Abstract
A 57-year-old female was referred to our department for treatment of a duodenal submucosal tumor (SMT), which had been growing over the last five years. Computed tomography demonstrated a marginally enhanced mass, measuring 36 mm in diameter, containing internal multiple hypovascular areas. Endoscopic ultrasonography-guided fine needle biopsy was performed using a 20-gauge core trap needle, and the specimens showed benign Brunner's glands. She underwent laparoscopic endoscopic cooperative surgery and the SMT was completely removed without any adverse events. Histology of the resected tumor showed Brunner's gland hyperplasia (BGH). BGH is generally a benign lesion. However, an accurate diagnosis is required to avoid overtreatment when it mimics malignancy.
Keywords: Brunner's gland hyperplasia, EUS-FNB, ESD, LECS
Introduction
Brunner's gland hyperplasia (BGH) is basically a benign polyp lesion that is mainly limited to the first part of the duodenum, and it frequently is associated with duodenal ulcer, gastric erosions, and a hypersecretory status (1). Making an accurate diagnosis of this polyp is usually easy based on the typical endoscopic findings; a submucosal tumor-like nodule with a smooth surface, with or without a secretary orifice, and cushion sign (soft). However, it is sometimes difficult to differentiate from neoplasia, when the size is large and/or growing in size. Laparotomies, including duodenectomy and pancreatoduodenectomy, have been considered as treatment choices for neoplasms of the duodenum, however, they are considered to be too invasive for nonneoplastic polyps. Therefore, an accurate diagnosis is required to avoid overtreatment.
We herein report a case of giant submucosal tumor (SMT) of the duodenum, which could be diagnosed as Brunner's gland hyperplasia using an endoscopic ultrasonography-guided fine needle biopsy (EUS-FNB), and thereafter duodenal laparoscopic endoscopic cooperative surgery (D-LECS) was successfully performed.
Case Report
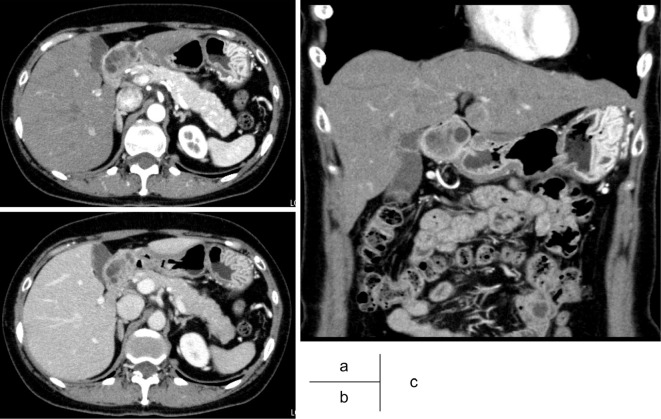
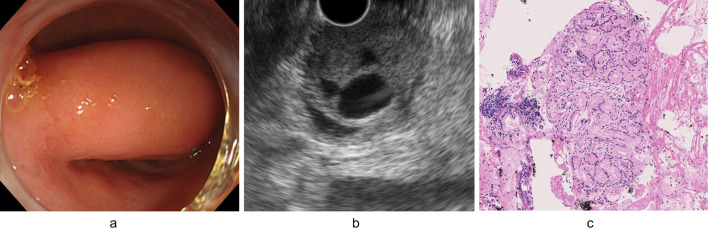
A 57-year-old female was referred to our hospital for treatment of an SMT of the duodenum, which was suspected to be a gastrointestinal stromal tumor (GIST) as it had been gradually growing over last five years. The laboratory data including tumor markers was all within the normal ranges. A barium contrast examination image of the upper gastrointestinal tract showed a protruding lesion at the duodenal bulbs, without any evidence of stiffening of the duodenal wall (Fig. 1). Computed tomography (CT) demonstrated a marginally enhanced nodular lesion, measuring 36 mm in diameter, containing internal multiple hypovascular areas (Fig. 2). Esophagogastroduodenoscopy revealed an SMT with cushion sign, but without any ulcerous lesion (Fig. 3a). EUS showed a low-echoic nodule, continuing to the deep mucosa and submucosa of the duodenum. Inside of this SMT, multiple echolucent areas were recognized, suggesting cystic lesions rather than necrosis of an originally solid lesion (Fig. 3b). BGH, inflammatory fibroid polyp, neuroendocrine tumor and GIST accompanied with central necrosis were included in the differential diagnoses. However, BGH was strongly suspected and neuroendocrine tumor (NET) or GIST were less likely based on the CT and EUS findings. To exclude malignancy and reach an accurate diagnosis, EUS-FNB was performed using a 20-gauge core trap needle. The specimens of EUS-FNB showed Brunner's gland with a duodenal epithelium without any sign of neoplasia (Fig. 3c). Immunostaining with Ki-67 revealed a non-neoplastic distribution and that with TP53 showed no overexpression.
Figure 1.
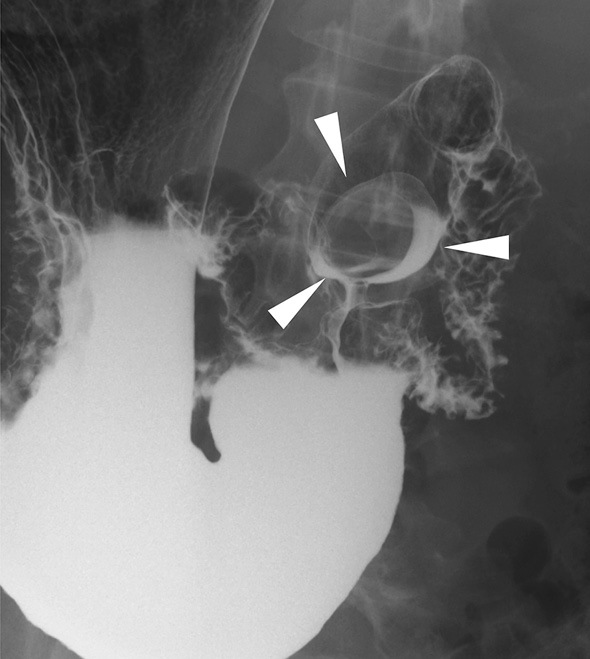
Contrast images of the upper gastrointestinal tract. The upper gastrointestinal series showed a protruding lesion at the duodenal bulbs (arrowheads), without any evidence of duodenal wall stiffening.
Figure 2.
Computed tomography (CT). CT demonstrated a marginally enhanced nodular lesion, measuring 36 mm in diameter, and containing internal multiple cystic lesions.
Figure 3.
Esophagogastroduodenoscopy and endoscopic ultrasonography (EUS) images. Esophagogastroduodenoscopy revealed a soft submucosal tumor (SMT) without any ulcerous lesions (a). EUS showed a low-echoic nodule, continuing to the deep mucosa and sub-mucosa of the duodenum. Inside this SMT, multiple cystic lesions were recognized (b). Specimens obtained by EUS-guided fine needle biopsy (FNB) showed Brunner’s glands without any neoplastic findings (Hematoxylin and Eosin staining, ×20) (c).
The patient requested resection, regardless of the neoplastic or non-neoplastic features, as the duodenal polyp had been gradually growing. The patient underwent D-LECS and the SMT was completely removed. Macroscopically, it was a 45 mm polypoid lesion, covered by a smooth mucosa, and containing multiple macroscopic and microscopic cystic lesions (Fig. 4a). Microscopically, this tumor consisted of proliferative Brunner's glands partially dilated in various sizes, but no neoplastic or malignant cells were observed (Fig. 4b). The mucus phenotype analyzed by immunohistochemistry was compatible with Brunner glands; MUC2 (-), MUC5AC (-), MUC6 (+), and CD10 (-). The histological diagnosis was Brunner's gland hyperplasia (hamartoma). There were no adverse events either during or after the resection, and the patient was thereafter successfully discharged.
Figure 4.
Pathological findings. Macroscopically, the tumor was a 45 mm polypoid lesion, covered by a smooth mucosa, and containing multiple macroscopic and microscopic cystic lesions (a). Microscopically, this tumor consisted of proliferative Brunner’s glands that were partially dilated in various sizes (Hematoxylin and Eosin staining, ×4) (b).
Discussion
BGH is generally a benign lesion of the duodenum which histologically consists of a mixture of acini, ducts, smooth muscle, adipose tissue, lymphoid tissue, and occasionally heterotopic pancreatic tissue (1). Not like frequently seen “Brunner's gland cysts” showing dome-like SMT (3-10 mm) (2), BGHs are mostly pedunculated polyps such as observed in the current case, and range in size from 0.7-12 cm (mean: 4 cm), and they account for 5-10% of all benign duodenal tumors (3). Most BGHs are incidentally detected during upper gastrointestinal examinations in middle-age examinees, but BGHs may rarely present with abdominal pain, anemia, duodenal obstruction, gastrointestinal hemorrhaging and ulcers, duodenal intussusception, or obstruction of the common bile duct or pancreatic duct (3-5). Although they are mostly benign polyps, a small fraction of these polyps has a malignant potential [1.1% (8 cases) of low-grade dysplasia, 0.7% (5 cases) of high-grade dysplasia, and 0.3% (2 cases) of invasive carcinoma, in 722 cases of BGHs], so that Brunner's gland hyperplasia-adenocarcinoma sequence has been proposed (6). The size of these BGHs with high-grade dysplasia and invasive cancer was 26 mm (range: 13-78 mm) and the shape of these lesions was typically flat-elevation with central depression (6).
The diagnosis of BGH can be difficult, when it grows or becomes a large SMT. The differential diagnoses may include benign masses (gastric and pancreatic heterotopias), periampullary adenocarcinomas, lymphoma, neuroendocrine tumors, mesenchymal tumors (GIST, leiomyomas, and leiomyosarcomas), and metastatic diseases. In previous reports, BGHs occasionally mimic malignancies of the duodenum and pancreas (7), as they can present with obstructive jaundice (4), duodenal irregular mucosa (5), and ulcer (3). For the diagnosis of BGHs, EUS is thought to be effective as they have several characteristic findings; i) involvement of the mucosa and submucosal layers; ii) variable echogenicity (mixed to hypoechoic); and iii) multiple cystic changes within the tumor (8, 9). Actually, in the current case, an image diagnosis by the previous hospital suggested GIST, but the EUS findings of the current tumor were quite compatible with BGHs (Fig. 3b). Multiple well-demarcated echolucent areas without any inside debris normally suggest pure cystic lesions rather than necrosis in the GIST or neuroendocrine tumor.
In addition to these image findings, histological evidence is essential to support the diagnosis of BGH. In this sense, a forceps biopsy is not sufficiently effective as the surface tissue of the SMT can not predict what is in inside. EUS-FNB is a method for sampling from the pancreatic masses (10), lymph nodes and gastrointestinal SMTs, and is thought to be most suitable in this case. To date, including our patient, four cases of BGHs diagnosed using EUS-guide fine needle aspiration (EUS-FNA) have been reported (Table) (7, 11, 12). The main purpose of this procedure was to exclude malignancy, and tissue fragments of proliferative Brunner's glands in combination with image findings can normally provide a fairly accurate diagnosis. Furthermore, recent advances in FNB needles enabled us to acquire large pieces of the target tissues (10, 13, 14). EUS-FNB using Franseen and Fork-tip needles (22-gauge) can provide excellent specimens by obtaining core biopsy specimens (14). One caution is, as written in above, there is a possibility of a tiny carcinoma component within the large BGH. Hence, if not resected, a careful follow up is needed, particularly for flat-elevated BGHs with central depression.
Table.
Reported Cases of Brunner’s Gland Hyperplasia Diagnosed Using EUS-FNA.
| No. | Reference | Age (y.o) | Sex | Complaint | Tumor size | Needle | Histological diagnosis by EUS-FNA | Treatment |
|---|---|---|---|---|---|---|---|---|
| 1 | 11 | 62 | male | epigastric pain | 100 mm | N.D. | no malignancy | PD |
| 2 | 7 | 53 | male | abdominal pain | 40 mm | N.D. | no malignancy, not GIST | follow up |
| 3 | 12 | 50 | female | anemia | 60 mm | 22G EZ-Shot | no malignancy, not GIST | local excision |
| 4 | Current case | 57 | female | no | 45 mm | 20G pro-core | no malignancy, Burunner’s grands | D-LECS |
EUS-FNA: endoscopic ultrasonography-guided fine needle aspiration, N.D.: not described, PD: pancreatoduodenectomy, GIST: gastrointestinal stromal tumor, D-LECS: duodenal laparoscopic endoscopic cooperative surgery
Asymptomatic small Brunner gland hamartomas usually require no treatment. However, treatment is suggested for large tumors, even if asymptomatic, to prevent the development of complications such as bleeding or obstruction (15). In this case, the size of BGH had gradually increased and the patient therefore requested the resection. Although EUS-FNB demonstrated no evidence of malignancy, the possibility of a minimal malignant component could not be ruled out. For these reasons, this BGH was resected.
Endoscopic resection is the treatment choice if the tumor is pedunculated and relatively small, whereas larger-sized lesions require open surgery. Recently, some case reports described endoscopic resection for a large BGH (15). However, duodenal endoscopic submucosal dissection (ESD) has a high risk of complications, such as perforation and bleeding (6-50 % and 0-7 %, respectively) due to the thinness of the duodenal wall and technical difficulties in the narrow duodenal lumen (16, 17). Furthermore, delayed perforations sometimes develop after duodenal ESD, but not during ESD (16, 18). The higher frequency of delayed perforation after duodenal ESD may be due to the thinness of the duodenal walls, coupled with proteolysis or chemical irritation due to pancreatic enzymes and bile juice (16, 19).
Recently, laparoscopic and endoscopic cooperative surgery (LECS) is a newly developed concept for tumor dissection of the gastrointestinal tract that was first investigated for the local resection of SMT (usually GIST) (20). In addition, some modified LECS procedures have also been developed for the dissection of malignant tumors. Furthermore, laparoscopic endoscopic cooperative surgery for duodenal tumors (D-LECS) has been developed as a new procedure, which can prevent perforations by reinforcing the ESD site (21). Otowa et al. reported on the efficacy of D-LECS, which made it possible to perform ESD for superficial non-ampullary duodenal epithelial tumors (21) safely without any associated perforations. Up to now, to the best of our knowledge, there has been no report on a BGH treated by D-LECS. However, we chose this method for the treatment a large BGH to enhance the safety after duodenal ESD.
Our current report demonstrated a case of large BGH of the duodenal bulbs which was accurately diagnosed by the combination of clinical images and EUS-FNB, and thereafter was successfully treatment by the minimally invasive and safe procedure of D-LECS.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Franzin G, Musola R, Ghidini O, Manfrini C, Fratton A. Nodular hyperplasia of Brunner's glands. Gastrointest Endosc 31: 374-378, 1985. [DOI] [PubMed] [Google Scholar]
- 2. Brown IS, Miller GC. Brunner's gland cyst: a clinicopathological study of 25 cases highlighting an underappreciated lesion. Pathology 49: 476-478, 2017. [DOI] [PubMed] [Google Scholar]
- 3. Nakabori T, Shinzaki S, Yamada T, et al. . Atypical duodenal ulcer and invagination caused by a large pedunculated duodenal Brunner's gland hamartoma. Gastrointest Endosc 79: 679-680: discussion 680, 2014. [DOI] [PubMed] [Google Scholar]
- 4. Hol JW, Stuifbergen WN, Teepen JL, van Laarhoven CJ. Giant Brunner's hamartomas of the duodenum and obstructive jaundice. An overview of the literature and suspicion of malignancy in a case. Dig Surg 24: 452-455, 2007. [DOI] [PubMed] [Google Scholar]
- 5. Judd S, Patel S, Levi E, Antaki F. Brunner's gland hamartoma: a rare cause of iron deficiency anaemia and report of an adapted colonic polyp resection technique. BMJ Case Rep 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sakurai T, Sakashita H, Honjo G, Kasyu I, Manabe T. Gastric foveolar metaplasia with dysplastic changes in Brunner gland hyperplasia: possible precursor lesions for Brunner gland adenocarcinoma. Am J Surg Pathol 29: 1442-1448, 2005. [DOI] [PubMed] [Google Scholar]
- 7. Arena M, Rossi S, Morandi E, Mangiavillano B, Franchi P. Brunner's glands hyperplasia: diagnosis with EUS-FNA for suspected pancreatic tumor involving the duodenum. JOP 13: 684-686, 2012. [DOI] [PubMed] [Google Scholar]
- 8. Gao YP, Zhu JS, Zheng WJ. Brunner's gland adenoma of duodenum: a case report and literature review. World J Gastroenterol 10: 2616-2617, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Martinez MA, Zyromski NJ, Luz LP. An unusual case of large symptomatic Brunner's gland adenoma: endoscopic ultrasound imaging. Endosc Ultrasound 4: 266-267, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Matsubayashi H, Matsui T, Yabuuchi Y, et al. . Endoscopic ultrasonography guided-fine needle aspiration for the diagnosis of solid pancreaticobiliary lesions: clinical aspects to improve the diagnosis. World J Gastroenterol 22: 628-640, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Stewart ZA, Hruban RH, Fishman EF, Wolfgang CL. Surgical management of giant Brunner's gland hamartoma: case report and literature review. World Journal of Surgical Oncology 7: 68, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Stoos-Veic T, Tadic M, Aralica G. EUS–FNA of Brunner's gland hamartoma: a case report. Cytopathology 24: 194-196, 2013. [DOI] [PubMed] [Google Scholar]
- 13. Hucl T, Wee E, Anuradha S, et al. . Feasibility and efficiency of a new 22G core needle: a prospective comparison study. Endoscopy 45: 792-798, 2013. [DOI] [PubMed] [Google Scholar]
- 14. Bang JY, Hebert-Magee S, Navaneethan U, Hasan MK, Hawes R, Varadarajulu S. Randomized trial comparing the Franseen and Fork-tip needles for EUS-guided fine-needle biopsy sampling of solid pancreatic mass lesions. Gastrointest Endosc 87: 1432-1438, 2018. [DOI] [PubMed] [Google Scholar]
- 15. Jung Y, Chung IK, Lee TH, et al. . Successful endoscopic resection of large pedunculated Brunner's gland hamartoma causing gastrointestinal bleeding arising from the pylorus. Case Rep Gastroenterol 7: 304-307, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kakushima N, Kanemoto H, Tanaka M. et al. Treatment for superficial non-ampullary duodenal epithelial tumors. World J Gastroenterol 20: 12501-12508, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yamamoto Y, Yoshizawa N, Tomida H. et al. Therapeutic outcomes of endoscopic resection for superficial non-ampullary duodenal tumor. Dig Endosc 26 (Suppl. 02): 50-56, 2014. [DOI] [PubMed] [Google Scholar]
- 18. Marques J, Baldaque-Silvia F, Pereira P. et al. Endoscopic mucosal resection and endoscopic submucosal dissection in the treatment of sporadic nonampullary duodenal adenomatous polyps. World J Gastrointest Endosc 7: 720-727, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jung JH, Choi KD, Ahn JY, et al. . Endoscopic submucosal dissection for sessile, nonampullary duodenal adenomas. Endoscopy 45: 133-135, 2013. [DOI] [PubMed] [Google Scholar]
- 20. Hiki N, Nunobe S, Matsuda T, et al. . Laparoscopic endoscopic cooperative surgely. Dig Endosc 27: 197-204, 2015. [DOI] [PubMed] [Google Scholar]
- 21. Otowa Y, Kanaji S, Morita Y, et al. . Safe management of laparoscopic endoscopic cooperative surgery for superficial non-ampullary duodenal epithelial tumors. Endosc Int Open 05: E1153-E1158, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]