Abstract
We herein report a case of autoimmune pulmonary alveolar proteinosis (PAP) diagnosed after one-time exposure to silica powder. Owing to the misuse of a silica-containing fire extinguisher and the inhalation of large amounts of its powder, the patient experienced prolonged cough and visited our hospital. The findings of chest computed tomography and surgical lung biopsy specimens led to the diagnosis of PAP. Interestingly, the presence of anti-GM-CSF antibody was detected; therefore, both autoimmune characteristics and exposure to large amounts of silica may have caused the development of PAP in this patient. This case provides important insight into the mechanisms leading to the onset of PAP.
Keywords: pulmonary alveolar proteinosis, anti-GM-CSF antibody, dust, silica, fire extinguisher, elemental analysis
Introduction
Pulmonary alveolar proteinosis (PAP), first described by Rosen et al. in 1958 (1), is a rare disease characterized by the accumulation of surfactant-derived lipids and proteins in the alveolar spaces due to a disruption of surfactant homeostasis. Based on the etiology, it can be classified into three clinical forms, namely autoimmune, secondary, and congenital. In autoimmune PAP (APAP), neutralizing autoantibody against granulocyte/macrophage colony-stimulating factor (GM-CSF) causes the dysfunction of the alveolar macrophages and neutrophils, thereby resulting in the development of APAP (2, 3).
An international epidemiological survey conducted by Inoue et al. in 2008 revealed that >90% of patients with PAP were diagnosed with APAP, among whom 23% had histories of dust inhalation (4). However, the mechanism of the underlying development of APAP, particularly by exposure to dust, remains unknown.
We herein report the case of a patient with APAP who was diagnosed after one-time exposure to large amounts of silica powder from a fire extinguisher and discuss the effect of dust exposure on the development of this disease.
Case Report
A 76-year-old Japanese woman misused a fire extinguisher and continued to discharge its powder for about 10 sec in a room without a window, thereby inhaling a large amount of dust in early October 2015. She had been asymptomatic for 2 weeks after the incident; however, she had been coughing since mid-October. She visited a local outpatient clinic in late October; chest radiography showed a diffuse infiltrate shadow (Supplementary material). She showed no improvement after the administration of cough suppressants by the clinic, and thereafter visited another clinic in early December. Chest computed tomography (CT) showed diffuse infiltrate shadows in the bilateral lung fields, and she was hospitalized at the Sapporo Medical University Hospital in late December.
She had been working in the seafood processing industry since 18 years of age, and had no history of smoking and also no occupational or environmental exposure to dust. While previously she had WPW syndrome and dyslipidemia, there was no history of hematological diseases, autoimmune diseases, inflammatory bowel disease, or pulmonary infection. On examinations before the incident, chest radiographs showed no abnormalities in 2006, 2011, and 2012; her lactate dehydrogenase (LDH) level was within the normal range at 227 U/L [cutoff (CO) <240] in 2007.
At the time of hospitalization, she was conscious, with a temperature of 96.3°F (35.7℃), pulse of 78 beats per min, blood pressure of 128/70 mmHg, and oxygen saturation of 91% (room air) and without cyanosis and clubbed fingers. Fine crackles could be weakly heard in the back and lower lung fields upon chest auscultation.
Laboratory evaluation at the time of hospitalization revealed that her LDH, surfactant protein (SP)-A, and Krebs von den Lungen-6 (KL-6) levels were elevated at 294 U/L (CO<240), 77.9 ng/mL (CO<43.8), and 1,709 U/mL (CO<500), respectively. However, her white blood cell (WBC) count, C-reactive protein (CRP), and SP-D levels were within the normal range at 6.1×103/μL (CO<9.8×103), 0.10 mg/dL (CO<0.30), and 86.9 ng/mL (CO<110), respectively.
A respiratory physiological examination revealed a gas exchange disturbance. An arterial blood gas analysis showed the partial pressure of oxygen (PaO2) to have decreased (62.9 Torr), while the alveolar arterial oxygen gradient (A-aDO2) was elevated (39.2 Torr). Additionally, the minimum oxygen saturation in the 6-min walk test was decreased (89%). Pulmonary function tests showed the diffusion capacity for carbon monoxide (DLCO) to have decreased (68.6%) and the vital capacity (VC) remained (120.0%).
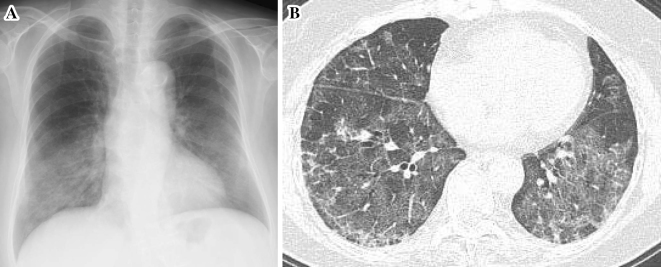
A radiological examination of the chest showed diffuse infiltrate shadows (Fig. 1A), and high-resolution CT showed multifocal ground glass opacities in the bilateral lower lobe dominance (Fig. 1B). Owing to the characteristic geographic distribution, intralobular and interlobular septa thickening, and crazy-paving appearance, PAP was suspected.
Figure 1.
(A) Chest radiography at hospitalization (late December 2015) shows diffuse infiltrate shadows in the bilateral lower lung fields. (B) Chest high resolution computed tomography at hospitalization shows bilateral ground glass opacities and intralobular and interlobular septal thickening, which were most prominent in the lower lobes.
Bronchoalveolar lavage (BAL) was performed on hospital day 2 from the right B5, and a recovery rate of 43.3% was obtained. Her BAL fluid (BALF) was colorless and transparent; it was not characteristic of PAP. A BALF evaluation showed the total cell number and the cell concentration to have decreased to 0.416×107 and 0.640×105/mL, respectively. The differential WBC count for macrophage, lymphocyte, neutrophils, eosinophils, and basophils was 77.8%, 19.6%, 2.6%, 0.0%, and 0.0%, respectively, showing a slight elevation of lymphocyte proportion. Among the T lymphocytes, the CD4/CD8 ratio was 1.8. The levels of SP-A, SP-D, and KL-6 in BALF were 1,665, 550 ng/mL, and 519 U/mL, respectively. Finally, no bacteria were detected in BALF.
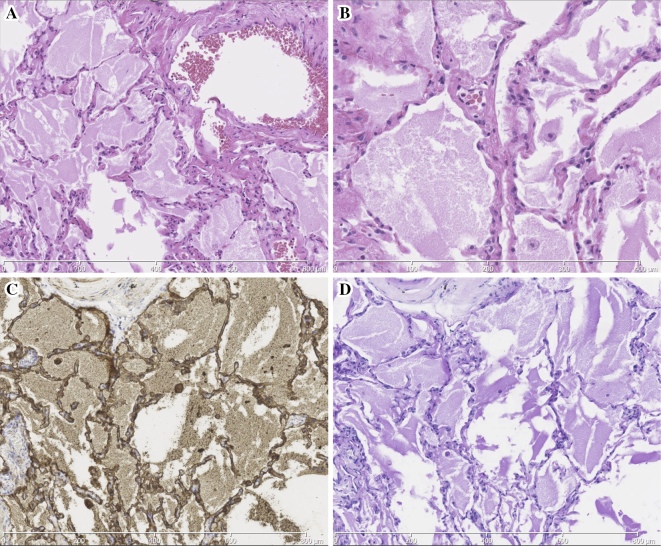
A transbronchial lung biopsy was performed on the patient on hospital day 3, and samples were collected from the right S3, S4, and S8; small amount of granular substances was present in the alveolar spaces, however, these findings did not enable us to make a definite diagnosis. Therefore, a surgical lung biopsy was performed in late January 2016 from the left S5, S6, and S9. A histopathological evaluation revealed the alveolar spaces to be filled with anti-SP-A antibody (PE10)/periodic acid-Schiff (PAS)-positive eosinophilic granular substances and there were some foamy macrophages locally (Fig. 2), and these findings were consistent with the characteristics of PAP. An evaluation using a polarizing microscope revealed that hyaline-like structure suggesting of silicotic nodules were not present. In addition, there were no findings indicating any another disease such as lung injury.
Figure 2.
Histopathological sections of a surgical lung biopsy show the filling of the alveolar spaces with eosinophilic granular substances with (A, B) Hematoxylin and Eosin staining (A, magnification ×100; B, ×200), which were (C) PE 10 and (D) PAS positive (magnification ×100). These finding were consistent with PAP. PAP: pulmonary alveolar proteinosis, PAS: periodic acid-Schiff, PE 10: anti-SP-A antibody
Interestingly, the serum level of anti-GM-CSF antibody at her first visit to our hospital was found to be positive at 15.0 μg/mL. Based on these results, she was diagnosed with APAP which may have been caused by her autoimmune characteristics and her exposure to large amounts of dust.
We speculated that she would recover without any therapeutic intervention, such as whole lung lavage or GM-CSF therapy, because the effect of exposure to dust would be temporary. Therefore, we decided to follow up the patient without any specific treatment. Although her condition remained unchanged at first, infiltrate shadows on chest images seemed to improve in mid-March and then completely disappeared in late May. The serum biomarker levels at each time-point are summarized in Table. In addition, she was able to return to her normal work from April 2016 and did not change her living environment.
Table.
Blood Biomarkers at Each Time-point.
| Late May 2007 | Late Oct. 2015 | Late Dec. 2015 | Mid Mar. 2016 | Late May 2016 | |
|---|---|---|---|---|---|
| WBC (/µL) | 3,600 | 6,170 | 4,800 | 5,200 | 4,500 |
| LDH (U/L) | 227 | 288 | 292 | 223 | 209 |
| CRP (mg/dL) | 0.29 | 0.59 | 0.14 | <0.10 | <0.10 |
| SP-A (ng/mL) | NA | NA | 77.9 | 57.7 | 41.5 |
| SP-D (ng/mL) | NA | NA | 86.9 | 89.1 | 56.4 |
| KL-6 (U/mL) | NA | NA | 1,709 | 2,462 | 709 |
| GM-CSF Ab (µg/mL) | NA | NA | 15.0 | NA | NA |
WBC: white blood cell, CRP: C-reactive protein, LDH: lactate dehydrogenase, SP-A: surfactant protein A, SP-D: surfactant protein D, KL-6: Krebs von den Lungen-6, GM-CSF Ab: anti-GM-CSF antibody, NA: not available
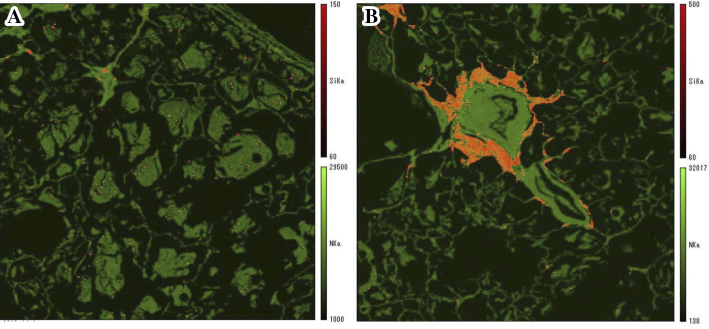
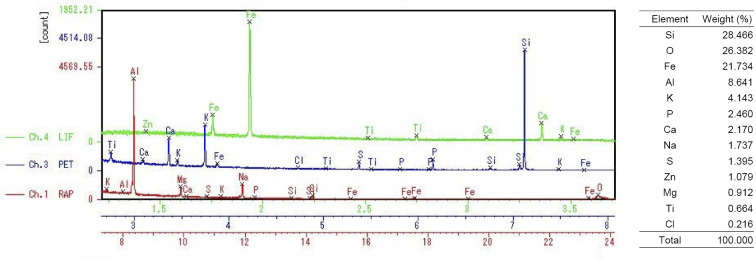
An elemental analysis was performed on lung tissue specimens obtained by a surgical biopsy using the Electron Probe Micro Analyzer (EPMA; EPMA-1610, Shimadzu, Kyoto, Japan); which revealed the deposition of silicon (Si) in the alveolar spaces (Fig. 3A) and around the bronchioles (Fig. 3B), together with oxygen (O). A semi-quantitative analysis showed two peaks of Si and O at a ratio of Si to O, suggestive of silicon dioxide (silica) (Fig. 4). In addition, their simultaneous presence on the specimen at a similar high molecular weight ratio strongly suggests that silica powder was directly inhaled into the lung (5). Even in healthy lung tissues, Si can be detected on the airway surface of the bronchiole and alveolar walls (6); however, it is not as abundant as was observed in the present case. These results supported the notion that silica powder from the fire extinguisher had been inhaled into the lung.
Figure 3.
An elemental analysis of lung tissue specimens using an Electron Probe Micro Analyzer. (A) The deposition of silicon (Si) was detected in the alveolar spaces, and (B) Si is also observed in a large proportion around the bronchioles. The deposition of Si is shown in yellow.
Figure 4.
An elemental analysis of lung tissue around the bronchioles using the Electron Probe Micro Analyzer. Si: silicon, O: oxygen, Fe: iron, Al: aluminum, K: potassium, P: phosphorus, Ca: calcium, Na: sodium, S: sulfur, Zn: zinc, Mg: magnesium, Ti: titanium, Cl: Chlorine, RAP: rubidium acid phthalate, PET: pentaerythritol, LIF: lithium fluoride
Discussion
Our patient would have been diagnosed as secondary PAP (SPAP) using the previous disease classification (i.e., before anti-GM-CSF antibody was measurable) because it had been diagnosed after dust inhalation from a fire extinguisher. SPAP has been reported to be caused by hematological diseases, including myelodysplastic syndrome, lymphoma and leukemia, Behçet’s disease, autoimmune diseases, and mycobacterium infections (7) and the inhalation of dust, including silica, aluminum (Al), and titanium (8-11). However, because our patient was also positive for anti-GM-CSF antibody, she was thus diagnosed with APAP rather than SPAP based on the diagnostic flow chart of Inoue et al. (4).
Another report of dust-related PAP with anti-GM-CSF antibody discussed a case caused by occupational exposure to indium-tin oxide for 4 months (12). Additionally, anti-GM-CSF antibodies were reported to be positive in many patients with pneumoconiosis who merged PAP (13). However, there are no reports on PAP with anti-GM-CSF antibody detected after one-time exposure to dust. It is believed that the one-time exposure of silica does not always result in PAP. Thus, she might have had some onset factor, although she was asymptomatic before dust exposure from the fire extinguisher. Taken together, she might have potential characteristic of APAP. Unfortunately, the serum of the patient prior to the dust exposure was unavailable; nevertheless, this case can be highly significant for understanding the mechanism underlying the development of APAP.
During APAP, anti-GM-CSF antibody causes a deterioration of the functions of alveolar macrophages, such as their migratory and phagocytic ability, and, thereby, results in a decrease in the surfactant elimination capacity (2, 3). Moreover, a report has suggested that in pulmonary alveolar microlithiasis, the presence of calcium phosphate stones causes surfactant accumulation via the dysfunction of alveolar macrophages (14). Considering these studies, it is possible that the deteriorated alveolar macrophages reach their elimination capacity limit upon dust exposure. Consistent with this, in the large cohort of 223 APAP patients studied in Inoue et al., 23% of the patients had a history of dust inhalation (4). Our case showed that large amounts of dust exposure, even just one-time, may cause PAP.
Although many cases of SPAP induced by dust inhalation have been reported previously, most of them were reported before anti-GM-CSF antibody became measurable. In Japan, one case of dust-related PAP without anti-GM-CSF antibody was reported after the autoantibody became measurable (15). However, even in this case, the serum level of anti-GM-CSF antibodies exceeded the cutoff level at 1 year after exposure; in addition, in vitro studies revealed that the serum from this patient had an inhibitory effect on GM-CSF signaling (15). These findings suggest that the measurement of anti-GM-CSF antibody is necessary even in cases of dust-related PAP.
We found just one case of PAP induced by the contents of a fire extinguisher, which was reported by Kim et al. (16). The patient in this case had been working at a facility manufacturing gas-type fire extinguishers using hydrofluorocarbon (HF) and developed PAP after repeated exposure to fire extinguisher spray. In our case, the main components of the fire extinguisher were silica, ammonium phosphate, and ammonium sulfate, but not HF. Therefore, our case is the first report of PAP diagnosed after the inhalation of a powder-type fire extinguisher. Patients with PAP or patients with anti-GM-CSF antibody-positive should therefore be extremely careful while using such fire extinguishers.
In our case, the ratio of elements detected by EPMA was high in the order of Si, O, iron (Fe), and Al (Fig. 4). Adachi et al. showed that the intratracheal exposure of rats to silica increased the surfactant synthesis and secretion from the alveolar type II cells and impaired the surfactant removal system (17). Regarding Fe, it has been reported that patients with PAP have a disorder of iron homeostasis, and the hemosiderin-laden macrophages in their lung tissue tend to contain large amounts of iron (18, 19).
Crystalline silica and silicotic nodules are observed in the lungs of patients who repeatedly inhale silica, and such patients are diagnosed with chronic silicosis. We did not find these pathological findings in our patient using a polarizing microscope. Although conditions with a rapid onset are called acute silicosis or silicoproteinosis, patients with exposure shorter than 1 year are extremely rare. To our knowledge, the shortest exposure period in case reports of PAP induced by dust exposure, irrespective of silica, is several weeks (20, 21). Although Xiao et al. reported four cases of dust-related PAP without anti-GM-CSF antibody, these patients were diagnosed with silicoproteinosis, which is clinical and radiologically different from typical PAP, and had a very poor prognosis (22).
Some patients with PAP in whom BALF was colorless and transparent, similar to that in our patient, have been reported (23-25). They had milder symptoms and the extent of shadows on the chest images was not so intense. Similarly, in our patient, the extent of a shadow observed on right S4 where BAL was performed was mild, which may have resulted in the BALF findings.
The levels of SP-A, SP-D, and KL-6 in sera and BALF in this patient were significantly lower than those in patients with PAP reported previously (26-28). Although BALF can be explained by the above paragraph, the sera remain unknown. One possible explanation is: due to the inhalation of fire extinguisher powder, the disease developed relatively rapidly; as a result, production of SP-A, SP-D, and KL-6 from alveolar type II cells were insufficient, and leakage of them into the blood was difficult.
It is presumed that silica from the fire extinguisher still remained in the lung due to the lack of any removal mechanism. Nonetheless, PAP in this patient recovered without therapeutic intervention. There is a possibility that the phagocytic capacity could have been complemented by macrophages newly recruited to the alveolus. If so, the anti-GM-CSF antibody might not have inhibited the differentiation and proliferation of alveolar macrophages completely in this patient. Similarly, in the cohort study by Inoue et al., 28.2% of asymptomatic APAP participants at enrollment had experienced a spontaneous improvement since the onset (4).
In our case, we speculated that dust exposure had led to the development of PAP in a patient with autoimmune characteristics. Conversely, reports have shown that dust exposure induces anti-GM-CSF antibody as an epiphenomenon (29, 30). In our case, the serum of the patient prior to dust exposure was unavailable, and, thus, the limitation of determining when anti-GM-CSF antibodies were developed remains. In the future, the further accumulation of such cases is needed. In conclusion, our case study provides significant insight into the effect of dust exposure on the development of APAP.
Written informed consent for the publication of the clinical details and images was obtained from the patient.
The authors state that they have no Conflict of Interest (COI).
Financial Support
The work of the EPMA was supported by JSPS KAKENHI Grant Number JP17K09635.
Supplementary Materials
Chest radiography in late October 2015.
(A) Low (×50) and (B) high (×200) magnification of histopathological sections of Fig. 2A.
Acknowledgement
The authors would like to thank Niigata University for the EPMA of the specimens.
References
- 1. Rosen SH, Castleman B, Liebow AA, Enzinger FM, Hunt RTN. Pulmonary alveolar proteinosis. N Engl J Med 258: 1123-1142, 1958. [DOI] [PubMed] [Google Scholar]
- 2. Kitamura T, Tanaka N, Watanabe J, et al. Idiopathic pulmonary alveolar proteinosis as an autoimmune disease with neutralizing antibody against granulocyte/macrophage colony-stimulating factor. J Exp Med 190: 875-880, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Uchida K, Beck DC, Yamamoto T, et al. GM-CSF autoantibodies and neutrophil dysfunction in pulmonary alveolar proteinosis. N Engl J Med 356: 567-579, 2007. [DOI] [PubMed] [Google Scholar]
- 4. Inoue Y, Trapnell BC, Tazawa R, et al. Characteristics of a large cohort of patients with autoimmune pulmonary alveolar proteinosis in Japan. Am J Respir Crit Care Med 177: 752-762, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Takada T, Moriyama H, Suzuki E. Elemental analysis of occupational and environmental lung diseases by electron probe microanalyzer with wavelength dispersive spectrometer. Respir Investig 52: 5-13, 2014. [DOI] [PubMed] [Google Scholar]
- 6. Moriyama H, Kobayashi M, Takada T, et al. Two-dimensional analysis of elements and mononuclear cells in hard metal lung disease. Am J Respir Crit Care Med 176: 70-77, 2007. [DOI] [PubMed] [Google Scholar]
- 7. Ishii H, Tazawa R, Kaneko C, et al. Clinical features of secondary pulmonary alveolar proteinosis: pre-mortem cases in Japan. Eur Respir J 37: 465-468, 2011. [DOI] [PubMed] [Google Scholar]
- 8. Xipell JM, Ham KN, Price CG, Thomas DP. Acute silicolipoproteinosis. Thorax 32: 104-111, 1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Huaringa AJ, Francis WH. Pulmonary alveolar proteinosis: a case report and world literature review. Respirol Case Rep 4: e00201, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Miller RR, Churg AM, Hutcheon M, Lom S. Pulmonary alveolar proteinosis and aluminum dust exposure. Am Rev Respir Dis 130: 312-315, 1984. [DOI] [PubMed] [Google Scholar]
- 11. Keller CA, Frost A, Cagle PT, Abraham JL. Pulmonary alveolar proteinosis in a painter with elevated pulmonary concentrations of titanium. Chest 108: 277-280, 1995. [DOI] [PubMed] [Google Scholar]
- 12. Cummings KJ, Kreiss K, Roggli VL. Pulmonary alveolar proteinosis in workers at an indium processing facility. Am J Respir Crit Care Med 182: 578-579, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nishimura M, Yamaguchi E, Takahashi A, et al. Clinical significance of serum anti-GM-CSF autoantibody levels in autoimmune pulmonary alveolar proteinosis. Biomark Med 12: 151-159, 2018. [DOI] [PubMed] [Google Scholar]
- 14. Saito A, Nikolaidis NM, Amlal H, et al. Modeling pulmonary alveolar microlithiasis by epithelial deletion of the Npt2b sodium phosphate cotransporter reveals putative biomarkers and strategies for treatment. Sci Transl Med 7: 313ra181, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hisata S, Moriyama H, Tazawa R, Ohkouchi S, Ichinose M, Ebina M. Development of pulmonary alveolar proteinosis following exposure to dust after the Great East Japan Earthquake. Respir Investig 51: 212-216, 2013. [DOI] [PubMed] [Google Scholar]
- 16. Kim Y, Shin J, Kang S, et al. Pulmonary alveolar proteinosis induced by hydrofluoric acid exposure during fire extinguisher testing. J Occup Med Toxicol 10: 4-6, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Adachi H, Hayashi H, Sato H, Dempo K, Akino T. Characterization of phospholipids accumulated in pulmonary-surfactant compartments of rats intratracheally exposed to silica. Biochem J 262: 781-786, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shimizu Y, Matsuzaki S, Dobashi K, et al. Elemental analysis of lung tissue particles and intracellular iron content of alveolar macrophages in pulmonary alveolar proteinosis. Respir Res 12: 88, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Persson HL, Vainikka LK. Lysosomal iron in pulmonary alveolar proteinosis: a case report. Eur Respir J 33: 673-679, 2009. [DOI] [PubMed] [Google Scholar]
- 20. Marchiori E, Souza CA, Barbassa TG, Escuissato DL, Gasparetto EL, Souza AS. Silicoproteinosis: high-resolution CT findings in 13 patients. Am J Roentgenol 189: 1402-1406, 2007. [DOI] [PubMed] [Google Scholar]
- 21. Sauni R, Järvenpää R, Iivonen E, Nevalainen S, Uitti J. Pulmonary alveolar proteinosis induced by silica dust? Occup Med 57: 221-224, 2007. [DOI] [PubMed] [Google Scholar]
- 22. Xiao YL, Xu KF, Li Y, et al. Occupational inhalational exposure and serum GM-CSF autoantibody in pulmonary alveolar proteinosis. Occup Environ Med 72: 504-512, 2015. [DOI] [PubMed] [Google Scholar]
- 23. Inui N, Chida K, Suda T, et al. A case of pulmonary alveolar proteinosis presenting with peripheral infiltrates. Nihon Kokyuki Gakkai Zasshi (J Jpn Respir Soc) 37: 333-336, 1999(in Japanese). [PubMed] [Google Scholar]
- 24. Takato H, Nakatsumi Y, Inuzuka K, et al. Early stage pulmonary alveolar proteinosis detected by chest CT scan in a medical examination. Nihon Kokyuki Gakkai Zasshi (J Jpn Respir Soc) 45: 277-281, 2007(in Japanese). [PubMed] [Google Scholar]
- 25. Yamasaki K, Yoshii C, Nishida C, et al. Early case of idiopathic pulmonary alveolar proteinosis positive for serum anti-GM-CSF antibody. Nihon Kokyuki Gakkai Zasshi (J Jpn Respir Soc) 46: 712-716, 2008(in Japanese). [PubMed] [Google Scholar]
- 26. Honda Y, Takahashi H, Shijubo N, Kuroki Y, Akino T. Surfactant protein-A concentration in bronchoalveolar lavage fluids of patients with pulmonary alveolar proteinosis. Chest 103: 496-499, 1993. [DOI] [PubMed] [Google Scholar]
- 27. Honda Y, Kuroki Y, Matsuura E, et al. Pulmonary surfactant protein D in sera and bronchoalveolar lavage fluids. Am J Respir Crit Care Med 152: 1860-1866, 1995. [DOI] [PubMed] [Google Scholar]
- 28. Takahashi T, Munakata M, Suzuki I, Kawakami Y. Serum and bronchoalveolar fluid KL-6 levels in patients with pulmonary alveolar proteinosis. Am J Respir Crit Care Med 158: 1294-1298, 1998. [DOI] [PubMed] [Google Scholar]
- 29. Costabel U, Nakata K. Pulmonary alveolar proteinosis associated with dust inhalation:not secondary but autoimmune? Am J Respir Crit Care Med 181: 427-428, 2010. [DOI] [PubMed] [Google Scholar]
- 30. Uzmezoglu B, Simsek C, Gulgosteren S, Gebesoglu BE. Does dust-associated pulmonary alveolar proteinosis represent an autoimmune disorder? Am J Ind Med 60: 591-597, 2017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Chest radiography in late October 2015.
(A) Low (×50) and (B) high (×200) magnification of histopathological sections of Fig. 2A.