Abstract
A 71-year-old woman with congenital rubella syndrome (CRS) presented with prolonged cough. No physical findings suggested the presence of any connective tissue diseases. Chest computed tomography showed ground-glass opacities and consolidations in the bilateral lower lobes. She had elevated serum Krebs von den Lungen-6, hypoxemia and positive serum anti-Jo-1 antibody. Bronchoalveolar lavage fluid revealed lymphocytosis with a decreased CD4/CD8 ratio. A transbronchial lung biopsy specimen revealed organizing pneumonia. Based on a diagnosis of interstitial pneumonia with autoimmune features (IPAF), systemic corticosteroids were administered, and a good outcome was obtained. A possible relationship between CRS and IPAF is herein discussed.
Keywords: congenital rubella syndrome, interstitial pneumonia, autoimmunity
Introduction
Congenital rubella syndrome (CRS) in newborns is caused by a rubella infection during the first trimester of pregnancy. Manifestations of CRS include hearing impairment, cataracts, congenital heart diseases such as pulmonary stenosis and persistent ductus arteriosus and mental retardation (1, 2). In addition, interstitial pneumonia arising in infants with CRS has been reported (3-6). However, interstitial pneumonia in an adult patient with CRS has never been reported as far as we can ascertain.
This case report describes a rare case of anti-Jo-1 antibody-positive interstitial pneumonia in an elderly patient with CRS and the possible relationship between CRS and anti-Jo-1 antibody-positive interstitial pneumonia is herein discussed.
Case Report
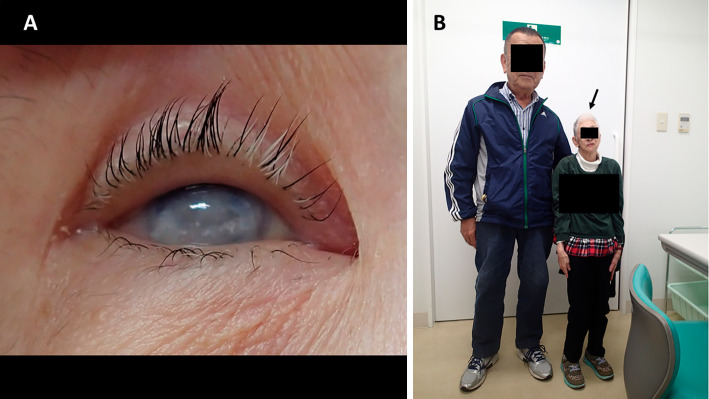
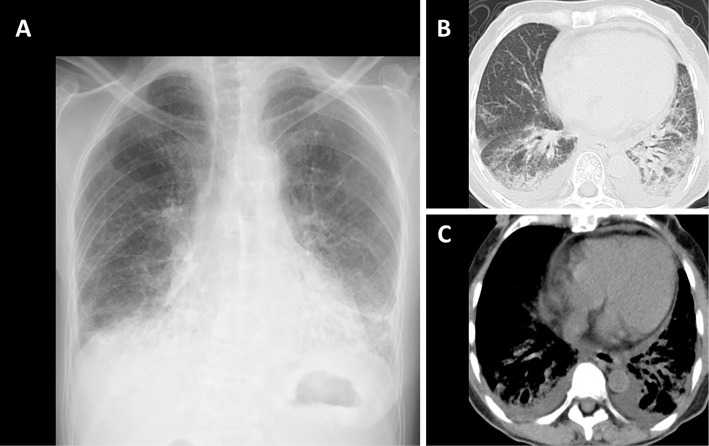
A 71-year-old woman was referred to our hospital for detailed assessment of a cough that had persisted for two months. She had never smoked. She had endured epilepsy, hearing loss, blurred vision, and mental retardation since childhood and had been treated by a general physician. The etiology of these symptoms was unknown at that time. She had been clinically diagnosed with CRS based on characteristic manifestations that were observed at 40 years of age, when she was taken by ambulance to a general hospital with status epilepticus. She had been medicated with carbamazepine since then. She was operated on for left cataracts at 63 years of age, but her vision did not improve substantially. She had no signs of connective tissue diseases (skin rash, arthritis, muscle weakness, Raynaud phenomenon) throughout the course. Her mother died of lung cancer at 80 years of age, without any known history of rubella infection. The physical findings were as follows: height 123.0 cm, weight 20.0 kg. She was afebrile on admission, but fever appeared soon thereafter, and she also had a cataract in her right eye, dwarfism and microcephaly (Fig. 1). Fine crackles were audible in the bilateral lower lung lobes, however, no heart murmurs were audible. No physical findings of skin rash, arthritis, proximal muscle weakness or Raynaud phenomenon suggestive of connective tissue diseases or congenital heart failure (pitting edema of the lower extremities and jugular venous distention) were found. Laboratory data showed leukocytosis (11,300/μL) with neutrophilia (77.6%), elevated serum levels of lactate dehydrogenase (402 U/L), C-reactive protein (3.16 mg/dL), immunoglobulin G (1,713 mg/dL), Krebs von den Lungen-6 (723 U/mL), surfactant protein-D (163.0 ng/mL), anti- Jo-1 antibody (≥550 U/mL) and hypoxemia (arterial oxygen partial pressure, 53.8 mmHg under room air). The serum creatine kinase and aldolase, as well as plasma brain natriuretic peptide levels were normal. Pulmonary function tests could not be carried out because of the patient's hearing loss, blurred vision and reduced mental capacity. The electrocardiography and echocardiography findings were normal. Chest radiography showed ground-glass shadows in the bilateral lower lung fields (Fig. 2A). Chest computed tomography (CT) showed ground-glass opacities and consolidations in bilateral lower lobes (Fig. 2B) and some bilateral pleural and pericardial effusions (Fig. 2C). Analyses of bronchoalveolar lavage fluid (BALF) revealed a cell count of 1.6×105/mL, with a cell differential of 18.0% macrophages, 47.0% lymphocytes, 24.0% neutrophils, 10.0% eosinophils, 1.0% basophils and a CD4/CD8 ratio of 1.0. No microorganisms were detected in BALF cultures. A transbronchial biopsy specimen obtained from the left lower lung lobe revealed alveolar septal thickening, inflammatory cell infiltration and organizing pneumonia (Fig. 3).
Figure 1.
Physical features of the patient. Right eye and whole-body images show the presence of a cataract (A), dwarfism and microcephaly (B, arrow).
Figure 2.
Chest radiography findings at the initial presentation. Ground-glass shadows are observed in the bilateral lower lung fields (A). Chest CT shows ground-glass opacities and consolidations in the bilateral lower lobes (B) and small volumes of bilateral pleural effusion and pericardial effusion (C).
Figure 3.
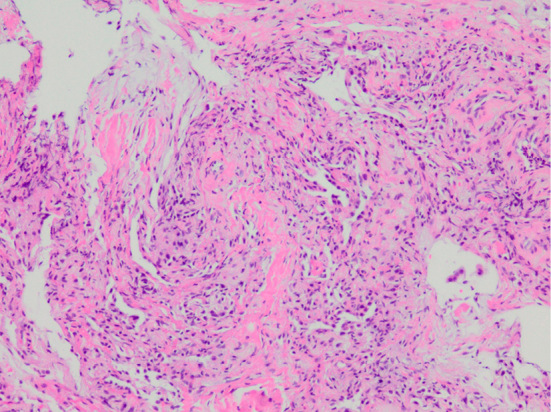
A transbronchial lung biopsy specimen of the left lower lobe. An image shows alveolar septal thickening, inflammatory cell infiltration, and organizing pneumonia (Hematoxylin and Eosin staining, ×100).
Based on a diagnosis of anti-Jo-1 antibody-positive interstitial pneumonia, systemic corticosteroid therapy with prednisolone 20 mg per day was started, which relieved her symptoms. The chest radiology findings of interstitial pneumonia as well as pleural and pericardial effusions, hypoxemia, elevated serum lactate dehydrogenase, C-reactive protein, Krebs von den Lungen-6 and surfactant protein-D improved. Prednisolone was tapered four months later to 10 mg daily and interstitial pneumonia did not recur thereafter.
Discussion
Interstitial pneumonia is an uncommon complication of CRS. Mizuno et al. summarized reports of 13 infants with interstitial pneumonia associated with CRS including one that they experienced (6). Only four of these 13 infants survived, indicating that the prognosis for interstitial pneumonia associated with CRS is poor. The pathological findings of interstitial pneumonia associated with CRS include fibrosing interstitial pneumonia and desquamative interstitial pneumonia (3, 4). The pathophysiology of interstitial pneumonia associated with CRS remains unclear. However, an immunological mechanism such as the pulmonary deposition of immunoglobulin M complexes containing rubella antigen- rather than rubella virus infection of pulmonary tissue has been considered (5).
Our patient was diagnosed with CRS in adulthood based on the characteristic clinical features of impaired hearing, cataracts, dwarfism, microcephaly and mental retardation. Although a history of her mother having been infected with rubella was not evident and her serum levels of rubella-specific immunoglobulin M determined soon after birth was unavailable, the clinical diagnosis of CRS was essentially confirmed from the characteristic clinical manifestations (1, 2). Pulmonary hypertension due to congenital heart diseases is closely associated with mortality in patients with CRS (7). This patient has remained alive for over 70 years probably due to the absence of congenital heart diseases. Interstitial pneumonia associated with adult CRS has never been previously reported. To the best of our knowledge, this the first case report of interstitial pneumonia in an adult patient with CRS. The pathophysiology of interstitial pneumonia in this patient is unclear. Previous reports indicate that the rubella virus is usually eliminated in childhood (2), and the interstitial pneumonia in this patient developed at the age of 71 years. This suggests that rubella infection was not directly associated with interstitial pneumonia in this patient. However, the findings of several studies have suggested a relationship between CRS in childhood and adulthood and autoimmune diseases, such as diabetes, thyroiditis and scleroderma (8-11). Although the mechanism of the association between autoimmune diseases and CRS remains unclear, some studies have suggested that a decreased regulatory T lymphocyte function is involved, which could facilitate the autoimmune response and thus lead to the development of autoimmune diseases in patients with CRS (11). We speculate that anti-Jo-1 antibody-positive interstitial pneumonia might have developed through this pathway in our patient, although a coincidental association between CRS and interstitial pneumonia could not be fully ruled out. Our patient was positive for serum anti-Jo-1 antibody and chest CT revealed an organizing pneumonia pattern accompanied by pleural and pericardial effusions. These radiological findings resolved under systemic corticosteroid therapy, suggesting that the pleural and pericardial effusions as well as the interstitial pneumonia originated from an immunological process, although we were unable to biochemically and cytologically assess the effusions. Previous reports have described unusual pleural and pericardial effusions associated with antisynthetase syndrome (12-15). Interstitial pneumonia with autoimmune features was diagnosed in our patient based on the serological and radiological findings (16). Previous studies have suggested that viral infection is involved in the development of connective tissue diseases (17-19). To clarify the relationship between interstitial pneumonia and CRS in adulthood, further investigations will be needed.
In conclusion, we herein described the first known case of anti-Jo-1 antibody-positive interstitial pneumonia in an elderly patient with CRS. An autoimmune response in CRS might have been involved in the development of interstitial pneumonia in this patient, as well as other autoimmune diseases, such as diabetes. thyroiditis and scleroderma in CRS, although this remains speculative. The fact that CRS persists is regrettable because it can be prevented by vaccination against rubella. Physicians should therefore strive to prevent rubella infection.
The authors state that they have no Conflict of Interest (COI).
Acknowledgement
We thank the patient and her family for providing written informed consent and cooperating with this case report.
References
- 1. Sugishita Y, Shimatani N, Katow S, Takahashi T, Hori N. Epidemiological characteristics of rubella and congenital rubella syndrome in the 2012-2013 epidemics in Tokyo, Japan. Jpn J Infect Dis 68: 159-165, 2015. [DOI] [PubMed] [Google Scholar]
- 2. Sugishita Y, Akiba T, Sumitomo M, et al. Shedding of rubella virus among infants with congenital rubella syndrome born in Tokyo, Japan, 2013-2014. Jpn J Infect Dis 69: 418-423, 2016. [DOI] [PubMed] [Google Scholar]
- 3. Boner A, Wilmott RW, Dinwiddie R, et al. Desquamative interstitial pneumonia and antigen-antibody complexes in two infants with congenital rubella. Pediatrics 72: 835-839, 1983. [PubMed] [Google Scholar]
- 4. Franklin SL, Kelley R. Congenital rubella and interstitial pneumonitis. Clin Pediatr (Phila) 40: 101-103, 2001. [DOI] [PubMed] [Google Scholar]
- 5. Sanchez MO, Chang AB. Congenital rubella pneumonitis complicated by Pneumocystis jiroveci infection with positive long term respiratory outcome: a case report and literature review. Pediatr Pulmonol 44: 1235-1239, 2009. [DOI] [PubMed] [Google Scholar]
- 6. Mizuno Y, Yokoi K, Suzuki S. Congenital rubella syndrome with death from interstitial pneumonia. Pediatr Int 58: 490-493, 2016. [DOI] [PubMed] [Google Scholar]
- 7. Toizumi M, Motomura H, Vo HM, et al. Mortality associated with pulmonary hypertension in congenital rubella syndrome. Pediatrics 134: e519-e526, 2014. [DOI] [PubMed] [Google Scholar]
- 8. Clarke WL, Shaver KA, Bright GM, Rogol AD, Nance WE. Autoimmunity in congenital rubella syndrome. J Pediatr 104: 370-373, 1984. [DOI] [PubMed] [Google Scholar]
- 9. Ginsberg-Fellner F, Witt ME, Fedun B, et al. Diabetes mellitus and autoimmunity in patients with the congenital rubella syndrome. Rev Infect Dis 7: S170-S176, 1985. [DOI] [PubMed] [Google Scholar]
- 10. Español T, Pascual C, Huguet P, Caragol I, Hernandez M, Bertran JM. Circumscribed scleroderma in congenital rubella syndrome with hypogammaglobulinemia. Allergy 53: 1005-1006, 1998. [DOI] [PubMed] [Google Scholar]
- 11. Palacin PS, Castilla Y, Garzón P, Figueras C, Castellví J, Español T. Congenital rubella syndrome, hyper-IgM syndrome and autoimmunity in an 18-year-old girl. J Paediatr Child Health 43: 716-718, 2007. [DOI] [PubMed] [Google Scholar]
- 12. Mogulkoc N, Kabasakal Y, Ekren PK, Bishop PW. An unusual presentation of anti-Jo-1 syndrome, mimicking lung metastases, with massive pleural and pericardial effusions. J Clin Rheumatol 12: 90-92, 2006. [DOI] [PubMed] [Google Scholar]
- 13. Sugie K, Tonomura Y, Ueno S. Characterization of dermatomyositis with coexistence of anti-Jo-1 and anti-SRP antibodies. Intern Med 51: 799-802, 2012. [DOI] [PubMed] [Google Scholar]
- 14. Miyata M, Fukaya E, Takagi T, et al. Two patients with polymyositis or dermatomyositis complicated with massive pleural effusion. Intern Med 37: 1058-1063, 1998. [DOI] [PubMed] [Google Scholar]
- 15. Saito G, Kono M, Tsutsumi A, et al. Anti-PL-7 antisynthetase syndrome with eosinophilic pleural effusion. Intern Med 57: 2227-2232, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fischer A, Antoniou KM, Brown KK, et al. ; “ERS/ATS Task Force on Undifferentiated Forms of CTD-ILD”.. An official European Respiratory Society/American Thoracic Society research statement: interstitial pneumonia with autoimmune features. Eur Respir J 46: 976-987, 2015. [DOI] [PubMed] [Google Scholar]
- 17. Zampieri S, Ghirardello A, Iaccarino L, et al. Polymyositis-dermatomyositis and infections. Autoimmunity 39: 191-196, 2006. [DOI] [PubMed] [Google Scholar]
- 18. Arcangeletti MC, Maccari C, Vescovini R, et al. A paradigmatic interplay between human cytomegalovirus and host immune system: possible involvement of viral antigen-driven CD8+ T cell responses in systemic sclerosis. Viruses 10: E508, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Friedrich SK, Lang PA, Friebus-Kardash J, Duhan V, Bezgovsek J, Lang KS. Mechanisms of lymphatic system-specific viral replication and its potential role in autoimmune disease. Clin Exp Immunol 195: 64-73, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]