beroud C, Tuffery-Giraud S, Matsuo M, Hamroun D, Humbertclaude V, et al. Multiexon skipping leading to an artificial DMD protein lacking amino acids from exons 45 through 55 could rescue up to 63% of patients with Duchenne muscular dystrophy. Hum Mutat. 2007;28:196–202. doi: 10.1002/humu.20428. [DOI] [PubMed] [Google Scholar]
- 137.Aoki Y, Yokota T, Nagata T, Nakamura A, Tanihata J, et al. Bodywide skipping of exons 45-55 in dystrophic mdx52 mice by systemic antisense delivery. Proc Natl Acad Sci U S A. 2012;109:13763–8. doi: 10.1073/pnas.1204638109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Gee P, Xu H, Hotta A. Cellular Reprogramming, Genome Editing, and Alternative CRISPR Cas9 Technologies for Precise Gene Therapy of Duchenne Muscular Dystrophy. Stem Cells Int. 2017;2017 doi: 10.1155/2017/8765154. 8765154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Bengtsson NE, Hall JK, Odom GL, Phelps MP, Andrus CR, et al. Muscle-specific CRISPR/Cas9 dystrophin gene editing ameliorates pathophysiology in a mouse model for Duchenne muscular dystrophy. Nat Commun. 2017;8 doi: 10.1038/ncomms14454. 14454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Tabebordbar M, Zhu K, Cheng JKW, Chew WL, Widrick JJ, et al. In vivo gene editing in dystrophic mouse muscle and muscle stem cells. Science. 2016;351:407–11. doi: 10.1126/science.aad5177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zhang Y, Long C, Li H, McAnally JR, Baskin KK, et al. CRISPR-Cpf1 correction of muscular dystrophy mutations in human cardiomyocytes and mice. Sci Adv. 2017;3:e1602814. doi: 10.1126/sciadv.1602814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Handschin C, Mortezavi A, Plock J, Eberli D. External physical and biochemical stimulation to enhance skeletal muscle bioengineering. Advanced drug delivery reviews. 2015;82:83–168. doi: 10.1016/j.addr.2014.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kodaka Y, Rabu G, Asakura A. Skeletal Muscle Cell Induction from Pluripotent Stem Cells. Stem Cells Int. 2017;2017:1376151. doi: 10.1155/2017/1376151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sienkiewicz D, Kulak W, Okurowska-Zawada B, Paszko-Patej G, Kawnik K. Duchenne muscular dystrophy: current cell therapies. Ther Adv Neurol Disord. 2015;8:166–77. doi: 10.1177/1756285615586123. [DOI] [PMC free article] [PubMed] [Google Scholar]