Abstract
Vasculature is the network of blood vessels of an organ or body part that allow for the exchange of nutrients and waste to and from every cell, thus establishing a circulatory equilibrium. Vascular health is at risk from a variety of conditions that includes disease and trauma. In some cases, medical therapy can alleviate the impacts of the condition. Intervention is needed in other instances to restore the health of abnormal vasculature. The main approaches to treat vascular conditions are endovascular procedures and open vascular reconstruction that often requires a graft to accomplish. However, current vascular prostheses have limitations that include size mismatch with the native vessel, risk of immunogenicity from allografts and xenografts, and unavailability of autografts. In this review, we discuss efforts in bioprinting, an emerging method for vascular reconstruction. This includes an overview of 3D printing processes and materials, graft characterization strategies and the regulatory aspects to consider for the commercialization of 3D bioprinted vascular prostheses.
Current strategies to address vascular disease & trauma
Vascular disease can arise directly in vasculature such as the buildup of plaque in carotid artery disease or emerge as a side effect from another health condition such as diabetes that can lead to atherosclerosis [1, 2]. Vascular trauma can result from a blunt injury that is associated with a crushed or stretched blood vessel or from a penetrating injury that is due to a punctured, torn or severed blood vessel [1]. Depending on the case, endovascular procedures or open vascular reconstruction are current viable approaches of intervention.
Endovascular procedures
Endovascular procedures are percutaneous, minimally invasive alternatives to open surgery, thus potentially of lower morbidity and mortality risk [3]. These procedures encompass angioplasty and stenting, rely on imaging techniques and are carried out by experts using catheters and other specialized and miniature instruments [3, 4]. Application of these procedures has been reported for a variety of vascular conditions from mesenteric ischemia and iliac artery aneurysms to renal artery occlusive disease [3]. An example of taking an endovascular approach is to treat occlusive disease of the brachiocephalic arteries. The procedure can involve imaging by performing an angiogram starting from a micropuncture system to gain femoral access, then inserting a sheath and a catheter guided by ultrasound and stent deployment [3]. Because endovascular procedures are percutaneous, the approach can be perceived as low risk [3]. The intervention for a safe outcome depends on the case at hand and considerations of multiple factors such as the targeted location and the size of vasculature as well as the available resources and expertise [3, 5].
Open vascular reconstruction
Open vascular reconstruction is needed for various conditions including plaque removal, shortening or straightening of kinks and even after endovascular therapy fails [3]. Reconstruction has been performed in efforts to preserve lower extremity limbs after surgical excision of soft tissue sarcoma, to treat patients with pancreatic cancer and other tumor resections [6–8]. Different types of procedures are encompassed in reconstruction that includes endarterectomy, extra-anatomic bypass, and interposition grafting [3]. A carotid enterectomy operation, for example, can involve incision into the stenotic region of the artery, use of a shunt to temporarily re-direct blood flow to then remove plaque [3]. To seal the opening in the artery, a patch-like graft may then be used, which can be from both synthetic (e.g. Dacron) or a naturally derived source (e.g. bovine pericardium) [3]. In other instances, and depending on the severity of the vascular condition, reconstruction may require a graft. The graft source can be autologous (e.g. saphenous vein), an allograft (e.g. CryoVein® derived from cadaver saphenous vein), a xenograft (e.g. Contegra® pulmonary valve conduit derived from bovine jugular vein), a synthetic material (e.g. polytetrafluoroethylene, PTFE) or a combination (e.g. Hancock® porcine valve Dacron conduit) [3, 8–11].
A main concern for the vascular graft is the size including the diameter and length, which when mismatched with the native vessel can lead to complications [3]. In adults, the replacement of vessels with diameters less than 6 mm is problematic because the smaller synthetic conduits have poor patency rates [12, 13]. Autologous grafts are not always available or accessible, and the harvesting presents risks of donor site morbidity and complications that can be amplified in patients often suffering from disease [13]. The graft fit is also a challenge for pediatric patients given the smaller conduit sizes and expected vessel outgrowth [14, 15]. Often, the lack of patient matched grafts and emergency repairs call for the construction of improvised grafts from sheets such as bovine patches [16, 17]. A production technique that has gained traction because of the ease in customization is additive manufacturing, also referred to as three-dimensional (3D) printing. In 3D printing, a computer-aided design (CAD) model of the product guides the automated layer by layer material build process. Thus, a change to the size of the product simply involves making digital modifications to the CAD model rather than physical changes to the production equipment. 3D printing with biological materials is an active area of research with potential to develop patient matched grafts using biodegradable materials for vascular tissue engineering. In this review, we present the underlying processes of 3D printing technology and discuss the emerging efforts toward its use for developing vascular prostheses. This work also highlights the performance and testing methods for bioprinted grafts and the United States Food and Drug Administration (FDA) guidelines to consider for prospective graft commercialization.
Brief overview of vasculature
Vascular biology
The vascular system has numerous functions in maintaining homeostasis, the most essential is the transport of oxygen and nutrients to tissues throughout the body [18]. The arteries carry blood away from the heart and branch into smaller arterioles [19]. These vessels eventually branch off into capillary beds where the interchange of blood and tissue takes place [19]. Capillaries recombine into venules and develop further into veins, which transport blood from the body back to the heart [20]. Arteries and arterioles generally have thick walls with small lumens and are round in appearance to support the high-pressure blood flow coming from the heart (Figure 1) [20]. Veins and venules are further from the blood flowing from the heart and have thin walls with large lumens which result in a flat appearance (Figure 1) [20]. This is to accommodate the lower blood pressure found further from the heart and allows for more blood to flow with less resistance [20].
Figure 1.
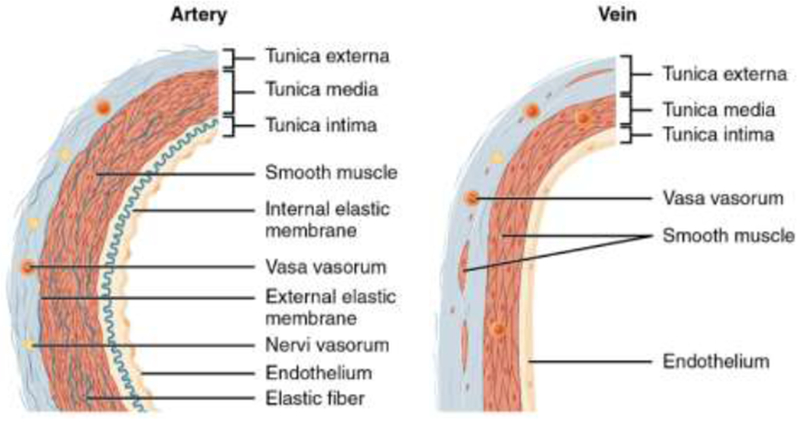
Blood vessel structural features depicting the thicker wall of the artery relative to the vein because of the higher blood flow pressure in the arteries. Reproduced from [164] with permission. Download for free at http://cnx.org/contents/14fb4ad7-39a1-4eee-ab6e-3ef2482e3e22@8.24.
Vascular histology
Blood vessels are comprised of living cells and their extracellular matrix [20]. These cells require an exchange of nutrients and produce waste [20]. Arteries and veins both share three discrete layers known as the tunica intima, tunica media, and tunica externa (also known as the adventitia) [21, 22]. The tunica intima is comprised mainly of epithelial and connective layers of tissue and is lined by the endothelium, a layer which runs continuously throughout the entire circulatory system [21, 22]. Next, the tunica media, or mid-layer of vessels, is much larger in arteries than veins (Figure 1). This mid-layer is made up of smooth muscle cells arranged in circular sheets that are supported by connective tissue composed of elastic and collagen fibers [21, 22]. These muscle sheets can be allowed to contract or relax based on signals from the nerves within the vessel allowing for vasoconstriction or vasodilation [20, 23]. The final layer, the tunica externa, is comprised of a layer of connective tissue made of collagenous and elastic fibers [20, 23]. This layer is generally the thickest in veins and in some small population of large arteries [20, 23]. The externa provides strength to keep the vessels relatively in the same spot and protects vessels from bursting [20, 23]. In larger vessels, the tunica externa’s integrity and function is maintained by the “vasa vasorum”, a microvascular circuit that facilitates the exchange of nutrients and waste [20].
Capturing the specific histological features of native vasculature has been a challenge in vascular graft development. An early effort with a Dacron scaffold strove to design the vessel layers of intima, media and adventitia using endothelial cells, smooth muscle cells with a collagenous matrix, and fibroblasts, respectively [24]. Still, the burst pressure of this vessel was about ten-fold lower than the human saphenous vein [24–26]. Thereafter, a range of techniques that include cell sheet engineering, electrospinning and tubular molding have been used toward developing freestanding vascular conduits [26, 27]. Overall, the studies seldom focus on the intricacies of the histological features and rather develop simplistic tubular structures from one or two materials or cell types. For example, a key proof-of-concept study [28], focused on testing a tissue-engineered vascular grafts in a large animal model that was molded using fibrin gels and seeded with fibroblast cells. Others efforts have focused on preserving the topological features by decellularizing native vasculature [29] and even the human placenta [30]. Using bioprinting to mimic the vascular histological features is even less explored compared to other methods [31]. An attempt includes a double layered vascular tube consisting of smooth muscle and fibroblast cells that was positioned horizontally on the platform and used agarose as support for 3D printing [32]. As with other techniques, bioprinting efforts of multilayered vessel walls that used hydrogels and synthetic polymers have not been specifically designed to mimic the properties of different layers of native vessel [33–36]. In one work [37], endothelial and smooth muscle cells printed in proximity were shown to form cell-cell junctions and resulted in the formation of a structure like the lumen. Thus, accounting for the vascular histology, to design the tunica intima, tunica media, and tunica externa may be promising for the next generation of vascular prostheses. Overall, the bioengineering of vascular histological properties will require the consideration of both the composition and the fiber arrangement in each vessel layer.
Bioprinting of vascular grafts
3D bioprinting processes toward vascular graft development
The basic underlying concept of additive manufacturing is to develop a product through addition of material (liquid, solid, powder) layer by layer, but the processes to achieve this can differ [38]. The American Society for Testing and Materials (ASTM) has categorized these technologies into seven processes: material extrusion, material jetting, powder bed fusion, binder jetting, direct energy deposition, vat photopolymerization, and sheet lamination [39]. Bioprinting efforts thus far have only been based on some of these processes. In most cases, the transformation to a bioprinting process mainly involves hardware adaptation for biological materials that can range from combinations of cells, biomolecules, and biomaterials. Below we provide an overview of 3D printing process categories identified by the ASTM standard (ISO/ASTM 52900) [39, 40] and highlight examples, if any, of efforts toward bioprinting vascular grafts.
Material extrusion
Material extrusion (Figure 2A) is an “additive manufacturing process in which a material is selectively dispensed through a nozzle” [39]. The dispensed layer, which is usually a thermoplastic melt, then cools and serves as foundation to build on the next layer [41]. Fused deposition modeling (FDM) is a popular material extrusion process and these 3D printers are accessible for purchase by consumers at the household level [42]. This is because of the lowering costs of FDM printers, the components of which are often also made by FDM printers (e.g. RepRaps), using common hardware, and run on open source software [42, 43]. Extrusion bio-printing (Table 1) has been applied for tissue engineering typically using syringe-based extruders without the heating elements of standard FDM printers that can damage biological materials [32, 44–47]. The printing solutions often contain cell-laden biopolymers or hydrogels. In one study, the extrusion bioprinting of a hydrogel was followed by a cross-linking step based on calcium ions to produce vascular structures [48]. Bioprinting based on the extrusion process is one of the most common methods used to develop 3D tissues due to a straightforward implementation and well characterized protocols [44, 46]. However, these tissues tend to lack the cell-density needed to successfully survive in vivo integration and have additional concerns of biocompatibility and biodegradation [44, 46]. Although less common, the extrusion bio-printing technique has also been used to create tissue without biomaterials. The scaffold-free approach enables the use of higher cell-densities, which can increase cell-cell contact and cellular signaling within a tissue and has been shown to have more accurate biomimicry [32, 49, 50]. One study incorporated spheroids into the bioink solution rather than using a free cell-suspension to make tissue strands [51]. Spheroids, or spherical conglomerations of cells typically around 300 μm–800 μm are generated from cell cultures for bioprinting [50]. A printer has even been specifically designed to pick up spheroids by vacuum suction and transfer them with high precision onto a needle array for compaction and maturation [45, 52, 53]. Although not exactly an extrusion process, reports show that three-dimensional tissues can be synthesized using this spheroid printer and the constructs can be vascularized with the use of endothelial cells [45, 52, 53]. Spheroids can also be used to express angiogenic factors through transfection of the cells [54–57]. Still, many of the biomaterial-free engineered tissues have weak mechanical properties and can be easily degraded without a structural support [45, 52–58].
Figure 2.
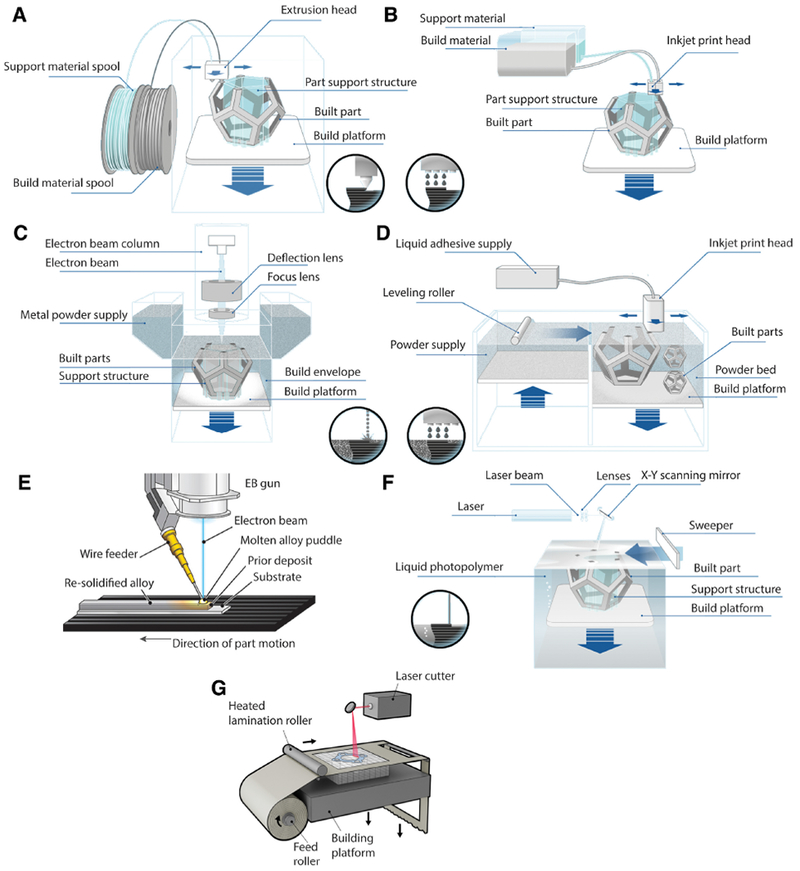
3D printing process categories of the American Society for Testing and Materials (ASTM). (A) Material extrusion. Adapted with permission from [165]. (B) Material jetting. Adapted with permission from [165]. (C) Powder bed fusion, specifically the EBM process. Adapted with permission from [165]. (D) Binder jetting. Adapted with permission from [165]. (E) Directed energy deposition. Adapted with permission and photo courtesy of Sciaky, Inc. [166]. (F) Vat photopolymerization, specifically the SLA process. Adapted with permission from [165]. (G) Sheet lamination, specifically the LOM process. Adapted with permission from Manufacturing Guide [167].
Table 1.
Bioprinting methods that have been used to develop vascular grafts and vascularized constructs.
| Vascular graft 3D printing strategies | 3D printing ASTM process category | Material | Advantages | Disadvantages | Reference |
|---|---|---|---|---|---|
| Extrusion bioprinting | Material extrusion | Synthetic and natural biomaterials | Commonly used method with well characterized procedure. Printing resolution up to 200 μm |
Bioinks have low cell densities and pressure of nozzle may be harmful to cells | 32, 47, 49 |
| Biomaterial-free extrusion bioprinting | None (cells & spheroids) | Increase cell-cell contact, cellular signaling, biomimicry, preservation of cellular fates | Poor mechanical properties | 32, 50–53 | |
| Inkjet bioprinting | Material jetting | Synthetic and natural biomaterials | Single-cell printing resolution | Low cell density designs. Challenge to implement due to clogging of nozzles | 47, 61, 62 |
| Laser-assisted bioprinting | Not applicable | Synthetic and natural biomaterials | Micro-scale printing resolution & additional computer assisted controls | Less common method. Requires rapid gelation of materials after printing, thus tedious process | 47, 65, 66 |
Material jetting
Material jetting (Figure 2B) is an “additive manufacturing process in which droplets of build material are selectively deposited” [39]. The process is also referred to as ink-jet or drop-on-demand printing and applicable to waxy polymers and acrylic photopolymers that have a viscosity in the 20 cP to 40 cP range and can form droplets [59]. These are deposited with high spatial resolution and then transformed to solid by either evaporation or through a reaction that proceeds upon application of an energy source, for example, heat or UV light [60]. Ink-jet bio-printing (Table 1) also uses hydrogel solutions and was designed to print with a single cell resolution in controlled droplets [61, 62]. This technique has been used to horizontally print alginate tubular constructs and bifurcations [63, 64]. The method is promising for printing microstructures and has been used to create a variety of tissue constructs; however, has yet to be used to create functional vasculature. Another material jetting technique that has a drop on demand approach is laser assisted bioprinting (Table 1), which has been leveraged to deposit a cell-laden solution with micro-scale resolution [65, 66]. The benefit of this technique is the addition of computer assisted controls with the ability to create microtissues [65, 66]. These newer technologies may become applicable in the bioprinting of capillary-like structures in the future.
Powder bed fusion
Powder bed fusion (Figure 2C) is an “additive manufacturing process in which thermal energy selectively fuses regions of a powder bed” [39]. This process can be used to additively manufacture metals, ceramics, polymers and composites parts. The energy source that joins the powder can vary with the technique. For example, the electron beam melting (EBM) process uses an electron beam while a laser beam is used for the selective laser sintering (SLS) technique [67]. Once a layer fuses, the next layer of powder is spread by a roller [68]. Powder bed fusion processes have thus far not been applied toward making vascular grafts, the extent of the technology for tissue engineering has encompassed mostly orthopedic products [69, 70]. Titanium has also been studied for making dental implants using powder bed fusion techniques [71]. The process has even been used as a technique to functionalize a biocompatible titanium alloy (Ti-6A1–4V) with copper that has potential as antimicrobial agent [72]. Other metals such as zinc have also been considered for developing biodegradable implants by SLS [73]. For orthopedic products many of which are load bearing, metal is the material of choice.
Binder jetting
Binder jetting (Figure 2D) is an “additive manufacturing process in which a liquid bonding agent is selectively deposited to join powder materials” [39]. The powder bed is lowered after printing a layer of binder and a new layer of powder is typically rolled onto the bed [74]. In contrast, the powder bed fusion process uses a thermal energy source (e.g. laser) to melt and join the powder particles [74]. The term 3D printing (3DP) was initially coined for the binder jetting process, but now synonymous for all additive manufacturing processes [74]. Binder jetting has been shown to produce parts from polymers, metals, ceramics, and composites [67, 74], yet the process has not been of focus toward 3D printing vascular prostheses. The application of binder jetting in medicine has been toward developing structures for bone implants [75, 76]. This includes the fabrication of porous bone prototypes using polyethylene that was sterilized and had promising cytotoxicity results after testing with fibroblast cells [77]. To demonstrate the production of a complex structure with binder jetting, the process was employed to develop a denture framework [78]. A metal powder was used to make the denture and overall, the sintering conditions affected the resulting density of the product [78].
Direct energy deposition
Direct energy deposition (Figure 2E) is an “additive manufacturing process in which focused thermal energy is used to fuse materials by melting as they are being deposited” [39]. The process is mainly used on metal powder [79]. The difference with the powder bed fusion process that also uses an energy source to join powder is that in directed energy deposition, the powder is not spread and rather being actively dispensed by streaming [79]. This process has not been used for bioprinting applications, but the resulting structures are of interest for orthopedic use [80]. For example, directed energy deposition has been investigated for making components with the alloy Ti-6A1–4V [81]. This titanium alloy is of interest in medical designs [82]. Additional studies that investigate the implications of directed energy deposition to produce a part directly for a medical application can determine the advantages of this technique.
Vat photopolymerization
Vat photopolymerization (Figure 2F) is an “additive manufacturing process in which liquid photopolymer in a vat is selectively cured by light-activated polymerization” [39]. Stereolithography (SLA or SL) is a well-known vat photopolymerization technique that produces parts with high accuracy and thus coveted for biomedical applications including dentistry and surgical guides [83–86]. These parts often require post-processing [87, 88]. Although limited to photo-crosslinkable resins, material advances have expanded the application of SLA technology to photopolymer composites with ceramics [42]. In one study [89], a photocurable resin developed for the SLA technique was used to produce bifurcated structures and tubes with diameter smaller than 2 mm, which were tested for biocompatibility. Huber et al. [90] used SLA to 3D print porous tubular structures that were functionalized with biomolecules and resulted in the formation of an endothelial monolayer after cell seeding. The application of vat polymerization toward bioprinting vascular grafts should be accompanied by methods to assess the removal of cytotoxic substances such as uncured resin from the process [90].
Sheet lamination
Sheet lamination (Figure 2G) is an “additive manufacturing process in which sheets of material are bonded to form a part” [39]. The process is amenable to different materials from papers and plastics to metals and ceramics [91]. An example is laminated object manufacturing (LOM), which involves lamination of sheets of paper, typically within the 0.07 mm to 0.2 mm thickness range, and cut into the CAD based shape with CO2 laser [91]. The paper can be adhesive backed, but other bonding mechanism such as thermal, clamping and ultrasonic welding have been feasible [91]. Interchanging the order of process steps that is either stacking first and then cutting or vice versa have also been possible [91]. The sheet lamination process has not been leveraged for bioprinting. However, a cell-based tissue development method is cell-sheet engineering [58]. This method uses a thermoresponsive nanofiber polymer to detach entire sheets of cells with the associated extracellular matrix from cell culture [58]. Following this step, multiple sheets may be stacked to create a tissue [58]. The prospective automation and systematization of this cell sheet approach to make tubular structures may be a step toward the application of sheet lamination for bioprinting vascular grafts.
Aside from process classifications, the ASTM standard (ISO/ASTM 52900) provides an overview of differences in material type, feedstock, distribution, and other aspects of additive manufacturing [39]. This standard together with others [92] established for additive manufacturing are resources that serve to maintain consistent terminology for product development and regulation. Emerging techniques may lead to changes in these standards. Future bioprinting techniques may involve hybrids of multiple processes or lead to the development of new methods that may not align with the underlying principles of the current ASTM processes. For example, a new printing method has recently been described that uses projected light to solidify liquid resin all at once instead of layer by layer [93]. Still, herein we strove to classify the bioprinting techniques as best as possible according to their underlying engineering principles. Other than vascular prostheses, several strategies have been used to promote vascularization in tissue constructs, which is beyond the scope of this review on vascular grafts. In brief, a main example of a 3D bioprinted tissue construct [94] involved embedding multiple materials such as a sacrificial ink to design a vascular network within a hydrogel construct. The same group [95] then used this multi-material printing strategy, including a temperature sensitive fugitive ink, to design thick vascularized tissues (> 1 cm), maintained in culture for ~6 weeks. Other reviews [27, 31, 33, 96] provide insight on various aspect of bioprinting vascular constructs and present additional perspectives on the bioprinting of vascular grafts.
Materials used for 3D bioprinting of vascular grafts
Graft materials currently on the market are derived from both synthetic and natural sources. Commercially available grafts from synthetic materials are typically knitted or woven [97]. Examples include Uni-Gaft®, GORE-TEX®, and FUSION that are made with the polymers polyethylene terephthalate (PET, Dacron®), expanded polytetrafluoroethylene (ePTFE, Teflon®), and a combination of both, respectively [97–101]. A graft derived from a natural source includes the Artegraft®, which is produced from bovine collagen [102]. Some grafts are hybrids of synthetic and natural sources, an example is AlboGraft® that combines bovine skin collagen and polyester filaments [103]. These materials may be used for 3D printing vascular grafts since the technology has been adapted for different ink types [38]. For instance, the FDM process that intakes standard rigid thermoplastic filament was modified to produce flexible structures from a heat curable silicone gel [104, 105]. Other efforts that pushed the boundaries of materials for additive manufacturing involved the 3D printing of glass [106], fiber-reinforcement [107], and even magnetic [108] inks. Vascular graft materials for 3D printing should importantly be biocompatible and non-thrombogenic and have relevant mechanical integrity and hemodynamic properties for the given application [13, 109–112]. Most research on 3D printing of vascular graft have used biodegradable inks, taking a tissue engineering approach toward regenerating vascular tissue. For example, SLA technology was used to develop a biodegradable vascular graft from poly(propylene) (PPF) with 1 mm inner diameter and 150 μm wall thickness that was maintained in vivo up to 6 months [113]. In another instance, a 3D printed template made by the SLA process was used to develop a patient matched tissue-engineered vascular graft (TEVG) by electrospinning biodegradable polymers [114]. The TEVG was subsequently tested in a large animal model [114]. Other printing efforts for tissue engineering vascular grafts used cell-laden inks [115], but overall 3D printed conduits from biodegradable materials or otherwise have not yet attained commercial stature.
Assessment of bioprinted vascular grafts
Developing vascular grafts is an iterative process that requires in vitro and subsequent in vivo characterization efforts. Few studies have extensively characterized bioprinted grafts as these are a new technological advancement. Many bioprinted vessels lack the appropriate mechanical properties to withstand in vivo hydrostatic pressures and the strain involved in surgical grafting procedures. Here we highlight the testing methods to reduce the risk of failure for vascular grafts that also apply to bioprinted grafts. The characterizations, summarized in Table 2, are grouped under the risk categories for vascular prosthesis proposed by the U.S. FDA [116] toward an outlook for clinical approval.
Table 2.
Techniques to assess vascular grafts based on FDA identified risks for vascular prostheses.
| Risks for vascular prostheses identified by FDA116 | Examples of vascular graft assessment techniques | Reference | |
|---|---|---|---|
| in vitro | in vivo | ||
| Thrombosis, Embolic Events, Occlusion, & Stenosis | • Cell seeding & response to heparin •Cell penetration in graft material •Microscopy •Simulation & flow loop models |
• Implantation in animal model •Diagnostic techniques •Monitoring & immunostaining |
56, 116, 120, 121, 123 |
| Leakage & Graft Disruption | • Microfluidic platform with cultured endothelial cell • Flow loop models |
• Implantation in animal model • Dye injection & optical density measurement • Microscopic analysis of explanted grafts |
124–127 |
| Biocompatibility, Allergic Reaction | •Cell attachment, proliferation, penetration & viability • Gene expression of macrophages and cytokines |
• Response to immunosuppressive & antibacterial drugs •Monitoring & biochemical testing •Gene expression of macrophages & cytokines |
135, 137–139 |
| Aneurysm | • Computational flow dynamic (CFD) analysis •Flow chamber model |
• Animal model for aneurysm •Imaging |
142–145 |
| Infection/Sterility | • Autoclaving | • Hoechst fluorescent staining for cytoplasmic DNA | 153, 154, 156 |
| Performance | • Mechanical testing • Flow loop models |
• Implantation in animal model • Imaging • Histological analysis |
56, 123, 135 |
Thrombosis, Embolic Events, Occlusion, & Stenosis
Immunostaining, cell penetration studies, simulations, macroscopy and other visual inspection techniques represent in vitro methods to determine graft occlusion [51, 104]. Vascular stenosis has been linked to the proliferation of vascular smooth muscle cells (VSMCs) [117, 118]. An in vitro technique that Refson et al. [117] used to assess graft patency involved evaluating the response of VSMCs on the graft to heparin that reduces cell proliferation [119]. Similarly, other studies have shown that immediate thrombosis, vascular rejection, and loss of vascular integrity is associated with endothelial cell activation present on the implanted grafts [56, 120, 121]. Thus, in one in vivo study, engineered blood vessels were intentionally grafted without being endothelialized [56, 120, 121]. Vessel thrombogenicity is characterized in vivo based on the implantation results, if the vessel causes coagulation or a local blood clot due to poor healing processes post-implantation [122]. Doppler signaling can reveal graft failures, which has been shown to occur in the first few days from occlusion and thrombosis formation attributed to the collagen matrix present in the vessels [56]. In another study [123] that developed pulsatile myocardial tubes, observations of the animals following implantation showed no thrombosis. This study reflects the advantage of using cells derived from the same animal species and biodegradable scaffolds [123]. In bypass surgeries, a patient’s own blood vessels currently remain the best material for grafting [56, 122]
Leakage & Graft Disruption
An example of characterizations recommended by the FDA to assess the risk of leakage are tests of graft porosity and water permeability [116]. An FDA recommended graft disruption test is the suture retention strength [116]. In one study [124], an in vitro assay to study blood vessel permeability has been suggested by culturing endothelial cells in a three-dimensional, microfluidic, platform and measuring the flux of two fluorescent dyes. Radu et al. [125] developed an in vivo method to assess blood vessel permeability that relied on optical density measurements of an injected dye to determine the amount captured by tissue. In another study [56], histological analysis was used to determine intramural blood infiltrations of cell-based grafts transplanted intrafemorally. Leakage was not found to reduce vessel patency and relative vessel architecture was maintained after seven days [56]. Other analysis of explanted grafts [126, 127] can provide further insight on graft leakage and disruption. Efforts to develop leak-proof grafts include the incorporation of collagen within Dacron [128]. Since then, other studies have focused on strategies that involve incorporating different materials together to “seal” and prevent graft leakage [129–131]. Other studies have taken cues from the textile industry to develop techniques that include weaving, knitting or braiding that produce patterns that may be suitable to withstand leaks [132]. Thus, the inclusion of multiple materials or a finely-tuned design pattern can be promising to prevent leaks and disruptions in bioprinted grafts.
Biocompatibility, Allergic Reaction
A concern with implanting synthesized materials that do not originate from the host is immune system activation, which leads to tissue rejection and allergic reactions [122, 133–137]. Lee et al. [135] assessed the biocompatibility of electrospun vascular grafts from both natural and synthetic biodegradable polymers in vitro by looking at cell viability and mitochondrial metabolic activity. To test the biocompatibility of polyurethane based biodegradable vascular grafts in vitro, the grafts were seeded with fibroblast and macrophage cells to then determine the macrophage gene expression [138]. These were also compared with in vivo macrophage and cytokine expressions and the study also involved an analysis of the type of proliferated cells and cell infiltration [138]. Immunosuppressive drugs have been used as a strategy to prevent allergic reaction when observing the in vivo performance of a graft [139]. Even with immunosuppression, endothelial cells can express the major histocompatibility complex (MHC) class II proteins [140]. The mismatch of these proteins on a vascular graft is known to lead to poor in vivo performance and complications [140, 141].
Aneurysm
Many aneurysms are believed to occur from inflammatory processes at locations of hemodynamic shear stress [142]. To study the effect of flow stresses on endothelial cells lining the vasculature, Kanko et al. [143] developed an in vitro intracranial aneurysm model that was subject to computational flow dynamics (CFD) analysis. The model was based on a patient-specific vasculature 3D printed in silicone, coated with fibronectin and cultured with endothelial cells [143]. Nowicki et al. [142] compared endothelial cell phenotype across an in vitro flow chamber model of a straight artery, a bifurcation and a bifurcation aneurysm. In vivo, magnetic resonance imaging (MRI) was used to detect abdominal aortic aneurysm based on the collagen content in a mouse model [144]. The aneurysm in this model was chemically induced, other animal models of aneurysm are made by physical techniques or use genetically predisposed animals [145]. Overall, aneurysm formations are a rare complication of certain types of grafts, including Dacron grafts used in various surgical procedures [146, 147]. Currently, bioprinted grafts have no record of aneurysms formation after in vivo implantation [146, 147]. This is likely because these grafts have not been tested long-term in vivo. In one study [113], biodegradable grafts (n = 6) printed using the SLA techniques were implanted for a six month period in mice without signs of aneurysms. Mechanical mismatch between the graft and native tissue has been identified as a cause of aneurysms [148]. In one study [149], collagen was crosslinked to increase the mechanical strength of tubes (< 1 mm diameter) made by molding and assessed as potential vascular grafts. The graft’s burst pressure had improved (75 mm Hg collagen as is versus 1300 mm Hg crosslinked collagen) along with the compliance, but the values were comparable to the vein, and did not match the artery [149, 150]. Finding a biomaterial processable by 3D printing that has mechanical properties matching the targeted native vessel remains a challenge to address for progress in using bioprinting to make vascular grafts. Overall, Bozeghrane et al. [151] recommended standardized multicenter studies to improve the assessment of endovascular devices for aneurysm therapy.
Infection Sterility
Sterility of surgical implants is a necessity in clinical practice. An example of ensuring a modern standard of sterility is through autoclaving, which has been used to sterilize hydrogels for bioprinting vascular-like structures [152]. Jia et al. [153] sterilized gelatin methacryloyl (GelMA) based inks for bioprinting a vascular construct. Sterilization practices should also be applied to the additive manufacturing system to maintain bioprinted graft sterility [154]. In order to reduce the risk of bacterial infection, one study 3D printed small diameter vascular grafts with antibacterial properties by using FDA approved biodegradable inks coated with nitric oxide (NO) [155]. In vivo, graft infection may be observed in situ by using Hoechst fluorescent staining for cytoplasmic DNA [156]. Overall, limiting the use of synthetic material is known to reduce the risk of foreign body reaction and graft infection [122]
Performance
An in vitro assessment of vascular graft performance includes mechanical characterization, which for electrospun nanofiber scaffolds has been reported as tensile tests [135]. L’heureux et al. [56] designed an in vitro system to study the integrity of a cell-based blood vessel for tissue engineering that was circulated with phosphate-buffered saline and pressurized successively. In vivo, implanted femoral grafts in mongrel dogs were assessed after seven days using angiography, explantation of the grafts revealed a 50% patency rate for the six grafts [56]. The successful grafts showed no signs of degradation, tearing, or dilation and the mechanical strength was attributed to the collagenous matrix present in the adventitia [56]. In another effort [123], pulsatile myocardial tubes were transplanted in place of the abdominal aorta of athymic rats, and survived a four-week observation period. The performance evaluation included histological analysis and transmission electron microscopy, which showed the beating tubes were composed of cardiac tissues resembling the native cardiovascular tissue [123]
Commercialization of bioprinted vascular grafts
Regulation of medical products and devices in the United States and vascular grafts on the market
The U.S. FDA is an agency within the U.S. Department of Health and Human Services (HHS) that oversees more than $2.5 trillion products [157]. These are food, drugs, biologies, medical devices, electronic, cosmetics, veterinary, and tobacco products [157]. The FDA’s mandate is to protect the public health by ensuring the safety and effectiveness of these products while supporting innovation [157]. To achieve this, the FDA issues regulations that are federal laws some of which are established based on the Federal Food, Drug, and Cosmetic Act (FD&C Act) that was enacted by congress [157]
The vascular grafts currently on the market are considered medical devices by the FDA [157]. Medical devices are administered by the Office of Medical Products and Tobacco [157]. The latter has different centers including the Center for Devices and Radiological Health (CDRH), the Center for Drug Evaluation and Research (CDER) and the Center for Biologies Evaluation and Research (CBER) [157]. At present, CDRH oversees the regulation of vascular grafts and classifies medical devices into three categories based on the “intended use”, the “indications for use” and the risk level to the patient or user [157]. From lowest to highest risk, these categories are Class I, II and III medical devices that shape the type of application to file for receiving clearance from the FDA to market (Figure 3) [157]. In most cases, Class I and Class II devices require filing a Premarket Notification (PMN), also referred as 510(k), to notify the FDA of an intent to market [157]. A 510(k) is granted if the device can be shown to be equivalent to a device already marketed in either of the three classification categories [157]. In some cases, especially for Class I devices, a 510(k) clearance may not be needed, but an application for the device must still be filed to the FDA toward obtaining an exemption for a 510(k) clearance [157]. For Class III devices defined as “those that sustain or support human life” a 510(k) clearance may not suffice, and these require filing for a Premarket Approval (PMA) clearance [157]. To receive a PMA, the medical device requires substantial backing of “sufficient valid scientific evidence to assure that the device is safe and effective for its intended use(s)” [157]
Figure 3.
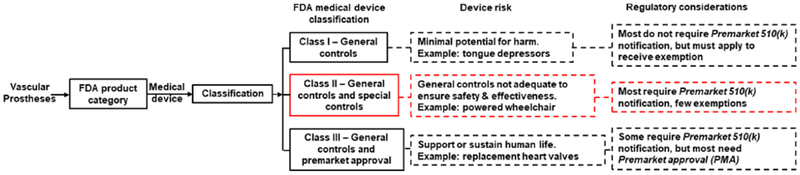
FDA framework for the regulation of medical devices highlighting the regulatory pathway for vascular prostheses currently on the market. Specifically, current vascular prostheses are categorized as Class II medical devices with “special controls”and should align with the nonbinding recommendations provided by the FDA in the “Guidance Document for Vascular Prostheses 510(k) Submissions - Guidance for Industry and FDA staff’ [116, 157]
Vascular grafts from materials such as PET or PTFE with biological or synthetic coatings, for example intended to gain vascular access, are typically Class II medical devices with “special controls” (Figure 3) [157]. The special controls require additional measures such as complying with the FDA’s “Guidance Document for Vascular Prostheses 510(k) Submissions - Guidance for Industry and FDA staff’ [116, 157]. The guidance identifies potential risks and associated control measures, for example to comply with standards for endovascular prostheses such as provided by ANSI/AAMI ISO-25539–1 [157, 158]
Overall the FDA guidance applies to vascular graft prosthesis of 6 mm in diameter and larger [157]. Following the guidance may also reduce the classification level for vascular grafts that are less than 6 mm from Class III to Class II devices [157]. Still, the guidance was not developed for vascular grafts made entirely of materials derived from animals and that are intended for coronary or neurovasculature, which are likely Class III devices [157]. With progress toward the next generation of vascular grafts, other FDA centers such as CDER and CEBR may begin to have a regulatory role, for example to approve drug-eluting vascular grafts or cell-seeded ones for tissue engineering.
Technical considerations for commercializing 3D printed vascular grafts
To receive FDA clearance, a 3D printed vascular graft has to abide by the same procedure as medical devices and products regardless of the fabrication technique [157]. The type of clearance will then depend on the risk-based classification of the vascular prosthesis [157]. In December 2017, the FDA issued a “Guidance for Industry and Food and Drug Administration Staff’ on the “Technical Considerations for Additively Manufactured Medical Devices” [157, 159]. Unlike its regulations, the FDA’s guidance publications are not legally binding and only express the agency’s perspective on a given topic [157, 159, 160]. Still, the guidance is a tool to facilitate meeting the Quality System (QS) requirements of the 3D printed graft by providing testing and characterization approaches relevant to the fabrication method [157, 159, 160]. In particular, a section of the guidance outlines Design and Manufacturing Considerations while another part focuses on Device Testing Considerations [157, 159, 160]. The Design and Manufacturing Considerations covers overall aspects and patient-matched device design, software workflow, material controls, post-processing, process validation and acceptance activities, and quality data [159]. An example of Device Testing Considerations are the provision of device description, mechanical testing, dimensional measurements, material characterization, residue removal and sterilization, and biocompatibility [159]. The FDA has held a webinar [160] and provided a review [161] in support of this guidance. Collaborations have also taken place with agencies such as the National Institute of Standards and Technology (NIST) [162]. Other resources on FDA’s outlook on 3D printed medical products include an overview by Christensen and Rybicki [163]. This review covers the applications of 3D printing in medicine through categories that include anatomical models, modified anatomical models, and virtual surgical planning with surgical templates [163]
Conclusion
Vascular grafts are tools for open vasculature reconstruction procedures that are commercially available from both synthetic or natural sources. Challenges in using current vascular prostheses to treat vascular disease or trauma include i) the size mismatch between the native conduit and the graft, ii) the inability of the graft to evolve, which is needed for some patients, especially the pediatric population that can outgrow the prosthesis, iii) the poor accessibility or the lack of availability of autologous grafts, and iv) the potential of immunogenic response for xenografts and synthetic grafts. Bioprinting has emerged as a promising avenue to develop vascular grafts. As an additive manufacturing technique, bioprinting presents an automated approach to develop on demand patient-matched grafts based on medical images. These grafts can often serve for tissue engineering with the use of biodegradable materials that serve as temporary scaffolds to support the regrowth of the native tissue vessel. Advances in bioprinting have included the development of biologic inks from combinations of cells, biomaterials and biomolecules with accompanying hardware and software adaptations. Here, we provided a snapshot of current examples of efforts to bioprint vascular grafts and the testing methods developed to assess the risk of graft failure. Thus far, the FDA has not approved a 3D bioprinted vascular graft for clinical treatment. Still, we presented the current regulatory framework for vascular grafts on the market and provided the guidance to consider toward receiving approval for vascular prostheses. Along with the innovations, future efforts toward the realization of patient-matched bioprinted vascular grafts should focus on validation, reproducibility and systematization throughout the process life cycle. A framework to assess bioprinted vascular grafts that includes in vitro, then in vivo and involve long-term follow-up can support translational efforts and facilitate comparisons across studies. This framework should build on the current characterization techniques used for bioprinted vascular grafts and include assessment methods to reduce the risks associated with vascular grafts identified by the FDA (leakage, biocompatibility, aneurysm, infection, and performance). Systematization can be supported by the integration of artificial intelligence for material and process parameter selection during bioprinting and the implementation of standard testing protocols.
Acknowledgments
All authors have read the journal’s policy on disclosure of potential conflicts of interest and have no conflicts to declare. All authors have read the journal’s authorship agreement and that the manuscript has been reviewed by and approved by all named authors.
List of Abbreviations:
- 3D
Three-dimensional
- CAD
computer-aided design
- PTFE
polytetrafluoroethylene
- FDA
United States Food and Drug Administration
- ASTM
American Society for Testing and Materials
- EBM
electron beam melting
- SLS
selective laser sintering
- SLA or SL
stereolithography
- LOM
laminated object manufacturing
- MEMS
microelectromechanical systems
- VEGF
vascular endothelial growth factor
- bFGF
basic fibroblast growth factor
- PDGF
platelet-derived growth factor
- PDMS
polydimethyl siloxane
- PGS
poly(glycerol sebacate)
- PET
polyethylene terephthalate
- ePTFE
expanded polytetrafluoroethylene
- FDM
fused deposition modeling
- TEVG
tissue-engineered vascular graft
- PPF
poly(propylene)
- VSMCs
vascular smooth muscle cells
- CFD
computational flow dynamics
- GelMA
gelatin methacryloyl
- HHS
U.S. Department of Health and Human Services
- FD&C Act
Federal Food, Drug, and Cosmetic Act
- CDRH
Center for Devices and Radiological Health
- CDER
Center for Drug Evaluation and Research
- CBER
Center for Biologics Evaluation and Research
- PMN
Premarket Notification
- PMA
Premarket Approval
- NIST
National Institute of Standards and Technology
- 3DP
3D printing
- MRI
magnetic resonance imaging
- NO
nitric oxide
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Vascular Trauma: Society for Vascular Surgery (SVS); 2018. [11/September/2018], Available from: vascular.org. [Google Scholar]
- 2.Luscher TF, Creager MA, Beckman JA, Cosentino F. Diabetes and Vascular Disease. Circulation. 2003;108(13): 1655–61. [DOI] [PubMed] [Google Scholar]
- 3.Hans SS, Weaver MR, Bove PG, Long GW. Endovascular and open vascular reconstruction: a practical approach. Boca Raton: CRC Press; 2017. p. p. [Google Scholar]
- 4.Endovascular versus surgical treatment in patients with carotid stenosis in the Carotid and Vertebral Artery Transluminal Angioplasty Study (CAVATAS): a randomised trial. The Lancet. 2001. ;357(9270): 1729–37. [PubMed] [Google Scholar]
- 5.Wiebers DO. Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. The Lancet. 2003;362(9378):103–10. [DOI] [PubMed] [Google Scholar]
- 6.Bianchi C, Ballard JL, Bergan JH, Killeen J. Vascular reconstruction and major resection for malignancy. Archives of Surgery. 1999; 134(8):851–5. [DOI] [PubMed] [Google Scholar]
- 7.Nishinari K, Krutman M, Aguiar S Junior, Pignataro BS, Yazbek G, Zottele Bomfim GA, Teivelis MP, Wolosker N. Surgical outcomes of vascular reconstruction in soft tissue sarcomas of the lower extremities. Journal of Vascular Surgery. 2015;62(l):143–9. [DOI] [PubMed] [Google Scholar]
- 8.Sgroi MD, Narayan RR, Lane JS, Demirjian A, Kabutey N-K, Fujitani RM, Imagawa DK. Vascular reconstruction plays an important role in the treatment of pancreatic adenocarcinoma. Journal of Vascular Surgery. 2015;61(2):475–80. [DOI] [PubMed] [Google Scholar]
- 9.Contegra® Pulmonary Valved Conduit Medtronic; 2018. [11/November/2018], Available from: www.medtronic.com.
- 10.P Carney J, M Zhang L, J Larson J, T Lahti M, A Robinson N, P Dalmasso A, W Bianco R. The Hancock® Valved Conduit for Right Ventricular Outflow Tract Reconstruction in Sheep for Assessing NewDevices 2017. 472–80 p. [PubMed]
- 11.Zehr BP, Niblick CJ, Downey H, Ladowski JS. Limb Salvage With CryoVein Cadaver Saphenous Vein Allografts Used for Peripheral Arterial Bypass: Role of Blood Compatibility. Annals of Vascular Surgery. 2011. ;25(2): 177–81. [DOI] [PubMed] [Google Scholar]
- 12.Peripheral Vascular Surgery for Large Vessel Vasculitis. Inflammatory Diseases of Blood Vessels.
- 13.Pashneh-Tala S, MacNeil S, Claeyssens F. The Tissue-Engineered Vascular Graft-Past, Present, and Future. Tissue engineering Part B, Reviews. 2015;22(1):68–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amaro E, Pophal S, Zoldos J. Vascular Reconstruction in a Neonate after Iatrogenic Injury during Cardiac Catheterization. Plastic and Reconstructive Surgery - Global Open. 2017;5(12):el600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Min S-K, Cho S, Kim H-Y, Kim SJ. Pediatric Vascular Surgery Review with a 30-Year-Experience in a Tertiary Referral Center. Vascular specialist international. 2017;33(2):47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kohler C, Attigah N, Demirel S, Zientara A, Weber M, Schwegler I. A technique for a self-made bifurcated graft with bovine pericardial patch in infectious vascular reconstruction. Journal of Vascular Surgery Cases and Innovative Techniques. 2016;2(4):158–60. [Google Scholar]
- 17.Stather PW, Howard AQ. A Novel Technique for Bifurcated Bovine Plus Omniflow Aortic Graft Reconstruction. European Journal of Vascular and Endovascular Surgery. 2017;53(1): 104. [DOI] [PubMed] [Google Scholar]
- 18.August K The Anatomy and Physiology of Capillaries. New Haven: Yale University Press; 1922. [Google Scholar]
- 19.Dale HH. The Oliver-Sharpey Lectures On the Activity of the Capillary Blood Vessels, and its Relation to Certain Forms of Toxaemia: Delivered before the Royal College of Physicians of London. British medical journal. 1923;1(3259):1006–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thiriet M Anatomy and Physiology of the Circulatory and Ventilatory Systems. New York: Springer; 2014. [Google Scholar]
- 21.Clark JM, Glagov S. Transmural organization of the arterial media. The lamellar unit revisited. Arteriosclerosis. 1985;5(1): 19–34. [DOI] [PubMed] [Google Scholar]
- 22.Mirea O, Donoiu I, Ple§ea IE. Arterial aging: a brief review. Rom J Morphol Embryol. 2012;53(3):473–7. [PubMed] [Google Scholar]
- 23.Prince EA, Ahn SH. Basic vascular neuroanatomy of the brain and spine: what the general interventional radiologist needs to know. Seminars in interventional radiology. 2013;30(3):234–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weinberg C, Bell E. A blood vessel model constructed from collagen and cultured vascular cells. Science. 1986;231(4736): 397–400. [DOI] [PubMed] [Google Scholar]
- 25.Konig G, McAllister TN, Dusserre N, Garrido SA, Iyican C, Marini A, Fiorillo A, Avila H, Wystrychowski W, Zagalski K, Maruszewski M, Jones AL, Cierpka L, de la Fuente LM, L’Heureux N. Mechanical properties of completely autologous human tissue engineered blood vessels compared to human saphenous vein and mammary artery. Biomaterials. 2009;30(8): 1542–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Song HHG, Rumma RT, Ozaki CK, Edelman ER, Chen CS. Vascular Tissue Engineering: Progress, Challenges, and Clinical Promise. Cell Stem Cell. 2018;22(3):340–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vyas C, Pereira R, Huang B, Liu F, Wang W, Bartolo P. Engineering the vasculature with additive manufacturing. Current Opinion in Biomedical Engineering. 2017;2:1–13. [Google Scholar]
- 28.Syedain Z, Reimer J, Lahti M, Berry J, Johnson S, Tranquillo RT. Tissue engineering of acellular vascular grafts capable of somatic growth in young lambs. Nature communications. 2016;7:12951-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin C-H, Hsia K, Ma H, Lee H, Lu J-H. In Vivo Performance of Decellularized Vascular Grafts: A Review Article. International journal of molecular sciences. 2018; 19(7):2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schneider KH, Enayati M, Grasl C, Walter I, Budinsky L, Zebic G, Kaun C, Wagner A, Kratochwill K, Redl H, Teuschl AH, Podesser BK, Bergmeister H. Acellular vascular matrix grafts from human placenta chorion: Impact of ECM preservation on graft characteristics, protein composition and in vivo performance. Biomaterials. 2018;177:14–26. [DOI] [PubMed] [Google Scholar]
- 31.Datta P, Ayan B, Ozbolat IT. Bioprinting for vascular and vascularized tissue biofabrication. Acta Biomaterialia. 2017;51:1–20. [DOI] [PubMed] [Google Scholar]
- 32.Norotte C, Marga FS, Niklason LE, Forgacs G. Scaffold-free vascular tissue engineering using bioprinting. Biomaterials. 2009;30(30):5910–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hoch E, Tovar GEM, Borchers K. Bioprinting of artificial blood vessels: current approaches towards a demanding goal. European Journal of Cardio-Thoracic Surgery. 2014;46(5):767–78. [DOI] [PubMed] [Google Scholar]
- 34.Skardal A, Zhang J, McCoard L, Oottamasathien S, Prestwich GD. Dynamically Crosslinked Gold Nanoparticle - Hyaluronan Hydrogels. Advanced Materials. 2010;22(42):4736–40. [DOI] [PubMed] [Google Scholar]
- 35.Skardal A, Zhang J, McCoard L, Xu X, Oottamasathien S, Prestwich GD. Photocrosslinkable hyaluronan-gelatin hydrogels for two-step bioprinting. Tissue engineering Part A. 2010;16(8):2675–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Skardal A, Zhang J, Prestwich GD. Bioprinting vessel-like constructs using hyaluronan hydrogels crosslinked with tetrahedral polyethylene glycol tetracrylates. Biomaterials. 2010;31(24):6173–81. [DOI] [PubMed] [Google Scholar]
- 37.Wu PK, Ringeisen BR. Development of human umbilical vein endothelial cell (HUVEC) and human umbilical vein smooth muscle cell (HUVSMC) branch/stem structures on hydrogel layers via biological laser printing (BioLP). Biofabrication. 2010;2(1):014111. [DOI] [PubMed] [Google Scholar]
- 38.Chua CK, Wong CH, Yeong WY. Chapter Five - Material Characterization for Additive Manufacturing In: Chua CK, Wong CH, Yeong WY, editors. Standards, Quality Control, and Measurement Sciences in 3D Printing and Additive Manufacturing: Academic Press; 2017. p. 95–137. [Google Scholar]
- 39.International A ASTMISO/ASTM52900–15 Standard Terminology for Additive Manufacturing - General Principles - Terminology. West Conshohocken, PA: 2015. [Google Scholar]
- 40.Mohamed OA, Masood SH, Bhowmik JL. Optimization of fused deposition modeling process parameters: a review of current research and future prospects. Advances in Manufacturing. 2015;3(l):42–53. [Google Scholar]
- 41.Wang X, Jiang M, Zhou Z, Gou J, Hui D. 3D printing of polymer matrix composites: A review and prospective. Composites Part B: Engineering. 2017;110:442–58. [Google Scholar]
- 42.Yun JS, Park T-W, Jeong YH, Cho JH. Development of ceramic-reinforced photopolymers for SLA 3D printing technology. Applied Physics A. 2016;122(6):629. [Google Scholar]
- 43.Wittbrodt BT, Glover AG, Laureto J, Anzalone GC, Oppliger D, Irwin JL, Pearce JM. Life-cycle economic analysis of distributed manufacturing with open-source 3-D printers. Mechatronics. 2013;23(6):713–26. [Google Scholar]
- 44.Hinton TJ, Jallerat Q, Palchesko RN, Park JH, Grodzicki MS, Shue HJ, Ramadan MH, Hudson AR, Feinberg AW. Three-dimensional printing of complex biological structures by freeform reversible embedding of suspended hydrogels. Sci Adv. 2015;1(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ong CS, Fukunishi T, Zhang H, Huang CY, Nashed A, Blazeski A, DiSilvestre D, Vricella L, Conte J, Tung L. Biomaterial-free three-dimensional bioprinting of cardiac tissue using human induced pluripotent stem cell derived cardiomyocytes. Scientific reports. 2017;7(1):4566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu W, DeConinck A, Lewis JA. Omnidirectional Printing of 3D Microvascular Networks. Advanced Materials. 2011;23(24):H178–H83. [DOI] [PubMed] [Google Scholar]
- 47.Pati F, Jang J, Lee JW, Cho D-W. Extrusion bioprinting Essentials of 3D Biofabrication and Translation: Elsevier; 2015. p. 123–52. [Google Scholar]
- 48.Tabriz AG, Hermida MA, Leslie NR, Shu W. Three-dimensional bioprinting of complex cell laden alginate hydrogel structures. Biofabrication. 2015;7(4):045012. [DOI] [PubMed] [Google Scholar]
- 49.Bertassoni LE, Cardoso JC, Manoharan V, Cristino AL, Bhise NS, Araujo WA, Zorlutuna P, Vrana NE, Ghaemmaghami AM, Dokmeci MR, Khademhosseini A. Direct-write bioprinting of cellladen methacrylated gelatin hydrogels. Biofabrication. 2014;6(2):024105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jakab K, Norotte C, Damon B, Marga F, Neagu A, Besch-Williford CL, Kachurin A, Church KH, Park H, Mironov V, Markwald R, Vunjak-Novakovic G, Forgacs G. Tissue engineering by self-assembly of cells printed into topologically defined structures. Tissue Eng Part A. 2008;14(3):413–21. [DOI] [PubMed] [Google Scholar]
- 51.Yu Y, Moncal KK, Li JQ, Peng WJ, Rivero I, Martin JA, Ozbolat IT. Three-dimensional bioprinting using self-assembling scalable scaffold-free “tissue strands” as a new bioink. Scientific Reports. 2016;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moldovan NI, Hibino N, Nakayama K. Principles of the Kenzan method for robotic cell spheroid-based three-dimensional bioprinting. Tissue Engineering Part B: Reviews. 2017;23(3):237–44. [DOI] [PubMed] [Google Scholar]
- 53.Ong CS, Yesantharao P, Hibino N. 3D and 4D Scaffold-Free Bioprinting. 3D and 4D Printing in Biomedical Applications: Process Engineering and Additive Manufacturing. 2019:317–42. [Google Scholar]
- 54.Egana JT, Fierro FA, Kruger S, Bornhauser M, Huss R, Lavandero S, Machens HG. Use of human mesenchymal cells to improve vascularization in a mouse model for scaffold-based dermal regeneration. Tissue Eng Part A. 2009; 15(5): 1191–200. [DOI] [PubMed] [Google Scholar]
- 55.Geiger F, Lorenz H, Xu W, Szalay K, Kasten P, Claes L, Augat P, Richter W. VEGF producing bone marrow stromal cells (BMSC) enhance vascularization and resorption of a natural coral bone substitute. Bone. 2007;41(4):516–22. [DOI] [PubMed] [Google Scholar]
- 56.L’heureux N, Paquet S, Labbe R, Germain L, Auger FA. A completely biological tissue-engineered human blood vessel. TheFASEB Journal. 1998;12(l):47–56. [DOI] [PubMed] [Google Scholar]
- 57.Yang J, Zhou W, Zheng W, Ma Y, Lin L, Tang T, Liu J, Yu J, Zhou X, Hu J. Effects of myocardial transplantation of marrow mesenchymal stem cells transfected with vascular endothelial growth factor for the improvement of heart function and angiogenesis after myocardial infarction. Cardiology. 2007; 107(1): 17–29. [DOI] [PubMed] [Google Scholar]
- 58.Elloumi-Hannachi I, Yamato M, Okano T. Cell sheet engineering: a unique nanotechnology for scaffold-free tissue reconstruction with clinical applications in regenerative medicine. J Intern Med. 2010;267(1): 54–70. [DOI] [PubMed] [Google Scholar]
- 59.Gibson I, Rosen D, Stucker B. Material Jetting. Additive Manufacturing Technologies: 3D Printing, Rapid Prototyping, and Direct Digital Manufacturing. New York, NY: Springer New York; 2015. p. 175–203. [Google Scholar]
- 60.He Y, Wildman RD, Tuck CJ, Christie SDR, Edmondson S. An Investigation of the Behavior of Solvent based Polycaprolactone ink for Material Jetting. Scientific Reports. 2016;6:20852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nakamura M, Kobayashi A, Takagi F, Watanabe A, Hiruma Y, Ohuchi K, Iwasaki Y, Horie M, Morita I, Takatani S. Biocompatible inkjet printing technique for designed seeding of individual living cells. Tissue Eng. 2005; 11 (11–12): 1658–66. [DOI] [PubMed] [Google Scholar]
- 62.Nishiyama Y, Henmi C, Iwanaga S, Nakagawa H, Yamaguchi K, Akita K, Mochizuki S, Takiura K, Nakamura M. Inkjet three-dimensional digital fabrication for biological tissue manufacturing: analysis of alginate microgel beads produced by inkjet droplets for three dimensional tissue fabrication. Journal of Imaging Science and Technology. 2008;52(6):60201-1--6. [Google Scholar]
- 63.Xu C, Christensen K, Zhang Z, Huang Y, Fu J, Markwald RR. Predictive compensation-enabled horizontal inkjet printing of alginate tubular constructs. Manufacturing Letters. 2013; 1 (1):28–32. [Google Scholar]
- 64.Christensen K, Xu C, Chai W, Zhang Z, Fu J, Huang Y. Freeform inkjet printing of cellular structures with bifurcations. Biotechnology and Bioengineering. 2015; 112(5): 1047–55. [DOI] [PubMed] [Google Scholar]
- 65.Guillotin B, Souquet A, Catros S, Duocastella M, Pippenger B, Bellance S, Bareille R, Remy M, Bordenave L, Amedee J. Laser assisted bioprinting of engineered tissue with high cell density and microscale organization. Biomaterials. 2010;31(28):7250–6. [DOI] [PubMed] [Google Scholar]
- 66.Yan J, Huang Y, Chrisey DB. Laser-assisted printing of alginate long tubes and annular constructs. Biofabrication. 2013;5(1):015002. [DOI] [PubMed] [Google Scholar]
- 67.Calignano F, Manfredi D, Ambrosio EP, Biamino S, Lombardi M, Atzeni E, Salmi A, Minetola P, Iuliano L, Fino P. Overview on Additive Manufacturing Technologies. Proceedings of the IEEE. 2017; 105(4): 593–612. [Google Scholar]
- 68.Gibson I, Rosen D, Stucker B. Powder Bed Fusion Processes Additive Manufacturing Technologies: 3D Printing, Rapid Prototyping, and Direct Digital Manufacturing. New York, NY: Springer New York; 2015. p. 107–45. [Google Scholar]
- 69.Shirazi SFS, Gharehkhani S, Mehrali M, Yarmand H, Metselaar HSC, Adib Kadri N, Osman NAA. A review on powder-based additive manufacturing for tissue engineering: selective laser sintering and inkjet 3D printing. Science and technology of advanced materials. 2015;16(3):033502-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wysocki B, Maj P, Sitek R, Buhagiar J, Kurzydlowski KJ, Swiqszkowski W. Laser and Electron Beam Additive Manufacturing Methods of Fabricating Titanium Bone Implants. Applied Sciences. 2017;7(7):657. [Google Scholar]
- 71.Mangano F, Chambrone L, van Noort R, Miller C, Hatton P, Mangano C. Direct Metal Laser Sintering Titanium Dental Implants: A Review of the Current Literature. International Journal of Biomaterials. 2014;2014:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Krakhmalev P, Yadroitsev I, Yadroitsava I, De Smidt O. Functionalization of Biomedical Ti6A14V via In Situ Alloying by Cu during Laser Powder Bed Fusion Manufacturing. Materials. 2017;10(10):1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Demir AG, Monguzzi L, Previtali B. Selective laser melting of pure Zn with high density for biodegradable implant manufacturing. Additive Manufacturing. 2017;15:20–8. [Google Scholar]
- 74.Gibson I, Rosen D, Stucker B. Binder Jetting Additive Manufacturing Technologies: 3D Printing, Rapid Prototyping, and Direct Digital Manufacturing. New York, NY: Springer New York; 2015. p. 205–18. [Google Scholar]
- 75.Hong D, Chou D-T, Velikokhatnyi 01, Roy A, Lee B, Swink I, Issaev I, Kuhn HA, Kumta PN. Binder-jetting 3D printing and alloy development of new biodegradable Fe-Mn-Ca/Mg alloys. Acta Biomaterialia. 2016;45:375–86. [DOI] [PubMed] [Google Scholar]
- 76.Khalyfa A, Vogt S, Weisser J, Grimm G, Rechtenbach A, Meyer W, Schnabelrauch M. Development of a new calcium phosphate powder-binder system for the 3D printing of patient specific implants. Journal of Materials Science: Materials in Medicine. 2007;18(5):909–16. [DOI] [PubMed] [Google Scholar]
- 77.Suwanprateeb J, Chumnanklang R. Three-dimensional printing of porous polyethylene structure using water-based binders. Journal of Biomedical Materials Research Part B: Applied Biomaterials. 2006;78B(1): 138–45. [DOI] [PubMed] [Google Scholar]
- 78.Mostafaei A, Stevens EL, Ference JJ, Schmidt DE, Chmielus M. Binder jetting of a complexshaped metal partial denture framework. Additive Manufacturing. 2018;21:63–8. [Google Scholar]
- 79.Gibson I, Rosen D, Stucker B. Directed Energy Deposition Processes Additive Manufacturing Technologies: 3D Printing, Rapid Prototyping, and Direct Digital Manufacturing. New York, NY: Springer New York; 2015. p. 245–68. [Google Scholar]
- 80.Nakano T, Ishimoto T. Powder-based Additive Manufacturing for Development of Tailor-made Implants for Orthopedic Applications. Kona Powder Part J. 2015(32):75–84. [Google Scholar]
- 81.Carroll BE, Palmer TA, Beese AM. Anisotropic tensile behavior of Ti-6A1–4V components fabricated with directed energy deposition additive manufacturing. Acta Materialia. 2015;87:309–20. [Google Scholar]
- 82.Bertol LS, Jrtnior WK, Silva FPd, Aumund-Kopp C. Medical design: Direct metal laser sintering of Ti-6A1–4V. Materials & Design. 2010;31(8):3982–8. [Google Scholar]
- 83.Al-Imam H, Gram M, Benetti AR, Gotfredsen K. Accuracy of stereolithography additive casts used in a digital workflow. The Journal of Prosthetic Dentistry. 2018;119(4): 580–5. [DOI] [PubMed] [Google Scholar]
- 84.Azari A, Nikzad S. The evolution of rapid prototyping in dentistry: a review. Rapid Prototyping Journal. 2009;15(3):216–25. [Google Scholar]
- 85.Melchels FPW, Feijen J, Grijpma DW. A review on stereolithography and its applications in biomedical engineering. Biomaterials. 2010;31(24):6121–30. [DOI] [PubMed] [Google Scholar]
- 86.Sammartino G, Valle AD, Marenzi G, Gerbino S, Martorelli M, di Lauro AE, di Lauro F. Stereolithography in Oral Implantology A Comparison of Surgical Guides. Implant Dentistry. 2004;13(2): 133–9. [DOI] [PubMed] [Google Scholar]
- 87.Bhushan B, Caspers M. An overview of additive manufacturing (3D printing) for microfabrication. Microsystem Technologies. 2017;23(4): 1117–24. [Google Scholar]
- 88.Kim H-C, Lee S-H. Reduction of post-processing for stereolithography systems by fabrication-direction optimization. Computer-Aided Design. 2005;37(7):711–25. [Google Scholar]
- 89.Meyer W, Engelhardt S, Novosel E, Elling B, Wegener M, Kruger H. Soft Polymers for Building up Small and Smallest Blood Supplying Systems by Stereolithography. Journal of functional biomaterials. 2012;3(2):257–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Huber B, Engelhardt S, Meyer W, Kruger H, Wenz A, Schonhaar V, Tovar GEM, Kluger PJ, Borchers K. Blood-Vessel Mimicking Structures by Stereolithographic Fabrication of Small Porous Tubes Using Cytocompatible Polyacrylate Elastomers, Biofunctionalization and Endothelialization. Journal of functional biomaterials. 2016;7(2):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gibson I, Rosen D, Stucker B. Sheet Lamination Processes Additive Manufacturing Technologies: 3D Printing, Rapid Prototyping, and Direct Digital Manufacturing. New York, NY: Springer New York; 2015. p. 219–44. [Google Scholar]
- 92.Wong CH. Standards, Quality Control and Measurement Sciences in 3D Printing and Additive Manufacturing. In: Yeong WY, Chua CK, editors. [Google Scholar]
- 93.Kelly BE, Bhattacharya I, Heidari H, Shusteff M, Spadaccini CM, Taylor HK. Volumetric additive manufacturing via tomographic reconstruction. Science. 2019:eaau7114. [DOI] [PubMed] [Google Scholar]
- 94.Kolesky DB, Truby RL, Gladman AS, Busbee TA, Homan KA, Lewis JA. 3D Bioprinting of Vascularized, Heterogeneous Cell-Laden Tissue Constructs. Advanced Materials. 2014;26(19):3124–30. [DOI] [PubMed] [Google Scholar]
- 95.Kolesky DB, Homan KA, Skylar-Scott MA, Lewis JA. Three-dimensional bioprinting of thick vascularized tissues. Proceedings of the National Academy of Sciences. 2016;113(12):3179–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jafarkhani M, Salehi Z, Aidun A, Shokrgozar MA. Bioprinting in Vascularization Strategies. Iranian biomedical journal. 2019;23(1):9–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kambic HE, Kantrowitz A, Sung P, editors. Vascular graft update : safety and performance 1986; Philadelphia, Penn.: ASTM. [Google Scholar]
- 98.FUSION Vascular Graft: MAQUET Holding B.V. & Co. KG; 2018. [11/December/2018]. Available from: www.maquet.com.
- 99.Uni-Graft® K DV: B. Braun Melsungen AG 2018. [11/December/2018]. Available from: www.bbraun. com. [Google Scholar]
- 100.GORE-TEX® Vascular Grafts: W. L. Gore & Associates; 2018. [11/December/2018]. Available from: www.goremedical.com.
- 101.Jensen LP, Lepantalo M, Fossdal JE, Roder OC, Jensen BS, Madsen MS, Grenager O, Fasting H, Myhre HO, B^kgaard N, Nielsen OM, Helgstrand U, Schroeder TV. Dacron or PTFE for Above-knee Femoropopliteal Bypass. A Multicenter Randomised Study. European Journal of Vascular and Endovascular Surgery. 2007;34(1):44–9. [DOI] [PubMed] [Google Scholar]
- 102.Artegraft® Collagen Vascular Graft Bovine Carotid Artery Graft (BCA): Artegraft, Inc; 2018. [November/13/2018]. Available from: www.artegraft.com.
- 103.AlboGraft® Polyester Vascular Graft: LeMaitre Vascular, Inc; 2018. [11/December/2018]. Available from: www.lemaitre.com.
- 104.Abdollahi S, Davis A, Miller JH, Feinberg AW. Expert-guided optimization for 3D printing of soft and liquid materials. Plos One. 2018;13(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hinton TJ, Hudson A, Pusch K, Lee A, Feinberg AW. 3D Printing PDMS Elastomer in a Hydrophilic Support Bath via Freeform Reversible Embedding. Acs Biomater Sci Eng. 2016;2(10):1781–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kotz F, Arnold K, Bauer W, Schild D, Keller N, Sachsenheimer K, Nargang TM, Richter C, Helmer D, Rapp BE. Three-dimensional printing of transparent fused silica glass. Nature. 2017;544:337. [DOI] [PubMed] [Google Scholar]
- 107.Gantenbein S, Masania K, Woigk W, Sesseg JPW, Tervoort TA, Studart AR. Three-dimensional printing of hierarchical liquid-crystal-polymer structures. Nature. 2018;561(7722):226–30. [DOI] [PubMed] [Google Scholar]
- 108.Kim Y, Yuk H, Zhao R, Chester SA, Zhao X. Printing ferromagnetic domains for untethered fast-transforming soft materials. Nature. 2018;558(7709):274–9. [DOI] [PubMed] [Google Scholar]
- 109.Conduits for Vascular Reconstruction in the Pediatric Patient WebMD LLC; 2017 [November/14/2018]. Available from: emedicine.medscape.com/article/1018266-overview.
- 110.Ravi S, Chaikof EL. Biomaterials for vascular tissue engineering. Regenerative Medicine. 2010;5(1):107–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Schmidt CE, Baier JM. Acellular vascular tissues: natural biomaterials for tissue repair and tissue engineering. Biomaterials. 2000;21(22):2215–31. [DOI] [PubMed] [Google Scholar]
- 112.Shojaee M, Bashur CA. Compositions Including Synthetic and Natural Blends for Integration and Structural Integrity: Engineered for Different Vascular Graft Applications. Advanced Healthcare Materials. 2017;6(12):1700001. [DOI] [PubMed] [Google Scholar]
- 113.Melchiorri AJ, Hibino N, Best CA, Yi T, Lee YU, Kraynak CA, Kimerer LK, Krieger A, Kim P, Breuer CK, Fisher JP. 3D-Printed Biodegradable Polymeric Vascular Grafts. Advanced healthcare materials. 2016;5(3):319–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Fukunishi T, Best CA, Sugiura T, Opfermann J, Ong CS, Shinoka T, Breuer CK, Krieger A, Johnson J, Hibino N. Preclinical study of patient-specific cell-free nanofiber tissue-engineered vascular grafts using 3-dimensional printing in a sheep model. The Journal of Thoracic and Cardiovascular Surgery. 2017;153(4):924–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Laura E, Peter YY. Additive Manufacturing of Vascular Grafts and Vascularized Tissue Constructs. Tissue Engineering Part B: Reviews. 2017;23(5):436–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Guidance Document for Vascular Prostheses 510(k) Submissions - Guidance for Industry and FDA Staff: Center for Devices and Radiological Health (CDRH); 2000 [January/27/2019]. Available from: www.fda.gov/MedicalDevices/ucm073681.htm.
- 117.Refson JS, Schachter M, Patel MK, Hughes AD, Munro E, Chan P, Wolfe JH, Sever PS. Vein graft stenosis and the heparin responsiveness of human vascular smooth muscle cells. Circulation. 1998;97(25):2506–10. [DOI] [PubMed] [Google Scholar]
- 118.Sindermann JR, March KL. Heparin Responsiveness In Vitro as a Prognostic Tool for Vascular Graft Stenosis. Circulation. 1998;97(25):2486–90. [DOI] [PubMed] [Google Scholar]
- 119.Gilotti AC, Nimlamool W, Pugh R, Slee JB, Barthol TC, Miller EA, Lowe-Krentz LJ. Heparin responses in vascular smooth muscle cells involve cGMP-dependent protein kinase (PKG). Journal of cellular physiology. 2014;229(12):2142–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Allaire E, Mandet C, Bruneval P, Bensenane S, Becquemin JP, Michel JB. Cell and extracellular matrix rejection in arterial concordant and discordant xenografts in the rat. Transplantation. 1996;62(6):794–803. [DOI] [PubMed] [Google Scholar]
- 121.Hoopes CW, Platt JF. Molecular strategies for clinical xenotransplantation in cardiothoracic surgery. Semin Thorac Cardiovasc Surg. 1996;8(2):156–74. [PubMed] [Google Scholar]
- 122.Chlupac J, Filova E, Bacakova L. Blood vessel replacement: 50 years of development and tissue engineering paradigms in vascular surgery. Physiol Res. 2009;58 Suppl 2:S119–39. [DOI] [PubMed] [Google Scholar]
- 123.Sekine H, Shimizu T, Yang J, Kobayashi E, Okano T. Pulsatile myocardial tubes fabricated with cell sheet engineering. Circulation. 2006;114(1 Suppl):I87–93. [DOI] [PubMed] [Google Scholar]
- 124.van Duinen V, van den Heuvel A, Trietsch SJ, Lanz HL, van Gils JM, van Zonneveld AJ, Vulto P, Hankemeier T. 96 perfusable blood vessels to study vascular permeability in vitro. Scientific Reports. 2017;7(1):18071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Radu M, Chernoff J. An in vivo assay to test blood vessel permeability. Journal of visualized experiments : JoVE. 2013(73):e50062–e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Chakfe N, Riepe G, Dieval F, Le Magnen J-F, Wang L, Urban E, Beaufigeau M, Durand B, Imig H, Kretz J-G. Longitudinal ruptures of polyester knitted vascular prostheses. Journal of Vascular Surgery. 2001;33(5):1015–21. [DOI] [PubMed] [Google Scholar]
- 127.Tanaka H, Okada K, Yamashita T, Kawanishi Y, Matsumori M, Okita Y. Disruption of the Vascular Prosthesis Caused by Aortic Calcification After Replacement of the Thoracoabdominal Aortic Aneurysm. The Annals of Thoracic Surgery. 2006;82(3):1097–9. [DOI] [PubMed] [Google Scholar]
- 128.Quinones-Baldrich WJ, Moore WS, Ziomek S, Chvapil M. Development of a “leak-proof,” knitted Dacron vascular prosthesis. Journal of Vascular Surgery. 1986;3(6):895–903. [DOI] [PubMed] [Google Scholar]
- 129.Drury JK, Ashton TR, Cunningham JD, Maini R, Pollock JG. Experimental and Clinical Experience with a Gelatin Impregnated Dacron Prosthesis. Annals of Vascular Surgery. 1987; 1(5): 542–7. [DOI] [PubMed] [Google Scholar]
- 130.Prager M, Polterauer P, Bohmig H-J, Wagner O, Fiigl A, Kretschmer G, Plohner M, Nanobashvili J, Huk I. Collagen versus gelatin-coated Dacron versus stretch polytetrafluoroethylene in abdominal aortic bifurcation graft surgery: Results of a seven-year prospective, randomized multicenter trial. Surgery. 2001;130(3):408–14. [DOI] [PubMed] [Google Scholar]
- 131.Vohra R, Drury JK, Shapiro D, Shenkin A, Pollock JG. Sealed versus Unsealed Knitted Dacron Prostheses: a Comparison of the Acute Phase Protein Response. Annals of Vascular Surgery. 1987;1(5):548–51. [DOI] [PubMed] [Google Scholar]
- 132.Singh C, Wong CS, Wang X. Medical Textiles as Vascular Implants and Their Success to Mimic Natural Arteries. Journal of functional biomaterials. 2015;6(3):500–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kidane AG, Burriesci G, Edirisinghe M, Ghanbari H, Bonhoeffer P, Seifalian AM. A novel nanocomposite polymer for development of synthetic heart valve leaflets. Acta biomaterialia. 2009;5(7):2409–17. [DOI] [PubMed] [Google Scholar]
- 134.Kim B-S, Mooney DJ. Development of biocompatible synthetic extracellular matrices for tissue engineering. Trends in biotechnology. 1998;16(5):224–30. [DOI] [PubMed] [Google Scholar]
- 135.Lee SJ, Yoo JJ, Lim GJ, Atala A, Stitzel J. In vitro evaluation of electrospun nanofiber scaffolds for vascular graft application. Journal of Biomedical Materials Research Part A. 2007;83A(4):999–1008. [DOI] [PubMed] [Google Scholar]
- 136.Shum-Tim D, Stock U, Hrkach J, Shinoka T, Lien J, Moses MA, Stamp A, Taylor G, Moran AM, Landis W. Tissue engineering of autologous aorta using a new biodegradable polymer. The Annals of thoracic surgery. 1999;68(6):2298–304. [DOI] [PubMed] [Google Scholar]
- 137.Hernandez-Richter T, Schardey HM, Lohlein F, Heiss MM, Redondo-Miiller M, Hammer C, Schildberg FW. The Prevention and Treatment of Vascular Graft Infection With a Triclosan (Irgasan)-bonded Dacron Graft: an Experimental Study in the Pig. European Journal of Vascular and Endovascular Surgery. 2000;20(5):413–8. [DOI] [PubMed] [Google Scholar]
- 138.Enayati M, Eilenberg M, Grasl C, Riedl P, Kaun C, Messner B, Walter I, Liska R, Schima H, Wojta J, Podesser BK, Bergmeister H. Biocompatibility Assessment of a New Biodegradable Vascular Graft via In Vitro Co-culture Approaches and In Vivo Model. Annals of biomedical engineering. 2016;44(11):3319–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Halloran PF. Immunosuppressive drugs for kidney transplantation. New England Journal of Medicine. 2004;351(26):2715–29. [DOI] [PubMed] [Google Scholar]
- 140.Epperson DE, Pober JS. Antigen-presenting function of human endothelial cells. Direct activation of resting CD8 T cells. J Immunol. 1994;153(12):5402–12. [PubMed] [Google Scholar]
- 141.Wagner E, Roy R, Marois Y, Douville Y, Guidoin R. Fresh venous allografts in peripheral arterial reconstruction in dogs. Effects of histocompatibility and of short-term immunosuppression with cyclosporine A and mycophenolate mofetil. J Thorac Cardiovasc Surg. 1995; 110(6): 1732–44. [DOI] [PubMed] [Google Scholar]
- 142.Nowicki KW, Hosaka K, He Y, McFetridge PS, Scott EW, Hoh BL. Novel high-throughput in vitro model for identifying hemodynamic-induced inflammatory mediators of cerebral aneurysm formation. Hypertension (Dallas, Tex : 1979). 2014;64(6): 1306–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kaneko N, Mashiko T, Namba K, Tateshima S, Watanabe E, Kawai K. A patient-specific intracranial aneurysm model with endothelial lining: a novel in vitro approach to bridge the gap between biology and flow dynamics. J Neurointerv Surg. 2018;10(3):306–+. [DOI] [PubMed] [Google Scholar]
- 144.Klink A, Heynens J, Herranz B, Lobatto ME, Arias T, Sanders HMHF, Strijkers GJ, Merkx M, Nicolay K, Fuster V, Tedgui A, Mallat Z, Mulder WJM, Fayad ZA. In vivo characterization of a new abdominal aortic aneurysm mouse model with conventional and molecular magnetic resonance imaging. Journal of the American College of Cardiology. 2011;58(24):2522–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Tsui JC. Experimental models of abdominal aortic aneurysms. The open cardiovascular medicine journal. 2010;4:221–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.May J, Stephen M. Multiple aneurysms in Dacron velour graft. Archives of Surgery. 1978;113(3):320–1. [DOI] [PubMed] [Google Scholar]
- 147.Hallin RW. Complications with the mandril-grown (Sparks) dacron arterial graft. Am Surg. 1975;41(9):550–4. [PubMed] [Google Scholar]
- 148.Carrabba M, Madeddu P. Current Strategies for the Manufacture of Small Size Tissue Engineering Vascular Grafts. Frontiers in Bioengineering and Biotechnology. 2018;6(41). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kumar VA, Caves JM, Haller CA, Dai E, Liu L, Grainger S, Chaikof EL. Acellular vascular grafts generated from collagen and elastin analogs. Acta biomaterialia. 2013;9(9):8067–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Li X, Xu J, Nicolescu CT, Marinelli JT, Tien J. Generation, Endothelialization, and Microsurgical Suture Anastomosis of Strong 1 -mm-Diameter Collagen Tubes. Tissue engineering Part A. 2017;23(7–8):335–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Bouzeghrane F, Naggara O, Kallmes DF, Berenstein A, Raymond J. In Vivo Experimental Intracranial Aneurysm Models: A Systematic Review. American Journal of Neuroradiology. 2010;31(3):418–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Suntornnond R, Tan EYS, An J, Chua CK. A highly printable and biocompatible hydrogel composite for direct printing of soft and perfusable vasculature-like structures. Scientific Reports. 2017;7(1): 16902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Jia W, Gungor-Ozkerim PS, Zhang YS, Yue K, Zhu K, Liu W, Pi Q, Byambaa B, Dokmeci MR, Shin SR, Khademhosseini A. Direct 3D bioprinting of perfusable vascular constructs using a blend bioink. Biomaterials. 2016;106:58–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Cui H, Nowicki M, Fisher JP, Zhang LG. 3D Bioprinting for Organ Regeneration. Advanced healthcare materials. 2017;6(1): 10.1002/adhm.201601118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kabirian F, Ditkowski B, Zamanian A, Hoylaerts MF, Mozafari M, Heying R. Controlled NO-Release from 3D-Printed Small-Diameter Vascular Grafts Prevents Platelet Activation and Bacterial Infectivity. Acs Biomater Sci Eng. 2019. [DOI] [PubMed] [Google Scholar]
- 156.Chen T In situ detection of mycoplasma contamination in cell cultures by fluorescent Hoechst 33258 stain. Experimental cell research. 1977;104(2):255–62. [DOI] [PubMed] [Google Scholar]
- 157.U.S. Food and Drug Administration Silver Spring: U.S. Department of Health and Human Services; 2019. [January/14/2019], Available from: www.fda.gov.
- 158.ANSEAAMI/ ISO 25539–1: 2003/(R)2014 Cardiovascular implants — Endovascular devices — Part 1: Endovascular prostheses Arlington, VA: Association for the Advancement of Medical Instrumentation (AAMI); 2003 [January/14/2019], Available from: my.aami.org/aamiresources/previewfiles/2553901_1404_preview.pdf. [Google Scholar]
- 159.Technical Considerations for Additive Manufactured Medical Devices: Guidance for Industry and Food and Drug Administration Staff Center for Devices and Radiological Health (CDRH); 2017 [January/14/2019], Available from: www.fda.gov/downloads/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/UCM499809.pdf.
- 160.Di Prima MCJ, Anderson J, Kiarashi N. FDA Webinar-Technical Considerations for Additive Manufactured Medical Devices 2018 [January/14/2019], Available from: www.fda.gov/downloads/Training/CDRHLearn/UCM592927.pdf.
- 161.Di Prima M, Coburn J, Hwang D, Kelly J, Khairuzzaman A, Ricles L. Additively manufactured medical products -the FDA perspective. 3D Printing in Medicine. 2016;2(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Measurement Science Roadmap for Polymer-Based Additive Manufacturing (3D Printing) workshop Gaithersburg, Maryland: National Institute of Standards and Technology (NIST); 2016 [January/14/2019], Available from: www.nist.gov/news-events/events/2016/06/measurement-science-roadmap-polymer-based-additive-manufacturing. [Google Scholar]
- 163.Christensen A, Rybicki FJ. Maintaining safety and efficacy for 3D printing in medicine. 3D Printing in Medicine. 2017;3(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.OpenStax. Anatomy & Physiology: OpenStax CNX; February 26, 2016. [Available from: http://cnx.org/contents/14fb4ad7-39a1-4eee-ab6e-3ef2482e3e22@8.24.
- 165.Overview over 3D printing technologies Zurich, Switzerland: 2018 [4-September-2019], Available from: www.additively.com. [Google Scholar]
- 166.Electron Beam Additive Manufacturing (EBAM®) Chicago, IL: Sciaky Inc.; 2019. [4/September/2019], Available from: www.sciaky.com/additive-manufacturing/wire-am-vs-powder-am. [Google Scholar]
- 167.Laminated Object Manufacturing, LOM Stockholm, Sweden: Manufacturing Guide Sweden AB; [4/September/2019], Available from: www.manufacturingguide.com/en/laminated-object-manufacturing-lom. [Google Scholar]


