Conspectus
Calprotectin (CP) is a versatile player in the metal-withholding innate immune response, a process termed “nutritional immunity.” CP is a heterooligomer of the polypeptides S100A8 and S100A9, and it houses two transition-metal-binding sites at its S100A8/S100A9 heterodimer interface. During infection, CP is released from host cells and sequesters “bioavailable” transition metal ions in the extracellular space, thereby preventing microbial acquisition of these essential nutrients. For many years, the role of CP in nutritional immunity was interpreted in the contexts of Mn(II) and Zn(II) limitation, but recent work has broadened our understanding of its contributions to this process. We uncovered that CP provides a form of nutritional immunity that has previously received little attention: the battle between host and microbe for ferrous iron (Fe(II)). In this Account, we present our current understanding of Fe(II) coordination by CP and its role in Fe(II) withholding, as well as considerations for future discovery.
Nutritional immunity was first described in the context of host-microbe competition for ferric iron (Fe(III)). The battle for Fe(II) has received comparably little attention because the abundance of Fe(II) at infection sites and the importance of Fe(II) acquisition for microbial pathogenesis was recognized only recently. Several years ago, we discovered that human CP sequesters Fe(II) at its His6 site with sub-picomolar affinity, and thus hypothesized that it provides a means for Fe(II) limitation by the host during microbial infection. Fe(II) coordination by CP is unprecedented in biology because of its novel hexahistidine coordination sphere and its high-affinity binding which surpasses that of other known Fe(II)-binding proteins. CP is also capable of shifting the Fe redox equilibrium by stabilizing Fe(II) in aerobic solution, and can thereby sequester Fe in both reducing and non-reducing environments. These coordination chemistry studies allowed us to hypothesize that CP provides a means for Fe(II) limitation by the host during microbial infection. While investigating this putative Fe(II)-sequestering function, we discovered that CP withholds Fe from diverse bacterial pathogens. Recent studies by our lab and others of the bacterial pathogens Pseudomonas aeruginosa and Acinetobacter baumannii have shown that, by preventing sufficient Fe acquisition, CP induces Fe starvation responses in these organisms. As a result, CP affects bacterial virulence and metabolism. We also elucidated a complex interplay between CP and secondary metabolites produced by P. aeruginosa during the competition for Fe. Our work provides a foundation for understanding how CP affects Fe homeostasis during microbial infection. We believe that understanding how bacterial physiology is altered when challenged with Fe(II) withholding by CP will likely reveal crucial determinants of bacterial survival within the host.
Graphical Abstract
Introduction
Calprotectin (CP) is known for its role in the metal-withholding innate immune response, a process termed “nutritional immunity.”1-2 This protein was first identified because of its abundance in the tissues of patients afflicted with inflammatory disorders.3-7 The name CP comes from two defining characteristics that were revealed during initial studies: its ability to bind calcium and antimicrobial acitivity.8-9 Early on, researchers found that the antimicrobial activity of CP was attenuated with the addition of Zn(II), providing the first clue to its contributions to the biology of transition metal ions.10 Subsequently, two seminal studies — the crystallographic structural evaluation of the Ca(II)-bound protein11 and a compelling report that CP sequesters Mn(II) at infection sites12 — motivated our lab to investigate its biological coordination chemistry. As a result of our studies and those of others, we now appreciate that CP is a remarkable and functionally versatile component of nutritional immunity.
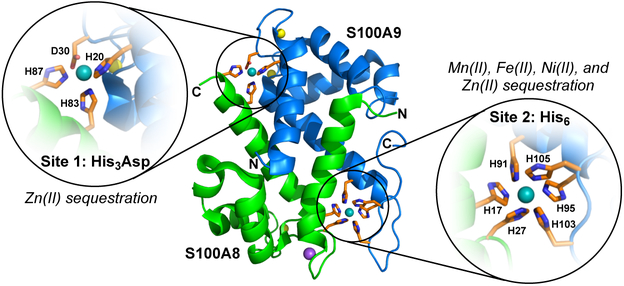
CP is a heterooligomer of two S100 polypeptides: S100A8 and S100A9. Each polypeptide possesses two EF-hand Ca(II)-binding domains. In addition, two transition-metal-binding sites form at the S100A8/S100A9 heterodimer interface: a His3Asp motif and a His6 motif (Figure 1).13 In human CP, the His3Asp site is composed of H83 and H87 of S100A8 and H20 and D30 of S100A9. The His6 site is formed by H17 and H27 of S100A8 and H91, H95, H103 and H105 of S100A9. The His3Asp site is selective for Zn(II), whereas the His6 site sequesters Mn(II), Fe(II), Ni(II), and Zn(II) with high affinity (Figure 1).13 CP also sequesters Cu, but further coordination chemistry studies are required to define the relevant site(s) and whether the protein preferentially binds Cu(II) or Cu(I).14 Various types of white blood cells and epithelial cells produce CP, and the protein is particularly abundant in neutrophils where it is reported to constitute ≥40% of total cytoplasmic protein.3, 15-16 In the current working model, which focuses the sequestration of nutrient metal ions in the extracellular space, CP is stored in the cytoplasm, which has low levels of Ca(II) (i.e. nanomolar) under resting conditions. Upon release into the extracellular space, CP encounters high levels of Ca (≈2 mM),17 binds Ca(II) at the EF-hand domains, and undergoes an oligomeric change from a heterodimer to a heterotetramer.18 In its Ca(II)-bound heterotetrameric form, CP exhibits greater antimicrobial activity attributable to the enhanced transition-metal affinities at both binding sites.13
Figure 1.
Crystal structure of Ni(II)-, Ca(II), and Na(I)-bound CP-Ser (PDB 5WIF).19 CP-Ser is composed of the S100A8(C42S) and S100A9(C3S) subunits. This variant has been employed for many metal-binding and microbiology studies.20 A heterodimer unit is taken from the structure of the heterotetramer. S100A8 is shown in green; S100A9 is shown in blue; Ni(II)-binding residues are shown in orange; Ni(II) is shown in teal; Ca(II) is shown in yellow; Na(I) is shown in purple. The N- and C-termini of S100A8 and S100A9 are labeled. The His3Asp site is shown expanded on the left of the dimer, and the His6 site is shown expanded on the right of the dimer.
In this Account, we focus on recent studies that uncovered the Fe(II)-withholding function of CP. We present our current understanding of Fe(II) coordination by CP and how CP impacts microbial Fe homeostasis. Our early investigations revealed that CP sequesters Fe(II) with high affinity at its biologically-unique His6 site 21 and shifts the redox equilibrium of Fe from Fe(III) to Fe(II) in solution.22 Moreover, contributions from our lab and others have provided compelling evidence that CP (i) inhibits microbial Fe acquisition,21, 23-25 (ii) induces Fe starvation responses in bacterial pathogens,23-24 and (iii) affects pathways that are important for survival and virulence as a consequence of Fe limitation.23-24 Our work also revealed that microbial metabolites modulate the Fe(II)-sequestering ability of CP.22-23 Taken together, our investigations of CP and Fe provide a foundation for future studies directed at elucidating the effect of this host-defense protein on Fe homeostasis in diverse microbial pathogens.
Discovery of Fe(II) sequestration by CP
Initially, the contributions of CP to nutritional immunity were only considered in the contexts of Mn(II) and Zn(II) withholding.13 Although several reports indicated that CP neither binds Fe nor contributes to Fe homeostasis,12, 26 and thus did not link CP to an Fe-withholding innate immune response, two lines of thought motivated our exploration of its Fe-sequestering properties.21 First, based on seminal studies of Mn(II) and Zn(II) sequestration at the His6 site, and the coordination chemistry principles defined by Irving-Williams series, we reasoned that CP coordinates divalent metal ions that fall between Mn(II) and Zn(II) on the periodic table at this site.21, 27 Second, from the perspective of microbial metabolism, CP possesses antibacterial activity against both Mn-centric or Fe-centric bacteria,26-28 which provided a clue that CP may inhibit the growth of Fe-centric microbes by withholding Fe.
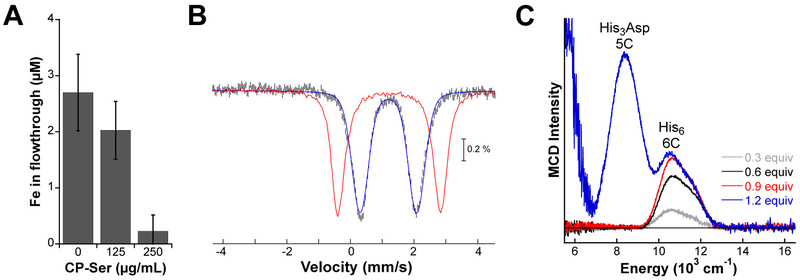
We first conducted an unbiased evaluation of metal binding by determining which metals CP20 depletes from bacterial growth medium. This assay revealed that CP depletes medium of Fe, Ni, and Cu in addition to Mn and Zn.21 Moreover, enhanced Fe depletion occurred in the presence of an exogenous reducing agent, suggesting that CP prefers to bind Fe under reducing conditions that favor the +2 oxidation state (Figure 2a). By examining CP variants that have the coordinating residues of either metal-binding site mutated to alanine, we determined that the His6 site was responsible for Fe depletion. These observations motivated our biophysical investigations of how CP coordinates Fe and whether this property has implications for microbial physiology.21, 23
Figure 2.
Fe(II) binding by CP.21, 30 (A) Depletion of Fe from Tris:TSB medium [62:38 20 mM Tris, 100 mM NaCl, pH 7.5:Tryptic soy broth (TSB) medium] supplemented with ~3 mM β-mercaptoethanol and ≈2 mM Ca(II) by CP-Ser.21 The mean and SDM are reported (n = 5). (B) The 4.2 K/53 mT Mössbauer spectrum for 57Fe(II)-bound CP-Ser prepared with excess Ca(II) and 0.83 equiv of 57Fe(II) sulfate per CP heterodimer is shown as black vertical parts.21 The simulation of this spectrum as a single quadrupole doublet with an isomer shift (δ) of 1.20 mm/s and a quadrupole splitting parameter (ΔEQ) of 1.78 mm/s is shown as the blue line. The Mössbauer spectrum of 57Fe(II) sulfate in 50 mM Tris, pH 7.5 is shown as the red line.21 (C) The 5 K, 7 T NIR MCD spectra for the titration of CP-Ser with Fe(II) in the presence of excess Ca(II).30 Panel C was reproduced with permission from ref. 30. Copyright 2017 the Royal Society of Chemistry.
Fe(II) coordination by CP
Both the ferric [Fe(III)] and ferrous [Fe(II)] oxidation states of Fe are common in biology. Building upon our initial metal-depletion studies, we investigated the binding preference of CP for Fe(III) vs. Fe(II), and found that CP binds Fe(II) but has negligible affinity for Fe(III) under conditions of low Ca(II).21, 29 In collaboration with the Krebs laboratory, we employed Mössbauer spectroscopy to study the Fe(II)-binding characteristics of CP and ΔHis3Asp. This study demonstrated that both proteins bind high-spin Fe(II) in an octahedral coordination sphere with essentially identical isomer shifts, indicating that the His6 site is the major Fe(II)-binding site in CP (Figure 2b).21 We note that the Mössbauer spectroscopy samples contained ~0.8 equiv of Fe(II) per heterodimer and excess Ca(II), which resulted in only the His6 site of CP being populated with Fe(II). Subsequent analyses using magnetic circular dichroism (MCD) spectroscopy, performed in collaboration with the Neidig laboratory, further supported the Fe(II)-His6 coordination motif.30 This Fe(II) coordination sphere expands the known coordination motifs of nonheme Fe proteins.21, 30 Moreover, we extended the MCD spectroscopy studies to samples prepared with varying Ca(II) and Fe(II) concentrations. This effort revealed that the His3Asp site binds Fe(II) in a distorted five-coordinate Fe(II) geometry. Both the Fe(II)-binding titrations monitored by MCD spectroscopy (Figure 2c)30 and the initial metal-depletion assays21 indicated that the His3Asp site has a lower Fe(II) affinity than the His6 site, and we currently have no evidence supporting a role for the His3Asp site in Fe(II) withholding.
The Fe(II)-binding affinity of the His6 site was evaluated by competing CP against ZP1, a metal-ion sensor with an apparent Kd,Fe(II) = 2.2 ± 0.3 pM for Fe(II) at pH 7.0.21 These competition titrations demonstrated that CP was unable to compete with ZP1 in the absence of Ca(II) and that CP outcompeted the sensor in the presence of excess Ca(II). These results showed that Ca(II) enhances the affinity of CP for Fe(II), and indicated that the His6 site of CP binds Fe(II) with sub-picomolar affinity.21 Relative to other characterized Fe(II)-binding proteins, which generally exhibit Kd values in the high nanomolar to low micromolar range,31 the affinity of the His6 site for Fe(II) is remarkably high. The high affinity of this site for Fe(II) and other divalent metal ions is at least partially attributable to the ability of the site to effectively “trap” divalent metal ions via the flexible S100A9 C-terminal tail, which encapsulates the bound metal ion and shields it from solvent.30, 32 Indeed, MCD spectroscopic analyses of CP variants lacking the coordinating histidines of the S100A9 C-terminal tail (H103A and H105A variants) revealed a six-coordinate Fe(II) center with a bound hydroxide or water molecule in place of the missing histidine ligand.30 Furthermore, ZP1 competition experiments indicated that H103 and H105 are necessary for high-affinity Fe(II) binding at the His6 site.
Multiple nutrient metal ions can be found at infection sites, and the His6 site of CP also sequesters Mn(II), Ni(II), and Zn(II). To determine the thermodynamic preference of CP for binding one metal ion over another, we performed metal substitution experiments. We found that the His6 site exhibits a thermodynamic preference of Kd, Mn(II) > Kd, Fe(II) > Kd, Zn(II) > Kd, Ni(II).19, 21 Nevertheless, these experiments also indicated slow exchange at the His6 site, suggesting that it may serve as a kinetic trap, binding whichever metal ion it first encounters and preventing its dissociation. In this case, the most abundant metal ions would be preferentially bound at the His6 site. The relative contributions of thermodynamics and kinetics to metal sequestration by CP under a variety of conditions is an important avenue for future investigation.
CP affects Fe redox equilibrium
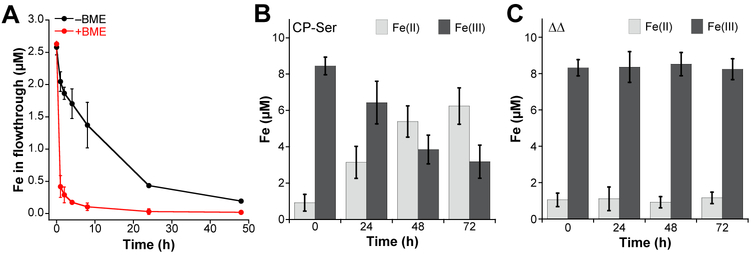
Whereas Fe(II) is highly susceptible to oxidation to Fe(III) in aerobic and oxidative environments, it can persist in anaerobic and reducing environments.33 Our initial Fe-binding studies indicated that CP would sequester Fe(II) in environments where Fe(II) is expected to be abundant, such as an anaerobic niche within the human host.21 Nonetheless, we also found that CP slowly depleted Fe from microbial growth medium under aerobic conditions in the absence of an exogenous reductant (Figure 3a).21-22 This observation motivated us to study the effect of CP on Fe redox speciation, which revealed that CP can shift the Fe redox equilibrium from Fe(III) to Fe(II) under aerobic conditions. For instance, when CP is added to an aerobic buffered solution of ferric citrate, the Fe redox speciation changes over time such that Fe(II) becomes the dominant redox state in solution (Figure 3b).22 A variant of CP lacking residues of the His3Asp and His6 sites (ΔΔ) did not affect the [Fe(II])/[Fe(III)] ratio over time, suggesting that Fe(II) sequestration allows for Fe(II) to accumulate (Figure 3c). Thus, CP is able to shift the Fe redox equilibrium by binding and stabilizing Fe(II) in aerobic non-reducing conditions. This result led us to speculate that CP may withhold Fe(II) in a range of oxygen availabilities and redox environments.
Figure 3.
CP sequesters Fe(II) under aerobic conditions and shifts Fe redox equilibrium to favor Fe(II).22 (A) Depletion of Fe from Tris:TSB medium (62:38 20 mM Tris, 100 mM NaCl, pH 7.5:TSB medium) supplemented with 2 mM Ca(II) by 10.5 μM CP-Ser in the absence or presence of ~3 mM β-mercaptoethanol (BME). The mean and SDM are reported (n = 3). (B & C) Fe(III) citrate (10 mM) was incubated with 10.5 μM (B) CP-Ser or (C) ΔΔ variant in the presence of 2 mM Ca(II) and the Fe redox speciation was monitored by the ferrozine assay (75 mM HEPES, 100 mM NaCl, pH 7.0 at 30 °C, 150 rpm).22 The mean and SDM are reported (n = 6). Panels B and C were reproduced with permission from ref. 22. Copyright 2017 the Royal Society of Chemistry.
CP inhibits microbial Fe uptake
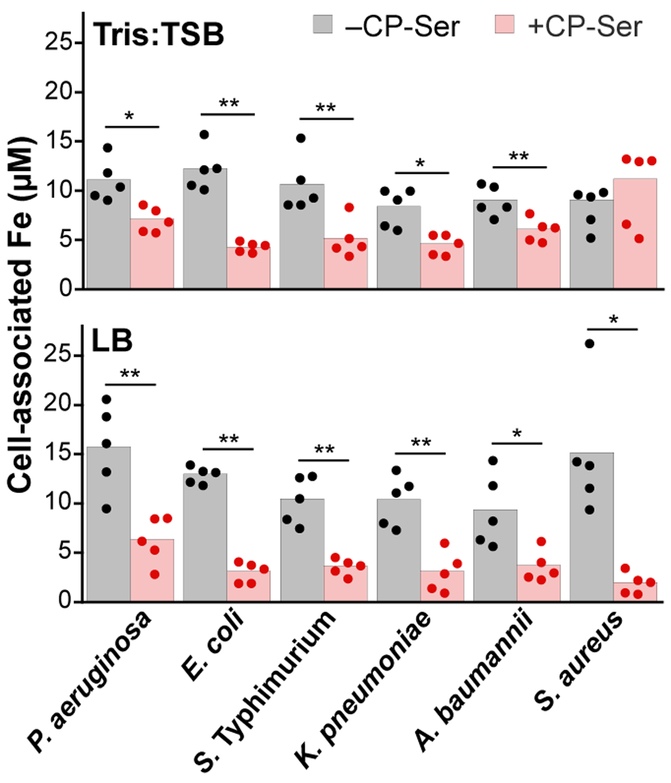
We first evaluated the ability of CP to block Fe uptake by two Fe-centric bacterial species, Escherichia coli and Pseudomonas aeruginosa, under reducing conditions where Fe(II) is the dominant oxidation state.21 Using an 55Fe-uptake assay, we found that CP inhibits Fe acquisition by both organisms.21 Recently, an independent report also presented CP-mediated inhibition of Fe uptake by E. coli under reducing conditions.25 After observing that CP shifts the Fe redox equilibrium from Fe(III) to Fe(II) under aerobic conditions, we reasoned that CP may be capable of inhibiting bacterial Fe uptake during aerobic culture. Indeed, we observed that CP can inhibit Fe uptake by P. aeruginosa, E. coli, Salmonella enterica serovar Typhimurium, Klebsiella pneumoniae, A. baumannii, and Staphylococcus aureus during aerobic culture and in the absence of an exogenous reductant (Figure 4).23 We also observed that CP-mediated inhibition of Fe-uptake is medium-dependent, especially for S. aureus. Our work indicated that CP inhibits Fe uptake by S. aureus during growth in LB medium, but not in Tris:TSB medium. Previous independent investigations also found that that CP does not inhibit Fe uptake by S. aureus in Tris:TSB-based medium.34-35 The reported effect of CP on Fe acquisition by A. baumannii has also varied,23-24, 36 which may also result from different media conditions.37 Although previous work indicated that CP does not inhibit Fe uptake by A. baumannii in RPMI-based media,36 it was recently reported that CP reduces Fe-uptake by ~75% in an LB-based medium,24 in agreement with our metal-uptake data.23 Taken together, these studies establish the ability of CP to inhibit Fe uptake by microbes in vitro, and whether this activity occurs in vivo during infection warrants thorough examination.
Figure 4.
Analysis of cell-associated Fe levels shows that CP inhibits Fe uptake by several bacterial pathogens during aerobic culture.23 Bacteria (P. aeruginosa PA14, E. coli UTI89, S. Typhimurium ATCC 14028, K. pneumoniae ATCC 13883, A. baumannii ATCC 17978, and S. aureus USA300 JE2) were grown in Tris:TSB or LB medium in the absence or presence of 10 μM CP-Ser (in Tris:TSB) or 20 μM CP-Ser (in LB) at 37°C for 8 h. Cell-associated Fe corresponds to the concentration of Fe in an OD600 = 10 cell suspension (n = 5, *P < 0.05; **P < 0.01). Reproduced with permission from ref. 23. Copyright 2019 the American Society for Biochemistry and Molecular Biology
CP induces bacterial Fe starvation
Fe is critical for the viability and virulence of many microbial pathogens, and the roles of Fe in P. aeruginosa biology and pathogenesis have been particularly well studied. P. aeruginosa is adept at overcoming host-mediated Fe deprivation and expresses several machineries for acquiring Fe(II) and Fe(III) ions as well as heme during infection.38-39 Fe starvation induces the production of PrrF small regulatory RNAs by P. aeruginosa, which reduce the metabolic requirement of P. aeruginosa for Fe when this nutrient is scarce.40 This process, referred to as the Fe-sparing response,41 is central to P. aeruginosa survival during Fe starvation and is therefore required for successful infection.42
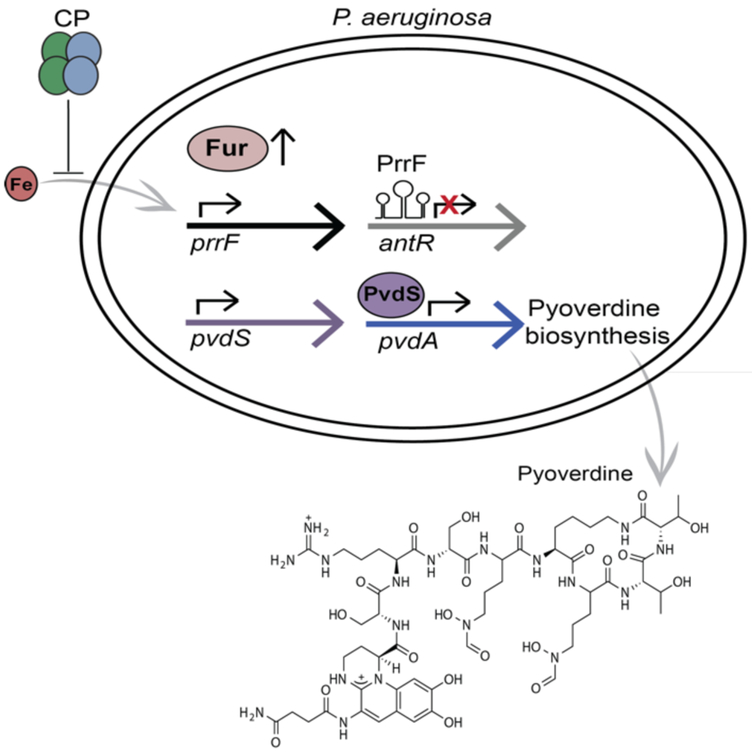
Our work, in collaboration with the Oglesby-Sherrouse laboratory, demonstrated that CP promotes Fe starvation responses in P. aeruginosa.23 We selected P. aeruginosa to study the effect of CP on bacterial Fe homeostasis due to its clinical relevance, its co-localization with CP in the cystic fibrosis lung,43 and its aforementioned responses to Fe starvation. When P. aeruginosa intracellular Fe is low, repression of genes encoding Fe acquisition systems and the PrrF sRNAs by the ferric uptake regulator (Fur) is relieved. PrrF negatively regulates antR, which encodes a regulatory protein that controls the degradation of the metabolite anthranilate. In turn, anthranilate serves as a precursor for a class of P. aeruginosa metabolites called alkyl-quiniolones that are important for signaling and virulence.44-45 We found that CP inhibits the translation of antR, indicating that P. aeruginosa initiates an Fe-sparing response in the presence of CP (Figure 5).23 Repression of transcription ofpvdS, which encodes a sigma factor needed for production of the siderophore pyoverdine, is also relieved when intracellular Fe is low. We observed that CP induces the transcription of pvdS and production of pyoverdine, supporting a Fur-mediated Fe starvation response in P. aeruginosa (Figure 5).23
Figure 5.
CP induces Fe starvation responses in P. aeruginosa.23 CP inhibits Fe uptake, and apo-Fur derepresses the transcription of prrF and pvdS. Subsequently produced PrrF sRNAs repress antR translation, and PvdS promotes pyoverdine biosynthesis. As a result, antR translation is inhibited by CP, and pyoverdine production is promoted by CP.
The altered production of AntR and pyoverdine is indicative of an Fe-starvation response, and may have far-reaching consequences for P. aeruginosa survival and virulence in the human host. The levels of AntR and pyoverdine indirectly regulate the biosynthesis of several P. aeruginosa virulence factors, including endoprotease PrpL, exotoxin A, and alkyl-quinolones45-46 Moreover, CP inhibits production of phenazines, redox-cycling secondary metabolites that are important for P. aeruginosa virulence.23, 43 We also found that Fe depletion, but not Mn or Zn depletion, inhibits phenazine production in P. aeruginosa, indicating that CP inhibits phenazine production via Fe(II) sequestration. This analysis revised a prior explanation of how CP inhibits the production of phenazines, which was based on its Mn(II)- and Zn(II)-sequestering properties.43 The mechanism by which Fe limitation results in reduced phenazine production is currently unknown and warrants further investigation. Overall, by inducing an Fe-starvation response in P. aeruginosa, CP affects several pathways that are implicated in its virulence and may thus affect the ability of this organism to cause disease in the host.
Recent independent work demonstrated that CP also induces an Fe-starvation response in A. baumannii.24 RNAseq analysis of A. baumannii exposed to CP indicated increased transcriptional levels of genes involved in the global Fe-starvation response. Genes that were upregulated in the presence of CP included those encoding the ferrous iron uptake system (feoAB) and proteins involved in the biosynthesis, utilization, and uptake of the siderophore acinetobactin. Higher levels of acinetobactin were also detected in supernatants of CP-treated cultures. Analysis of the cellular proteome upon CP exposure showed that CP decreases production of Fe-utilizing proteins including the [4Fe-4S] cluster protein fumarase and proteins involved in Fe-S cluster biogenesis, indicative of an Fe-sparing response. This study also revealed that challenge of A. baumannii with CP inhibits flavin biosynthesis, which may result from CP-mediated Fe limitation given the well documented metabolic crosstalk between Fe and riboflavin in both eukaryotes and prokaryotes.47 Taken together, these independent studies of P. aeruginosa and A. baumannii by two research groups indicate that CP elicits Fe-starvation responses in two bacterial pathogens that cause human disease and affects pathways that are central to cellular metabolism and virulence by limiting Fe.
Bacterial metabolites affect Fe(II) sequestration by CP
At sites of infection, CP is released into the complex chemical milieu of the extracellular space that includes multiple host and microbial factors that affect Fe speciation by coordinating the ion or altering its redox state. Microbes secrete siderophores to scavenge Fe(III) and compete with host Fe(III)-binding proteins lactoferrin and transferrin.48 Siderophores inhibit Fe(II)-sequestration by CP in solution, presumably by coordinating Fe(III) with high affinity, stabilizing the Fe(III) oxidation state, and making the metal ion unavailable (Figure 6).22-23 Thus, we expect that siderophores that CP encounters at a site of infection will attenuate its antimicrobial activity when Fe(III) is the dominant redox state.
Figure 6.
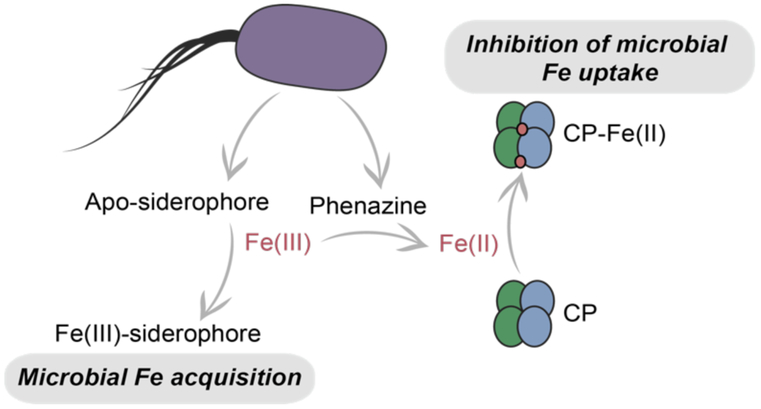
Effect of siderophores and phenazines on P. aeruginosa Fe homeostasis and Fe(II) sequestration by CP. 23
In some contexts, microbes depend on Fe(II) during infection and produce specialized machinery to acquire this metal ion. In particular, P. aeruginosa is capable of promoting Fe(II) availability by secreting phenazines to reduce Fe(III) to Fe(II) in the extracellular space.49 P. aeruginosa thereby provides Fe(II) for uptake via its Fe(II) uptake (Feo) ATP-binding cassette transporter.50 However, our recent work indicated that the redox-cycling capacity of phenazines may aid the host innate immune response by promoting Fe(II) sequestration by CP. The phenazine pyocyanin enhanced Fe depletion from microbial growth medium by CP (Figure 6).22-23 In aerobic culture, the phenazines produced by P. aeruginosa also enhanced Fe withholding by CP.23 Moreover, CP did not inhibit Fe uptake by a P. aeruginosa mutant defective in phenazine production (PA14 Δphz).23 CP-mediated inhibition of antR expression was reduced in the Δphz strain, suggesting that CP is less able to induce an Fe-starvation response in this non-phenazine producing strain.23 CP remained capable of inducing pyoverdine production in the Δphz strain, but to a lesser extent than that observed in its parent PA14 strain. Taken together, these studies indicate that phenazines facilitate Fe(II) sequestration by CP, and thus may enhance the efficacy of the innate immune response toward P. aeruginosa by aiding Fe(II) withholding by this innate immune protein. We note that aiding the innate immune response is unlikely to be an evolved role of phenazines; these molecules have several important functions including contributing to virulence and antibiotic resistance in the human host, and promoting nutrient acquisition for soil-dwelling pseudomonads.51-53
Broadly, these studies highlight that microbial metabolites that modulate metal speciation in the extracellular environment likely also alter the functional capacity innate immune factors, in this case by attenuating (siderophores) or promoting (phenazines) Fe(II) sequestration by CP. Such complex interplay between host factors and microbial metabolites will undoubtedly vary with the unique chemical composition of each infection site and metabolic profile of each microbe, requiring further elucidation on a case-by-case basis.
Conclusions and perspectives
Here, we provide an account of our current understanding of Fe(II) sequestration by CP and its effects on microbial Fe homeostasis. Our work demonstrates high-affinity Fe(II) coordination by CP at its His6 site, the capacity of this site to shift the redox equilibrium of Fe to favor Fe(II) under aerobic conditions, and CP-mediated withholding of Fe from microbes. Furthermore, our studies and those of others establish that CP induces Fe starvation responses in P. aeruginosa and A. baumannii, two bacterial pathogens of significant clinical concern, and affects several pathways that are important for survival and virulence in these organisms as a result of Fe limitation. Moving forward, we believe that studying microbial responses to Fe limitation by CP will provide valuable insight into their survival and virulence strategies when confronted by Fe(II) withholding by the host.
To the best of our knowledge, the discovery that CP withholds Fe(II) from a variety of bacterial pathogens provides the first evidence of a metal-sequestering innate immune protein that can contribute to an Fe(II)-withholding response. Fe(III) withholding is the paradigm for nutritional immunity.1, 54 In contrast to Fe(III), the competition for Fe(II) between host and pathogen was unappreciated for many years, likely due to uncertainty about the relevance of Fe(II) at infection sites, which are generally considered to be oxidative environments. However, recent studies provide a compelling picture for the importance of Fe(II) during infection.55-57 For instance, several murine models of infection have indicated the essentiality of Fe(II) uptake via the Feo system.56, 58 Additionally, two recent analyses of Fe levels revealed that Fe(II) is a significant component of Fe at infection sites.55, 57 The importance of Fe(II) for microbial pathogenesis in oxygen-limited niches of the host suggests that CP may limit the ability of microbes to colonize in these environments.
The studies described in this Account set the stage for investigating the effect of CP on bacterial Fe homeostasis in vivo, including work that leverages murine models of infection. Recent biochemical and functional evaluation of murine CP (mCP) demonstrated that it depletes Fe from microbial growth medium and is capable of stabilizing Fe(II).59-60 Thus, we reason that mCP may affect bacterial Fe homeostasis in a manner similar to human CP, a possibility that should certainly be further explored. To the best of our knowledge, reported murine model studies have given little consideration to the possibility of CP contributing to Fe(II) withholding and Fe homeostasis. This scenario is understandable because the prevailing notion in the field for many years was that CP sequesters only Mn(II) and Zn(II), which shaped study design and data interpretation.12, 36, 43, 61-62 We also note that, despite extensive work, the metal-sequestering function of CP has been examined in only a limited number of murine infection models compared to the array of possibilities that exist.12, 36, 43, 61-62 To date, these studies have focused on acute infection models where Fe(II) may be a less relevant player than Fe(III). In contrast, many chronic infections are characterized by the formation of biofilms, which exhibit steep oxygen gradients and increased dependency on Fe(II).55, 63-65 Specific to studies of P. aeruginosa infection, we expect that maximal Fe(II) withholding by CP will be observed during chronic infection due to (i) high phenazine levels and low pyoverdine levels, both of which favor Fe(II) sequestration by CP,23 and (ii) lower oxygen levels in biofilms that are characteristic of chronic P. aeruginosa infection.63-65 One caveat when comparing human and murine metal-withholding is that the repertoire of host-defense factors found in these two mammals differs. For instance, S100A12, an abundant metal-sequestering protein deployed by human neutrophils, is not produced by mice. Thus, it is possible that metal sequestration by CP is modulated differently in humans and other mammals depending on the composition and interplay of the arsenal of metal-sequestering proteins.66 Taken together, we believe that prior murine model studies do not preclude a role for Fe(II) sequestration by CP in vivo, and that a thorough analysis of Fe(II) withholding by CP in vivo is highly warranted.
Our work has also illuminated a complex interplay between microbial and host metal-chelating factors. By stabilizing Fe(III) in solution, microbial siderophores prevent Fe(II) sequestration by CP. Conversely, P. aeruginosa-produced phenazines are capable of promoting Fe(II) sequestration by CP by reducing Fe(III) to Fe(II) in solution. Together, these results indicate that the efficacy of CP in starving an organism of Fe in vivo will depend on the metabolic profile of the organism. Further studies with other metabolites involved in metal homeostasis will expand our understanding of how these molecules affect metal sequestration by CP.
In closing, we believe that our investigations of Fe(II) sequestration by CP are informative for multiple sub-disciplines. These contributions set the stage for evaluating the implications of Fe(II) withholding for a diversity of microbial pathogens, as well as in vivo evaluation of Fe(II) withholding by CP. This work has expanded our understanding of CP beyond Mn(II) and Zn(II) sequestration, and further investigations are necessary to elucidate (i) how CP impacts Fe homeostasis in a diversity of microbial pathogens, (ii) the interplay between CP and various nutrient metal ions in vivo, and (iii) how specific environmental conditions affect its function.13, 67-68 We very much look forward to future explorations of the contribution of Fe(II) sequestration by CP to the mammalian innate immune response and microbial pathogenesis.
Acknowledgements
We thank our past and current lab members and collaborators for invaluable contributions to elucidating Fe(II) sequestration by calprotectin. We thank the National Institutes of Health (R01 GM118695 and R01 GM126376) for supporting our current work on calprotectin. E.M.Z. is a recipient of an NSF Graduate Research Fellowship.
Biography
Elizabeth M. Nolan is an Associate Professor of Chemistry at MIT. Her research group investigates the bioinorganic chemistry of the host–microbe interaction and infectious disease.
Emily M. Zygiel received her BS in biochemistry from Stonehill College and is currently a chemistry graduate student in the Nolan lab at MIT. Her dissertation research focuses on effect of CP on metal homeostasis in bacterial pathogens including P. aeruginosa.
References
- 1.Weinberg ED, Nutritional immunity. Host’s attempt to withold iron from microbial invaders. J. Am. Med. Assoc. 1975, 231, 39–41. [DOI] [PubMed] [Google Scholar]
- 2.Hood MI; Skaar EP, Nutritional immunity: transition metals at the pathogen–host interface. Nat. Rev. Microbiol 2012, 10, 525–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wilkinson MM; Busuttil A; Hayward C; Brock DJH; Dorin JA; Van Heyningen V, Expression pattern of two cystic fibrosis-associated calcium binding proteins in normal and abnormal tissues. J. Cell Sci 1988, 91, 221–230. [DOI] [PubMed] [Google Scholar]
- 4.Bullock S; Hayward C; Manson J; Brock DJH; Raeburn JA, Quantitative immunoassays for diagnosis of carrier detection in cystic fibrosis. Clin. Genet 1982, 21, 336–341. [DOI] [PubMed] [Google Scholar]
- 5.Dale I; Fagerhol MK; Naesgaard I, Purification and partial characterization of highly immunogenic human leucocyte protein, the L1 antigen. Eur. J. Biochem 1983, 134, 1–6. [DOI] [PubMed] [Google Scholar]
- 6.Fagerhol MK; Dale I; Andersson T, Release and quantitation of a leucocyte derived protein (L1). Scand. J. Haematol 1980, 24, 393–398. [Google Scholar]
- 7.Odink K; Cerletti N; Brüggen J; Clerc RG; Tarcsay L; Zwaldo G; Gerhards G; Schlegel R; Sorg C, Two calcium-binding proteins in filtrate macrophages of rheumatoid arthritis. Nature 1987, 330, 80–82. [DOI] [PubMed] [Google Scholar]
- 8.Andersson KB; Sletten K; Berntzen HB; Dale I; Brandtzaeg P; Jellum E; Fagerhol MK, The leucocyte L1 protein: identity with the cystic fibrosis antigen and the calcium binding MRP-8 and MRP-14 macrophage components. Scand. J. Immunol 1988, 28, 241–245. [DOI] [PubMed] [Google Scholar]
- 9.Steinbakk M; Naess-Andresen C-F; Lingaas E; Dale I; Brandtzaeg P; Fagerhol MK, Antimicrobial actions of calcium binding leukocyte L1 protein, caprotectin. Lancet 1990, 336, 763–765. [DOI] [PubMed] [Google Scholar]
- 10.Sohnle PG; Collins-Lech C; Wiessner JH, The zinc-reversible antimicrobial activity of neutrophil lysates and abscess fluid supernatants. J. Infect. Dis 1991, 164, 137–142. [DOI] [PubMed] [Google Scholar]
- 11.Korndörfer IP; Brueckner F; Skerra A, The crystal structure of the human (S100A8/S100A9)2 heterotetramer, calprotectin, illustrates how conformational changes of interacting α-helices can determine specific association of two EF-hand proteins. J. Mol. Biol 2007, 370, 887–898. [DOI] [PubMed] [Google Scholar]
- 12.Corbin BD; Seeley EH; Raab A; Feldmann J; Miller MR; Torres VJ; Anderson KL; Dattilo BM; Dunman PM; Gerads R; Caprioli RM; Nacken W; Chazin WJ; Skaar EP, Metal chelation and inhibition of bacterial growth in tissue abscesses. Science 2008, 319, 962–965. [DOI] [PubMed] [Google Scholar]
- 13.Zygiel EM; Nolan EM, Transition metal sequestration by the host-defense protein calprotectin. Annu. Rev. Biochem 2018, 87, 621–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Besold AN; Gilston BA; Radin JN; Ramsoomair C; Culbertson EM; Li CX; Cormack BP; Chazin WJ; Kehl-Fie TE; Culotta VC, Role of calprotectin in withholding zinc and copper from Candida albicans. Infect. Immun 2018, 86, e00779–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brandtzaeg P; Dale I; Fagerhol MK, Distribution of a formalin-resistant myelomonocytic antigen (L1) in human tissues. Am. J. Clin. Pathol 1987, 87, 681–699. [DOI] [PubMed] [Google Scholar]
- 16.Johne B; Fagerhol MK; Lyberg T; Prydz H; Brandtzaeg P; Naess-Andresen CF; Dale I, Functional and clinical aspects of the myelomonocyte protein calprotectin. J. Clin. Pathol: Mol. Pathol 1997, 50, 113–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brini M; Ottolini D; Calì T; Carafoli E, Calcium in health and disease. Met. Ions Life Sci 2013, 20, 87–93. [DOI] [PubMed] [Google Scholar]
- 18.Leukert N; Vogl T; Strupat K; Reichelt R; Sorg C; Roth J, Calcium-dependent tetramer formation of S100A8 and S100A9 is essential for biological activity. J. Mol. Biol 2006, 359, 961–72. [DOI] [PubMed] [Google Scholar]
- 19.Nakashige TG; Zygiel EM; Drennan CL; Nolan EM, Nickel sequestration by the host-defense protein human calprotectin. J. Am. Chem. Soc 2017, 139, 8828–8836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.The majority of metal-binding and antimicrobial activity studies of CP described in this Accounts were performed with a S100A8(C42S)/S100A9(C3S) variant named CP-Ser. In experiments reported to date, this variant shows comparable metal-binding properties and antimicrobial activity to CP. We specify CP-Ser in figure panels and captions for which this variant was employed.
- 21.Nakashige TG; Zhang B; Krebs C; Nolan EM, Human calprotectin is an iron-sequestering host-defense protein. Nat. Chem. Biol 2015, 11, 765–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakashige TG; Nolan EM, Human calprotectin affects the redox speciation of iron. Metallomics 2017, 9, 1086–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zygiel EM; Nelson CA; Brewer LK; Oglesby-Sherrouse AG; Nolan EM, The innate immune protein human calprotectin induces iron starvation responses in Pseudomonas aeruginosa. J. Biol. Chem 2019, 294, 3549–3562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang J; Lonergan ZR; Gonzalez-Gutierrez G; Nairn BL; Maxwell CN; Zhang Y; Andreini C; Karty JA; Chazin WJ; Trinidad JC; Skaar EP; Giedroc DP, Multi-metal restriction by calprotectin impacts de novo flavin biosynthesis in Acinetobacter baumannii. Cell Chem. Biol 2019, 26, 745–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Besold AN; Culbertson EM; Nam L; Hobbs RP; Boyko A; Maxwell CN; Chazin WJ; Marques AR; Culotta VC, Antimicrobial action of calprotectin that does not involve metal witholding. Metallomics 2018, 10, 1728–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Damo SM; Kehl-Fie TE; Sugitani N; Holt ME; Rathi S; Murphy WJ; Zhang Y; Betz C; Hench L; Fritz G; Skaar EP; Chazin WJ, Molecular basis for manganese sequestration by calprotectin and roles in the innate immune response to invading bacterial pathogens. Proc. Natl. Acad. Sci. U.S.A 2013, 110, 3841–3846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brophy MB; Hayden JA; Nolan EM, Calcium ion gradients modulate the zinc affinity and antibacterial activity of human calprotectin. J. Am. Chem. Soc 2012, 134, 18089–18100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lisher JP; Giedroc DP, Manganese acquisition and homeostasis at the host-pathogen interface. Front. Cell Infect. Microbiol 2013, 3, 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.The ability of CP to retain Fe(III) and Fe(II) was evaluated using analytical size exclusion chromatography (SEC). CP-Ser was preincubated with 5 equivalents of Fe(II) or Fe(III). Samples were prepared and eluted in 75 mM HEPES, 100 mM NaCl, pH 7.0 in the absence of Ca(II). Only Fe(II) was retained under these conditions.
- 30.Baker TM; Nakashige TG; Nolan EM; Neidig ML, Magnetic circular dichroism studies of iron(II) binding to human calprotectin. Chem. Sci 2017, 8, 1369–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cotruvo JA Jr.; Stubbe J, Metallation and mismetallation of iron and manganese proteins in vitro and in vivo: the class I ribonucleotide reductases as a case study. Metallomics 2012, 4, 1020–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gagnon DM; Brophy MB; Bowman SEJ; Stich TA; Drennan CL; Britt RD; Nolan EM, Manganese binding properties of human calprotectin under conditions of high and low calcium: X-ray crystallographic and advanced electron paramagnetic resonance spectroscopic analysis. J. Am. Chem. Soc 2015, 137, 3004–3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pantopoulos K; Porwal SK; Tartakoff A; Devireddy L, Mechanisms of mammalian iron homeostasis. Biochemistry 2012, 51, 5705–5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Radin JN; Zhu J; Brazel EB; McDevitt CA; Kehl-Fie TE, Synergy between nutritional immunity and independent host defenses contributes to the importance of the MntABC manganese transporter during Staphylococcus aureus infection. Infect. Immun 2018, 87, e00642–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Radin JN; Kelliher JL; Párraga Solórzano PK; Kehl-Fie TE, The two-component system ArlRS and alterations in metabolism enable Staphylococcus aureus to resist calprotectin-induced manganese starvation. PLoS Pathog 2016, 12, e1006040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hood MI; Mortensen BL; Moore JL; Zhang Y; Kehl-Fie TE; Sugitani N; Chazin WJ; Caprioli RM; Skaar EP, Identification of an Acinetobacter baumannii zinc acquisition system that facilitates resistance to calprotectin-mediated zinc sequestration. PLoS Pathog 2012, 8, e1003068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.For metal-inventory experiments presented in Ref. 36, A. baumannii was cultured in a medium consisting of 20 % CP buffer (20 mM Tris, 100 mM NaCl, 10 mM β-mercaptoethanol, 3 mM CaCl2,) and 80% Chelex-treated RPMI supplemented with 0.1 mM CaCl2, 1 mM MgSO4, and 10 μM FeSO4, and 50 μM Mn. The Mn salt was not specified. It was observed that CP promotes Fe uptake by A. baumannii ATCC 17978. As reported in Ref. 23, we observed that CP treatement significantly reduces Fe uptake by A. baumannii ATCC 17978 in Tris:TSB or LB supplemented with 2 mM CaCl2. Thus, we reason that CP more effectively inhibits Fe uptake by A. baumannii in Tris:TSB or LB-based media than in RPMI based media.
- 38.Cornelis P; Dingemans J, Pseudomonas aeruginosa adapts its iron uptake strategies in function of the type of infections. Front. Cell Infect. Microbiol 2013, 3, doi: 10.3389/fcimb.2013.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nguyen AT; Oglesby-Sherrouse AG, Spoils of war: iron at the crux of clinical and ecological fitness of Pseudomonas aeruginosa. Biometals 2015, 28, 433–443. [DOI] [PubMed] [Google Scholar]
- 40.Wilderman PJ; Sowa NA; FitzGerald DJ; FitzGerald PC; Gottesman S; Ochsner UA; Vasil ML, Identification of tandem duplicate regulatory small RNAs in Pseudomonas aeruginosa involved in iron homeostasis. Proc. Nat. Acad. Sci. U.S.A 2004, 101, 9792–9797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Massé E; Vanderpool CK; Gottesman S, Effect of RhyB small RNA on global iron use in Escherichia coli. J. Bacteriol 2005, 187, 6962–6971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reinhart AA; Oglesby-Sherrouse AG, Regulation of Pseudomonas aeruginosa virulence by distinct iron sources. Genes 2016, 7, 7120126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wakeman CA; Moore JL; Noto MJ; Zhang Y; Singleton MD; Prentice BM; Gilston BA; Doster RS; Gaddy JA; Chazin WJ; Caprioli RM; Skaar EP, The innate immune protein calprotectin promotes Pseudomonas aeruginosa and Staphylococcus aureus interaction. Nat. Commun 2016, 7, 11951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Djapgne L; Panja S; Brewer LK; Gans JH; Kane MA; Woodson SA; Oglesby-Sherrouse AG, The Pseudomonas aeruginosa PrrF1 and PrrF2 small regulatory RNAs promote 2-alkyl-4-quinolone production through redundant regulation of the antR mRNA. J. Bacteriol 2018, 200, e00704–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oglesby AG; Farrow JM 3rd; Lee J-H; Tomaras AP; Greenberg EP; Pesci EC; Vasil ML, The influence of iron on Pseudomonas aeruginosa physiology: a regulatory link between iron and quorum sensing. J. Biol. Chem 2008, 283, 15558–15567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Minandri F; Imperi F; Frangipani E; Bonchi C; Visaggio D; Facchini M; Pasquali P; Bragonzi A; Visca P, Role of iron uptake systems in Pseudomonas aeruginosa virulence and airway infection. Infect. Immun 2016, 84, 2324–2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sepúveda Cisternas I; Salazar JC; García-Angulo VA, Overview on the bacterial iron-riboflavin metabolic axis. Front. Microbiol 2018, 9, 1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hider RC; Kong X, Chemistry and biology of siderophores. Nat. Prod. Rep 2010, 27, 637–657. [DOI] [PubMed] [Google Scholar]
- 49.Wang Y; Newman DK, Redox reactions of phenazine antibiotics with ferric (hydr)oxides and molecular oxygen. Environ. Sci. Technol 2008, 42, 2380–2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang Y; Wilks JC; Danhorn T; Ramos I; Croal L; Newman DK, Phenazine-1-carboxylic acid promotes bacterial biofilm development via ferrous iron acquisition. J. Bacteriol 2011, 193, 3606–3617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Recinos DA; Sekedat MD; Hernandez A; Cohen TS; Sakhtah H; Prince AS; Price-Whelan A; Dietrich LE, Redundant phenazine operons in Pseudomonas aeruginosa exhibit environment-dependent expression and differential roles in pathogenicity. Proc. Natl. Acad. Sci. U.S.A 2012, 109, 19420–19425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hernandez ME; Kappler A; Newman DK, Phenazines and other redox-active antibiotics promote microbial mineral reduction. Appl. Environ. Microbiol 2004, 70, 921–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schiessl KT; Hu F; Jo J; Nazia SZ; Wang B; Price-Whelan A; Min W; Dietrich LEP, Phenazine production promotes antibiotic tolerance and metabolic heterogeneity in Pseudomonas aeruginosa biofilms. Nat. Commun. 2019, 10, 762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cassat JE; Skaar EP, Iron in infection and immunity. Cell Host Microbe 2013, 13, 509–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hunter RC; Asfour F; Dingemans J; Osuna BL; Samad T; Malfroot A; Cornelis P; Newman DK, Ferrous iron is a significant component of bioavailable iron in cystic fibrosis airways. MBio 2013, 4, e00557–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lau CK; Krewulak KD; Vogel HJ, Bacterial ferrous iron transport: the Feo system. FEMS Microbiol. Rev. 2015, 40, 273–298. [DOI] [PubMed] [Google Scholar]
- 57.Aron AT; Heffern MC; Lonergan ZR; Vander Wal MN; Blank BR; Spangler B; Zhang Y; Park HM; Stahl A; Renslo AR; Skaar EP; Chang CJ, In vivo bioluminescence imaging of labile iron accumulation in a murine model of Acinetobacter baumannii infection. Proc. Nat. Acad. Sci. U.S.A 2017, 114, 12669–12674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stojiljkovic I; Cobeljic M; Hantke K, Escherichia coli K-12 ferrous iron uptake mutants are impaired in their ability to colonize the mouse intenstine. FEMS Microbiol. Lett 1993, 108, 111–116. [DOI] [PubMed] [Google Scholar]
- 59.Hadley RC; Gu Y; Nolan EM, Initial biochemical and functional evaluation of murine calprotectin reveals Ca(II)-dependence and its ability to chelate multiple nutrient transition metal ions. Biochemistry 2018, 57, 2846–2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hadley RC; Nolan EM, Preparation and iron redox speciation study of the Fe(II)-binding antimicrobial protein calprotectin In Calcium-binding proteins of the EF-hand superfamily, Heizmann C, Ed. Humana Press: New York, NY, 2019; Vol. 1929, pp 397–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Diaz-Ochoa VE; Lam D; Lee CS; Klaus S; Behnsen J; Liu JZ; Chim N; Nuccio S-P; Rathi SG; Mastroianni JR; Edwards RA; Jacobo CM; Cerasi M; Battistoni A; Ouellette AJ; Goulding CW; Chazin WJ; Skaar EP; Raffatellu M, Salmonella mitigates oxidative stress and thrives in the inflamed gut by evading calprotectin-mediated manganese sequestration. Cell Host Microbe 2016, 19, 814–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kehl-Fie TE; Chitayat S; Hood MI; Damo S; Restrepo N; Garcia C; Munro KA; Chazin WJ; Skaar EP, Nutrient metal sequestration by calprotectin inhibits bacterial superoxide defense, enhancing neutrophil killing of Staphylococcus aureus. Cell Host Microbe 2011, 10, 158–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wessel AK; Arshad TA; Fitzpatrick M; Connell JL; Bonnecaze RT; Shear JB; Whiteley M, Oxygen limitation within a bacterial aggregate. MBio 2014, 5, e00992–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Worlitzsch D; Tarran R; Ulrich M; Schwab U; Cekici A; Meyer KC; Birrer P; Bellon G; Berger J; Weiss T; Botzenhart K; Yankaskas JR; Randell S; Boucher RC; Doring G, Effects of reduced mucus oxygen concentration in airway Pseudomonas infections of cystic fibrosis patients. J. Clin. Invest 2002, 109, 317–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cowley ES; Kopf SH; LaRiviere A; Ziebis W; Newman DK, Pediatric cystic fibrosis sputum can be chemically dynamic, anoxic, and extremely reduced due to hydrogen sulfide formation. MBio 2015, 4, e00767–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cunden LS; Gaillard A; Nolan EM, Calcium ions tune the zinc-sequestering properties and antimicrobial activity of human S100A12. Chem. Sci 2016, 7, 1338–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stephan JR; Yu F; Costello RM; Bleier BS; Nolan EM, Oxidative post-translational modifications accelerate proteolytic degradation of calprotectin. J. Am. Chem. Soc 2018, 140, 17444–17455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hoskin TS; Crowther JM; Cheung J; Epton MJ; Sly PD; Elder PA; Dobson RCJ; Kettle AJ; Dickerhof N, Oxidative cross-linking of calprotectin occurs in vivo, altering its structure and susceptibility to proteolysis. Redox Biol 2019, 24, 101202. [DOI] [PMC free article] [PubMed] [Google Scholar]