Abstract
A 21-year-old woman presented with renal dysfunction during macrohematuria. A kidney biopsy revealed IgA nephropathy with a small percentage of crescent formation and macrohematuria-associated tubular injury. Macrohematuria-associated acute kidney injury could explain her renal dysfunction. However, she was seropositive for myeloperoxidase (MPO)-anti-neutrophil cytoplasmic antibody (ANCA) and showed fibrin deposition around one arteriole. Corticosteroids and mycophenolate mofetil were administered as for ANCA vasculitis, and the serum creatinine, abnormal urinalysis and MPO-ANCA titer all gradually ameliorated. The presence of extra-glomerular vasculitis, which was probably induced by ANCA, suggested that MPO-ANCA was an exacerbating factor for her prolonged renal dysfunction. This condition has so far only rarely been addressed in ANCA-positive IgA nephropathy.
Keywords: acute kidney injury, corticosteroid, IgA nephropathy, macrohematuria, mycophenolate mofetil, myeloperoxidase-ANCA
Introduction
IgA nephropathy (IgAN), which is defined by IgA deposition in the glomerular mesangium, is the most common form of glomerulonephritis worldwide (1). It is reported that approximately 35-42% of IgAN patients have a history of macrohematuria before kidney biopsy (2). IgAN during macrohematuria sometimes shows acute kidney injury (AKI), which is caused by macrohematuria-associated tubular injury, crescent formation (usually affecting more than 50% of glomeruli) or other causes (3, 4). Macrohematuria-associated AKI is induced by acute tubular necrosis, which is promoted by hemoglobin released from intratubular obstructed red-blood-cell (RBC) casts (5). Hemoglobin is a strong oxidant with potent pro-inflammatory properties which may sometimes lead to tubular cell injury, resulting in AKI (6). Crescentic IgAN can cause immune-complex type of rapidly progressive glomerulonephritis. According to the Kidney Disease Improving Global Outcomes (KDIGO) clinical practice guidelines for glomerulonephritis (3), continuous supportive care, as in other types of acute tubular necrosis, is recommended for macrohematuria-associated AKI, and corticosteroids and cyclophosphamide therapy are recommended for crescentic IgA nephropathy.
Anti-neutrophil cytoplasmic antibodies (ANCAs) are commonly associated with vasculitis and pauci-immune crescentic glomerulonephritis in the kidney (7). It is rare, but there are a number of reports about patients of ANCA-positive IgAN associated with various percentages of crescent formation, showing clinical manifestations of hematuria and/or proteinuria with or without an aggravated renal function (8-11). However, a patient with ANCA-positive IgAN associated with macrohematuira-associated AKI has not yet been previously reported. Some investigators reported that aggressive immunosuppressive therapy like ANCA-associated glomerulonephritis was effective for the treatment of ANCA-positive IgAN cases (9, 11). However, nephritogenicity of ANCA in patients with ANCA-positive IgAN has not yet been clearly elucidated (8, 10).
We herein report a rare case with MPO-ANCA-positive IgAN presenting with renal dysfunction during macrohematuria. Renal dysfunction at the time of the first examination may have been caused by macrohematuria-associated AKI. Without any signs of systemic vasculitis it is difficult to determine the pathogenicity of ANCA in IgAN which sometimes shows a small percentage of crescent formation. A kidney biopsy in our patient showed an extra-glomerular renal vasculitic lesion, thus, intensive immunosuppressive therapy was introduced in a manner similar to the treatment of ANCA-associated glomerulonephritis.
Case Report
A 21-year-old woman was admitted to our hospital to evaluate renal dysfunction during macrohematuria. Positive occult blood was identified in a urine specimen without any blood test abnormalities at a health check-up one year previously, but occult blood in her urine was negative at a local clinic. She had experienced a fever of 38.0 °C and macrohematuria (brown colored urine) 3 weeks before the admission. The macrohematuria soon disappeared. She visited a local clinic 1 day before admission because she had experienced some slight abdominal pain for 6 days and vomiting, a fever of 39.0℃ and macrohematuria for one day. 3+ occult blood with >100 RBC/high power field and 2+ protein in urine, serum creatinine of 1.9 mg/dL and C-reactive protein of 3.75 mg/dL were found. She was referred to our hospital the next day. She had a medical history of atopic dermatitis and allergic rhinitis.
On admission, she had almost no symptoms. Her body temperature was 36.7 °C, height 155.2 cm, weight 55.9 kg and blood pressure of 106/64 mmHg. She had no findings regarding her tonsils, skin, joints or neuromuscular system. The laboratory data are listed in the Table. The patient showed macrohematuria with high percentage of dysmorphic RBC, positive urinary RBC cast, proteinuria of 1.16 g/g creatinine, a slight increase in the markers for proximal tubular injury, hypoalbuminemia and renal dysfunction. Immunological findings showed an increase in the IgA level, normocomplementemia, negative hepatitis B and C serologies, negative anti-nuclear antibody and positive MPO-ANCA. Chest X-ray was normal and computed tomography images showed slightly swollen kidneys, but no pulmonary or gastrointestinal findings.
Table.
Laboratory Data on Admission.
| Urine | ||
| Protein | 2+ | |
| Occult blood | 3+ | |
| Red blood cell | >100 | /high power field |
| Dysmorphic red blood cells | >80 | % |
| White blood cell | 10-19 | /high power field |
| Red-blood-cell cast | positive | |
| Protein | 1.16 | g/g creatinine |
| NAG | 23.1 | U/L (0.7 to 11.2) |
| α1-microglobulin | 58.0 | mg/L (<11.9) |
| Complete blood count | ||
| WBC | 6,100 | /µL |
| Hb | 10.2 | g/dL |
| MCV | 88.4 | fL |
| MCHC | 34.5 | pg |
| Platelet | 34.5×104 | /µL |
| Blood chemistry | ||
| Total protein | 6.8 | g/dL |
| Albumin | 3.4 | g/dL |
| Urea nitrogen | 21.4 | mg/dL |
| Creatinine | 2.35 | mg/dL |
| Aspartate aminotransferase | 14 | IU/L |
| Alanine aminotransferase | 5 | IU/L |
| Total bilirubin | 0.39 | mg/dL |
| Alkaline phosphatase | 153 | IU/L |
| γ-glutamyltransferase | 13 | IU/L |
| Lactate dehydrogenase | 181 | IU/L |
| Na | 140 | mEq/L |
| K | 4.2 | mEq/L |
| Cl | 105 | mEq/L |
| Triglyceride | 94 | mg/dL |
| LDL cholesterol | 97 | mg/dL |
| Fe | 28 | μg/dL |
| TIBC | 280 | μg/dL |
| Ferritin | 190.3 | ng/mL |
| estimated GFR | 23.5 | mL/min/1.73m2 |
| Immunologic test | ||
| IgG | 1,420 | mg/dL |
| IgA | 366 | mg/dL |
| IgM | 145 | mg/dL |
| CH50 | >60 | U/mL |
| C3 | 124 | mg/dL |
| C4 | 31 | mg/dL |
| C-reactive protein | 2.93 | mg/dL |
| Antinuclear antibody | ×40> | |
| Anti-DNA antibody | 1.0 | IU/mL (<9.0) |
| MPO-ANCA | 12.5 | U/mL (<3.4) |
| PR3-ANCA | 1.0 | U/mL (<3.4) |
| Cryoglobulin | negative | |
| ASO | 131.0 | U/mL |
| HBs antigen | negative | |
| HCV antibody | negative |
NAG: N-acetyl-β-D-glucosaminidase, TIBC: total iron binding capacity, GFR: glomerular filtration rate, MPO-ANCA: myeloperoxidase-anti-neutrophil cytoplasmic antibody, PR3-ANCA: proteinase 3-anti-neutrophil cytoplasmic antibody, HBs: hepatitis B surface antigen, HCV: hepatitis C virus, ASO: anti-streptolysin O. The values in parentheses show the normal range
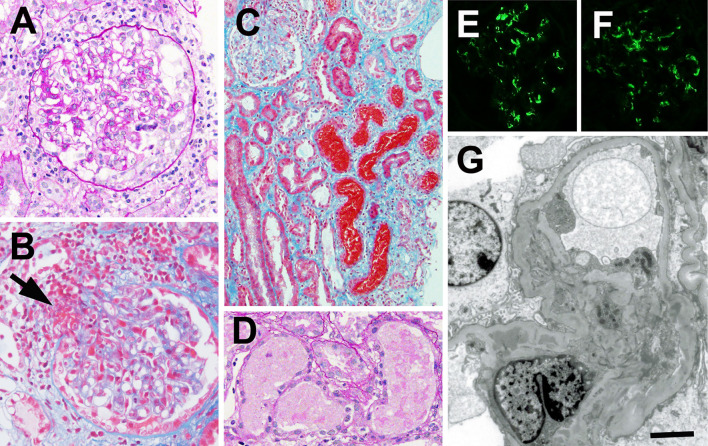
A kidney biopsy was performed on the 5th day of admission and showed small cellular crescents (Fig. 1A) in 2 (11.7%) out of 17 obtained glomeruli and focal fibrin deposition with capillary wall disruption in 4 glomeruli. Most glomeruli showed mild to moderate mesangial proliferation (Fig. 1A), suggesting that the glomerular changes could not be explained by ANCA-associated glomerulonephritis. Fibrin deposition was found around one arteriole at a vascular pole of glomerulus (Fig. 1B), thus suggesting arteriolar vasculitis, which resembled ANCA-associated vasculitis. Moderate to severe inflammatory cell infiltration was observed with mild fibrosis in 20 to 30% tubulointerstitial areas with many tubules filled with RBC casts (Fig. 1C). Signs of acute tubular injuries were observed mainly in the tubules filled with RBC casts (Fig. 1D). Immunofluorescence revealed granular mesangial deposits of IgA (Fig. 1E), light chain κ, light chain λ and C3 (Fig. 1F). IgG, IgM and C1q were negative. IgA was negative along the arteriolar walls. Electron microscopy showed electron dense deposits in the mesangial areas (Fig. 1G).
Figure 1.
Kidney biopsy findings. A: A light micrograph shows a glomerulus with mesangial proliferation and cellular crescent formation. Periodic acid-Schiff staining. Original magnification ×400. B: Fibrin deposition is found around the arteriole at vascular pole of glomerulus (arrow). Elastica-Masson staining. Original magnification ×200. C: Many tubules with flattened epithelial cells are obstructed with red-blood-cell casts. There is inflammatory cell infiltration around the injured tubules. Elastica-Masson staining. Original magnification ×200. D: Acute tubular injuries (dilation of the lumen, flattening and desquamation of the lining epithelial cells and cytoplasmic degeneration) are found in the tubules filled with red-blood-cell casts. Periodic acid-Schiff staining. Original magnification ×400. E and F: Immunofluorescent findings show positive granular staining for IgA (E) and C3 (F) in the mesangial areas. G: Electron micrograph of a glomerulus. Electron dense deposits are found in the mesangial areas. Bar=2.0 μm
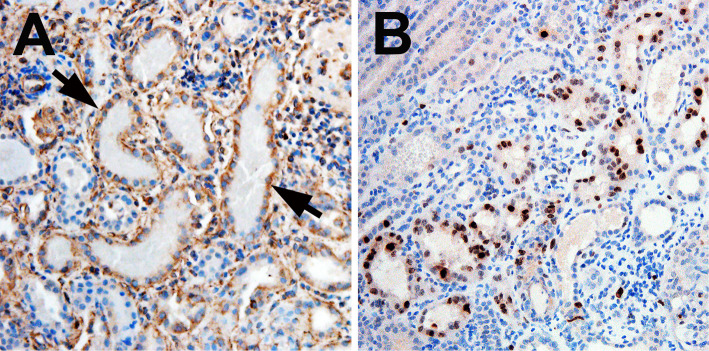
Further examination of renal tissues by immunohistochemistry showed vimentin-positive degenerating or Ki67-positive regenerating epithelial cells in the tubules mainly filled with RBC casts (Fig. 2A and B), indicating acute tubular injury and tubular cell regeneration. These findings confirmed that RBC cast formation could cause AKI in our patient. Our patient was diagnosed to have IgAN with macrohematuira-associated AKI and MPO-ANCA-associated renal arteriolar vasculitis.
Figure 2.
Indirect immunohistochemistry findings for vimentin (a marker of tubular injury and dedifferentiation) (A) and Ki67 (a marker of cell proliferation) (B). A: The simplified tubules with red-blood-cell casts show basal or cytoplasmic patterns of vimentin staining in epiethlial cells (arrows). B: Ki67 is expressed in some epithelial cells in tubules with or without red-blood-cell casts. A and B: Original magnification ×200. The primary antibodies, including murine monoclonal anti-vimentin antibody (clone V9, Sigma Aldrich, St Louis, USA), or murine monoclonal anti-human Ki67 antibody (clone MIB-1, Dako Denmark A/S, Glostrup, Denmark) were used.
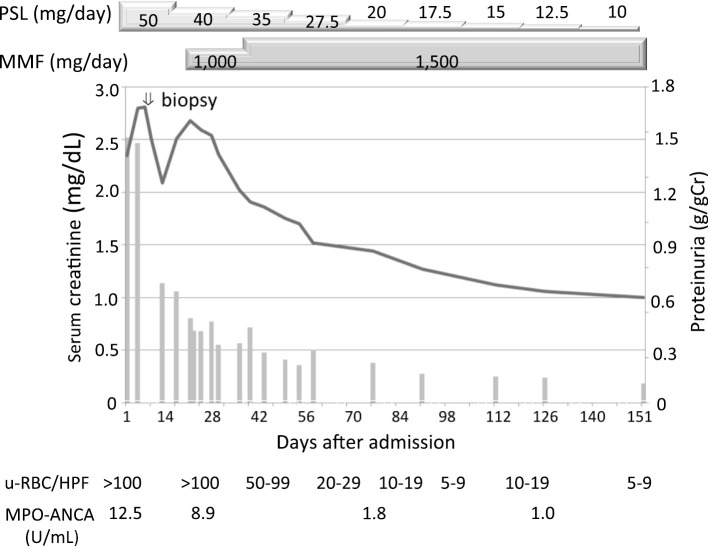
The clinical course after hospital admission is shown in Fig. 3. At 2 days after the kidney biopsy, 50 mg/day prednisolone was introduced. Macrohematuria disappeared 2 days after the start of treatment. After confirming the renal pathology, 1,000 mg/day of mycophenolate mofetil (MMF) were initiated. The dose of MMF was increased to 1,500 mg/day, then prednisolone was gradually tapered. The MPO-ANCA level normalized and proteinuria became negative by 2 months after the start of treatment. High-grade hematuria improved to low-grade hematuria of RBC 5 to 9/HPF in urinary sediments and renal dysfunction improved to 0.87 mg/dL with 10 mg/day prednisolone and 1,500 mg/day MMF by 8 months after the start of treatment.
Figure 3.
Clinical course after hospital admission. Line graph; serum creatinie, Bar graph; proteinuria, PSL: prednisolone, MMF: mycophenolate mophetil, Cr: creatinine, u-RBC/HPF: urinary red blood cells per high-power field, MPO-ANCA: myeloperoxidase-anti-neutrophil cytoplasmic antibody
Discussion
We encountered a patient with IgAN and seropositive MPO-ANCA presenting with AKI during macrohematuria. Given the presence of arteriolar vasculitis, IgA vasculitis with nephritis should thus be included in the differential diagnosis (7). However, we felt that this diagnosis is unlikely because she did not have the typical clinical signs of IgA vasculitis, such as purpura and arthritis. She had abdominal pain, but it was slight, not colicky, and a fecal occult blood test was negative. Histologically, IgA nephritis and IgA vasculitis with nephritis are indistinguishable. Although IgA vasculitis is a form of small vessel vasculitis, the occurrence of arteritis involving the interlobular arteries is rare. For instance, Pillebout et al. noted necrotizing and granulomatous arteritis involving interlobular arteries in only two cases out of 250 adults with IgA vasculitis with nephritis (12). As extra-glomerular renal vasculitis is exceptional in both InAN and IgA vasculitis, MPO-ANCA-associated vasculitis is thus considered to better explain the extra-glomerular renal vasculitis in our case. It was reported that the percentage of crescents was usually <25% with cellular, small, and segmental in IgAN who had macrohematuria-associated AKI, but it was not thought to be the cause of AKI (5). The glomerular changes and renal arteriolar vasculitis in our patient were not severe enough to explain the cause of renal dysfunction, such as rapidly progressive glomerulonephritis, whereas acute tubular injury associated with RBC casts could better explain the cause of renal dysfunction as macrohematuria-associated AKI at the time of kidney biopsy. The condition of MPO-ANCA-positive IgAN presenting with macrohematuria-associated AKI and ANCA-associated renal vasculitis has so far only rarely been described.
We decided to treat the patient with corticosteroids and MMF, in a manner similar to the treatment for ANCA associated glomerulonephritis. The KDIGO clinical practice guidelines for glomerulonephritis recommended corticosteroids and cyclophosphamide for AKI caused by crescentic IgAN (3). Instead of cyclophosphamide, we used MMF because that we wanted to avoid a risk of ovarian failure by cyclophosphamide and MMF has been reported to be effective for both ANCA associated glomerulonephritis and IgAN (13-15).
In macrohematuria-associated AKI in IgAN, the average duration of hematuria is 15-60 days and the mean time to peak serum creatinine is 6-15 days after the onset of macrohematuria, then the serum creatinine level decreased 2-45 days after macrohematuria cessation (16, 17). Gutiérrez et al. reported that approximately 25% of all patients with macrohematuria-associated AKI did not completely recover to baseline serum creatinine levels after the resolution of the episode of macrohematuria (5). According to a multivariate analysis, a duration of macrohematuria longer than 15 days was reported to be the only statistically significant variable for the risk of an incomplete recovery of renal function (5). Although there are no randomized controlled trials, early corticosteroid administration shortened the duration of macrohematuria and decreased the risk of chronic renal failure in these patients (5, 18). Our patient was expected to show a rapid and complete recovery of her renal function in terms of macrohematuria-associated AKI, however, the renal function was gradually ameliorated in association with a decrease in the MPO-ANCA titer and an improvement of both hematuria and proteinuria. The clinical course of our patient suggested that renal dysfunction was mainly caused by macrohematuria-associated AKI and that the progression of ANCA-associated renal vasculitis after a kidney biopsy might have contributed to her prolonged renal dysfunction.
There are a number of reports about patients with IgAN and seropositive ANCA (8-11). The causal relationship between IgAN and ANCA is not clear, however, most of the patients are diagnosed with an overlapping condition of IgAN and ANCA associated glomerulonephritis, and many patients are also associated with commonly extra-renal signs and/or symptoms of vasculitis. However, there were some patients who did not show any systemic symptoms among the patients with ANCA-positive IgAN. There was a case with ANCA-positive IgAN who had neither focal necrotization nor crescent formation in the kidney (19). A retrospective cohort study reported that ANCA-positive IgAN patients with systemic symptoms had a lower cumulative renal survival rate compared with both ANCA-positive IgAN patients without systemic symptoms and ANCA-negative IgAN patients (10). Another study showed that ANCA-positive IgAN patients had more severe clinical and histological features when compared with ANCA-negative IgAN patients and they were comparable to ANCA-associated systemic vasculitis patients, and their renal outcomes were relatively better with aggressive immunosuppressive therapy in the short term (9).
Xie et al. reported that an evaluation of extra-renal systemic symptom according to the Birmingham Vasculitis Activity Score (BVAS) item is a readily available clinical tool to predict the pathogenetic role of ANCA in IgAN (10). However, it is difficult to distinguish nephritogenic ANCA from non-nephritogenic ANCA in patients with IgAN. The pathogenicity of ANCA might depend on epitope specificity (20) and determination of the epitope specificity could thus help to distinguish pathogenic ANCA from non-pathogenic ANCA at the laboratory level. Establishing a simple and easy laboratory procedure to detect pathogenic ANCA is therefore required.
In summary, renal histology is essential to evaluate the pathophysiology of renal dysfunction during macrohematuriain in patients with IgAN. In cases demonstrating IgAN with macrohematuria-associated AKI and seropositive ANCA, the positive findings of extra-glomerular vasculitic lesions in the tubulointerstitial areas is useful for evaluating the nephritogenicity of accompanying serum ANCA. At present aggressive immunosuppressive treatment may be allowed or even recommended for any patients with ANCA-positive IgAN because ANCA-associated glomerulonephritis without appropriate treatment can potentially demonstrate a poor prognosis.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Li LS, Liu ZH. Epidemiologic data of renal diseases from a single unit in China. Analysis based on 13,519 renal biopsies. Kidney Int 66: 920-923, 2004. [DOI] [PubMed] [Google Scholar]
- 2. Le W, Liang S, Chen H, et al. Long-term outcome of IgA nephropathy patients with recurrent macroscopic hematuria. Am J Nephrol 40: 43-50, 2014. [DOI] [PubMed] [Google Scholar]
- 3. Kidney Disease Improving Global Outcomes (KDIGO) clinical practice guideline for glomerulonephritis. Kidney Int Suppl 2: 209-217, 2012. [Google Scholar]
- 4. Yuzawa Y, Yamamoto R, Takahashi K, et al. Evidence-based clinical practice guidelines for IgA nephropathy 2014. Clin Exp Nephrol 20: 511-535, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gutiérrez E, González E, Hernández E, et al. Factors that determine an incomplete recovery of renal function in macrohematuria-induced acute renal failure of IgA nephropathy. Clin J Am Soc Nephrol 2: 51-57, 2007. [DOI] [PubMed] [Google Scholar]
- 6. Tracz MJ, Alam J, Nath KA. Physiology and pathophysiology of heme: implications for kidney disease. J Am Soc Nephrol 18: 414-420, 2007. [DOI] [PubMed] [Google Scholar]
- 7. Jennette JC, Falk RJ, Bacon PA, et al. 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum 65: 1-11, 2013. [DOI] [PubMed] [Google Scholar]
- 8. Huang X, Wang Y, Xie L, et al. IgA nephropathy with anti-neutrophil cytoplasmic antibody seropositivity. Clin Nephrol 84: 156-164, 2015. [DOI] [PubMed] [Google Scholar]
- 9. Yang YZ, Shi SF, Chen YQ, et al. Clinical features of IgA nephropathy with serum ANCA positivity: a retrospective case-control study. Clin Kidney J 8: 482-488, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Xie L, He J, Liu X, et al. Clinical value of systemic symptoms in IgA nephropathy with ANCA positivity. Clin Rheumatol 37: 1953-1961, 2018. [DOI] [PubMed] [Google Scholar]
- 11. Bantis C, Stangou M, Schlaugat C, et al. Is presence of ANCA in crescentic IgA nephropathy a coincidence or novel clinical entity? A case series. Am J Kidney Dis 55: 259-268, 2010. [DOI] [PubMed] [Google Scholar]
- 12. Pillebout E, Thervet E, Hill G, Alberti C, Vanhille P, Nochy D. Henoch-Schönlein Purpura in adults: outcome and prognostic factors. J Am Soc Nephrol 13: 1271-1278, 2002. [DOI] [PubMed] [Google Scholar]
- 13. Chen Y, Gao E, Yang L, et al. Long-term outcome of mycophenolate mofetil treatment for patients with microscopic polyangiitis: an observational study in Chinese patients. Rheumatol Int 36: 967-974, 2016. [DOI] [PubMed] [Google Scholar]
- 14. Chen Y, Li Y, Yang S, Li Y, Liang M. Efficacy and safety of mycophenolate mofetil treatment in IgA nephropathy: a systematic review. BMC Nephrol 15: 193, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bhandari D, Jhaveri KD, Shah HH. Complete remission of nephrotic syndrome and acute kidney injury in crescentic IgA nephropathy: role of mycophenolate sodium. Indian J Nephrol 26: 370-372, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Praga M, Gutierrez-Millet V, Navas JJ, et al. Acute worsening of renal function during episodes of macroscopic hematuria in IgA nephropathy. Kidney Int 28: 69-74, 1985. [DOI] [PubMed] [Google Scholar]
- 17. Kveder R, Lindic J, Ales A, Kovac D, Vizjak A, Ferluga D. Acute kidney injury in immunoglobulin A nephropathy: potential role of macroscopic hematuria and acute tubulointerstitial injury. Ther Apher Dial 13: 273-277, 2009. [DOI] [PubMed] [Google Scholar]
- 18. Feith GW, Assmann KJ, Wetzels JF. Acute renal failure in patients with glomerular diseases: a consequence of tubular cell damage caused by haematuria? Neth J Med 61: 146-150, 2003. [PubMed] [Google Scholar]
- 19. Koratala A, Zeng X, Kazory A. ANCA-positive IgA nephropathy without necrotising or crescentic glomerulonephritis: a clinical conundrum. BMJ Case Rep: 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Roth AJ, Ooi JD, Hess JJ, et al. Epitope specificity determines pathogenicity and detectability in ANCAassociated vasculitis. J Clin Invest 123: 1773-1783, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]