Abstract
Structural adaptations in brain regions involved in domain-general cognitive control are associated with life-long bilingualism and may contribute to the executive function advantage of bilinguals over monolinguals. To the degree that these adaptations support bilingualism, their disruption by Alzheimer’s disease (AD) may compromise the ability to maintain proficiency in two languages, particularly in the less proficient, or nondominant, language that has greater control demands. The present study assessed this possibility in Spanish-English bilinguals with AD (n = 21) and cognitively normal controls (n = 30) by examining the brain correlates of dominant versus nondominant language performance on the Multilingual Naming Test (MINT), adjusting for age and education. There were no significant structural correlates of naming performance for either language in controls. In patients with AD, dominant language MINT performance was associated with cortical thickness of the entorhinal cortex and middle temporal gyrus, consistent with previous findings of temporal atrophy and related decline of naming abilities in AD. Nondominant language MINT performance, in contrast, was correlated with thickness of the left caudal anterior cingulate cortex (ACC), a central cognitive control region involved in error monitoring and task switching. The relationship between naming in the nondominant language and ACC in patients with AD but not in controls may reflect increased reliance on the ACC for nondominant language use in the face of atrophy of other control network components. The results are consistent with the possibility that the increased burden nondominant language use places on cognitive control systems compromised in AD may account for faster nondominant than dominant language decline in AD.
Keywords: Bilingualism, Cognitive Control, Alzheimer’s disease, Structural Neuroimaging, Anterior Cingulate Cortex
1. Introduction
Mounting evidence suggests that bilingualism is associated with the delayed onset of cognitive decline due to Alzheimer’s disease (AD) by an average of 4-5 years (Bialystok, Craik, & Freedman, 2007; Craik, Bialystok, & Freedman, 2010; Chertkow et al., 2010; Schweizer, Ware, Fischer, Craik, & Bialystok, 2012; for review see Bak & Alladi, 2014; Guzmán-Vélez & Tranel, 2015). This type of cognitive reserve is often attributed to the executive control advantage of bilinguals compared to monolinguals, which may result from increased demands associated with needing to manage and switch between activation of two languages in a single cognitive system (Gold, 2015; Green & Abutalebi, 2013; for review see Bialystok, 2011, 2017; Kroll & Bialystok, 2013). In their seminal neurocognitive language control model Abutalebi and Green (2007) identified the domain-general control structures involved in the dynamic process of bilingual language production, including the anterior cingulate cortex (ACC), prefrontal cortex (PFC), inferior parietal lobule (IPL), and basal ganglia. In their more recent Adaptive Control Hypothesis, Green and Abutalebi (2013) suggested that this network is adaptive to specific language contexts – with control regions more involved in some than in other interactional contexts (e.g., they propose less control is needed in conversations with dense code switching). Nevertheless, the very need to monitor which type of language use is appropriate for each conversation could itself produce adaptations within control regions, in turn resulting in advantages compared to monolinguals in non-linguistic executive control functions such as response inhibition, working memory, and task-switching (e.g., a bilingual advantage on the flanker task associated with more efficient functional activation of the ACC; Abutalebi et al., 2012; for review see Dong & Li, 2015; but see Paap & Greenberg, 2013; Lehtonen et al., 2018).
Structural neuroimaging studies in cognitively normal bilinguals support this view, suggesting that bilingualism may produce structural adaptations (i.e., increased grey matter density or volume) in a range of regions associated with domain-general cognitive control (Abutalebi & Green, 2016; Green & Abutalebi, 2013; Mechelli et al., 2004). Structural changes in the inferior frontal gyrus (IFG; part of the PFC) correlate with second language training (Stein et al., 2012), and thickness of the IFG is related to age of second language acquisition in bilinguals (Klein, Mok, Chen, & Watkins, 2014). Second language proficiency and bilingualism appears to similarly affect the left IPL in young adults (Mechelli et al., 2004) and cognitively normal older bilinguals (Abutalebi, Canini, Della Rosa, Green, & Weekes, 2015). Aging bilinguals also have increased grey matter volume in the ACC (Abutalebi, Guidi, et al., 2015), a structure whose grey matter density was previously reported to correlate with better conflict monitoring in bilinguals (Abutalebi et al., 2012). A recent study also identified greater temporal pole volume in aging bilinguals compared to monolinguals, and reported a correlation between volume of this structure and second language proficiency (Abutalebi et al., 2014). This is consistent with other reports of the protective effect of bilingualism on age-related temporal pole atrophy (Olsen et al., 2015). While not part of the domain-general control network (Green & Abutalebi, 2013; Niendam et al., 2012), the temporal poles function as general semantic hubs activated during lexical retrieval (Patterson, Nestor, & Rogers, 2007).
Although structural advantages (e.g., thicker cortex and higher grey matter density) in domain-general control regions persist in multilinguals with AD or Mild Cognitive Impairment (MCI) (Duncan et al., 2018), many of these same brain regions are subject to atrophy as the disease progresses (McDonald et al., 2012; Sabuncu et al., 2011; Westman et al., 2011). If bilingualism is supported by these adaptations, their disruption by AD may compromise the ability to maintain proficiency in two languages, particularly in the less proficient, or nondominant, language (Ivanova, Salmon, & Gollan, 2014; Mendez, Perryman, Pontón, & Cummings, 1999). Consistent with this possibility, it has been reported that bilinguals with AD gradually lose the ability to maintain fluency in two languages (Obler, Albert, & Lozowick, 1986), regress to strongly preferring the language that is first-learned and dominant (Ardila & Ramos, 2008), and have increased unintended intrusions of the dominant language into the second-learned nondominant language (Mendez et al., 1999). In addition, Ivanova and colleagues (2014) found that non-balanced Spanish-English bilinguals with AD exhibited greater longitudinal decline in their nondominant language over a 3-year period. These effects may, however, be specific to bilinguals with a clearly dominant language since studies of balanced Spanish-Catalan bilinguals (i.e. who were similarly proficient in both languages) with AD showed similar declines in both languages cross-sectionally as well as longitudinally (Calabria et al., 2017; Costa et al., 2012).
Greater decline in the nondominant than dominant language in non-balanced bilinguals with AD may be related to atrophy of domain-general cognitive control regions and accompanying decline in executive control functions (Chang et al., 2010; McDonald et al., 2012; Pa et al., 2009; for review see Nowrangi, Lyketsos, Rao, & Munro, 2014). Retrieval of words in the nondominant language is more difficult and less automatic than in the dominant language, and therefore more likely to be affected by the well-known deficits in executive control that occur in AD (Balota, Watson, Duchek, & Ferraro, 1999; Nebes, Martin, & Horn, 1984; Ober, Shenaut, & Reed, 1995). The present study assessed this possibility in Spanish-English bilinguals with AD and cognitively normal controls by examining the brain correlates of dominant versus nondominant language performance on the Multilingual Naming Test (MINT), a picture naming task designed for use in multiple languages (Gollan, Weissberger, Runnqvist, Montoya, & Cera, 2012; Ivanova, Salmon, & Gollan, 2013). We hypothesized that naming ability in the nondominant language would be associated with structural integrity in cognitive control areas previously associated with bilingualism in healthy older adults. Naming ability in the dominant language, in contrast, would be less reliant on the domain-general control network and be more likely to correlate with the structural integrity of temporal lobe structures associated with semantic memory.
2. Methods
The research protocol was reviewed and approved by the Institutional Review Board (IRB) at the University of California, San Diego and informed consent to participate in the study was obtained at the point of entry into the ADRC longitudinal study from all patients or their caregivers consistent with California State law.
2.1. Participants
The study included 21 Spanish-English bilingual participants clinically diagnosed with AD and 30 cognitively normal (NC) Spanish-English bilingual participants from the University of California, San Diego (UCSD) Shiley Marcos Alzheimer’s Disease Research Center (ADRC). Participants were diagnosed by a panel consisting of at least one neurologist and one neuropsychologist using systematic guidelines for clinical diagnosis based on the most up to date published research criteria (Albert et al., 2011; McKhann et al., 2011). Groups did not differ in age, gender distribution, APOE ε4 allele frequency, or dominant language spoken (see Table 1). Education, expressed continuously as the cumulative years of formal education, was greater in the NC group (p < 0.001). On average, the AD group had lower scores on global cognition measures such as the Mini-Mental State Exam (MMSE; p < 0.001) and the Mattis Dementia Rating Scale (DRS; p < 0.001), as well as a worse confrontation naming performance in their dominant and nondominant languages (MINT; p < 0.001 and p < 0.01 respectively). The bilingual index (a measure of degree of bilingualism that is described in detail below) revealed a small but significant difference between the groups such that the AD patients were relatively less bilingual (p = 0.04). Participants with AD were also more functionally impaired as judged by the Functional Assessment Questionnaire (FAQ; p < 0.001).
Table 1:
Demographics and cognitive function in participants with AD and in cognitively normal controls
| AD (n = 21) |
NC (n = 30) |
P value | |
|---|---|---|---|
| Age (years) | 72.0 ± 12.5 | 72.5 ± 8.2 | 0.89 |
| Gender: | |||
| Male | 11 (52%) | 9 (30%) | 0.15 |
| Female | 10 (48%) | 21 (70%) | |
| Education (years) | 10.0 ± 4.4 | 14.2 ± 3.4 | < 0.001 |
| APOE ε4 allele number a | |||
| 0 ε4 alleles | 11 (55%) | 21 (70%) | 0.22 |
| 1 ε4 alleles | 7 (35%) | 9 (30%) | |
| 2 ε4 alleles | 2 (10%) | 0 (0%) | |
| Dominant Language: | |||
| English | 9 (43%) | 18 (60%) | 0.27 |
| Spanish | 12 (57%) | 12 (40%) | |
| MMSE (/30) | 20.6 ± 4.5 | 28.3 ± 1.6 | < 0.001 |
| DRS Total (/144) | 109.8 ± 14.8 | 136.5 ± 4.8 | < 0.001 |
| Dominant MINT (/68) | 57.4 ± 6.5 | 63.8 ± 3.3 | < 0.001 |
| Nondominant MINT (/68) | 29.2 ± 17.4 | 42.1 ± 16.3 | 0.01 |
| Bilingual Index (/1) | 0.50 ± 0.3 | 0.70 ± 0.3 | 0.04 |
| FAQ a (/30) | 18.5 ± 7.9 | 0.6 ± 1.6 | < 0.001 |
Values presented as Mean ± SD for continuous variables or n (%) for discrete variables
Abbreviations: MMSE = Mini Mental State Exam, DRS = Mattis Dementia Rating Scale, FAQ = Functional Assessment Questionnaire, MINT = Multi-Lingual Naming Test, AD = Alzheimer’s Disease, NC = Cognitively Normal Controls
Bold indicate significance at a p < 0.05 threshold
Missing Data: 1 AD subject missing APOE4 genotype, 1 NC subject missing FAQ
2.2. Procedures
Participants were tested on the Multilingual Naming Test (MINT) as part of their annual standardized ADRC neuropsychological evaluation. Testing was performed by a Spanish-English bilingual psychometrician. The MINT (Gollan et al., 2012; Ivanova et al., 2013) consists of 68 black-and-white line drawings of objects that are presented one at a time to be named. The drawings are graded in difficulty, with the easiest drawings presented first. The MINT was designed to assess both dominant and nondominant languages; thus it includes a greater proportion of medium to high frequency items than is typical for naming tests designed for monolinguals (e.g., the Boston Naming Test; Kaplan, 1983). Participants first named all the pictures in their dominant language, and then in their nondominant language. If an individual encountered difficulty identifying an object, a semantic or phonemic cue was provided. Testing was discontinued after six consecutive failed naming trials. The number of spontaneous correct responses and correct responses following a semantic cue were summed to give a total score (which ranges from 0 to 68).
Bilingualism Index scores were calculated by dividing the score in whichever language produced the lower score (nondominant language) by the score in the language which produced a higher score (dominant language). Index scores range from 0–1 and measure the extent to which knowledge of each language is similar, ignoring direction of dominance and absolute ability level (Gollan, Salmon, Montoya, & da Pena, 2010; Gollan et al., 2012).
2.3. Image Analysis
2.3.1. MRI Aquisition
High-resolution, three-dimensional, T1-weighted images were acquired for each subject (TE: 2.8 ms/3.8 ms; TR: 6.5 ms/8.5 ms; TI: 600 ms/500 ms; flip angle: 8°/10° matrix: 256 × 256; voxel size: 0.9375 mm × 0.9375 mm × 1.2000 mm; values separated by ‘/’ are for 3.0 T data/1.5 T data). Respiratory effort and heart rate were monitored with a pressure transducer (BioPac Systems Inc., Goleta, CA) and a pulse oximeter (BioPac Systems and InVivo, Orlando, FL), respectively.
2.3.2. MRI Processing
A model of each subject’s cortical surface was reconstructed from the T1-weighted MRI scan using FreeSurfer (version 6.0), an automated image processing pipeline that is documented and freely available online (http://surfer.nmr.mgh.harvard.edu/). The technical details of these methods have been described in prior publications (e.g., Dale, Fischl, & Sereno, 1999; Fischl & Dale, 2000; Fischl, Liu, & Dale, 2001; Fischl et al., 2001, 2002, 2004; Fischl, Sereno, & Dale, 1999; Fischl, Sereno, Tootell, & Dale, 1999). Following completion of the automated FreeSurfer pipeline, all scans were reviewed to assess the quality of skull stripping and ensure that cortical surfaces followed the gray and white matter boundaries. Where needed, manual edits were performed to improve accuracy of segmentation. This involved correction of pial surface misplacement (e.g., inclusion of non-brain tissue) and errors in white matter segmentation. This editing procedure was conducted by operators blinded to the diagnostic status of the participants. Final surfaces were visually inspected to search for gross errors; none were found in the present data set.
The surface cortical model was then anatomically parcellated using the Desikan-Killiany atlas and standard FreeSurfer tools (Desikan et al., 2006; Fischl et al., 2004). This process assigns each point (vertex) on the native surface to the most probable anatomical label (e.g., inferior parietal, precentral, parahippocampal, etc.) based on registration to a probabilistic atlas of surface folding patterns and on the observed surface geometry at that location of the native surface (Fischl et al., 2004). Automated parcellation by this method has been shown to be comparable to manual labeling (Fischl et al., 2004). The parcellation for each subject was visually inspected to search for gross errors; none were found in the present data set.
2.3.3. Selection of Regions of Interest
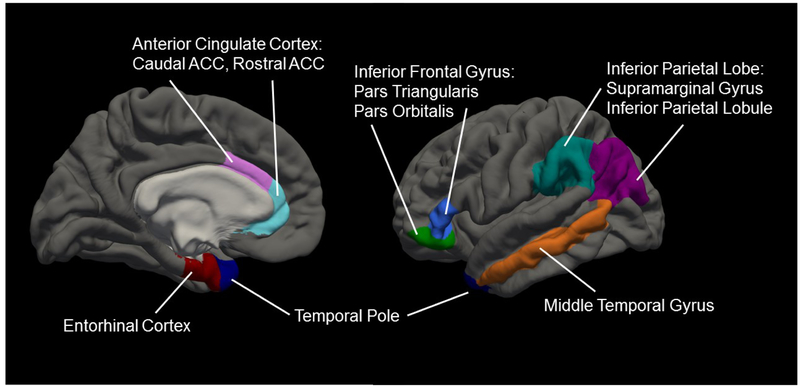
Regions of interest (ROIs) for this analysis were selected from the literature, targeting: 1) cognitive control regions demonstrating a bilingualism effect in older adults including the rostral and caudal ACC (Abutalebi, Guidi, et al., 2015), temporal poles (Abutalebi et al., 2014; Olsen et al., 2015), inferior parietal lobule (angular gyrus) and supramarginal gyrus (Abutalebi, Canini, et al., 2015; Mechelli et al., 2004), and inferior frontal gyrus (pars orbitalis and triangularis per Klein et al., 2014; Stein et al., 2012); 2) the middle temporal gyrus whose volume has been shown to correlate with confrontation naming in AD (Grossman et al., 2004); and 3) entorhinal cortex which shows the greatest degree of cortical thinning in early AD (Bakkour, Morris, & Dickerson, 2009; Dickerson et al., 2009, 2001; McEvoy et al., 2009; Ries et al., 2008). Figure 1 visually displays these regions per the Desikan-Killiany atlas on the pial surface of the FreeSurfer “fsaverage” brain.
Figure 1. Regions of Interest.
Regions of interest selected for the analysis overlaid on the pial surface of the standard FreeSurfer “fsaverage” brain.
2.4. Statistical Analysis
Demographics, clinical characteristics, and cognitive performance were compared using ANOVA for continuous variables and Fisher Exact Tests for categorical variables. Average cortical thicknesses for each ROI were extracted from FreeSurfer and subjected to a linear regression with dominant MINT score, nondominant MINT score, years of education, and age in years as predictors. The Kolmogorov-Smirnov test demonstrated that within each group, our variables did not deviate from normality (D range 0.10 - 0.14; all p > 0.6), while a variance inflation factor (VIF) of less than 3 for each covariate indicated that no significant co-linearity was present. For each of the models used, no autocorrelation was observed with the Durbin-Watson test (d range 1.72 – 2.27, all p > 0.16), and heteroscedasticity was ruled out with the Goldfeld-Quandt test (GQ range 0.38 – 1.20, all p > 0.34). Partial correlations were calculated for each variable, controlling for the others, from the regression model. We sampled 18 regions (9 ROIs bilaterally), with corrections for multiple comparisons carried out using the Benjamini-Hochberg (Benjamini & Hochberg, 1995; Storey, Taylor, & Siegmund, 2004) procedure to adjust for a false discovery rate (FDR) < 0.05. Results that survived this correction are bolded in tables. Analyses were performed using the R statistical software package (v 3.5.1, R Core Team, 2015).
3. Results
3.1. Cortical thickness differences between AD and NC
Compared to the cognitively normal bilingual controls, bilinguals with AD exhibited cortical thinning in bilateral entorhinal, middle temporal, temporal pole, and inferior parietal and supramarginal cortices. In addition, bilateral pars orbitalis and the right pars triangularis of the IFG, as well as the left rostral ACC were significantly thinner among patients (all FDR-adjusted p < 0.05, Table 2).
Table 2:
Regional cortical thicknesses of patients with AD and cognitively normal adults
| AD Mean ± SD |
NC Mean ± SD |
p-value | |
|---|---|---|---|
| Temporal Lobe | |||
| L. Entorhinal | 2.8 ± 0.5 | 3.2 ± 0.3 | 0.001 |
| R. Entorhinal | 2.9 ± 0.4 | 3.3 ± 0.3 | < 0.001 |
| L. Middle Temporal | 2.5 ± 0.2 | 2.7 ± 0.2 | < 0.001 |
| R. Middle Temporal | 2.4 ± 0.2 | 2.7 ± 0.1 | < 0.001 |
| L. Temporal Pole | 3.2 ± 0.3 | 3.4 ± 0.3 | 0.002 |
| R. Temporal Pole | 3.2 ± 0.4 | 3.5 ± 0.3 | 0.005 |
| Inferior Frontal Gyrus | |||
| L. Pars Orbitalis | 2.4 ± 0.2 | 2.6 ± 0.2 | 0.005 |
| R. Pars Orbitalis | 2.4 ± 0.2 | 2.6 ± 0.2 | 0.030 |
| L. Pars Triangularis | 2.2 ± 0.2 | 2.2 ± 0.1 | 0.363 |
| R. Pars Triangularis | 2.2 ± 0.1 | 2.3 ± 0.2 | 0.015 |
| Anterior CingulateCortex | |||
| L. Rostral ACC | 2.4 ± 0.2 | 2.6 ± 0.3 | 0.008 |
| R. Rostral ACC | 2.7 ± 0.2 | 2.8 ± 0.3 | 0.318 |
| L. Caudal ACC | 2.6 ± 0.3 | 2.5 ± 0.3 | 0.396 |
| R. Caudal ACC | 2.4 ± 0.3 | 2.5 ± 0.3 | 0.074 |
| Inferior Parietal Lobe | |||
| L. Supramarginal | 2.2 ± 0.2 | 2.3 ± 0.2 | 0.005 |
| R. Supramarginal | 2.1 ± 0.2 | 2.3 ± 0.1 | < 0.001 |
| L. Inferior Parietal | 2.1 ± 0.2 | 2.2 ± 0.1 | 0.002 |
| R. Inferior Parietal | 2.1 ± 0.2 | 2.3 ± 0.2 | < 0.001 |
Bold indicates significant differences after adjustment using the Benhamini-Hochberg procedure for a false discovery rate (FDR) < 0.05
Abbreviations: AD = Alzheimer’s Disease, NC = Cognitively Normal Controls, ACC = Anterior Cingulate Cortex
3.2. Partial correlations for dominant and nondominant MINT
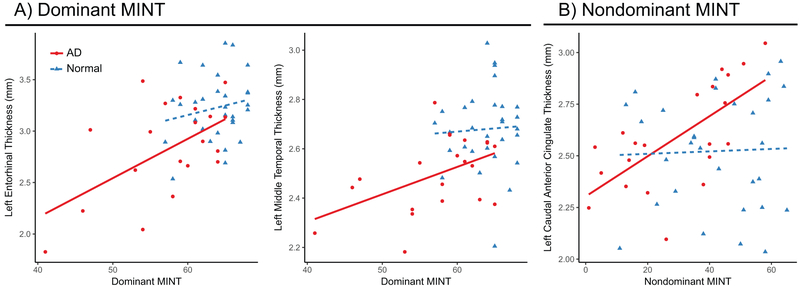
Partial correlations were used to examine the unique contributions of dominant and nondominant MINT scores, adjusted for age and education, on cortical thickness in each of our target ROIs in AD patients and NC controls separately. In the NC group, despite a larger sample size, no significant correlations emerged with any regions sampled (Supplemental Table 1). In the AD group, dominant language MINT performance showed a robust positive correlation with left entorhinal cortical thickness (partial r = 0.63, FDR adjusted p < 0.05; Figure 2A) and left middle temporal thickness (partial r = 0.63, FDR adjusted p < 0.05). In contrast, nondominant language MINT was positively associated only with left caudal ACC thickness (partial r = 0.68, FDR-adjusted p < 0.05; Figure 2B).
Figure 2. Relationship with Cortical Thickness.
Scatterplots of the significant correlations of A) dominant MINT with cortical thickness left temporal regions, and B) nondominant MINT with cortical thickness of the left caudal ACC. Patients with AD are red circles, while normal controls are in blue triangles.
3.3. Correlations with the Bilingual Index
The bilingual index provides a measure of relative degree of bilingualism by taking the ratio of nondominant to dominant naming ability. When using the bilingual index measure in place of the nondominant MINT in the regression, the pattern of results was unchanged, such that there was a correlation with only left caudal ACC thickness (partial r = 0.68, FDR-adjusted p < 0.05; Supplemental Figure 1). Three AD patients had bilingual index scores that were less than 10%, lower than any NC participants. When excluding these three cases, the correlation strengthened to a partial r of 0.70 (FDR-adjusted p < 0.05; Supplemental Figure 2).
4. Discussion
The goal of this study was to examine the distinct structural correlates of dominant and nondominant picture naming ability in bilinguals with AD. Previous work suggested that bilingualism is associated with increased grey matter density, thickness, or volume in cognitive control regions in healthy individuals (Abutalebi & Green, 2016), but it is unknown if decline in language ability in either or both languages is related to the integrity of these regions in AD.
Our analysis was specifically designed to address this question by subjecting the average cortical thickness for each a-priori defined region of interest to a regression analysis with both dominant and nondominant confrontation naming performance on the MINT, controlling for age and education. This approach allowed us to examine correlates of naming ability in the two languages in the AD brain, and revealed reliable differences between them.
As expected, thickness of the left entorhinal cortex and left middle temporal cortex correlated with dominant MINT performance. This result suggests that ability to name pictures in the dominant language primarily taps semantic processes that are known to be impaired in AD in conjunction with early atrophy and decreased metabolic activity in the temporal lobes (Domoto-Reilly, Sapolsky, Brickhouse, Dickerson, & Initiative, 2012; Galton et al., 2001; Melrose et al., 2009). The middle temporal gyrus in particular has been shown to be the common correlate of confrontation naming in AD, frontotemporal dementia, and corticobasal degeneration (Grossman et al., 2004). This region, along with the entorhinal cortex, exhibited significant thinning in AD compared to healthy controls (Table 2), suggesting that atrophy of these structures may compromise dominant language naming abilities. Our finding of a robust association between dominant MINT scores and cortical thickness in patients, but not in elderly control participants (Figure 2A), further suggests that ability to name pictures in the dominant language may reflect global cognitive impairment.
The nondominant language, in contrast, was positively correlated with thickness of the left caudal ACC in AD patients. The ACC has been postulated to be a common locus for domain-general cognitive control, particularly related to task monitoring and conflict/error detection (Aarts, Roelofs, & van Turennout, 2008; Carter & van Veen, 2007; Kerns et al., 2004). The ACC is recruited in tasks that require bilingual language control—e.g., language switching in production (e.g., Branzi, Della Rosa, Canini, Costa & Abutalebi, 2016; Guo, Liu, Misra, & Kroll, 2011; for review see Abutalebi & Green, 2007) and in auditory comprehension (Abutalebi et al., 2007; Blanco-Elorrieta & Pylkkänen, 2016; 2017), and has increased volume in aging bilinguals compared to monolinguals (Abutalebi, Guidi, et al., 2015). Branzi et al. (2016) reported increased ACC activity when using the nondominant language in comparison to the dominant language, and Abutalebi et al. (2008) specifically identified activation in the left ACC during use of the nondominant language. These fMRI studies suggest that the dorsal ACC region may be more engaged when processing a weaker language. Previous literature also suggests overlap between language control and cognitive control functions, with bilinguals showing activation in the ACC across both verbal and nonverbal tasks (e.g., Anderson, Chung-Fat-Yim, Bellana, Luk, & Bialystok, 2018; Weissberger, Gollan, Bondi, Clark, & Wierenga, 2015; for review see Hervais-Adelman, Davis, Johnsrude, Taylor, & Carlyon, 2011). For example, Abutalebi and colleagues (2012) found that bilinguals used the ACC more efficiently than monolinguals on a flanker task (a visual test of response inhibition), and bilinguals’ flanker task performance correlated with both grey matter density and BOLD response in the ACC.
Our finding of the association of nondominant language performance with thickness of the ACC in AD patients lends support to the idea that the same brain regions that are enhanced by bilingual language use are also important for the maintenance of bilingualism in AD. Indeed, the bilingual index (an objective measure of bilingualism) is at least as strongly related to ACC thickness as the nondominant language (Supplemental Figure 1 and 2). However, the correlation between bilingual ability and ACC thickness is apparent only in patients and not controls, despite the lack of a difference in the mean ACC thicknesses between the groups. This suggests that this region did not exhibit significant atrophy in our AD patients. Because all of the other major hubs of the cognitive control network (namely the IFG and IPL) did show severe atrophy in AD (Table 2), we speculatively interpret these findings as evidence that atrophy in these alternate control structures places a greater burden on the ACC, such that integrity of this last control hub becomes essential for the maintenance of bilingualism (and specifically nondominant language use). This relationship is masked by intact cognitive systems in normal controls. This is in line with the suggestion of Gold (2015) that bilingualism confers cognitive reserve through effects on the cognitive control networks rather than explicit memory systems, either through direct structural changes (see Abutalebi & Green, 2016) or development of alternative pathways which could be recruited to compensate for early disruptions.
Several limitations of the present study should be noted. First, the sample size was relatively small, precluding a voxel-wise whole-brain analysis. We instead chose to focus on regions of interest from the literature which may introduce bias and miss unexpected results. Given this smaller sample size, caution should be taken in interpreting any null findings, and future replication with different groups of bilinguals is warranted. Second, the AD and NC groups differed in education by approximately 4 years. While we adjusted for education in regression analyses, the potential for nonlinear interactions between brain changes, education and bilingualism warrants further study. This point is emphasized by the results of Gollan and colleagues (2011) who found that degree of bilingualism was associated with age of AD diagnosis only for participants with low education level. This suggests an upper limit to the extent to which cognitive reserve can function to delay dementia.
In contrast to these limitations, the sample was a well-characterized group of Spanish-English bilinguals who had undergone thorough clinical and neuropsychological evaluations that included objective measures of language ability in both languages. The use of the MINT administered in both languages allowed measurement of language ability as a continuous variable for each language, and allowed degree of bilingualism to be measured in an objective manner on a continuous scale. This approach provides significant advantages over most previous studies that performed group comparisons by treating bilingualism as a dichotomous variable based upon the participant’s self-report of their proficiency (which is not as powerful or reliable as the MINT in measuring language proficiency; Tomoschuk, Ferreira, & Gollan, 2018).
5. Conclusions
We examined cortical structural correlates of dominant and nondominant language abilities in patients with AD and cognitively normal adults, controlling for age and education. Correlations between dominant language naming performance and measures of cortical thickness were identified in the left entorhinal cortex and left middle temporal gyrus. These results are consistent with the temporal lobe atrophy and decline in naming that occur in AD. In contrast, we found a robust correlation between nondominant language naming performance and thickness of the left caudal ACC, a region associated with conflict monitoring and language switching. This association was only apparent in bilinguals with AD, and suggests that AD-related atrophy may increase reliance on the ACC for the control requirements of nondominant language use.
Supplementary Material
Table 3.
Partial Correlations of Dominant and Nondominant Language with Cortical Thickness in AD
| Dominant MINT | Nondominant MINT | |||
|---|---|---|---|---|
| Partial r | p- value | Partial r | p- value | |
| Temporal Lobe | ||||
| L. Entorhinal | 0.63 | 0.005 | −0.38 | 0.115 |
| R. Entorhinal | 0.54 | 0.021 | −0.37 | 0.134 |
| L. Middle Temporal | 0.63 | 0.005 | −0.36 | 0.142 |
| R. Middle Temporal | 0.28 | 0.257 | −0.29 | 0.248 |
| L. Temporal Pole | 0.34 | 0.162 | 0.12 | 0.640 |
| R. Temporal Pole | 0.42 | 0.082 | −0.10 | 0.707 |
| Inferior Frontal Gyrus | ||||
| L. Pars Orbitalis | 0.09 | 0.718 | −0.10 | 0.701 |
| R. Pars Orbitalis | −0.03 | 0.903 | −0.22 | 0.376 |
| L. Pars Triangularis | 0.34 | 0.164 | −0.16 | 0.519 |
| R. Pars Triangularis | 0.21 | 0.405 | −0.35 | 0.151 |
| Anterior CingulateCortex | ||||
| L. Rostral ACC | 0.19 | 0.452 | −0.03 | 0.900 |
| R. Rostral ACC | −0.08 | 0.761 | 0.02 | 0.946 |
| L. Caudal ACC | 0.07 | 0.782 | 0.68 | 0.002 |
| R. Caudal ACC | −0.47 | 0.048 | 0.03 | 0.906 |
| Inferior Parietal Lobe | ||||
| L. Supramarginal | 0.47 | 0.049 | −0.36 | 0.144 |
| R. Supramarginal | 0.27 | 0.273 | −0.34 | 0.165 |
| L. Inferior Parietal | 0.45 | 0.062 | −0.39 | 0.109 |
| R. Inferior Parietal | 0.50 | 0.037 | −0.42 | 0.082 |
Bold indicates significant differences after adjustment using the Benhamini-Hochberg procedure for a false discovery rate (FDR) < 0.05
Abbreviations: AD = Alzheimer’s Disease, ACC = Anterior Cingulate Cortex
Highlights.
Dominant language naming was associated with temporal cortical thickness in AD
Nondominant language naming correlated with thickness of the left caudal ACC in AD
No structural correlates of naming were apparent in controls for either language
Results suggests increased reliance on the ACC for nondominant language use in AD
Acknowledgements
We wish to thank the UCSD Shiley-Marcos Alzheimer’s Disease Research Center faculty and staff. This work was supported by the National Institutes of Health (P50 AG05131) and the National Institute on Deafness and Other Communication Disorders (011492). AS was additionally funded by a Ruth L. Kirschstein National Research Service Award (NRSA) Individual Predoctoral Fellowship (1F31AG058379-01). There were no conflicts of interest in completing this research.
Footnotes
Declarations of Interest
DSS reports no disclosures. AS reports no disclosures. DPS serves as a consultant for Aptinx, Inc. and Biogen MA, Inc. DG serves as editor for Alzheimer’s Research and Therapy, and as a paid consultant on Data Safety Monitoring Boards for Pfizer, Inc., Elan, Inc., and Balance Pharmaceuticals, Inc. JBB has served on advisory boards for Elan, Bristol-Myers Squibb, Avanir, Novartis, Genentech, and Eli Lilly and holds stock options in CorTechs Labs, Inc and Human Longevity, Inc. THG reports no disclosures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aarts E, Roelofs A, & van Turennout M (2008). Anticipatory activity in anterior cingulate cortex can be independent of conflict and error likelihood. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 28(18), 4671–4678. 10.1523/JNEUROSCI.4400-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abutalebi J, Annoni J-M, Zimine I, Pegna AJ, Seghier ML, Lee-Jahnke H, … Khateb A (2008). Language control and lexical competition in bilinguals: an event-related FMRI study. Cerebral Cortex (New York, N.Y.: 1991), 18(7), 1496–1505. 10.1093/cercor/bhm182 [DOI] [PubMed] [Google Scholar]
- Abutalebi J, Brambati SM, Annoni J-M, Moro A, Cappa SF, & Perani D (2007). The neural cost of the auditory perception of language switches: An event-related functional magnetic resonance imaging study in bilinguals. Journal of Neuroscience, 27, 13762–13769. 10.1523/JNEUROSCI.3294-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abutalebi J, Canini M, Della Rosa PA, Green DW, & Weekes BS (2015). The neuroprotective effects of bilingualism upon the inferior parietal lobule: A Structural Neuroimaging Study in Aging Chinese Bilinguals. Journal of Neurolinguistics, 33, 3–13. 10.1016/jjneuroling.2014.09.008 [DOI] [Google Scholar]
- Abutalebi J, Canini M, Della Rosa PA, Sheung LP, Green DW, & Weekes BS (2014). Bilingualism protects anterior temporal lobe integrity in aging. Neurobiology of Aging, 35(9), 2126–2133. [DOI] [PubMed] [Google Scholar]
- Abutalebi J, Della Rosa PA, Green DW, Hernandez M, Scifo P, Keim R, … Costa A (2012). Bilingualism tunes the anterior cingulate cortex for conflict monitoring. Cerebral Cortex (New York, N.Y.: 1991), 22(9), 2076–2086. 10.1093/cercor/bhr287 [DOI] [PubMed] [Google Scholar]
- Abutalebi J, & Green D (2007). Bilingual language production: The neurocognition of language representation and control. Journal of Neurolinguistics, 20(3), 242–275. 10.1016/j.jneuroling.2006.10.003 [DOI] [Google Scholar]
- Abutalebi J, & Green DW (2016). Neuroimaging of language control in bilinguals: neural adaptation and reserve. Bilingualism: Language and Cognition, 19(4), 689–698. 10.1017/S1366728916000225 [DOI] [Google Scholar]
- Abutalebi J, Guidi L, Borsa V, Canini M, Della Rosa PA, Parris BA, & Weekes BS (2015). Bilingualism provides a neural reserve for aging populations. Neuropsychologia, 69, 201–210. 10.1016/j.neuropsychologia.2015.01.040 [DOI] [PubMed] [Google Scholar]
- Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, … Phelps CH (2011). The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & Dementia, 7(3), 270–279. 10.1016/j.jalz.2011.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JAE, Chung-Fat-Yim A, Bellana B, Luk G, & Bialystok E (2018). Language and cognitive control networks in bilinguals and monolinguals. Neuropsychologia, 117, 352–363. https://doi.Org/10.1016/j.neuropsychologia.2018.06.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardila A, & Ramos E (2008). Normal and abnormal aging in bilinguals. Dementia & Neuropsychologia, 2(4), 242–247. 10.1590/S1980-57642009DN20400002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bak TH, & Alladi S (2014). Can being bilingual affect the onset of dementia? Future Neurology, 9(2), 101–103. 10.2217/fnl.14.8 [DOI] [Google Scholar]
- Bakkour A, Morris JC, & Dickerson BC (2009). The cortical signature of prodromal AD Regional thinning predicts mild AD dementia. Neurology, 72(12), 1048–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balota DA, Watson JM, Duchek JM, & Ferraro FR (1999). Cross-modal semantic and homograph priming in healthy young, healthy old, and in Alzheimer’s disease individuals. Journal of the International Neuropsychological Society: JINS, 5(7), 626–640. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, & Hochberg Y (1995). Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society. Series B (Methodological), 289–300. [Google Scholar]
- Bialystok E (2011). Reshaping the mind: The benefits of bilingualism. Canadian Journal of Experimental Psychology/Revue Canadienne de Psychologie Experimental, 65(4), 229–235. 10.1037/a0025406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bialystok E (2017). The bilingual adaptation: How minds accommodate experience. Psychological Bulletin, 143(3), 233–262. 10.1037/bul0000099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bialystok E, Craik FIM, & Freedman M (2007). Bilingualism as a protection against the onset of symptoms of dementia. Neuropsychologia, 45(2), 459–464. 10.1016/j.neuropsychologia.2006.10.009 [DOI] [PubMed] [Google Scholar]
- Blanco-Elorrieta E, & Pylkkänen L (2016). Bilingual language control in perception versus action: MEG reveals comprehension control mechanisms in anterior cingulate cortex and domain-general control of production in dorsolateral prefrontal cortex. Journal of Neuroscience, 36, 290–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco-Elorrieta E, & Pylkkänen L (2017). Bilingual language switching in the laboratory versus in the wild: The spatiotemporal dynamics of adaptive language control. Journal of Neuroscience, 37, 9022–9036. 10.1523/JNEUROSCI.0553-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branzi FM, Della Rosa PA, Canini M, Costa A, & Abutalebi J (2016). Language control in bilinguals: monitoring and response selection. Cerebral Cortex, 26(6), 2367–2380. [DOI] [PubMed] [Google Scholar]
- Calabria M, Cattaneo G, Marne P, Hernández M, Juncadella M, Gascón-Bayarri J, … Costa A (2017). Language deterioration in bilingual Alzheimer’s disease patients: A longitudinal study. Journal of Neurolinguistics, 43, 59–74. 10.1016/j.jneuroling.2016.06.005 [DOI] [Google Scholar]
- Carter CS, & van Veen V (2007). Anterior cingulate cortex and conflict detection: an update of theory and data. Cognitive, Affective & Behavioral Neuroscience, 7(4), 367–379. [DOI] [PubMed] [Google Scholar]
- Chang Y-L, Bondi MW, Fennema-Notestine C, McEvoy LK, Hagler DJ, Jacobson MW, & Dale AM (2010). Brain substrates of learning and retention in mild cognitive impairment diagnosis and progression to Alzheimer’s disease. Neuropsychologia, 48(5), 1237–1247. https://doi.Org/10.1016/j.neuropsychologia.2009.12.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chertkow H, Whitehead V, Phillips N, Wolfson C, Atherton J, & Bergman H (2010). Multilingualism (but not always bilingualism) delays the onset of Alzheimer disease: evidence from a bilingual community. Alzheimer Disease and Associated Disorders, 24(2), 118–125. 10.1097/WAD.0b013e3181ca1221 [DOI] [PubMed] [Google Scholar]
- Costa A, Calabria M, Marne P, Hernández M, Juncadella M, Gascón-Bayarri J, … Reñé R (2012). On the parallel deterioration of lexico-semantic processes in the bilinguals’ two languages: Evidence from Alzheimer’s disease. Neuropsychologia, 50(5), 740–753. 10.1016/j.neuropsychologia.2012.01.008 [DOI] [PubMed] [Google Scholar]
- Craik FIM, Bialystok E, & Freedman M (2010). Delaying the onset of Alzheimer disease. Neurology, 75(19), 1726–1729. 10.1212/WNL.0b013e3181fc2a1c [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale AM, Fischl B, & Sereno MI (1999). Cortical surface-based analysis: I. Segmentation and surface reconstruction. Neuroimage, 9(2), 179–194. [DOI] [PubMed] [Google Scholar]
- Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, … Hyman BT (2006). An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage, 31(3), 968–980. [DOI] [PubMed] [Google Scholar]
- Dickerson BC, Feczko E, Augustinack JC, Pacheco J, Morris JC, Fischl B, & Buckner RL (2009). Differential effects of aging and Alzheimer’s disease on medial temporal lobe cortical thickness and surface area. Neurobiology of Aging, 30(3), 432–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson BC, Goncharova I, Sullivan MP, Forchetti C, Wilson RS, Bennett DA, & Beckett LA (2001). MRI-derived entorhinal and hippocampal atrophy in incipient and very mild Alzheimer’s diseased☆. Neurobiology of Aging, 22(5), 747–754. [DOI] [PubMed] [Google Scholar]
- Domoto-Reilly K, Sapolsky D, Brickhouse M, Dickerson BC, & Initiative ADN (2012). Naming impairment in Alzheimer’s disease is associated with left anterior temporal lobe atrophy. Neuroimage, 63(1), 348–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y, & Li P (2015). The Cognitive Science of Bilingualism. Language and Linguistics Compass, 9(1), 1–13. 10.1111/Inc3.12099 [DOI] [Google Scholar]
- Duncan HD, Nikelski J, Pilon R, Steffener J, Chertkow H, & Phillips NA (2018). Structural brain differences between monolingual and multilingual patients with mild cognitive impairment and Alzheimer disease: Evidence for cognitive reserve. Neuropsychologia, 109, 270–282. 10.1016/j.neuropsychologia.2017.12.036 [DOI] [PubMed] [Google Scholar]
- Fischl B, & Dale AM (2000). Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proceedings of the National Academy of Sciences, 97(20), 11050–11055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Liu A, & Dale AM (2001). Automated manifold surgery: constructing geometrically accurate and topologically correct models of the human cerebral cortex. IEEE Transactions on Medical Imaging, 20(1), 70–80. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, …Klaveness S (2002). Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron, 33(3), 341–355. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, & Dale AM (1999). Cortical surface-based analysis: II: inflation, flattening, and a surface-based coordinate system. Neuroimage, 9(2), 195–207. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Tootell RB, & Dale AM (1999). High-resolution intersubject averaging and a coordinate system for the cortical surface. Human Brain Mapping, 8(4), 272–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Van Der Kouwe A, Destrieux C, Halgren E, Ségonne F, Salat DH, … Kennedy D (2004). Automatically parcellating the human cerebral cortex. Cerebral Cortex, 14(1), 11–22. [DOI] [PubMed] [Google Scholar]
- Galton CJ, Patterson K, Graham K, Lambon-Ralph MA, Williams G, Antoun N, … Hodges JR (2001). Differing patterns of temporal atrophy in Alzheimer’s disease and semantic dementia. Neurology, 57(2), 216–225. [DOI] [PubMed] [Google Scholar]
- Gold BT (2015). Lifelong bilingualism and neural reserve against Alzheimer’s disease: A review of findings and potential mechanisms. Behavioural Brain Research, 281, 9–15. 10.1016/j.bbr.2014.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollan TH, Salmon DP, Montoya RI, & da Pena E (2010). Accessibility of the nondominant language in picture naming: A counterintuitive effect of dementia on bilingual language production. Neuropsychologia, 48(5), 1356–1366. 10.1016/j.neuropsychologia.2009.12.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollan TH, Salmon DP, Montoya RI, & Galasko DR (2011). Degree of bilingualism predicts age of diagnosis of Alzheimer’s disease in low-education but not in highly educated Hispanics. Neuropsychologia, 49(14), 3826–3830. 10.1016/j.neuropsychologia.2011.09.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollan TH, Weissberger GH, Runnqvist E, Montoya RI, & Cera CM (2012). Self-ratings of spoken language dominance: A Multilingual Naming Test (MINT) and preliminary norms for young and aging Spanish-English bilinguals. Bilingualism: Language and Cognition, 15(3), 594–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green DW, & Abutalebi J (2013). Language control in bilinguals: The adaptive control hypothesis. Journal of Cognitive Psychology, 25(5), 515–530. 10.1080/20445911.2013.796377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman M, McMillan C, Moore P, Ding L, Glosser G, Work M, & Gee J (2004). What’s in a name: voxel-based morphometric analyses of MRI and naming difficulty in Alzheimer’s disease, frontotemporal dementia and corticobasal degeneration. Brain, 127(3), 628–649. [DOI] [PubMed] [Google Scholar]
- Guo T, Liu H, Misra M, & Kroll JF (2011). Local and global inhibition in bilingual word production: fMRI evidence from Chinese-English bilinguals. NeuroImage, 56, 2300–2309. 10.1016/j.neuroimage.2011.03.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzmán-Vélez E, & Tranel D (2015). Does bilingualism contribute to cognitive reserve? Cognitive and neural perspectives. Neuropsychology, 29(1), 139–150. 10.1037/neu0000105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hervais-Adelman AG, Davis MH, Johnsrude IS, Taylor KJ, & Carlyon RP (2011). Generalization of perceptual learning of vocoded speech. Journal of Experimental Psychology. Human Perception and Performance, 37(1), 283–295. 10.1037/a0020772 [DOI] [PubMed] [Google Scholar]
- Ivanova I, Salmon DP, & Gollan TH (2013). The Multilingual Naming Test in Alzheimer’s Disease: Clues to the Origin of Naming Impairments. Journal of the International Neuropsychological Society, 19(3), 272–283. 10.1017/S1355617712001282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanova I, Salmon DP, & Gollan TH (2014). Which Language Declines More? Longitudinal versus Cross-sectional Decline of Picture Naming in Bilinguals with Alzheimer’s Disease. Journal of the International Neuropsychological Society, 20(5), 534–546. 10.1017/S1355617714000228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan E (1983). The assessment of aphasia and related disorders (Vol. 2). Lippincott Williams & Wilkins. [Google Scholar]
- Kerns JG, Cohen JD, MacDonald AW, Cho RY, Stenger VA, & Carter CS (2004). Anterior cingulate conflict monitoring and adjustments in control. Science (New York, N.Y.), 303(5660), 1023–1026. 10.1126/science.1089910 [DOI] [PubMed] [Google Scholar]
- Klein D, Mok K, Chen J-K, & Watkins KE (2014). Age of language learning shapes brain structure: A cortical thickness study of bilingual and monolingual individuals. Brain and Language, 131, 20–24. 10.1016/j.bandl.2013.05.014 [DOI] [PubMed] [Google Scholar]
- Kroll JF, & Bialystok E (2013). Understanding the consequences of bilingualism for language processing and cognition. Journal of Cognitive Psychology, 25(5), 497–514. 10.1080/20445911.2013.799170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehtonen M, Soveri A, Laine A, Järvenpää J, de Bruin A, & Antfolk J (2018). Is bilingualism associated with enhanced executive functioning in adults? A meta-analytic review. Psychological Bulletin, 144(4), 394–425. 10.1037/bul0000142 [DOI] [PubMed] [Google Scholar]
- McDonald CR, Gharapetian L, McEvoy LK, Fennema-Notestine C, Hagler DJ, Holland D, & Dale AM (2012). Relationship between regional atrophy rates and cognitive decline in mild cognitive impairment. Neurobiology of Aging, 33(2), 242–253. 10.1016/j.neurobiolaging.2010.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEvoy LK, Fennema-Notestine C, Roddey JC, Hagler DJ Jr, Holland D, Karow DS, … Dale AM (2009). Alzheimer disease: quantitative structural neuroimaging for detection and prediction of clinical and structural changes in mild cognitive impairment. Radiology, 251(1), 195–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Kawas CH, … Phelps CH (2011). The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & Dementia, 7(3), 263–269. 10.1016/j.jalz.2011.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechelli A, Crinion JT, Noppeney U, O’Doherty J, Ashburner J, Frackowiak RS, & Price CJ (2004). Neurolinguistics: Structural plasticity in the bilingual brain. Nature, 431(7010), 757 10.1038/431757a [DOI] [PubMed] [Google Scholar]
- Melrose RJ, Campa OM, Harwood DG, Osato S, Mandelkern MA, & Sultzer DL (2009). The neural correlates of naming and fluency deficits in Alzheimer’s disease: an FDG-PET study. International Journal of Geriatric Psychiatry: A Journal of the Psychiatry of Late Life and Allied Sciences, 24(8), 885–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez MF, Perryman KM, Pontón MO, & Cummings JL (1999). Bilingualism and Dementia. The Journal of Neuropsychiatry and Clinical Neurosciences, 11(3), 411–412. https://doi.Org/10.1176/jnp.11.3.411 [DOI] [PubMed] [Google Scholar]
- Nebes RD, Martin DC, & Horn LC (1984). Sparing of semantic memory in Alzheimer’s disease. Journal of Abnormal Psychology, 93(3), 321. [DOI] [PubMed] [Google Scholar]
- Niendam TA, Laird AR, Ray KL, Dean YM, Glahn DC, & Carter CS (2012). Meta-analytic evidence for a superordinate cognitive control network subserving diverse executive functions. Cognitive, Affective & Behavioral Neuroscience, 12(2), 241–268. 10.3758/s13415-011-0083-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowrangi MA, Lyketsos C, Rao V, & Munro CA (2014). Systematic Review of Neuroimaging Correlates of Executive Functioning: Converging Evidence From Different Clinical Populations. The Journal of Neuropsychiatry and Clinical Neurosciences, 26(2), 114–125. 10.1176/appi.neuropsych.12070176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ober BA, Shenaut GK, & Reed BR (1995). Assessment of associative relations in Alzheimer’s disease: Evidence for preservation of semantic memory. Aging, Neuropsychology, and Cognition, 2(4), 254–267. [Google Scholar]
- Obler L, Albert M, & Lozowick S (1986). The Aging Bilingual In Vaid J (Ed.), Language Processing in Bilinguals: Psycholinguistic and Neuropsychological Perspectives (pp. 221–231). Hillsdale, NJ: Erlbaum. [Google Scholar]
- Olsen RK, Pangelinan MM, Bogulski C, Chakravarty MM, Luk G, Grady CL, & Bialystok E (2015). The effect of lifelong bilingualism on regional grey and white matter volume. Brain Research, 1612, 128–139. 10.1016/j.brainres.2015.02.034 [DOI] [PubMed] [Google Scholar]
- Pa J, Boxer A, Chao LL, Gazzaley A, Freeman K, Kramer J, … Johnson JK (2009). Clinical-Neuroimaging Characteristics of Dysexecutive Mild Cognitive Impairment. Annals of Neurology, 65(4), 414–423. 10.1002/ana.21591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paap KR, & Greenberg ZI (2013). There is no coherent evidence for a bilingual advantage in executive processing. Cognitive Psychology, 66(2), 232–258. 10.1016/j.cogpsych.2012.12.002 [DOI] [PubMed] [Google Scholar]
- Patterson K, Nestor PJ, & Rogers TT (2007). Where do you know what you know? The representation of semantic knowledge in the human brain. Nature Reviews Neuroscience, 8(12), 976–987. 10.1038/nrn2277 [DOI] [PubMed] [Google Scholar]
- Ries ML, Carlsson CM, Rowley HA, Sager MA, Gleason CE, Asthana S, & Johnson SC (2008). Magnetic resonance imaging characterization of brain structure and function in mild cognitive impairment: a review. Journal of the American Geriatrics Society, 56(5), 920–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabuncu MR, Desikan RS, Sepulcre J, Yeo BTT, Liu H, Schmansky NJ, … Initiative, for the A. D. N. (2011). The Dynamics of Cortical and Hippocampal Atrophy in Alzheimer Disease. Archives of Neurology, 68(8), 1040–1048. 10.1001/archneurol.2011.167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer TA, Ware J, Fischer CE, Craik FIM, & Bialystok E (2012). Bilingualism as a contributor to cognitive reserve: Evidence from brain atrophy in Alzheimer’s disease. Cortex, 48(8), 991–996. https://doi.Org/10.1016/j.cortex.2011.04.009 [DOI] [PubMed] [Google Scholar]
- Stein M, Federspiel A, Koenig T, Wirth M, Strik W, Wiest R, … Dierks T (2012). Structural plasticity in the language system related to increased second language proficiency. Cortex, 48(4), 458–465. 10.1016/j.cortex.2010.10.007 [DOI] [PubMed] [Google Scholar]
- Storey JD, Taylor JE, & Siegmund D (2004). Strong control, conservative point estimation and simultaneous conservative consistency of false discovery rates: a unified approach. Journal of the Royal Statistical Society: Series B (Statistical Methodology), 66(1), 187–205. [Google Scholar]
- Tomoschuk B, Ferreira VS, & Gollan TH (2018). When a seven is not a seven: Self-ratings of bilingual language proficiency differ between and within language populations. Bilingualism: Language and Cognition, 1–21. 10.1017/S1366728918000421 [DOI] [Google Scholar]
- Weissberger GH, Gollan TH, Bondi MW, Clark LR, & Wierenga CE (2015). Language and task switching in the bilingual brain: Bilinguals are staying, not switching, experts. Neuropsychologia, 66, 193–203. 10.1016/j.neuropsychologia.2014.10.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westman E, Simmons A, Muehlboeck J-S, Mecocci P, Vellas B, Tsolaki M, … Wahlund L-O (2011). AddNeuroMed and ADNI: Similar patterns of Alzheimer’s atrophy and automated MRI classification accuracy in Europe and North America. NeuroImage, 58(3), 818–828. 10.1016/j.neuroimage.2011.06.065 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.