Abstract
A 37-year-old man developed abdominal pain and the frequency of severe abdominal pain steadily increased to once a month. He was therefore admitted to our hospital. Abdominal CT showed bowel obstruction. It revealed transient stenosis in the small intestine. There were no symptoms such as fever or weight loss, it seemed unlikely that the patient had inflammatory bowel disease. Considering the history of recurrent abdominal pain, Familial Mediterranean Fever (FMF) was considered. As a result, a genetic analysis revealed mutations in exons 3 and 8 of the MEFV gene. We herein report the first known case of FMF with transient small bowel stenosis in Japan.
Keywords: Familial Mediterranean fever, small bowel stenosis, inflammatory bowel disease, colchicine
Introduction
Familial Mediterranean fever (FMF) is an autoimmune disease caused by mutations of the MEFV gene that is characterized by periodic fever associated with peritonitis, pleuritis, and arthritis. So far, reports on more than 4,000 patients with FMF have been published worldwide. Approximately 500 FMF patients have been reported in Japan, and the number of such patients has recently tended to increase (1). Patients who have FMF usually present to hospital with abdominal pain and fever. Because these are common symptoms, FMF patients are often followed up without an accurate diagnosis being made. The association of small bowel stenosis with FMF has been reported overseas, but not in Japan. We herein report a Japanese FMF patient with small bowel stenosis in whom the correct diagnosis was made only 2 years after the onset of symptoms.
Case Report
A 37-year-old man with no relevant medical history or family history developed abdominal pain about every 3 months from 2016. The cause of this symptom could not be identified by his local doctor. Under a diagnosis of acute gastroenteritis, he was treated with an antibiotic and a medication for intestinal dysfunction, and the symptoms improved after a few days. However, the patient returned to his local clinic because the frequency of abdominal pain steadily increased to once a month from around August 2017 and the pain had also become more severe. On examination, bowel obstruction was diagnosed and the patient was transported to the Department of Gastroenterology at our hospital by ambulance.
On admission, physical revealed prominent rebound tenderness in the left lower quadrant of the abdomen and the supraumbilical region. Laboratory tests showed the WBC to be 8,400/μL (87.5% neutrophils), while CRP was 24.78 mg/dL, and procalcitonin (PCT) was 0.85 ng/mL. A plain abdominal X-ray film revealed air-fluid levels in the small bowel (Fig. 1). Abdominal CT showed a slight dilation of the bowel and the accumulation of fluid (Fig. 2).
Figure 1.
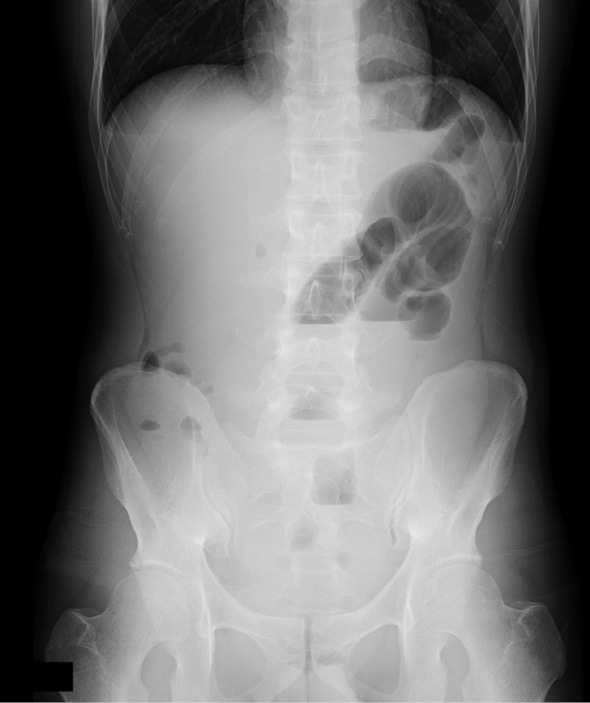
An abdominal X-ray film obtained on admission. Air-fluid levels are noted in the small bowel.
Figure 2.
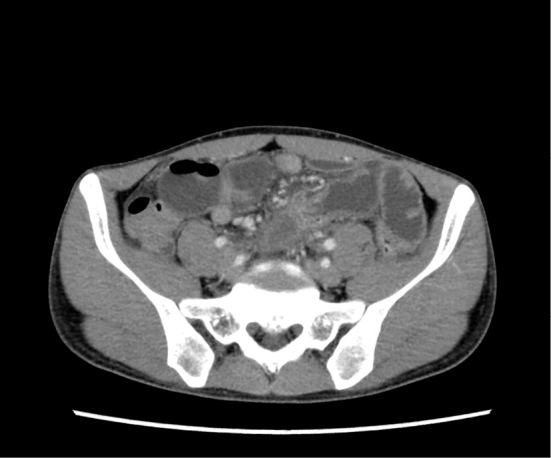
An abdominal CT scan obtained on admission. Slight dilation of the intestinal tract and accumulation of fluid are noted. Part of the intestinal tract has formed a loop, suggesting the mechanism of obstruction.
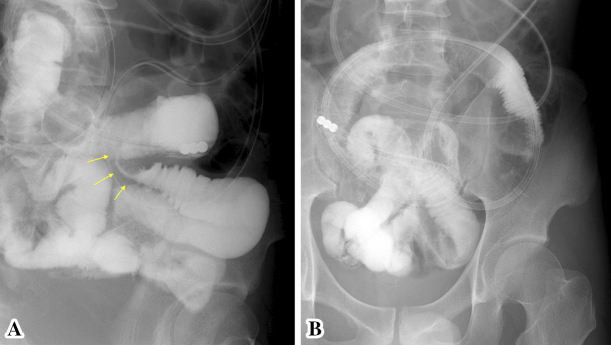
After hospitalization, he was placed on nothing per os and received intravenous infusion. Because PCT was positive, the administration of levofloxacin (500 mg/day) was started to treat a possible bacterial infection. On the next day, the patient's abdominal symptoms resolved and CRP improved rapidly. On hospital day 6, patency capsule endoscopy was performed because conditions such as Crohn's disease were suspected. On day 7, he developed abdominal fullness and an abdominal X-ray film revealed air-fluid levels in the small bowel again. Bowel obstruction due to the patency capsule was suspected, so a long tube that could reach the small bowel was inserted for decompression and small bowel imaging revealed jejunal stenosis (Fig. 3A). Small bowel imaging was repeated after a few days, and the stenosis was no longer visualized (Fig. 3B).
Figure 3.
A showed contrast-enhanced image through the ileus tube. Stenosis of the small bowel is revealed. B showed contrast-enhanced image through the ileus tube 3 days after A. The previous small bowel stenosis has resolved.
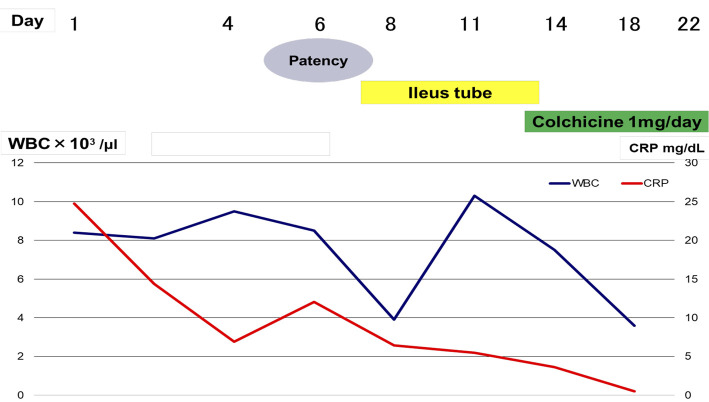
Because there were no systemic symptoms such as fever, weight loss, or joint pain, it seemed unlikely that the patient had inflammatory bowel disease like Crohn's disease. Considering the history of recurrent abdominal pain, FMF was considered to require a differential diagnosis and the oral administration of colchicine (1 mg/day) was initiated as diagnostic treatment. We summarized the clinical course in Fig. 4. Subsequently, a definite diagnosis of FMF was made because a genetic analysis revealed mutations in exons 3 and 8 of the MEFV gene (the causative gene of FMF). The patient's symptoms resolved after starting colchicine therapy, and he was thereafter successfully discharged from the hospital. At present, he is being followed as an outpatient without any recurrence of symptoms on colchicine therapy.
Figure 4.
Clinical Course.
Discussion
It is thought that there are more than 100,000 FMF patients around the world. The prevalence of this disease is higher in the Mediterranean region, where it is estimated to be 1 in 200 of the general population (2). Recently, many FMF patients have also been reported in Japan. Onset usually occurs during childhood in other countries, but patients aged over 20 years account for nearly 40% of the total in Japan and the incidence of adult FMF is higher than in other countries. The ranking of symptoms due to FMF is as follows: fever (95.5%)>abdominal pain (62.7%)>chest pain (35.8%)>joint pain (31.3%)>skin rash (7.5%). The incidence of abdominal pain is lower in Japan than in other countries. FMF is diagnosed based on the Tel-Hashomer criteria. According to these criteria, FMF is classified into two types (complete or incomplete) depending on the severity of symptoms and the grade and periodicity of fever (Table) (3).
Table.
Diagnostic Criteria for Familial Mediterranean Fever [3].
| Major criteria typical attack | Minor criteria |
|---|---|
| 1. Peritonitis (generalized) | 1. Incomplete attacks affecting one or more sites |
| 2. Pleurisy (unilateral) or pericarditis | -Abdomen |
| -Lungs | |
| -Joints | |
| 3. Monoarthritis (hip,knee,ankle) | 2. Exertion-related leg pain |
| 4. Isolated fever | 3. Response to colchicine |
| *axillary temperature >38°C, duration | |
| 12-72 h and ≥three attacks |
The diagnosis requires least one of the major criteria or at least two of the minor criteria.
The present patient had recurrent abdominal pain and the intestinal tract showed enlarged loops suggesting obstruction on abdominal CT. In addition, small bowel imaging revealed stenosis. Based on these findings, we initially suspected inflammatory bowel disease such as Crohn's disease. However, repeat small bowel imaging failed to visualize any stenosis and the patient's history included periodic attacks of abdominal pain without other symptoms such as weight loss or melena, so FMF was considered in the differential diagnosis although he only had episodic abdominal pain without typical fever. When diagnostic treatment with colchicine was initiated, his symptoms improved. Accordingly, a clinical diagnosis of incomplete FMF was made, followed by a definite diagnosis of FMF after a mutation of the MEFV gene was confirmed.
In patients with complete FMF, the duration of fever is less than 3 days and the temperature is often 38℃ or higher. On the other hand, fever may only last a few hours in incomplete FMF and the temperature may remain under 38℃. In addition, serositis is not typical and nonspecific symptoms such as joint pain and muscle pain may occur. Therefore, an MEFV gene assay is an important adjunct for making an accurate diagnosis of FMF. In patients with incomplete FMF, the frequency of an exon 10 mutation is low, while exons 1, 2, 3, and 5 are often involved. A mutation of exon 3 was noted in our patient, which is consistent with previous reports (4).
The main pathology of FMF is serosal inflammation, but severe abdominal pain associated with peritoneal irritation may also occur. In Crohn's disease, full-thickness inflammation from the mucosa to the serosa occurs throughout the whole digestive tract, including the small bowel, leading to symptoms such as severe abdominal pain. Owing to this similarity of symptoms between FMF and Crohn's disease, some FMF patients have been misdiagnosed and treated for inflammatory bowel disease. It has been reported that MEFV gene expression is increased in the inflamed bowel mucosa of patients with inflammatory bowel disease (5), suggesting the possibility that FMF may be associated with mucosal lesions as well as serosal lesions, which could make the diagnosis of FMF even more difficult.
Small bowel stenosis has already been reported in patients with FMF from outside Japan (6). Small bowel stenosis can be classified as inflammatory or tumoral depending on its etiology. When stenosis is caused by FMF, peritonitis and increased intraperitoneal exudate lead to the development of intestinal adhesions (7). In our patient, the bowel obstruction resolved spontaneously within about 3 days, suggesting that the stenosis was ascribable to mucosal swelling secondary to inflammation rather than organic obstruction, such as adhesions or a tumor.
Conclusion
We herein reported an adult patient with FMF and small bowel stenosis. If a patient presents with periodical recurrent abdominal pain, FMF should be considered in the differential diagnosis and a detailed history should be obtained.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Ogita C, Matsui K, Kisida D, et al. A retrospective analysis of 7 cases of familial mediterranean fever. Jpn J Clin Immunol 40: 21-27, 2017. [DOI] [PubMed] [Google Scholar]
- 2. Ben-Chetrit E, Touitou I. Familial Mediterranean fever in the world. Arthritis Rheum 61: 1447-1453, 2009. [DOI] [PubMed] [Google Scholar]
- 3. Livneh A, Langevitz P, Zemer D, et al. Criteria for the diagnosis of familial Mediterranean fever. Arthritis Rheum 40: 1879-1885, 1997. [DOI] [PubMed] [Google Scholar]
- 4. Ryan JG, SL Masters, MG Booty, et al. Clinical features and functional significance of the P369S/R408Q variant in pyrin, the familial Mediterranean fever protein. Ann Rheum Dis 69: 1383-1388, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Villani AC, Lemire M, Louis E, et al. Genetic variation in the familial Mediterranean fever gene (MEFV) and risk for Crohn's disease and ulcerative colitis. PLoS One 4: e7154, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ciftci AO, Tanyel FC, Büyükpamukçu N, Hiçsönmez A. Adhesive small bowel obstruction caused by familial Mediterranean fever: the incidence and outcome. J Pediatr Surg 30: 577-579, 1995. [DOI] [PubMed] [Google Scholar]
- 7. Kaşifoğlu T, Cansu DU, Korkmaz C. Frequency of abdominal surgery in patients with familial Mediterranean fever. Intern Med 48: 523-526, 2009. [DOI] [PubMed] [Google Scholar]