Abstract
The ventromedial hypothalamic nucleus (VMN) is a vital component of the neural circuitry that governs glucostasis. Norepinephrine (NE) governs VMN gluco-inhibitory γ-aminobutyric acid (GABA) and gluco-stimulatory nitric oxide (NO) transmission. Sex-specific insulin-induced hypoglycemic (IIH) patterns of VMN GABA signaling are estrogen receptor-alpha (ERα)- and -beta (ERβ)-dependent. Current research utilized combinatory immunocytochemistry, laser-microdissection, and Western blot techniques in a pharmacological approach to address the hypothesis that ERα and/or -β mediate sex-dimorphic VMN GABAergic and/or nitrergic nerve cell receptivity to NE and estradiol during IIH. The impact of these ER on expression of the pyruvate recycling pathway marker proteins glutaminase (GLS) and malic enzyme-1 (ME-1) was also examined. Both VMN neuron populations express ERα, ERβ, and G protein-coupled estrogen receptor-1 (GPER), along with alpha1, alpha2, and beta1 adrenergic receptor (AR) proteins. NO neurons exhibited ERα/β-dependent (beta1 AR, GPER) and -independent (alpha1 AR) sex differences in receptor protein responses to hypoglycemia. Similarly, sex-dimorphic effects of IIH on alpha1 AR, alpha2 AR, and ERα profiles in GABA neurons involve ERα/β. These ERs also underlie divergent adjustments in gluco-regulatory nerve cell GLS and ME-1 protein expression in hypoglycemic males and females. Sex-specific nitrergic and GABAergic nerve cell sensitivity to NE and E, respectively, during IIH may contribute to sex-contingent patterns of neurotransmitter signaling.
Keywords: laser-catapult microdissection, glutamate decarboxylase65/67, neuronal nitric oxide synthase, MPP, PHTPP, glutaminase
1. Introduction
The ventromedial hypothalamic nucleus (VMN) integrates nutrient, endocrine, and neurochemical indicators of metabolic state to shape glucose counter-regulation [Watts and Donovan, 2010; Donovan and Watts, 2014]. Insulin-induced hypoglycemia (IIH) is an unrelenting complication of requisite strict glycemic management of type I diabetes mellitus [Cryer, 2013, 2014]. In diabetes patients, hypoglycemic neuro-glucopenia poses a significant risk of neural dysfunction as energy supply is inadequate to maintain critical nerve cell functions [Auer, 1986; Auer and Siesjo, 1993]. The hypothalamus coordinates counter-active autonomic, neuroendocrine, and behavioral outflow during hypoglycemia that collectively reverses glucose decline [Chan and Sherwin, 2013]. Dedicated metabolic-sensory neurons in the VMN provide a dynamic readout of cellular energy by increasing (‘fuel-inhibited’) or decreasing (‘fuel-excited’) synaptic firing as ambient energy substrate levels fall [Oomura et al., 1969; Ashford et al., 1990; Silver and Erecinska, 1998]. Neurochemical effectors of ventromedial hypothalamic energy imbalance include γ-aminobutyric acid (GABA), which suppresses hypersecretion of glucagon and epinephrine during hypoglycemia [Chan et al., 2006], and nitric oxide (NO), which augments counter-regulatory hormone output [Fioramonti et al., 2011; Routh et al., 2014].
The catecholamine neurotransmitter norepinephrine (NE) is an important gluco-regulatory signal to the ventromedial hypothalamus, where NE levels increase in response to hypoglycemia-associated reductions in tissue glucose levels and in turn stimulate GABA release in that location [Beverly et al., 2000, 2001]. Recent studies show that exogenous NE governs the expression of biosynthetic enzyme markers of GABA (glutamate decarboxylase65/67; GAD65/67) and nitric oxide (neuronal nitric oxide synthase; nNOS) neurotransmission in the male rat VMN [Ibrahim et al., 2019]. Noradrenergic control of VMN neuron energy status is achieved, at least in part, by indirect mechanisms involving regulation of astrocyte glycogen breakdown to glucosyl units for conversion to the transportable energy substrate L-lactate [Mahmood et al., 2019]. However, NE may additionally act by direct mechanisms to control GABA and nitrergic signaling as these cells express alpha1 (α1), alpha2 (α2), and beta1 (β1) adrenoreceptor (AR) proteins [Ibrahim et al., 2019]. The gonadal steroid hormone estradiol imposes sex-contingent control of VMN gluco-regulatory signaling [Mahmood et al., 2018]. Estrogen receptor-alpha (ERα) and -beta (ERβ) act to inhibit VMN nNOS protein profiles during IIH in each sex, but promote up-regulation of GAD65/67 expression in hypoglycemic female rats only. Current research utilized high spatial-resolution microdissection/high-sensitivity molecular analytical techniques to investigate the premise that VMN GABA and/or nitrergic neurons exhibit sex-dimorphic patterns of basal and/or hypoglycemia-associated expression of one or more AR variants, and that demonstrable differences in cellular receptivity to NE are mediated by ERα and/or -β input. Here, adult testes-intact males and estradiol-implanted ovariectomized (OVX) female rats were pretreated by intracerebroventricular (icv) administration of the ERα antagonist 1,3-Bis(4-hydroxyphenyl)-4-methyl-5-[4-(2-piperidinylethoxy)phenol]-1H-pyrazole dihydrochloride (MPP), ERβ antagonist 4-[2-phenyl-5,7-bis(trifluoromethyl)pyrazolo[1,5-a]pyrimidin-3-yl]phenol (PHTPP), or vehicle prior to onset of IIH. VMN neurons identified by immunocytochemical labeling as GAD65/67- or nNOS-immunoreactive (-ir)-positive were laser-catapult microdissected for Western blot analysis of α1AR, α2AR, and β1AR proteins. Each neuron population was also evaluated for expression of ERα, ERβ, and the plasma membraneassociated ER G protein-coupled estrogen receptor-1 (GPER) proteins to determine if estradiol regulation of GABAergic and nitrergic nerve cell responsiveness to NE may involve input to these cells by one or more distinctive ER.
There is mounting interest in the concept of brain neuron metabolism of non-glucose substrates during hypoglycemia as a compensatory mechanism to offset diminished energy yield from glucose. Glutamine and glutamate are potential cerebral energy substrates during glucose deficiency as brain levels of these amino acids decline during IIH [Behar et al., 1985]. Glutaminolysis is an energy-yielding pathway that utilizes glutamine metabolism via the pyruvate recycling pathway [Cerdan, 2017] to support tricarboxylic acid (TCA) cycle function. Complete glutamine oxidation in the TCA cycle involves entrance and exit of glutamate in the form of alpha-ketoglutarate and malate, respectively, followed by processing of pyruvate to acetyl-CoA. Gluco-deprivation is postulated to up-regulate the pyruvate recycling pathway in brain neurons [Amaral, 2013]. Studies here investigated the secondary hypothesis that basal and/or hypoglycemia-associated patterns of expression of the rate-limiting pyruvate recycling pathway enzymes glutaminase (GLS) and NADP-dependent malic enzyme-1 (ME-1) in VMN GABA and nitrergic neurons may differ between male and female rats, and that sex-specific adjustments in one or both proteins in response to IIH may be mediated by ERα and/or -β signaling.
2. Results
2.1. VMN nitrergic and GABAergic neuron immunocytochemical labeling and laser-catapult Microdissection
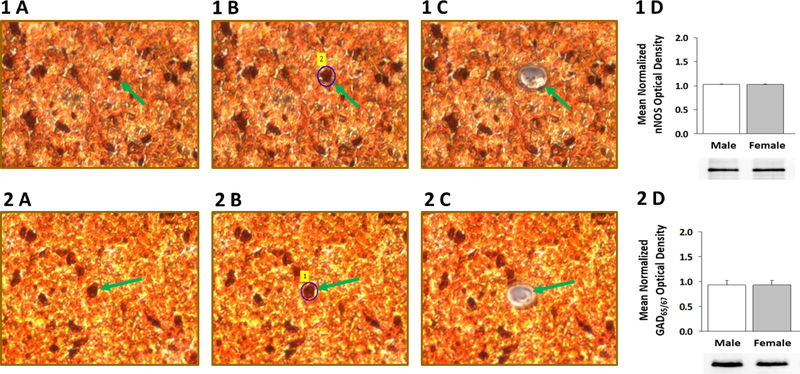
Nerve cells in VMH tissue sections were identified by nNOS- (Figure 1A) or GAD65/67-(Figure 2A)-immunoreactivity prior to laser-catapult microdissection [left-hand column]; representative labeled neurons are indicated by green arrows. Middle and right-hand columns illustrate actions, including sequential positioning of a continuous laser cut (shown in blue in Figures 1B and 2B) around individual neurons, which result in efficacious removal of each cell without destruction of surrounding tissue and minimal inclusion of adjacent tissue (Figures 1C and 2C). Immunoblots shown in in Figures 1D and 2D indicate that nNOS and GAD65/67 proteins are expressed in neurons immunostained for these respective proteins.
Figure 1. Laser-Catapult Microdissection of Immunolabeled Ventromedial Hypothalamic Nucleus (VMN) Nitrergic- or γ-Aminobutyric Acid (GABA) Neurons: Western Blot Confirmation of Accuracy of Immunocytochemical Identification of Neurotransmitter Phenotype.
VMN neurons were identified in situ for neuronal nitric oxide (nNOS)- [top row; Panel 1 A] or glutamate decarboxylase65/67 (GAD65/67)-[bottom row; Panel 2 A] immunoreactivity (-ir); representative nNOS- or GAD65/67-ir-positive neurons is indicated by green arrows. Areas shown in Panel 1 A and 2 A were re-photographed after positioning of a continuous laser track (depicted in blue) around a single nNOS-ir [Panel 1 B; green dashed arrow] or GAD65/67-ir neuron [Panel 2 B; green dashed arrow] and subsequent ejection of that cell by laser pulse [Panels 1 C and 2 C]. Note that this microdissection technique causes negligible destruction of surrounding tissue and minimal inclusion of adjacent tissue. Panels 1 D and 2 D show that nNOS or GAD65/67 protein is expressed in pure VMN nerve cell samples identified immunocytochemically for nNOS or GAD immunoreactivity, respectively.
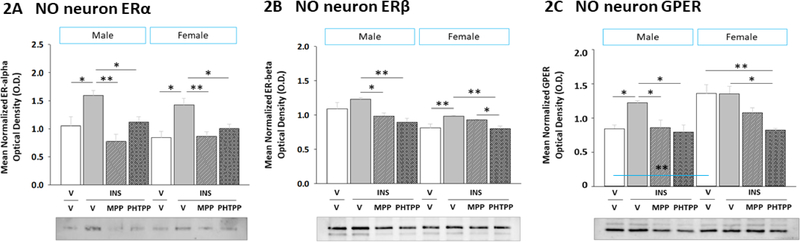
Figure 2. Effects of Intracerebroventricular (icv) Administration of the ERα Antagonist 1,3-Bis(4-hydroxyphenyl)-4-methyl-5-[4-(2-piperidinylethoxy)phenol]-1H-pyrazole dihydrochloride (MPP) or the ERβ Antagonist 4-[2-phenyl-5,7-bis(trifluoromethyl)pyrazolo[1,5-a]pyrimidin-3-yl]phenol (PHTPP) on VMN Nitrergic Neuron Estrogen Receptor-Alpha (ERα), Estrogen Receptor-Beta (ERβ), and G Protein-Coupled Estrogen Receptor (GPER) Protein Expression in Insulin-Induced Hypoglycemic (IIH) Male versus Female Rats.
Pooled lysates of laser-catapult microdissected VMN nNOS-immunoreactive (-ir) neurons created for each treatment group were evaluated by Western blot for ERα [Panel A; male data at left, female data at right], ERβ [Panel B; male data at left, female data at right], and GPER [Panel C; male data at left, female data at right] protein expression in groups of vehicle-pretreated male or female rats injected subcutaneously (sc) with either vehicle (solid white bars; n=6 males, n=6 females) or neutral protamine Hagedorn insulin (INS; 10.0 U/kg bw; solid gray bars; n=6 males, n=6 females) and groups of INS-injected animals of either sex that were pretreated with MPP (diagonal-striped gray bars; n=6 males, n=6 females) or PHTPP (cross-hatched gray bars; n=6 males, n=6 females). Data depict mean normalized protein O.D. ± S.E.M. Data were analyzed by two-way ANOVA for sex versus treatment. *p <0.05; **p <0.01; ***p <0.001.
2.2. Hypoglycemic patterns of ERα, ERβ, and GPER protein expression in VMN nitrergic neurons: regulation by ERα and ERβ
The present project investigated the premise that VMN gluco-stimulatory nitrergic and gluco-inhibitory GABAergic neurons express one or both nuclear ER and/or the membrane ER GPER, and that ERα and/or -β mediate sex-dimorphic adjustments in these receptor profiles during hypoglycemia. Figure 2 depicts ERα (Figure 2A) (Sex main effect: F(1,40)= 1.42; p = 0.25; Treatment main effect: F(3,40)= 12.74; p < 0.0001; Sex treatment interaction: F(3,40)= 0.62; p = 0.61), ERβ (Figure 2B) (Sex main effect: F(1,40)= 24.43; p < 0.0001; Treatment main effect: F(3,40)= 9.89; p < 0.0001; Sex treatment interaction: F(3,40)= 2.66; p = 0.07), and GPER (Figure 2C) (Sex main effect: F(1,40)= 13.47; p < 0.0001; Treatment main effect: F(3,40)= 10.99; p < 0.0001; Sex treatment interaction: F(3,40)= 2.83; p = 0.07) protein profiles in nNOS-immunoreactive (-ir) neurons harvested from male and female rats pretreated by icv administration of the selective ERα antagonist MPP, the selective ERβ antagonist PHTPP, or vehicle ahead of IIH. Data were analyzed by two-way ANOVA for sex versus treatment. Plasma glucose levels did not differ vehicle-treated control groups, e.g. male V/V versus female V/V (Table 1). In both male and female rats, circulating glucose was decreased to equivalent levels among INS-injected groups, irrespective of pretreatment. Data indicate that male and female V/V groups showed no sex differences in VMN NO neuron ERα or -β protein content, but elevated baseline GPER expression in females versus males (Table 2). IIH stimulated ERα and -β expression [V/INS versus V/V] in NO neurons in each sex, responses that were ER-dependent [MPP/INS and PHTTP/INS versus V/INS]. Hypoglycemia also caused an MPP- or PHTPP-preventable increase in GPER profiles in hypoglycemic males, but not females. These results indicate sex-dimorphic GPER, but not nuclear ER protein responses to IIH in VMN nitrergic neurons.
Table 1.
Impact of Estrogen Receptor-Alpha or -Beta Antagonism on Insulin-Induced Hypoglycemia in Male versus Female Rats
| Plasma Glucose Levels (mg/dL) |
||||
|---|---|---|---|---|
| Vehicle sca | Insulin sc | |||
| Vehicle icvb | Vehicle icv | MPPc icv | PHTPPd icv | |
| V/V e | V/INSf | MPP/INS | PHTPP/INS | |
| Male | 126.6 ± 5.5 | 71.4 ± 3.8g | 73.5 ± 3.2g | 66.5 ± 4.1g |
| Female | 157.4 ± 3.8 | 69.2 ± 3.6g | 64.8 ± 3.4g | 63.5 ± 3.6g |
subcutaneous
intracerebroventricular
1,3-Bis(4-hydroxyphenyl)-4-methyl-5-[4-(2-piperidinylethoxy)phenol]-1H-pyrazole dihydrochloride
4-[2-phenyl-5,7-bis(trifluoromethyl)pyrazolo[1,5-a]pyrimidin-3-yl]phenol
n=6 animals per group
insulin
p<0.001 versus V/V; data were analyzed by two-way ANOVA for sex versus treatment.
Table 2.
Summary of Effects of Insulin-Induced Hypoglycemia (IIH) on Ventromedial Hypothalamic Nucleus Nitrergic Neuron Adrenergic Receptor (AR), Estrogen Receptor (ER), and Pyruvate Recycling Enzyme Protein Expression in Male versus Female Rats: Role of ER-Alpha and ER-Beta
| MALE | FEMALE | |||||||
|---|---|---|---|---|---|---|---|---|
| Vehicle sca |
Insulin sc |
Vehicle sc |
Insulin sc |
|||||
| Vehicle icvb | Vehicle icv | MPPc icv | PHTPPd icv | Vehicle icv | Vehicle icv | MPP icv | PHTPP icv | |
| V/V n=6 | V/INSe n=6 | MPP/INS n=6 | PHTPP/INS n=6 | V/V n=6 | V/INS n=6 | MPP/INS n=6 | PHTPP/INS | |
| Alpha1-AR | N.C.f vs V/V | ↓g vs V/INS | N.C. vs V/INS | ↓ vs V/V | N.C. vs V/INS | N.C. vs V/INS | ||
| Alpha2-AR | ↑ vs V/V | ↓ vs V/INS N l | ↓ vs V/INS | ↑ vs V/V | N.C. vs V/INS | ↓ vs V/INS | ||
| Beta1-AR | ↑ vs V/V | ↓ vs V/INS N | ↓ vs V/INS N | ↓ vs V/V | ↑ vs V/INS N | ↑ vs V/INS N | ||
| ER-alpha | ↑ vs V/V | ↓ vs V/INS N | ↓ vs V/INS N | ↑ vs V/V | ↓ vs V/INS N | ↓ vs V/INS N | ||
| ER-beta | ↑ vs V/V | ↓ vs V/INS N | ↓ vs V/INS N | ↑ vs V/V | ↓ vs V/INS N | ↓ vs V/INS N | ||
| GPERh | ↑ vs V/V | ↓ vs V/INS N | ↓ vs V/INS N | ↑ vs. Male V/V | N.C. vs V/V | N.C. vs V/INS | ↓ vs V/INS | |
| MCT2h | N.C. vs V/V | N.C. vs V/INS | ↑ vs V/INS | N.C. vs V/V | N.C. vs V/INS | ↑ vs V/INS | ||
| GLSj | ↑ vs V/V | ↓ vs V/INS N | ↓ vs V/INS N | ↑ vs. Male V/V | ↓ vs V/V | ↓ vs V/INS | ↓ vs V/INS | |
| ME-1k | N.C. vs V/V | ↑ vs V/INS | ↑ vs V/INS | ↓ vs V/V | ↑ vs V/INS N | ↑ vs V/INS N | ||
subcutaneous
intracerebroventricular
1,3-Bis(4-hydroxyphenyl)-4-methyl-5-[4-(2-piperidinylethoxy)phenol]-1H-pyrazole dihydrochloride
4-[2-phenyl-5,7-bis(trifluoromethyl)pyrazolo[1,5-a]pyrimidin-3-yl]phenol
insulin
no change
arrows indicate a significant difference of at least p<0.05
G protein-coupled estrogen receptor-1
monocarboxylate transporter-2
glutaminase
malic enzyme-1
N normalized relative to V/V.
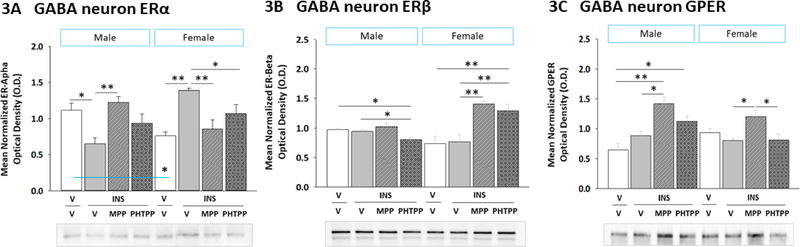
2.3. ERα and ERβ regulation of hypoglycemic patterns of ERα, ERβ, and GPER protein expression in VMN GABAergic neurons
Figure 3 illustrates effects of MPP versus PHTPP on VMN GABAergic nerve cell ERα (Figure 3A) (Sex main effect: F(1,40)= 6.68; p = 0.02; Treatment main effect: F(3,40)= 4.64; p = 0.02; Sex treatment interaction: F(3,40)= 32.73; p < 0.0001), ERβ (Figure 3B) (Sex main effect: F(1,40)= 2.99; p = 0.10; Treatment main effect: F(3,40)= 6.89; p = 0.002; Sex treatment interaction: F(3,40)= 8.17; p = 0.001), and GPER (Figure 3C) (Sex main effect: F(1,40)= 1.54; p = 0.23; Treatment main effect: F(3,40)= 13.49; p < 0.0001; Sex treatment interaction: F(3,40)= 4.32; p = 0.02) protein expression in hypoglycemic male and female rats. Data were analyzed by two-way ANOVA for sex versus treatment. Male and female V/V controls exhibited dissimilar baseline ERα profiles, as these were higher in males, but no differences in ERβ or GPER protein content (Table 3). IIH caused suppression or augmentation of GABA nerve cell ERα expression in males versus females, respectively, responses that were reversed by MPP pretreatment. While IIH did not modify GABAergic neuron ERβ or GPER profiles in either sex, data show that ERβ imposes a stimulatory or inhibitory tone on ERβ levels in males versus females, whereas ERα input exerts a positive tonus on GPER content in both sexes. Overall effects of IIH on ER and GPER expression in VMN nitrergic neurons render this population sensitive to estradiol in each sex, whereas GABA neurons become less receptive to estradiol in males, but more sensitive to this hormone in females.
Figure 3. Effects of ERα or ERβ Blockade on VMN GABAergic Nerve Cell ERα, ERβ, and GPER Protein Expression in Hypoglycemic in Male versus Female Rats.
VMN GAD65/67-ir neurons were evaluated by Western blot for ERα [Panel A; male data at left, female data at right], ERβ [Panel B; male data at left, female data at right], and GPER [Panel C; male data at left, female data at right] protein expression in vehicle-pretreated euglycemic controls (solid white bars; n=6 males, n=6 females) controls and vehicle- (solid gray bars; n=6 males, n=6 females), MPP- (diagonal-striped bars; n=6 males, n=6 females), or PHTPP (cross-hatched gray bars; n=6 males, n=6 females)-pretreated INS (10.0 U/kg bw, sc) - injected animals. Data depict mean normalized protein O.D. measures ± S.E.M. Data were analyzed by two-way ANOVA for sex versus treatment. *p <0.05; **p <0.01; ***p <0.001.
Table 3.
Summary of Effects of Insulin-Induced Hypoglycemia (IIH) on Ventromedial Hypothalamic Nucleus GABAergic Neuron Adrenergic Receptor (AR), Estrogen Receptor (ER), and Pyruvate Recycling Enzyme Protein Expression in Male versus Female Rats: Role of ER-Alpha and ER-Beta
| MALE |
FEMALE |
|||||||
|---|---|---|---|---|---|---|---|---|
| Vehicle sca |
Insulin sc |
Vehicle sc |
Insulin sc |
|||||
| Vehicle icv b | Vehicle icv | MPPc icv | PHTPPd icv | Vehicle icv | Vehicle icv | MPP icv | PHTPP icv | |
| V/V n=6 | V/INSe n=6 | MPP/INS n=6 | PHTPP/INS n=6 | V/V n=6 | V/INS n=6 | MPP/INS n=6 | PHTPP/INS n=6 | |
| Alpha1-AR | ↓f vs V/V | ↑ vs V/INS Ng | N.C.h vs V/INS | N.C. vs V/V | ↑ vs V/INS | N.C. vs V/INS | ||
| Alpha2-AR | N.C. vs V/V | N.C. vs V/INS | N.C. vs V/INS | ↓ vs V/V | ↑ vs V/INS N | ↑ vs V/INS N | ||
| Beta1-AR | ↓ vs V/V | ↑ vs V/INS N | N.C. vs V/INS | ↓ vs V/V | N.C. vs V/INS | N.C. vs V/INS | ||
| ER-alpha | ↓ vs V/V | ↑ vs V/INS N | N.C. V/INS | ↓ vs. Male V/V | ↑ vs V/V | ↓ vs V/INS N | ↓ vs V/INS N | |
| ER-beta | ↑ vs V/V | ↓ vs V/INS N | ↓ vs V/INS N | ↑ vs V/V | ↓ vs V/INS N | ↓ vs V/INS N | ||
| GPERi | N.C. vs V/V | N.C. vs V/INS | ↓ vs V/INS | N.C. vs V/V | ↑ vs V/INS | N.C. vs V/INS | ||
| MCT2j | N.C. vs V/V | N.C. vs V/INS | ↑ vs V/INS | ↑ vs V/V | N.C. vs V/INS | ↑ vs V/INS | ||
| GLSk | ↓ vs V/V | ↑ vs V/INS N | N.C. vs V/INS N | ↓ vs V/V | N.C. vs V/INS | ↑ vs V/INS N | ||
| ME-1l | ↑ vs V/V | N.C. vs V/INS | ↓ vs V/INS N | N.C. V/INS | N.C. vs V/INS | ↑ vs V/INS | ||
subcutaneous
intracerebroventricular
1,3-Bis(4-hydroxyphenyl)-4-methyl-5-[4-(2-piperidinylethoxy)phenol]-1H-pyrazole dihydrochloride
4-[2-phenyl-5,7-bis(trifluoromethyl)pyrazolo[1,5-a]pyrimidin-3-yl]phenol
insulin
arrows indicate a significant difference of at least p<0.05
N normalized relative to V/V
no change
G protein-coupled estrogen receptor-1
monocarboxylate transporter-2
glutaminase
malic enzyme-1
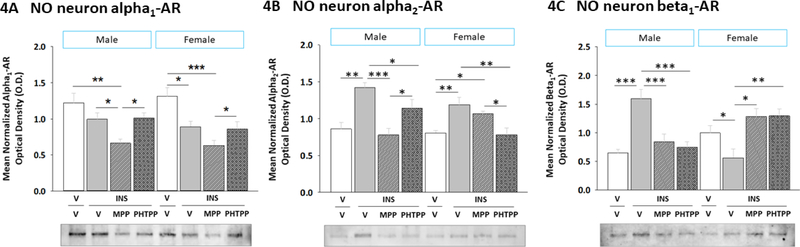
2.4. Effects of IIH on α1AR, α2AR, and β1AR protein expression in VMN nitrergic neurons: regulation by ERα and ERβ
Current research investigated whether ERα- and/or -β mediate sex-dimorphic effects on adrenergic receptor (AR) protein profiles in VMN gluco-regulatory neuron populations during IIH. Data in Figure 4 depict effects of icv MPP or PHTPP delivery on hypoglycemia-associated patterns of α1AR (Figure 4A) (Sex main effect: F(1,40)= 0.58; p = 0.45; Treatment main effect: F(3,40)= 13.95; p < 0.0001; Sex treatment interaction: F(3,40)= 0.59; p = 0.62), α2AR (Figure 4B) (Sex main effect: F(1,40)= 2.4; p = 0.13; Treatment main effect: F(3,40)= 9.82; p < 0.0001; Sex treatment interaction: F(3,40)= 2.42; p = 0.01), and β1AR (Figure 4C) (Sex main effect: F(1,40)= 25.92; p < 0.000; Treatment main effect: F(3,40)= 3.36; p = 0.03; Sex treatment interaction: F(3,40) = 14.79; p < 0.0001) protein expression in VMN nNOS-ir neurons harvested from testes-intact male or estradiol-implanted OVX female rats. Data were analyzed by two-way ANOVA for sex versus treatment. NO neuron α1AR, α2AR, or β1AR protein levels were equivalent between V/V males and V/V females. IIH did not modify α1AR levels in males, but decreased this profile in females. In hypoglycemic rats, MPP decreased α1AR protein expression compared to V-pretreated animals [males: MPP/INS versus V/V and V/INS; females: MPP/INS versus V/V]. Nitrergic nerve cell α2AR protein levels were elevated during IIH [V/INS versus V/V] in both sexes; this protein profile was normalized by MPP in males or by PHTPP pretreatment in females. IIH caused opposing adjustments in β1AR expression in hypoglycemic male versus female rats. In each sex, this response was prevented by either MPP or PHTPP pretreatment. Data reveal that VMN NO neurons exhibit ER-dependent β1AR and ER-independent α1AR protein responses to hypoglycemia. Hypoglycemia may contribute to increased nitregic neuron sensitivity to NE in male, but decreased receptivity to NE in females.
Figure 4. Role of ERα and ERβ in Sex-Specific VMN Nitrergic Neuron Alpha1 Adrenergic Receptor (α1AR), Alpha2 AR (α2AR), and Beta1 AR (β1AR) Protein Responses to IIH.
Bars depict for each sex mean normalized nitrergic neuron α1AR [Panel A], α2AR [Panel B], and β1AR [Panel C] protein O.D. measures ± S.E.M. according to the following treatment groups: V/V (white bars; n=6 males, n=6 females), V/INS (10.0 U/kg bw, sc; solid gray bars; n=6 males, n=6 females), MPP/INS (diagonal-striped gray bars; n=6 males, n=6 females), and PHTPP/INS (cross-hatched gray bars; n=6 males, n=6 females). Data were analyzed by two-way ANOVA for sex versus treatment. *p<0.05; **p<0.01; ***p<0.001.
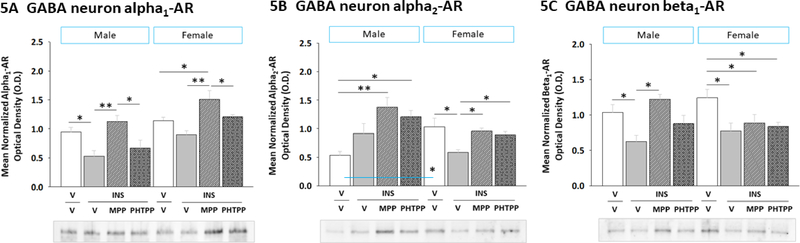
2.5. ERα and ERβ regulation of hypoglycemic patterns of α1AR, α2AR, and β1AR protein expression in VMN GABAergic neurons
Figure 5 illustrates effects of MPP versus PHTPP on VMN GABAergic nerve cell α1AR (Figure 5A) (Sex main effect: F(1,40)= 22.47; p < 0.000; Treatment main effect: F(3,40)= 10.13; p < 0.0001; Sex treatment interaction: F(3,40)= 0.74; p = 0.53), α2AR (Figure 5B) (Sex main effect: F(1,40)= 2.33; p = 0.14; Treatment main effect: F(3,40)= 5.05; p = 0.009; Sex treatment interaction: F(3,40)= 5.63; p = 0.006), and β1AR (Figure 5C) (Sex main effect: F(1,40)= 0.044; p = 0.96; Treatment main effect: F(3,40)= 6.94; p = 0.002; Sex treatment interaction: F(3,40)= 4.19; p = 0.03) protein expression in hypoglycemic male and female rats. Data were analyzed by two-way ANOVA for sex versus treatment. V/V males exhibited lower α2AR protein levels compared to V/V females, whereas α1AR and β1AR profiles were equivalent between sexes. MPP reversed hypoglycemic suppression of GABA neuron α1AR profiles in male rats [MPP/I versus V/I], whereas either MPP or PHTPP pretreatment averted this decline in females. Hypoglycemic diminution of GABA neuron β1AR protein content involved ER signaling in males, but not females. Results point to ER involvement in sex-dimorphic hypoglycemic effects on GAD-ir nerve cell α1AR and α2AR protein expression. Hypoglycemia-associated patterns of AR protein expression in VMN GABA neurons may result in diminished responsiveness to NE in both sexes.
Figure 5. Impact of ERα or ERβ Antagonism on VMN GABAergic Nerve Cell α1AR, α2AR, and β1AR Protein Expression in Hypoglycemic Male versus Female Rats.
Data illustrate for each sex mean normalized GABAergic neuron α1AR [Panel A], α2AR [Panel B], and β1AR [Panel C] protein O.D. measures ± S.E.M. for groups of male and female rats treated as follows: V/V (white bars; n=6 males, n=6 females), V/INS (10.0 U/kg bw, sc; solid gray bars; n=6 males, n=6 females), MPP/INS (diagonalstriped gray bars; n=6 males, n=6 females), and PHTPP/INS (cross-hatched gray bars; n=6 males, n=6 females). Data were analyzed by two-way ANOVA for sex versus treatment. *p<0.05; **p<0.01; ***p<0.001.
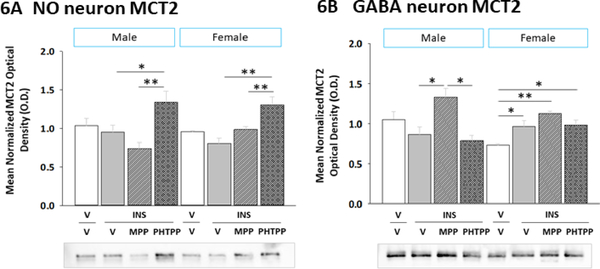
2.6. ERα and ERβ regulation of VMN NO and GABA neuron MCT2 protein expression during IIH
Neuronal uptake of the oxidizable energy fuel L-lactate is achieved by MCT2 transporter function. Here, effects of ERα or ERβ blockade on VMN NO (Figure 6A) (Sex main effect: F(1,40)= 0.002; p = 0.961; Treatment main effect: F(3,40)= 10.36; p = 0.002; Sex treatment interaction: F(3,40)= 1.72; p = 0.19), and GABA (Figure 6B) (Sex main effect: F(1,40)= 1.06; p = 0.32; Treatment main effect: F(3,40)= 9.65; p = 0.001; Sex treatment interaction: F(3,40)= 5.31; p = 0.01) nerve cell MCT2 protein content were evaluated by two-way ANOVA for sex versus treatment (Figure 6). Baseline MCT2 expression in both neuron populations was similar among male and female subjects. IIH did not alter this protein profile in either gluco-regulatory neuron population in either sex.
Figure 6. Effects of MPP or PHTPP Pretreatment on VMN Nitrergic and GABAergic Neuron Monocarboxylate Transporter-2 (MCT2) Protein Expression in Hypoglycemic Male versus Female Rats.
Pooled lysates of nNOS- or GAD-ir neurons were created for each treatment group for Western blot analysis of MCT2 expression. Data show nitrergic [Panel A] and GABAergic [Panel B] neuron mean normalized MCT2 protein O.D. measures ± S.E.M. for groups of male (at left) and female (at right) animals treated as follows: V/V (n=6 males, n=6 females), V/INS (10.0 U/kg bw, sc; n=6 males, n=6 females), MPP/INS (n=6 males, n=6 females), and PHTPP/INS (n=6 males, n=6 females). Data were analyzed by two-way ANOVA for sex versus treatment. *p<0.05; **p<0.01; ***p<0.001.
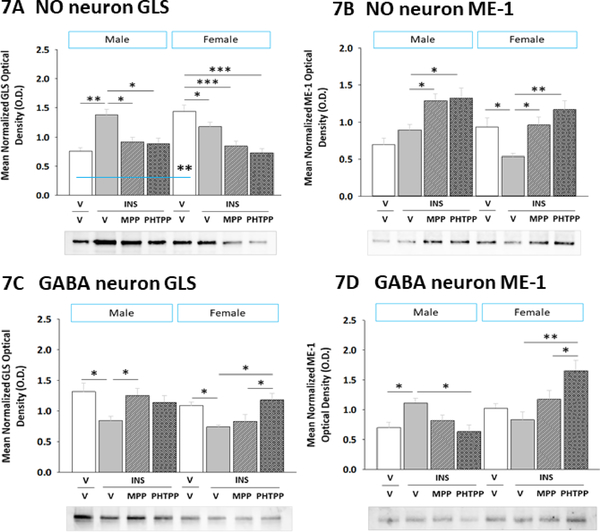
2.7. Effects of IIH on VMN NO and GABA neuron GLT and ME-1 protein profiles: regulation by ERα and ERβ
Present studies investigated the premise that VMN gluco-regulatory neurons express the pyruvate recycling pathway biomarker enzyme proteins GLS and ME-1, and that ER may mediate sex differences in these protein profiles during hypoglycemia. Data were analyzed by two-way ANOVA for sex versus treatment. In Figure 7, data show that baseline nNOS-ir neuron GLS (Figure 7A) (Sex main effect: F(1,40)= 0.28; p = 0.600; Treatment main effect: F(3,40)= 6.84; p = 0.001; Sex treatment interaction: F(3,40)= 10.85; p < 0.0001), but not ME-1 (Figure 7B) (Sex main effect: F(1,40)= 3.72; p = 0.066; Treatment main effect: F(3,40)= 11.74; p = 0.001; Sex treatment interaction: F(3,40)= 3.36; p = 0.03) protein content was elevated in V/V females versus V/V males. IIH caused MPP- or PHTPP-reversible augmentation of GLS profiles in nitrergic nerve cells in male, but not female rats. Conversely, NO nerve cell ME-1 content was diminished by ER-dependent mechanisms in females, but not males during hypoglycemia. Figures 7C and 7D illustrate GLS (Sex main effect: F(1,40)= 3.36; p = 0.08; Treatment main effect: F(3,40)= 5.13; p = 0.007; Sex treatment interaction: F(3,40)= 1.50; p = 0.23) and ME-1 (Sex main effect: F(1,40)= 25.91; p < 0.0001; Treatment main effect: F(3,40)= 3.35; p = 0.034; Sex treatment interaction: F(3,40)= 14.79; p < 0.0001) patterns of expression, respectively, in VMN GABAergic neurons. Data show that ERα suppresses GLS in hypoglycemic males, whereas ERβ inhibits this profile in females. Meanwhile, GABA neuron ME-1 profiles were elevated by ERβ signaling in hypoglycemic males, but were refractory to hypoglycemia in females. These results implicate ER in sex differences in GLS and ME-1 expression protein expression in nitrergic and GABAergic neurons, in that order, during hypoglycemia.
Figure 7. Effects of MPP or PHTPP Pretreatment on VMN Nitrergic and GABAergic Neuron Glutaminase (GLS) and Malic Enzyme-1 (ME-1) Protein Expression in Hypoglycemic Male versus Female Rats.
Pooled lysates of nNOS- or GAD-ir neurons were created for each treatment group for Western blot analysis of GLS and ME-1. Data depict nitrergic nerve cell GLS [Panel A] and ME-1 [Panel B] and GABAergic neuron GLS [Panel C] and ME-1 [Panel D] profiles in groups of male (at left) and female (at right) rats treated by V/V (n=6 males, n=6 females), V/INS (10.0 U/kg bw, sc; n=6 males, n=6 females), MPP/INS (n=6 males, n=6 females), or PHTPP/INS (n=6 males, n=6 females). Bars indicate mean normalized protein O.D. measures ± S.E.M. Data were analyzed by two-way ANOVA for sex versus treatment. *p<0.05; **p<0.01; ***p<0.001.
3. Discussion
This research addressed the hypothesis that in each sex, VMN gluco-stimulatory NO and gluco-inhibitory GABA neurons are direct substrates for NE and estradiol action, and that ERα and/or -β impose sex-dimorphic control of AR, ER, and pyruvate recycling pathway enzyme protein expression in these distinctive brain cell populations during IIH. Results show that multiple AR (alpha1, alpha2, and beta1 AR) and ER (ERα, ERβ, and GPER) proteins are measurable in each neuron population. NO neurons exhibited ERα/β-dependent (beta1 AR, GPER) and -independent (alpha1 AR) sex differences in receptor protein responses to IIH, while sex-dimorphic hypoglycemic regulation of alpha1 AR, alpha2 AR, and ERα profiles in GAD cells involves ERα/β. In male rats, dissimilar effects of IIH on nitrergic versus GABAergic neuron AR and ER expression may render NO neurons more sensitive to NE and estradiol, while diminishing GABA nerve cell receptivity to these signals. In females, on the other hand, hypoglycemia-associated changes in NO and GABA neuron ER and AR receptor profiles may cause both cell populations to become more sensitive to estradiol but less responsive to NE. Sex-specific ERα/β-dependent adjustments in gluco-regulatory nerve cell GLS protein content imply that amino acid oxidation may be correspondingly up- or down-regulated in NO neurons in hypoglycemic male versus female rats, whereas GABA neurons in the male may generate pyruvate from substrates other than glutamate and glutamine.
Current studies present novel proof that VMN nitrergic and GABAergic neurons are direct substrates for estradiol action, likely involving ER-specific signal transduction mechanisms. Data show that ERα/β signaling drives IIH up-regulation of NO nerve cell ERα and -β expression in each sex, but enhances GPER profiles in males only. In GABA neurons, IIH reduced (male) or elevated (females) ERα content, but not ERβ or GPER protein profiles. Data support the view that IIH may increase VMN nitrergic neuron sensitivity to estradiol in both sexes, but impair (males) or augment (females) GABA neuron receptiveness to estradiol. Outcomes do not reveal if hypoglycemia alters expression of nuclear versus plasma membrane ERα and -β proteins in these neurons, nor it is clear whether estradiol regulates gene transcription in these cells by genomic, e.g. direct/indirect control of gene expression through nuclear ER contact with DNA estrogen response elements or modulation of activity of other transcription factors, and/or non-genotropic mechanisms, e.g. rapid cytoplasmic or nuclear hormone actions mediated by plasma membrane-associated nuclear ER control of signal transduction pathway activity. Since VMN neurons were reported to contain ERα but not ERβ mRNA, while both gene transcripts are expressed in other hypothalamic metabolic loci that project to the VMN [Shughrue et al., 1997], the experimental design used here involved icv administration of MPP or PHTPP to deliver these drugs to ERs expressed in the VMN as well as upstream/afferent structures. Thus, it is possible that observed effects of either ER antagonist may partially reflect actions on extra-VMN ER-expressing targets in addition to VMN nitrergic or GABA neurons.
VMN NO neurons exhibited up-regulated α2AR profiles in both sexes during hypoglycemia, alongside divergent sex-specific adjustments in α1 (unaltered in males/decreased in females) and β1AR (increased in males/decreased in females) protein profiles. In these neurons, ERs mediate sex-contingent β1AR and sex-independent α2AR responses to IIH. VMN GABA neurons showed sex-specific changes in α1AR (decreased in males/no change in females) and α2AR (no change in males/decrease in females) proteins during IIH, as well as sex-unrelated suppression of β1AR expression. In males, ERα signaling drives hypoglycemic suppression of GABA neuron α1AR and β1AR protein profiles, whereas ERα/β mediate diminution of GABA α2AR but not β1AR profiles in females. Collectively, these data imply that hypoglycemia may blunt VMN GABAergic neuron sensitivity to NE in each sex. Evidence that VMN GAD65/67 expression is up-regulated in hypoglycemic female rats [Mahmood et al., 2018] may signify enhanced AR-initiated downstream signal transduction activity in GABA neurons in that sex.
Glutaminolysis of the amino acids glutamine and glutamate provides carbon to maintain TCA activity during waning glucose metabolism [Lewis et al., 1974; Behar et al., 1985; Amaral et al., 2011]. Upon GLS-catalyzed hydrolysis of glutamine to glutamate, the glutamate carbon skeleton enters and exits the TCA cycle in the form of alpha-ketoglutarate and malate/oxaloacetate, respectively. ME-1 action completes pyruvate recycling by converting malate to pyruvate. Data here show that IIH acts via ERα/β signaling to stimulate (males) or inhibit (females) VMN NO nerve cell GLS protein expression; these ERs also suppress ME-1 profiles in females. These findings suggest that in males, hypoglycemia may augment glutaminolysis and subsequent diversion of glutamine and glutamate for energy production, whereas regeneration of pyruvate from these amino acids may be suppressed in females. It remains unclear if and how sex-contingent adjustments in glutaminolysis impact gauges of neuronal metabolic stability, for example, AMP-activated protein kinase. Current data also show that IIH suppressed GLS profiles in VMN GABAergic neurons in each sex, but did enhance GABA ME-1 protein expression in male rats alone. The latter findings imply that during hypoglycemia, GABA neurons in males may utilize malate derived independently of glutamine for re-entry into the TCA. Further work is needed to examine whether AR input to these neuron populations regulates glutaminolysis and pyruvate recycling during IIH.
Neuronal aerobic respiration is supported by astrocyte-to-nerve cell trafficking of the oxidizable glycolytic end-product L-lactate [Pellerin et al., 1998]. Glial (MCT1)- and neuron (MCT2)-specific monocarboxylate transporters (MCT) transfer this substrate fuel between cell compartments [Broer et al., 1997]. Ventromedial hypothalamic lactate deficiency enhances glucose counter-regulation as local lactate infusion suppresses glucagon and catecholamine secretion during hypoglycemia [Borg et al., 2003], by mechanisms involving intensified GABAergic transmission [Chan et al., 2006]. IIH diminishes whole-VMN MCT2 levels in male, but not female rats, suggestive of sex differences in net nerve cell lactate uptake in this structure [Mahmood et al., 2018]. Current findings that VMN nitrergic neuron MCT2 protein levels were unaffected by IIH in either sex infer that rate of lactate uptake by these cells, at least over the current time frame between insulin injection and sacrifice, is not different from euglycemia. Additional work is needed to determine if lactate utilization and NO nerve cell energy stability are correlated. In contrast, hypoglycemia increased MCT2 protein expression in VMN GABAergic neurons from female, but not male rats; interestingly, up-regulated MCT2 in the former sex was not mediated by ERα/β signaling. These findings underscore the importance of investigative approaches that permit discriminative analysis of individual nerve cell populations, as region-wide measurements are likely to obscure differential neurotransmitter-specific responses.
ERα/β signaling is reported to inhibit VMN nNOS protein expression in both sexes, whereas ERβ alone up-regulates GAD65/67 profiles in the female VMN [Mahmood et al., 2018]. Further studies are needed to determine if NO nerve cell ERα and -β mediate this decline in nNOS as both ER profiles are up-regulated by hypoglycemia. Hypoglycemic repression of gluco-stimulatory NO signaling likely infers a positive gain in energy state in NO neurons; however, further experiments are required to verify this assumption. Current outcomes imply that enhanced glutamine utilization for energy production may contribute, in part, to nitrergic neuron energy stability in males, but not females. Mechanisms of ERβ augmentation of GAD65/67 protein in female rat GABA neurons are less clear as no AR or ER protein profile in GABA neurons is regulated by ERβ alone. A possible explanation is that ERα and/or GPER signaling may be a passive requirement for ERβ stimulation of this biosynthetic enzyme profile.
Current research did not determine how MPP or PHTPP might affect patterns of AR or ER protein expression in the absence of hypoglycemia. Thus, data do not shed light on whether, in each sex, ERα and -β regulation of NO or GABA neuron sensitivity to NE and estradiol differs during eu- versus hypoglycemia. The likelihood that observed drug effects reported here may reflect, to some degree, additive effects of IIH and ER antagonist cannot be discounted. Present findings do not identify the ligand(s) that activates VMN nerve cell ERα and -β signaling in either sex. While it is plausible that these receptors interact with estradiol metabolized from testosterone by aromatase enzyme action in both the periphery and VMN, these ER may also be activated by ligand derived by local de novo neurosteroid synthesis.
In summary, VMN neurons characterized by gluco-stimulatory or -inhibitory function were obtained by high-resolution cellular-level microdissection techniques from male and female rats for characterization of ERα and -β regulation of adrenergic and estrogen receptor, amino acid oxidation pathway enzyme, and aerobic respiration fuel transporter protein expression during IIH. These ERs exert sex-dimorphic effects on hypoglycemic patterns of AR subtype protein expression in nitrergic and GABAergic neurons, and govern sex-specific GLS and ME-1 protein profiles in hypoglycemic male versus female rats. In male rats, nitrergic and GABAergic neurons may become correspondingly more or less sensitive to both NE and estradiol during hypoglycemia, whereas both nerve cell populations may exhibit increased receptivity to estradiol, alongside decreased responsiveness to NE in females. Differential receptivity to these critical regulatory stimuli, supported by present outcomes, likely contribute to sex-dirmorphic patterns of gluco-regulatory transmitter signaling during IIH.
4. Experimental Procedure
4.1. Experimental Design
Adult male and female Sprague Dawley (3–4 months of age; Envigo, Houston, TX) were housed in shoe-box cages containing Aspen Sani chip bedding (Envigon), 2–3 per cage, according to sex, under a 14 hr light/10 hr dark cycle (lights on at 05.00 h). Animals had free access to standard laboratory rat chow and water, and were acclimated to daily handling. All surgical and experimental protocols were conducted in accordance with NIH guidelines for care and use of laboratory animals, and approved by the ULM Institutional Animal Care and Use Committee [IACUC approval number: 16AUG-KPB-1]. Surgeries were performed under aseptic, sterile conditions in the College of Pharmacy Vivarium rat surgery suite, between 13.00 and 16.00 hr on scheduled days. On day 1, animals were implanted with a PE-20 cannula into the left lateral ventricle (LV) [Singh and Briski, 2005], under ketamine/xylazine (0.1 mL/100 g bw; 90 mg ketamine:10 mg xylazine/mL; Henry Schein Inc., Melville, NY) anesthesia. While under anesthesia, females were also bilaterally OVX [Briski and Sylvester, 1988]. After surgery, rats were injected subcutaneously (sc) with ketoprofen (1 mg/kg body weight) and intramuscularly with enrofloxacin (10 mg/0.1 mL), treated by topical application of 0.25% bupivacaine to closed incisions, then transferred to individual cages. On day 7, female rats were implanted with a sc silastic capsule (i.d. 0.062 in/o.d. 0.125 in.; 10 mm/100 g bw) containing 30 ug 17β estradiol-3-benzoate/mL safflower oil, under isoflurane anesthesia. This steroid replacement regimen yields approximate plasma E concentrations of 22 pg/ml [Briski et al., 2001], replicating circulating hormone levels characteristic of metestrus in 4-day cycling animals [Butcher et al., 1974]. The current experimental design sought to standardize plasma estradiol levels in female subjects to avoid potential variability due to differences in endogenous circulating hormone levels at individual stages of the estrous cycle. Animals were randomly assigned to treatment groups. At either 08.45 or 08.55 hr on day 10, animals of each sex were divided into four treatment groups, and injected to the LV with the vehicle dimethyl sulfoxide (V) (groups 1 and 2; n=6 male and n-6 females/group), the ERα antagonist MPP (10 μM/200 nL [Mahmood et al., 2018]; Tocris/Bio-Techne Corp., Minneapolis, MN) (group 3; n=6 males, n=6 females), or the ERβ antagonist PHTPP (10 μM/200 nL [Mahmood et al., 2018]; Tocris) (group 4; n=6 males, n=6 females). At 9:00 or 09.10 hr on day 10, animals in group 1 were injected sc with sterile insulin diluent (V; Eli Lilly & Co., Indianapolis, IN); at the same time, groups 2–4 were treated by injection of neutral protamine Hagedorn insulin (INS; 10.0 U/kg bw; Butler Schein Animal Health, Dublin, OH). Drug administration occurred in the absence of anesthesia. Rats were sacrificed at 10:00 or 10.10 hr by rapid decapitation in the absence of anesthesia outside their housing room; uniform timing between icv pretreatment and INS injection (15 min) and between INS injection and sacrifice (1 hr) was maintained throughout the experiment. No animals were excluded from the study due to surgical complications. Each brain was individually snap-frozen in liquid nitrogen-cooled isopentane for storage at −80°C. Plasma was stored at −20°C.
4.2. VMN GABA and NO Neuron Immunolabeling and Laser-Catapult Microdissection
A series of ten consecutive μm-thick frozen sections were cut at successive 300 μm intervals over the length of the VMN, and mounted on PEN membrane-coated slides (Carl Zeiss Microscopy, LLC, Thornwood, NY). After acetone fixation, washing in Tris-buffered saline (TBS; Sigma Aldrich, St. Louis, MO), pH 7.4, and blocking with 5.0% normal goat serum (Vector Laboratories, Inc., Burlingame, CA) in TBS containing 0.05% Triton X-100 (TX-100), immunocytochemical identification of GABAergic or nitrergic was accomplished by 48 hr (4°C) incubation of tissues with rabbit primary antibodies raised against GAD65/67 (prod. no. ABN904, 1:1500; MilliporeSigma, Burlington, MA) or nNOS (prod. no. NBP1–39681, 1:500; Novus Biologicals, LLC, Littleton, CO) [Ibrahim et al., 2019]. After sequential 1 hr exposure to goat anti-rabbit biotinylated secondary antibody (prod. no. BA-1000, 1:000, Vector Laboratories, Burlingame, CA) diluted in 1.5% normal goat serum in TBS containing 0.05% TX-100 (Sigma Aldrich), followed by RTU Vectastain Elite ABC-HRP reagent (prod. no. PK-7100, Vector Lab.) incubation, GAD65/67 and nNOS-immunoreactive (ir)-positive neurons were visualized using Vector ImmPACT DAB peroxidase substrate kit reagents (prod. no. SK-4105; Vector Lab.). Individual immunolabeled neurons were harvested from sections using a Zeiss P.A.L.M. UV-A microlaser IV, and collected into lysis buffer (2.0% sodium dodecyl sulfate (SDS; VWR Intl., Radnor, PA), 0.05 M dithiothreitol (Sigma Aldrich), 10.0% glycerol (Sigma Aldrich), 1.0 mM EDTA (Sigma Aldrich), 60 mM Tris-HCl (Sigma Aldrich), pH 7.2).
4.3. GABA and NO Neuron Western Blot Analyses
Within each treatment group, triplicate pools of n=50 GAD65/67- or nNOS-ir nerve cell lysates were prepared for separation of individual target proteins in BioRad TGX 10–12% stain-free gels (prod. no. 161–0183, Bio-Rad Laboratories Inc., Hercules CA) [Ibrahim et al., 2019]. After electrophoresis, gels were UV light-activated (1 min) in a Bio-Rad ChemiDoc TM Touch Imaging System before transblotting (30 V, overnight at 4°C; Towbin buffer) to 0.45-μm PVDF membranes (ThermoFisherScientific; Waltham, MA). After blocking with Tris-buffer saline (TBS), pH 7.4, containing 0.1% Tween-20 (VWR) and 2% bovine serum albumin (MP Biomedicals, LLC, Solon, OH), membranes were incubated overnight (4°C) with primary antisera raised in rabbit against α1AR/ADRA1A (prod. no. NB100–78585, 1:1,000; Novus Biol.), α2AR/ADRA3A (prod. no. NBP2–22452, 1:1,000; Novus Biol.), ERβ/NR3A2 (prod. no. NB120–3577, 1:1,000; Novus Biol.), GPER/GPR30 (prod. no. NLS 4271, 1:1,000; Novus Biol.), monocarboxylate transporter-2 (MCT2) (AB3540P, 1:1500; MilliporeSigma), GLS2 (NBP1–89766, 1:1000; Novus Biol.) or ME1 (NBP1–32398, 1:1200; Novus Biol.); in mouse against ERα/NR3A1 (prod. no. NB300560, 1:1,000; Novus Biol.), or in goat against β1AR/ADRB1 (prod. no. NB600–978, 1:2,000; Novus Biol.). Membranes were then incubated for 1 hr with peroxidase-conjugated goat anti-rabbit (prod. no. NEF812001EA, 1:5000; PerkinElmer, Waltham, MA), rabbit anti-goat (prod. no. AP106P, 1:5000; MilliporeSigma) or goat anti-mouse (prod. no. NEF822001EA, 1:6000; PerkinElmer) secondary antibodies before exposure to Supersignal West Femto chemiluminescent substrate (prod. no. 34095; ThermoFisherScientific, Rockford, IL). Membrane buffer washes and antibody incubations were carried out by Freedom Rocker™ Blotbot® automation (Next Advance, Inc., Troy NY). Chemiluminescence band optical density (O.D.) values were normalized to total in-lane protein using Image Lab™ 6.0.0 software (Bio-Rad). Precision plus protein molecular weight dual color standards (prod. no. 161–0374, Bio-Rad) were included in each Western blot analysis. Verification of accurate collection of nNOS- and GAD65/67-ir was performed by Western blot analysis of nNOS or GAD65/67 protein expression using antibodies described above.
4.4. Blood Glucose Measurements
Trunk blood glucose was measured with an ACCU-CHECK Aviva Plus glucometer (Roche Diagnostics USA, Indianapolis, IN), as described [Kale et al., 2006].
4.5. Statistics
Mean glucose values and normalized protein O.D. data were analyzed between groups by two-way ANOVA and Student-Newman-Keuls post hoc test. Differences of p<0.05 were considered significant. Graphical representation was constructed using Sigma plot 10.0.3.
Highlights.
Norepinephrine acts on the ventromedial hypothalamic nucleus (VMN) to control counter-regulation.
Estrogen receptor-alpha (ERα)- and -beta (ERβ) control VMN gluco-inhibitory signaling.
ERα- or -β antagonist was delivered to rats of each sex before insulin-induced hypoglycemia (IIH).
VMN γ-aminobutyric acid (GABA) and nitric oxide (NO) neurons were analyzed by Western blot.
GABA and NO neurons exhibit ER-dependent sex-specific reactivity to NE and estradiol during IIH.
5. Acknowledgements
This research was funded by National Institutes of Health grant DK 109382.
Abbreviations
- α1AR
alpha1 adrenergic receptor
- α2AR
alpha2 adrenergic receptor
- β1AR
beta1 adrenergic receptor
- ERα
estrogen receptor-alpha
- ERβ
estrogen receptor-beta
- GABA
γ-aminobutyric acid
- GAD65/67
glutamate decarboxylase65/67
- GLT
glutaminase
- GPER
G protein-coupled estrogen receptor 1
- INS
insulin
- IIH
insulin-induced hypoglycemia
- MCT2
monocarboxylate transporter-2
- ME-1
NADP-dependent malic enzyme-1
- MPP
1,3-Bis(4-hydroxyphenyl)-4-methyl-5-[4-(2-piperidinylethoxy)phenol]-1H-pyrazole dihydrochloride
- NE
norepinephrine
- NO
nitric oxide
- nNOS
neuronal nitric oxide synthase
- OVX
ovariectomy
- PHTPP
4-[2-phenyl-5,7-bis(trifluoromethyl)pyrazolo[1,5-a]pyrimidin-3-yl]phenol
- VMN
ventromedial hypothalamic nucleus
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
6. References
- Amaral AI, 2013. Effects of hypoglycaemia on neuronal metabolism in the adult brain: role of alternative substrates to glucose. J. Inherit. Metab. Dis, 36: 621–634. [DOI] [PubMed] [Google Scholar]
- Amaral AI, Teixeira AP, Hakonsen BI, Sonnewald U, Alves PM, 2011. A comprehensive metabolic profile of cultured astrocytes using isotopic transient metabolic flux analysis and C-labeled glucose. Front. Neuroenerg 2011, 3: 5 10.3389/fnene.2011.00005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashford MLJ, Boden PR, Treherne JM, 1990. Glucose-induced excitation of hypothalamic neurons is mediated by ATP-sensitive K+ channels. Pfugers Arch. 415, 479–483. [DOI] [PubMed] [Google Scholar]
- Auer RN, 1986. Progress review: hypoglycemic brain damage. Arch. Intern. Med 159, 281–284. [DOI] [PubMed] [Google Scholar]
- Auer RN, Siesjo BK, 1993. Hypoglycemia: brain neurochemistry and neuropathology. Baill. Clin. Endocrinol. Metab 7, 611–625. [DOI] [PubMed] [Google Scholar]
- Behar KL, den Hollander JA, Petroff OA, Hetherington HP, Prichard JW, Shulman RG, 1985. Effect of hypoglycemic encephalopathy upon amino acids, high-energy phosphates, and pH in the rat brain in vivo: detection by sequential 1H and 31P NMR spectroscopy. J. Neurochem, 44: 1045–1055. [DOI] [PubMed] [Google Scholar]
- Beverly JL, de Vries MG, Beverly MF, Arseneau LM, 2000. Norepinephrine mediates glucoprivic-induced increase in GABA in the ventromedial hypothalamus of rats. Amer. J. Physiol. Regul. Integr. Comp. Physiol 279, R990–R996. [DOI] [PubMed] [Google Scholar]
- Beverly JL, de Vries MG, Bouman SD, Arseneau LM, 2001. Noradrenergic and GABAergic systems in the medial hypothalamus are activated during hypoglycemia. Amer. J. Physiol. Regul. Integr. Comp. Physiol 280, R563–R569. [DOI] [PubMed] [Google Scholar]
- Borg MA, Tamborlane WV, Shulman GI, Sherwin RS, 2003. Local lactate perfusion of the ventromedial hypothalamus suppresses hypoglycemic counterregulation. Diabetes 52, 663–666. [DOI] [PubMed] [Google Scholar]
- Briski KP, Sylvester PW, 1988. Effects of specific acute stressors on LH release in ovariectomized and ovariectomized estrogen-treated female rats. Neuroendocrinology 47, 194–202. [DOI] [PubMed] [Google Scholar]
- Bröer S, Rahman B, Pellegri G, Pellerin L, Martin JL, Verleysdonk S, Hamprecht B, Magistretti PJ, 1997. Comparison of lactate transport in astroglial cells and monocarboxylate transporter 1 (MCT 1) expressing Xenopus laevis oocytes. Expression of two different monocarboxylate transporters in astroglial cells and neurons. J. Biol. Chem 272, 30096–30102. [DOI] [PubMed] [Google Scholar]
- Butcher RL, Collins WE, Fugo NW, 1974. Plasma concentrations of LH, FSH, progesterone, and estradiol-17beta throughout the 4-day estrous cycle of the rat. Endocrinology 94, 1704–1708. [DOI] [PubMed] [Google Scholar]
- Cerdan S, 2017. Twenty-seven years of cerebral pyruvatre recycling. J. Neurochem 42, 1621–1628. [DOI] [PubMed] [Google Scholar]
- Chan O, Sherwin R, 2013. Influence of VMN fuel sensing on hypoglycemic responses. Trends Endocrinol. Metab 24, 616–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan O, Zhu W, Ding Y, McCrimmon RJ, Sherwin RS, 2006. Blockade of GABA(A) receptors in the ventromedial hypothalamus further stimulates glucagon and sympathoadrenal but not the hypothalamo-pituitary-adrenal response to hypoglycemia. Diabetes 55, 1080–1087. [DOI] [PubMed] [Google Scholar]
- Donovan CM, Watts AG, 2014. Peripheral and central glucose sensing in hypoglycemic detection. Physiology 29, 314–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fioramonti X, Marsollier N, Song Z, Fakira KA, Patel RM, Brown S, Duparc T, Pica-Mendez A, Sanders NM, Knauf C, Valet P, McCrimmon RJ, Beuve A, Magnan C, Routh VH, 2010. Ventromedial hypothalamic nitric oxide production is necessary for hypoglycemia detection and counterregulation. Diabetes 59, 519–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim MMH, Alhamami HN, Briski KP, 2019. Norepinephrine regulation of ventromedial hypothalamic nucleus metabolic transmitter biomarker and astrocyte enzyme and receptor expression: role of 5’-AMP-activated protein kinase. Brain Research 2019, January 7 pii: S0006–8993(19)30018–6. doi: 10.1016/j.brainres.2019.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kale AY, Paranjape SA, Briski KP, 2006. I.c.v. administration of the nonsteroidal glucocorticoid receptor antagonist, CP4–72555, prevents exacerbated hypoglycemia during repeated insulin administration. Neuroscience 140, 555–565. [DOI] [PubMed] [Google Scholar]
- Lewis LD, Ljunggren B, Norberg K, Siesjo BK, 1974. Changes in carbohydrate substrates, amino acids, and ammonia in the brain during insulin-induced hypoglycemia. J. Neurochem 23, 659–671. [DOI] [PubMed] [Google Scholar]
- Mahmood ASMH, Bheemanapally K, Mandal SK, Ibrahim MMH, Briski KP, 2019. Norepinephrine control of ventromedial hypothalamic nucleus glucoregulatory neurotransmitter expression in the female rat: role monocarboxylate transporter function. Mol. Cell. Neurosci 95, 51–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmood ASMH, Uddin MM, Mandal SK, Ibrahim MMH, Alhamami HN, Briski KP, 2018. Sex differences in forebrain estrogen receptor regulation of hypoglycemic patterns of counter-regulatory hormone secretion and ventromedial hypothalamic nucleus gluco-regulatory neurotransmitter and astrocyte glycogen metabolic enzyme expression. Neuropeptides 18, 72:65–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oomura Y, Ono H, Ooyama H, Wayner MJ, 1969. Glucose and osmosensitive neurons of the rat hypothalamus. Nature 222, 282–284. [DOI] [PubMed] [Google Scholar]
- Pellerin L, Pellegri G, Bittar PG, Charnay Y, Bouras C, Martin JL, 1998. Evidence supporting the existence of an activity-dependent astrocyte-neuron lactate shuttle. Dev. Neurosci 20, 291–299. [DOI] [PubMed] [Google Scholar]
- Routh VH, Hao L, Santiago AM, Sheng Z, Zhou C, 2014. Hypothalamic glucose sensing: making ends meet. Front. Syst. Neurosci 8, 236. doi: 10.3389/fnsys.2014.00236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakya M, Shrestha PK, Briski KP, 2018. Hindbrain 5’-adenosine monophosphate-activated protein kinase mediates short-term food deprivation inhibition of the gonadotropin-releasing hormone-luteinizing hormone axis: role of nitric oxide. Neuroscience 383, 46–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver IA, Erecińska M, 1998. Glucose-induced intracellular ion changes in sugar-sensitive hypothalamic neurons. J. Neurophysiol 79, 1733–1745. [DOI] [PubMed] [Google Scholar]
- Singh SR, Briski KP, 2004. Septopreoptic mu opioid receptor mediation of hindbrain glucoprivic inhibition of reproductive neuroendocrine function in the female rat. Endocrinology 145, 5322–5331. [DOI] [PubMed] [Google Scholar]
- Watts AG, Donovan CM, 2010. Sweet talk in the brain: glucosensing, neural networks, and hypoglycemic counterregulation. Front. Neuroendocrinol 31, 32–43. [DOI] [PMC free article] [PubMed] [Google Scholar]