Abstract
Background:
In low-income countries, there are multiple barriers for children with epilepsy (CWE) to attend school. We examined potentially modifiable associations with poor school performance in CWE in the West African Republic of Guinea.
Methods:
CWE of school age were recruited using public announcements and a clinical register of people with epilepsy at the Ignace Deen Hospital in Conakry in 2018. A team of Guinean and U.S. neurologists and neurologists-in-training interviewed each CWE and parent for his/her epilepsy history, household finances, educational attainment level, and stigma using the Stigma Scale of Epilepsy (SSE). Each child was also tested using the Wechsler Nonverbal Scale of Ability (WNV). Low school performance was defined as either not attending school or being held back a grade level at least once. Potential predictors of low school performance were analyzed.
Findings:
Of 128 CWE (mean age 11.6 years, 48.4% female), 11.7% (n=15) never attended school, 23.3% (n=30) dropped out, and 64.8% (n=83) were currently enrolled. Of CWE attending school, 46.9% (n=39) were held back a grade level. Overall, 54 children were defined as low-performers (42%). >100 lifetime seizures (odds ratio (OR)=8.81; 95% CI=2.51,37.4; p=0.001) and lower total WNV score (OR=0.954; 95% CI=0.926,0.977; p<0.001) were significantly associated with poor school performance in separate models, when controlling for potential confounders. Given the strong relationship between seizure freedom and school performance, we estimated that 38 additional CWE (33.6%) could become high performers if all CWE were adequately treated to achieve the lifetime seizure category of <10 seizures and could be cognitively intact again. Models examining SSE and household wealth quintile were not significantly associated with school performance.
Conclusions:
Higher lifetime seizures and lower WNV score were significantly associated with low school performance in CWE in Guinea. In spite of our conservative definition of high school performance (attending without failing) and risk of referral bias at an academic center where patients were allowed to self-refer, we demonstrate that seizure control in this setting is could increase the number of CWE who could attend and stay in school.
Keywords: Epilepsy, Education, Global Health, Pediatrics, Cognition, Stigma
1. Introduction1
More than 90% of people with epilepsy in low-income countries (LICs) are younger than 20 years old[1]. In Sub-Saharan Africa (SSA), access to primary school is already limited for many children by poverty, armed conflict, and a variety of other socioeconomic and political factors. Among CWE in SSA, school attendance and performance are understudied but may represent the best opportunity for CWE to break a cycle of disenfranchisement.
CWE in SSA have increased rates of school dropout, more missed days of school, and decreased participation in extracurricular sports[2,3]. Most published research has focused on the influence of teachers’ beliefs and perceptions: in both Nigeria [4–7] and Ethiopia [8,9], misperceptions among teachers on epilepsy were common. There have been few studies investigating barriers to education from the perspective of CWE and their families. To our knowledge, only five such studies in SSA exist. In Sierra Leone, a structured interview of 50 CWE and their caregivers reported a 20% rate of school drop out[2]. All of the students who dropped out cited reasons related to their seizures, including poor seizure control, learning disabilities, and perceived stigma from classmates and teachers. Two studies from Tanzania found that learning and behavioral problems contributed significantly to school drop-out rates[3,10]. Two population-based studies have been done: in Gabon, CWE who remained in school were more likely to have high sociability scores, take anti-epileptic drugs (AED), and have been seen by a medical specialist[11]. In rural Kenya, more than 50% of CWE did not attend school regularly with both higher seizure frequency and cognitive impairment predicting worse attendance [12].
Studies in other low-income countries have shown that learning disorders increase the likelihood of school drop-out, and poorer performance in school [13]. Since recurrent seizures may contribute to developmental delays and learning disorders, this is one of several potential mechanisms through which epilepsy could affect education.
The Guinea Epilepsy Project is a prospective study of PWE. 95% of the cohort, comprised of more than 50% children, had at least one seizure in the prior year [14]. Even among the 50% of the cohort currently taking an AED, 97% had a seizure in the prior year, implying poor seizure control. Upon enrollment to the cohort, 30% of school-aged CWE reported no education. This was recognized to be higher than the national average. In 2014 the World Bank Reported the gross enrollment ratio for children attending primary school in Guinea was 93[15]. As education rates are highest in Conakry, where the study took place [16], one would expect that schooling rate to exceed the national average. Aside from this study from our group, we are unaware of other formal research on CWE and school attendance in Guinea.
We performed a cross-sectional study on CWE on factors that were assumed to impact school performance. We hypothesized that four patient-focused factors would relate to a child’s ability to attend and perform well in school: seizure control, cognitive ability, household finances, and perceived stigma. We sought to better understand which factors had the greatest impact on education in CWE in Guinea, aiming to identify potentially modifiable for future interventions.
2. Methods
2.1. Ethics Approvals:
Approval was granted by the institutional review board at Ignace Deen Hospital (IDH) in Conakry, Republic of Guinea and the Partners Human Research Committee at the Massachusetts General Hospital in Boston, USA. Informed written consent was obtained from children and a parent. Thumbprints were used in the place of signatures for low-literacy parents when needed. Mature children provided assent.
2.2. Setting:
Guinea is a West African country of 12.7 million people, with an average life expectancy of 60 years in 2017[15]. In Guinea, education is separated into préprimaire (essentially preschool--ages 3–7 years), primaire (lower secondary--ages 7–12), collège (secondary--ages 13–16) and lycée (advanced secondary--ages 17–19)[17]. Schooling beginning at primary school is mandatory and receives public funding. The official language of Guinea is French, however other local languages, including Susu, Malinke, and Pular, are commonly spoken[18]. IDH is one of three national hospitals in Guinea and is located in the capital city of Conakry. IDH has a neurology department with four faculty neurologists and a postgraduate neurology residency program. All enrollment was performed in August and September of 2018 in collaboration with a team of physicians at IDH.
2.3. Participants:
Eligible study participants were identified by physicians at IDH when they were clinically evaluated and kept on a study register. CWE throughout Guinea could self-refer to the study through advertisements on local and national radio and television. The study was advertised as a study on epilepsy, and not as related to schooling or cognition. Children within 1 month of 4 to 19 years of age were eligible for enrollment. Although préprimaire begins at 3 years, our cognitive assessment was not validated for this age, so we began enrollment at 3 years 11 months. Epilepsy was defined as two or more unprovoked seizures, at least 24 hours apart. Each child was evaluated by one U.S. neurologist and one African neurology resident and/or neurologist. Children with exclusively febrile seizures or non-epileptic events in the absence of epileptic seizures were excluded. Participants received a formal clinical consultation with a neurologist and AEDs free of charge. Customary entrance fees were waived by the hospital in order to reach participants who lacked the ability to pay. Each participant or parent was reimbursed a total of 100,000 Guinean francs (approximately 12 USD) for travel.
2.4. Interview:
Children were interviewed with a parent or caretaker. Older teenagers who presented alone were interviewed independently. Interviews were conducted by a West African resident physician fluent in the participant’s preferred language. Children and/or their next of kin, were interviewed using structured questionnaires on their epilepsy and medical history, school experiences, family educational history, and household finances. Survey instruments are available as Appendices 1 (English, for review purposes only) and 2 (French). We included areas that had previously been associated with poor school outcomes in CWE in SSA based upon a current review of the literature [2,3,10,11]. Participants with missing or inconsistent responses that were time sensitive were called for clarification within one month of enrollment. Responses that were not time sensitive were confirmed within four months of enrollment.
2.5. Cognitive Assessment:
CWE were given the two-scale version of the Wechsler nonverbal assessment of ability (WNV)[19]. This test was chosen because it is applicable in cross cultural settings and does not rely on language. The WNV has been validated in non-English speaking populations in the United States[19], Democratic Republic of the Congo[20], and Malaysia[21]. The two-scale version of the test was administered by a U.S.-based investigator with translation of verbal instructions by a West African resident physician fluent in the participant’s preferred language. We used the full scale score for the two-subtest version in our assessment. Scores range from 30 to 170 with a score of 100 corresponding to the 50th percentile.
2.6. Stigma Evaluation:
Parents and children completed the Stigma Scale of Epilepsy (SSE)[22]. This is a 24-question survey that has been validated to assess stigma. It has been used in numerous international settings and has been validated in SSA [23]. Each question was scored on a scale from one (not at all) to four (totally). All questions were translated into French by a local francophone. The SSE was adjusted based upon the total number of questions answered on a total scale from 1–100% with 100% implying more stigma, in accordance with the scoring system used in the original validation study[22]. As there are no validated cutoff points for differentiating mild versus moderate stigma, the composite score was used as a summary measure in our analysis.
2.7. Financial Survey:
Children’s caretakers completed a financial questionnaire using the Demographic and Health Survey model household assets questionnaire[24] designed by the United States Agency for International Development (USAID). This instrument assesses different aspects of daily living, including electricity, housing, and possessions. Questions were analyzed for variation across participants. Water source, toilet, bank account, electronics, transportation, and mosquito net use had significant variation. Items including electricity, telephone and boat ownership were dropped due to a lack of variation. Participants were given a score for each item, which was ranked in ascending order. Then, polychoric principal component analysis was performed on all of the ordered and ranked asset covariates to extract a single principal component which was deemed the “wealth score.” Participants were divided into quintiles across the sample based upon their wealth score.
2.8. Analysis:
Variables of interest were described according to their mean and standard deviation for normal distributions or median and interquartile range for non-normal distributions. For the WNV assessment, raw test scores were age adjusted by converting them to a t-score using the standard scoring manual[19].. t-scores on the two sub-tests were combined for a composite WNV test score.
The sample was separated into “high performers,” (HP) who had attended school and had never been held back a grade, and “low performers,” (LP) who had either dropped out of school or been held back from at least one grade at least once. Logistic regression models were constructed with school performance as the primary outcome variable. Directed acyclic graphs were drawn to select potential confounders in each model. Four models were analyzed: one for each primary explanatory variable of interest (lifetime seizure estimate, WNV score, stigma score, and familial wealth status). A p-value of 0.05/4=0.0125 was used to correct for multiple comparisons in the method of Bonferroni. All statistical calculations were conducted using the programming language R (Vienna, Austria).
3. Results:
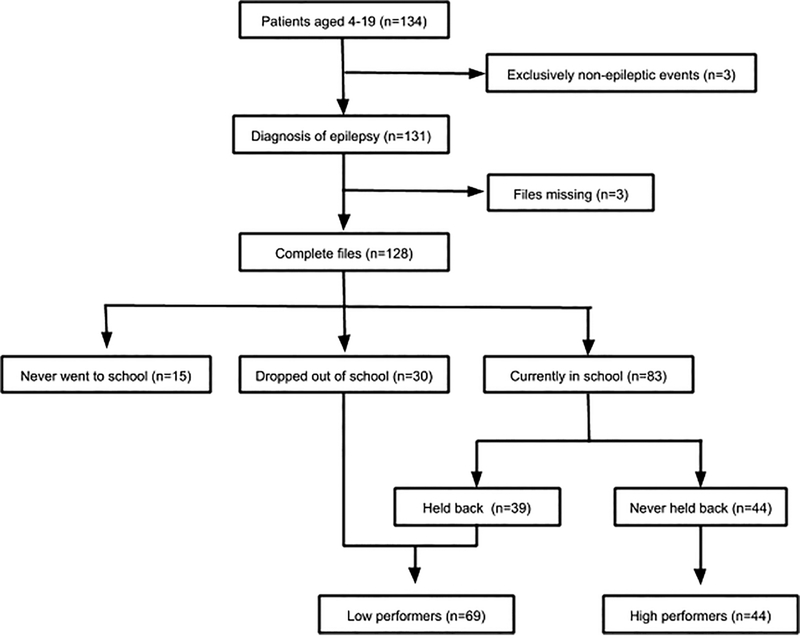
134 children in the appropriate age range presented to IDH during the study enrollment period and received the WNV assessment. Three children were later excluded because of a diagnosis of non-epileptic spells based on clinical evaluation by a neurologist. Three additional children were excluded because of incomplete data (Figure 1). The final sample included 128 CWE participants whose demographic and clinical characteristics are detailed in Table 1.
Figure 1.
Participant flowsheet {see separate document}
Table 1.
Clinical and Demographic Characteristics of included Children with Epilepsy (n=128)
| Value | % on (n) |
|---|---|
| Age, mean (SD) | 11.6 (4.54) |
| Gender, %F (n) | 48.4 (62) |
| On anti-epileptic drug, % on (n) | 60.2 (77) |
| Loss of consciousness with seizure, %with (n) | 80.5 (103) |
| Head imaging, %with (n)* | 37.5 (48) |
| WNV median (25th, 75th percentile) | 51 (3.25, 66.5) |
| Median age of first seizure (25th, 75th percentile) | 4 (1, 9) |
| Median age diagnosed (25th, 75 percentile) | 7 (4–12) |
| Seizure Characteristics, %with (n)** | |
| Loss of consciousness | 80.5 (103) |
| Falling with tonic/clonic movements | 72.7 (93) |
| Movement of a single limb | 11.7 (15) |
| Unusual sensations | 4.69 (6) |
| Staring spells | 51.6 (66) |
| Number of AEDs Used | |
| Zero | 39.8 (51) |
| One | 51.6 (66) |
| Two | 7.0 (9) |
| Three | 1.6 (2) |
| Type of AED used*** | |
| Phenobarbital | 17.2 (22) |
| Carbamazepine | 19.5 (25) |
| Valproic Acid | 19.5 (25) |
| Levetiracetam**** | 12.5 (16) |
| Diazepam | 1.6 (2) |
Imaging was not part of our study, so it was not reviewed by study staff. Based upon parent report, 9 children had abnormal imaging, 14 had normal imaging, and 25 did not disclose results.
One participant only did not fully respond to seizure characteristic questions and only responded to LOC.
Participants on multiple AEDs were counted once for each AED they were taking
While Levetiracetam is not typically available in Guinea, physicians with the Guinea Epilepsy Project had prescribed levetiracetam to participants during a prior trip. All participants on levetiracetam had received it from our group, with the exception of one participant who received it from relatives abroad.
3.1. Epilepsy Characteristics
The median age of first seizure was 4 years (25th, 75th percentile 1, 9 years). Average age of diagnosis was 7 years (25th, 75th percentile 4, 12 years). 68.8% (n=88) of CWE had at least one seizure in the prior 3 months. 80.5% (n=103) had seizures associated with loss of consciousness. 72.7 (93) had seizures with falling and tonic clonic movements of the limbs suggestive of primary or secondary generalized seizures (Table 1). Two were noted to have partial seizures consistent with Benign Rolandic Epilepsy with Centrotemporal Spikes. One had staring spells consistent with absence epilepsy. 61.7% (n=79) were taking an AED and 37.5% (n=48) had head imaging. In 9 cases imaging was reported as abnormal. The most common AEDs taken were carbamazepine 19.5% (n=25) and valproic acid 19.5% (n=25) (Table 1).
3.2. School Status
Of the 128 CWE, 11.7% (n=15) had never attended school, 23.3% (n=30) previously attended school and had dropped out, and 64.8% (n=83) were currently in school (Table 2). Of CWE currently attending school 46.9% (n=39) had been held back a grade level at least once and 53.0% (n=44) had never been held back. Due to the small number of children who had never attended school, and our observation that many of these children had severe cognitive and attentional issues that would preclude them from attending school such as epileptic encephalopathies, we excluded them from further analyses. Reasons for school drop-out and no schooling are in Table 3.
Table 2.
Characteristics of high school performers, low performers, and children who never went to school
| Variable | No School (n=15) | Low Performers (n=69) | High Performers (n=44) |
|---|---|---|---|
| Age, median, years (25th, 75th percentile) | 7.9 (5.0–11.1) | 12.24 (9.64–16.14) | 9.91 (7.19–14.92) |
| Gender, %F (n) | 40.0 (6) | 47.8 (33) | 52.3 (23) |
| WNV, median (25th, 75th percentile) | 0.00 (0.00–18.50) | 46.0 (30.0–57.0) | 67.0 (51.0–77.0) |
| Lifetime seizures, %(n) | |||
| <10 | 6.67 (1) | 5.8 (4) | 29.5 (13) |
| 10–50 | 13.3 (2) | 17.4 (12) | 27.3 (12) |
| 50–100 | 0.00 (0) | 17.4 (12) | 11.4 (5) |
| >100 | 80.0 (12) | 59.4 (41) | 31.8 (14) |
| Family wealth quintile | |||
| First | 20.0 (3) | 23.1 (16) | 11.4 (5) |
| Second | 33.3 (5) | 26.1 (18) | 13.6 (6) |
| Third | 13.3 (2) | 15.9 (11) | 25.0 (11) |
| Fourth | 20.0 (3) | 21.7 (15) | 22.7 (10) |
| Fifth | 13.3 (2) | 13.0 (9) | 27.3 (12) |
| SSE, mean (standard deviation) | 53.5 (14.1) | 51.8 (14.9) | 49.9 (14.0) |
| Most recent seizure: | |||
| 24 hours | 26.7 (4) | 15.9 (11) | 9.09 (4) |
| One week | 40.0 (6) | 28.9 (20) | 6.82 (3) |
| One month | 13.3 (2) | 11.6 (8) | 27.3 (12) |
| 3 months | 0.00 (0) | 18.8 (13) | 6.82 (3) |
| 6 months | 0.00 (0) | 7.25 (5) | 13.6 (6) |
| 9 months | 0.00 (0) | 7.25 (5) | 15.9 (7) |
| One year | 0.00 (0) | 2.90 (2) | 9.09 (4) |
| >1 year | 6.67 (1) | 7.25 (5) | 9.09 (4) |
| Highest parental education | |||
| No school | 20.0 (3) | 20.3 (14) | 2.27 (1) |
| Primary | 13.3 (2) | 4.35 (3) | 4.54 (2) |
| Some secondary | 6.67 (1) | 10.1 (7) | 4.54 (2) |
| Full secondary | 6.67 (1) | 5.80 (4) | 4.54 (2) |
| High school | 13.3 (2) | 11.6 (8) | 18.2 (8) |
| University | 40.0 (6) | 47.8 (33) | 65.9 (29) |
| Anti-epileptic drug, % (n) | 33.3 (5) | 69.6 (48) | 59 (26) |
| Age at first seizure, median (25th, 75th percentile) | 0.75 (0.50–2.25) | 6.0 (1.5–10.0) | 4.0 (1.0–7.25) |
| Age diagnosed, median (25th, 75th percentile) | 3.0 (0.54–5.0) | 9.0 (5.0–13.0) | 7.0 (4.0–11.0) |
Table 3.
Reasons for dropping out of school or never going to school
| Reason | Never attended school (n=15) | Dropped out (n=30) |
|---|---|---|
| Parental pressure/decision | 46.7 (7) | 40.0 (12) |
| Pressure from teachers | 6.67 (1) | 40.0 (12) |
| Pressure from classmates | 6.67 (1) | 23.3 (7) |
| Fear of injury | 20.0 (3) | 46.7 (14) |
| School was too difficult | 26.7 (4) | 33.3 (10) |
| Needed to work or help at home | 6.67 (1) | 3.33 (1) |
| Behavioral problems | 0.00 (0) | 3.33 (1) |
| Developmental delay | 46.7 (7) | 3.33 (1) |
| Other* | 20.0 (3) | 20.0 (6) |
Reasons listed under “other” included, speech difficulties (n=2), embarrassment or trouble with seizures (n=4), shame and difficulty concentrating (n=1), difficulty walking (n=1), could not play (n=2)
3.3. High and Low Academic Performers
There were 38.9% (n=44) HP and 61.0% (n=69) LP. HP were a median age of 9.9 years old (25th, 75th percentile 7.2, 14.9 years), 52.3% (n=23) female, and 59% (n=26) were on an AED. LP were a median of age of 12.2 (25th, 75th percentile 9.6, 16.1), 47.8% (n=33) female and 69.6% (n=48) were on an anti-epileptic drug. Descriptive statistics of HP and LP are in Table 2. Since HP were younger than LP, age was adjusted in subsequent regression analyses. HP reported on average a higher class rank and lower class difficulty than LP (Table 4).
Table 4.
Experiences of Children with Epilepsy who Attend School
| Characteristic | Low Performers (n=69) | High Performers (n-44) |
|---|---|---|
| School rank, % (n) | ||
| Top of the class | 5.80 (4) | 11.4 (5) |
| Above average | 18.8 (13) | 36.4 (16) |
| Average | 31.9 (22) | 36.4 (16) |
| Below average | 26.1 (18) | 6.82 (3) |
| Bottom of the class | 17.4 (12) | 0.00 (0) |
| School difficulty, % (n) | ||
| Extremely difficult | 40.6 (28) | 6.82 (3) |
| Sometimes difficult | 20.3 (14) | 45.0 (11) |
| Average difficulty | 13.0 (9) | 15.9 (7) |
| Not very difficult | 14.5 (10) | 29.5 (13) |
| Easy | 11.6 (8) | 2.27(1) |
| Participates in sports, % (n)* | 54.4 (37) | 72.7 (32) |
| Teachers informed of epilepsy, % (n)* | ||
| All | 73.1 (49) | 53.4 (23) |
| Some | 13.4 (9) | 32.6 (14) |
| None | 13.4 (9) | 14.0 (6) |
| Students informed of epilepsy, % (n)* | ||
| All | 68.2 (45) | 43.2 (19) |
| Some | 12.1 (8) | 29.5 (13) |
| None | 19.6 (13) | 27.3 (12) |
One low performer did not respond to questions about sports participation. Two low performers and 1 high performer did not respond to questions about whether or not they had informed teachers of their epilepsy. Three low performers did not respond to questions about whether or not they had informed classmates of their epilepsy
3.4. Stigma Scores
The mean stigma score in our sample was 51.1 points (standard deviation=14.5 points), which is higher than the mean stigma score in the Brazilian validation study of 46 points [22].
3.5. WNV Score
Median WNV score was 51, which corresponds to a percentile of 0.1%. Distribution of WNV scores across the sample is reported in Table 5.
Table 5.
Distribution of WNV scores
| Category | Percentage (n) |
|---|---|
| Very Superior | 0 (0) |
| Superior | 0 (0) |
| High average | 0 (0) |
| Average | 4.7 (6) |
| Low average | 6.3 (8) |
| Borderline | 10.9 (14) |
| Extremely low | 78.1 (100) |
3.6. Multivariate Analysis
In model 1, having greater than 100 total lifetime seizures was significantly associated with school performance while controlling for age and wealth quintile (odds ratio (OR)=8.81, 95% CI=2.51,37.41, p=0.001). Having between 50–100 seizures did not meet our threshold after Bonferroni correction (OR=6.56, 95% CI=1.44,35.75, p=0.020). In model 2, total WNV score was significantly associated with school performance. Individuals who scored worse on the WNV were more likely to be LPs (OR=0.954, 95% CI=0.926,0.978, p<0.001). In models 3 and 4, neither stigma (OR=0.993, 95% CI 0.957–1.029, p=0.693) nor wealth quintile (OR=0.846, 95% CI 0.586–1.212, p=0.364) were significantly independently associated with school performance (Table 6).
Table 6.
Clinical and Demographic Associations with High versus Low Performing Children with Epilepsy in School
| Model | Odds Ratio (95% CI) | p-value |
|---|---|---|
| Model 1 | ||
| Total Seizures: <10 (ref) | ||
| 10–50 | 0.244 (0.821–15.0) | 0.105 |
| 50–100 | 6.56 (1.44–35.8) | 0.020 |
| >100 | 8.81 (2.51–37.4) | 0.001 |
| Age | 1.11 (1.01–1.23) | 0.041 |
| Wealth Quintile | 0.736 (0.531–1.00) | 0.057 |
| Model 2 | ||
| Wechsler Nonverbal Test Score (WNV) | 0.954 (0.926–0.977) | <0.001 |
| Age | 1.14 (1.00–1.31) | 0.051 |
| Parental education: none (ref) | ||
| Primary | 0.040 (0.001–0.823) | 0.047 |
| Some Secondary | 0.249 (0.008–4.13) | 0.350 |
| Full Secondary | 0.108 (0.003–2.15) | 0.162 |
| High School | 0.056 (0.002–0.597) | 0.034 |
| University | 0.074 (0.003–0.571) | 0.033 |
| Most recent seizure: 24 hours (ref) | ||
| One week | 5.37 (0.620–59.0) | 0.141 |
| One month | 0.718 (0.098–5.24) | 0.740 |
| 3 months | 4.12 (0.540–37.0) | 0.181 |
| 6 months | 0.635 (0.069–5.63) | 0.680 |
| 9 months | 1.08 (0.130–9.15) | 0.942 |
| One year | 0.082 (0.004–1.13) | 0.074 |
| Greater than one year | 1.77 (0.160–21.4) | 0.641 |
| Model 3 | ||
| Stigma Score | 0.993 (0.957–1.03) | 0.693 |
| Age | 1.11 (0.990–1.26) | 0.078 |
| Parental Education: none (ref) | ||
| Primary | 0.135 (0.004–1.03) | 0.178 |
| Some secondary | 0.340 (0.011–5.66) | 0.461 |
| Full secondary | 0.239 (0.008–4.44) | 0.339 |
| High school | 0.096 (0.005–0.691) | 0.061 |
| University | 0.109 (0.991–1.26) | 0.497 |
| WNV | 0.966 (0.944–0.985) | 0.001 |
| Total Seizures: <10 (ref) | ||
| 10–50 | 5.11 (1.09–30.1) | 0.049 |
| 50–100 | 5.64 (1.03–37.9) | 0.055 |
| >100 | 7.48 (1.72–40.7) | 0.011 |
| Model 4 | ||
| Wealth Quintile | 0.845 (0.586–1.21) | 0.364 |
| Age | 1.01 (0.989–1.23) | 0.086 |
| Parental education: none (ref) | ||
| Primary | 0.268 (0.008–5.11) | 0.395 |
| Some secondary | 0.275 (0.010–4.22) | 0.360 |
| Full secondary | 0.176 (0.006–2.97) | 0.236 |
| High school | 0.133 (0.006–1.14) | 0.102 |
| University | 0.147 (0.007–1.01) | 0.096 |
| Total Seizures: <10 (ref) | ||
| 10–50 | 3.74 (0.907–18.4) | 0.081 |
| 50–100 | 6.32 (1.32–36.5) | 0.027 |
| >100 | 8.77 (2.39–39.6) | 0.002 |
The outcome of school dropout versus attending school, whether failing a grade or not, was performed (Appendix 3). CWE from wealthier families were less likely to drop out of school.
Given the impact of seizure control on school performance and the missed opportunity for seizure control in Guinea compared to higher income settings, we used model 1 to estimate the number of CWE who could become HP if their seizures were treated to achieve a lifetime seizure burden of <10, assuming other forms of cognitive delay were not present. Based upon our model, 38 additional CWE (33.6%) could become HP if all CWE were treated to the seizure category of <10 seizures, bringing the total percentage of HP from 38.9% (n=44) to 72.6% (n=82).
4. Discussion
Our study shows that educational challenges are common for CWE in Guinea. Over 10% of the CWE in our study had no schooling, over 20% had dropped out of school, and over 40% of those who were still attending school had been held back at least one grade. One of the Millennium Development Goals determined by the United Nations is universal primary school education by 2015 [25]. This study shows that epilepsy is a barrier to this milestone in some children in settings like Guinea.
Our study demonstrates that more lifetime seizures and poor cognitive test scores are associated with poor school performance among CWE. Multiple other studies in SSA have reported that learning difficulties and poor seizure control lead children to drop out of school [2,3,10,11]. However, due to a larger sample size, we have been able test this hypothesis while controlling for other variables. We hypothesized that a higher total lifetime seizure count leads to poorer cognitive performance and therefore worse school performance. However, it is also possible that underlying cortical dysfunction leads to both seizures as well as cognitive impairment. If the latter scenario is true, treatment with AEDs would be less powerful at preventing poor school outcomes.
Overall our CWE participants performed poorly on the WNV, with percentile scores ranging from <0.1 to 58. The WNV was chosen because it is designed to be a culturally sensitive test that does not rely a primary language. Although it has been used in other international settings [20,21], the overall poor performance of our sample leads to questions on whether the WNV is valid in a Guinean population. The study was advertised as a study of EEG and epilepsy, and CWE were only informed of cognitive testing during the consent process, making it unlikely that we obtained a sample referred directly for greater cognitive impairment. Our results are similar to a previous study of a convenience sample of CWE in the Democratic Republic of the Congo, in which 73.3% of CWE had a WNV total raw score <70, which corresponds to the first percentile [20]. Using the same criteria, 72.4% of our cohort have a WNV <70. The WNV score appears consistent across populations in the region and correlates with success in school in both locations, suggesting that it is an acceptable measure in this population.
Unlike prior studies, we did not see an association between school performance and epilepsy-related stigma. The prior studies that have reported this association used self-report and structured interviews to evaluate both the degree of epilepsy-related stigma and reasons for school dropout [2,3,10]. Our study differs in that we used an epilepsy-specific scale that has been validated in SSA and elsewhere and in that we controlled for factors such as wealth in our analysis of contributors to poor school performance. Since very young children could not answer stigma questions themselves, and parents often wanted to answer on behalf of their children, it is difficult to disaggregate the impact of perceived stigma by a child versus stigma of a parent or both in the present study.
No significant association was found between wealth quintile and poor school performance. To our knowledge, no prior studies have examined this association for CWE in SSA. Our expectation was that poverty, independent of epilepsy, would negatively affect access to education. A subset of 77 participants responded to questions about their monthly expenditures on school. Among these participants, school cost on average of 122,527 Guinean Francs per month, roughly 13.50 USD. Across a 9-month school year, this amounts to roughly 15% of the average gross national income of a person in Guinea (790 USD) [26]. Unsurprisingly, when children were grouped based on whether or not they had dropped out of school, wealthier children were found to be more likely to attend school.
We did not observe any gender-related differences in school performance. Girls comprised 52.3% of HP and 47.7% of LP. While gender was not a primary variable of interest, this finding is surprising considering the gender gap in education in Guinea. In 2014, the most recently reported year, the World Bank published a female-to-male ratio of completion of enseignement primaire as 0.66 in Guinea [27]. That same year, the mean female-to-male completion rate in other low-income nations was 0.82. This could indicate that, in this population, medical issues and cognitive deficits among CWE overshadow differences due to gender disparities.
While 61.7% of our sample reported taking AEDs, we did not control for this in our analyses. The majority of CWE were on sub-therapeutic doses of anti-epileptic drugs. Use of AEDs was likely higher in our sample than among CWE in Guinea in general since many patients had received AEDs upon our recommendation during a prior study period, and because our method of recruitment selected for families and children actively seeking medical care versus traditional healing [28].
4.1. Limitations
Our study sample is not population-based. 72.6% (n=82) of the participants included in our analysis were from Conakry, the largest city in Guinea, which is home to just 15.8% of the total Guinean population [29]. According to the World Bank’s 2014 data, the gross enrollment ratio for children attending primary school in Guinea was 93 [15]. However, children in urban environments are more likely to attend school [30].
We included CWE with multiple different etiologies and types of epilepsy. Due to the lack of prior medical records and limited access to EEG, and neuroimaging, it was impossible to consistently make syndromic diagnoses. All patients were seen by both a U.S.-based neurologist and a Guinean neurology resident or neurologist, but it was beyond the scope of our study to perform a full etiologic work up on each participant. Clinical data and histories were carefully recorded. To our knowledge, this limitation is shared by all prior studies exploring associations between epilepsy and education in SSA. Nearly all patients in this study had generalized seizures with loss of consciousness. Our population may have had disproportionately worse epilepsy and poor seizure control, as the families in our sample were seeking care at a medical center, i.e. referral bias. Since the only prior recent studies of epilepsy in Guinea are from our research group, we cannot compare our patients’ epilepsy characteristics to epilepsy epidemiology in Guinea more broadly.
While we had a larger sample than most prior studies, we did not have a large enough sample to stratify our results by subgroups of age. Future research should investigate factors based on age, which would better allow targeted interventions. Recruiting children at the age of entry to school would allow us to observe factors affecting education early, when interventions would likely have the greatest impact.
Our study used a self-reported measure of school performance—a combination of self-reported school dropout and being held back a grade. We selected these measures because they were considered to be objective and could be accurately conveyed through self-report. It is possible that parents wanted to either inflate or deflate their child’s degree of education or exaggerated their degree of success or impairment in school.
Finally, parents and children responded to the questionnaires together. This may have influenced participants’ willingness to answer sensitive questions. In an effort to mitigate any misunderstandings related to phrasing or interpretation, we telephoned all participants with discordant answers after the study visit to clarify their responses.
4.2. Study Strengths
We focused on modifiable factors, making our results potentially actionable. If our findings are confirmed, they could directly influence policy decisions. We recognize that not all of the factors we looked, such as familial finances and cognition, are easily or completely modifiable. However, even these variables could be influenced through relatively simple interventions, such as improved educational funding or improved seizure control respectively.
Our result that poor seizure control and cognitive impairment are associated with school performance demonstrates the necessity of improving access to AEDs for CWE. Prior research has attempted to improve school performance through stigma reduction programs [31–33]. While stigma reduction programs are important, our study demonstrates that in Guinea, one of the world’s poorest countries, inadequate seizure control is likely a greater contributor to educational disparities for CWE. Our modeling predicted better seizure treatment could improve education outcomes for as many as 38 children—increasing the number of HP by approximately 33%. Even with optimum seizure care, not all children would achieve fewer than 10 lifetime seizures. However, this finding is useful for policy discussions and demonstrates the critical importance of provision of adequate and consistent supplies of AEDs and improving access to AEDs from an early age.
Additional strengths of our study include our relatively large sample size: 128 patients total and 113 children who had attended school for some period of time. This allowed us to control for multiple explanatory variables. Prior studies have also used a historical diagnosis of epilepsy or a pediatrician’s assessment, while diagnoses in our sample were made by board-certified neurologists from both the U.S.A. and Guinea. Thus, the number of children with pseudo-seizures alone is likely lower in our cohort.
4.3. Conclusions
There are significant barriers to achieving even a basic education for CWE in Guinea. Associations between poor educational outcomes and both poor seizure control and cognitive difficulties were notable. One important and urgent intervention for CWE is early and aggressive seizure control to prevent both seizures and cognitive limitations that arise from poorly controlled epilepsy.
Supplementary Material
Highlights.
Educational outcomes are poor for children with epilepsy in Guinea
>30% of children were not currently attending school
>40% of those in school had been held back at least once
More lifetime seizures, and worse cognition were associated with poor educational status.
5. Funding and Disclosures:
This study was supported by an R21 grant from the National Institutes of Health (5R21NS098886), the Charles Hood Foundation (#2017P001214) and the Fondation Pierre Fabre.
The authors have no conflicts of interest directly related to this study. Dr. Mateen has received personal fees from Genentech, Biogen, and Oxford Pharmagenesis Ltd. outside of the scope of this work
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Abbreviations used in this paper: Anti-epileptic drug (AED), Children with epilepsy (CWE), High performer (HP), Ignace Deen Hospital (IDH), Low income country (LIC), Low performer (LP), Stigma scale of epilepsy (SSE), Sub-Saharan Africa (SSA), Weschler Nonverbal Assessment of Ability (WNV).
6. References
- [1].Ba-Diop A, Marin B, Druet-Cabanac M, Ngoungou EB, Newton CR, Preux PM. Epidemiology, causes, and treatment of epilepsy in sub-Saharan Africa. Lancet Neurol. 2014;13: 1029–1044. doi: 10.1016/S1474-4422(14)70114-0.Epidemiology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Ali DB, Tomek M, Lisk DR. The effects of epilepsy on child education in Sierra Leone. Epilepsy Behav. 2014;37:236–240. doi: 10.1016/j.yebeh.2014.07.007. [DOI] [PubMed] [Google Scholar]
- [3].Quereshi C, Standing HC, Swai A, Hunter E, Walker R, Owens S. Barriers to access to education for young people with epilepsy in Northern Tanzania: A qualitative interview and focus group study involving teachers, parents and young people with epilepsy. Epilepsy Behav. 2017;72:145–149. doi: 10.1016/j.yebeh.2017.04.005. [DOI] [PubMed] [Google Scholar]
- [4].Akpan MU Ikpeme EE Utuk E-OE Teachers’ knowledge and attitudes towards seizure disorder: A comparative study of urban and rural school teachers in Akwa Ibom State, Nigeria. Niger J Clin Pract. 2013;16:365–370. doi: 10.4103/1119-3077.113465. [DOI] [PubMed] [Google Scholar]
- [5].Mustapha A, Odu O, Akande O. Knowledge, attitude and perception of epilepsy among secondary school teachers in Nigeria: a community-based study. Niger J Clin Pract. 2013;16:12–18. doi: 10.4103/1119-3077.106709. [DOI] [PubMed] [Google Scholar]
- [6].Owolabi LF, Shehu NM, Owolabi SD. Epilepsy and education in developing countries: A survey of school teachers’ knowledge about epilepsy and their attitude towards students with epilepsy in Northwestern Nigeria. Pan Afr Med J. 2014;18:255. doi: 10.11604/pamj.2014.18.255.3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ojinnaka NC. Teachers’ perception of epilepsy in Nigeria: A community-based study. Seizure. 2002;11:386–391. doi: 10.1053/seiz.2001.0664. [DOI] [PubMed] [Google Scholar]
- [8].Berhe T, Yihun B, Abebe E, Abera H. Knowledge, attitude, and practice about epilepsy among teachers at Ethio-National School, Addis Ababa, Ethiopia. Epilepsy Behav. 2017;70:150–153. doi: 10.1016/j.yebeh.2017.02.009. [DOI] [PubMed] [Google Scholar]
- [9].Gebrewold MA, Enquselassie F, Teklehaimanot R, Gugssa SA. Ethiopian teachers: Their knowledge, attitude and practice towards epilepsy. BMC Neurol 2016;16:1–8. doi: 10.1186/s12883-016-0690-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Mushi D, Burton K, Mtuya C, Gona JK, Walker R, Newton CRJC. Perceptions, social life, treatment and education gap of Tanzanian children with epilepsy: a community-based study. Epilepsy Behav. 2013;23(3):224–229. doi: 10.1016/j.yebeh.2011.12.003.Perceptions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ibinga E, Ngoungou EB, Olliac B, et al. Impact of epilepsy on children and parents in Gabon. Epilepsy Behav. 2015;44:110–116. doi: 10.1016/j.yebeh.2014.12.035. [DOI] [PubMed] [Google Scholar]
- [12].Munyoki G, Edwards T, White S., et al. Clinical and neurophysiological features of active convulsive epilepsy in rural Kenya: a population based study. Epilepsia. 2010;51: 2370–2376. doi: 10.1111/j.1528-1167.2010.02653.x.Clinical. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Patel V, Flisher AJ, Nikapota A, Malhotra S. Promoting child and adolescent mental health in low and middle income countries. 2008;3:313–34. doi: 10.1111/j.1469-7610.2007.01824.x. [DOI] [PubMed] [Google Scholar]
- [14].Jang M, Sakadi F, Tassiou NR, et al. Impact of poorly controlled epilepsy in the Republic of Guinea. Seizure. 2018;61:71–77. doi: 10.1016/j.seizure.2018.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Guinea. The World Bank. Published 2017. https://data-worldbank-org.ezp-prod1.hul.harvard.edu/country/Guinea (accessed October 18, 2018)
- [16].Bah MC, Bangoura MA. Troisieme Recensement General de La Population et de l’habitation; 2017. http://www.statguinee.org/images/Publications/INS/RGPH3/RGPH3_education.pdf (accessed April 10, 2019)
- [17].Country at a Glance - Guinea. The World Bank, Education Statistics. Published 2018. http://datatopics.worldbank.org/education/country/guinea (accessed January 27, 2019).
- [18].The World Factbook – Guinea. The Central Intelligence Agency. https://www.cia.gov/library/publications/the-world-factbook/geos/gv.html. (accessed March 28, 2019).
- [19].Wechsler D, Naglieri J. Wechsler Nonverbal Assessment of Ability. San Antonio: Harcourt Assessment; 2006. [Google Scholar]
- [20].Matonda-ma-Nzuzi T, Mampunza Ma Miezi S, Mpembi MN, et al. Factors associated with behavioral problems and cognitive impairment in children with epilepsy of Kinshasa, Democratic Republic of the Congo. Epilepsy Behav. 2018;78:78–83.doi: 10.1016/j.yebeh.2017.08.030. [DOI] [PubMed] [Google Scholar]
- [21].Nurliyana AR, Mohd Nasir MT, Zalilah MS, Rohani A. Dietary patterns and cognitive abiliy among 12- to 13 year-old adolescents in Selangor, Malaysia. Public Health Nutr. 2015;18:303–12. doi: 10.1017/S1368980014000068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Fernandes PT, Salgado PCB, Noronha ALA, Sander JW, Li LM. Stigma scale of epilepsy: Validation process. Arq Neuropsiquiatr. 2007;65:35–42. doi: 10.1590/S0004-282X2007001000006. [DOI] [PubMed] [Google Scholar]
- [23].Elafros MA, Bowles RP. Reexamining epilepsy-associated stigma : validation of the Stigma Scale of Epilepsy in Zambia. Qual Life Res 2015:1483–9. doi: 10.1007/s11136-014-0868-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Demographic and Health Surveys Model Household Questionnaire. United States Agency for International Development. Published 2017. https://dhsprogram.com/pubs/pdf/DHSQ7/DHS7_Household_QRE_EN_16Mar2017_DHSQ7.pdf (accessed October 19, 2018).
- [25].Millennium Development Goal 2. United Nations, Millennium Development Goals. Published 2000. http://www.un.org/millenniumgoals/education.shtml (accessed January 29, 2019).
- [26].Guinea: Databank. The World Bank. https://data.worldbank.org/country/guinea (accessed January 24, 2018).
- [27].Guinea: Gender Data Portal. The World Bank. http://datatopics.worldbank.org/gender/country/guinea (accessed April 9, 2019).
- [28].Anand P, Carlos G, Sakadi F, Rahamatou N, Abdoul BDH, Aissatou KB et al. Epilepsy and traditional healers in the Republic of Guinea: A mixed methods study.Epilepsy Behav. 2019;92:276–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Annuaire Statistique 2016. Published 2017. Institute National de la Statistique, République de Guinée. http://www.statguinee.org/images/Publications/INS/annuelles/INS_annuaire_2016.pdf (accessed April 9, 2019)
- [30].Guinea Core USAID Profile. Published 2005. https://www.usaid.gov/guinea Accessed October 19, 2018
- [31].Kaddumukasa M, Kaddumukasa MN, Buwembo W, et al. Epilepsy misconceptions and stigma reduction interventions in sub-Saharan Africa, a systematic review. Epilepsy Behav. 2018;85:21–7. doi: 10.1016/j.yebeh.2018.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Adjei P, Akpalu A, Laryea R, et al. Beliefs on epilepsy in Northern Ghana. Epilepsy Behav. 2013;29:316–321. doi: 10.1016/j.yebeh.2013.07.034. [DOI] [PubMed] [Google Scholar]
- [33].Tekle-Haimanot R, Pierre-Marie P, Daniel G, Worku DK, Belay HD, Gebrewold MA. Impact of an educational comic book on epilepsy-related knowledge, awareness, and attitudes among school children in Ethiopia. Epilepsy Behav.2016;61:218–223. doi: 10.1016/j.yebeh.2016.05.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.