Abstract
In children with congenital heart defects, surgical correction often involves the use of valves, patches or vascular conduits to establish anatomic continuity. Due to the differences between the pediatric and adult populations, tissue reconstruction in pediatric patients requires a substantially different approach from those in adults. Cardiovascular anatomy of children with congenital heart defect vary, which requires tailored surgical operations for each patient. Since grafts used in these palliative surgeries are sensitive to the local hemodynamic environments, their geometries need to be precisely designed to ensure long-term performance. Tissue engineered vascular grafts (TEVGs) have made tremendous progress over the past decade, but it remains difficult to fabricate patient- and operation-specific vascular grafts. This review summarizes historical milestones of TEVG development for repairing pediatric congenital defects and current clinical outcomes. We also highlight ongoing works on 3D bioprinting of TEVGs with complex geometries and address the current limitations of each technique. Although 3D bioprinted vascular grafts with appropriate functions are yet to be developed, some of the current researches are promising to create better patient specific tissue engineered vascular grafts in the future.
Keywords: Congenital Heart Defect, 3D bioprinting, Vascular Conduit, Biomaterials, Hydrogels
1. Introduction
Congenital heart defects (CHDs) are the most common birth defect in the United States, with the prevalence of approximately 6 in 1000 live births when accounting for moderate and severe forms of CHD[1]. Annual cost attributed to CHD-related pediatric hospitalizations was approximately $5.6 billion[2]. Roughly half of all newborns with CHDs require surgical intervention to restore cardiovascular function to prevent the development of complications such as arrhythmia[3], cyanosis[4], congestive heart failure[5] and developmental disabilities[6]. Vascular conduits or patches are widely used in surgical correction of CHDs such as tricuspid atresia, pulmonary atresia, hypoplastic left heart syndrome, tetralogy of fallot, transposition of the great arteries, truncus arteriosus, and other complex anatomic defects [7]. The most common procedure to treat single ventricle heart defect is the Fontan operation, which aims to direct system venous return to pulmonary artery by using a vascular graft to separate systemic and pulmonary blood flow [8,9]. However, the ideal graft for this procedure has yet to developed. Commercial synthetic grafts made of polyethylene terephthalate (Dacron) or polytetrafluoroethylene (PTFE or Gore-Tex), while successful in larger vessel (> 6mm) repairs, yield poor clinical results for small diameter applications (< 5 mm) due to their high incidence of thrombosis [7,10]. In addition, synthetic grafts are unable to adapt to the growth of children; repeated revision surgery may be necessary, increasing the risk of infections. On the other hand, tissue grafts (autografts, allografts or xenografts) can adapt with the growth of pediatric patients but are not without their own limitations. Cryopreserved allografts induced immune response from the pediatric recipients, which could result in immune-mediated injury to the implants[11]. There is also a risk of disease transmission and chronic inflammation[12]. Autologous saphenous vein grafts are the current gold stand for vascular repair for elderly patients, but suitable donor vessels are limited, especially in pediatric patients.
Limitations of current clinically available graft approaches motivated the development of living and functional vascular grafts. Tissue engineered vascular grafts (TEVGs) holds great potential in improving the treatment of pediatric patients with CHDs. Since Weinberg and Bell’s seminal work on the first collagen-based TEVGs in 1986 [13], TEVGs have demonstrated positive results in clinical trials [14,15]. Despite significant progress in the field of vascular tissue engineering, fabricating patient geometry specific graft remain an ongoing challenge. Since each patient have unique anatomy and physiology, individualized treatment and surgical procedure is necessary. Furthermore, important parameters that regulate blood pressure and flow can change drastically when children grow, therefore, the geometry of grafts would play an integral role in the local hemodynamics of the surgical domain [16,17]. Recent advancement in additive manufacturing have made it possible to print biocompatible materials, cells and extracellular proteins into complex 3-dimensional tissues. Bioprinting uses a computer-controlled device to accurately deposit desired printing material and cells in a layer and by layer fashion (x, y and z direction)[18]. Bioprinting of TEVGs has recently gained momentum as a potential method to fabricate patient-specific vascular grafts. In this review, we will highlight popular methods of TEVG fabrications and their clinical outcomes in CHD repair. Then will examine current progress in 3D bioprinting of patient specific TEVGs.
2. Cell Sources for Vascular Tissue Engineering
Fabrication of a tissue engineered vascular graft requires the use of cells that recapitulate the native tissue organization. These cells need to be able to proliferate, produce load-bearing matrix and reorganize as the scaffold degrades. Traditionally, autologous primary cells were studied as cell sources for vascular tissue engineering. Pioneers in the field of small diameter vascular tissue engineering seeded endothelial cells into the lumen of synthetic grafts to improve their patency. These cells, which make up the tunica intima of native vessels, modulate coagulation, platelet adhesion, inflammation and barrier function. Seeding endothelial cells onto the luminal surface vascular grafts improved patency and reduced thrombogenicity when compared to non-seeded grafts[19,20]. In addition, conditioning endothelial cells under fluid flow generated monolayers that aligned parallel to the fluid shear and improved retention of endothelization[21,22].
The tunica media contains dense concentric layers of smooth muscle cells (SMC) that regulate the caliber of the blood vessel via vasoconstriction and vasodilation. These cells also synthesize the extracellular matrix protein – elastin - which contributes to the elasticity and compliance of blood vessels. Therefore, the success of SMC-based vascular grafts is evaluated based on the degree of elastin synthesis. The challenge with using SMC in tissue engineering is that they lose their contractile phenotype when isolated from native tissue; only contractile SMC can produce mature elastin fibers. Cyclic mechanical stretching of smooth muscle cells has been shown to promote cell proliferation[23], extracellular matrix synthesis[24,25], and cell alignment [26].
The tunica adventitia provides rigidity and shape to the blood vessel and acts as a sheath that holds it in place. Fibroblasts, the major cells found in the tunica adventitia, synthesis several different extracellular matrix proteins and play a crucial role in wound healing. In the context of vascular tissue engineering, most studies focused on collagen synthesis[27-29]. TGF-β and ascorbic acid supplementation has been shown to promote collagen synthesis and stabilize collagen fibers, leading to vascular conduits with better mechanical properties[30]. Similar to SMCs, fibroblasts align and produce more extracellular matrix under pulsatile mechanical stimulation. Fibroblast-based vascular conduits can achieve physiological burst pressures after 9 weeks of pulsatile stimulation[31,32].
Although primary cells have been widely studied for tissue engineering, it may not be possible to obtain sufficient cells from a newborn patient. Expansion of primary cells can be time consuming, which limits their clinical translation. Over-expansion of primary cells in vitro may also lead to cell de-differentiation or even senescence. Therefore, stem cells became an attractive cell sources for vascular tissue engineering due to their proliferative capacity and ability differentiate into different cell types. Mesenchymal stem cells (MSC) can be found in umbilical cords, amniotic fluid and Wharton’s jelly, making them an ideal cell source for pediatric vascular tissue engineering. MSC can differentiate into smooth muscle cells in response to growth factors[33], extracellular proteins and cyclic mechanical strain[34]. In addition, MSC have immunomodulatory properties and that promote host cell recruitment and vascular remodeling[35], which would reduce culture time of the grafts and improve long term patency.
3. Conventional Methods of Fabricating Vascular Grafts
Weinberg and Bell’s seminal work reported the fabrication of the first collagen-based TEVG [13]. They created a physiological and functional graft by casting and gelling a collagen solution seeded with bovine smooth muscle cells in a cylindrical mold. Then fibroblasts were deposited around the tube to create the tunica adventitia and endothelial cells were seeded into the lumen. Even though the cells remodeled and displayed appropriate barrier functions, the graft failed to reach appropriate mechanical strength. This study inspired the investigation of many other natural polymers such as fibrin [37], elastin [38] and silk fibroin [39]. However, the lack of physiologically relevant elasticity and strength in natural polymer-based TEVGs are hurdles that need to be overcome. One way to improve the mechanical strengths of TEVGs was to apply mechanical stimuli to the grafts during in vitro culture. Niklason and Langer devised the first pulsatile perfusion system used for TEVG culture. Burst pressure, vessel wall thickness and suture strength of TEVGs all improved under pulsatile stimulation[40]. Syedain and colleagues cultured fibroblast-laden fibrin conduits in a similar pulsatile circumferential stretch system and created grafts with burst pressures of up to 1600 mmHg after 7-9 weeks in culture[31]. Using ovine fibroblasts, the same group was able to create TEVGs with burst pressures exceeding 4000 mmHg after decelluarization[32]. These values are comparable to burst pressures native saphenous veins (~1600 mmHg) and native internal mammary arteries (~3200 mmHg)[41]
To address the mechanical limitations of natural materials, Langer’s lab pioneered the use of degradable synthetic polymers for vascular engineering using polyglycolic acid (PGA) fiber meshes as scaffolds [42]. Biodegradable synthetic polymer is an attractive material for vascular engineering due to its tunable mechanical properties[43], high reproducibility and abundance. Synthetic biodegradable polymer scaffolds have since been investigated as the primary method to fabricate TEVGs [44] and reached clinical trials for the treatment of CHD in both Japan [14,15] and the United States.
Of all manufacturing techniques used in TEVG fabrication, electrospinning has received the most attention due to its ease of fabrication and control of scaffold features, abundance of available materials, and the capability to produce conduits with high surface area to volume ratio[44,45]. High voltage is applied to a polymer solution as it is extruded through a syringe needle. When the surface tension of the polymer droplet is overcome by electrostatic repulsion, a jet of entangled polymer chain forms and accelerates across the electric field towards a grounded substrate, forming micro- or nanofibers. Natural materials, such as collagen and elastin, can be mixed with synthetic polymers solution to create vascular scaffolds that are both strong and biomimetic [39,46,47]. Huang et al further demonstrated the versatility of electrospinning when they fabricated electrospun PCL conduits with 3 different layers consisting of different fiber orientations and pore sizes [48]. Electrospinning onto a mandrel is suitable for fabricating uniform diameter cylindrical grafts, but creating more sophisticated shapes has been difficult. Recently, bifurcating conduits have been fabricated using electrospinning methods, but assembling and disassembling of a custom mandrel is required and distribution of fibers is not uniform [49]. Making patient-specific vascular grafts with any desired geometry using electrospinning methods remains a challenge.
Current clinical requirements of TEVGs can be summarized as follows: 1) non-thrombogenic 2) autologous 3) long-term patency 4) mechanical properties (burst pressure, suture retention, ultimate tensile strength, compliance, etc.) comparable to native tissue.
4. Clinical Outcomes
Current clinical requirements of TEVGs can be summarized as follows: 1) non-thrombogenic 2) autologous 3) long-term patency 4) mechanical properties (burst pressure, suture retention, ultimate tensile strength, compliance, etc.) comparable to native tissue. A group in Japan underwent a successful clinical trial and fulfilled these requirements. Shinoka et al performed the first implantation of TEVG for pediatric CHD repair in 1999. The patient was a 4-year old girl who had previously undergone a Fontan procedure. A 2-cm thrombosed segment of her pulmonary artery was replaced with a TEVG made with polycaprolactone-polylactic acid copolymer reinforced with woven polyglycolic fibers. The graft was seeded with autologous vascular cells 10 days prior to implantation. The patient experienced no postoperative complications and the TEVG remained patent after 7 months. Following the success of the procedure, the same group continued to pioneer the first human TEVG clinical trial on pediatric patients with CHD. 42 patients between the age of 1 to 24 (median age of 5.5 years) were implanted with TEVG seeded with autologous bone marrow cells between 2001 to 2004. During the midterm follow up (mean of 16 months), no complications associated with the grafts, such as thrombosis, rupture, calcification or stenosis were observed [14]. The group continued to monitor the patients and published late-term clinical outcomes (mean of 5.8 years) of their trial. There was no mortality associated with the implanted TEVGs. In addition, there were no signs of infection, graft rupture, aneurysm formation or calcification. However, there was graft stenosis in 6 patients. They successfully underwent angioplasty and stent placement and recovered. One patient had partial mural thrombosis but recovered after warfarin anticoagulation treatment. Their results demonstrated that TEVGs is a feasible alternative to synthetic grafts, which have risks of thrombosis, for treatment of congenital heart diseases. Since these TEVGs were seeded with autologous cells, there is no need for immunosuppressive therapy. Their results also suggest that mechanism behind graft stenosis should be investigated. Phase I clinical trials using TEVGs as extra cardiac total cavopulmonary connection for the treatment of single ventricle cardiac anomaly have also been completed in the United States, but the results have yet to be published.
5. 3D Bioprinting of Vascular Grafts
4.1. Hemodynamic models of TEVGs
Since physiology and anatomy vary from patient to patient, hemodynamics in the local environment would differ as well. Therefore, TEVGs used in surgical procedures should be tailor-made for each patient. It has been suggested through computational modeling that bifurcating Y-shaped grafts can improve hemodynamic efficiency by optimizing flow and diminishing power loss [50,51]. Similarly, Marsden and associates demonstrated that Y-shaped grafts were more energy efficient and distributed blood flow more evenly to both lungs when compared to conventional grafts[52]. These results point to notion that bifurcating TEVGs may improve the long-term outcomes of Fontan conduits. However, it is difficult to fabricate bifurcating grafts using conventional methods such as molding or electrospinning. There is a recent paradigm shift towards 3D bioprinting - fabrication of computer-designed tissue constructs by depositing successive layers of bioink, usually a mixture of live cells and biomaterials, until the entire construct is created. 3D bioprinting offers a potential way to fabricate patient-specific grafts with complex geometries. It also offers other advantages such as accurate distribution of different cell types and materials, providing a versatile platform to create biomimetic vascular tissue.
4.2. Inkjet bioprinting
The hardware used in inkjet-based bioprinting is similar to a commercial desktop inkjet printer. It is a non-contact process in which droplets of bioink are deposited onto a substrate using thermal, piezoelectric or electromagnetic actuators. This technique is most suitable for the deposition of low viscosity materials (<30 cP), such as collagen, fibrin, and PEG-based bioinks[53]. The low viscosity of these bioinks allows cells to be printed with minimal cell death because of the low shear forces. Bioink droplets can be deposited rapidly with high resolution[54]. In addition, droplet sizes and distribution can be precisely controlled[55]. Despite its high speed in the x-y direction, it is not suitable to fabricate structures in the vertical direction; therefore, inkjet bioprinting is a not widely investigated as a method to fabricate TEVGs. To address this issue, Xu et al assembled an inkjet bioprinting platform capable of freeform printing of alginate bioink [56]. Alginate droplets were dispensed unto a motorized platform that gradually lowers the printed layer into a crosslinking solution. Each submerged layer is sequentially crosslinked into calcium alginate and becomes the substrate for the next layer (Figure 1). Using this method, the group fabricated 3-dimensional, fibroblast-laden tubular vascular constructs with zig-zag [57] and bifurcation [58]. The cells maintained high viability after the printing process (>90%).
Figure 1.
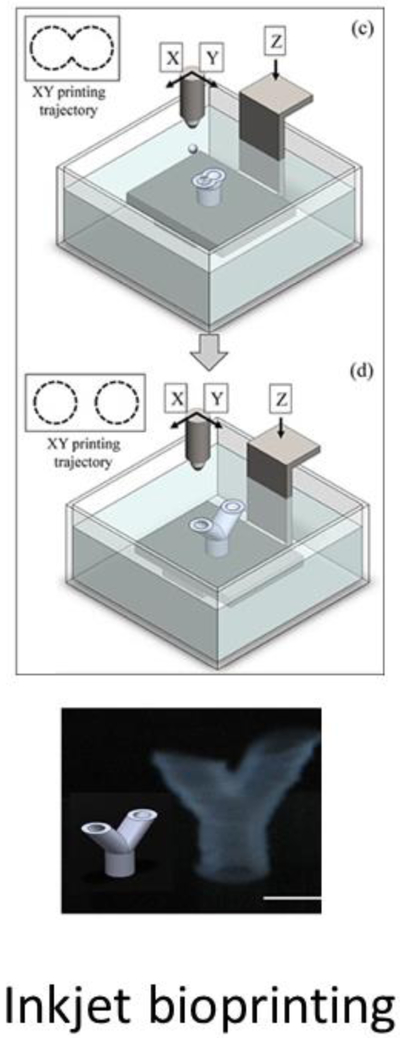
Inkjet bioprinting of a bifurcating vascular graft (Christensen et al, 2015)
4.3. Extrusion-based bioprinting
In an extrusion based-bioprinter, material is dispensed through motor-controlled nozzles either pneumatically or mechanically. Materials used in extrusion bioprinting usually exhibit shear-thinning behavior[59-63] – flows like liquid during extrusion but remain stationary after the material is deposited. This allows the material to stack on top of each other, layer by layer. In general, extrusion-based bioprinting builds a 3D structure more rapidly than other methods but have limitations in resolution due to nozzle size and material properties.
Freeform reversible embedding of suspended hydrogel (FRESH) is a popular extrusion-based method to fabricate freestanding, 3D structures using low-viscosity materials[64]. Soft materials are oftentimes not suitable for extrusion-based bioprinting because they flow upon contact with the substrate and collapse after few layers of printing. To address these limitations, low viscosity hydrogel inks (alginate, collagen and fibrin) were extruded into a slurry of gelatin microparticles using an extrusion-based 3D bioprinter. The slurry behaves likes a Bingham plastic, which held the hydrogel inks in place during the printing process but allowed the nozzle to move around without resistance. The hydrogel inks crosslink and solidify inside the support slurry bath. After printing, the embedded products were heated to 37 °C and released from the liquified slurry. Perfusable branching coronary arteries with lumen diameter of 1 to 3 mm and wall thickness of < 1mm were printed using FRESH (Figure 2). Using a similar concept, Bhattacharjee et al printed vascular networks embedded in granular gel medium consisting of Carbopol microgels. Carbopol is high molecular weight, crosslinked polyacrylic acid polymers used as thickening and stabilizing agent in the cosmetic industry. High resolution (wall thickness of 100 μm) printing and precise distribution of materials and material was achieved using this technique. In addition, they have demonstrated the versatility of the technique by printing different materials such as photocrosslinkable polyvinyl alcohol, PDMS elastomer, methanol-microspheres and cell suspension.
Figure 2.
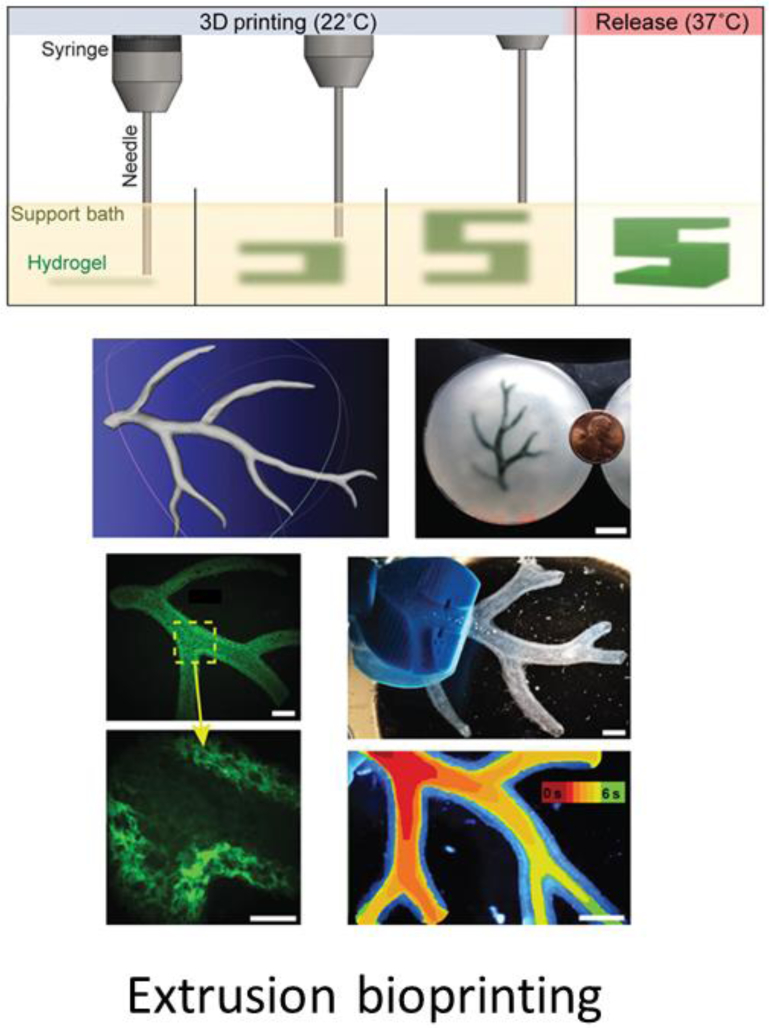
Perfusable branching vascular structure fabricated using FRESH (Hinton et al, 2015).
Gao et al combined elements of organ weaving and extrusion-based bioprinting to fabricate alginate-based vascular conduits containing multilevel fluidic channels [65]. Hollow cell-laden alginate filaments were extruded through coaxial nozzles onto a rotating mandrel to form a tubular conduit. The resulting product was multilevel microchannels, with a level containing smooth muscle cells and the other containing fibroblasts, concentric to the main macro-channel. Then, endothelial cells were seeded into the inner wall of the main channel. The conduit displayed highly organized distribution of the 3 different cell types and maintained high cell viability (over 90%) after 7 days in culture. The group envisioned the possibility of culturing the conduit in a bioreactor that would allow for mechanical and chemical stimulation. The microchannels would allow for the delivery of growth factors or drugs to the layer of choice.
Not all extrusion bioprinting require the use of hydrogel bioinks. Norotte et al developed a scaffold-free method to bioprint vascular tissue [66]. Multicellular cell aggregates were created by loading pelleted cells into capillary micropipettes. These cellular spheroids were mechanically extruded concomitantly with agarose rods used as sacrificial molding templates, to create branched vascular structures. Using the same technique, double layered vascular tubes containing smooth muscle cells and fibroblasts, representing the tunica media and tunica adventitia, were also fabricated. The limitation of this method currently lies in resolution. With a minimal feature size of 300 μm, it is not suitable for small-diameter blood vessel tissue engineering. Furthermore, scaffold-free structures require a large quantity of cells, which could make the process very expensive and time consuming.
Overall, extrusion based bioprinting allows for rapid vertical fabrication of 3D structures and the versatility of printing with multiple materials and cell types. However, its limitation lies in its relatively low resolution compared to other bioprinting methods[67]. Additionally, cells experience significant shear stress during extrusion [68], resulting in cell lysis and apoptosis. Therefore, optimization of printing parameters [69] and modifications bioink rheological properties [61,63,70,71] are the current focus of extrusion-based bioprinting research.
4.4. Laser direct-write
In a laser direct-write system, print ribbons are coated with cell encapsulated bioinks. A pulsed laser beam is transmitted through the ribbon to propel the bioink unto a motorized receiving substrate[72]. Bifurcating graft structures have also been created using this method. Alginate based fibroblast suspension was printed using a matrix-assisted pulse-laser direct write (MAPLE DW) system [73]. Each printed layer was sequentially submerged into a crosslinking calcium chloride solution (Figure 3). Wall thickness of printed conduit could be modulated by adjusting the step size of the substrate in the z-direction. Resulting Y-shaped vascular conduits have a lumen diameter of 5 mm and wall thickness of 1.3mm. However, the cell viability of printed structures was around 64%, which was attributed due to mechanical stress during droplet formation and landing on the substrate. Other studies have also reported potential cell death due to thermal injury and UV damage using laser direct-write, but the degree of these effects remain inconclusive. It has been suggested that laser fluence [74,75], impact-induced cell damage [76], bioink properties [75] and cell type [77,78] all play a role in post-print cell viability. Therefore, comprehensive optimization procedures need to be performed for each material/cell type to achieve successful printing results.
Figure 3.

Fibroblast-laden bifurcating TEVG fabricated using MAPLE-DW (Xiong et al, 2015)
4.5. Stereolithography
Stereolithography bioprinters use a digital light projector to cure bioink resins plane-by-plane and the solidified bioink is progressively lifted by a motor platform. Since the resin is crosslinked plane-by-plane, printing speed does not increase for complex features. There is also no need for material extrusion through nozzles, which decreases shear-related cell death[79]; the resulting tissue constructs generally maintains high cell viability (>85%) across different cell types[80-82]. This method is capable of creating high resolution features of 50-100 μm using popular hydrogels such as polyethylene glycol-diacrylate (PEGDA)[83] and gelatin methacrylate (GelMA)[82]. Bifurcating acellular vascular conduits were created using digital light stereolithography[84]. 3D images of aorta were obtained using MRI or CT scan and custom graft that matched the physiology was designed using CAD software. Polyproylene fumarate (PPF) was UV crosslinked layer by layer to create acellular, polymeric grafts that matched the CAD design. This graft was implanted as inferior vena cava interposition graft in mice. All grafts remained patent over 6 months and no thrombosis, aneurysm or stenosis were observed. In addition, endothelial cell and smooth muscle cells populated the surface of the grafts, suggesting that the scaffold supports neotissue formation. Hydrogel-based, cell-laden, bifurcating grafts have also been created using stereolithograhic bioprinting[85]. Methacrylated poly(ethylene glycol-co-depsipeptide) was mixed with cell-adhesion RGDS peptide and endothelial cells and printed using light projection stereolithography (Figure 4). The encapsulated endothelial cells maintained high viability and proliferated over 10 days in vitro. However, the scaffold degraded rapidly in vitro over the course of 24 days (up to 66%) and mechanical stiffness of the scaffold drastically decreased. This is a common limitation of hydrogel-based systems and addition of peptides further decreases crosslinking density, making the hydrogel degrade more rapidly. Current hydrogel-based TEVG will not withstand the hemodynamic forces at the surgical site. Future research direction should focus on the development of novel biocompatible materials that maintains its strength and elasticity over time. Furthermore, it is only possible to print one material at a time using stereolithography, so it would not be possible to recapitulate the 3-layered structure of native vascular tissue.
Figure 4.

Hydrogel-based, HUVEC-laden, bifurcating TEVG containing cell adhesion peptides were fabricated using light projection stereolithography (Elomaa et al, 2015).
6. Summary and Future Perspectives
Due to the differences between the pediatric and adult populations, tissue reconstruction in pediatric patients require a substantially different approach from those in adults. For example, synthetic grafts that are frequently used in adult large vessel repair lack growth potential. As a result, multiple surgeries are often needed if used in children. Their tendency of thrombosis also requires life-long anti-coagulation, which poses another disadvantage in pediatric age group. These limitations support the clear notion that a living, biologic tissue which can remodel and grow with the child is the key to solve these problems. Toward this goal, tissue engineered vascular grafts hold great promise to solve these challenges, and significant progresses have been made in the past decade. However, the current tissue engineered vascular grafts are mostly made as tubular constructs, which cannot meet the demanding requirement of wide array of anatomies in children with cardiovascular defects in a patient-specific manner. To create vascular conduit that matches the exact size and geometry of individual patient, 3-D bioprinting is a promising approach.
Current 3-D printing is successful of making stiff biomaterial scaffold (without cells) using various polymers. Some polymeric tissue engineered vascular grafts have already achieved some successful results in the clinical settings. Due to variations in patient’s physiology and specificity of each surgical intervention, there is a need to fabricate patient-specific tissue engineered vascular grafts (Figure 5). Computational models can assist in the design of the grafts by predicting local hemodynamics around the surgical site, creating grafts that minimizes turbulence and diminish power loss. These geometries (Figure 6) can be accurately fabricated using a plethora of 3D bioprinting technics, each with their distinct advantages and limitations (Table. 1). Many groups have also demonstrated the possibility of using 3D printing to create complex geometries such as curvatures and bifurcations, shapes that are difficult to fabricate using conventional electrospinning techniques.
Figure 5.
Proposed workflow of patient-specific TEVG fabrication.
Figure 6.
Various geometries of interest in TEVG design. A) Cylindrical electrospun conduit B) Vascular conduit with multiple angles C) Vascular conduit with multiple branches fabricated using inkjet bioprinting[58]. D) Hollow shell and core shell structures created using coaxial nozzles[92]. E) Vascular conduit with multilevel microfluidic channels created via coaxial extrusion onto a rotating mandrel[65]. F) Triple-layer electrospun vascular conduit with different materials and fiber alignment[48] G) Scaffold free vascular graft composed of uniform cell aggregates[66].
Table. 1.
Summary of advantages and limitations of different bioprinting techniques, in the context of vascular tissue engineering.
| Type | Advantages | Limitations | Resolution | Cell Viability |
|---|---|---|---|---|
| Inkjet | Low viscosity bioink, compatible with many available hydrogel precursors, low cost | Very difficult to build vertically, requires predictive compensation for 3D structures | Medium-high | ~90% (high) |
| Extrusion | Rapid fabrication of 3D structures, no need for supporting bath/slurry, capable of printing with multiple materials/cell types simultaneously in one print | Cells experience relatively high shear stress, require bioink with shear-thinning properties | Medium (>100 μm) | 80%-90% (medium) |
| Laser direct-write | Precise spatial distribution, very high resolution | Can only print with one bioink at a time, impact-induced cell damage, risk of thermal injury and UV damage, high cost | Highest (10 μm) | 64%-85% (lowest) |
| Stereolithography | Plane by plane fabrication, capable of making very complex geometries with high resolution and reproducibility | Can only print with one bioink at a time, cells experience UV damage | High (50-100 μm) | >85% (highest) |
However, polymeric grafts, even after seeded with cells on the surface, are still not ideal for pediatric population. For example, these grafts lack suitable elastic properties and stay in the body for very long time due to slow degradation rate. Their degradation byproducts may also lead to inflammation and thrombosis. These limitations suggest that a soft tissue bioprinting with live cells inside the scaffolds may be a better approach. Nevertheless, due to technical difficulties, in soft tissue bioprinting which requires live cells inside the scaffolds, there are still significant challenges. Current 3-D bioprinting of soft tissue uses hydrogel materials such as collagen, fibrin, PEG, alginate, gelatin, hyaluronic acid, etc. The bioprinted constructs from these hydrogel materials, however, are very fragile and are not able to withstand the surgical manipulation and high pulse pressure when implanted in vivo. Most cell-laden structures were fabricated using alginate or PEG-based materials. Even though these materials are biocompatible, they do not provide a bioactive environment that allows for tissue remodeling.
The limitations of bioprinting technology lies in the availability of suitable biomaterials. Therefore, it is much needed to develop new bioprintable materials for vascular graft construction. There are currently ongoing works to functionalize synthetic biomaterials[86-90]. Future efforts will make these materials more bioactive, better degradation profile in response to cellular environment, as well as make them printable. In addition, developing materials with superior mechanical properties such as elasticity is also a promising approach to tackle this challenge[68,91]. The elastic material will allow us to engineer a vascular graft to match the native tissue biomechanics and geometries therefore avoid the problem of compliance mismatch and offer a better hemodynamic profile.
Acknowledgements
We thank the support by grants from American Heart Association Scientist Development Grant (12SDG12050083 to G.D.), National Institute of Health (R21HL102773, R01HL118245 to G.D., R21HD090680 to G.D. and Y.H.) and National Science Foundation (CBET-1263455, CBET-1350240 to G.D., CAREER award #1554835 to Y.H.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
All authors have read the journal's policy on disclosure of potential conflicts of interest and the authors have none to declare.
All authors have read the journal's authorship agreement and that the manuscript has been reviewed by and approved by all named authors.
References:
- [1].Hoffman JI, Kaplan S, J. Am. Coll. Cardiol 2002, 39, 1890. [DOI] [PubMed] [Google Scholar]
- [2].Simeone RM, Oster ME, Cassell CH, Armour BS, Gray DT, Honein MA, Birth Defects Res. Part A Clin. Mol. Teratol 2014, 100, 934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Hanash CR, Crosson JE, J. Emerg. Trauma. Shock 2010, 3, 251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Varan B, Tokel K, Yilmaz G, Arch. Dis. Child. 1999, 81, 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kay JD, Colan SD, Graham TP, et al. , Am. Heart J 2001, 142, 923. [DOI] [PubMed] [Google Scholar]
- [6].Marino BS, Lipkin PH, Newburger JW, Peacock G, Gerdes M, Gaynor JW, Mussatto KA, Uzark K, Goldberg CS, Johnson WH, Li J, Smith SE, Bellinger DC, Mahle WT, American Heart Association Congenital Heart Defects Committee, Council on Cardiovascular Disease in the Young, Council on Cardiovascular Nursing, and Stroke Council, Circulation 2012, 126, 1143. [DOI] [PubMed] [Google Scholar]
- [7].Patterson JT, Gilliland T, Maxfield MW, Church S, Naito Y, Shinoka T, Breuer CK, Regen. Med 2012, 7, 409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Fontan F, Baudet E, Thorax 1971, 26, 240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Jones MB, Crit. Care Nurse 2018, 38, e1. [DOI] [PubMed] [Google Scholar]
- [10].Bennion RS, Williams RA, Stabile BE, Fox MA, Owens ML, Wilson SE, Surg. Gynecol. Obstet 1985, 160, 239. [PubMed] [Google Scholar]
- [11].Shaddy RE, Hunter DD, Osborn KA, Lambert LM, Minich LL, Hawkins JA, McGough EC, Fuller TC, Circulation 1996, 94. [DOI] [PubMed] [Google Scholar]
- [12].Boneva RS, Folks TM, Chapman LE, Clin. Microbiol. Rev 2001, 14, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Weinberg CB, Bell E, Science 1986, 231, 397. [DOI] [PubMed] [Google Scholar]
- [14].Shin’oka T, Matsumura G, Hibino N, Naito Y, Watanabe M, Konuma T, Sakamoto T, Nagatsu M, Kurosawa H, J. Thorac. Cardiovasc. Surg 2005, 129, 1330. [DOI] [PubMed] [Google Scholar]
- [15].Hibino N, McGillicuddy E, Matsumura G, Ichihara Y, Naito Y, Breuer C, Shinoka T, J. Thorac. Cardiovasc. Surg 2010, 139, 431. [DOI] [PubMed] [Google Scholar]
- [16].Conover T, Hlavacek AM, Migliavacca F, Kung E, Dorfman A, Figliola RS, Hsia T-Y, Taylor A, Khambadkone S, Schievano S, de Leval M, Hsia T-Y, Bove E, Dorfman A, Baker GH, Hlavacek A, Migliavacca F, Pennati G, Dubini G, Marsden A, Vignon-Clementel I, Figliola R, McGregor J, J. Thorac. Cardiovasc. Surg 2018, 155, 712. [DOI] [PubMed] [Google Scholar]
- [17].Marsden AL, Reddy VM, Shadden SC, Chan FP, Taylor CA, Feinstein JA, Congenit. Heart Dis 2010, 5, 104. [DOI] [PubMed] [Google Scholar]
- [18].Du X, IOP Conf. Ser. Mater. Sci. Eng 2018, 301, 012023. [Google Scholar]
- [19].Schmidt SP, Hunter TJ, Sharp WV, Malindzak GS, Evancho MM, J. Vasc. Surg 1984, 1, 434. [PubMed] [Google Scholar]
- [20].De Visscher G, Mesure L, Meuris B, Ivanova A, Flameng W, Acta Biomater. 2012, 8, 1330. [DOI] [PubMed] [Google Scholar]
- [21].Ott MJ, Ballermann BJ, Surgery 1995, 117, 334. [DOI] [PubMed] [Google Scholar]
- [22].Dardik A, Liu A, Ballermann BJ, J. Vasc. Surg 1999, 29, 157. [DOI] [PubMed] [Google Scholar]
- [23].Birukov KG, Shirinsky VP, Stepanova OV, Tkachuk VA, Hahn AWA, Resink TJ, Smirnov VN, Mol. Cell. Biochem 1995, 144, 131. [DOI] [PubMed] [Google Scholar]
- [24].Kulik TJ, Alvarado SP, J. Cell. Physiol 1993, 157, 615. [DOI] [PubMed] [Google Scholar]
- [25].Sumpio BE, Banes AJ, Link WG, Johnson G, Arch. Surg 1988, 123, 1233. [DOI] [PubMed] [Google Scholar]
- [26].Kim B-S, Nikolovski J, Bonadio J, Mooney DJ, Nat. Biotechnol 1999, 17, 979. [DOI] [PubMed] [Google Scholar]
- [27].Huynh TN, Tranquillo RT, Ann. Biomed. Eng 2010, 38, 2226. [DOI] [PubMed] [Google Scholar]
- [28].Hirai J, Matsuda T, Cell Transplant. 1996, 5, 93. [DOI] [PubMed] [Google Scholar]
- [29].L’Heureux N, Dusserre N, Konig G, Victor B, Keire P, Wight TN, Chronos NAF, Kyles AE, Gregory CR, Hoyt G, Robbins RC, McAllister TN, Nat. Med 2006, 12, 361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Patel B, Xu Z, Pinnock CB, Kabbani LS, Lam MT, Sci. Rep 2018, 8, 3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Syedain ZH, Meier LA, Bjork JW, Lee A, Tranquillo RT, Biomaterials 2011, 32, 714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Syedain ZH, Meier LA, Lahti MT, Johnson SL, Tranquillo RT, Tissue Eng. Part A 2014, 20, 1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Gong Z, Calkins G, Cheng E, Krause D, Niklason LE, Tissue Eng. Part A 2009, 15, 319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Gong Z, Niklason LE, Methods Mol. Biol 2011, 698, 279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Ziegelhoeffer T, Fernandez B, Kostin S, Heil M, Voswinckel R, Helisch A, Schaper W, Circ. Res 2004, 94, 230. [DOI] [PubMed] [Google Scholar]
- [36].Eoh JH, Shen N, Burke JA, Hinderer S, Xia Z, Schenke-Layland K, Gerecht S, Acta Biomater. 2017, 52, 49. [DOI] [PubMed] [Google Scholar]
- [37].Swartz DD, Russell JA, Andreadis ST, AJP Hear. Circ. Physiol 2004, 288, H1451. [DOI] [PubMed] [Google Scholar]
- [38].McKenna KA, Hinds MT, Sarao RC, Wu P-C, Maslen CL, Glanville RW, Babcock D, Gregory KW, Acta Biomater. 2012, 8, 225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Marelli B, Achilli M, Alessandrino A, Freddi G, Tanzi MC, Faré S, Mantovani D, Macromol. Biosci 2012, 12, 1566. [DOI] [PubMed] [Google Scholar]
- [40].Niklason LE, Gao J, Abbott WM, Hirschi KK, Houser S, Marini R, Langer R, Science 1999, 284, 489. [DOI] [PubMed] [Google Scholar]
- [41].Konig G, McAllister TN, Dusserre N, Garrido SA, Iyican C, Marini A, Fiorillo A, Avila H, Wystrychowski W, Zagalski K, Maruszewski M, Jones AL, Cierpka L, de la Fuente LM, L’Heureux N, Biomaterials 2009, 30, 1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Mooney DJ, Mazzoni C, Breued C, McNamara K, Hem D, Vacanti J, Langer R, Eiomateriak 1996, 17, 115. [DOI] [PubMed] [Google Scholar]
- [43].Austin MJ, Rosales AM, Biomater. Sci 2019, 7, 490. [DOI] [PubMed] [Google Scholar]
- [44].Hasan A, Memic A, Annabi N, Hossain M, Paul A, Dokmeci MR, Dehghani F, Khademhosseini A, Acta Biomater. 2014, 10, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Rocco KA, Maxfield MW, Best CA, Dean EW, Breuer CK, Tissue Eng. Part B Rev 2014, 20, 628. [DOI] [PubMed] [Google Scholar]
- [46].Stitzel J, Liu J, Lee SJ, Komura M, Berry J, Soker S, Lim G, Van Dyke M, Czerw R, Yoo JJ, Atala A, Biomaterials 2006, 27, 1088. [DOI] [PubMed] [Google Scholar]
- [47].Lee SJ, Heo DN, Park JS, Kwon SK, Lee JH, Lee JH, Kim WD, Kwon IK, Park SA, Phys. Chem. Chem. Phys 2015, 17, 2996. [DOI] [PubMed] [Google Scholar]
- [48].Huang R, Gao X, Wang J, Chen H, Tong C, Tan Y, Tan Z, Ann. Biomed. Eng 2018, 46, 1254. [DOI] [PubMed] [Google Scholar]
- [49].Tejeda-Alejandre R, Lara-Padilla H, Mendoza-Buenrostro C, Rodriguez CA, Dean D, Procedia CIRP 2017, 65, 207. [Google Scholar]
- [50].Soerensen DD, Pekkan K, de Zelicourt D, Sharma S, Kanter K, Fogel M, Yoganathan AP, Ann. Thorac. Surg 2007, 83, 2182. [DOI] [PubMed] [Google Scholar]
- [51].Kanter KR, Haggerty CM, Restrepo M, de Zelicourt DA, Rossignac J, Parks WJ, Yoganathan AP, J. Thorac. Cardiovasc. Surg 2012, 144, 383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Marsden AL, Bernstein AJ, Reddy VM, Shadden SC, Spilker RL, Chan FP, Taylor CA, Feinstein JA, J. Thorac. Cardiovasc. Surg 2009, 137, 394. [DOI] [PubMed] [Google Scholar]
- [53].Saunders RE, Derby B, Int. Mater. Rev 2014, 59, 430. [Google Scholar]
- [54].Roth E, Xu T, Das M, Gregory C, Hickman J, Boland T, Biomaterials 2004, 25, 3707. [DOI] [PubMed] [Google Scholar]
- [55].Nishiyama Y, Nakamura M, Henmi C, Yamaguchi K, Mochizuki S, Nakagawa H, Takiura K, J. Biomech. Eng 2009, 131, 035001. [DOI] [PubMed] [Google Scholar]
- [56].Xu C, Chai W, Huang Y, Markwald RR, Biotechnol. Bioeng 2012, 109, 3152. [DOI] [PubMed] [Google Scholar]
- [57].Xu C, Zhang Z, Christensen K, Huang Y, Fu J, Markwald RR, J. Manuf. Sci. Eng 2014, 136, 061020. [Google Scholar]
- [58].Christensen K, Xu C, Chai W, Zhang Z, Fu J, Huang Y, Biotechnol. Bioeng 2015, 112, 1047. [DOI] [PubMed] [Google Scholar]
- [59].Peak CW, Stein J, Gold KA, Gaharwar AK, n.d., DOI 10.1021/acs.langmuir.7b02540. [DOI] [PubMed]
- [60].Markstedt K, Mantas A, Tournier I, Martínez Ávila H, Hägg D, Gatenholm P, Biomacromolecules 2015, 16, 1489. [DOI] [PubMed] [Google Scholar]
- [61].He Y, Yang F, Zhao H, Gao Q, Xia B, Fu J, Sci. Rep 2016, 6, 29977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Paxton N, Smolan W, Böck T, Melchels F, Groll J, Jungst T, Biofabrication 2017, 9, 044107. [DOI] [PubMed] [Google Scholar]
- [63].Gao T, Gillispie GJ, Copus JS, PR AK, Seol Y-J, Atala A, Yoo JJ, Lee SJ, Biofabrication 2018, 10, 034106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Hinton TJ, Jallerat Q, Palchesko RN, Park JH, Grodzicki MS, Shue H-J, Ramadan MH, Hudson AR, Feinberg AW, Sci. Adv 2015, 1, e1500758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Gao Q, Liu Z, Lin Z, Qiu J, Liu Y, Liu A, Wang Y, Xiang M, Chen B, Fu J, He Y, ACS Biomater. Sci. Eng 2017, 3, 399. [DOI] [PubMed] [Google Scholar]
- [66].Norotte C, Marga FS, Niklason LE, Forgacs G, Biomaterials 2009, 30, 5910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Derakhshanfar S, Mbeleck R, Xu K, Zhang X, Zhong W, Xing M, Bioact. Mater 2018, 3, 144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Xu C, Lee W, Dai G, Hong Y, ACS Appl. Mater. Interfaces 2018, 10, 9969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Kyle S, Jessop ZM, Al-Sabah A, Whitaker IS, Adv. Healthc. Mater 2017, 6, 1700264. [DOI] [PubMed] [Google Scholar]
- [70].Paxton N, Smolan W, Böck T, Melchels F, Groll J, Jungst T, Biofabrication 2017, 9, 044107. [DOI] [PubMed] [Google Scholar]
- [71].Chung JHY, Naficy S, Yue Z, Kapsa R, Quigley A, Moulton SE, Wallace GG, Biomater. Sci 2013, 1, 763. [DOI] [PubMed] [Google Scholar]
- [72].Schiele NR, Corr DT, Huang Y, Raof NA, Xie Y, Chrisey DB, Biofabrication 2010, 2, 032001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Xiong R, Zhang Z, Chai W, Huang Y, Chrisey DB, Biofabrication 2015, 7, 045011. [DOI] [PubMed] [Google Scholar]
- [74].Lin Y, Huang G, Huang Y, Tzeng T-RJ, Chrisey DB, Tissue Eng. Part C. Methods 2010, DOI 10.1089/ten.TEC.2009.0606. [DOI] [PubMed] [Google Scholar]
- [75].Yan J, Chrisey DB, Gudapati H, Huang Y, Chrisey DB, 2014, DOI 10.1088/1758-5082/6/3/035022. [DOI] [PubMed] [Google Scholar]
- [76].Wang W, Huang Y, Grujicic M, Chrisey DB, J. Manuf. Sci. Eng 2008, 130, 021012. [Google Scholar]
- [77].Raof NA, Schiele NR, Xie Y, Chrisey DB, Corr DT, Biomaterials 2011, 32, 1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Hopp B, Smausz T, Szab G, Kolozsvri L, Kafetzopoulos D, Fotakis C, Ngrdi A, Opt. Eng 2012, 51, 014302. [Google Scholar]
- [79].Melchels FPW, Feijen J, Grijpma DW, Biomaterials 2010, 31, 6121. [DOI] [PubMed] [Google Scholar]
- [80].Lin H, Zhang D, Alexander PG, Yang G, Tan J, Cheng AW-M, Tuan RS, Biomaterials 2013, 34, 331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Chan V, Zorlutuna P, Jeong JH, Kong H, Bashir R, n.d., DOI 10.1039/c004285d. [DOI]
- [82].Gauvin R, Chen Y-C, Lee JW, Soman P, Zorlutuna P, Nichol JW, Bae H, Chen S, Khademhosseini A, Biomaterials 2012, 33, 3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Wang Z, Abdulla R, Parker B, Samanipour R, Ghosh S, Kim K, Biofabrication 2015, 7, 045009. [DOI] [PubMed] [Google Scholar]
- [84].Melchiorri AJ, Hibino N, Best CA, Yi T, Lee YU, Kraynak CA, Kimerer LK, Krieger A, Kim P, Breuer CK, Fisher JP, Adv. Healthc. Mater 2016, 5, 319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Elomaa L, Pan C-C, Shanjani Y, Malkovskiy A, Seppälä JV, Yang Y, J. Mater. Chem. B 2015, 3, 8348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Ali S, Saik JE, Gould DJ, Dickinson ME, West JL, Biores. Open Access 2013, 2, 241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Shu XZ, Ghosh K, Liu Y, Palumbo FS, Luo Y, Clark RA, Prestwich GD, J. Biomed. Mater. Res 2004, 68A, 365. [DOI] [PubMed] [Google Scholar]
- [88].V Sridhar B, Brock JL, Silver JS, Leight JL, Randolph MA, Anseth KS, Adv. Healthc. Mater 2015, 4, 702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Lutolf MP, Lauer-Fields JL, Schmoekel HG, Metters AT, Weber FE, Fields GB, Hubbell JA, Proc. Natl. Acad. Sci. U. S. A 2003, 100, 5413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Weber LM, Hayda KN, Haskins K, Anseth KS, Biomaterials 2007, 28, 3004. [DOI] [PubMed] [Google Scholar]
- [91].Weng L, Gouldstone A, Wu Y, Chen W, Biomaterials 2008, 29, 2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Ouyang L, Highley CB, Sun W, Burdick JA, Adv. Mater 2017, 29, 1604983. [DOI] [PubMed] [Google Scholar]




