Abstract
Although a good deal is known about the genetics and pathophysiology of Parkinson’s disease (PD), and information is emerging about its cause, there are no pharmacological treatments shown to have a significant, sustained capacity to prevent or attenuate the ongoing neurodegenerative processes. However, there is accumulating clinical results to suggest that physical exercise is such a treatment, and studies of animal models of the dopamine (DA) deficiency associated with the motor symptoms of PD further support this hypothesis. Exercise is a non-pharmacological, economically practical, and sustainable intervention with little or no risk and with significant additional health benefits. In this study, we investigated the long-term effects of voluntary exercise on motor behavior and brain biochemistry in the transgenic MitoPark mouse PD model with progressive degeneration of the DA systems caused by DAT- driven deletion of the mitochondrial transcription factor TFAM in DA neurons. We found that voluntary exercise markedly improved behavioral function, including overall motor activity, narrow beam walking, and rotarod performance. There was also improvement of biochemical markers of nigrostriatal DA input. This was manifested by increased levels of DA measured by HPLC, and of the DA membrane transporter measured by PET. Moreover, exercise increased oxygen consumption and, by inference, ATP production via oxidative phosphorylation. Thus, exercise augmented aerobic mitochondrial oxidative metabolism vs glycolysis in the nigrostriatal system. We conclude that there are clear-cut physiological mechanisms for beneficial effects of exercise in PD.
Keywords: Parkinson’s Disease, Exercis, PET, Mitochondria, Dopamine
Introduction
Parkinson’s disease (PD) is a progressive neurodegenerative condition in the central nervous system, characterized by dopamine (DA) neuron loss in the nigrostriatal system and other neurodegenerative processes that may differ between different types of PD. Clinical symptoms include resting tremor, rigidity, akinesia, and disturbances of postural reflexes, and affect some 10 million people worldwide (Sethi, 2002).
Exercise is currently often used as an important part of the treatment for PD (Lauze et al., 2016; Shen et al., 2016). Although both motor and non-motor symptoms may affect PD patients’ ability to participate in, and/or impact the outcomes of exercise, most PD patients can respond to exercise interventions similarly to subjects of matching age who do not suffer from PD. Increasing evidence also indicates that exercise improves specific PD motor symptoms and reduces DA neuron loss in PD animal models (Shi et al., 2017; Tillerson et al., 2001). This may involve several mechanisms, including angiogenesis (Pereira et al., 2007; Pianta et al, 2019), increased mitochondrial function, neurogenesis (Watson et al, 2015; Yasuhara et al, 2007) and enhanced neuronal plasticity (Cho et al., 2013; Svensson et al., 2015). In addition, neurotrophic factors that play a crucial role in changes in brain plasticity and neurogenesis are induced by exercise (McAllister, 1999; Zigmond et al., 2012; Zigmond and Smeyne, 2014).
Here we use the MitoPark mouse PD model in which DA neurons are targeted for respiratory chain dysfunction. This is accomplished by dopamine transporter (DAT) promoter-driven removal of the crucial mitochondrial transcription factor TFAM (Ekstrand et al., 2007). Such mice develop progressive Parkinsonian symptoms over several months and exhibit neuropathology similar to idiopathic PD. Intraneuronal inclusions develop sequentially within substantia nigra pars compacta (SNc) and ventral tegmental area (VTA) DA neurons, followed by progressive degeneration of the mesencephalic DA projections to the forebrain with subsequent loss of DA in the striatum (Ekstrand et al 2007). As in PD, DA neurons in SNc in this mouse model degenerate before, and more completely than, those in VTA, even though both neuronal types are targeted for mitochondrial disruption. As in PD, L-DOPA medications in the mouse model have profound normalizing effects on phenotypes dependent on DA neurotransmission at 20-weeks and even at 30 weeks, albeit lesser in the latter “post- honeymoon” phase of L-DOPA treatment. Furthermore, even the “wearing off” of L-DOPA is apparent in this slowly progressing model (Galter et al 2010, Shan et al 2015, Gellhaar et al 2015). Using the MitoPark mouse model we now describe the long-term effects of voluntary exercise on motor behavior and brain biochemistry.
Results
Behavioral Studies.
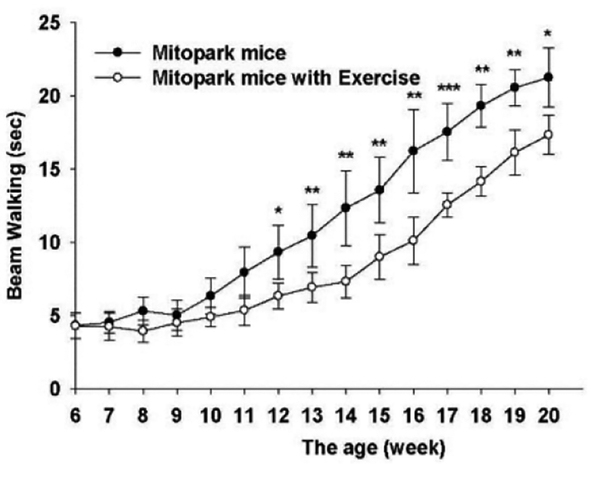
The behavioral studies detailed below are from two series of mice, studied in Sweden and in Taiwan, respectively (see Experimental Procedures). We have previously reported that by 10–12 weeks of age MitoPark animals already show a significant reduction in overall motor activity and rotarod performance (Ekstrand et al., 2007). Importantly, as shown in Fig. 1, given access to a running wheel, MitoPark animals actually run more than wild type mice during the first 10–11 weeks of life if the exercise is started early (5–6 weeks of age) (Series 1). Given the severe phenotype of these animals, however, there is soon a progressive reduction in running wheel activity (Fig. 2). Behavioral recordings were carried out weekly with mice from 6 to 20 weeks of age, after which animals were sacrificed for biochemical measurements. Behavioral measurements showed that exercise improved motor performance in MitoPark animals as evidenced by beam walking (Fig. 3) and rotarod performance (Fig. 4A-C). When normalized to wild type animals (normalized to 100%, data not shown), sedentary MitoPark mice performed significantly worse on the rotarod than wild type mice, whereas exercising MitoPark mice did not (Fig. 4D).
Fig. 1.
Wheel running in MitoPark mice and wild type littermates. After 12 weeks, and at later stages, MitoPark mice run progressively less than WT animals (see also Ekstrand et al 2007) p<0.05 weeks 6–9.
Fig. 2.
A. Exercising MitoPark mice were fed in the home cage which also contained the running wheel. B. Running wheel rotations per day of MitoPark mice (N = 7). C. A schematic graph of behavioral tests for MitoPark mice with and without access to running wheel exercise. We investigated DA neuron activity by PET scanning and behavior at the indicated times (7 MitoPark mice in each group)
Fig 3.
Effect of exercise on time to cross a beam. Stars indicate significances between exercised and non-exercised MitoPark mice. *** P < 0.001, ** P < 0.01, * P < 0.05.
Fig. 4.
Effects of exercise on motor coordination of MitoPark mice determined using the rotarod test. The latency (A), the rotational velocity (B) and the distance (C) of MitoPark mice compared with MitoPark-Exercise mice. *** P < 0.001, ** P < 0.01, * P < 0.05. (D) Rotarod data from MitoPark mice with and without exercise normalized to wild type animals *p<0.05 (7 MitoPark mice in each group).
PET study of DAT.
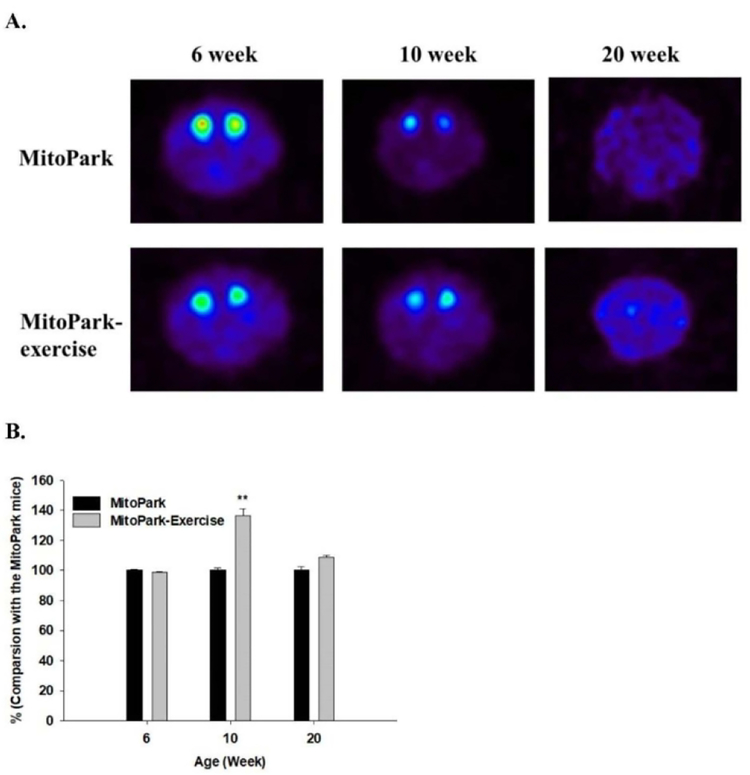
The behavioral data were complemented by PET imaging at weeks 6, 10, and 20 (Fig. 5). There was a transient but significant increase in striatal DAT binding at 10 weeks. Again, because of the severity of the phenotype, this was no longer apparent at 20 weeks.
Fig. 5.
Dopamine neuron activity determined by PET using a DAT ligand. A. Examples of the distribution of [18F]-FE-PE2 in MitoPark mice without and with exercise 6, 10, and 20 weeks after intravenous administration. B. Ligand binding in striatum in MitoPark mice with exercise compared with MitoPark mice without exercise. ** P < 0.01(5 MitoPark mice in each group)
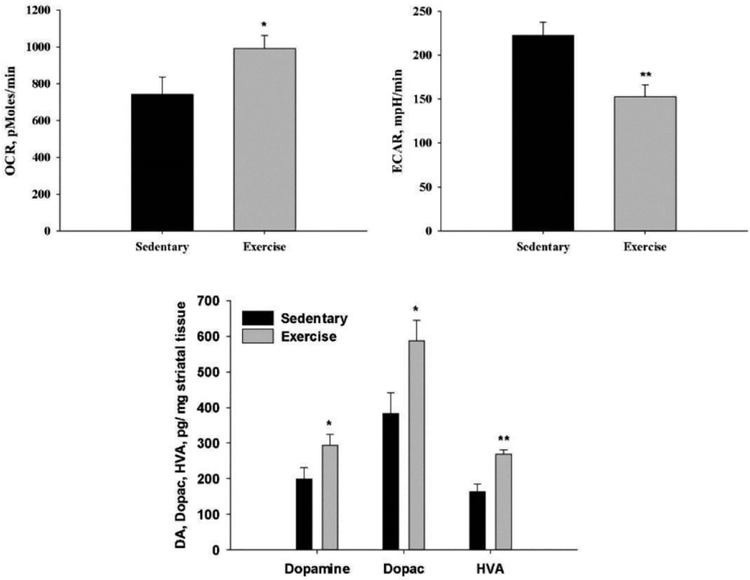
Postmortem respiratory assay. Biochemical observations after sacrifice of 20 weeks old animals support the effects of exercise in the MitoPark PD model. As shown in Fig 6 (top) there is more oxidative phosphorylation (OCR) for ATP generation and less use of glycolysis (ECAR) for ATP generation in exercised, compared to sedentary MitoPark mice.
Fig. 6.
Bioenergetics, DA and DA metabolites in striatum from sedentary and exercised MitoPark mice. Top: Seahorse assays (7 MitoPark mice in each group) of mitochondrial respiration rate (oxygen consumption rate, OCR) (left) and glycolytic rate (extracellular acidification rate, ECAR) (right). Bottom: Levels of Dopamine, Dopac and HVA from 5 MitoPark mice in each group. ** P < 0.01, * P < 0.05.
Levels of DA and its metabolites.
HPLC showed that there were small but significant increases in the levels of DA and its metabolites in the exercised compared to sedentary MitoPark mice (Fig. 6 bottom).
Discussion
Physical exercise is an economical, practical, and relatively safe approach to achieve neuroprotective and neurorestorative effects in PD. There are robust clinical data supporting positive effects of aerobic exercise in PD (Fang et al., 2018; Fiorelli et al., 2019; Oliveira de Carvalho et al., 2018; Park et al., 2014). Similarly, there are well-documented positive effects of aerobic exercise in genetic and toxin-induced PD models in rodents using both behavioral and cellular indices of nigrostriatal DA activity. Animal models show that exercise promotes mitochondrial efficiency, upregulates antioxidant mechanisms, reduces inflammation, triggers angiogenesis, and neurogenesis, increases neurotrophic factors and produces synaptogenesis (Choe et al., 2012; da Costa et al., 2017; Hsueh et al., 2018; Jang et al., 2018; Koo et al., 2017; Real et al., 2017; Tajiri et al., 2010; Wi et al., 2018; Zigmond et al., 2012).
Our present results are compatible with previous data showing that physical exercise constitutes an effective intervention also in other neurodegenerative diseases and attenuates disease progression (Ahlskog, 2011; Alonso-Frech et al., 2011; Sutoo and Akiyama, 2003). The mechanisms contributing to these phenomena may not only derive from peripheral effects of acute exercise, including increasing cardiac output and cerebral blood flow (Paillard et al., 2015), but may also derive directly from CNS effects on neurobiological mechanisms, including increases in angiogenesis (Pianta et al, 2019), neurogenesis, synaptogenesis, and neurotransmitter synthesis in cerebral areas involved in cognition and mobility in PD (Radak et al., 2010; Zigmond et al., 2012).
Neurogenesis specifically is enhanced by exercise as evidenced by increased cell proliferation in known brain neurogenic niches, which coincides with neuronal gene expression (Watson et al, 2015; Yasuhara et al, 2007). Such exercise-induced neurogenesis and behavioral function stands as a potent therapy for many brain disorders characterized by impaired neurogenesis in motor and cognitive abnormalities.
Physical exercise activates antioxidant enzymes and reduces chronic oxidative stress. Exercise also stimulates mitochondrial biogenesis, and there is up-regulation of mitophagy in PD patients (Monteiro-Junior et al., 2015). However, while exercise may lead to improved mitochondrial quality control through autophagy, as well as mitochondrial renewal through biogenesis that would be beneficial in prematurely ageing mice with pathologically enhanced mtDNA mutation rates (Safdar et al., 2011), exercise is less likely to exert similar positive effects long-term at the mitochondrial level in MitoPark mice, due to the lack of the mitochondrial transcription factor TFAM in the DA neurons. It follows that the demonstration of positive effects in our specific PD model, in which mitochondria in DA neurons cannot be rescued, may also be achieved by mechanisms other than direct support of the DA system.
Exercise also stimulates trophic factor synthesis (BDNF, GDNF, FGF-2, IGF-1, among others), which promotes neuroplasticity, decreases neural apoptosis (Monteiro-Junior et al., 2015), and alters dopaminergic neurotransmission (Petzinger et al., 2007; Tillerson et al., 2003). Exercise may exert neuroprotective effects or enhance neuronal survival by increasing neurotrophic factor availability (Gerecke et al., 2012; Gerecke et al., 2010; Hsueh et al., 2018; Tuon et al., 2012). Physical training also elevates intracellular defenses against ROS. This in turn increases the capacity of DA neurons to deliver transmitter (Tajiri et al., 2010; Zigmond et al., 2009).
Mitochondrial dysfunction and energy failure are implicated as the cause of death of DA neurons in PD (Dauer and Przedborski, 2003; Dawson and Dawson, 2003; Ellis et al., 2005; Mizuno et al., 1989; Shen and Cookson, 2004; Ved et al., 2005). Toxins used to model PD, such as MPTP and rotenone, impair respiratory chain function by inhibiting complex I (Betarbet et al., 2000; Langston et al., 1983; Mizuno et al., 1987; Sherer et al., 2003; Smeyne and Jackson-Lewis, 2005). In further support for a “mitochondrial hypothesis” for PD pathophysiology, Bender et al (2006) reported higher levels of mitochondrial DNA deletions in nigral neurons from PD patients. Moreover, both Bender et al (2006) and Kraytsberg et al (2006) reported higher levels of mitochondrial DNA deletions in nigral neurons in aged humans with sharp elevations starting shortly before age 70. This correlates with age being a known risk factor for PD.
Several genes (Parkin, Pink-1, DJ-1, LRRK2) implicated in PD are considered important for mitochondrial function (Shen and Cookson, 2004; Valente et al., 2004; West et al., 2005). To further study the role of such genes, transgenic mouse models are used to knockout normal alleles or overexpress mutant alleles. However, such models in mice tend to fail to recapitulate the full behavioral and pathological features of PD, and some models have produced conflicting or inconclusive data. Despite these shortcomings, studies of transgenic mice have identified mitochondrial dysfunction as possibly underlying the slow degeneration in PD.
Interpretation of results from more traditional experiments with neurotoxins are complicated by additional pharmacological effects in DA neurons, effects on non-DA cell types, or both. Targeting mitochondrial respiratory chain function as used in the current study leads to a strikingly PD-like phenotype, with respect to both behavior and progressive neuropathology. Because the MitoPark mouse model is purely genetic, the degenerative events are inevitable when there is no intervention such as exercise. However, the symptoms occur only after reaching adulthood, as in the vast majority of cases of PD, which provides a long window of opportunity for studies of pathophysiology and tests of treatment strategies. Because the genetic defect and its consequences are controlled from the outset, intervention can begin at any time, even presymptomatically. Those properties are becoming more relevant for clinical interventions as genetic risk factors are further unraveled.
Summary
In this study, we investigated the long-term effects of voluntary exercise on motor behavior and biochemistry in the MitoPark mouse PD model with progressive degeneration of the DA systems. We found that voluntary exercise markedly improved overall motor activity, narrow beam walking, and rotarod performance. There was also improvement of biochemical markers of nigrostriatal DA input. This was manifested by increased levels of DA measured by HPLC and of the DA membrane transporter measured by PET. Moreover, exercise increased oxygen consumption and, by inference, ATP production via oxidative phosphorylation. Thus, exercise augmented aerobic mitochondrial oxidative metabolism vs glycolysis in striatum. We conclude that exercise activates physiological mechanisms with beneficial effects in PD.
Experimental Procedures
Animals.
The breeding scheme for generating MitoPark mice has been described previously (Ekstrand et al., 2007; Galter et al., 2010; Good et al., 2011). Briefly, animals on a C57BL6 background, in which the DAT promoter was used to drive cre-recombinase expression, were crossed with mice in which the TFAM gene had been loxP-flanked. MitoPark mice used in these experiments were heterozygous for DAT-cre expression (DAT/DATcre) and homozygous for the loxP-flanked Tfam gene (TfamloxP/TfamloxP), ensuring complete removal of TFAM. Two sets of studies on exercise were carried out. In one series, MitoPark mice were compared with an equal number of wild type littermates with both groups housed individually in cages equipped with running wheels. These animals were subsequently studied on the rotarod to evaluate motor coordination and motor learning. In a second series, MitoPark mice were individually housed in cages with or without running wheels to compare exercising and sedentary animals both with slowly degenerating DA neurons. Behavioral, PET, and biochemical studies were carried out. For this second study, 14 MitoPark mice were randomly assigned to the exercise and non-exercise groups.
All mice were housed with water and standard food ad libitum at 25 °C in a 12 h/12 h light/dark cycle. The first series of animals was maintained and studied at the Department of Neuroscience, Karolinska Institute. All experimental procedures in this study were approved by the Northern Stockholm Animal Ethics Committee. The second series of animals was maintained and studied in the Laboratory Animal Center (LAC) of the National Defense Medical Center (NDMC) in Taipei. This animal study was approved by the Institutional Animal Care and Use Committees (IACUC) of the NDMC and Taipei Medical University (TMU). Animal protocol numbers were IACUC-15–270, 16–269 and 17–298.
Exercise.
Exercising MitoPark mice were housed in open acrylic plastic cages. Each cage contained one stainless steel, hollow running wheel (diameter, 12 cm). The rotation numbers were recorded by magnetic (Series 1) or infrared sensors (Series 2).
Beam walk.
This test was used to determine fine motor coordination and balance. Mice were trained for 3 days to walk along a narrow Plexiglas beam (100 cm long, 0.5 cm wide) towards a home cage located at one end of the beam. The mean time to walk across the beam was used as a measure of motor coordination. Three trials with 30 min intervals were done at each age that was tested.
Rotarod.
A rotating rod instrument (Rotarod, Ugo Basile, Washington D.C, US) was used to further validate motor coordination. The accelerating protocol started at a speed of 5 rpm and reached 40 rpm within 300 seconds. Three measures of performance were taken: 1) time to fall, 2) rotarod rotation velocity at falling, and 3) distance travelled on the rotarod before falling. Three trials were given at each age with a 15 minute interval between trials.
Catecholamine measurements.
Levels of DA, DOPAC (3, 4-dihydroxyphenylacetic acid) and HVA (homovanillic acid) in dorsolateral striatal brain samples were determined by treatment with 200 mM KCl followed by several rounds of physical disruption by trituration. The disrupted slices were then freeze-thawed, sonicated, and filtered at 0.2 μm. DA, DOPAC, and HVA contents were analyzed using an HPLC-EC system (ESA, Chelmsford, MA)(Yuan et al., 2002). On the HPLC chromatograms, DA, DOPAC, and HVA were separate peaks that were quantified by comparing their heights to those of DA, DOPAC, and HVA standard curves using linear regression analysis.
Mitochondrial Oxygen Consumption.
A respiratory assay (Seahorse XF Analyzer, Agilent) was used to measure oxygen consumption rate (OCR) and extracellular acidification rate (ECAR) according to previously published methods (Fried et al., 2014). Nigrostriatal tissue from MitoPark mice was dissected on ice after anesthesia with 3% isoflurane. The tissue was kept in ice-cold aCSF solution (124 mM NaCl, 2.5 mM KCl, 2.0 mM MgSO4, 1.25 mM KH2PO4, 10 mM glucose, 4 mM sucrose, and 2.5 mM CaCl2 adjusted to pH to 7.4). Before the respiratory assay, we dissected the tissue into approximately 1.5 × 1.5 × 0.5 mm3 pieces and placed them in the center of meshes. The mesh with tissue was gently loaded into a well filled with 37°C aCSF. A microplate with these wells was then incubated at 37°C for 30 minutes and aCSF was calibrated for temperature and pH equilibration. The assay protocol was executed following a continuous procedure of 3-min mix, 3-min wait, and 2-min measure sequences for 5 cycles. OCR and ECAR values were normalized to protein concentration.
PET Imaging.
We used the DAT radiotracer [18F]-FE-PE2I (Schou et al., 2009) (14.8–18.5 MBq; 0.4–0.5 mCi), to evaluate presence of DA neuron elements in the mice. The radiotracer was synthesized in the Positron Emission Tomography (PET) Center of the Department of Nuclear Medicine of Tri-Service General Hospital. We delivered [18F]-FE-PE2I to MitoPark mice by tail vein injections at 6, 10, and 20 weeks of age. The mice were anesthetized by passive inhalation of isoflurane/oxygen (5% isoflurane for induction and 2% for maintenance) (Abbott Laboratories Ltd., Maidenhead, UK). PET imaging (PET R4 scanner, Concorde MicroSystems, Knoxville, TN, USA) was performed for 30 minutes after [18F]-FE-PE2 injections. Images of striatum were analyzed using appropriate software (ASIPro VM 6.3.3.1 Concorde MicroSystems).
Statistical Analysis
Evaluations were undertaken in an observer blinded manner. Statistical analyses were performed with Student’s t-test and one-way or two-way ANOVA. Significance was inferred at p≤ 0.05 or less, and is noted within each Figure legend. Data are presented as mean ± SEM values throughout.
Highlights.
Voluntary exercise markedly improves behavioral function in the MitoPark mouse PD model with progressive degeneration of the DA systems.
Exercise increased levels of DA and the DA membrane transporter, measured by HPLC and PET, respectively.
Exercise increases oxygen consumption and, by inference, ATP production via oxidative phosphorylation.
Exercise augments aerobic mitochondrial oxidative metabolism over glycolysis in the nigrostriatal system.
Acknowledgement
This study was supported in part by (i) the Ministry of Science and Technology, Grant numbers MOST 101–2632-B-038 −001 -MY3, MOST 106-2314-B-038-029 and MOST 107-2314-B-038- 063; (ii) USPHS, NIH, NS094152; (iii) Swedish Research Council, Swedish Brain Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest and Author Disclosure Statement
LO is a co-owner of a company that owns commercial rights to the MitoPark mouse. The other authors have no conflict of interest.
References
- Ahlskog JE (2011). Does vigorous exercise have a neuroprotective effect in Parkinson disease? Neurology 77, 288–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso-Frech F, Sanahuja JJ, and Rodriguez AM (2011). Exercise and physical therapy in early management of Parkinson disease. Neurologist 17, S47–53. [DOI] [PubMed] [Google Scholar]
- Betarbet R, Sherer TB, MacKenzie G, Garcia-Osuna M, Panov AV, and Greenamyre JT (2000). Chronic systemic pesticide exposure reproduces features of Parkinson’s disease. Nat Neurosci 3, 1301–1306. [DOI] [PubMed] [Google Scholar]
- Cho HS, Shin MS, Song W, Jun TW, Lim BV, Kim YP, and Kim CJ (2013). Treadmill exercise alleviates short-term memory impairment in 6-hydroxydopamine-induced Parkinson’s rats. J Exerc Rehabil 9, 354–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe MA, Koo BS, An GJ, and Jeon S (2012). Effects of Treadmill Exercise on the Recovery of Dopaminergic Neuron Loss and Muscle Atrophy in the 6-OHDA Lesioned Parkinson’s Disease Rat Model. Korean J Physiol Pharmacol 16, 305–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Costa RO, Gadelha-Filho CVJ, da Costa AEM, Feitosa ML, de Araujo DP, de Lucena JD, de Aquino PEA, Lima FAV, Neves KRT, and de Barros Viana GS (2017). The Treadmill Exercise Protects against Dopaminergic Neuron Loss and Brain Oxidative Stress in Parkinsonian Rats. Oxid Med Cell Longev 2017, 2138169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauer W, and Przedborski S (2003). Parkinson’s disease: mechanisms and models. Neuron 39, 889–909. [DOI] [PubMed] [Google Scholar]
- Dawson TM, and Dawson VL (2003). Molecular pathways of neurodegeneration in Parkinson’s disease. Science 302, 819–822. [DOI] [PubMed] [Google Scholar]
- Ekstrand MI, Terzioglu M, Galter D, Zhu S, Hofstetter C, Lindqvist E, Thams S, Bergstrand A, Hansson FS, Trifunovic A, et al. (2007). Progressive parkinsonism in mice with respiratory-chain-deficient dopamine neurons. Proc Natl Acad Sci U S A 104, 1325–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis CE, Murphy EJ, Mitchell DC, Golovko MY, Scaglia F, Barcelo-Coblijn GC, and Nussbaum RL (2005). Mitochondrial lipid abnormality and electron transport chain impairment in mice lacking alpha-synuclein. Mol Cell Biol 25, 10190–10201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang X, Han D, Cheng Q, Zhang P, Zhao C, Min J, and Wang F (2018). Association of Levels of Physical Activity With Risk of Parkinson Disease: A Systematic Review and Meta-analysis. JAMA Netw Open 1, e182421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorelli CM, Ciolac EG, Simieli L, Silva FA, Fernandes B, Christofoletti G, and Barbieri FA (2019). Differential Acute Effect of High-Intensity Interval or Continuous Moderate Exercise on Cognition in Individuals With Parkinson’s Disease. J Phys Act Health 16, 157–164. [DOI] [PubMed] [Google Scholar]
- Fried NT, Moffat C, Seifert EL, and Oshinsky ML (2014). Functional mitochondrial analysis in acute brain sections from adult rats reveals mitochondrial dysfunction in a rat model of migraine. Am J Physiol Cell Physiol 307, C1017–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galter D, Pernold K, Yoshitake T, Lindqvist E, Hoffer B, Kehr J, Larsson NG, and Olson L (2010). MitoPark mice mirror the slow progression of key symptoms and L-DOPA response in Parkinson’s disease. Genes, brain, and behavior 9, 173–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellhaar S, Marcellino D, Abrams MB, and Galter D (2015). Chronic L-DOPA induces hyperactivity, normalization of gait and dyskinetic behavior in MitoPark mice. Genes, brain, and behavior 14, 260–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerecke KM, Jiao Y, Pagala V, and Smeyne RJ (2012). Exercise does not protect against MPTP-induced neurotoxicity in BDNF haploinsufficient mice. PLoS One 7, e43250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerecke KM, Jiao Y, Pani A, Pagala V, and Smeyne RJ (2010). Exercise protects against MPTP-induced neurotoxicity in mice. Brain research 1341, 72–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good CH, Hoffman AF, Hoffer BJ, Chefer VI, Shippenberg TS, Backman CM, Larsson NG, Olson L, Gellhaar S, Galter D, et al. (2011). Impaired nigrostriatal function precedes behavioral deficits in a genetic mitochondrial model of Parkinson’s disease. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 25, 1333–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsueh SC, Chen KY, Lai JH, Wu CC, Yu YW, Luo Y, Hsieh TH, and Chiang YH (2018). Voluntary Physical Exercise Improves Subsequent Motor and Cognitive Impairments in a Rat Model of Parkinson’s Disease. Int J Mol Sci 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang Y, Kwon I, Song W, Cosio-Lima LM, Taylor S, and Lee Y (2018). Modulation of mitochondrial phenotypes by endurance exercise contributes to neuroprotection against a MPTP-induced animal model of PD. Life Sci 209, 455–465. [DOI] [PubMed] [Google Scholar]
- Koo JH, Cho JY, and Lee UB (2017). Treadmill exercise alleviates motor deficits and improves mitochondrial import machinery in an MPTP-induced mouse model of Parkinson’s disease. Exp Gerontol 89, 20–29. [DOI] [PubMed] [Google Scholar]
- Langston JW, Ballard P, Tetrud JW, and Irwin I (1983). Chronic Parkinsonism in humans due to a product of meperidine-analog synthesis. Science 219, 979–980. [DOI] [PubMed] [Google Scholar]
- Lauze M, Daneault JF, and Duval C (2016). The Effects of Physical Activity in Parkinson’s Disease: A Review. Journal of Parkinson’s disease 6, 685–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lusis SA (1997). Pathophysiology and management of idiopathic Parkinson’s disease. J Neurosci Nurs 29, 24–31. [DOI] [PubMed] [Google Scholar]
- Maetzler W (2014). Comment: why do nondopaminergic features in Parkinson disease matter? Neurology 82, 417. [DOI] [PubMed] [Google Scholar]
- McAllister AK (1999). Subplate neurons: a missing link among neurotrophins, activity, and ocular dominance plasticity? Proc Natl Acad Sci U S A 96, 13600–13602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno Y, Ohta S, Tanaka M, Takamiya S, Suzuki K, Sato T, Oya H, Ozawa T, and Kagawa Y (1989). Deficiencies in complex I subunits of the respiratory chain in Parkinson’s disease. Biochem Biophys Res Commun 163, 1450–1455. [DOI] [PubMed] [Google Scholar]
- Mizuno Y, Sone N, and Saitoh T (1987). Effects of 1-methyl-4-phenyl-1,2,3,6- tetrahydropyridine and 1-methyl-4-phenylpyridinium ion on activities of the enzymes in the electron transport system in mouse brain. J Neurochem 48, 1787–1793. [DOI] [PubMed] [Google Scholar]
- Monteiro-Junior RS, Cevada T, Oliveira BR, Lattari E, Portugal EM, Carvalho A, and Deslandes AC (2015). We need to move more: Neurobiological hypotheses of physical exercise as a treatment for Parkinson’s disease. Med Hypotheses 85, 537–541. [DOI] [PubMed] [Google Scholar]
- Oliveira de Carvalho A, Filho ASS, Murillo-Rodriguez E, Rocha NB, Carta MG, and Machado S (2018). Physical Exercise For Parkinson’s Disease: Clinical And Experimental Evidence. Clin Pract Epidemiol Ment Health 14, 89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paillard T, Rolland Y, and de Souto Barreto P (2015). Protective Effects of Physical Exercise in Alzheimer’s Disease and Parkinson’s Disease: A Narrative Review. J Clin Neurol 11, 212–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park A, Zid D, Russell J, Malone A, Rendon A, Wehr A, and Li X (2014). Effects of a formal exercise program on Parkinson’s disease: a pilot study using a delayed start design. Parkinsonism & related disorders 20, 106–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira AC, Huddleston DE, Brickman AM, Sosunov AA, Hen R, McKhann GM, Sloan R, Gage FH, Brown TR, and Small SA (2007). An in vivo correlate of exercise- induced neurogenesis in the adult dentate gyrus. Proc Natl Acad Sci U S A 104, 5638–5643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petzinger GM, Walsh JP, Akopian G, Hogg E, Abernathy A, Arevalo P, Turnquist P, Vuckovic M, Fisher BE, Togasaki DM, et al. (2007). Effects of treadmill exercise on dopaminergic transmission in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned mouse model of basal ganglia injury. The Journal of neuroscience : the official journal of the Society for Neuroscience 27, 5291–5300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pianta S, Lee JY, Tuazon JP, Castelli V, Mantohac LM, Tajiri N, and Borlongan CV (2019). A Short Bout of Exercise Prior to Stroke Improves Functional Outcomes by Enhancing Angiogenesis. Neuromolecular Med. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radak Z, Hart N, Sarga L, Koltai E, Atalay M, Ohno H, and Boldogh I (2010). Exercise plays a preventive role against Alzheimer’s disease. Journal of Alzheimer’s disease : JAD 20, 777–783. [DOI] [PubMed] [Google Scholar]
- Real CC, Garcia PC, and Britto LRG (2017). Treadmill Exercise Prevents Increase of Neuroinflammation Markers Involved in the Dopaminergic Damage of the 6-OHDA Parkinson’s Disease Model. J Mol Neurosci 63, 36–49. [DOI] [PubMed] [Google Scholar]
- Safdar A, Bourgeois JM, Ogborn DI, Little JP, Hettinga BP, Akhtar M, Thompson JE, Melov S, Mocellin NJ, Kujoth GC, et al. (2011). Endurance exercise rescues progeroid aging and induces systemic mitochondrial rejuvenation in mtDNA mutator mice. Proc Natl Acad Sci U S A 108, 4135–4140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schou M, Steiger C, Varrone A, Guilloteau D, and Halldin C (2009). Synthesis, radiolabeling and preliminary in vivo evaluation of [18F]FE-PE2I, a new probe for the dopamine transporter. Bioorg Med Chem Lett 19, 4843–4845. [DOI] [PubMed] [Google Scholar]
- Sethi KD (2002). Clinical aspects of Parkinson disease. Curr Opin Neurol 15, 457–460. [DOI] [PubMed] [Google Scholar]
- Shan L, Diaz O, Zhang Y, Ladenheim B, Cadet JL, Chiang YH, Olson L, Hoffer BJ, and Backman CM (2015). L-Dopa induced dyskinesias in Parkinsonian mice: Disease severity or L-Dopa history. Brain research 1618, 261–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J, and Cookson MR (2004). Mitochondria and dopamine: new insights into recessive parkinsonism. Neuron 43, 301–304. [DOI] [PubMed] [Google Scholar]
- Shen X, Wong-Yu IS, and Mak MK (2016). Effects of Exercise on Falls, Balance, and Gait Ability in Parkinson’s Disease: A Meta-analysis. Neurorehabilitation and neural repair 30, 512–527. [DOI] [PubMed] [Google Scholar]
- Sherer TB, Betarbet R, Testa CM, Seo BB, Richardson JR, Kim JH, Miller GW, Yagi T, Matsuno-Yagi A, and Greenamyre JT (2003). Mechanism of toxicity in rotenone models of Parkinson’s disease. The Journal of neuroscience : the official journal of the Society for Neuroscience 23, 10756–10764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi K, Liu X, Qiao D, and Hou L (2017). Effects of Treadmill Exercise on Spontaneous Firing Activities of Striatal Neurons in a Rat Model of Parkinson’s Disease. Motor control 21, 58–71. [DOI] [PubMed] [Google Scholar]
- Smeyne RJ, and Jackson-Lewis V (2005). The MPTP model of Parkinson’s disease. Brain Res Mol Brain Res 134, 57–66. [DOI] [PubMed] [Google Scholar]
- Sutoo D, and Akiyama K (2003). Regulation of brain function by exercise. Neurobiol Dis 13, 1–14. [DOI] [PubMed] [Google Scholar]
- Svensson M, Lexell J, and Deierborg T (2015). Effects of Physical Exercise on Neuroinflammation, Neuroplasticity, Neurodegeneration, and Behavior: What We Can Learn From Animal Models in Clinical Settings. Neurorehabilitation and neural repair 29, 577–589. [DOI] [PubMed] [Google Scholar]
- Tajiri N, Yasuhara T, Shingo T, Kondo A, Yuan W, Kadota T, Wang F, Baba T, Tayra JT, Morimoto T, et al. (2010). Exercise exerts neuroprotective effects on Parkinson’s disease model of rats. Brain research 1310, 200–207. [DOI] [PubMed] [Google Scholar]
- Tillerson JL, Caudle WM, Reveron ME, and Miller GW (2003). Exercise induces behavioral recovery and attenuates neurochemical deficits in rodent models of Parkinson’s disease. Neuroscience 119, 899–911. [DOI] [PubMed] [Google Scholar]
- Tillerson JL, Cohen AD, Philhower J, Miller GW, Zigmond MJ, and Schallert T (2001). Forced limb-use effects on the behavioral and neurochemical effects of 6- hydroxydopamine. The Journal of neuroscience : the official journal of the Society for Neuroscience 21, 4427–4435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuon T, Valvassori SS, Lopes-Borges J, Luciano T, Trom CB, Silva LA, Quevedo J, Souza CT, Lira FS, and Pinho RA (2012). Physical training exerts neuroprotective effects in the regulation of neurochemical factors in an animal model of Parkinson’s disease. Neuroscience 227, 305–312. [DOI] [PubMed] [Google Scholar]
- Valente EM, Abou-Sleiman PM, Caputo V, Muqit MM, Harvey K, Gispert S, Ali Z, Del Turco D, Bentivoglio AR, Healy DG, et al. (2004). Hereditary early-onset Parkinson’s disease caused by mutations in PINK1. Science 304, 1158–1160. [DOI] [PubMed] [Google Scholar]
- Ved R, Saha S, Westlund B, Perier C, Burnam L, Sluder A, Hoener M, Rodrigues CM, Alfonso A, Steer C, et al. (2005). Similar patterns of mitochondrial vulnerability and rescue induced by genetic modification of alpha-synuclein, parkin, and DJ-1 in Caenorhabditis elegans. J Biol Chem 280, 42655–42668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson N, Ji X, Yasuhara T, Date I, Kaneko Y, Tajiri N, and Borlongan CV (2015). No pain, no gain: lack of exercise obstructs neurogenesis. Cell Transplant 24, 591–597. [DOI] [PubMed] [Google Scholar]
- West AB, Moore DJ, Biskup S, Bugayenko A, Smith WW, Ross CA, Dawson VL, and Dawson TM (2005). Parkinson’s disease-associated mutations in leucine-rich repeat kinase 2 augment kinase activity. Proc Natl Acad Sci U S A 102, 16842–16847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wi S, Lee JW, Kim M, Park CH, and Cho SR (2018). An Enriched Environment Ameliorates Oxidative Stress and Olfactory Dysfunction in Parkinson’s Disease with alpha- Synucleinopathy. Cell Transplant 27, 831–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuhara T, Hara K, Maki M, Matsukawa N, Fujino H, Date I, and Borlongan CV (2007). Lack of exercise, via hindlimb suspension, impedes endogenous neurogenesis. Neuroscience 149, 182–191. [DOI] [PubMed] [Google Scholar]
- Yuan J, Cord BJ, McCann UD, Callahan BT, and Ricaurte GA (2002). Effect of depleting vesicular and cytoplasmic dopamine on methylenedioxymethamphetamine neurotoxicity. J Neurochem 80, 960–969. [DOI] [PubMed] [Google Scholar]
- Zigmond MJ, Cameron JL, Hoffer BJ, and Smeyne RJ (2012). Neurorestoration by physical exercise: moving forward. Parkinsonism & related disorders 18 Suppl 1, S147–150. [DOI] [PubMed] [Google Scholar]
- Zigmond MJ, Cameron JL, Leak RK, Mirnics K, Russell VA, Smeyne RJ, and Smith AD (2009). Triggering endogenous neuroprotective processes through exercise in models of dopamine deficiency. Parkinsonism & related disorders 15 Suppl 3, S42–45. [DOI] [PubMed] [Google Scholar]
- Zigmond MJ, and Smeyne RJ (2014). Exercise: is it a neuroprotective and if so, how does it work? Parkinsonism & related disorders 20 Suppl 1, S123–127. [DOI] [PubMed] [Google Scholar]