Abstract
A 70-year-old woman was admitted to our hospital due to difficulty in moving her limbs. She had trismus and a necrotic and contaminated wound in her left lower leg. A diagnosis of tetanus was confirmed and intensive care was started. On the second day, her blood pressure fell and a ST segment elevation on electrocardiography (ECG) was detected. She was diagnosed with takotsubo cardiomyopathy by echocardiogram and improved undergoing conservative therapy.
Keywords: tetanus, takotsubo cardiomyopathy, tetanus severity score
Introduction
Tetanus is a nervous system disorder often accompanied by autonomic dysfunction in severe cases. Takotsubo cardiomyopathy (TTC) is characterized by the transient systolic dysfunction of the apical and mid segments of the left ventricle in the absence of obstructive coronary artery lesions. Although the etiology of TTC is unknown, catecholamines probably play a role in its development. Comorbidities of tetanus and TTC have rarely been reported in the literature. TTC is an important complication resulting from autonomic dysfunction in case of severe tetanus. Therefore, careful monitoring and adequate evaluations of the heart condition is essential during the clinical course of TTC.
Case Report
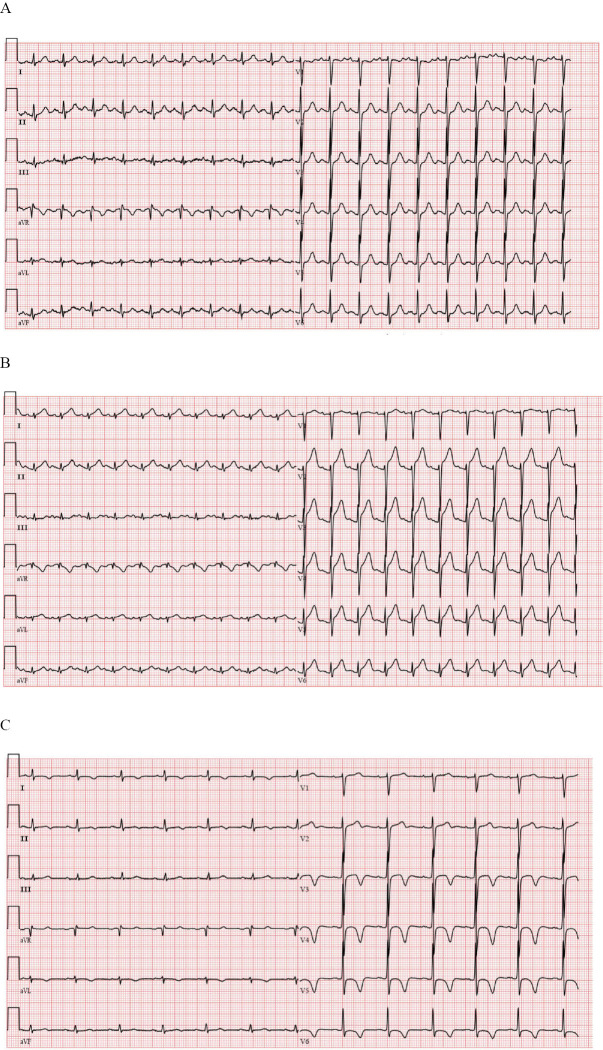
A 70-year-old woman was brought to our hospital by ambulance due to difficulty in moving her limbs. The patient reported malaise for two days and the inability to move her limbs on the day prior to admission. She was an agriculturist and had an unremarkable medical history except for hypertension. On admission, she was tachycardic (114 beats per minute), blood pressure was 166/91 mmHg, while all other vital signs were normal. Her cervical lymph nodes were not palpable, but she had a blank expression and could not open her mouth on instruction (Fig. 1). Furthermore, a wound that was partly necrotic and contaminated with mud was observed in her left lower leg (Fig. 2). This wound had been caused by an injury resulting from a fall that had occurred a week before admission and the patient had not sought medical treatment for it. Given the clinical presentation, and the fact that the patient had never taken a tetanus vaccine, we suspected that she had tetanus. Electrocardiography (ECG) showed sinus tachycardia (113 beat per minute)(Fig. 3A). Laboratory tests showed increased levels of inflammatory markers, namely C-reactive protein (CRP) (3.51 mg/dL) and fibrinogen (556.2 mg/dL), and slight renal dysfunction, as indicated by elevated urea nitrogen (UN) (25.5 mg/dL) and creatinine (0.94 mg/dL) levels.
Figure 1.
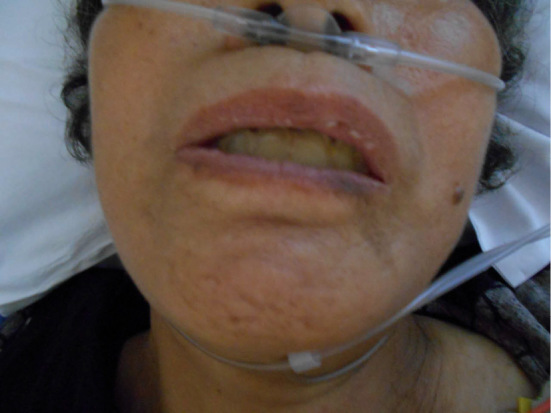
Facial appearance on admission: she could not open her mouth. Trismus was considered.
Figure 2.
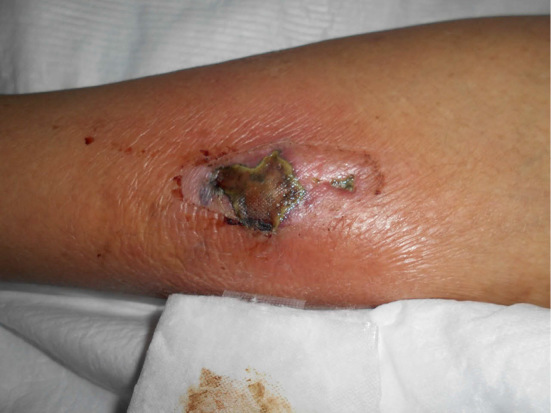
Image of the left lower leg wound observed on admission.
Figure 3.
A: Electrocardiogram on admission; almost normal except for sinus tachycardia. B: Electrocardiogram at the onset of TTC; ST segment elevation was seen on V2 to V6 leads. C: ECG at the 33rd day: T wave inversion on the I, aVL, V3 to V6 leads, and prolonged QTc (479ms) was seen.
The patient was transferred to the intensive care unit (ICU). Nasotracheal intubation was performed using a bronchofiberscope, and mechanical ventilation was started. A dose of 3,000 units of intravenous human tetanus immune globulin (HTIG) was immediately administered and wound debridement was performed.
As an infection with Clostridium tetani was suspected, the patient was treated with Penicillin G, and the continuous administration of propofol was started with an aim to increase the patient's sedation (Richmond agitation-sedation scale (RASS) score was 4 to 5).
On the second day, systemic muscle spasms were observed, which were difficult to control by increasing the propofol dosage alone. Thus, the continuous administration of midazolam (1.5 mg/kg/hour) and the intermittent administration of diazepam (10 mg per time) were initiated. Moreover, her blood pressure fell to 80 mmHg and paroxysmal tachycardia worsened (130 to 150 beat per minutes). ECG showed a ST segment elevation on the V2 to V6 leads (Fig. 3B), and the troponin I levels rose to 6.810 ng/mL. At transthoracic echocardiogram was then performed, revealing severe diffuse hypokinesis on the apical and mid portions, and the basal segment contraction was well preserved (Fig. 4). Therefore, acute coronary syndrome was suspected. The ejection fraction (EF) was 23%. As the muscle spasms were uncontrollable, coronary angiography was difficult to perform. She was diagnosed with TTC, based on the characteristic motion abnormality observed in the left ventricular wall. In laboratory tests, the serum catecholamine levels were elevated: adrenaline (268 pg/mL), noradrenaline (1,078 pg/mL) and dopamine (137 pg/mL) levels (reference values: ≤100 pg/mL, 100-450 pg/mL and ≤20 pg/mL, respectively). Conservative treatment was initiated, with continuous catecholamine administration, to support cardiac contraction and increase blood pressure. Due to excessive sweating, she developed pre-renal failure and tachycardia persisted. Therefore, a high amount of infusion (3,000 to 4,000 mL per day in total) was administered for seven days. Due to renal dysfunction, Penicillin G was replaced with metronidazole, with the concomitant use of β-lactam antibiotics to treat aspiration pneumonia complications. On the 10th day, the echocardiogram showed an improvement in the motion abnormality in the LV wall and catecholamine was discontinued after three days. Since the frequency of muscle spasms decreased on the 14th day, midazolam treatment was discontinued on the 19th day. Nevertheless, sweating and tachycardia persisted and were attributed to the sympathetic nerve hyperactivity caused by the C. tetani infection. Dexmedetomidine was then introduced to control these symptoms. On the 26th day, gram-positive bacilli, with a drumstick-like form, were detected in the wound microbiological culture. Molecular identification, performed by sequencing of the 16S ribosomal RNA intergenic spacer region, confirmed the presence of a C. tetani infection and the patient was diagnosed with tetanus (Fig. 5). After obtaining an improvement in the autonomic disorder and trismus, mechanical ventilation was discontinued on the 31st day. ECG at the 33th day showed T wave inversion on the I, aVL, V3 to V6 leads, and prolonged QTc (Fig. 3C). She had no neurological sequelae and was transferred to a general ward to participate in a rehabilitation program.
Figure 4.
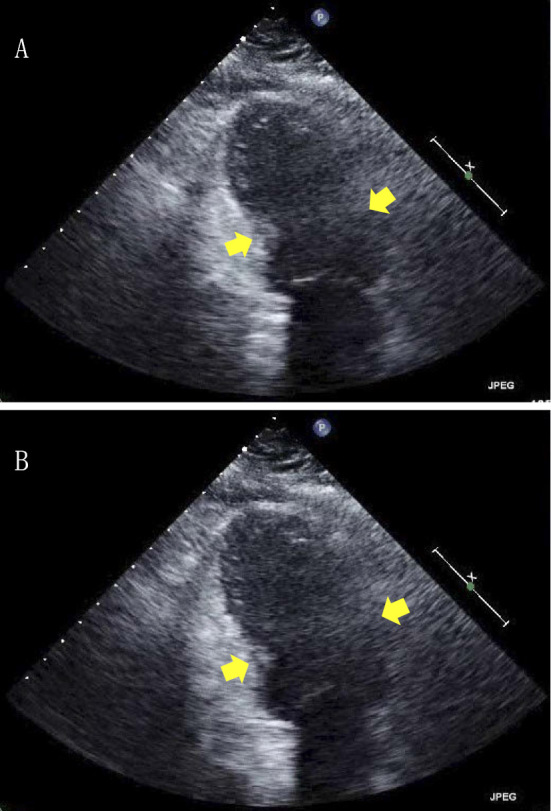
Echocardiogram on the second day (A: systolic, B: diastolic.). Apical ballooning of LV and diffuse severe hypokinesis on the apical and mid potions was seen, but the basal contraction was spared, characteristic of TTC.
Figure 5.
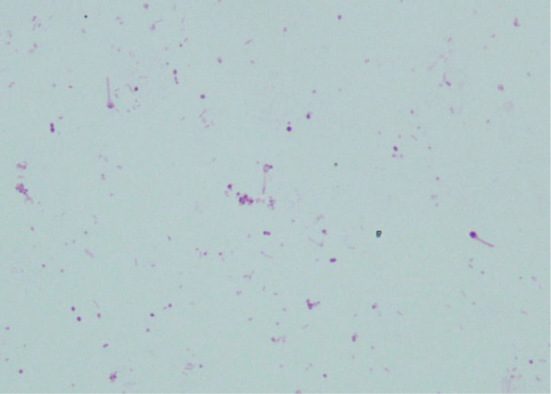
C. tetani detected in wound culture. Drumstick-like gram-negative bacilli are seen.
Discussion
Tetanus is a nervous system disorder, caused by infection with the gram-positive bacillus C. tetani and characterized by rigidity, muscle spasms and autonomic dysfunction. C. tetani is a ubiquitous bacterium, normally found in soil, and opportunistically infects the human body through wounded tissue. The secretion of tetanus toxin leads to tetanus, which is the clinical syndrome (1).
In Japan, due to the widespread vaccination with tetanus toxoid among children, the incidence of tetanus has declined remarkably in the last years. According to the National Institute of Infectious Diseases, in 2016, 129 cases of tetanus were reported, among which 101 were patients aged 65 years or older born at a time when tetanus vaccination was not widespread (2). Tetanus toxin is transferred from the peripheral nerves to the central nerve system, predominantly affecting inhibitory neurons and inhibiting the release of glycine and gamma-aminobutyric acid (GABA) (3-6). Interneurons in the alpha motor neurons are affected first, impairing the inhibitory control of the motor neurons. Later, preganglionic sympathetic neurons in the lateral horns and in the parasympathetic centers are also affected (7), leading to the clinical symptoms, such as muscle stiffness, spasm, and autonomic dysfunction, manifested as sweating, tachycardia and hypertension. In addition, autonomic dysfunction is a common symptom associated with severe tetanus.
In the past, respiratory failure due to spasm and respiratory muscle disorder accounted for most causes of death. With the advent of mechanical ventilation, a dramatic decrease was observed in the mortality rate due to respiratory failure, while sudden cardiac death, caused by autonomic dysfunction, has become more common. Trujillo et al. reported that 40% of deaths in tetanus, after the introduction of ICU care, were due to sudden cardiac death, and 15% were due to respiratory complications (8). Therefore, it is important to stabilize the patient's autonomic state and keep track of the patient's vital signs.
TTC is a disease exhibiting an acute left ventricular apical ballooning of unknown cause. The contraction abnormality occurs mainly in the left ventricle, and dynamic obstruction of the left ventricular outflow tract (pressure gradient difference, acceleration of blood flow, or systolic cardiac murmurs) is also observed. TTC often mimics acute coronary syndrome, because of the abnormalities on ECG (ST segment elevations, negative T-wave in multiple leads) or clinical symptoms such as sudden chest pain or dyspnea. However, an obstruction of coronary artery is not involved and a wall motion abnormality tend to completely improve in the majority of the patients within a month (9). Its name derives from the visual appearance of the heart on left ventriculography, which is similar to an octopus pod, or “takotsubo”, in Japanese (10). TTC commonly affects older women. In a review of 10 small prospective series, women with a mean age of 61 to 76 years accounted for 80 to 100% of all affected subjects (11). Another important feature of TTC is its association with emotional or physical stress factors (12,13). According to previous reports, several factors, such as catecholamine-induced microvascular spasm (13), direct catecholamine-associated myocardial toxicity (14), and coronary artery dysfunction (15) are thought to be related with the onset of TTC. However, the definite mechanism of TTC pathogenesis has not yet been clearly elucidated.
The patient described in the present report developed TTC during the course of severe tetanus. Sudden cardiac death is a feature of severe tetanus and, although the cause is unclear, a sudden loss of the sympathetic drive, catecholamine-induced cardiac damage, and increased parasympathetic tone may play a role (1). It appears that all these factors have catecholamine as a common factor with respect to the pathogenesis of TTC. Our patient had increased serum catecholamine levels, and was diagnosed with TTC on the second day. Thus, these catecholamines probably played a role in the developmentof TTC. TTC has been reported after a wasp bites (16) a jellyfish sting (17), as well as following a snake bites (18). It is uncertain, in these previous reports, whether the toxin given by these creatures directly injured the myocardium (19). Tonomura et al. reported a case of TTC in a patient with botulism (20). In this report, it was considered that the Botulinum toxin had induced autonomic dysfunction, and the hypercontraction of the left ventricle increases mechanical wall stress in the apex through β-receptor activation, resulting in Takotsubo syndrome (10). This mechanism is seems to be similar to the pathogenesis of TTC induced by the tetanus toxin.
Tetanus complicated with TTC is uncommon and it has only been reported in severe tetanus cases to date. We found only six cases in the literature (including ours) reporting this complication (Table 1) (21-25). All cases were female patients and the mean age was 70.83 years old (range: 44-80). The period from tetanus diagnosis to TTC onset ranged from zero to four days. Autonomic dysfunction usually starts several days after the spasms and then persists for one to two weeks (1). Thus, TTC onset coincides with autonomic dysfunction in the reported cases. As tetanus complicated with TTC is a very rare condition, the prognosis is uncertain. The patients in all reported cases (including ours) recovered well, but fatal cases of tetanus that had not been diagnosed as TTC might be present. The tetanus severity score (TSS), which predicts the outcome of tetanus, was proposed in 2006 (Table 2). Using a cut-off value of 8 points, the sensitivity and specificity values for the tetanus death were 77% and 85%, respectively (26). TSS can be evaluated in 5 cases, which is higher than 8 points in 3 cases. Although the number of cases is limited, the complication of tetanus and TTC may be associated with high risk for mortality. Therefore, for appropriate care and management of tetanus, electrocardiograms and echocardiography, as well as a careful monitoring of the blood pressure and heart rate, are crucial for the early diagnosis of TTC.
Table 1.
Reported Cases of Tetanus Complicated with TTC.
| References | sex | age | days* | TSS*** | outcome |
|---|---|---|---|---|---|
| [15] | F | 80 | 3 | N/A** | recovery |
| [16] | F | 76 | 0 | 12 | recovery |
| [17] | F | 44 | 3 | 0 | recovery |
| [18] | F | 76 | 4 | -1 | recovery |
| [19] | F | 79 | 3 | 11 | recovery |
| our case | F | 70 | 2 | 15 | recovery |
* The period from tetanus to TTC onset.
** N/A: not available.
*** TSS: tetanus severity score.
Table 2.
TSS: Tetanus Severity Score[20].
| Score | |
|---|---|
| Age(year) | |
| ≤70 | 0 |
| 71-80 | 5 |
| >80 | 10 |
| Time from first symptom to admission (days) | |
| ≤2 | 0 |
| 3-5 | -5 |
| >5 | -6 |
| Difficulty breathing on admission | |
| No | 0 |
| Yes | 4 |
| Co-existing medical conditions | |
| Fit and well | 0 |
| Minor illness or injury | 3 |
| Moderately severe illness | 5 |
| Severe illness not immediately life threatening | 5 |
| Immediately life-threatening illness | 9 |
| Entry site | |
| Internal or injection | 7 |
| Other (including unknown) | 0 |
| Highest systolic blood pressure recorded during first day in hospital (mmHg) | |
| ≥130 | 0 |
| 131-140 | 2 |
| >140 | 4 |
| Highest heart rate recorded during first day in hospital (bpm) | |
| ≤100 | 0 |
| 101-110 | 1 |
| 111-120 | 2 |
| >120 | 4 |
| Lowest heart rate recorded during first day in hospital (bpm) | |
| ≤110 | 0 |
| >110 | -2 |
| Highest temperature recorded during first day in hospital (°C) | |
| ≤38.5 | 0 |
| 38.6-39 | 4 |
| 39.1-40 | 6 |
| >40 | 8 |
In conclusion, tetanus complicated with TTC is a rare condition. All cases reported so far were successfully treated, but fatal cases may occur because cardiac sudden death is common cause of death in tetanus patients. We emphasize that abnormal blood pressure and pulse rate, and an elevation of the ST segments on ECG may be noticed not only in acute coronary syndrome but also in TTC. Therefore, careful monitoring is quite important in the treatment of tetanus.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Cook TM, Protheroe RT, Handel JM. Tetanus a review of the literature. Br J Anaesth 87: 477-487, 2001. [DOI] [PubMed] [Google Scholar]
- 2. National Institute of Infectious Diseases. [cited 2018 Nov. 1]. Available at https://www.niid.go.jp/niid/en/.
- 3. Collingridge GL, Davies J. The in vitro inhibition of GABA release by tetanus toxin. Neuropharmacology 21: 851-855, 1982. [DOI] [PubMed] [Google Scholar]
- 4. Crossley K, Irvine P, Warren JB, Lee BK, Mead K. Tetanus and diphtheria immunity in urban Minnesota adults. JAMA 242: 2298-3000, 1979. [PubMed] [Google Scholar]
- 5. Curtis DR, De Groat WC. Tetanus toxin and spinal inhibition. Brain Res 10: 208-212, 1968. [DOI] [PubMed] [Google Scholar]
- 6. Curtis DR, Felix D, Game CJA, McCulloch RM. Tetanus toxin and the synaptic release of GABA. Brain Res 51: 358-362, 1973. [DOI] [PubMed] [Google Scholar]
- 7. Bleck TP. Tetanus: dealing with the continuing clinical challenge. J Crit Ill 2: 41-52, 1987. [Google Scholar]
- 8. Trujillo MH, Castillo A, Espana J, Manzo A, Zerpa R. Impact of intensive care management on the prognosis of tetanus. Analysis of 641 cases. Chest 92: 63-65, 1987. [DOI] [PubMed] [Google Scholar]
- 9. Kawai S, Kitabatake A, Tomoike H, et al. Guidelines for diagnosis of takotsubo (ampulla) cardiomyopathy. Cric J 71: 990-992, 2007. [DOI] [PubMed] [Google Scholar]
- 10. Nyui N, Yamanaka O, Nakayama R, et al. ‘Tako-Tsubo’ transient ventricular dysfunction: a case report. Ipn Cric J 64: 715-719, 2000. [DOI] [PubMed] [Google Scholar]
- 11. Akashi YJ, Goldstein DS, Barbaro G, et al. Takotsubo cardiomyopathy: a new form of acute, reversible heart failure. Circulation 118: 2754-2762, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sharkey SW, Windenburg DC, Lesser JR, et al. Natural history and expansive clinical profile of stress (tako-tsubo) cardiomyopathy. J Am Coll Cardiol 55: 333-341, 2010. [DOI] [PubMed] [Google Scholar]
- 13. Gianni M, Dentali F, Grandi AM, Sumner G, Hiralal R, Lonn E. Apical ballooning syndrome or takotsubo cardiomyopathy: a systematic review. Eur Heart J 27: 1523-1529, 2006. [DOI] [PubMed] [Google Scholar]
- 14. Nef HM, Möllmann H, Kostin S, et al. Tako-Tsubo cardiomyopathy: intraindividual structural analysis in the acute phase and after functional recovery. Eur Heart J 28: 2456-2464, 2007. [DOI] [PubMed] [Google Scholar]
- 15. Tsuchihashi K, Ueshima K, Uchida T, et al. Transient left ventricular apical ballooning without coronary artery stenosis: a novel heart syndrome mimicking acute myocardial infarction. Angina Pectoris-Myocardial Infarction Investigations in Japan. J Am Coll Cardiol 38: 11-18, 2001. [DOI] [PubMed] [Google Scholar]
- 16. Jairam A, Kumar RS, Ghosh AK, et al. Delayed Kounis syndrome and acute renal failure after wasp sting. Int J Cardiol 138: e12-e14, 2010. [DOI] [PubMed] [Google Scholar]
- 17. Bianchi R, Torella D, Spaccarotella C, et al. Mediterranean jellyfish sting-induced Tako-Tsubo cardiomyopathy. Eur Heart J 32: 18, 2011. [DOI] [PubMed] [Google Scholar]
- 18. Murase K, Takagi K. Takotsubo cardiomyopathy in a snake bite victim: a case report. Pan Afr Med J 13: 51, 2012. [PMC free article] [PubMed] [Google Scholar]
- 19. Lykourgos-Christos A, Sophia A, Ioannis S, et al. Transient reverse takotsubo cardiomyopathy following a spider bite in greece a case report. Medicine 94: e457, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tonomura S, Kakehi Y, Sato M, et al. Takotsubo-like myocardial dysfunction in a patient with botulism. Intern Med 56: 2925-2927, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. vanBeek T, Knockaert D. Tetanus and tako-tsubo: case report. Neth J Crit Care 15: 19-23, 2011. [Google Scholar]
- 22. Osada J, Kuroki K, Nakamura S, et al. Takotsubo myopathy associated with tetanus. J Cardiol Jpn Ed 2: 226-231, 2008. [Google Scholar]
- 23. Jung JM, Kim YH, Park MH, et al. Takotsubo cardiomyopathy following severe tetanus. Neurology Asia 17: 75-78, 2012. [Google Scholar]
- 24. Sueyoshi S, Chitose S, Nagata K, et al. A case of generalized tetanus with an initial complaint of dysphagia. The Larynx Japan 27: 24-30, 2015. [Google Scholar]
- 25. Shirokawa M, Shibuya Y, Mitsusada M, et al. Tetanus case isolating clostridium tetani from scab. JJAAM 19: 279-282, 2008. [Google Scholar]
- 26. Thwaites CL, Yen LM, Glover C, et al. Predicting the clinical outcome of tetanus: the tetanus severity score. Trop Med Int Health 11: 279-287, 2006. [DOI] [PubMed] [Google Scholar]