Abstract
Wound chronicity due to intrinsic and extrinsic factors perturbs adequate lesion closure and reestablishment of the protective skin barrier. Immediate and proper care of chronic wounds is necessary for a swift recovery and a reduction of patient vulnerability to infection. Advanced therapies supplemented with standard wound care procedures have been clinically implemented to restore aberrant tissue; however, these treatments are ineffective if local vasculature is too compromised to support minimally-invasive strategies. Autologous ―flaps‖, which are tissues equipped with their own hierarchical vascular supply, can be harvested from one region of the patient and transplanted to the wound where it is reperfused upon microsurgical anastomosis to appropriate recipient vessels. Despite the success of autologous flap transfer, these procedures are extremely invasive, incur obligatory donor-site morbidity, require sufficient donor-tissue availability as well as microsurgical expertise and specialized equipment. 3D-bioprinting modalities, such as extrusion-based bioprinting, can be used to address the clinical constraints of autologous flap transfer, primarily addressing donor-site morbidity and tissue availability. This advancement in regenerative medicine allows the biofabrication of heterogenous tissue structures with high shape fidelity and spatial resolution to generate biomimetic constructs with the anatomically-precise geometries of native tissue to ensure tissue-specific function. Yet, meaningful progress towards clinical application has been limited by the lack of vascularization required to meet the nutrient and oxygen demands of clinically relevant tissue volumes. Thus, various criteria for the fabrication of functional tissues with hierarchical, patent vasculature must be considered when implementing 3D-bioprinting technologies for deep, chronic wounds.
Keywords: angiogenesis, wound healing, bioprinting, blood flow, flap graft, regenerative medicine, flap graft, stem cells, microenvironment
1. Introduction
Deep, chronic wounds following infection, severe burns and trauma, diabetes, or tumor resection are characterized by the exposure of cutaneous tissues underneath the skin. The limited capacity of deep wounds to restore functional tissue within an efficient timeframe can ultimately cause permanent disfigurement, debilitation, or even death. Nearly 6.5 million Americans are burdened by the chronicity of non-healing, cutaneous wounds, with an annual cost of $25 billion dollars for treatment (Sen et al., 2009). Unlike acute wound healing, which spontaneously restores injured tissue within a predictable timeframe, deep wounds are complicated by severely compromised vasculature, diminishing the success rate of adjunctive therapies or tissue graft implantation and revascularization for complete rejuvenation. Delivery of an autologous tissue flap, with intact and patent vascular networks, to the wound site provides a reliable solution for the reconstruction of deep, non-healing wounds. However, these highly invasive surgical procedures have obligatory donor-site morbidity, which can exacerbate patient debilitation post-operatively, and may require a large supply of unavailable donor tissue. Novel tissue engineering strategies coupled with stem cell therapy can be used to address this clinical concern through the biofabrication of functional and vascularized neo-pedicles.
In native tissues, defined vascular organization exists to ensure that organ viability and functionality are achieved. Tissue flaps used for autologous transplantation may require layers of healthy skeletal bone, muscle, fascia, subcutaneous fat, and skin equipped with its own vasculature, regardless of size. Importantly, this vasculature is hierarchical, and the harvested flap requires an intact pedicle comprised of an arterial inflow vessel and at least one venous outflow vessel, which are microsurgically anastomosed to recipient vessels at the wound site. Establishment of tissue-specific properties will be necessary for the replacement of deranged tissues with a viable alternative. However, variations in niche specificity and cell occupancy of distinct tissue limits the ability to engineer a flap with tissue-specific properties. 3D-bioprinting has been investigated as a prospective strategy for the development of cellular scaffolds with precise vascular design and geometry, biological and mechanical properties, and robust structural integrity and architecture (Ning and Chen, 2017). Common modalities of 3Dbioprinting vascularized constructs are categorized as laser-assisted, inkjet, and extrusionbased. However, the successful use of bioprinting technology to fabricate cellularized constructs for deep wounds are highly dependent on bioink composition, polymerization mechanism, substrate stiffness, bioink rheology, cell source, and construct preconditioning. Thus, identification and exploitation of the key biomolecular and biophysical regulators that support hierarchical neovascularization of distinct tissues can inspire innovative approaches to engineer a functional thick tissue flap for chronic wound repair.
Noteworthy factors that facilitate angiogenic sprouting in the perivascular niche are attributed to mural support cells, extracellular matrix (ECM) stiffness and composition, and acute microenvironmental stresses to local regions of the vascular endothelium. Mesenchymal stromal cells (MSCs) and other support mural cells (e.g. pericytes, smooth muscle cells, and adipose progenitor cells) have been shown to promote microvessel formation of endothelial cells (ECs) via enhanced paracrine signaling in 2D and 3D co-culture systems (Armulik et al., 2005; Bowers et al., 2015; Caplan and Dennis, 2006; Watt et al., 2013). When ECs are subjected to local microenvironmental stresses, such as hypoxia, hemodynamic flow, and inflammation, capillary network formation, structure, and integrity are improved (Hsiai and Wu, 2008; Logsdon et al., 2014). Additionally, the formation and patency of endothelial microvasculature is highly regulated by the stiffness and composition of the ECM that ECs and other mural support cells reside in (LaValley and Reinhart-King, 2014).
In this review, we will briefly discuss the classification of deep, chronic wounds and the physiological aberrations that contribute to inadequate tissue restoration. We will also summarize the key considerations that must be addressed to fabricate cellularized constructs, using extrusion-based bioprinting, with tissue-specific functionality (e.g. skin, muscle, fascia, and bone) and hierarchical vasculature for chronic wound healing. Lastly, we will highlight the biological and biophysical nuances of the local microenvironment that directly prime and modulate neovascularization within 3D tissues.
2. Classification and Contributing Factors of Deep, Chronic Wound Repair
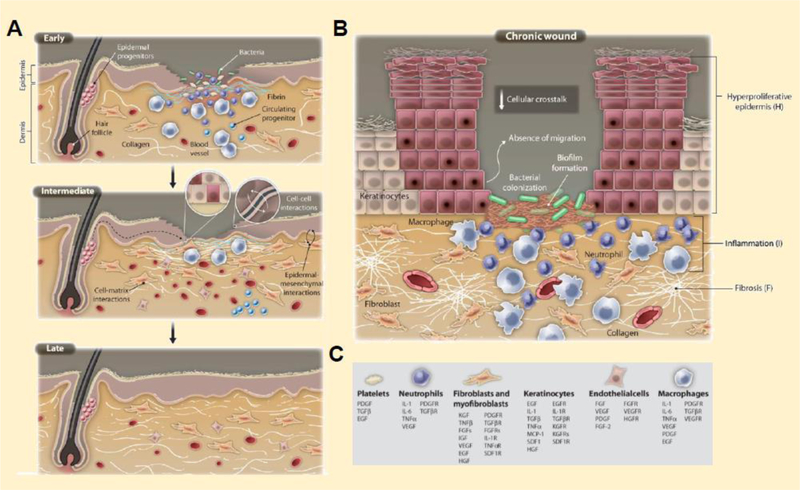
Wound repair involves an intricate and highly coordinated series of events that enable lesion closure and tissue regeneration within a predicted timeframe (Figure 1A). The general mechanisms that govern acute wound healing are categorized into the following four stages: hemostasis, inflammation, proliferation, and remodeling (Guo and DiPietro, 2010). In brief, damage to the skin prompts vasoconstriction of leaky microvessels to reduce blood flow and trigger fibrin clot formation at the injury site to mitigate excessive blood loss (Bonar et al., 2017; Ellis et al., 2018). Proinflammatory cytokines released from platelet granules of the fibrin clot and resident macrophages attract circulating neutrophils, macrophages, and T-lymphocytes to the wound bed for the removal of cell debris, infectious agents, and necrotic tissue, while stimulating the anti-inflammatory response to promote angiogenesis, dermal tissue regeneration, and wound closure (Ali and Rosenblum, 2017; Brancato and Albina, 2011; Ellis et al., 2018; Eming et al., 2014; Guo and DiPietro, 2010; MacLeod and Mansbridge, 2016; Ramasastry, 2005; Sorg et al., 2017). Although the native response to acute injury is finelytuned, this phenomenon may be disrupted due to complications accompanied by metabolic syndromes, radiation therapy, infection, ischemia, and prolonged inflammation, leading to chronic wound development (Figure 1B). Erroneous response to native wound healing processes can extend recovery time, resulting in the development and expansion of chronic, non-healing ulcers, which are further distinguished as pressure-induced, diabetic, venous, or arterial-insufficient wounds (Table 1).
Figure 1.
Wound healing physiology and pathology. During (A) acute wound repair, injured microvessels constrict and induce fibrin clot formation to prevent excessive blood loss. The combination of leaky blood vessels and pathogenic breach of the skin barrier elicits inflammation, triggering the recruitment and infiltration of immune cells. Lymphocytes, macrophages, and neutrophils support the removal of cell debris and microbes, while promoting neoangiogenesis in the developing tissue and re-epithelialization over the defect site. However, if inflammation persists for an extended period of time, chronic wounds may form, increasing susceptibility to infection and tissue degeneration (B). The key cell players and cytokine regulators present during wound repair are indicated by the legend (C). (Eming et al., 2014)
Table 1.
Characteristic traits and therapies for the 4 types of chronic wounds
| Ulcer Type | Anatomic Locations | Characteristics and Symptoms | Etiology and Risk Factors | Adjunctive Therapies | References |
|---|---|---|---|---|---|
| Pressure Ulcers | • Pressure points and bony protuberan ces • Hip, lower back, buttock, foot heel, ankle, back of head, shoulder, elbow, inner knee |
• Discolored and tender wound bed • Blistered and swollen skin about the ulcer • Ruptured skin, subcutis exposure, lymphatic drainage |
• Sustained pressure, friction, or shear stress applied to local tissue regions • Susceptibility increased due to cardiometabolic and perivascular diseases, immobility, and old age |
• Negative pressure wound therapy • Hyperbaric oxygen therapy • Electrotherapy |
Bluestein and Javaheri, 2008; Ontario Health Technology Advisory Committee in October 2008; National Institute for Health and Care Excellence 2013; Boyko et al., 2018; Prevention and managemen t, 2014 |
| Diabetic Foot Ulcers | • Lower extremities • Typically on the big toe or at the bottom of the foot |
• Redness, swelling, and irritation • Subcutis exposure • Foul odor and foot drainage • Eschar formation • Gangrene • Numbness • Callus formation |
• Sensory neuropathy • Compromised microvasculature and poor circulation • Irregular gait mechanics • Hyperglycemia • Diabetes and obesity |
• Hyperbaric oxygen therapy • Maggot therapy • Autologous platelet-rich plasma therapy |
Waniczek et al. 2013 Alexiadou et al. 2019 Armstrong et al. 2017 Wu et al., 2007 Bus et al., 2016 Monteriro-Soares et al., 2012 |
| Venous Ulcers | • Between the knee and the ankle • Medial or lateral malleolus (sides of the ankle) or other lateral bony prominences of the leg |
• Rash or dry skin • Itchy and burning sensation about the lesion • Discoloration • Foul odor • Redness and swelling • Lower extremity edema • Exposed subcutis |
• Varicose veins • Cardiometabolic syndromes • Smoking • Venous hypertension • Previous leg injuries • Poor blood circulation |
• Vascular surgery • Compression therapy |
Grey et al., 2006 Collins and Seraj, 2010 Werchek, 2010 Lim et al., 2018 Alavi et al. 2016 |
| Arterial Insufficie nt Ulcers | • Lower extremities at sites of poor arterial circulation • Typically on the lateral side of the leg between or above the ankle and the toes |
• Thin, dry, taut skin • Episodic claudication • Blockage of main arteries • Painful, demarcated wounds • Necrotic tissue and slough • Minimal exudate • Delayed capillary refill • Exposed subcutis |
• Peripheral vascular disease • Cardiometabolic syndromes Compromised microvascular flow • Chronic ischemia |
• Hyperbaric oxygen therapy • Angioplasty |
Hedayati et al., 2015 Grey et al., 2016 Bhutani and Vishwanath, 2012 |
Continuous tissue compression, particularly at highly-sensitive pressure points or bony protuberances, can disrupt blood flow and nutrient transfer to local subcutaneous regions, increasing the risk of pressure-induced ulcer (PIU) formation and growth (Figure 2A; 19). Pressure ulcers can present as acute, non-blanchable erythema with skin intact and vasculature or manifest into full-thickness tissue loss and derangement of the underlying muscle, fascia, bone and connective tissue (Bluestein and Javaheri, 2008a; Boyko et al., 2018; Ontario et al., 2008). Current preventative strategies to mitigate the occurrence of pressure-induced wounds in immobilized or heavily debilitated patients require the use of soft pads and frequent repositioning of the patient to ameliorate prolonged periods of pressure (Lyder and Ayello, 2008). However, when a pressure ulcer has developed, standard wound care procedures, including pressure offload, selective gauze and topical treatment, necrotic tissue debridement, infection minimization, supplemented with adjunctive pressure removal techniques, such as negative wound pressure therapy, are used to heal severe pressure ulcers.
Figure 2.
Chronic wounds are classified into 4 major categories: (A) Pressure ulcers, (B) diabetic foot ulcers, (C) venous ulcers, and (D) arterial-insufficient ulcers. (Duci et al., 2013; Besse et al., 2011; Rubin et al., 2008; Grey et al., 2006)
Similar to PIU, diabetic foot ulcers (DFU) typically develop in regions of high pressure such as the plantar metatarsal heads of the foot and are induced due to neuropathy, peripheral ischemia, and irregular foot biomechanics (Figure 2B; Alexiadou and Doupis, 2012; Armstrong et al., 2017; Sindhu, 2018). In general, large vessels above the ankle remain healthy and viable in patients with diabetes mellitus; however, the microvasculature below the ankle can become severely compromised and irreparable. In neuropathic-related DFU development, the loss of pain sensation compromises the loaded regions ability to detect aversive stimuli caused by gait abnormalities, allowing it to undergo repetitive pathological stresses and subsequent ulceration (Alexiadou and Doupis, 2012; Armstrong et al., 2017; Bus et al., 2016; Monteiro- Soares et al., 2012; Wu et al., 2007). DFU are also acquired by DM patients with peripheral vascular diseases. In these cases, inadequate microvessel perfusion at local regions of high pressure can impede adequate nutrient diffusion and barrier protection, which subsequently induce tissue necrosis and defective infection clearance, respectively (Alexiadou and Doupis, 2012; Armstrong et al., 2017; Wu et al., 2007). Adjunctive therapies supplemented with necrotic tissue debridement, pressure alleviation, infection mitigation, and vascular reconstruction can be performed on the appropriate candidates (Sindhu, 2018; Wu et al., 2007). However, persistent ulcer infection can progress to an adjacent limb, potentially increasing susceptibility to the lifethreatening infection, gangrene, which requires amputation for patient survival.
In addition to diabetes-induced ulcerations, venous perforator incompetence due to sustained hypertension, particularly in the lower extremities between the midcalf and the ankle, contributes to the development of venous ulcers, characterized by hyperpigmented and granulated tissue surrounded by eczematous skin (Figure 2C; Collins and Seraj, 2010; Grey et al., 2006; Lim et al., 2018; O’Meara et al., 2009; Werchek, 2010). Expansion of the venous walls allows excess fluid and sera proteins to enter the surrounding interstitium, causing edema, and triggers capillary deposition of a fibrin cuff and entrapment of leukocytes. This increases proinflammatory cytokine secretion and chronic inflammation, while preventing adequate diffusion of oxygen and proteins to the subcutaneous tissue, leading to tissue necrosis and ulceration (Collins and Seraj, 2010; Crawford et al., 2017; Lim et al., 2018; Werchek, 2010). Venous incompetence is exacerbated by immobility, deranged calf muscle pumping, congenital defects or valvular dysfunction; and patients afflicted by old age, obesity, deep vein thrombosis, or previous leg injuries are at high risk for developing lower extremity venous ulcers (Werchek, 2010). Current treatment options, including compression therapy or endovenous procedures, are aimed at maintaining a moist environment for the healing wound, while minimizing infection and alleviating edema about the injury.
Although the characteristics of venous and arterial ulcers are comparable, arterial insufficient ulcers (AIU) are primarily distinguished from venous ulcers due to the presence of pain and lack of edema at the lesion site. Arterial ulcers commonly develop in the lower extremities of patients with significant peripheral vascular damage at the lower extremities; however, they can occur in local regions subjected to repetitive trauma or induced pathological pressure (Grey et al., 2006; Hedayati et al., 2015). AIU is caused by the chronic ischemia of tissue which are distinguished as painful and demarcated wounds of necrotic tissue, slough, and minimal exudate (Figure 2D; (Grey et al., 2006). In general, aberrant perfusion of the peripheral arteries caused by macrovessel restriction damages capillary function, depriving the skin and subcutaneous tissues from adequate oxygen and nutrients (Forster and Pagnamenta, 2015; Grey et al., 2006; Hedayati et al., 2015). This persistent low blood supply at local tissue regions contributes to necrosis, ulceration, and eventually infection. Interventional strategies developed to mitigate ulcer expansion and tissue loss are centered around improving tissue perfusion via (endo)surgical revascularization, nutrition, pain management, patient education, infection clearance, and topical treatment (Greer et al., 2013; Grey et al., 2006; Nelson and Bradley, 2007).
3. Tissue-Specific Flaps for Deep, Chronic Wounds
The emergence of advanced therapies supplemented with standard wound care procedures have been implemented to promote chronic lesion closure within a timely manner. Growth factor therapy (GFT) accelerates the healing of deranged tissues through local or topical delivery of restorative factors in the lesioned area (Han, 2016; Piaggesi et al., 2018). However, there is a high cost associated with growth factor usage, and the external application of these reparative cytokines is limited by the swift degradation rate, high dose requirement and reapplication, and low permeation through the wound (Park et al., 2017). In stem cell therapy (SCT), autologous mesenchymal stem cells (MSCs) derived from bone marrow or adipose lipoaspirates has been shown to improve wound granulation, lesion closure, and tissue restoration in deep, chronic wounds (Badiavas et al., 2007, 2003; Dash et al., 2009; Falanga et al., 2007; Garcia-Olmo et al., 2008; Lee et al., 2011; Rigotti et al., 2007). However, patients with severe microvascular deficiencies and chronic inflammation may not support cell-based treatment to repair these defects in a time- and cost-efficient manner (Duscher et al., 2016, 2015; Mester et al., 2017). Lastly, artificial skin substitutes with decellularized or cellularized matrices can be used to promote host cell infiltration, proliferation, differentiation, and matrix remodeling for adequate wound closure (Duscher et al., 2015; Han, 2016; Herskovitz et al., 2016; Nicholas and Yeung, 2017; Piaggesi et al., 2018). However, these options are limited by the host immunological response to allogeneic and xenogeneic materials, degradation rate of biological matrices, cell type source, isolation and seeding methods, and compromised biofunctionality of soluble and insoluble ECM constituents (Nicholas and Yeung, 2017; Piaggesi et al., 2018). The wound bed vasculature of late-stage chronic wounds may also be too deranged to support revascularization of transplanted matrices or skin substitutes. In this case, surgical procedures involving the transfer of a tissue flap or pedicle will be required to reconstruct the tissue at the wound site.
Autologous tissue flap transfer relies on the successful resection and transplantation of a vascular pedicle derived from a healthy, autologous source either adjacent or distant to the defect site for sufficient wound repair (Horch et al., 2017; Simman, 2009). Tissue flaps are equipped with their own hierarchical vasculature and do not require vascular ingrowth from the underlying wound bed to receive adequate nutrients and oxygen since they are already adequately perfused (Basu, 2016; Dunn, 2006). Tissue flaps that restore the anatomical and physiological integrity of severely compromised tissue are beneficial to patients who suffer from microvascular inadequacies and advanced ulcers that penetrate the subcutis. However, it should be noted that the injured tissue can never achieve the same properties and appearance as the native tissue upon transplantation. Therefore, the essential requirements for donor-site flap selection must prioritize optimal tissue aesthetics and functionality (Tschoi et al., 2005).
Distinct vascular properties of potential donor sites also dictate its use for sufficient wound repair. Thus, tissue flaps are classified by donor site proximity, structure and composition, and origin of blood supply. Tissue flaps identified by donor-site proximity can be local, regional, or distal. Autologous local and regional flaps are derived from anatomical locations adjacent to and near the defect site, respectively, and maintain their original source of blood supply (Dunn, 2006). However, distant flaps are harvested from a different location of the body, and the vascular pedicle, which contains the feeding artery and draining veins are microsurgically anastomosed to recipient vascular supply at the defect site (Dunn, 2006). Tissue flaps can contain a variety of tissues including skin-only and muscle-only, or the underlying musculocutaneous, fasciocutaneous, and osteocutaneous regions of donor tissue, or any combination thereof (Basu, 2016; Geddes et al., 2003; Kim, 2005; Saint-Cyr et al., 2009). Tissue flaps can also be categorized as random or axial depending due to their origin of blood supply. In random pattern skin flaps, the dermal-subdermal plexus is harvested from a local donor site and transplanted to the wound bed to replenish the healthy skin and subcutaneous fat via small, unnamed blood vessels (Fujioka, 2014; McGregor and Morgan, 1973). Axial tissue flaps, however, may contain skin, subcutaneous fat, muscle or bone, and can either be transplanted regionally connected to its original vascular source, or distally, as a free flap, reconnected to the new blood vasculature near the wound (Fujioka, 2014; McGregor and Morgan, 1973).
Candidate regions for autologous donor tissue must meet a set of criteria to repair the functional and aesthetic needs of tissue-specific wounds with severely compromised vasculature. As previously described, deep cutaneous wounds are slow healing and require interventional methods for full recovery. Wound chronicity can cause any combination of subcutaneous fat, muscle, fascia, or bone to be exposed, increasing the patients’ susceptibility to infection if left untreated. Therefore, adequate tissue reconstruction through surgical methods requires knowledge regarding the properties of the specific donor-site tissue in relation to the native tissue properties at the recipient site. This is not only crucial for the successful transplantation an autologous flap, but also for the biofabrication of neo-pedicles using tissue engineering strategies. The biostructural and mechanical properties of bone, muscle, fat, skin, and connective tissue (e.g. tendon, fascia, and ligaments) are important considerations that must be accounted for when treating deep, chronic wounds (Figure 3). Insight on these parameters streamlines the selection process for choosing potential donor tissues that successfully recapitulate the former properties and mechanics of the healthy tissue. For instance, subjects afflicted by craniofacial congenital defect, such as oral clefts, require local flaps for proper palate reconstruction to improve speech, realign soft palate muscles, and minimize maxillary disturbances (Agrawal, 2009). On the other hand, breast cancer patients who have undergone a mastectomy, require the transplantation of fleshy and fatty tissue from the abdomen or buttock to reestablish the original aesthetic and contour of breast tissue. (Healy and Allen, 2014). Therefore, tissue engineers can benefit from the understanding of autologous donor-tissue flap properties to fabricate neo-pedicles that are capable for rejuvenating specific, chronic wounds (Table 2).
Figure 3.
Tissue-specific mechanics regulate cell differentiation. Distinct tissues acquire unique mechanical properties that contribute to their overall function. Compliant tissues (e.g. brain and lungs) exhibit a lower elastic modulus, indicative of tissue stiffness, whereas, rigid tissues (e.g. bone) demonstrate a greater elastic modulus. (Cox and Erler, 2011)
Table 2.
Donor-site characteristics and flap considerations for the reconstruction of distinct soft tissues. (L: local flaps; R: regional flaps; D: distal flaps)
| Injured Recipien t Site | Defect Causes | Major Considerations for Tissue Reconstruction | Donor-Site Characteristics | Typical Flaps | Key Donor-Site Regions | References |
|---|---|---|---|---|---|---|
| Facial (Cheek) Injury | • Traumatic injury • Congenital anomaly • Tumor resection • Lymph node dissection |
• Tissue pigment, character, and texture matching • Minimal scarring |
• Pliable skin for external coverage and internal cheek lining • Low subcutaneous fat content • Re-establish mandible lining |
L: advancement, rotational, transposition flaps R: myocutaneous major flap D: microvascular free flap |
L: lateral facial planes R: lateral facial planes, upper chest D: radial forearm, anterolateral thigh, scapular, lateral arm |
Heller et al., 2008; Pilsl et al., 2012 |
| Breast Injury | • Tumor resection • Radiation therapy |
• Re-establish breast contour, texture, and lift • Nipple reconstructi on • Minimal scarring |
• Sufficient subcutaneous fat volume • Adequate muscle tissue and strength • Pliable skin • Well-defined vascularity |
R: transverse rectus abdominis myocutaneous (TRAM) pedicle; latissimus dorsi (LD) pedicle D: deep inferior epigastric perforator (DIEP) free flap; Superficial inferior epigastric artery (SIEA) free flap |
R: lower abdomen, lateral side of the middle back D: buttock region, Inner thigh |
Lee and Sheckter, 2018 Dayan and Allen, 2017 Pinel-Giroux et al., 2013 |
| Lower Back (Sacrum) Tissue Injury | • Pressure ulcers or bed sores | • Soft tissue defect coverage • Weight-bearing support in supine position |
• Pliable skin with sufficient tensile properties | L: Limberg (rhomboid), bilateral and unilateral rotation and advancement flaps with VY closure, musculocutaneous s or fasciocutaneous transverse flap | L: gluteus maximus, lower back axial to the defect site | Oksman et al.,2018 Chasmar 2007, Bamba et al., 2017 Borman and Maral, 2002 Hill et al., 1978 George et al., 2018 |
| Leg (knee) and Arm (elbow) Injury | • Venous ulcers, arterial insufficient ulcers, severe burns, tumor excision | • Soft tissue defect coverage | • Thin pliable coverage with hairless skin (elbow) • Low subcutaneo us adipose content • Withstand flexion and extension about the elbow |
L: advancement, island, transposition flaps R: pedicles, posterior interosseous artery flap D: microvascular free flaps, SIEA |
L & R: lateral arm, radial forearm, LD D: radial forearm, groin, scapula, lateral arm, anterolateral thigh |
Griffin et al., 2014 Wu et al., 2015 Kamath et al., 2012 Shiwei et al.. 2007 |
| Hand, Foot, and Ankle Injury | • Diabetic foot ulcers, pressure ulcers, arterial insufficient ulcers, tumor resection | • Soft tissue defect coverage • Fat pad re-establishme nt for weightbearing regions of skeletal stability • Sensation preservation |
• Adequate skin thickness, nerve endings, and fat supply for weightbearing regions • Pliable skin with thin subcutaneous fat layer for non-weightbearing regions |
L: myocutanous VY flaps, fasciocutaneous sural artery (SA) flaps, transposition flap, propeller flap, perforator flap D: myocutaneous or fasciocutaneous free flap, pedicle, fascia flap, contralateral leg free flaps, SA free flap |
L: gastrocnemiu s muscle, radial forearm, ulnar nerve D: abdominus rectus, latissimus, anterolateral thigh, parascapular, radial forearm, groin, scapula, gastrocnemius |
Sato et al., 2017 Ring et al., 2016 Friedrich et al., 2009 |
4. Extrusion-Based Bioprinting to Fabricate Anatomically-Precise Tissues
Invasive surgeries that utilize autologous flaps are required to restore the function and appearance of chronic wounds with compromised vasculature; however, these strategies are not only limited by donor tissue availability, but also increase donor-site morbidity and infection susceptibility post-operatively. Tissue engineering approaches – namely, stem-cell based therapies supplemented with protective bio-scaffold design – overcome these hurdles by eliminating the need to harvest volumes of healthy, autologous donor tissue, that may otherwise be limited and unavailable (Mathew, 2016; Olson et al., 2011). Additionally, the expansion and fabrication of engineered tissues derived from a sampled biopsy reduces post-operative patient debilitation and infection vulnerability by decreasing donor-site morbidity (Mathew, 2016; Olson et al., 2011). Although tissue engineering can address these concerns, conventional approaches are limited by the lack of spatial control, patient-specificity, tissue heterogeneity, and reproducibility. 3D-bioprinting is a growing advancement in tissue engineering that offers a cost-effective and minimally invasive alternative to autologous flap transfer. In general, 3Dbioprinting fabricates geometrically-precise and heterogenous biological constructs equipped with viable cells, structural matrix proteins, and bioactive morphogens to guide tissue-specific cell behavior (Cui et al., 2012; Do et al., 2015). The potential of 3D-bioprinting can not only regulate bio-construct geometry with fine-tuned control, high spatial resolution, and reproducibility, but also recapitulate the heterogenous architecture and residing hierarchical vasculature of native, complex tissues unique to individuals (Vijayavenkataraman et al., 2018). The combined use of adult autologous progenitor cells, readily-available biomaterials with low immunogenicity, pro-angiogenic stimuli, and extrusion-based 3D-bioprinting (EBB) can produce organ-specific constructs with anatomical precision, replacing the use of autologous tissue flap for chronic wound repair.
Thick, bioprinted neo-pedicles with preexisting vasculature must utilize bioinks that not only permit neoangiogenesis, but also the formation of bone, muscle, fat, skin, and connective tissue for the adequate restoration of deep, chronic wounds. Additionally, the homeostatic function of unique tissues is highly dependent on their signature architectural organization, mechanical behavior, microenvironment heterogeneity, and residential cell populations. Guided by computer-aided designs or medically-acquired images, EBB technology utilizes pressure- or mechanically-driven dispensing systems to fabricate 3D cellular constructs with high shape fidelity, spatial resolution, and reproducibility (Figure 4A; Derakhshanfar et al., 2018; Ning and Chen, 2017; Pati et al., 2015; van Kogelenberg et al., 2018). Cylindrical filaments of bioink are deposited in a layer-by-layer fashion to generate mechanically robust and cell-compatible constructs that recapitulate the native structure and organization of healthy tissues (Pati et al., 2015; van Kogelenberg et al., 2018). Despite these advances, biofabrication methods are restricted to the development of thin tissue-engineered constructs that can facilitate the diffusion of oxygen, which falls between 100 and 200 microns (Radisic et al., 2006; Richards et al., 2017). To address this concern, the incorporation of multiscale vasculature within voluminous bioprinted tissues is required to ensure that healthy tissue-specific function is achieved and maintained upon implantation.
Figure 4.
Extrusion-based bioprinting can be used to fabricate hierarchical vascularized constructs. General schematic of pressure- or mechanically-driven EBB (A) and omnidirectional 3D-printing of bifurcated micro-channels within an hydrogel reservoir (B-G). (Murphy et al., 2014; Wu et al., 2010)
4.1. Microvessels
Bioprinted constructs must be equipped with macro-vessels that can anastomose with the host vasculature and withstand long-term hemodynamic flow. Blood vessel architecture is organized as three concentric layers or tunica intima, media, and adventitia, by which each layer serves a distinct purpose for maintaining cardiovascular homeostasis (Song et al., 2018). This hierarchical vascular organization within multi-level tissue eventually diverges into arterioles and venules, which bifurcate into capillary networks to facilitate nutrient transfer, oxygen diffusion, and waste removal throughout voluminous tissues (Figure 4B–G; Miri et al., 2018).
Antiquated methods of studying vascular tissue engineering have progressed from 2D systems to 3D-spheroid co-cultures, primarily focusing on the role of either one or any combination of the following attributes: angiogenic and pro-inflammatory cytokine supplementation, perivascular cell crosstalk, niche-specific structural ligands, and oxygen-tense conditions (Boyko et al., 2017; Bray and Werner, 2018; Irvin et al., 2014; Song et al., 2018). Although these studies have contributed to the development and progression of the field, these strategies are limited by their inability to support perfusion and achieve long-term stability. The use of microfluidic devices to tissue engineer vasculature have not only addressed the limitations of traditional 2D and 3D culture systems, but also includes another crucial parameter – shear stress – that governs the angiogenic potential of endothelial cells to generate microvessels in 3D culture systems (Akbari et al., 2017; Song et al., 2018; X. Wang et al., 2018). However, these strategies offer no control over the vascular directionality or hierarchy, potentially causing tortuous vasculature that resembles more of a tumor microenvironment. Additionally, the biomaterials used for initiating de novo sprout formation within 3D matrices are often purely biological, primarily fibrin or collagen, which are thrombogenic and mechanically inferior, rendering them ineffective for long-term stability in vivo. Lastly, microfluidic devices only account for the role of shear stress on angiogenesis, ignoring the influence of additional hemodynamic flow parameters, including cyclic stress, interstitial pressure, and vorticity. Therefore, there is a need to micropattern hierarchical, patent vasculature within a mechanically robust material that not only recapitulates native blood vessel architecture, but also supports true hemodynamics and capillary plexus formation between preestablish vasculature.
The stability of tissue-engineered neo-pedicles implanted into the wound bed of non-healing tissue is highly dependent on its ability to adjoin with the host vascular supply, promote host vascular integration, and permit adequate oxygen and nutrient diffusion through thick tissues. For tissue engineering applications, macro-channels embedded within bulk materials should mimic the relevant vessel geometry and architecture of native tissues. Through direct, indirect, or a combination of both EBB strategies, the high spatial resolution and fine control of 3Dbioprinting allows micropatterning of hierarchical 3D-vascularity that better recapitulates the native architecture of bifurcated networks within distinct tissues. Bioprinted vessels require endothelialization to prevent thrombosis and anastomosis with the host vasculature, where upon perfusion, vasculogenesis and subsequent angiogenesis and vessel pruning can occur (Datta et al., 2017). Although the surgical anastomosis of macrovessels to host vasculature is crucial for the survival of implanted neo-pedicles, biologically-induced microvessel anastomosis between host and graft microvasculature, in addition to inosculation between bifurcated channels, will also be critical for the full integration of implanted grafts. Several factors have been identified to accelerate microvessel anastomosis between host and graft vasculature, including cellular and biomolecular composition, inflammation, hemodynamics, hierarchical vascular architecture, and preestablished microvasculature (Table 3, Song et al., 2018). Albeit, since some studies provide contradictory evidence on the anastomotic potential of pre-vascularized and non-vascularized bioengineered constructs, the precise mechanisms that govern host-graft microvessel anastomosis remain unclear (Ben-Shaul et al., 2019; Lin et al., 2017; Mazio et al., 2019; Pattanaik et al., 2019).
Table 3.
Attributing factors of vessel anastomosis
| Attributing Factors | Major Findings | Limitations | References |
|---|---|---|---|
| Inflammation | • Non-inflammatory myeloid cells (e.g. macrophages, neutrophils, and monocytes) enhance graft-host anastomosis, host vasculature integration, and graft perfusion in the absence of thrombosis | • Inadequate blood supply to the center of the graft will induce tissue necrosis • Loss of endogenous vessels in the pre-vascularized construct may result in graft failure |
Fantin et al., 2010 Gerri et al., 2017 Lin et al., 2017 |
| Cellular and Biomolecular Components | • Fibroblast cocultured with ECs and bFGF supplementation accelerate vascular graft integration and graft-host anastomosis, typically in regions of low VEGFR1 expression in the vascular endothelium | • Mechanistic studies, involving niche-specific biomolecular, cellular, and biophysical components, are required to fully elucidate the precise mechanisms that contribute to graft-host anastomosis | Chen et al., 2010 Sekine et al., 2013 Nesmith et al., 2017 |
| Hemodynamics | • In comparison to normal flow, pulsatile vorticity at anastomotic ends of graft and host macrovasculature enhances endto-end joining, with negligent effects on platelet activation, decreasing monocyte adhesion and the potential for thrombus formation | • Additional hemodynamic flow properties have not been considered, including cyclic strain, wall shear stress, and interstitial pressure | Zhan et al., 2010 Chen et al., 2012 Ha et al., 2015 Zhang et al., 2016 |
| Prepatterned Vascular Hierarchy (Top-down approach) | • Prepatterned vasculature with defined geometry enhances graft integration and rescues ischemic tissue perfusion in comparison to un-patterned endothelium | • Spatial resolution of current 3D-bioprinting modalities is not precise enough to recapitulate the microarchitectural complexity of the capillary plexus | Baranski et al., 2013 Chaturvedi et al., 2015 Mirabella et al. 2017 Stevens et al., 2017 |
| Preestablished Microvasculature (Bottom-up approach) | • Bioengineered constructs equipped with stable, complex, and elongated vasculature accelerate graft-host microvessel anastomosis, via wrapping-and-tapping, in comparison to non-vascularized or poorly vascularized implants | • De novo formation of vasculature within bioengineered constructs is less regulated, potentially leading to tortuous vessel network formation, tumor-like vessel density, and lack of directionality • Occluded microvessels and improper graft perfusion may invoke necrotic tissue formation at the center of the graft |
Cheng et al., 2011 Koffler et al., 2011 Samuel et al., 2013 Franco et al., 2015 Heller et al., 2016 Asano et al., 2017 Sugden et al., 2017 Ben-Shaul et al., 2019 |
Bioprinted macrovessels with distinct geometry can also be perfused to initiate angiogenic sprouting from micropatterned vasculature, enhancing the anastomotic potential of prevascularized neo-pedicles for the long-term stability and graft integration. Thus, implementation of 3D-biotprinting technology to engineer multi-level vascularized neo-pedicles is of increasing interest to tissue engineers for deep, chronic wound recovery. Previous studies have verified that the fabrication of hierarchical and permeable vasculature within voluminous bioprinted tissues is feasible and can improve cell survival and activity throughout the bulk of tissueengineered constructs (Table 4; Figure 5; Datta et al., 2017; Jia et al., 2016; Kolesky et al., 2014; V. K. Lee et al., 2014; Miller et al., 2012; Miri et al., 2018; Richards et al., 2017). Despite these findings, it is crucial that the surrounding matrix about the fabricated lumen is not only mechanically robust to withstand rapid matrix modification and perfusion upon anastomosis, but also complaint enough to facilitate tip and stalk cell activation and angiogenic sprout formation from preestablished blood vessels for adequate bulk cell survival.
Table 4.
Vascular biofabrication using EBB
| Authors | Methodology | Major Findings | Limitations |
|---|---|---|---|
| Miller et al., 2012 | • EBB was used to fabricate rigid, filamentous networks of fugitive carbohydrate glass entombed in cellularized PEG- or agarose-based bulk material | • The viability and activity of bulk material resident cells can be maintained if adequate nutrient and oxygen diffusion is permissible by a leaky macrovasculature, which is a function of endothelialized lumen saturation | • Cells in the innermost region of the bulk mass did not remain viable after 3 days due to their limited accessibility to nutrients |
| Kolesky et al., 2014 | • Bifurcated, acellular lumens were fabricated in GelMA bulk hydrogels using a fugitive ink, Pluronic® | • Hollow lumens withstood perfusion of animal blood, and supported the attachment, survival, and proliferation HUVEC, HNDF, and 10T1/2 MSCs | •No information is provided on the effects of macrovessel permeability on nutrient diffusion and cell viability in bulk resident cell populations |
| Lee et al., 2014 | • GFP-HUVEC-laden fibrin matrix was printed and positioned between two parallel, gelatin-based channels, lined with RFP-HUVEC, and perfused to invoke de novo microvessel formation and matrix-channel anastomosis | • Biofabricated system facilitates sprout anastomosis between lumen HUVEC and bulk HUVEC, permitting perfusion of 10kDa dextran throughout the spontaneously formed capillary networks within the fibrin bulk | • These studies do not address whether or not nutrient diffusion through de novo capillary networks supports cell viability of non-vascular cells within the bulk material • Fibrin is thrombogenic and not mechanically robust enough to withstand implantation and perfusion in vivo |
| Jia et al., 2016 | • A multilayered coaxial system was used to print hollow, cellularized macro-lumens of biomimetic PEGTA and sodium alginate, with varying diameters and wall thickness | • Fabricated macrovessel supported HUVEC and MSC viability and proliferation, and matrix degradation over a 21day period at the lumen | • Implantation of a highly-organized, patent lumen into a defect site requires bulk material for long-term stability |
Figure 5.
Vascular biofabrication using EBB. (A) Schematic of bioprinted PEGTA/GelMA tubular constructs with multiple layers (i). Single, tenlayered tubes bioprinted with green fluorescent microbeads supports perfusion along a continuous lumen (ii). Fabricated lumens support colocalization of αSMA+-MSCs and CD31+-HUVEC after 14 (top) and 21 (bottom) days of culture (iii). (B) Optical image of bioprinted GelMA construct with interwoven channels upon sacrificing fugitive bioink (i). Cellularized channels containing bioprinted 10T1/2 fibroblasts-laden GelMA (blue), HDNF-laden GelMA (green), and endothelialized lumen with HUVEC (red) (ii). (C) Schematic of perfusion systems with (right) and without (left) lumen fabrication (i). Heat map depicting cell activity within each PEG-based construct (ii). Live/Dead analysis on bulk stromal fibroblasts within slab (left) or perfusable (right) agarose-based construct indicate perfusion support enhanced cell viability. (D) Schematic (top) and optical image (bottom) of a collagenous biofabricated construct within a custom-designed flow chamber (i). Live/Dead analysis of hepatocytes within the bulk of hydrogel indicate that confluency of the endothelial barrier at the lumen wall perturbs cell viability after 3 days (ii). (E) De novo formation of capillary networks between two parental channels after 2 (left) and 12 (right) days (i). Depiction of lumen HUVEC sprouting upon perfusion and inosculation with bulk material forming sprouts after 9 days (ii). Formation of patent capillary networks connected to larger parental channels as demonstrated by the 10kDa dextran diffusion through the fibrin bulk material (iii). (Jia et al., 2016; Kolesky et al., 2013; Miller et al., 2012; Lee et al., 2014)
5A: Jia W, Gunger-Ozkerim PS, Zhang YS et al. Direct 3D bioprinting of perfusable vascular constructs using a blend bioink. Biomaterials. 2016;106:58–68. doi:10.1016/j.biomaterials.2016.07.038.
5B: Kolesky DB, Truby RL, Gladman S et al. 3D Bioprinting of Vascularized, Heterogeneous Cell-Laden Tissye Constructs. Adv Mater. 2014;26:3124–3130. doi:10.1002/adma.201305506
5C: Miller JS, Stevens RK, Yang MT et al. Rapid casting of patterned vascular networks for perfusable engineered 3D tissues. Nat Mater. 2012;11(9):768–774. doi:10.1038/nmat3357
5D-E: Lee VK, Kim DY, Ngo H et al. Creating Perfused Functional Vascular Channels Using 3D Bio-Printing Technology. Biomaterials. 2014; 35(28): 8092–8102. doi:10.1016/j.biomaterials.2014.05.083.
4.2. Skin
Advanced therapies available to replenish deep, chronic wounds typically require healthy, viable skin tissue to ensure thermoregulation, absorption and excretion, sensation, and adequate protection from environmental pathogens. The epidermis, dermis, and hypodermis are three integrated layers of skin, each comprising of unique cell populations and accessory structures to support its full functionality. Although the epidermis is a keratinized sheet of stratified squamous epithelium that lacks vascularity and neuronal networks, the underlying dermal tissue contains many sensory neurons, blood and lymphatic vessels, hair follicles, and sweat glands (Betts et al., 2013). Underneath the dermis, the hypodermis is also highly innervated and vascularized. However, it is also equipped with melanocytes for epidermal pigmentation, fat storage for insulation and cushioning, and fascia for attachment to subcutaneous bone and muscle (Betts et al., 2013).
Conventional therapies to repair partial-thickness skin defects have been developed to stimulate dermal and epidermal tissue healing. Current methods of treatment include splitthickness and epithelial autografts, transplantable dressings derived from ECM proteins, growth factor delivery, and the use of autologous stem cells. Although these strategies have been shown to support the adequate healing of partial-thickness wounds, simple skin substitutes are limited by donor-site morbidity, donor tissue and stem cell availability, prolonged graft development, inefficient host-graft integration, spontaneous blistering, and adverse immune reactions to xenogenic or allogenic agents (Dreifke et al., 2015). To address these concerns, previous studies have demonstrated the capacity to engineer perfusable dermo-epidermal constructs that permit the integration of host lymphatic and circulatory vasculature upon implantation (Abaci et al., 2016; Bourland and Fradette, 2018; M. B. Chen et al., 2017; Dai et al., 2018; Frueh et al., 2017; Groeber et al., 2016; Marino et al., 2014; Mori et al., 2017; Redd et al., 2019; Sooppan et al., 2016). However, bioengineered skin substitutes still inaccurately represent the structure of epidermal and dermal tissue as consecutive layers of fibroblasts and keratinocytes in the absence of accessory glands, structures, and neuronal networks (Herskovitz et al., 2016; Yan et al., 2018). Since these simple skin substitutes still lack the architectural complexity of native skin, including relevant vasculature, the complete functionality of bioengineered skin is compromised.
In addition to the architectural complexity of skin, the vast heterogeneity of niche cell populations that constitute the epidermis, dermis, and hypodermis complicates the use of tissue engineering strategies for the repair of full-thickness skin defects. Complete restoration of deranged, deep tissue circulation will require technologies capable of fabricating predefined geometries of cell-laden biomaterials that support the development of hair follicles, sweat glands, sensory neurons, vasculature, and pigment cells within the skin (Abaci et al., 2018; Gledhill et al., 2015; Huang et al., 2010; Lugo et al., 2011; Muller et al., 2018). 3D-bioprinting technology not only has the potential improve the fabrication of pre-vascularized skin substitutes, but also the ability to recapitulate the structural complexity of native skin for its complete functionality. 3D-bioprinted skin, harboring prepatterned lymphatic and circulatory vasculature, accessory glands, structures, and neuronal networks, can be fabricated with either iPSCs or a heterogenous stem cell populations, capable of differentiating into to the various lineage-specific skin cell types, in restricted, predefined spaces. Recent developments in skin fabrication combine the use of stem cell therapy with EBB technology to reproduce patientspecific skin substitutes with geometric precision and preestablished, perfusable vasculature (Table 5; Figure 6; B. S. Kim et al., 2018; Pourchet et al., 2017; Skardal et al., 2012). Although these advancements are only capable of treating partial-thickness wounds, EBB offers promising resolutions to address the limitations of engineering full-thickness skin alternatives that recapitulate the architectural complexity and heterogeneity of native skin. It should also be noted that EBB strategies used for generating vascularized hypodermal tissue, including subcutaneous fat and superficial fascia, for the complete rejuvenation of full-thickness skin defects remains an understudied area of research.
Table 5.
Skin biofabrication using EBB
| Authors | Methodology | Major Findings | Limitations |
|---|---|---|---|
| Skardal et al., 2012 | • Cell-laden composite bioinks (fibrin and collagen), encapsulating amniotic-fluid stem cells (AFSCs) and BM-MSCs, were bioprinted, implanted over a murine mid-dorsal skin defect model, and assessed based on wound closure rate at 0, 7, and 14 days post-surgery | • Wound closure, contraction, and re-epithelialization of the defect site was accelerated in implanted cellular constructs when compared to acellular constructs • Robust microvessel integration into the implanted construct was enhanced, primarily due to AFSC cytokine secretion |
• Lack of cell integration from the implant to the wound bed indicates that tissue-engineered implants possess limited engraftment potential, compromising their long-term stability |
| Lee et al., 2014 | • Fibroblast-laden collagen constructs were 3D-bioprinted and keratinized to recapitulate the native structure of the epidermal-dermal junction • Bioengineered construct were cultured with the fabricated dermis submerged in media and the epidermal layer at the air-liquid interface |
• Bioengineered constructs resembled the morphological appearance and biological structure of native skin tissue in vitro | • Methods do not fully recapitulate the architectural complexity of skin, which requires the presence of hair follicles, pigment cells, sweat glands, blood vessels, and sensory neurons |
| Pourchet et al., 2017 | •Fibroblasts-laden composite bioinks (alginate, gelatin, and fibrinogen) were printed and cured onto a cooling plate to emulate dermal tissue, keratinized to mimic epidermal tissue, and conditioned for 26 days | • Bioengineered constructs exhibited comparable morphological and biological features as native skin tissue, with defined epidermal stratification and dermal tissue maturation within 3 weeks | • Vascularization of bioprinted skin flaps are required to maintain stable and functional tissue throughput the lifetime of the recipient •Studies lack information on the integrative potential (both vasculature and dermal tissue) of bioprinted skin in an in vivo defect model |
| Kim et al., 2018 | • Biomimetic scaffolds of native skin were printed using EBB, to fabricate the dermis (fibroblastladen S-dECM), and inkjet bioprinting, to fabricate the epidermi, (keratinocyte-laden culture medium) • Constructs were cultured until stratification and keratinization were achieved • EPC/ASC-laden S-dECM were printed and pre-vascularized prior to implantation in a cutaneous wound healing murine model |
•S-dECM maintained its endogenous biophysical and biomolecular features, and can serve as a bioink • EBB can print prevascularized S-dECM to promote wound healing in vivo • Co-encapsulation EPCs and ASCs in S-dECM bioink support rapid wound closure, re-epithelialization, and neovascularization of a full-thickness excisional wound |
• Biofabrication of functional skin for full-thickness defects will require the presence of tissue-engineered subcutaneous fat to replenish the hypodermis |
Figure 6.
Skin biofabrication using EBB. (A) Supplementation of fibrin-collagen bioink with MSCs or AFSCs alone augment wound closure rate (i), re-epithelization (ii), tissue thickening (iii), microvessel density and vessel diameter (iv). (B) Schematic of the maturation process of skin tissue (i). This biofabrication method for producing fibroblast-laden alginate-gelatin-fibrinogen hydrogels with a superficial keratinocyte monolayer recapitulates the native morphology of human skin tissue as depicted by the organized epidermis (red), dermal-epidermal junction (purple), and dermis (blue) (ii). (C) ECM structural ligands (collagen, GAGs, and hyaluronan) are maintained upon porcine dermal tissue decellularization and are capable of forming a bioink at 37℃ (i). Schematic of 3D-bioprinted human skin model construct (ii). Quantitative analysis depicting S-dECM retained significantly greater area and thickness of the original structure in comparison to collagen gels (iii). D10 histological images of epidermal thickening of collagen- or s-dECM-fabricated constructs (iv). Prevascularization of S-dECM skin patches using ASCs and EPCs accelerates wound closure with near native skin tissue aesthetics (v). (Skardal et al., 2012; Pourchet et al., 2017; Kim et al., 2018)
6A: Skardal A, Mack D, Kapetanovic E et al. Bioprinted Amniotic Fluid-Derived Stem Cells Accelerate Healing of Large Wounds. Stem Cells Translation Medicine. 2012;1:792–802
6B: Pourchet LJ, Thepot A, Albouy M et al. Human Skin 3D Bioprinting Using Scaffold-Free Approach. Adv Healthcare Mater. 2017;6:1601101. doi: 10.1002/adhm.201601101
6C: Kim BS, Kwon YW, Kong JS et al. 3D cell printing of in vitro stabilized skin model and in vivo pre-vascularized skin patch using tissue-specific extracellular matrix bioink: A step towards advanced skin tissue engineering. Biomaterials. 2018;168:38–53
4.3. Muscle and Fascia
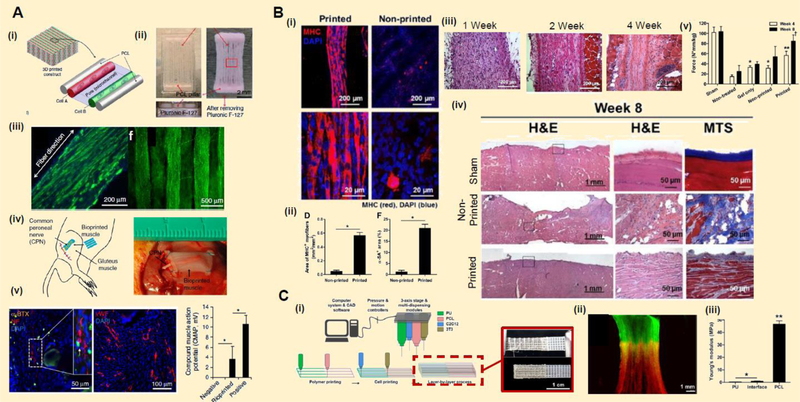
Deep, chronic wounds may also require the restoration of myocutaneous tissue defects. The development and implantation of thick, tissue-engineered muscle flaps for these relevant deep wounds is limited by the lack of vascularization and innervation necessary for the survival and function of bioengineered muscle during tissue growth. In muscle, multiple contractile, multinucleated myofibers, surrounded by endomysium, are assembled in parallel to form perimysium-encased muscle fascicles (Betts et al., 2013; Korthuis, 2011). These fascicles are further grouped into distinct muscles encased by a thick, collagenous epimysium, establishing tissue integrity and its hierarchical organization (Betts et al., 2013; Korthuis, 2011). In muscle vasculature, primary blood vessels extend along the axis of the muscle fascicle and bifurcate to form capillary networks that wrap around individual myofibers for adequate nutrient and oxygen diffusion, waste removal, and cell survival (Betts et al., 2013; Korthuis, 2011). Previous studies have shown that thick tissue-engineered muscle can be fabricated with preexisting vasculature, as well as support host vascular integration to replace deranged muscle and restore its function (Carosio et al., 2013; Juhas et al., 2014; Levenberg et al., 2005; Li et al., 2017; J. Liu et al., 2018; Sicari et al., 2014). Although these current strategies provide viable solutions to replace aberrant muscle, fine-tuned control achieved with EBB technology can better recapitulate the architectural complexity of distinct tissue, vascular hierarchical organization, and anatomical specificity of tissue-engineered muscle in comparison to preestablished culture methods. Advanced muscle tissue engineering strategies utilizing integrated tissue-organ printing systems have shown a promising capacity to fabricate concentric layers of tightly-packed and organized engineered myofibers and muscle-to-tendon units to achieve the native contractile properties of skeletal muscle (Table 6; Figure 8; Kang et al., 2016; J. H. Kim et al., 2018; Merceron et al., 2015). Although these studies demonstrate that printed muscle constructs can attain mechanical robustness and structural integrity to support neural and vascular network integration from the host, there is a lack of research on the biofabrication of pre-vascularized muscle tissue and relevant fascia using EBB technology.
Table 6.
Muscle biofabrication using EBB
| Authors | Methodology | Major Findings | Limitations |
|---|---|---|---|
| Merceron et al., 2015 | • Integrated tissue-organ printing (ITOP) was used to fabricate a linear MTU construct, containing alternating layers thermoplastic polymeric scaffolds and cellular-based bioinks • On one end, a polyurethane scaffold and myoblast-laden composite bioink of fibrinogen, gelatin, and hyaluronan were concurrently deposited to support muscle development • On the opposite end, a poly(ε-caprolactone) (PCL) scaffold and fibroblast-laden composite bioinks for tendon development were co-printed |
• After a week of in vitro culture, the MTU construct exhibited more elastic and rigid properties on the designated muscle and tendon sides, respectively, whereas the muscle-tendon interface region of the MTU attained intermediate mechanical properties of both tissues after a week in culture | • Biofabrication of avascular muscle tissue must be equipped with its own hierarchical vascular supply to support the longevity of implanted tissue, graft-host anastomosis, and host vascular integration |
| Kang et al., 2016 | • ITOP was used to print myoblast-laden composite bioinks (fibrinogen, gelatin, hyaluronan, and glycerol) supported by PCL pillars, which induce compaction by invoking cell directionality • Cellular constructs were matured for 7 days in myogenic culture conditions upon subcutaneous implantation in a murine gastrocnemius defect model |
• Constructs underwent contraction as myoblast began to extend along the length of the fabricated myofiber-like structure in vitro
• Implantation of mature, biofabricated muscle (7d maturation period) displayed sufficient muscle fiber organization, neuronal innervation, vascularization, and increased myogenic receptor expression after 2 weeks in vivo • Myofiber-like structures attained partial muscle function, invoking measurable action potentials upon electrical stimulation |
• Pre-vascularized bioengineered constructs may enhance the engraftment of implants and graft-host anastomosis • Tendon repair may be required to fully restore musculocutaneous wounds |
| Kim et al., 2018 | • ITOP was used to print spatially-organized, densely-packed, and aligned myofiber-like filaments of human muscle progenitor cell (hMPC)-laden composite (fibrinogen, gelatin, hyaluronan, and glycerol) bioinks supported by a PCL polymeric base • Bioengineered muscle was myogenically induced for 9 days for in vitro studies • Pre-conditioned bioengineered muscle was implanted in a rodent anterior tibialis defect model for 8 weeks |
• Bioprinted pre-myofiber demonstrated enhanced myogenic marker expression and structural alignment upon mechanical stimulation in comparison to non-printed constructs • 8-week explants revealed successful vascularization and innervation by host, and nearly full functional recovery of the deranged muscle |
• Avascular tissueengineered muscle is unsuitable for long-term in vivo functionality if blood supply is inadequate • Tendon repair may be required to fully restore musculocutaneous wounds |
Figure 8.
Bone Fabrication using EBB. (A) Cubic scaffolds of composite Laponite-alginate-methylcellulose (LAM) bioink with increasing layers of polymer maintain high shape fidelity upon printing (i). 7-day release profiles of BSA and VEGF from different scaffolds designs indicate that cytokine liberation from LAM scaffolds can be tuned for a specific application (ii). Mechanical properties of LAM scaffolds decrease within 3 weeks in cell culture conditions. (B) Schematic of computer-aided bioprinting procedure for producing cylindrical filaments of tissue-engineered bone with complex architecture, a perfusable lumen, and an increasing VEGF gradient from the outer region of the lumen to the construct perimeter (i-ii). 5% (w/v) GelMA with a low degree of efficiency (34.1%) and VEGF functionalization enhances angiogenic sprout length and branch points (iii) and MSC-EC colocalization (iv). Upon perfusion, the mineralization of biofabricated bone (v) and osteogenic-specific markers (collagen type I (col1), alkaline phosphatase activity (ALP), osteocalcin (OC), osteopontin (OP); vi) is enhanced comparison to static controls after 21 days. GelMA functionalization efficiency is positively correlated with the hydrogel degradation rate (vii). (C) Schematic of the biofabrication process for printing bone tissue with native structural architecture using EBB and laser-assisted bioprinting (i) Functionalized GelMA and polydopamine with VEGF (left) and BMP-2 (right), respectively (ii). Schematic of perfusion bioreactor system (iii). Dynamic flow enhances angiogenic sprouting and branching (iv), and osteogenic differentiation in HUVEC/hMSC cocultures encapsulated in biphasic materials (v). (Ahlfeld et al., 2017; Byambaa et al., 2017; Cui et al., 2016)
8A: Ahlfeld T, Cidonio G, Kilian Det al. Development of a clay based bioink for 3D cell printing for skeletal application. Biofabrication. 2017;9:034103
8B: Byambaa B, Annabi N, Yue K et al. Bioprinted Osteogenic and Vasculogenic Patterns for Engineering 3D Bone Tissue. Adv Healthcare Mater. 2017;6:1700015
8C: Cui Haitao, Zhu W, Nowicki M et al.. Hierarchical Fabrication of Engneered Vascularized Bone Biphasic construct via Dual 3D:Bioprinting: Integrating Regional Bioactive Factors into Architectural Design. Adv Healthc
Current methods to engineer functional muscle will also require the fabrication of fascia, a collagenous and fibrous connective tissue that supports muscle stabilization, attachment, enclosure, and separation (Gatt and Zito, 2019; Kumka and Bonar, 2012; Stecco et al., 2011). Superficial, deep, visceral, and parietal fascial tissue each serve an anatomically-specific function, which is regulated by the local biological and cellular composition, ECM structural integrity, and biomechanical properties (Findley et al., 2012; Gatt and Zito, 2019; Stecco et al., 2013, Stecco et al., 2011). Fascia is also composed of circulatory and lymphatic vasculature, as well as neuronal networks to fulfill nutritive demands, maintain interstitium homeostasis, and achieve proper motor function and tension distribution, respectively (Bordoni and Varacallo, 2019a; Klingler et al., 2014; Schleip et al., 2019). Although there are distinct types of fascia, the regeneration of superficial and deep fascia is of greater importance for tissue reconstruction and will play a crucial role in the fabrication of viable neo-pedicles that successfully integrate and adjoin with healthy tissues at the defect site (Stecco et al., 2013b). Autologous fasciocutaneous flaps, containing fascia, skin, and fat, can also be used to accelerate the restoration of deep tissue injuries with severely compromised vasculature; however, transplanted fascia derived from anatomically-incorrect locations can impede the proper mechanics and function of the restored tissue (Stecco et al., 2013b). Tissue engineering can help address these limitations by fabricating tissuespecific fascia with distinct mechanical properties, structural organization, and material composition comparable to that of the former native tissue. Previous studies have demonstrated the ability to fabricate MSC-laden fascia equivalents, derived from collagen and/or alginate, enhancing vascular integration, macrophage infiltration, collagen and elastin deposition, and fiber thickening, while attenuating hernia recurrence in a rat injury model (Ayala et al., 2015; Hung et al., 2014, Hung et al., 2010). However, this work primarily focuses on fascia reconstruction for the pelvic floor and the abdominal wall and may have variations in cellular composition and local material properties due to its classification and anatomical location. These studies are also limited by the use of mechanically inferior biomaterials to prepare fascia equivalents, which may not only limit tissue directionality for optimal tissue function, but also facilitate aberrant contraction and improper deformation upon matrix remodeling and tensile loading, respectively.
The integration of functional muscle within a myocutaneous defect will require the incorporation of superficial and/or deep fascia to support and stabilize the bioengineered muscle upon transplantation. The use of 3D-bioprinting can aid this process by regulating the intricate and spatial organization of myofibers, fascia, hypodermal tissue, vasculature, and neuronal networks. It may also be worthwhile to investigate variations in tissue-specific fascia composition and structural orientation to engineer fascial tissue with the appropriate mechanical properties. Overall, incorporation of superficial and deep fascia into functional, bioprinted skin and muscle will contribute to the successful stabilization and attachment of implanted neo-pedicles; however, the use of EBB technology to tissue-engineer vascularized muscle with fascia still remains unexplored.
4.4. Bone
Osteocutaneous defects may also arise if deep wounds persist to the underlying bone, requiring tissue-engineered intervention for effective repair; however, the structure and organization of individual bones are highly variable. Bone type, anatomical location, and cortical-to-trabecular tissue ratio dictate the overall macroscopic structure and tissue biomechanics of distinct bones. The microscopic organization of trabecular and cortical bone can be attributed to their respective basic subunits. In compact bone, individual osteons form concentric circles of calcified matrix, or lamellae, encasing niche resident cells between lamellar sheets, and surround lymphatic vessels, nervous tissue, and blood vessels within its core (Betts et al., 2013). The parallel configuration and assembly of osteons into dense, compact tissue improves its stiffness, enabling it to withstand compressive forces (Betts et al., 2013). On the other hand, spongy, cancellous bone is a marrow-filled, vascularized mesh of trabeculae that sits underneath cortical bone (Betts et al., 2013). Trabeculae consists of lamellar layers and residential bone cell populations that are oriented along stress lines to increase overall bone strength and balance (Betts et al., 2013).
Various tissue engineering approaches have been used to fabricate vascularized bone to achieve long-term viability and host integration for relevant bone defects (Dang et al., 2018; Sicari et al., 2014; Yin et al., 2019). Although current tissue engineering methods have made great progress in developing therapies for bone repair, these techniques are extremely limited by the lack of reproducibility, long-term stability, and anastomotic potential, rendering them ineffective for clinical translation (Brennan et al., 2013; Mercado-Pagán et al., 2015). In addition to these criteria, bone tissue engineering also requires the simultaneous fabrication and development of hierarchical bone and blood vessels, which is necessary for tissue-engineered bone integration. 3D-bioprinting of biomimetic scaffolds that recapitulate the native architecture of bone and its relevant vasculature will not only resolve these drawbacks, but also improve the overall mechanical function and biomaterial integration into patient-specific deep wounds (Zhang and Wang, 2019). Using EBB, much work has been done to fabricate biomimetic and perfusable bone-like structures that support cell proliferation and differentiation for aberrant bone repair (Table 7; Figure 9; (Ahlfeld et al., 2017; Byambaa et al., 2017; Cui et al., 2016; Kang et al., 2016)). However, the architectural complexity and microenvironment heterogeneity between individual bones cannot be achieved solely by EBB, requiring various 3D-bioprinting modalities to bioengineer vascularized bone.
Table 7.
Bone biofabrication using EBB
| Authors | Methodology | Major Findings | Limitations |
|---|---|---|---|
| Cui et al., 2016 | • ITOP combined with CAD modeling was used to recreate precise vascular and bone tissue complex architecture fabricated with a soft, EC-laden, VEGFincorporated GelMA hydrogel surrounded by a mechanically-rigid PLA scaffold with immobilized BMP-2 ligands | • Printed a patent construct that supported perfusion and stimulated vascular bone formation by coaxing luminal ECs and bulk MSC osteoblastic under flow conditions | • Requires various 3D-bioprinting modalities to generate a biomimetic scaffold with the precise, anatomical bone structure and hierarchical vascular architecture |
| Kang et al., 2016 | • An arbitrary mandibular fragment, derived from a traumatic craniofacial injury, was printed using a hAFSC-laden composite (fibrinogen, gelatin, hyaluronan, and glycerol ) bioink and exposed to osteogenic conditions for 28 days • Circular implants of hAFSC-laden composite bioinks were printed and preconditioned in defined osteogenic media for 10 days, and subsequently implanted into a murine calvarial defect model for assessment after 5 months |
• In the mandibular bone model, matrix calcification was observed upon 28 days of osteogenic differentiation in vitro • In the calvarial bone model, 5-month explants demonstrated host vascularization, mature bone and osteoid formation, and no necrotic tissue formation |
• Tissue-engineered bone architecture that recapitulates the native mandible is important but was not assessed • Constructs must be able to support host vascular integration for long-term survival, but this was not assessed in this study • Proper tissue mechanics that are physiologically relevant to the mandible would be necessary to assess the functionality of bioengineered bone |
| Ahlfeld et al., 2017 | • hMSC-encapsulated alginate and methylcellulose bioinks were combined with a nano-silicate clay, Laponite, to print rigid, geometrically complex architectures | • Addition of Laponite to bioink improved printability of hMSC-laden scaffolds with high shape fidelity • Cell-laden composite bioinks functionalized with the VEGF and BSA promoted optimal hMSC functionality, decreased scaffold stiffness indicative of matrix modification, and increased release of morphogens over 21 days |
• Constructs must be equipped with preexisting vasculature to support stable and functional bone tissue replacement for long-term stability |
| Byambaa et al., 2017 | • Print cylindrical filaments of low-efficient GelMA with EBB, encapsulating pericytes and HUVEC in highly-synthesized GelMA, functionalized with pro-osteoblastic silicate nanoparticles and increasing concentrations of VEGF | • Printed highly organized and perfusable bone-like structures that support cell migration, proliferation, osteoblastic differentiation of MSCs, matrix mineralization, colocalization of the HUVEC and pericytes on the lumen wall, and angiogenic sprout lengthening and branching | • Rapid degradation rate of perfused GelMA constructs limits its use for long-term studies, indicating that composite bioinks that maintain their mechanical robustness over an extended period of time will be required for tissue stability and functionality |
The biofabrication of functional tissues to repair chronic defects requires sufficient knowledge on the local regions of intended repair. The specific biological and mechanical demands of bone, muscle, fat, skin, and connective tissue must be considered when generating functional bio-constructs (Table 8). Bone composition, in particular, varies in strength and toughness depending on its classification, location, and cortical-to-trabecular bone ratio (Table 9). Tissue engineers must consider these differences for osteocutaneous wound repair as it would be important to distinguish the structural and mechanical properties that, for example, differentiate the sacrum from the sternum. Additionally, information on the cell types that reside in defined niches can help tailor tissue engineering approaches to coax targeted stem cell differentiation, cell behavior, and tissue function (Table 10). Overall, extrusion-based bioprinting applications provides a tool to engineer neo-pedicles with cell heterogeneity, hierarchical vasculature, architectural complexity, and mechanical stability. With these criteria met, the biomolecular and biophysical cues between tissue-specific stem cells, perivascular cell types, local microenvironmental stresses, and niche specificity can be tightly regulated to facilitate the development of highly vascularized tissues.
Table 8.
General structure, composition, function, and mechanics, of tissue-specific organs in full-thickness tissue flaps
| Tissue | Structure and Composition | Function | Relative Stiffness and Tissue Mechanics | Reference |
|---|---|---|---|---|
| Cortical Bone | • Dense, compact, osseous shell of concentric lamellae in parallel along the longitudinal axis (anisotropic) • Innervated tissue • Vascularized Haversian (parallel) and Volkmann’s (perpendicular) canals • Encased by periosteum (outer) and endosteum (inner) |
• Outer protective shell of bone • Contributes the most to overall mechanical properties of the bone • Withstand bending moments (combined shear, tension, and compression) generated by connected muscle |
• Bone toughness (due to collagen content) and strength (due to mineral content) depend on the bone thickness, location, and classification • More brittle and vulnerable to fracture |
Ott, 2018 Bankoff, 2012 Osterhoff et al., 2017 Augat and Schorlemmer, 2006 |
| Cancellous Bone | • Spongy, porous network of lamellae • Less dense, homogenous, and oriented than cortical bone (anisotropic) • Bone marrow and fat • Vascularized and innervated • High water content |
• Transfers mechanic load from cortical bone and serves as a shock absorber • Bear bulk of load in highly trabeculated tissue (e.g. vertebrae) • Withstand repetitive compressive axial loads |
• High surface-to-volume ratio allows tissue to support greater compressive loads and strain • Varies depending on bone type and location |
Oftadeh et al., 2015 Ott 2018 Osterhoff et al., 2017 Bankoff, 2012 |
| Muscle | • Cylindrical myofibers of serial sarcomeres encapsulated by collagenous sheaths and packaged in fascicles in parallel alignment • Vascularized and innervated |
• Sarcomere contractile elements facilitate muscle contraction to induce or restrict movement • Under voluntary and involuntary control • Tensile forces support muscle stiffening • Inertial forces support muscle bending and twisting • Hydrostatic forces support muscle lengthening and shorting |
• Tensile strength is greater in the orientation parallel to the longitudinal axis • Collagen density of muscle is less than tendon, contributing to its weaker stiffness |
Ting and Chiel, 2017 Korthius 2011 Broek et al., 2010 |
| Tendon | • Cylindrical collagen molecules arranged in a hierarchical, parallel alignment • Vascularized and innervated |
• Transmit forces generated by muscle to bone • Long tendons (e.g. hand flexors) for finer movement • Short tendons (e.g. Achilles) for power and endurance • Withstand large loads with little deformation |
• Acquires a greater tensile strength than muscle • Stiffness varies based on tendon diameter, length, and collagen orientation • Tensile strength is greater in the axial direction |
Varacallo, 2018 Bordoni and Thorpe and Screen, 2016 |
| Ligaments | • Cylindrical collagen molecules arranged in a hierarchical, parallel alignment • Less collagen content than tendons • Vascularized and innervated |
• Promote motion and stability of the musculoskeletal system • Secure joints between bone • Transmit tensile forces along the longitudinal axis of the tissue • Resist bone torsion |
• Less dense collagenous fascicles result in a toughness that is inferior to tendon • Increase in the cross-sectional area diameter increases tissue strength • Tensile strength is greater in the axial direction |
Massel,1999 Amis, 2004 |
| White Adipose Tissue | • Lipid-containing adipocytes in WAT exhibiting a round morphology within a collagenous matrix • Highly vascularized loose connective tissue covering skeletal muscle |
• Lipid storage and metabolism • Thermal insulation and mechanical protection • Supports load transfer to the underlying bone • Metabolic regulation through endocrine functions |
• Stiffness of adipose tissue depends on the anatomical location and the fatty acid composition of resident adipocytes | Trayhurn and Beattie, 2001 Alkhouli et al., 2013 Mariman and Wang 2010, Shoham and Gefen 2016 |
| Skin | • Avascular epidermis with nerve endings present • Vascularized dermis innervated by PNS sensory neurons • Dermis also contains hair follicles and varies in thickness depending on its location • Heterogeneous and anisotropic material |
• Barrier protection from external environment • Thermal regulation, prevent water loss, and UV protection |
• Tissue mechanics depend on the anatomical location • Dermal collagen contributes to the bulk of skin’s elastic properties • Intact skin is under a constant state of tension • Viscous properties require tissue fibers to align before tissue can withstands tensile deformation (hysteresis) |
Joodaki and Panzer 2018, Rognoni and Watt, 2018 |
Table 9.
Cortical-to-cancellous ratio composition of distinct bone types
| Bone Type | Structure and Composition | Function | Physiological Loads | Examples | Cortical-toCancellous Ratio | References |
|---|---|---|---|---|---|---|
| Long Bone | • Cylindrical and long • Longer in length than width • Cancellous bone surrounde d by a cortical outer shell |
• Locomotion with muscle contraction • Provide levers for movement |
• Tension • Compression • Torsion • Bending • Shear |
• Femur, tibia, fibula, humerus, radius, ulna, metacarpals, metatarsals, phalanges | Proximal/Dis tal (50:50) Central (95:5) | Bankoff, 2012 Clarke, 2008 |
| Short Bone | • Cube-shaped with equal length, width, and thickness • Cancellous bone surrounde d by a cortical outer shell |
• Provides stability and support • Limited motion |
• Tension • Compression |
• Wrist carpals, ankle tarsals | (40:60) | Clarke, 2008 |
| Flat Bone | • Thin and curved • Cancellous bone sandwiched between thin cortical bone |
• Protect internal vital organs • Serves as base for muscle |
• Tension | • Cranium, sternum, scapulae, pelvis, ilium, ribs | (45:55) | Clarke, 2008 Graeber and Nazim, 2007 |
| Irregular Bone | • Non-uniform bone of irregular shapes • Primarily cancellous bone encased by a thin cortical bone layer |
• Anchor points for nervous tissue • Provides stability and support |
• Tension • Compression • Torsion |
• Vertebrae, sacrum, mandible | (25:75) | Clarke, 2008 Cramer, 2014 |
| Sesamoid Bone | • Small and round bone embedded within tendon | • Protects tendons from severe compressive forces | • Tension • Compression |
• Patellae | (40:60) | Clarke, 2008 Toumi et al., 2006 Townsen d et al., 1976 |
Table 10.
Resident cell populations in tissue-specific niches
| Tissue | Cell Composition | ECM Composition | References |
|---|---|---|---|
| Bone | • Osteoblast, osteoclasts, osteocytes, osteoprogenitors mesenchymal stem cells, hematopoietic stem cells (BM), bone lining cells | • Inorganic salts (calcium, phosphate, hydroxyapatite) • Organic structural ligands (collagen, osteonectin, osteopontin, fibronectin) |
Ott, 2018 Osterhoff et al., 2017 Bankoff, 2012 Augat and Schorlemmer, 2006 Florencio-Silva et al., 2015 |
| Muscle | • Myocytes, myoblasts, muscle-derived stem cells, satellite cells, mesenchymal stem cells | • Collagen Type 1 • Proteoglycans • Glycosaminoglycans Glycoproteins |
Ting and Chiel, 2017 Korthuis, 2011 Broek et al., 2010 Gillies and Lieber, 2012 |
| Tendon | • Tenocytes and tenoblasts (tendon fibroblasts) | • Collagen type 1 • Proteoglycans • Glycoprotein • Glycosaminoglycans |
Bordoni and Varacallo, 2018 Thorpe and Screen, 2016 |
| Ligaments | • Fibrocytes and fibroblast | • Collagen type 1 • Elastin • Glycoproteins • Glycolipids |
Massel, 1999 |
| Adipose | • Adipocyte, pre-adipocytes, mesenchymal stem cells, fibroblasts | • Collagen type IV • Laminin • Fibronectin |
Alkhouli et al., 2013 Mariman and Wang, 2010 |
| Skin | • Keratinocytes, fibroblasts, melanocytes, Langerhans, Merkel’s cells, epithelial stem cells, hair follicle stem cells | • Collagen type I and III • Elastin • Hyaluronan |
Rognoni and Watt, 2018 |
5. Design Considerations for Vascularized Constructs using EBB
Structural replication of the native tissue at the defect site is crucial for the long-term retention, bio-integration, and proper biofunctionality of engineered neo-pedicles. Stem cell morphogenesis into targeted phenotypes, within their tissue-engineered spatial boundaries, will require greater microenvironmental control over a heterogenous cell population to mimic the true architectural complexity of native tissues. Strategic selection of biological agents (e.g. cell type, polymer selection, and pro-angiogenic cytokines) and acute microenvironmental stresses (e.g. hemodynamic flow, hypoxia, and inflammation) for printed vascularized tissue constructs, requires a thorough understanding of the cellular, mechanical, and biological components that regulate neoangiogenesis.
5.1. Cell Type and Derivation
Microvessel formation and maturation occur through three major processes: (1) vasculogenesis, the de novo formation of macrovessels from angiogenic progenitor cells; (2) angiogenesis, the sprouting of microvessels from preexisting blood vessels; and (3) vessel pruning, the random regression of excessive angiogenic sprouts that are no longer required. These highly regulated and sequential phenomena are crucial for the survival and function of cells that reside within voluminous tissue.
Peripheral vasculature is subdivided into the arterial and venous systems, by which arterial circulation is highly pressurized for adequate nutrient and oxygen delivery and waste removal to distal organs, whereas venous vasculature functions to transport large volumes of blood from the periphery to the heart under lower pressures (Tucker and Mahajan, 2018). The anatomical structure of both arterial and venous vasculature is arranged as concentric, cylindrical sheets of tunica intima, media, and adventitia. The innermost tunica intima consists of a polarized monolayer of endothelium bound to basement membrane structural ligands, including collagen, laminin, and proteoglycans (Datta et al., 2017; Ucuzian et al., 2010). The vascular endothelium is directly exposed to the cellular, protein, and mineral components of circulating blood, controlling cell infiltration and soluble ligand diffusion (Datta et al., 2017; Davis et al., 2011). Alternatively, the tunica media surrounds the intima and consists of multiple layers of tightly packed smooth muscle cells (SMCs), innervated by neurons to facilitate vessel contraction and dilatation in response to changes in physiological demands (Datta et al., 2017; Davis et al., 2011). Lastly, the tunica adventitia, or outermost vessel layer, contains fibroblasts embedded in a dense collagenous matrix to stabilize peripheral vasculature and maintain blood vessel functionality (Datta et al., 2017; Davis et al., 2011).
Prior to angiogenesis, pro-angiogenic stimuli deposited from perivascular and extravascular cell types activate luminal ECs to degrade the underlying matrix and burrow through the media and adventitia strata (Ucuzian et al., 2010). Subsequently, tip and stalk cell activation is initiated, prompting neovessel elongation toward distal tissue regions along a chemotactic gradient (Ucuzian et al., 2010). Crosstalk between ECs and mural support cells (e.g. pericytes, SMCs, fibroblasts, and MSCs) is not only crucial for tubule formation, but also vessel inosculation, anastomosis, and stabilization for adequate blood vessel functionality and tissue survival (Logsdon et al., 2014; Ucuzian et al., 2010).
5.1.1. Pericytes
Colocalization of pericytes to vascular endothelium is necessary to support capillary maturation and stabilization during development and wound healing. Neovascularization occurs in a stepwise process by which endothelial sprouting and migration are followed by pericyte recruitment and colocalization to the abluminal surface of newly-formed vasculature (Bichsel et al., 2015). Pericyte secretion of vascular endothelial growth factor (VEGF) guides EC sprouting and de novo tubule formation through bidirectional, angiopoietin (Ang)/Tie2-mediated pathways (Bergers and Song, 2005; Davis et al., 1996; Suri et al., 1996). Co-presentation of both ligands induces pro-angiogenic activity of Ang2, supporting EC proliferation and migration, and basal lamina remodeling (Eilken et al., 2017; Lobov et al., 2002). Recent studies also reveal that the expression of the Tie2 is not only specific to ECs but is also minimally expressed in pericytes, contributing to vascular maturation (Teichert et al., 2017).
Upon tubulogenesis, neovessel inosculation and pericyte recruitment to the vascular endothelium are required to modulate proper lumen geometry and stability through endothelialpericyte interactions; however, VEGF signaling is not sufficient to support these phenomena alone (Bichsel et al., 2015; Kim et al., 2015). Endothelial secretion of platelet-derived growth factor (PDGF) and epidermal growth factor (EGF) activate the motility, proliferation, and recruitment of pericyte progenitors to the vascular lumen surface for neovessel maturation (Stratman et al., 2009). These paracrine mediators also facilitate robust secretion of the basal lamina structural proteins, collagen, laminin, and fibronectin to support sufficient cell-ECM interactions (Bowers et al., 2015; Smith et al., 2013; Stratman et al., 2009). Although VEGF is known to support EC proliferation and migration during angiogenesis, its role in pericyte functionality offers deleterious effects on vessel maturation (Greenberg et al., 2008). In vascular pruning, EC apoptosis must occur to finalize the maturation process of nascent microvessels via anti-angiogenic, Ang2-mediated vessel regression and weakened endothelial-pericyte interactions (Augustin et al., 2009; Bergers and Song, 2005; Simonavicius et al., 2012). Vessel diameter and permeability to soluble ligands require direct contact between ECs, pericytes, and ECM structural ligands to support favorable lumen morphology, constriction, and barrier function through tightly-regulated VEGF and transforming growth factor beta-1 (TGF-β1)/SMADmediated signaling (Bichsel et al., 2015; Darland and D’Amore, 2001; Kim et al., 2015; Orlidge and D’Amore, 1987; Stratman et al., 2009; Waters et al., 2013; Zonneville et al., 2018). Although restricted lumen permeability is important for microvessel maintenance, the presence of circulating pro-inflammatory cytokines, a key feature of wound healing, increases vessel dilation and leakiness, through interfering with pericyte behavior, and supports angiogenesis via Notch/Jagged1 signaling (Kerkar et al., 2006; Tattersall et al., 2016). Overall, these studies indicate that finely-tuned crosstalk between ECs, pericytes, and the surrounding matrix is required for supporting proper tubulogenesis, matrix deposition, neovessel stabilization, and maturation through lumen diameter and permeability restrictions; and that inflammatory mediators can reverse this process by interfering with pericyte functionality and cell-cell interactions to support adequate wound healing.
5.1.2. Smooth muscle cells
Smooth muscle cells also maintain the capacity to support microvessel maturation and stability through enhancing endothelial migration, barrier permeability, and network formation via paracrine VEGF, PDGF, and TGF-β activity (Heydarkhan-Hagvall et al., 2003). HUVEC/SMCs 3D-cocultures improve endothelial tight junctions, cytoskeletal organization, and EC/SMC colocalization, preventing leakage of high molecular weight proteins from de novo sprouts (Dietrich and Lelkes, 2006; Joo et al., 2014; Kurzen et al., 2002). ECs seeded onto the surface of fibrin-encapsulated SMC aggregates facilitated unidirectional invasion and superior sprout length and density in relation to endothelial-coated acellular aggregates (Ucuzian et al., 2013). In vivo studies further demonstrate that co-transplantation of spatially-arranged ECs and SMCs into an ischemic animal model enhances VEGF secretion, impedes necrotic tissue progression, and improves neovascularization, cell migration, blood vessel patency, and tissue regeneration (Bak et al., 2016; Joo et al., 2014; Shudo et al., 2013). Despite these findings, recent work demonstrates that tissue-specific SMC derivation regulates endothelial sprouting and maturation differently (Bargehr et al., 2016).
Additional paracrine mediators have also been shown to support the formation, organization and stabilization of neovasculature. EC/SMC spheroids spontaneously reorganize to recapitulate the structural organization of native lumen structure, forming cell aggregates arranged as an SMC-core pellet surrounded by an EC monolayer in the presence of VEGF and Ang-2 (Korff et al., 2001). Further investigation revealed that pharmacological inhibition of Ang-2 disrupts the EC/SMC dissociation mechanisms crucial for angiogenesis, impeding vessel destabilization and subsequently, VEGF-induced endothelial sprouting (Molnar and Siemann, 2012). Previous work has also shown that microvessel formation and stabilization are regulated by sphingosine-1-phosphate (S1P)-induced EC angiogenesis and Notch3/Jagged1-mediated SMC phenotype switching, indicating that paracrine interactions between endothelial and smooth muscle cells support the differentiation and maintenance of both cell types for microvessel formation and maturation. (Bhattacharyya et al., 2014; Harvey et al., 2010; Jin et al., 2018; Liu et al., 2009; H. Wang et al., 2018; Williams et al., 2018).
5.1.3. Fibroblasts
Fibroblasts have also been shown to facilitate neovascularization by modulating EC behavior, tubule formation, and vessel regression. In 3D-cocultures, HMVEC overlaid with collagen-encapsulated human dermal fibroblasts (HDF) facilitated robust capillary network formation through enhanced EC survival, migration into the overlying matrix, and tubulogenesis via fibroblastic-mediated VEGF/VEGFR2 signaling (Velazquez et al., 2002). Additionally, coencapsulation of cardiac fibroblast and aortic ECs in a fibrin-spheroid model demonstrated superior EC proliferation, sprout extension, and vessel density in relation to EC/MSC constructs (Twardowski and Black, 2014). Not only are biomolecular ligands involved regulating neoangiogenesis, but also biostructural ligands play a significant and synergistic role in regulating endothelial and fibroblastic cell behavior. The combinatorial effects of bioactive BG ions and nanofibrous ligand structure and orientation supports robust VEGF secretion from HDFs and endothelial tubule formation in 3D cocultures (Xu et al., 2017). This work further confirmed that cocultured bio-scaffolds, with combined structural and chemical features, enhanced wound closure, neo-epidermis formation, and neovascularization in a chronic wound healing murine model, indicating the significance of these synergistic properties in potential skin tissue engineering strategies. These phenomena demonstrated by both in vitro and in vivo work can be attributed to the enhanced expression of genes and proteins critical to the development of functional blood vessels. Microarray analysis of EC/fibroblast cocultures revealed an upregulation in over 300 uniquely expressed genes, namely pro-angiogenic factors and receptors required for mural cell recruitment and vessel stabilization, αβ-integrins that support vessel wall integrity through cell and basement membrane adhesive interactions, and blood coagulation factors necessary for downstream maturation and functionality of developing neovessels (Lilly and Kennard, 2009). Further investigation of this work demonstrated that endothelial-fibroblast paracrine mechanisms upregulates these genes via Notch/Jagged1 signaling, suggesting that tightly-regulated crosstalk between ECs and fibroblasts is crucial for the proper formation and function of blood vessels. Although paracrine interactions between ECs and fibroblasts strengthen vessel integrity, variations in sera sources, cell derivation, and culture conditions evoke differences in neovessel development and maturation (Costa-Almeida et al., 2015; Eckermann et al., 2011). Overall, these studies conclude that a universal strategy cannot be applied to promote angiogenesis in vitro, and that precise culture systems and cytokine cocktails must be considered to optimize this phenomenon.
5.1.4. Mesenchymal stromal cells
Paracrine signals between MSCs and ECs have also been shown to enhance 3D neovascularization in vitro. MSCs can easily be harvested from a variety of tissues and maintain their innate proliferative, migratory, multipotent, and immunoregulatory capacity outside their native niche, making them an ideal cell source for regenerative medicine. These distinct features encourage neovascularization and subsequent vessel stabilization through endothelialMSC interactions, making MSCs suitable for chronic wound healing therapies. Global gene expression analysis of human BM-MSCs cocultured with ECs demonstrated an upregulation of the pro-angiogenic factors, cadherin-5 (Cad-5), Ang-4, CD34, platelet/endothelial cell adhesion molecule-1 (PECAM-1) and von Willebrand factor (vWF), and robust expression of Ang proteins, suggesting that ECs play a potential role in mediating MSC behavior and differentiation (Xue et al., 2013; Zhang et al., 2017). When BM-MSCs were cocultured with HUVEC in 3Dfibrin matrices, Ang-1 stimulation supported vasculature with restricted lumen diameter, and coaxed MSC differentiation down a mural support cell lineage pathway, as demonstrated by increased αSMA expression and EC-MSC colocalization (Jeon et al., 2014). In 3D-collagenous cultures of MVEC and adipose-derived stromal cells (ASC), disruption of Notch/Jagged1 signaling attenuated the expression stromal ligands, VEGF-A/B, and endothelial VEGFR1/3, but supported robust angiogenic behavior of MVEC under native conditions (Zhao et al., 2017). Additionally, HUVEC-MSC cocultures in 3D collagen-fibronectin matrices prompted stable vascular network architecture and cell-cell contact, yet immature plexus formation, via mesenchymal secretion of Ang-1/2 cytokines and hepatocyte growth factor (HGF), a known regulator of VEGF transcription and activity (Boyd et al., 2013). Although MSC demonstrate the capacity to act as a mural support cell type, previous work has shown that delayed exposure of MSC to endothelial cultures improves microvessel formation and develops better vasculature (McFadden et al., 2013). Support of stromal cells during neovascularization and vessel maturation identified in vitro is also recapitulated in vivo, suggesting that paracrine interactions that mediate endothelial behavior and sprouting angiogenesis coax MSC differentiation into mural support cells, and facilitate the engraftment of cellular constructs and host integration at the wound site (L. Chen et al., 2017; Ma et al., 2014; McFadden et al., 2013; Pedersen et al., 2014; Sueyama et al., 2017).
Although EC-MSC cocultures support these phenomena, the degree and efficiency of neovascularization is highly dependent on the tissue source of MSC derivation (Esteves et al., 2017; Verschueren et al., 2011). Discrepancies between tissue-specific MSC induction of vessel initiation and maturation exists; therefore, information regarding MSCs derived from one source cannot be extrapolated to MSCs derived from an alternative source. Work by Kachgal et al. has shown that tissue-specific MSCs promote angiogenesis through distinct paracrine mechanisms (Kachgal and Putnam, 2011; König et al., 2015; Morris et al., 2018). Although tissue-specific MSCs contribute to variations in pro-angiogenic potential, EC derivation may serve a less important role in mediating comparable degrees of neovascularization. MSC cocultured with ECs derived from umbilical cord, peripheral blood, and neonatal cord blood, demonstrated comparable differentiation potential, αSMA expression via ERK activation, cell-cell communication, and MSC colocalization to endothelial tubules (Goerke et al., 2012). Thus, concerns regarding the origin of ECs used for wound healing therapies may be more focused on utilizing minimally-invasive isolation procedures that guarantee high EC yield and expansion, whereas derivation of autologous MSCs from distinct tissue sources may require deeper consideration to meet the specific needs for individualized treatment.
5.2. Microenvironmental Stress
Bioengineered tissues must also acquire the appropriate biological and mechanical properties of distinct native tissue to be successful upon transplantation. Exposure to distinct growth factors and niche-specific matrix proteins are suboptimal for coaxing stem cells down lineage-specific paths and developing mechanically-robust, pre-vascularized constructs capable of long-term survival and tissue-specific functionality. Acute microenvironmental stress, including ischemia and inflammation, are occurrences that take place during healthy wound healing to support angiogenesis in areas of microvascular inadequacy. In addition to these biological phenomena, substrate mechanics and local hemodynamics are also important in mediating vascularization of newly developed tissues. Therefore, exploitation of the these proangiogenic stimuli can supplement current tissue engineering strategies to initiate and maintain stable, vascularized biomaterials for adequate wound recovery.
5.2.1. Hypoxia and Inflammation
The oxygenation of ischemic tissues via endothelial tubule formation is a critical step in the initiation and progression of wound healing. Under hypoxic conditions, perivascular stromal cells maintain their stemness, multipotency, and stability, while promoting pro-angiogenic factor secretion and limiting apoptotic behavior (Ahmed et al., 2016; Basciano et al., 2011; Burlacu et al., 2013; Tsai et al., 2011). Secretion of pro-angiogenic factors (VEGFA/B, EGF-1, HIF-1a, TGF-β1, BMP Ang-1, and FGF-2) accelerates vascular sprout formation and stabilization via paracrine signaling between ASCs and MVECs (Oberringer et al., 2018; Xie et al., 2016; L. Zhang et al., 2016; Zhang et al., 2017). Work by Li et al. revealed that microvessel and capillary formation are mediated by R-Ras/Akt activity promotes endothelial cytoskeletal stabilization in vitro and the reperfusion of ischemic tissue in vivo (X. Liu et al., 2018). Further investigation of oxygen levels on endothelial behavior confirmed that hypoxia regulates EC migration, whereas normoxia modulates cell adhesion, with comparable effects on EC proliferation (Burlacu et al., 2013). As acute wound healing mechanisms progresses, local hypoxic regions at the injured site are also crucial to the promotion of sufficient skin regeneration and lesion closure. Tang et al. demonstrated that oxygen-tense conditions activate epidermal stem cells (EpSCs), pushing these cells from a quiescent state to a proliferative state, via HIF-1α signaling in vitro, and that this activation diminishes healing time through accelerating EpSC proliferation and epidermis regeneration in vivo (Tang et al., 2018). Although oxygen-tense microenvironments support MSC stemness and local angiogenesis, chronic hypoxia can have deleterious effects on the maintenance of pericyte phenotype and recruitment to the vascular endothelium of established blood vessels. Silenced expression of HypERlnc, a hypoxia-induced long noncoding RNA, perturbs pericyte viability, proliferation, and behavior via Cas9-based transcriptional activation, reversing its differentiated state and increasing endothelium permeability in MVEC-pericyte Matrigel cocultures (Bischoff et al., 2017).
The inflammatory response is also a key regulator in the formation of neovessels during wound repair. Butoi et al. demonstrated that crosstalk between M1 macrophages and SMCs mediates matrix protein deposition, ECM maintenance, and neoangiogenesis. When M1 macrophages were cocultured with SMCs, the deposition of ECM structural ligands, collagen and elastin, was diminished while the expression and activity of MMP and VEGF were enhanced (Butoi et al., 2016). This study also confirmed the angiogenic potential of proinflammatory macrophages by examining endothelial sprout formation of EC-monocultures in pre-conditioned medium extracted from SMC/M1 cocultures, whereby robust secretion of VEGF into the conditioned medium augmented EC tubulogenesis. Additionally, Womba et al. sought to examine the proteomic and metabolic changes in MSCs upon acute injury and chronic disease under oxygen-tense and pro-inflammatory conditions. MSC 2D-cultures subjected to ischemia adapted to local environmental conditions, secreting proteins associated with autophagy, anaerobic metabolism, angiogenesis, and cell migration. However, inflammatory stimulation via interferon-gamma (INF-γ) of MSCs prompted injury containment through the active reduction of infection, inflammation, and tissue fibrosis (Wobma et al., 2018). Overall, these studies indicate that both ischemia and inflammation enhance the angiogenic behavior of endothelial cells under highly-regulated events; however, pathological conditions that prolong these phenomena may cause perturbations in microvascular maintenance and functionality.
5.2.2. Hemodynamic Flow: Shear Stress, Vorticity, Pressure, and Cyclic Strain
Fluid shear stress induced by blood flow is crucial for endothelial phenotype regulation, microvessel lumen formation, vascular network remodeling and homeostasis. Laminar shear stress applied to a lawn of ECs on a collagenous hydrogel, promoted cell invasion deep into the underlying matrix, increasing angiogenic sprout length, repeated bifurcations and endpoints, and tubular structure formation in relation to static controls (Kang et al., 2008; Ueda et al., 2004). This phenomenon is partially attributed to sphingosine-1-phosphate (S1P) activity, whereby wall shear stress stimulates S1P-induced EC sprouting, invasion, and vascular maintenance via PI3K/Akt signaling and MMP-2 activation. Additionally, mature microvessel equivalents of gelatin seeded with HUVEC and ASC exhibited enhanced cell viability, capillary density, and mural cell recruitment at the lumen wall of constructs subjected to pulsatile perfusion in comparison to rotating culture, which had shorter and interrupted capillary networks (Frerich et al., 2008). Blood-flow induced shear stress induced at the vascular endothelium is also important for mediating the behavior of perivascular smooth muscle cells. Previous work confirms that the co-presence of smooth muscle and endothelial cells stimulates SMC migratory potential and EPC activation. However, the application of physiological shear stress to the vascular endothelium diminishes this phenomenon through reduced MMP-2 and Akt activity, indicating a protective feature of the vessel wall against vascular pathogenesis (Sakamoto et al., 2006; Wang et al., 2006; Ye et al., 2008).
Computational analysis on vascular fluid dynamics has also contributed to the growing understanding of hemodynamic flow on angiogenic sprouting behavior. Using a multiscale modeling approach, Bazamara et al. investigated the tissue, cellular, and intracellular phenomena responsible for the initiation and maturation of capillary networks as it pertains to closed-loop blood flow. Upon sprout anastomosis, blood flow is initiated, and endothelial phenotype transitions from a quiescent state to an active state, stimulating the expression of proliferative and migratory signals and subsequent loop elongation (Bazmara et al., 2015). Further investigation revealed that shear-induced endothelial activation is necessary for loop maintenance and endothelium barrier function, and that perturbations in hemodynamic intracellular signaling paths inhibits pro-angiogenic factor expression, impeding angiogenesis and disrupting vascular integrity (Bazmara et al., 2016, 2015; Bussmann et al., 2011; Ogunrinade et al., 2002; Reneman et al., 2006).
Computational hemodynamics not only address the influence of local shear stress presented at the vascular endothelium, but also describe the effects of vortical flow, pressure differences, and cyclic tension on pro-angiogenic gene expression and regional vascular morphogenesis. Ghaffari et al. developed a fluid dynamics computational model to analyze the hemodynamic microenvironment during avian embryonic development and to predict the local regions of sprout formation from a perfused vascular network. Their work revealed that endothelial sprouts originate from regions of low pressure and elongate towards vessels of higher pressure, regardless of the overall magnitude of pressure difference. Additionally, it was demonstrated that sprout formation generally occurs in areas of low shear stress in the arterial plexus but not at the regions where channels converge, establishing local vorticity. This phenomenon was attributed to the changes in angular velocity experienced at the bifurcation site, rather than the recirculation of fluid. With this model, the location of sprout formation could be successfully predicted to occur at regions with low and positive pressure differentials with minimal shear stress and nonzero vortical flow (Ghaffari et al., 2015). Additional work by Gebala et al. describes pressure-driven endothelial blebbing, whereby flow-induced lumen expansion increases external vessel pressure, inducing spherical deformation of the apical endothelium and initiating sprout formation in developing tissues (Gebala et al., 2016).
In addition to hemodynamic shear stress, pressure, and vorticity, tensile strain invokes endothelial polarization and directional migration to initiate angiogenesis. When endothelial cells are exposed to blood pressure-induced cyclic strain, EC migration, tubule formation, and cell reorientation along the axis of mechanical loading are enhanced in comparison to unstrained EC cultures (Ceccarelli et al., 2012; Joung et al., 2006; Thodeti et al., 2009). This phenomenon is regulated by MMP activity, as they help remodel the surrounding matrix to promote endothelial migration and angiogenesis (Kim et al., 2008; Von Offenberg Sweeney et al., 2005). Additionally, the inability to reorient EC directionality, a feature of tumor capillary ECs, permits tortuous vascular network formation due to abnormally high levels GTPase Rho and ROCK expression (Ghosh et al., 2008). Although cyclic strain promotes the initiation of angiogenesis, directly applied periodic strains and loss of nuclear tension will perturb endothelial adhesion, network length, migration, and reorientation (Chancellor et al., 2010; Wilson et al., 2009). It should also be noted that mural support cells subjected to cyclic uniaxial strain augment the secretion pro-angiogenic and pro-inflammatory cytokines, enhancing endothelial and mural cell migration and network formation via Ang1-mediated Tie-1/2 signaling and Notch receptor expression (Charoenpanich et al., 2014, Charoenpanich et al., 2011; Morrow et al., 2007; Mousavizadeh et al., 2014; Yung et al., 2009). Altogether, these studies reveal the importance of various hemodynamic flow parameters, including shear stress, vorticity, pressure, and cyclic strain, on regulating microvessel morphogenesis and maintenance. However, the combinatorial effects of these hemodynamic flow parameters on angiogenesis remains unclear and is an area for potential research.
6. Future Outlook
Complete restoration of deep, chronic wounds within a timely manner are attenuated by the lack of viable and patent vasculature at the wound site. Advanced therapeutic strategies, in conjunction with standard wound care techniques, have been implemented to address this clinical concern. However, in severe cases, chronic wound bed vasculature may be too deranged due to prolonged infection or ischemia at local regions of repetitive trauma or pathological loads. Autologous flaps, equipped with its own vascular supply, can restore deep tissue wounds without the risk of immune rejection. The characteristics of tissue-specific defects including wound severity, location, size, composition, and vasculature must be considered when choosing donor-site tissue for transplantation to ensure the adequate tissue aesthetic and functional demands are met. Although autologous flaps are capable of restoring deep tissue wounds with compromised vasculature, these invasive procedures can be complicated by the limited availability of donor tissue, increased donor-site morbidity, and extended post-operative recovery time. Thus, tissue engineering can be utilized to fabricate vascularized neo-pedicles for chronic wound repair.
Previous work has begun to exploit the use of extrusion-based bioprinting technology to fabricate thick constructs with hierarchical vascular networks, which is crucial for adequate nutrient and oxygen diffusion throughout the bulk of voluminous, cell-laden constructs. Namely, EBB methods have shown success in the fabrication of functional bone, muscle, tendon, and skin for deep, chronic wounds. Despite these advances, successful tissue flap biofabrication will require printed constructs to recapitulate the various niches of each distinct tissue to restore subcutaneous, fasciocutaneous, musculocutaneous, or osteocutaneous defects. Proper selection of stem cell source, materials, conditioning, and bioprinting modalities will be crucial for the successful fabrication of thick, tissue-engineered neo-pedicles for deep, chronic wounds.
Cellularized bioinks, in particular, must attain niche-specific structural ligands that not only acquire appropriate substrate mechanics and architecture to invoke stem cell differentiation, but also permit de novo vasculogenesis and angiogenic sprouting throughout mechanically robust biomaterials. Since matrix stiffness plays a key role in regulating stem cell differentiation, optimizing the compliance of bioprinted ECM will be more critical in engineering vascularized constructs of hard tissue (e.g. bone), as opposed to soft tissue (e.g. fat). Appropriate stem cell type and source is also important for bioprinting vascularized tissues. Under optimal conditions, MSCs are capable of differentiating into the various cell types present in the vasculature, including pericytes and smooth muscle cells, as well as the distinct tissues present in pedicle flaps, including adipocytes, myoblasts, fibroblasts, and osteoblasts. In addition to cell source and substrate mechanics, optimal crosslinking methods for bioprinted tissues must be investigated to efficiently polymerize mechanically-tunable materials with high shape fidelity, while maintaining adequate cell viability. Bioink rheological properties used for EBB can be adjusted to print material with anatomical precision. Inferior bioinks can be supplemented with rheological additives (e.g. alginate) to increase viscosity, solely for extrusion purposes, and sacrificed upon polymerization to achieve structural integrity with high spatial resolution, while maintaining the appropriate microenvironmental stiffness.
EBB demonstrates the potential to recapitulate the precise architectural complexity of native tissues and their relevant vasculature. However, there are limitations in printing intricate, microscopic geometries, such as integrated capillary networks, with anatomical precision, high spatial resolution, and proper functionality. Current methods of vascular tissue engineering include the fabrication of either large, single macrovessels or impermeable angiogenic sprouts, networks, and capillary-like structures that support perfusion. Although these are key features of mature and stable blood vessels, such arterioles and venules, functional capillaries must be permeable to facilitate adequate nutrient transfer, oxygen diffusion, and waste removal for cell survival. To overcome this hurdle, printing of hierarchical vasculature that fully mimics precise cellular organization may not be necessary immediate upon fabrication. Instead, 3D-bioprinting can be used to engineer voluminous tissues of simpler complexity, while still achieving heterogeneous material properties and structure, as well as multiple cell types and variations in biochemical functionalization within predefined spaces. These bioengineered tissues can undergo biomolecular and mechanical conditioning, by which various cell types can be coaxed down their lineage-specific paths to reorganize into mature substructures and remodel their predefined microenvironment, achieving whole-tissue maturation. Dynamic perfusion of cellladen constructs harboring partial macrovasculature can be used to invoke angiogenesis and proper capillary network formation within complex tissues. Bioreactor designs that simultaneously promote the maturation of bifurcated vessels and capillaries, via hemodynamic flow, and mature bone or muscle formation, via cyclic loading or tension, may augment the growth and development of distinct, vascularized tissues. It should also be noted that most native tissues are equipped with lymphatic vessels and nerves, indicating that bioengineered neo-pedicles must either permit sufficient host innervation and lymphatic vascularization, or be printed with said predefined structures. Overall, a combination of various 3D-bioprinting modalities in tandem with the appropriate cells type selection, biostructural polymers, bioink rheology and curing methods, and construct preconditioning may be necessary to achieve this multi-layered, tissue-engineered neo-pedicle suitable for the adequate repair of deep, chronic wounds.
Figure 7.
Muscle biofabrication using EBB. (A) Schematic of 3Dbioprinted cell-laden hydrogels flanked by PCL polymer supporting filaments (i). Optical image of 3D-bioprinted muscle before (left) and after (right) the removal of fugitive ink, Pluronic F127 (ii). D7 live/dead analysis of myoblasts encapsulated in a composite fibrinogen-gelatin-hyaluronan structure, demonstrating cell alignment along the length of the printed fiber (iii). Subcutaneous implantation of bioprinted muscle facilitates host innervation, vascularization, and partial restoration of musclegenerated action potentials (iv-v). (B) D7 immunohistochemical images of myofiber formation depict bioprinted muscle constructs enhance myoblasts alignment and increase myogenic-specific marker (α-sarcomeric actin and myosin-heavy chain) expression in comparison to nonprinted muscle constructs (i-ii). Histological analysis of muscle construct explants after 2 weeks demonstrates an increase in cell density overtime (iii). H&E and MTS analysis on muscle construct explants after 8 weeks reveals comparable muscle volume retention and morphology in bioprinted tissues and sham models in relation to non-printed tissue (iv). Tetanic force of bioprinted muscle construct displayed the greatest recovery in muscle function in comparison to non-printed muscle constructs (v). (C) Schematic of 3D muscle-tendon unit printing system and optical images of the fabricated structure under tension (top) and at rest (bottom) (i). Fluorescently-labelled NIH/3T3 fibroblasts (red ) and C2C12 myoblasts (green) imaged after 7 days in culture presenting a well-defined intermediate zone (yellow) between fabricated muscle and tendon (ii). Mechanical properties of distinct acellular MTU regions under tension (iii). (Kang et al., 2016; Kim et al., 2018; Merceron et al., 2015)
7A: Kang HW, Lee SJ, Ko IK et al. A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nature Biotechnology. 2016;34(3):312322 doi:10.1038/nbt.3413
7B: Kim JH, Seol YJ, Ko IK et al. 3D Bioprinted Human skeletal Muscle Contructs for Muscle Restoration. Scientific Reports. 2018;8:12307. doi:10.1038/s41598–018-29968–5
7C: Merceron TK, Burt M, Seol YJ et al. A 3D bioprinted complex structure for engineering the muscle-tendon unit. Biofabrication. 2015;7:035003. doi:10.1088/1758-5090/7/3/035003
Acknowledgments
The project described was supported by the Empire State Stem Cell Fund through New York State Department of Health Contract # C30293GG. Opinions expressed here are solely those of the author and do not necessarily reflect those of the Empire State Stem Cell Board, the New York State Department of Health, or the State of New York. This project was also supported by the NIH AR070408, HL128745, and the NSF CMMI-1635712. All authors have read and understood this journal’s policy on the disclosure of potential conflicts of interests and declare that there are no potential conflicts of interest. All authors have also read and understood this journal’s authorship agreement for the publication of this manuscript, which has been reviewed and approved by all named authors.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abaci HE, Coffman A, Doucet Y, Chen J, Jacków J, Wang E, Guo Z, Shin JU, Jahoda CA, Christiano AM, 2018. Tissue engineering of human hair follicles using a biomimetic developmental approach. Nat. Commun 9, 5301 10.1038/s41467-018-07579-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abaci HE, Guo Z, Coffman A, Gillette B, Lee W, Sia SK, Christiano AM, 2016. Human Skin Constructs with Spatially Controlled Vasculature Using Primary and iPSC-Derived Endothelial Cells. Adv. Healthc. Mater 5, 1800–1807. 10.1002/adhm.201500936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agrawal K, 2009. Cleft palate repair and variations. Indian J. Plast. Surg. Off. Publ. Assoc. Plast. Surg. India 42, S102–S109. 10.4103/0970-0358.57197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahlfeld T, Cidonio G, Kilian D, Duin S, Akkineni AR, Dawson JI, Yang S, Lode A, Oreffo ROC, Gelinsky M, 2017. Development of a clay based bioink for 3D cell printing for skeletal application. Biofabrication 9, 034103. 10.1088/1758-5090/aa7e96 [DOI] [PubMed] [Google Scholar]
- 5.Ahmed NE-MB, Murakami M, Kaneko S, Nakashima M, 2016. The effects of hypoxia on the stemness properties of human dental pulp stem cells (DPSCs). Sci. Rep 6, 35476 10.1038/srep35476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Akbari E, Spychalski GB, Song JW, 2017. Microfluidic approaches to the study of angiogenesis and the microcirculation. Microcirculation 24, e12363 10.1111/micc.12363 [DOI] [PubMed] [Google Scholar]
- 7.Alexiadou K, Doupis J, 2012. Management of Diabetic Foot Ulcers. Diabetes Ther 3 10.1007/s13300-012-0004-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ali N, Rosenblum MD, 2017. Regulatory T cells in skin. Immunology 152, 372–381. 10.1111/imm.12791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alkhouli N, Mansfield J, Green E, Bell J, Knight B, Liversedge N, Tham JC, Welbourn R, Shore AC, Kos K, Winlove CP, 2013. The mechanical properties of human adipose tissues and their relationships to the structure and composition of the extracellular matrix. Am. J. Physiol.-Endocrinol. Metab 305, E1427–E1435. 10.1152/ajpendo.00111.2013 [DOI] [PubMed] [Google Scholar]
- 10.Amis AA, 2004. The Biomechanics of Ligaments, in: Poitout DG (Ed.), Biomechanics and Biomaterials in Orthopedics Springer London, London, pp. 550–563. 10.1007/978-1-4471-3774-0_48 [DOI] [Google Scholar]
- 11.Armstrong DG, Boulton AJM, Bus SA, 2017. Diabetic Foot Ulcers and Their Recurrence. N. Engl. J. Med 376, 2367–2375. 10.1056/NEJMra1615439 [DOI] [PubMed] [Google Scholar]
- 12.Armulik A, Abramsson A, Betsholtz C, 2005. Endothelial/pericyte interactions. Circ. Res 97, 512–523. 10.1161/01.RES.0000182903.16652.d7 [DOI] [PubMed] [Google Scholar]
- 13.Asano Y, Shimoda H, Okano D, Matsusaki M, Akashi M, 2017. Transplantation of three-dimensional artificial human vascular tissues fabricated using an extracellular matrix nanofilm-based cell-accumulation technique. J. Tissue Eng. Regen. Med 11, 1303–1307. 10.1002/term.2108 [DOI] [PubMed] [Google Scholar]
- 14.Augat P, Schorlemmer S, 2006. The role of cortical bone and its microstructure in bone strength. Age Ageing 35 Suppl 2, ii27–ii31. 10.1093/ageing/afl081 [DOI] [PubMed] [Google Scholar]
- 15.Augustin HG, Koh GY, Thurston G, Alitalo K, 2009. Control of vascular morphogenesis and homeostasis through the angiopoietin-Tie system. Nat. Rev. Mol. Cell Biol 10, 165–177. 10.1038/nrm2639 [DOI] [PubMed] [Google Scholar]
- 16.Ayala P, Caves J, Dai E, Siraj L, Liu L, Chaudhuri O, Haller C, J Mooney D, L Chaikof E, 2015. Engineered Composite Fascia for Stem Cell Therapy in Tissue Repair Applications. Acta Biomater 26 10.1016/j.actbio.2015.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Badiavas EV, Abedi M, Butmarc J, Falanga V, Quesenberry P, 2003. Participation of bone marrow derived cells in cutaneous wound healing. J. Cell. Physiol 196, 245–250. 10.1002/jcp.10260 [DOI] [PubMed] [Google Scholar]
- 18.Badiavas EV, Ford D, Liu P, Kouttab N, Morgan J, Richards A, Maizel A, 2007. Long-term bone marrow culture and its clinical potential in chronic wound healing. Wound Repair Regen. Off. Publ. Wound Heal. Soc. Eur. Tissue Repair Soc 15, 856–865. 10.1111/j.1524-475X.2007.00305.x [DOI] [PubMed] [Google Scholar]
- 19.Bak S, Ahmad T, Lee YB, Lee J, Kim EM, Shin H, 2016. Delivery of a Cell Patch of Cocultured Endothelial Cells and Smooth Muscle Cells Using Thermoresponsive Hydrogels for Enhanced Angiogenesis. Tissue Eng. Part A 22, 182–193. 10.1089/ten.TEA.2015.0124 [DOI] [PubMed] [Google Scholar]
- 20.Bamba R, Madden JJ, Hoffman AN, Kim JS, Thayer WP, Nanney LB, Spear ME, 2017. Flap Reconstruction for Pressure Ulcers: An Outcomes Analysis. Plast. Reconstr. Surg. Glob. Open 5 10.1097/GOX.0000000000001187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baranski JD, Chaturvedi RR, Stevens KR, Eyckmans J, Carvalho B, Solorzano RD, Yang MT, Miller JS, Bhatia SN, Chen CS, 2013. Geometric control of vascular networks to enhance engineered tissue integration and function. Proc. Natl. Acad. Sci. U. S. A 110, 7586–7591. 10.1073/pnas.1217796110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bargehr J, Low L, Cheung C, Bernard WG, Iyer D, Bennett MR, Gambardella L, Sinha S, 2016. Embryological Origin of Human Smooth Muscle Cells Influences Their Ability to Support Endothelial Network Formation. Stem Cells Transl. Med 5, 946–959. 10.5966/sctm.2015-0282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Basciano L, Nemos C, Foliguet B, de Isla N, de Carvalho M, Tran N, Dalloul A, 2011. Long term culture of mesenchymal stem cells in hypoxia promotes a genetic program maintaining their undifferentiated and multipotent status. BMC Cell Biol 12, 12 10.1186/1471-2121-12-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Basu A, 2016. Classification of flaps and application of the concept of vascular territories pp. 93–120.
- 25.Bazmara H, Soltani M, Sefidgar M, Bazargan M, Mousavi Naeenian M, Rahmim A, 2016. Blood flow and endothelial cell phenotype regulation during sprouting angiogenesis. Med. Biol. Eng. Comput 54, 547–558. 10.1007/s11517-015-1341-4 [DOI] [PubMed] [Google Scholar]
- 26.Bazmara H, Soltani M, Sefidgar M, Bazargan M, Mousavi Naeenian M, Rahmim A, 2015. The Vital Role of Blood Flow-Induced Proliferation and Migration in Capillary Network Formation in a Multiscale Model of Angiogenesis. PloS One 10, e0128878 10.1371/journal.pone.0128878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ben-Shaul S, Landau S, Merdler U, Levenberg S, 2019. Mature vessel networks in engineered tissue promote graft–host anastomosis and prevent graft thrombosis. Proc. Natl. Acad. Sci 116, 2955–2960. 10.1073/pnas.1814238116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bergers G, Song S, 2005. The role of pericytes in blood-vessel formation and maintenance. Neuro-Oncol 7, 452–464. 10.1215/S1152851705000232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Besse J-L, Leemrijse T, Deleu P-A, 2011. Diabetic foot: The orthopedic surgery angle. Orthop. Traumatol. Surg. Res 97, 314–329. 10.1016/j.otsr.2011.03.001 [DOI] [PubMed] [Google Scholar]
- 30.Betts JG, Desaix P, Johnson E, Johnson JE, Korol O, Kruse D, Poe B, Wise J, Womble M, Young KA, OpenStax College, 2013. Anatomy and Physiology OpenStax College, Rice University. [Google Scholar]
- 31.Bhattacharyya A, Lin S, Sandig M, Mequanint K, 2014. Regulation of vascular smooth muscle cell phenotype in three-dimensional coculture system by Jagged1-selective Notch3 signaling. Tissue Eng. Part A 20, 1175–1187. 10.1089/ten.TEA.2013.0268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bhutani S, Vishwanath G, 2012. Hyperbaric oxygen and wound healing. Indian J. Plast. Surg 45, 316 10.4103/0970-0358.101309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bichsel CA, Hall SRR, Schmid RA, Guenat OT, Geiser T, 2015. Primary Human Lung Pericytes Support and Stabilize In Vitro Perfusable Microvessels. Tissue Eng. Part A 21, 2166–2176. 10.1089/ten.TEA.2014.0545 [DOI] [PubMed] [Google Scholar]
- 34.Bischoff FC, Werner A, John D, Boeckel J-N, Melissari M-T, Grote P, Glaser SF, Demolli S, Uchida S, Michalik KM, Meder B, Katus HA, Haas J, Chen W, Pullamsetti SS, Seeger W, Zeiher AM, Dimmeler S, Zehendner CM, 2017. Identification and Functional Characterization of Hypoxia-Induced Endoplasmic Reticulum Stress Regulating lncRNA (HypERlnc) in Pericytes. Circ. Res 121, 368–375. 10.1161/CIRCRESAHA.116.310531 [DOI] [PubMed] [Google Scholar]
- 35.Bluestein D, Javaheri A, 2008a. Pressure Ulcers: Prevention, Evaluation, and Management 78, 9. [PubMed] [Google Scholar]
- 36.Bluestein D, Javaheri A, 2008b. Pressure Ulcers: Prevention, Evaluation, and Management - - American Family Physician. Am Fam Physician 78, 1186–1194. [PubMed] [Google Scholar]
- 37.Bonar RA, Lippi G, Favaloro EJ, 2017. Overview of Hemostasis and Thrombosis and Contribution of Laboratory Testing to Diagnosis and Management of Hemostasis and Thrombosis Disorders. Methods Mol. Biol. Clifton NJ 1646, 3–27. 10.1007/978-1-4939-7196-1_1 [DOI] [PubMed] [Google Scholar]
- 38.Bordoni B, Varacallo M, 2019a. Anatomy, Fascia, in: StatPearls. StatPearls Publishing, Treasure Island (FL). [PubMed] [Google Scholar]
- 39.Bordoni B, Varacallo M, 2019b. Anatomy, Tendons, in: StatPearls. StatPearls Publishing, Treasure Island (FL). [PubMed] [Google Scholar]
- 40.Borman H, Maral T, 2002. The gluteal fasciocutaneous rotation-advancement flap with V-Y closure in the management of sacral pressure sores. Plast. Reconstr. Surg 109, 2325–2329. [DOI] [PubMed] [Google Scholar]
- 41.Bourland J, Fradette J, 2018. 8 - Strategies to promote the vascularization of skin substitutes after transplantation, in: Marques AP, Pirraco RP, Cerqueira MT, Reis RL (Eds.), Skin Tissue Models Academic Press, Boston, pp. 177–200. 10.1016/B978-0-12-810545-0.00008-5 [DOI] [Google Scholar]
- 42.Bowers SLK, Meng C-X, Davis MT, Davis GE, 2015. Investigating human vascular tube morphogenesis and maturation using endothelial cell-pericyte co-cultures and a doxycycline-inducible genetic system in 3D extracellular matrices. Methods Mol. Biol. Clifton NJ 1189, 171–189. 10.1007/978-1-4939-1164-6_12 [DOI] [PubMed] [Google Scholar]
- 43.Boyd NL, Nunes SS, Krishnan L, Jokinen JD, Ramakrishnan VM, Bugg AR, Hoying JB, 2013. Dissecting the role of human embryonic stem cell-derived mesenchymal cells in human umbilical vein endothelial cell network stabilization in threedimensional environments. Tissue Eng. Part A 19, 211–223. 10.1089/ten.tea.2011.0408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boyko T, Longaker M, Yang G, 2017. Laboratory Models for the Study of Normal and Pathologic Wound Healing. Plast. Reconstr. Surg 139, 654–662. 10.1097/PRS.0000000000003077 [DOI] [PubMed] [Google Scholar]
- 45.Boyko TV, Longaker MT, Yang GP, 2018. Review of the Current Management of Pressure Ulcers. Adv. Wound Care 7, 57–67. 10.1089/wound.2016.0697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brancato SK, Albina JE, 2011. Wound macrophages as key regulators of repair: origin, phenotype, and function. Am. J. Pathol 178, 19–25. 10.1016/j.ajpath.2010.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bray LJ, Werner C, 2018. Evaluation of Three-Dimensional in Vitro Models to Study Tumor Angiogenesis. ACS Biomater. Sci. Eng 4, 337–346. 10.1021/acsbiomaterials.7b00139 [DOI] [PubMed] [Google Scholar]
- 48.Brennan MA, Davaine J-M, Layrolle P, 2013. Pre-vascularization of bone tissueengineered constructs. Stem Cell Res. Ther 4, 96 10.1186/scrt307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Broek RWT, Grefte S, Hoff J.W.V. den, 2010. Regulatory factors and cell populations involved in skeletal muscle regeneration. J. Cell. Physiol 224, 7–16. 10.1002/jcp.22127 [DOI] [PubMed] [Google Scholar]
- 50.Burlacu A, Grigorescu G, Rosca A-M, Preda MB, Simionescu M, 2013. Factors secreted by mesenchymal stem cells and endothelial progenitor cells have complementary effects on angiogenesis in vitro. Stem Cells Dev 22, 643–653. 10.1089/scd.2012.0273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bus SA, van Deursen RW, Armstrong DG, Lewis JEA, Caravaggi CF, Cavanagh PR, International Working Group on the Diabetic Foot, 2016. Footwear and offloading interventions to prevent and heal foot ulcers and reduce plantar pressure in patients with diabetes: a systematic review. Diabetes Metab. Res. Rev 32 Suppl 1, 99–118. 10.1002/dmrr.2702 [DOI] [PubMed] [Google Scholar]
- 52.Bussmann J, Wolfe SA, Siekmann AF, 2011. Arterial-venous network formation during brain vascularization involves hemodynamic regulation of chemokine signaling. Dev. Camb. Engl 138, 1717–1726. 10.1242/dev.059881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Butoi E, Gan AM, Tucureanu MM, Stan D, Macarie RD, Constantinescu C, Calin M, Simionescu M, Manduteanu I, 2016. Cross-talk between macrophages and smooth muscle cells impairs collagen and metalloprotease synthesis and promotes angiogenesis. Biochim. Biophys. Acta 1863, 1568–1578. 10.1016/j.bbamcr.2016.04.001 [DOI] [PubMed] [Google Scholar]
- 54.Byambaa B, Annabi N, Yue K, Trujillo-de Santiago G, Alvarez MM, Jia W, Kazemzadeh-Narbat M, Shin SR, Tamayol A, Khademhosseini A, 2017. Bioprinted Osteogenic and Vasculogenic Patterns for Engineering 3D Bone Tissue. Adv. Healthc. Mater 6 10.1002/adhm.201700015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Caplan AI, Dennis JE, 2006. Mesenchymal stem cells as trophic mediators. J. Cell. Biochem 98, 1076–1084. 10.1002/jcb.20886 [DOI] [PubMed] [Google Scholar]
- 56.Carosio S, Barberi L, Rizzuto E, Nicoletti C, Del Prete Z, Musarò A, 2013. Generation of eX vivo-vascularized Muscle Engineered Tissue (X-MET). Sci. Rep 3, 1420 10.1038/srep01420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ceccarelli J, Cheng A, Putnam AJ, 2012. Mechanical strain controls endothelial patterning during angiogenic sprouting. Cell. Mol. Bioeng 5, 463–473. 10.1007/s12195-012-0242-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chancellor TJ, Lee J, Thodeti CK, Lele T, 2010. Actomyosin tension exerted on the nucleus through nesprin-1 connections influences endothelial cell adhesion, migration, and cyclic strain-induced reorientation. Biophys. J 99, 115–123. 10.1016/j.bpj.2010.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Charoenpanich A, Wall ME, Tucker CJ, Andrews DMK, Lalush DS, Dirschl DR, Loboa EG, 2014. Cyclic tensile strain enhances osteogenesis and angiogenesis in mesenchymal stem cells from osteoporotic donors. Tissue Eng. Part A 20, 67–78. 10.1089/ten.TEA.2013.0006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Charoenpanich A, Wall ME, Tucker CJ, Andrews DMK, Lalush DS, Loboa EG, 2011. Microarray analysis of human adipose-derived stem cells in three-dimensional collagen culture: osteogenesis inhibits bone morphogenic protein and Wnt signaling pathways, and cyclic tensile strain causes upregulation of proinflammatory cytokine regulators and angiogenic factors. Tissue Eng. Part A 17, 2615–2627. 10.1089/ten.tea.2011.0107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chasmar LR, 2007. The versatile rhomboid (Limberg) flap. Can. J. Plast. Surg 15, 67–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chaturvedi RR, Stevens KR, Solorzano RD, Schwartz RE, Eyckmans J, Baranski JD, Stapleton SC, Bhatia SN, Chen CS, 2015. Patterning vascular networks in vivo for tissue engineering applications. Tissue Eng. Part C Methods 21, 509–517. 10.1089/ten.TEC.2014.0258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen L, Xing Q, Zhai Q, Tahtinen M, Zhou F, Chen Lili, Xu Y, Qi S, Zhao F, 2017. Pre-vascularization Enhances Therapeutic Effects of Human Mesenchymal Stem Cell Sheets in Full Thickness Skin Wound Repair. Theranostics 7, 117–131. 10.7150/thno.17031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen MB, Whisler JA, Fröse J, Yu C, Shin Y, Kamm RD, 2017. On-chip human microvasculature assay for visualization and quantification of tumor cell extravasation dynamics. Nat. Protoc 12, 865–880. 10.1038/nprot.2017.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen X, Aledia AS, Popson SA, Him L, Hughes CCW, George SC, 2010. Rapid anastomosis of endothelial progenitor cell-derived vessels with host vasculature is promoted by a high density of cotransplanted fibroblasts. Tissue Eng. Part A 16, 585–594. 10.1089/ten.TEA.2009.0491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen Z, Zhang X, Deng X, 2012. Swirling flow can suppress monocyte adhesion in the flow disturbance zones of the endovascular stent. Biorheology 49, 341–352. 10.3233/BIR-120622 [DOI] [PubMed] [Google Scholar]
- 67.Cheng G, Liao S, Kit Wong H, Lacorre DA, di Tomaso E, Au P, Fukumura D, Jain RK, Munn LL, 2011. Engineered blood vessel networks connect to host vasculature via wrapping-and-tapping anastomosis. Blood 118, 4740–4749. 10.1182/blood-2011-02-338426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Clarke B, 2008. Normal Bone Anatomy and Physiology. Clin. J. Am. Soc. Nephrol. CJASN 3, S131–S139. 10.2215/CJN.04151206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Collins LG, Seraj S, 2010. Diagnosis and Treatment of Venous Ulcers. Am. Fam. Physician 81, 989–996. [PubMed] [Google Scholar]
- 70.Costa-Almeida R, Gomez-Lazaro M, Ramalho C, Granja PL, Soares R, Guerreiro SG, 2015. Fibroblast-Endothelial Partners for Vascularization Strategies in Tissue Engineering. Tissue Eng. Part A 21, 1055–1065. 10.1089/ten.tea.2014.0443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cox TR, Erler JT, 2011. Remodeling and homeostasis of the extracellular matrix: implications for fibrotic diseases and cancer. Dis. Model. Mech 4, 165–178. 10.1242/dmm.004077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cramer GD, 2014. Chapter 2 - General Characteristics of the Spine, in: Cramer GD, Darby SA (Eds.), Clinical Anatomy of the Spine, Spinal Cord, and Ans (Third Edition). Mosby, Saint Louis, pp. 15–64. 10.1016/B978-0-323-07954-9.00002-5 [DOI] [Google Scholar]
- 73.Crawford JM, Lal BK, Durán WN, Pappas PJ, 2017. Pathophysiology of venous ulceration. J. Vasc. Surg. Venous Lymphat. Disord 5, 596–605. 10.1016/j.jvsv.2017.03.015 [DOI] [PubMed] [Google Scholar]
- 74.Cui H, Zhu W, Nowicki M, Zhou X, Khademhosseini A, Zhang LG, 2016. Hierarchical Fabrication of Engineered Vascularized Bone Biphasic Constructs via Dual 3D Bioprinting: Integrating Regional Bioactive Factors into Architectural Design. Adv. Healthc. Mater 5, 2174–2181. 10.1002/adhm.201600505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cui X, Boland T, D’Lima DD, Lotz MK, 2012. Thermal Inkjet Printing in Tissue Engineering and Regenerative Medicine. Recent Pat. Drug Deliv. Formul 6, 149–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dai N-T, Huang W-S, Chang F-W, Wei L-G, Huang T-C, Li J-K, Fu K-Y, Dai L-G, Hsieh P-S, Huang N-C, Wang Y-W, Chang H-I, Parungao R, Wang Y, 2018. Development of a Novel Pre-Vascularized Three-Dimensional Skin Substitute Using Blood Plasma Gel. Cell Transplant 27, 1535–1547. 10.1177/0963689718797570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dang M, Saunders L, Niu X, Fan Y, Ma PX, 2018. Biomimetic delivery of signals for bone tissue engineering. Bone Res 6, 25 10.1038/s41413-018-0025-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Darland DC, D’Amore PA, 2001. TGF beta is required for the formation of capillary-like structures in three-dimensional cocultures of 10T1/2 and endothelial cells. Angiogenesis 4, 11–20. [DOI] [PubMed] [Google Scholar]
- 79.Dash NR, Dash SN, Routray P, Mohapatra S, Mohapatra PC, 2009. Targeting nonhealing ulcers of lower extremity in human through autologous bone marrow-derived mesenchymal stem cells. Rejuvenation Res 12, 359–366. 10.1089/rej.2009.0872 [DOI] [PubMed] [Google Scholar]
- 80.Datta P, Ayan B, Ozbolat IT, 2017. Bioprinting for vascular and vascularized tissue biofabrication. Acta Biomater 51, 1–20. 10.1016/j.actbio.2017.01.035 [DOI] [PubMed] [Google Scholar]
- 81.Davis GE, Stratman AN, Sacharidou A, Koh W, 2011. Molecular basis for endothelial lumen formation and tubulogenesis during vasculogenesis and angiogenic sprouting. Int. Rev. Cell Mol. Biol 288, 101–165. 10.1016/B978-0-12-386041-5.00003-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Davis S, Aldrich TH, Jones PF, Acheson A, Compton DL, Jain V, Ryan TE, Bruno J, Radziejewski C, Maisonpierre PC, Yancopoulos GD, 1996. Isolation of Angiopoietin-1, a Ligand for the TIE2 Receptor, by Secretion-Trap Expression Cloning. Cell 87, 1161–1169. 10.1016/S0092-8674(00)81812-7 [DOI] [PubMed] [Google Scholar]
- 83.Dayan JH, Allen RJ, 2017. Lower Extremity Free Flaps for Breast Reconstruction. Plast. Reconstr. Surg 140, 77S–86S. 10.1097/PRS.0000000000003944 [DOI] [PubMed] [Google Scholar]
- 84.Derakhshanfar S, Mbeleck R, Xu K, Zhang X, Zhong W, Xing M, 2018. 3D bioprinting for biomedical devices and tissue engineering: A review of recent trends and advances. Bioact. Mater 3, 144–156. 10.1016/j.bioactmat.2017.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dietrich F, Lelkes PI, 2006. Fine-tuning of a three-dimensional microcarrier-based angiogenesis assay for the analysis of endothelial-mesenchymal cell co-cultures in fibrin and collagen gels. Angiogenesis 9, 111–125. 10.1007/s10456-006-9037-x [DOI] [PubMed] [Google Scholar]
- 86.Do A-V, Khorsand B, Geary SM, Salem AK, 2015. 3D Printing of Scaffolds for Tissue Regeneration Applications. Adv. Healthc. Mater 4, 1742–1762. 10.1002/adhm.201500168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dreifke MB, Jayasuriya AA, Jayasuriya AC, 2015. Current wound healing procedures and potential care. Mater. Sci. Eng. C Mater. Biol. Appl 48, 651–662. 10.1016/j.msec.2014.12.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Duci S, Arifi H, Selmani M, Mekaj A, M. Gashi M, A. Buja Z, H. Ismajli V, N. Kllokoqi A, T. Hoxha E, 2013. Surgical Treatment of 55 Patients with Pressure Ulcers at the Department of Plastic and Reconstructive Surgery Kosovo during the Period 2000–2010: A Retrospective Study 10.1155/2013/129692 [DOI] [PMC free article] [PubMed]
- 89.Dunn R, 2006. Grafts and flaps. Surg. Oxf., Plastic surgery 24, 27–32. 10.1383/surg.2006.24.1.27 [DOI] [Google Scholar]
- 90.Duscher D, Barrera J, Wong VW, Maan ZN, Whittam AJ, Januszyk M, Gurtner GC, 2016. Stem Cells in Wound Healing: The Future of Regenerative Medicine? A Mini-Review. Gerontology 62, 216–225. 10.1159/000381877 [DOI] [PubMed] [Google Scholar]
- 91.Duscher D, Neofytou E, Wong VW, Maan ZN, Rennert RC, Inayathullah M, Januszyk M, Rodrigues M, Malkovskiy AV, Whitmore AJ, Walmsley GG, Galvez MG, Whittam AJ, Brownlee M, Rajadas J, Gurtner GC, 2015. Transdermal deferoxamine prevents pressure-induced diabetic ulcers. Proc. Natl. Acad. Sci. U. S. A 112, 94–99. 10.1073/pnas.1413445112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Eckermann CW, Lehle K, Schmid SA, Wheatley DN, Kunz-Schughart LA, 2011. Characterization and modulation of fibroblast/endothelial cell co-cultures for the in vitro preformation of three-dimensional tubular networks. Cell Biol. Int 35, 1097–1110. 10.1042/CBI20100718 [DOI] [PubMed] [Google Scholar]
- 93.Eilken HM, Diéguez-Hurtado R, Schmidt I, Nakayama M, Jeong H-W, Arf H, Adams S, Ferrara N, Adams RH, 2017. Pericytes regulate VEGF-induced endothelial sprouting through VEGFR1. Nat. Commun 8, 1574 10.1038/s41467-017-01738-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ellis S, Lin EJ, Tartar D, 2018. Immunology of Wound Healing. Curr. Dermatol. Rep 7, 350–358. 10.1007/s13671-018-0234-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Eming SA, Martin P, Tomic-Canic M, 2014. Wound repair and regeneration: mechanisms, signaling, and translation. Sci. Transl. Med 6, 265sr6. 10.1126/scitranslmed.3009337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Esteves CL, Sheldrake TA, Dawson L, Menghini T, Rink BE, Amilon K, Khan N, Péault B, Donadeu FX, 2017. Equine Mesenchymal Stromal Cells Retain a Pericyte-Like Phenotype. Stem Cells Dev 26, 964–972. 10.1089/scd.2017.0017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Falanga V, Iwamoto S, Chartier M, Yufit T, Butmarc J, Kouttab N, Shrayer D, Carson P, 2007. Autologous bone marrow-derived cultured mesenchymal stem cells delivered in a fibrin spray accelerate healing in murine and human cutaneous wounds. Tissue Eng 13, 1299–1312. 10.1089/ten.2006.0278 [DOI] [PubMed] [Google Scholar]
- 98.Fantin A, Vieira JM, Gestri G, Denti L, Schwarz Q, Prykhozhij S, Peri F, Wilson SW, Ruhrberg C, 2010. Tissue macrophages act as cellular chaperones for vascular anastomosis downstream of VEGF-mediated endothelial tip cell induction. Blood 116, 829–840. 10.1182/blood-2009-12-257832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Findley T, Chaudhry H, Stecco A, Roman M, 2012. Fascia research – A narrative review. J. Bodyw. Mov. Ther 16, 67–75. 10.1016/j.jbmt.2011.09.004 [DOI] [PubMed] [Google Scholar]
- 100.Florencio-Silva R, Sasso G.R. da S., Sasso-Cerri E, Simões MJ, Cerri PS, 2015. Biology of Bone Tissue: Structure, Function, and Factors That Influence Bone Cells. BioMed Res. Int 2015 10.1155/2015/421746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Forster R, Pagnamenta F, 2015. Dressings and topical agents for arterial leg ulcers. Cochrane Database Syst. Rev CD001836. 10.1002/14651858.CD001836.pub3 [DOI] [PMC free article] [PubMed]
- 102.Franco CA, Jones ML, Bernabeu MO, Geudens I, Mathivet T, Rosa A, Lopes FM, Lima AP, Ragab A, Collins RT, Phng L-K, Coveney PV, Gerhardt H, 2015. Dynamic endothelial cell rearrangements drive developmental vessel regression. PLoS Biol 13, e1002125 10.1371/journal.pbio.1002125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Frerich B, Zückmantel K, Winter K, Müller-Dürwald S, Hemprich A, 2008. Maturation of capillary-like structures in a tube-like construct in perfusion and rotation culture. Int. J. Oral Maxillofac. Surg 37, 459–466. 10.1016/j.ijom.2008.01.014 [DOI] [PubMed] [Google Scholar]
- 104.Friedrich JB, Katolik LI, Vedder NB, 2009. Soft tissue reconstruction of the hand. J. Hand Surg 34, 1148–1155. 10.1016/j.jhsa.2009.04.035 [DOI] [PubMed] [Google Scholar]
- 105.Frueh FS, Später T, Lindenblatt N, Calcagni M, Giovanoli P, Scheuer C, Menger MD, Laschke MW, 2017. Adipose Tissue-Derived Microvascular Fragments Improve Vascularization, Lymphangiogenesis, and Integration of Dermal Skin Substitutes. J. Invest. Dermatol 137, 217–227. 10.1016/j.jid.2016.08.010 [DOI] [PubMed] [Google Scholar]
- 106.Fujioka M, 2014. Surgical Reconstruction of Radiation Injuries. Adv. Wound Care 3, 25–37. 10.1089/wound.2012.0405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Garcia-Olmo D, Garcia-Arranz M, Herreros D, 2008. Expanded adipose-derived stem cells for the treatment of complex perianal fistula including Crohn’s disease. Expert Opin. Biol. Ther 8, 1417–1423. 10.1517/14712598.8.9.1417 [DOI] [PubMed] [Google Scholar]
- 108.Gatt A, Zito PM, 2019. Anatomy, Skin, Fascias, in: StatPearls. StatPearls Publishing, Treasure Island (FL). [Google Scholar]
- 109.Gebala V, Collins R, Geudens I, Phng L-K, Gerhardt H, 2016. Blood flow drives lumen formation by inverse membrane blebbing during angiogenesis in vivo. Nat. Cell Biol 18, 443–450. 10.1038/ncb3320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Geddes CR, Morris SF, Neligan PC, 2003. Perforator flaps: evolution, classification, and applications. Ann. Plast. Surg 50, 90–99. 10.1097/01.SAP.0000032309.30122.55 [DOI] [PubMed] [Google Scholar]
- 111.George C, V, D., Balasubramanian, Santharam, 2018. Formation of reconstruction protocol for sacral pressure sore defects. Internaltional Journal of Medical and Health Research 4, 18–24. [Google Scholar]
- 112.Gerri C, Marín-Juez R, Marass M, Marks A, Maischein H-M, Stainier DYR, 2017. Hif-1α regulates macrophage-endothelial interactions during blood vessel development in zebrafish. Nat. Commun 8, 15492 10.1038/ncomms15492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ghaffari S, Leask RL, Jones EAV, 2015. Flow dynamics control the location of sprouting and direct elongation during developmental angiogenesis. Development 142, 4151–4157. 10.1242/dev.128058 [DOI] [PubMed] [Google Scholar]
- 114.Ghosh K, Thodeti CK, Dudley AC, Mammoto A, Klagsbrun M, Ingber DE, 2008. Tumor-derived endothelial cells exhibit aberrant Rho-mediated mechanosensing and abnormal angiogenesis in vitro. Proc. Natl. Acad. Sci. U. S. A 105, 11305–11310. 10.1073/pnas.0800835105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gillies AR, Lieber RL, 2011. Structure and function of the skeletal muscle extracellular matrix. Muscle Nerve 44, 318–331. 10.1002/mus.22094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gledhill K, Guo Z, Umegaki-Arao N, Higgins CA, Itoh M, Christiano AM, 2015. Melanin Transfer in Human 3D Skin Equivalents Generated Exclusively from Induced Pluripotent Stem Cells. PLOS ONE 10, e0136713 10.1371/journal.pone.0136713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Goerke SM, Plaha J, Hager S, Strassburg S, Torio-Padron N, Stark GB, Finkenzeller G, 2012. Human Endothelial Progenitor Cells Induce Extracellular SignalRegulated Kinase-Dependent Differentiation of Mesenchymal Stem Cells into Smooth Muscle Cells upon Cocultivation. Tissue Eng. Part A 18, 2395–2405. 10.1089/ten.tea.2012.0147 [DOI] [PubMed] [Google Scholar]
- 118.Graeber GM, Nazim M, 2007. The Anatomy of the Ribs and the Sternum and Their Relationship to Chest Wall Structure and Function. Thorac. Surg. Clin., Thoracic Anatomy, Part I 17, 473–489. 10.1016/j.thorsurg.2006.12.010 [DOI] [PubMed] [Google Scholar]
- 119.Greenberg JI, Shields DJ, Barillas SG, Acevedo LM, Murphy E, Huang J, Scheppke L, Stockmann C, Johnson RS, Angle N, Cheresh DA, 2008. A role for VEGF as a negative regulator of pericyte function and vessel maturation. Nature 456, 809–813. 10.1038/nature07424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Greer N, Foman NA, MacDonald R, Dorrian J, Fitzgerald P, Rutks I, Wilt TJ, 2013. Advanced wound care therapies for nonhealing diabetic, venous, and arterial ulcers: a systematic review. Ann. Intern. Med 159, 532–542. 10.7326/0003-4819-159-8-201310150-00006 [DOI] [PubMed] [Google Scholar]
- 121.Grey JE, Harding KG, Enoch S, 2006. Venous and arterial leg ulcers. BMJ 332, 347–350. 10.1136/bmj.332.7537.347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Griffin M, Hindocha S, Malahias M, Saleh M, Juma A, 2014. Flap Decisions and Options in Soft Tissue Coverage of the Upper Limb. Open Orthop. J 8, 409–414. 10.2174/1874325001408010409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Groeber F, Engelhardt L, Lange J, Kurdyn S, Schmid FF, Rücker C, Mielke S, Walles H, Hansmann J, 2016. A first vascularized skin equivalent as an alternative to animal experimentation. ALTEX - Altern. Anim. Exp 33, 415–422. 10.14573/altex.1604041 [DOI] [PubMed] [Google Scholar]
- 124.Guo S, DiPietro LA, 2010. Factors Affecting Wound Healing. J. Dent. Res 89, 219–229. 10.1177/0022034509359125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ha H, Choi W, Lee SJ, 2015. Beneficial fluid-dynamic features of pulsatile swirling flow in 45° end-to-side anastomosis. Med. Eng. Phys 37, 272–279. 10.1016/j.medengphy.2015.01.007 [DOI] [PubMed] [Google Scholar]
- 126.Han S-K, 2016. Growth Factor Therapy, in: Han S-K (Ed.), Innovations and Advances in Wound Healing Springer Berlin Heidelberg, Berlin, Heidelberg, pp. 201–213. 10.1007/978-3-662-46587-5_9 [DOI] [Google Scholar]
- 127.Harvey KA, Welch Z, Sliva D, Siddiqui RA, 2010. Role of Rho kinase in sphingosine 1-phosphate-mediated endothelial and smooth muscle cell migration and differentiation. Mol. Cell. Biochem 342, 7–19. 10.1007/s11010-010-0461-2 [DOI] [PubMed] [Google Scholar]
- 128.Healy C, Allen RJ, 2014. The Evolution of Perforator Flap Breast Reconstruction: Twenty Years after the First DIEP Flap. J. Reconstr. Microsurg 30, 121–126. 10.1055/s-0033-1357272 [DOI] [PubMed] [Google Scholar]
- 129.Hedayati N, Carson JG, Chi Y-W, Link D, 2015. Management of mixed arterial venous lower extremity ulceration: A review. Vasc. Med. Lond. Engl 20, 479–486. 10.1177/1358863X15594683 [DOI] [PubMed] [Google Scholar]
- 130.Heller L, Cole P, Kaufman Y, 2008. Cheek Reconstruction: Current Concepts in Managing Facial Soft Tissue Loss. Semin. Plast. Surg 22, 294–305. 10.1055/s-0028-1095888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Herskovitz I, Hughes OB, Macquhae F, Rakosi A, Kirsner R, 2016. Epidermal skin grafting: Epidermal skin grafting. Int. Wound J 13, 52–56. 10.1111/iwj.12631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Heydarkhan-Hagvall S, Helenius G, Johansson BR, Li JY, Mattsson E, Risberg B, 2003. Co-culture of endothelial cells and smooth muscle cells affects gene expression of angiogenic factors. J. Cell. Biochem 89, 1250–1259. 10.1002/jcb.10583 [DOI] [PubMed] [Google Scholar]
- 133.Hill HL, Brown RG, Jurkiewicz MJ, 1978. The transverse lumbosacral back flap. Plast. Reconstr. Surg 62, 177–184. [DOI] [PubMed] [Google Scholar]
- 134.Horch R, Weigand A, Wajant H, An R, Sun J-M, Arkudas A, 2017. Towards the future of plastic surgery: from flaps to microsurgery and regenerative medicine and biofabrication? 10.20517/2347-9264.2017.72 [DOI]
- 135.Hsiai TK, Wu JC, 2008. Hemodynamic forces regulate embryonic stem cell commitment to vascular progenitors. Curr. Cardiol. Rev 4, 269–274. 10.2174/157340308786349471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Huang S, Xu Y, Wu C, Sha D, Fu X, 2010. In vitro constitution and in vivo implantation of engineered skin constructs with sweat glands. Biomaterials 31, 5520–5525. 10.1016/j.biomaterials.2010.03.060 [DOI] [PubMed] [Google Scholar]
- 137.Hung M-J, Wen M-C, Huang Y-T, Chen G-D, Chou M-M, Yang VC, 2014. Fascia tissue engineering with human adipose-derived stem cells in a murine model: Implications for pelvic floor reconstruction. J. Formos. Med. Assoc. Taiwan Yi Zhi 113, 704–715. 10.1016/j.jfma.2013.04.017 [DOI] [PubMed] [Google Scholar]
- 138.Hung M-J, Wen MJ, Hung C-N, Ho ES-C, Chen G-D, Yang VC, 2010. Tissue-engineered fascia from vaginal fibroblasts for patientsneeding reconstructive pelvic surgery. Int. Urogynecology J 21, 1085–1093. 10.1007/s00192-010-1168-3 [DOI] [PubMed] [Google Scholar]
- 139.Irvin MW, Zijlstra A, Wikswo JP, Pozzi A, 2014. Techniques and assays for the study of angiogenesis. Exp. Biol. Med. Maywood NJ 239, 1476–1488. 10.1177/1535370214529386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Jeon JS, Bersini S, Whisler JA, Chen MB, Dubini G, Charest JL, Moretti M, Kamm RD, 2014. Generation of 3D functional microvascular networks with mural celldifferentiated human mesenchymal stem cells in microfluidic vasculogenesis systems. Integr. Biol. Quant. Biosci. Nano Macro 6, 555–563. 10.1039/c3ib40267c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Jia W, Gungor-Ozkerim PS, Zhang YS, Yue K, Zhu K, Liu W, Pi Q, Byambaa B, Dokmeci MR, Shin SR, Khademhosseini A, 2016. Direct 3D bioprinting of perfusable vascular constructs using a blend bioink. Biomaterials 106, 58–68. 10.1016/j.biomaterials.2016.07.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Jin F, Hagemann N, Sun L, Wu J, Doeppner TR, Dai Y, Hermann DM, 2018. High-density lipoprotein (HDL) promotes angiogenesis via S1P3-dependent VEGFR2 activation. Angiogenesis 21, 381–394. 10.1007/s10456-018-9603-z [DOI] [PubMed] [Google Scholar]
- 143.Joo HJ, Seo H-R, Jeong HE, Choi S-C, Park JH, Yu CW, Hong SJ, Chung S, Lim D-S, 2014. Smooth muscle progenitor cells from peripheral blood promote the neovascularization of endothelial colony-forming cells. Biochem. Biophys. Res. Commun 449, 405–411. 10.1016/j.bbrc.2014.05.061 [DOI] [PubMed] [Google Scholar]
- 144.Joodaki H, Panzer MB, 2018. Skin mechanical properties and modeling: A review. Proc. Inst. Mech. Eng. [H] 232, 323–343. 10.1177/0954411918759801 [DOI] [PubMed] [Google Scholar]
- 145.Joung IS, Iwamoto MN, Shiu Y-T, Quam CT, 2006. Cyclic strain modulates tubulogenesis of endothelial cells in a 3D tissue culture model. Microvasc. Res 71, 1–11. 10.1016/j.mvr.2005.10.005 [DOI] [PubMed] [Google Scholar]
- 146.Juhas M, Engelmayr GC, Fontanella AN, Palmer GM, Bursac N, 2014. Biomimetic engineered muscle with capacity for vascular integration and functional maturation in vivo. Proc. Natl. Acad. Sci 111, 5508–5513. 10.1073/pnas.1402723111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kachgal S, Putnam AJ, 2011. Mesenchymal stem cells from adipose and bone marrow promote angiogenesis via distinct cytokine and protease expression mechanisms. Angiogenesis 14, 47–59. 10.1007/s10456-010-9194-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kamath JB, Shetty MS, Joshua TV, Kumar A, Harshvardhan, Naik DM, 2012. Soft tissue coverage in open fractures of tibia. Indian J. Orthop 46, 462–469. 10.4103/0019-5413.97265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kang H, Bayless KJ, Kaunas R, 2008. Fluid shear stress modulates endothelial cell invasion into three-dimensional collagen matrices. Am. J. Physiol.-Heart Circ. Physiol 295, H2087–H2097. 10.1152/ajpheart.00281.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kang H-W, Lee SJ, Ko IK, Kengla C, Yoo JJ, Atala A, 2016. A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nat. Biotechnol 34, 312–319. 10.1038/nbt.3413 [DOI] [PubMed] [Google Scholar]
- 151.Kerkar S, Williams M, Blocksom JM, Wilson RF, Tyburski JG, Steffes CP, 2006. TNF-α and IL-1β Increase Pericyte/Endothelial Cell Co-Culture Permeability. J. Surg. Res 132, 40–45. 10.1016/j.jss.2005.06.033 [DOI] [PubMed] [Google Scholar]
- 152.Kim BS, Kwon YW, Kong J-S, Park GT, Gao G, Han W, Kim M-B, Lee H, Kim JH, Cho D-W, 2018. 3D cell printing of in vitro stabilized skin model and in vivo prevascularized skin patch using tissue-specific extracellular matrix bioink: A step towards advanced skin tissue engineering. Biomaterials 168, 38–53. 10.1016/j.biomaterials.2018.03.040 [DOI] [PubMed] [Google Scholar]
- 153.Kim HS, Sun X, Lee J-H, Kim H-W, Fu X, Leong KW, 2018. Advanced drug delivery systems and artificial skin grafts for skin wound healing. Adv. Drug Deliv. Rev 10.1016/j.addr.2018.12.014 [DOI] [PubMed]
- 154.Kim J, Chung M, Kim S, Jo DH, Kim JH, Jeon NL, 2015. Engineering of a Biomimetic Pericyte-Covered 3D Microvascular Network. PloS One 10, e0133880 10.1371/journal.pone.0133880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kim JH, Seol Y-J, Ko IK, Kang H-W, Lee YK, Yoo JJ, Atala A, Lee SJ, 2018. 3D Bioprinted Human Skeletal Muscle Constructs for Muscle Function Restoration. Sci. Rep 8 10.1038/s41598-018-29968-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kim JI, Cordova AC, Hirayama Y, Madri JA, Sumpio BE, 2008. Differential Effects of Shear Stress and Cyclic Strain on Sp1 Phosphorylation by Protein Kinase Cδ Modulates Membrane Type 1–Matrix Metalloproteinase in Endothelial Cells. Endothel. J. Endothel. Cell Res 15, 33–42. 10.1080/10623320802092260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kim JT, 2005. New nomenclature concept of perforator flap. Br. J. Plast. Surg 58, 431–440. 10.1016/j.bjps.2004.12.009 [DOI] [PubMed] [Google Scholar]
- 158.Klingler W, Velders M, Hoppe K, Pedro M, Schleip R, 2014. Clinical Relevance of Fascial Tissue and Dysfunctions. Curr. Pain Headache Rep 18, 439 10.1007/s11916-014-0439-y [DOI] [PubMed] [Google Scholar]
- 159.Koffler J, Kaufman-Francis K, Shandalov Y, Yulia S, Egozi D, Dana E, Pavlov DA, Daria AP, Landesberg A, Levenberg S, 2011. Improved vascular organization enhances functional integration of engineered skeletal muscle grafts. Proc. Natl. Acad. Sci. U. S. A 108, 14789–14794. 10.1073/pnas.1017825108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Kolesky DB, Truby RL, Gladman AS, Busbee TA, Homan KA, Lewis JA, 2014. 3D Bioprinting of Vascularized, Heterogeneous Cell-Laden Tissue Constructs. Adv. Mater 26, 3124–3130. 10.1002/adma.201305506 [DOI] [PubMed] [Google Scholar]
- 161.König J, Weiss G, Rossi D, Wankhammer K, Reinisch A, Kinzer M, Huppertz B, Pfeiffer D, Parolini O, Lang I, 2015. Placental mesenchymal stromal cells derived from blood vessels or avascular tissues: what is the better choice to support endothelial cell function? Stem Cells Dev 24, 115–131. 10.1089/scd.2014.0115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Korff T, Kimmina S, Martiny-Baron G, Augustin HG, 2001. Blood vessel maturation in a 3-dimensional spheroidal coculture model: direct contact with smooth muscle cells regulates endothelial cell quiescence and abrogates VEGF responsiveness. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol 15, 447–457. 10.1096/fj.00-0139com [DOI] [PubMed] [Google Scholar]
- 163.Korthuis RJ, 2011. Anatomy of Skeletal Muscle and Its Vascular Supply Morgan & Claypool Life Sciences. [PubMed] [Google Scholar]
- 164.Kumka M, Bonar J, 2012. Fascia: a morphological description and classification system based on a literature review. J. Can. Chiropr. Assoc 56, 179–191. [PMC free article] [PubMed] [Google Scholar]
- 165.Kurzen H, Manns S, Dandekar G, Schmidt T, Prätzel S, Kräling BM, 2002. Tightening of endothelial cell contacts: a physiologic response to cocultures with smoothmuscle-like 10T1/2 cells. J. Invest. Dermatol 119, 143–153. 10.1046/j.1523-1747.2002.01792.x [DOI] [PubMed] [Google Scholar]
- 166.LaValley DJ, Reinhart-King CA, 2014. Matrix stiffening in the formation of blood vessels. Adv. Regen. Biol 1, 25247 10.3402/arb.v1.25247 [DOI] [Google Scholar]
- 167.Lee GK, Sheckter CC, 2018. Breast Reconstruction Following Breast Cancer Treatment—2018. JAMA 320, 1277–1278. 10.1001/jama.2018.12190 [DOI] [PubMed] [Google Scholar]
- 168.Lee K, Silva EA, Mooney DJ, 2011. Growth factor delivery-based tissue engineering: general approaches and a review of recent developments. J. R. Soc. Interface 8, 153–170. 10.1098/rsif.2010.0223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Lee V, Singh G, Trasatti JP, Bjornsson C, Xu X, Tran TN, Yoo S-S, Dai G, Karande P, 2014. Design and fabrication of human skin by three-dimensional bioprinting. Tissue Eng. Part C Methods 20, 473–484. 10.1089/ten.TEC.2013.0335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Lee VK, Kim DY, Ngo H, Lee Y, Seo L, Yoo S-S, Vincent PA, Dai G, 2014. Creating perfused functional vascular channels using 3D bio-printing technology. Biomaterials 35, 8092–8102. 10.1016/j.biomaterials.2014.05.083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Levenberg S, Rouwkema J, Macdonald M, Garfein ES, Kohane DS, Darland DC, Marini R, van Blitterswijk CA, Mulligan RC, D’Amore PA, Langer R, 2005. Engineering vascularized skeletal muscle tissue. Nat. Biotechnol 23, 879–884. 10.1038/nbt1109 [DOI] [PubMed] [Google Scholar]
- 172.Li M-T, Ruehle MA, Stevens HY, Servies N, Willett NJ, Karthikeyakannan S, Warren GL, Guldberg RE, Krishnan L, 2017. * Skeletal Myoblast-Seeded Vascularized Tissue Scaffolds in the Treatment of a Large Volumetric Muscle Defect in the Rat Biceps Femoris Muscle. Tissue Eng. Part A 23, 989–1000. 10.1089/ten.TEA.2016.0523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Lilly B, Kennard S, 2009. Differential gene expression in a coculture model of angiogenesis reveals modulation of select pathways and a role for Notch signaling. Physiol. Genomics 36, 69–78. 10.1152/physiolgenomics.90318.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Lim CS, Baruah M, Bahia SS, 2018. Diagnosis and management of venous leg ulcers. BMJ 362, k3115. 10.1136/bmj.k3115 [DOI] [PubMed] [Google Scholar]
- 175.Lin R-Z, Lee CN, Moreno-Luna R, Neumeyer J, Piekarski B, Zhou P, Moses MA, Sachdev M, Pu WT, Emani S, Melero-Martin JM, 2017. Host non-inflammatory neutrophils mediate the engraftment of bioengineered vascular networks. Nat. Biomed. Eng 1, 0081 10.1038/s41551-017-0081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Liu H, Kennard S, Lilly B, 2009. NOTCH3 expression is induced in mural cells through an autoregulatory loop that requires endothelial-expressed JAGGED1. Circ. Res 104, 466–475. 10.1161/CIRCRESAHA.108.184846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Liu J, Saul D, Böker KO, Ernst J, Lehman W, Schilling AF, 2018. Current Methods for Skeletal Muscle Tissue Repair and Regeneration. BioMed Res. Int 2018 10.1155/2018/1984879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Liu X, Wan X, Kan H, Wang Y, Yu F, Feng L, Jin J, Zhang P, Ma X, 2018. Hypoxia-induced upregulation of Orai1 drives colon cancer invasiveness and angiogenesis. Eur. J. Pharmacol 832, 1–10. 10.1016/j.ejphar.2018.05.008 [DOI] [PubMed] [Google Scholar]
- 179.Lobov IB, Brooks PC, Lang RA, 2002. Angiopoietin-2 displays VEGF-dependent modulation of capillary structure and endothelial cell survival in vivo. Proc. Natl. Acad. Sci. U. S. A 99, 11205–11210. 10.1073/pnas.172161899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Logsdon EA, Finley SD, Popel AS, Mac Gabhann F, 2014. A systems biology view of blood vessel growth and remodelling. J. Cell. Mol. Med 18, 1491–1508. 10.1111/jcmm.12164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Lugo LM, Lei P, Andreadis ST, 2011. Vascularization of the Dermal Support Enhances Wound Re-Epithelialization by In Situ Delivery of Epidermal Keratinocytes. Tissue Eng. Part A 17, 665–675. 10.1089/ten.tea.2010.0125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Lyder CH, Ayello EA, 2008. Pressure Ulcers: A Patient Safety Issue, in: Hughes RG (Ed.), Patient Safety and Quality: An Evidence-Based Handbook for Nurses, Advances in Patient Safety Agency for Healthcare Research and Quality (US), Rockville (MD). [PubMed] [Google Scholar]
- 183.Ma J, Yang F, Both SK, Prins H-J, Helder MN, Pan J, Cui F-Z, Jansen JA, van den Beucken JJJP, 2014. In vitro and in vivo angiogenic capacity of BM-MSCs/HUVECs and AT-MSCs/HUVECs cocultures. Biofabrication 6, 015005. 10.1088/1758-5082/6/1/015005 [DOI] [PubMed] [Google Scholar]
- 184.MacLeod AS, Mansbridge JN, 2016. The Innate Immune System in Acute and Chronic Wounds. Adv. Wound Care 5, 65–78. 10.1089/wound.2014.0608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Mariman ECM, Wang P, 2010. Adipocyte extracellular matrix composition, dynamics and role in obesity. Cell. Mol. Life Sci 67, 1277–1292. 10.1007/s00018-010-0263-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Marino D, Luginbühl J, Scola S, Meuli M, Reichmann E, 2014. Bioengineering Dermo-Epidermal Skin Grafts with Blood and Lymphatic Capillaries. Sci. Transl. Med 6, 221ra14–221ra14. 10.1126/scitranslmed.3006894 [DOI] [PubMed] [Google Scholar]
- 187.Massel SR, 1999. Mechanical Properties of Biological Materials, in: Massel SR (Ed.), Fluid Mechanics for Marine Ecologists Springer Berlin Heidelberg, Berlin, Heidelberg, pp. 337–352. 10.1007/978-3-642-60209-2_10 [DOI] [Google Scholar]
- 188.Mathew A, 2016. Tissue Engineering: Principles, Recent Trends and the Future 10.1201/b19867-3 [DOI]
- 189.Mazio C, Casale C, Imparato G, Urciuolo F, Attanasio C, De Gregorio M, Rescigno F, Netti PA, 2019. Pre-vascularized dermis model for fast and functional anastomosis with host vasculature. Biomaterials 192, 159–170. 10.1016/j.biomaterials.2018.11.018 [DOI] [PubMed] [Google Scholar]
- 190.McFadden TM, Duffy GP, Allen AB, Stevens HY, Schwarzmaier SM, Plesnila N, Murphy JM, Barry FP, Guldberg RE, O’Brien FJ, 2013. The delayed addition of human mesenchymal stem cells to pre-formed endothelial cell networks results in functional vascularization of a collagen–glycosaminoglycan scaffold in vivo. Acta Biomater 9, 9303–9316. 10.1016/j.actbio.2013.08.014 [DOI] [PubMed] [Google Scholar]
- 191.McGregor IA, Morgan G, 1973. Axial and random pattern flaps. Br. J. Plast. Surg 26, 202–213. [DOI] [PubMed] [Google Scholar]
- 192.Medical Advisory Secretariat, 2009. Management of chronic pressure ulcers: an evidence-based analysis. Ont. Health Technol. Assess. Ser 9, 1–203. [PMC free article] [PubMed] [Google Scholar]
- 193.Mercado-Pagán ÁE, Stahl AM, Shanjani Y, Yang Y, 2015. Vascularization in bone tissue engineering constructs. Ann. Biomed. Eng 43, 718–729. 10.1007/s10439-015-1253-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Merceron TK, Burt M, Seol Y-J, Kang H-W, Lee SJ, Yoo JJ, Atala A, 2015. A 3D bioprinted complex structure for engineering the muscle-tendon unit. Biofabrication 7, 035003. 10.1088/1758-5090/7/3/035003 [DOI] [PubMed] [Google Scholar]
- 195.Mester A, Opincariu D, Benedek, Imre, Benedek, István, 2017. Stem Cell Therapy in Wound Healing 10.1515/jim-2017-0094 [DOI]
- 196.Miller JS, Stevens KR, Yang MT, Baker BM, Nguyen D-HT, Cohen DM, Toro E, Chen AA, Galie PA, Yu X, Chaturvedi R, Bhatia SN, Chen CS, 2012. Rapid casting of patterned vascular networks for perfusable engineered three-dimensional tissues. Nat. Mater 11, 768–774. 10.1038/nmat3357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Mirabella T, MacArthur JW, Cheng D, Ozaki CK, Woo YJ, Yang M, Chen CS, 2017. 3D-printed vascular networks direct therapeutic angiogenesis in ischaemia. Nat. Biomed. Eng 1 10.1038/s41551-017-0083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Miri AK, Khalilpour A, Cecen B, Maharjan S, Shin SR, Khademhosseini A, 2018. Multiscale bioprinting of vascularized models. Biomaterials 10.1016/j.biomaterials.2018.08.006 [DOI] [PMC free article] [PubMed]
- 199.Molnar N, Siemann DW, 2012. Inhibition of endothelial/smooth muscle cell contact loss by the investigational angiopoietin-2 antibody MEDI3617. Microvasc. Res 83, 290–297. 10.1016/j.mvr.2012.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Monteiro-Soares M, Boyko EJ, Ribeiro J, Ribeiro I, Dinis-Ribeiro M, 2012. Predictive factors for diabetic foot ulceration: a systematic review. Diabetes Metab. Res. Rev 28, 574–600. 10.1002/dmrr.2319 [DOI] [PubMed] [Google Scholar]
- 201.Mori N, Morimoto Y, Takeuchi S, 2017. Skin integrated with perfusable vascular channels on a chip. Biomaterials 116, 48–56. 10.1016/j.biomaterials.2016.11.031 [DOI] [PubMed] [Google Scholar]
- 202.Morris AD, Dalal S, Li H, Brewster LP, 2018. Human diabetic mesenchymal stem cells from peripheral arterial disease patients promote angiogenesis through unique secretome signatures. Surgery 163, 870–876. 10.1016/j.surg.2017.11.018 [DOI] [PubMed] [Google Scholar]
- 203.Morrow D, Cullen John P, Cahill Paul A, Redmond Eileen M, 2007. Cyclic Strain Regulates the Notch/CBF-1 Signaling Pathway in Endothelial Cells. Arterioscler. Thromb. Vasc. Biol 27, 1289–1296. 10.1161/ATVBAHA.107.142778 [DOI] [PubMed] [Google Scholar]
- 204.Mousavizadeh R, Khosravi S, Behzad H, McCormack RG, Duronio V, Scott A, 2014. Cyclic strain alters the expression and release of angiogenic factors by human tendon cells. PloS One 9, e97356 10.1371/journal.pone.0097356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Muller Q, Beaudet M-J, De Serres-Bérard T, Bellenfant S, Flacher V, Berthod F, 2018. Development of an innervated tissue-engineered skin with human sensory neurons and Schwann cells differentiated from iPS cells. Acta Biomater 82, 93–101. 10.1016/j.actbio.2018.10.011 [DOI] [PubMed] [Google Scholar]
- 206.Murphy SV, Atala A, 2014. 3D bioprinting of tissues and organs. Nat. Biotechnol 32, 773–785. 10.1038/nbt.2958 [DOI] [PubMed] [Google Scholar]
- 207.Navsaria P, Nicol A, Hudson D, Cockwill J, Smith J, 2013. Negative pressure wound therapy management of the ―open abdomen‖ following trauma: a prospective study and systematic review. World J. Emerg. Surg. WJES 8, 4 10.1186/1749-7922-8-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Nelson EA, Bradley MD, 2007. Dressings and topical agents for arterial leg ulcers. Cochrane Database Syst. Rev CD001836. 10.1002/14651858.CD001836.pub2 [DOI] [PubMed]
- 209.Nesmith JE, Chappell JC, Cluceru JG, Bautch VL, 2017. Blood vessel anastomosis is spatially regulated by Flt1 during angiogenesis. Dev. Camb. Engl 144, 889–896. 10.1242/dev.145672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Nicholas MN, Yeung J, 2017. Current Status and Future of Skin Substitutes for Chronic Wound Healing. J. Cutan. Med. Surg 21, 23–30. 10.1177/1203475416664037 [DOI] [PubMed] [Google Scholar]
- 211.Ning L, Chen X, 2017. A brief review of extrusion-based tissue scaffold bio-printing. Biotechnol. J 12 10.1002/biot.201600671 [DOI] [PubMed] [Google Scholar]
- 212.Oberringer M, Bubel M, Jennewein M, Guthörl S, Morsch T, Bachmann S, Metzger W, Pohlemann T, 2018. The role of adipose-derived stem cells in a selforganizing 3D model with regard to human soft tissue healing. Mol. Cell. Biochem 445, 195–210. 10.1007/s11010-017-3265-9 [DOI] [PubMed] [Google Scholar]
- 213.Oftadeh R, Perez-Viloria M, Villa-Camacho JC, Vaziri A, Nazarian A, 2015. Biomechanics and Mechanobiology of Trabecular Bone: A Review. J. Biomech. Eng 137, 0108021–01080215. 10.1115/1.4029176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Ogunrinade O, Kameya GT, Truskey GA, 2002. Effect of fluid shear stress on the permeability of the arterial endothelium. Ann. Biomed. Eng 30, 430–446. [DOI] [PubMed] [Google Scholar]
- 215.Oksman D, de Almeida OM, de Arruda RG, de Almeida MLM, do Carmo FS, 2018. Comparative study between fasciocutaneous and myocutaneous flaps in the surgical treatment of pressure ulcers of the sacral region. JPRAS Open 16, 50–60. 10.1016/j.jpra.2017.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Olson JL, Atala A, Yoo JJ, 2011. Tissue Engineering: Current Strategies and Future Directions. Chonnam Med. J 47, 1–13. 10.4068/cmj.2011.47.1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.O’Meara S, Cullum NA, Nelson EA, 2009. Compression for venous leg ulcers. Cochrane Database Syst. Rev CD000265. 10.1002/14651858.CD000265.pub2 [DOI] [PubMed]
- 218.Ontario, Ministry of Health and Long-Term Care, Medical Advisory Secretariat, 2008. Behavioural interventions for urinary incontinence in community-dwelling seniors: an evidence-based analysis. Medical Advisory Secretariat, Toronto. [PMC free article] [PubMed] [Google Scholar]
- 219.Orlidge A, D’Amore PA, 1987. Inhibition of capillary endothelial cell growth by pericytes and smooth muscle cells. J. Cell Biol 105, 1455–1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Osterhoff G, Morgan EF, Shefelbine SJ, Karim L, McNamara LM, Augat P, 2016. Bone mechanical properties and changes with osteoporosis. Injury 47, S11–S20. 10.1016/S0020-1383(16)47003-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Ott SM, 2018. Cortical or Trabecular Bone: What’s the Difference? Am. J. Nephrol 47, 373–375. 10.1159/000489672 [DOI] [PubMed] [Google Scholar]
- 222.Park JW, Hwang SR, Yoon I-S, 2017. Advanced Growth Factor Delivery Systems in Wound Management and Skin Regeneration. Mol. Basel Switz 22 10.3390/molecules22081259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Pati F, Jang J, Lee JW, Cho D-W, 2015. Chapter 7 - Extrusion Bioprinting, in: Atala A, Yoo JJ (Eds.), Essentials of 3D Biofabrication and Translation Academic Press, Boston, pp. 123–152. 10.1016/B978-0-12-800972-7.00007-4 [DOI] [Google Scholar]
- 224.Pattanaik S, Arbra C, Bainbridge H, Dennis SG, Fann SA, Yost MJ, 2019. Vascular Tissue Engineering Using Scaffold-Free Prevascular Endothelial–Fibroblast Constructs. BioResearch Open Access 8, 1–15. 10.1089/biores.2018.0039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Pedersen TO, Blois AL, Xue Y, Xing Z, Sun Y, Finne-Wistrand A, Lorens JB, Fristad I, Leknes KN, Mustafa K, 2014. Mesenchymal stem cells induce endothelial cell quiescence and promote capillary formation. Stem Cell Res. Ther 5, 23 10.1186/scrt412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Piaggesi A, Låuchli S, Bassetto F, Biedermann T, Marques A, Najafi B, Palla I, Scarpa C, Seimetz D, Triulzi I, Turchetti G, Vaggelas A, 2018. Advanced therapies in wound management: cell and tissue based therapies, physical and bio-physical therapies smart and IT based technologies. J. Wound Care 27, S1–S137. 10.12968/jowc.2018.27.Sup6a.S1 [DOI] [PubMed] [Google Scholar]
- 227.Pilsl U, Anderhuber F, Rzany B, 2012. Anatomy of the Cheek: Implications for Soft Tissue Augmentation: Dermatol. Surg 38, 1254–1262. 10.1111/j.1524-4725.2012.02382.x [DOI] [PubMed] [Google Scholar]
- 228.Pinel-Giroux FM, El Khoury MM, Trop I, Bernier C, David J, Lalonde L, 2013. Breast Reconstruction: Review of Surgical Methods and Spectrum of Imaging Findings. RadioGraphics 33, 435–453. 10.1148/rg.332125108 [DOI] [PubMed] [Google Scholar]
- 229.Pourchet LJ, Thepot A, Albouy M, Courtial EJ, Boher A, Blum LJ, Marquette CA, 2017. Human Skin 3D Bioprinting Using Scaffold-Free Approach. Adv. Healthc. Mater 6 10.1002/adhm.201601101 [DOI] [PubMed] [Google Scholar]
- 230.Pria Bankoff AD, 2012. Biomechanical Characteristics of the Bone, in: Goswami T (Ed.), Human Musculoskeletal Biomechanics. InTech; 10.5772/19690 [DOI] [Google Scholar]
- 231.Radisic M, Malda J, Epping E, Geng W, Langer R, Vunjak-Novakovic G, 2006. Oxygen gradients correlate with cell density and cell viability in engineered cardiac tissue. Biotechnol. Bioeng 93, 332–343. 10.1002/bit.20722 [DOI] [PubMed] [Google Scholar]
- 232.Ramasastry SS, 2005. Acute wounds. Clin. Plast. Surg 32, 195–208. 10.1016/j.cps.2004.12.001 [DOI] [PubMed] [Google Scholar]
- 233.Redd MA, Zeinstra N, Qin W, Wei W, Martinson A, Wang Y, Wang RK, Murry CE, Zheng Y, 2019. Patterned human microvascular grafts enable rapid vascularization and increase perfusion in infarcted rat hearts. Nat. Commun 10 10.1038/s41467-019-08388-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Reneman RS, Arts T, Hoeks APG, 2006. Wall shear stress--an important determinant of endothelial cell function and structure--in the arterial system in vivo. Discrepancies with theory. J. Vasc. Res 43, 251–269. 10.1159/000091648 [DOI] [PubMed] [Google Scholar]
- 235.Richards D, Jia J, Yost M, Markwald R, Mei Y, 2017. 3D Bioprinting for Vascularized Tissue Fabrication. Ann. Biomed. Eng 45, 132–147. 10.1007/s10439-016-1653-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Rigotti G, Marchi A, Galiè M, Baroni G, Benati D, Krampera M, Pasini A, Sbarbati A, 2007. Clinical treatment of radiotherapy tissue damage by lipoaspirate transplant: a healing process mediated by adipose-derived adult stem cells. Plast. Reconstr. Surg 119, 1409–1422; discussion 1423–1424. 10.1097/01.prs.0000256047.47909.71 [DOI] [PubMed] [Google Scholar]
- 237.Ring A, Kirchhoff P, Goertz O, Behr B, Daigeler A, Lehnhardt M, Harati K, 2016. Reconstruction of Soft-Tissue Defects at the Foot and Ankle after Oncological Resection. Front. Surg 3 10.3389/fsurg.2016.00015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Rognoni E, Watt FM, 2018. Skin Cell Heterogeneity in Development, Wound Healing, and Cancer. Trends Cell Biol 28, 709–722. 10.1016/j.tcb.2018.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Saint-Cyr M, Schaverien MV, Rohrich RJ, 2009. Perforator flaps: history, controversies, physiology, anatomy, and use in reconstruction. Plast. Reconstr. Surg 123, 132e–145e. 10.1097/PRS.0b013e31819f2c6a [DOI] [PubMed] [Google Scholar]
- 240.Sakamoto N, Ohashi T, Sato M, 2006. Effect of fluid shear stress on migration of vascular smooth muscle cells in cocultured model. Ann. Biomed. Eng 34, 408–415. 10.1007/s10439-005-9043-y [DOI] [PubMed] [Google Scholar]
- 241.Samuel R, Daheron L, Liao S, Vardam T, Kamoun WS, Batista A, Buecker C, Schäfer R, Han X, Au P, Scadden DT, Duda DG, Fukumura D, Jain RK, 2013. Generation of functionally competent and durable engineered blood vessels from human induced pluripotent stem cells. Proc. Natl. Acad. Sci. U. S. A 110, 12774–12779. 10.1073/pnas.1310675110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Sato T, Yana Y, Ichioka S, 2017. Free flap reconstruction for diabetic foot limb salvage. J. Plast. Surg. Hand Surg 51, 399–404. 10.1080/2000656X.2017.1285782 [DOI] [PubMed] [Google Scholar]
- 243.Schleip R, Gabbiani G, Wilke J, Naylor I, Hinz B, Zorn A, Jäger H, Breul R, Schreiner S, Klingler W, 2019. Fascia Is Able to Actively Contract and May Thereby Influence Musculoskeletal Dynamics: A Histochemical and Mechanographic Investigation. Front. Physiol 10, 336 10.3389/fphys.2019.00336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Sekine H, Shimizu T, Sakaguchi K, Dobashi I, Wada M, Yamato M, Kobayashi E, Umezu M, Okano T, 2013. In vitro fabrication of functional three-dimensional tissues with perfusable blood vessels. Nat. Commun 4, 1399 10.1038/ncomms2406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Sen CK, Gordillo GM, Roy S, Kirsner R, Lambert L, Hunt TK, Gottrup F, Gurtner GC, Longaker MT, 2009. Human Skin Wounds: A Major and Snowballing Threat to Public Health and the Economy. Wound Repair Regen. Off. Publ. Wound Heal. Soc. Eur. Tissue Repair Soc 17, 763–771. 10.1111/j.1524-475X.2009.00543.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Shiwei B, Mingyong Y, 2007. Cross-leg Pedicled Free Scapular Flap for the Repair of Extensive Soft Tissue Defect with Tibial Bone Exposure. Wounds Res 19, 46–50. [PubMed] [Google Scholar]
- 247.Shoham N, Gefen A, 2016. The Biomechanics of Fat: From Tissue to a Cell Scale, in: Kassab GS, Sacks MS (Eds.), Structure-Based Mechanics of Tissues and Organs Springer US, Boston, MA, pp. 79–92. 10.1007/978-1-4899-7630-7_5 [DOI] [Google Scholar]
- 248.Shudo Y, Cohen JE, Macarthur JW, Atluri P, Hsiao PF, Yang EC, Fairman AS, Trubelja A, Patel J, Miyagawa S, Sawa Y, Woo YJ, 2013. Spatially oriented, temporally sequential smooth muscle cell-endothelial progenitor cell bi-level cell sheet neovascularizes ischemic myocardium. Circulation 128, S59–68. 10.1161/CIRCULATIONAHA.112.000293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Sicari BM, Dearth CL, Badylak SF, 2014. Tissue engineering and regenerative medicine approaches to enhance the functional response to skeletal muscle injury. Anat. Rec. Hoboken NJ 2007 297, 51–64. 10.1002/ar.22794 [DOI] [PubMed] [Google Scholar]
- 250.Simman R, 2009. Letter from the editor in chief. J. Am. Coll. Certif. Wound Spec 1, 104 10.1016/j.jcws.2009.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Simonavicius N, Ashenden M, van Weverwijk A, Lax S, Huso DL, Buckley CD, Huijbers IJ, Huijber IJ, Yarwood H, Isacke CM, 2012. Pericytes promote selective vessel regression to regulate vascular patterning. Blood 120, 1516–1527. 10.1182/blood-2011-01-332338 [DOI] [PubMed] [Google Scholar]
- 252.Sindhu S, 2018. An Overview on Diabetic Foot Ulcer (DFU): Mini Review. Diabetes Case Rep 03 10.4172/2572-5629.1000134 [DOI] [Google Scholar]
- 253.Skardal A, Mack D, Kapetanovic E, Atala A, Jackson JD, Yoo J, Soker S, 2012. Bioprinted amniotic fluid-derived stem cells accelerate healing of large skin wounds. Stem Cells Transl. Med 1, 792–802. 10.5966/sctm.2012-0088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Smith AO, Bowers SLK, Stratman AN, Davis GE, 2013. Hematopoietic Stem Cell Cytokines and Fibroblast Growth factor-2 Stimulate Human Endothelial Cell-Pericyte Tube Co-Assembly in 3D Fibrin Matrices under Serum-Free Defined Conditions. PLOS ONE 8, e85147 10.1371/journal.pone.0085147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Song H-HG, Rumma RT, Ozaki CK, Edelman ER, Chen CS, 2018. Vascular Tissue Engineering: Progress, Challenges, and Clinical Promise. Cell Stem Cell 22, 340–354. 10.1016/j.stem.2018.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Sooppan R, Paulsen SJ, Han J, Ta AH, Dinh P, Gaffey AC, Venkataraman C, Trubelja A, Hung G, Miller JS, Atluri P, 2016. In Vivo Anastomosis and Perfusion of a Three-Dimensionally-Printed Construct Containing Microchannel Networks. Tissue Eng. Part C Methods 22, 1–7. 10.1089/ten.TEC.2015.0239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Sorg H, Tilkorn DJ, Hager S, Hauser J, Mirastschijski U, 2017. Skin Wound Healing: An Update on the Current Knowledge and Concepts. Eur. Surg. Res. Eur. Chir. Forsch. Rech. Chir. Eur 58, 81–94. 10.1159/000454919 [DOI] [PubMed] [Google Scholar]
- 258.Stecco C, Macchi V, Porzionato A, Duparc F, De Caro R, 2011. The fascia: the forgotten structure. Ital. J. Anat. Embryol. Arch. Ital. Anat. Ed Embriologia 116, 127–138. [PubMed] [Google Scholar]
- 259.Stecco C, Tiengo C, Stecco A, Porzionato A, Macchi V, Stern R, De Caro R, 2013a. Fascia redefined: anatomical features and technical relevance in fascial flap surgery. Surg. Radiol. Anat. SRA 35, 369–376. 10.1007/s00276-012-1058-0 [DOI] [PubMed] [Google Scholar]
- 260.Stecco C, Tiengo C, Stecco A, Porzionato A, Macchi V, Stern R, De Caro R, 2013b. Fascia redefined: anatomical features and technical relevance in fascial flap surgery. Surg. Radiol. Anat 35, 369–376. 10.1007/s00276-012-1058-0 [DOI] [PubMed] [Google Scholar]
- 261.Stevens KR, Scull MA, Ramanan V, Fortin CL, Chaturvedi RR, Knouse KA, Xiao JW, Fung C, Mirabella T, Chen AX, McCue MG, Yang MT, Fleming HE, Chung K, de Jong YP, Chen CS, Rice CM, Bhatia SN, 2017. In situ expansion of engineered human liver tissue in a mouse model of chronic liver disease. Sci. Transl. Med 9, eaah5505 10.1126/scitranslmed.aah5505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Stratman AN, Malotte KM, Mahan RD, Davis MJ, Davis GE, 2009. Pericyte recruitment during vasculogenic tube assembly stimulates endothelial basement membrane matrix formation. Blood 114, 5091–5101. 10.1182/blood-2009-05-222364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Sueyama Y, Kaneko T, Ito T, Kaneko R, Okiji T, 2017. Implantation of Endothelial Cells with Mesenchymal Stem Cells Accelerates Dental Pulp Tissue Regeneration/Healing in Pulpotomized Rat Molars. J. Endod 43, 943–948. 10.1016/j.joen.2017.01.035 [DOI] [PubMed] [Google Scholar]
- 264.Sugden WW, Meissner R, Aegerter-Wilmsen T, Tsaryk R, Leonard EV, Bussmann J, Hamm MJ, Herzog W, Jin Y, Jakobsson L, Denz C, Siekmann AF, 2017. Endoglin controls blood vessel diameter through endothelial cell shape changes in response to haemodynamic cues. Nat. Cell Biol 19, 653–665. 10.1038/ncb3528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Suri C, Jones PF, Patan S, Bartunkova S, Maisonpierre PC, Davis S, Sato TN, Yancopoulos GD, 1996. Requisite Role of Angiopoietin-1, a Ligand for the TIE2 Receptor, during Embryonic Angiogenesis. Cell 87, 1171–1180. 10.1016/S0092-8674(00)81813-9 [DOI] [PubMed] [Google Scholar]
- 266.Tang D, Zhang J, Yan T, Wei J, Jiang X, Zhang D, Zhang Q, Jia J, Huang Y, 2018. FG-4592 Accelerates Cutaneous Wound Healing by Epidermal Stem Cell Activation via HIF-1α Stabilization. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol 46, 2460–2470. 10.1159/000489652 [DOI] [PubMed] [Google Scholar]
- 267.Tattersall IW, Du J, Cong Z, Cho BS, Klein AM, Dieck CL, Chaudhri RA, Cuervo H, Herts JH, Kitajewski J, 2016. In vitro modeling of endothelial interaction with macrophages and pericytes demonstrates Notch signaling function in the vascular microenvironment. Angiogenesis 19, 201–215. 10.1007/s10456-016-9501-1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Teichert M, Milde L, Holm A, Stanicek L, Gengenbacher N, Savant S, Ruckdeschel T, Hasanov Z, Srivastava K, Hu J, Hertel S, Bartol A, Schlereth K, Augustin HG, 2017. Pericyte-expressed Tie2 controls angiogenesis and vessel maturation. Nat. Commun 8, 16106 10.1038/ncomms16106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Thodeti CK, Matthews B, Ravi A, Mammoto A, Ghosh K, Bracha AL, Ingber DE, 2009. TRPV4 channels mediate cyclic strain-induced endothelial cell reorientation through integrin-to-integrin signaling. Circ. Res 104, 1123–1130. 10.1161/CIRCRESAHA.108.192930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Thorpe CT, Screen HRC, 2016. Tendon Structure and Composition, in: Ackermann PW, Hart DA (Eds.), Metabolic Influences on Risk for Tendon Disorders, Advances in Experimental Medicine and Biology Springer International Publishing, Cham, pp. 3–10. 10.1007/978-3-319-33943-6_1 [DOI] [PubMed] [Google Scholar]
- 271.Ting LH, Chiel HJ, 2017. Muscle, Biomechanics, and Implications for Neural Control, in: Hooper SL, Büschges A (Eds.), Neurobiology of Motor Control John Wiley & Sons, Inc, Hoboken, NJ, USA, pp. 365–416. 10.1002/9781118873397.ch12 [DOI] [Google Scholar]
- 272.Toumi H, Higashiyama I, Suzuki D, Kumai T, Bydder G, McGonagle D, Emery P, Fairclough J, Benjamin M, 2006. Regional variations in human patellar trabecular architecture and the structure of the proximal patellar tendon enthesis. J. Anat 208, 47–57. 10.1111/j.1469-7580.2006.00501.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Townsend PR, Miegel RE, Rose RM, 1976. Structure and function of the human patella: the role of cancellous bone. J. Biomed. Mater. Res 10, 605–611. 10.1002/jbm.820100417 [DOI] [PubMed] [Google Scholar]
- 274.Trayhurn P, Beattie JH, 2001. Physiological role of adipose tissue: white adipose tissue as an endocrine and secretory organ. Proc. Nutr. Soc 60, 329–339. [DOI] [PubMed] [Google Scholar]
- 275.Tsai C-C, Chen Y-J, Yew T-L, Chen L-L, Wang J-Y, Chiu C-H, Hung S-C, 2011. Hypoxia inhibits senescence and maintains mesenchymal stem cell properties through down-regulation of E2A-p21 by HIF-TWIST. Blood 117, 459–469. 10.1182/blood-2010-05-287508 [DOI] [PubMed] [Google Scholar]
- 276.Tschoi M, Hoy EA, Granick MS, 2005. Skin Flaps. Clin. Plast. Surg 32, 261–273. 10.1016/j.cps.2004.11.005 [DOI] [PubMed] [Google Scholar]
- 277.Tucker WD, Mahajan K, 2018. Anatomy, Blood Vessels, in: StatPearls. StatPearls Publishing, Treasure Island (FL). [PubMed] [Google Scholar]
- 278.Twardowski RL, Black LD, 2014. Cardiac fibroblasts support endothelial cell proliferation and sprout formation but not the development of multicellular sprouts in a fibrin gel co-culture model. Ann. Biomed. Eng 42, 1074–1084. 10.1007/s10439-014-0971-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Ucuzian AA, Bufalino DV, Pang Y, Greisler HP, 2013. Angiogenic endothelial cell invasion into fibrin is stimulated by proliferating smooth muscle cells. Microvasc. Res 90, 40–47. 10.1016/j.mvr.2013.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Ucuzian AA, Gassman AA, East AT, Greisler HP, 2010. Molecular mediators of angiogenesis. J. Burn Care Res. Off. Publ. Am. Burn Assoc 31, 158–175. 10.1097/BCR.0b013e3181c7ed82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Ueda A, Koga M, Ikeda M, Kudo S, Tanishita K, 2004. Effect of shear stress on microvessel network formation of endothelial cells with in vitro three-dimensional model. Am. J. Physiol.-Heart Circ. Physiol 287, H994–H1002. 10.1152/ajpheart.00400.2003 [DOI] [PubMed] [Google Scholar]
- 282.van Kogelenberg S, Yue Z, Dinoro JN, Baker CS, Wallace GG, 2018. ThreeDimensional Printing and Cell Therapy for Wound Repair. Adv. Wound Care 7, 145–155. 10.1089/wound.2017.0752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Velazquez OC, Snyder R, Liu Z-J, Fairman RM, Herlyn M, 2002. Fibroblastdependent differentiation of human microvascular endothelial cells into capillary-like 3dimensional networks. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol 16, 1316–1318. 10.1096/fj.01-1011fje [DOI] [PubMed] [Google Scholar]
- 284.Verschueren JHM, Post MWM, de Groot S, van der Woude LHV, van Asbeck FWA, Rol M, 2011. Occurrence and predictors of pressure ulcers during primary inpatient spinal cord injury rehabilitation. Spinal Cord 49, 106–112. 10.1038/sc.2010.66 [DOI] [PubMed] [Google Scholar]
- 285.Vijayavenkataraman S, Yan W-C, Lu WF, Wang C-H, Fuh JYH, 2018. 3D bioprinting of tissues and organs for regenerative medicine. Adv. Drug Deliv. Rev., 3DBioprinting and Micro-/Nano-Technology: Emerging Technologies in Biomedical Sciences 132, 296–332. 10.1016/j.addr.2018.07.004 [DOI] [PubMed] [Google Scholar]
- 286.Von Offenberg Sweeney N, Cummins PM, Cotter EJ, Fitzpatrick PA, Birney YA, Redmond EM, Cahill PA, 2005. Cyclic strain-mediated regulation of vascular endothelial cell migration and tube formation. Biochem. Biophys. Res. Commun 329, 573–582. 10.1016/j.bbrc.2005.02.013 [DOI] [PubMed] [Google Scholar]
- 287.Wang H, Huang H, Ding S-F, 2018. Sphingosine-1-phosphate promotes the proliferation and attenuates apoptosis of Endothelial progenitor cells via S1PR1/S1PR3/PI3K/Akt pathway. Cell Biol. Int 42, 1492–1502. 10.1002/cbin.10991 [DOI] [PubMed] [Google Scholar]
- 288.Wang H, Huang L, Qu MJ, Yan ZQ, Liu B, Shen BR, Jiang ZL, 2006. Shear stress protects against endothelial regulation of vascular smooth muscle cell migration in a coculture system. Endothel. J. Endothel. Cell Res 13, 171–180. [DOI] [PubMed] [Google Scholar]
- 289.Wang X, Sun Q, Pei J, 2018. Microfluidic-Based 3D Engineered Microvascular Networks and Their Applications in Vascularized Microtumor Models. Micromachines 9 10.3390/mi9100493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Waniczek D, Kozowicz A, Muc-Wierzgoń M, Kokot T, Świętochowska E, Nowakowska-Zajdel E, 2013. Adjunct Methods of the Standard Diabetic Foot Ulceration Therapy. Evid.-Based Complement. Altern. Med. ECAM 2013 10.1155/2013/243568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Waters JP, Kluger MS, Graham M, Chang WG, Bradley JR, Pober JS, 2013. In vitro self-assembly of human pericyte-supported endothelial microvessels in threedimensional coculture: a simple model for interrogating endothelial-pericyte interactions. J. Vasc. Res 50, 324–331. 10.1159/000353303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Watt SM, Gullo F, van der Garde M, Markeson D, Camicia R, Khoo CP, Zwaginga JJ, 2013. The angiogenic properties of mesenchymal stem/stromal cells and their therapeutic potential. Br. Med. Bull 108, 25–53. 10.1093/bmb/ldt031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Werchek S, 2010. Diagnosis and treatment of venous leg ulcers. Nurse Pract 35, 46 10.1097/01.NPR.0000390438.98004.be [DOI] [PubMed] [Google Scholar]
- 294.Williams PA, Campbell KT, Silva EA, 2018. Alginate hydrogels of varied molecular weight distribution enable sustained release of sphingosine-1-phosphate and promote angiogenesis. J. Biomed. Mater. Res. A 106, 138–146. 10.1002/jbm.a.36217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Wilson CJ, Kasper G, Schütz MA, Duda GN, 2009. Cyclic strain disrupts endothelial network formation on Matrigel. Microvasc. Res 78, 358–363. 10.1016/j.mvr.2009.08.002 [DOI] [PubMed] [Google Scholar]
- 296.Wobma HM, Tamargo MA, Goeta S, Brown LM, Duran-Struuck R, Vunjak-Novakovic G, 2018. The influence of hypoxia and IFN-γ on the proteome and metabolome of therapeutic mesenchymal stem cells. Biomaterials 167, 226–234. 10.1016/j.biomaterials.2018.03.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Wu J, Wu J, Gong X, Ding Z, Lin B, Chen Z, Guo Z, 2016. Repairing Pretibial and Foot Soft Tissue Defects With Reverse Transplantation of the Medial Crural Fasciocutaneous Flap. Int. J. Low. Extrem. Wounds 15, 34–40. 10.1177/1534734615597864 [DOI] [PubMed] [Google Scholar]
- 298.Wu SC, Driver VR, Wrobel JS, Armstrong DG, 2007. Foot ulcers in the diabetic patient, prevention and treatment. Vasc. Health Risk Manag 3, 65–76. [PMC free article] [PubMed] [Google Scholar]
- 299.Wu W, DeConinck A, Lewis JA, 2011. Omnidirectional printing of 3D microvascular networks. Adv. Mater. Deerfield Beach Fla 23, H178–183. 10.1002/adma.201004625 [DOI] [PubMed] [Google Scholar]
- 300.Xie Q, Xie J, Zhong J, Cun X, Lin S, Lin Y, Cai X, 2016. Hypoxia enhances angiogenesis in an adipose-derived stromal cell/endothelial cell co-culture 3D gel model. Cell Prolif 49, 236–245. 10.1111/cpr.12244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Xu Yachen, Peng J, Dong X, Xu Yuhong, Li H, Chang J, 2017. Combined chemical and structural signals of biomaterials synergistically activate cell-cell communications for improving tissue regeneration. Acta Biomater 55, 249–261. 10.1016/j.actbio.2017.03.056 [DOI] [PubMed] [Google Scholar]
- 302.Xue Y, Xing Z, Bolstad AI, Van Dyke TE, Mustafa K, 2013. Co-culture of human bone marrow stromal cells with endothelial cells alters gene expression profiles. Int. J. Artif. Organs 36, 650–662. 10.5301/ijao.5000229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Yan W-C, Davoodi P, Vijayavenkataraman S, Tian Y, Ng WC, Fuh JYH, Robinson KS, Wang C-H, 2018. 3D bioprinting of skin tissue: From pre-processing to final product evaluation. Adv. Drug Deliv. Rev., 3D-Bioprinting and Micro-/Nano-Technology: Emerging Technologies in Biomedical Sciences 132, 270–295. 10.1016/j.addr.2018.07.016 [DOI] [PubMed] [Google Scholar]
- 304.Ye C, Bai L, Yan Z-Q, Wang Y-H, Jiang Z-L, 2008. Shear stress and vascular smooth muscle cells promote endothelial differentiation of endothelial progenitor cells via activation of Akt. Clin. Biomech. Bristol Avon 23 Suppl 1, S118–124. 10.1016/j.clinbiomech.2007.08.018 [DOI] [PubMed] [Google Scholar]
- 305.Yin S, Zhang W, Zhang Z, Jiang X, 2019. Recent Advances in Scaffold Design and Material for Vascularized Tissue-Engineered Bone Regeneration. Adv. Healthc. Mater 0, 1801433. 10.1002/adhm.201801433 [DOI] [PubMed] [Google Scholar]
- 306.Yung YC, Chae J, Buehler MJ, Hunter CP, Mooney DJ, 2009. Cyclic tensile strain triggers a sequence of autocrine and paracrine signaling to regulate angiogenic sprouting in human vascular cells. Proc. Natl. Acad. Sci. U. S. A 106, 15279–15284. 10.1073/pnas.0905891106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Zhan F, Fan Y, Deng X, Xu Z, 2010. The beneficial effect of swirling flow on platelet adhesion to the surface of a sudden tubular expansion tube: its potential application in endto-end arterial anastomosis. ASAIO J. Am. Soc. Artif. Intern. Organs 1992 56, 172–179. 10.1097/MAT.0b013e3181d0ea15 [DOI] [PubMed] [Google Scholar]
- 308.Zhang B, Montgomery M, Chamberlain MD, Ogawa S, Korolj A, Pahnke A, Wells LA, Massé S, Kim J, Reis L, Momen A, Nunes SS, Wheeler AR, Nanthakumar K, Keller G, Sefton MV, Radisic M, 2016. Biodegradable scaffold with built-in vasculature for organ-on-a-chip engineering and direct surgical anastomosis. Nat. Mater 15, 669–678. 10.1038/nmat4570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Zhang L, Xing Q, Qian Z, Tahtinen M, Zhang Z, Shearier E, Qi S, Zhao F, 2016. Hypoxia Created Human Mesenchymal Stem Cell Sheet for Prevascularized 3D Tissue Construction. Adv. Healthc. Mater 5, 342–352. 10.1002/adhm.201500744 [DOI] [PubMed] [Google Scholar]
- 310.Zhang S, Wang H, 2019. Current Progress in 3D Bioprinting of Tissue Analogs. SLAS Technol. Transl. Life Sci. Innov 24, 70–78. 10.1177/2472630318799971 [DOI] [PubMed] [Google Scholar]
- 311.Zhang Y, Xia X, Yan J, Yan L, Lu C, Zhu X, Wang T, Yin T, Li R, Chang HM, Qiao J, 2017. Mesenchymal stem cell-derived angiogenin promotes primodial follicle survival and angiogenesis in transplanted human ovarian tissue. Reprod. Biol. Endocrinol. RBE 15, 18 10.1186/s12958-017-0235-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Zhao D, Xue C, Lin S, Shi S, Li Q, Liu M, Cai X, Lin Y, 2017. Notch Signaling Pathway Regulates Angiogenesis via Endothelial Cell in 3D Co-Culture Model. J. Cell. Physiol 232, 1548–1558. 10.1002/jcp.25681 [DOI] [PubMed] [Google Scholar]
- 313.Zonneville J, Safina A, Truskinovsky AM, Arteaga CL, Bakin AV, 2018. TGF-β signaling promotes tumor vasculature by enhancing the pericyte-endothelium association. BMC Cancer 18 10.1186/s12885-018-4587-z [DOI] [PMC free article] [PubMed] [Google Scholar]