CONSPECTUS:
Indazoles are an important class of nitrogen heterocycles because of their excellent performance in biologically relevant applications, such as in chemical biology and medicinal chemistry. In these applications, convenient synthesis using commercially available and diverse building blocks is highly desirable. Within this broad class, 2H-indazoles are relatively underexploited when compared to 1H-indazole, perhaps because of regioselectivity issues associated with the synthesis of 2H-indazoles. This Account describes our unfolding of the synthetic utility of the Davis–Beirut reaction (DBR) for the construction of 2H-indazoles and their derivatives; parallel unfoldings of mechanistic models for these interrelated N–N bond forming reactions are also summarized.
The Davis–Beirut reaction is a robust method that exploits the diverse chemistries of a key nitroso imine or nitroso benzaldehyde intermediate generated in situ under redox neutral conditions. The resulting N–N bond-forming heterocyclization between nucleophilic and electrophilic nitrogens can be leveraged for the synthesis of multiple classes of indazoles and their derivatives, such as simple or fused indazolones, thiazolo-indazoles, 3-alkoxy-2H-indazoles, 2H-indazole N-oxides, and 2H-indazoles with various substitutions on the ring system or the nitrogens. These diverse products can all be synthesized under alkaline conditions and the various strategies for accessing these heterocycles are discussed. Alternatively, we have also developed methods involving mild photochemical conditions for the nitrobenzyl → aci-nitro → nitroso imine sequence. Solvent consideration is especially important for modulating the chemistry of the reactive intermediates in these reactions; the presence of water is critically important in some cases, but water’s beneficial effect has a ceiling because of the alternative reaction pathways it enables. Fused 2H-indazoles readily undergo ring opening reactions to give indazolones when treated with nucleophiles or electrophiles. Furthermore, palladium-catalyzed cross coupling, the Sonagashira reaction, EDC amide coupling, 1,3-dipolar cycloadditions with nitrile oxides, copper-catalyzed alkyne–azide cycloadditions (click reaction), as well as copper-free click reactions, can all be used late-stage to modify 2H-indazoles and indazolones. The continued development and applications of the Davis–Beirut reaction has provided many insights for taming the reactivity of highly reactive nitro and nitroso groups, which still has a plethora of underexplored chemistries and challenges. For example, there is currently a limited number of nonfused 2H-indazole examples containing an aryl substitution at nitrogen. This is caused by relatively slow N–N bond formation between N-aryl imine and nitroso reactants, which allows water to add to the key nitroso imine intermediate causing imine bond cleavage to be a competitive reaction pathway rather than proceeding through the desired N–N bond-forming heterocyclization.
Graphical abstract

INTRODUCTION
The broad interest in nitrogen heterocycle methodology development is a direct result of their exceptional properties and widespread applications in multiple areas of chemical research. As such, reactions for accessing various heterocyclic architectures from diverse building blocks are valuable. The indazole core is composed of a 6–5 fused ring system with an N–N bond embedded in the 5-membered ring (Figure 1). Indazoles are highly privileged in pharmaceutical applications (Figure 2)1 because of their structural similarities2 with indoles,3 benzimidazoles,4 and pyrazoles.5 Similar to the synthesis of pyrazoles, regioselectivity is an important consideration when selecting synthetic routes to indazoles, especially for the more challenging 2H-indazole scaffold.6 In general, the synthesis of indazoles and their derivatives are enabled by the use of hydrazine and its derivatives, often involving protecting groups,6,7 because methods for forming N–N bonds are scarce compared to those for forming C–N bonds. Hydrazine’s hazards are well documented in the literature and advances dedicated to improving their safety profile are still being made.7e,8 Alternatively, N–N bonds may be constructed using methods involving redox manipulation.9 One well-known reductive method for accessing 2H-indazoles is the Cadogan reaction.10 Although the reaction conditions were traditionally harsh for the Cadogan reaction (>150 °C), recent advances have made this transformation milder.11 However, even these modern variations allow only minimal substitution at C3.11
Figure 1.
Indazole ring system.
Figure 2.
Biologically valuable indazoles.
Our reinvestigation of a reported method for preparing anthranils from o-nitrobenzyl compounds12 resulted in a structure reassignment from anthranils to indazolones, which was the hydrolysis product of a 3-alkoxy 2H-indazole precursor.13 This initial study ultimately culminated in the discovery of the Davis–Beirut reaction (DBR), which has since emerged as a robust and reliable method for building the 2H-indazole core under redox neutral conditions (Scheme 1). The initially proposed mechanism of the Davis–Beirut reaction involves conversion of o-nitrobenzyl amine 1 to highly reactive nitroso imine intermediate 2, which triggers an N–N bond forming heterocyclization to form 2H-indazole 3. This Account summarizes our recent efforts on expanding both the utility and mechanistic understanding of the Davis–Beirut reaction.
Scheme 1.
Typical Davis–Beirut Reaction Products
APPLICATIONS OF THE DAVIS–BEIRUT REACTION
Our initial survey of this indazole-forming reaction (Scheme 1)14 identified several generalities, as well as substrate scope limitations. For example, using alkyl amines and primary alcohols typically resulted in good indazole yields. On the other hand, using secondary alcohols, allyl/propargyl alcohols, or anilines drastically reduced the efficiency of the reaction. It was noted in this initial report that changing the alcohol solvent properties noticeably influenced the progression of the Davis–Beirut reaction.
This lead-in work was followed up by a reoptimization study of Davis–Beirut reaction conditions. It was found that the addition of water to the reaction mixture dramatically increased the 2H-indazole yield—from 27% with anhydrous n-propanol to 65% with 15% water added (Scheme 2).15 This beneficial effect was also observed when the solvent was methanol or ethanol. However, when the amount of added water surpassed 20–25%, the yield of the reaction sharply decreased. At 50% added water, the yield of the Davis–Beirut reaction carried out in methanol, ethanol, and 1-propanol dropped to 40%, 28%, and 15%, respectively.
Scheme 2.
Effect of Water on the Davis–Beirut Reaction
Despite using these newly optimized reaction conditions, the synthesis of N-aryl substituted 2H-indazoles 3 was still poor yielding. However, by using the most reactive solvent, methanol, and by making the nitro-containing ring more electron-poor, yields of the N-aryl products 4 were significantly boosted (Scheme 3A).16 Although useful insights were gained about the electronic preference of the nitroso intermediate in the Davis–Beirut reaction, this strategy for accessing N-aryl indazoles restricts the substrate scope and limits the Davis–Beirut reaction’s overall synthetic utility.
Scheme 3.
Improving the N-Aryl Davis–Beirut Reaction
Even though N-alkyl amines reliably delivered the corresponding indazole products, the low reactivity of anilines in the Davis–Beirut reaction was disappointing. It was reasoned that an intramolecular version of the reaction would more smoothly deliver the N-aryl indazole products due to a favorable cascade of ring closing reactions (5 → 6, Scheme 3B).17 Indeed, when 2-aminobenzyl alcohol derivatives were used as substrates, polycyclic indazole products were isolated in up to 90% yield—likely because the internal nucleophile thwarted the unfolding of the nonproductive pathways.
While sulfur is known to reduce both nitro and nitroso groups, multiple classes of thio-containing polycyclic indazoles were successfully synthesized via the Davis–Beirut reaction.18 This suggests that the nitroso intermediate is relatively short-lived when the nucleophile is tethered due to a fast cyclization cascade (8 → 9; Scheme 3C). It should also be noted that these thiol reactions were not as straightforward as their oxygen counterpart, largely due to thioaminal formation impeding the synthesis of starting material 7. This issue could be circumvented, however, by trityl protection of the thiol prior to reductive amination. The trityl group was easily removed using TFA/Et3SiH, followed by treatment with KOH to initiate the Davis–Beirut reaction and delivery of the corresponding thiazolo/thiazino/thiazepino-2H-indazoles.
These 6–5–6–6-fused indazoles tolerate a number of different late-stage modifications (Scheme 4), which grants a certain degree of synthetic flexibility when planning their synthesis/applications. For example, a Sonagashira coupling sequence followed by dipolar cycloadditions can be used to further functionalize the Davis–Beirut product (10; Scheme 4A); when these ring systems are decorated with a carboxylic acid, they can be modified late-stage using EDC coupling (11; Scheme 4B).17b
Scheme 4.
Various Reactions of 2H-Indazoles
On the other hand, these polycyclic indazoles are not stable toward alkoxide19 or thiolate20 nucleophiles for extended times (Scheme 4C–E), as these fused ring system undergo ring-opening reactions when treated with these nucleophiles. Increasing the steric bulk around the electrophilic carbon of 12 (see site of reaction denoted with an asterisk in Scheme 4C) significantly decreased the rate of this ring-opening, as demonstrated by the reduced yield of 14 versus 13. Likewise, when polycyclic indazole was heated in the presence of potassium iodide, the iodide nucleophile facilitated a rearrangement of the 6–5–6–6 fused indazole system (15) to a 6–5–5–6 fused indazolone system (16; Scheme 4D). Considering the high electron density at the N1-position, it was then hypothesized that various electrophiles would be suitable reaction partners for 2H-indazoles. For example, reaction of 17 with propargyl bromide gives indazolone intermediate 18, which could be trapped by azide (Scheme 4E). The close proximity of the alkyne and azide in 19 enabled an intramolecular cycloaddition to occur under copper-free conditions to generate 20, a structurally complex indazolone containing a fused 7-membered ring.21 Also, treatment of 21 with various alkylating, acylating, and sulfonylating reagents gave the corresponding 1-substituted indazolones in 70–95% yield (Scheme 4F). In particular, indazolone 22 (R = propargyl) was useful for facile late-stage generation of a library of triazole-containing analogues using intermolecular copper catalyzed alkyne–azide cycloaddition.22
The proposed mechanism of the Davis–Beirut reaction involves addition of an oxygen nucleophile to nitroso imine intermediate 2 (Scheme 5A).13 This mechanistic model was studied computationally16 and found to be consistent with experimental results. However, this simple mechanistic model did not fully explain why the nucleophilic species prefers to be alkoxide/alcohol rather than hydroxide/water. Indeed, indazolones are not formed directly in the typical Davis–Beirut reaction. Through additional mechanistic work,23 it was found that hydroxide/water does add to nitroso imine 2 to give hemiaminal 23, but was unable to deliver indazolone directly in the presence of alcohol/alkoxide due to unproductive C–N bond cleavage24 generating nitrosobenzaldehyde 24 (Scheme 5A). This competition between C–N bond cleavage and heterocycle formation is most apparent in the double Davis–Beirut reaction (Scheme 5B), where bisindazole 25 was only formed in 6% yield while monoindazole 26 was obtained in 36% yield. In contrast, when the two oxygen nucleophiles are tethered to diamine starting material 27, this problem was alleviated; double Davis–Beirut spiro-fused bisindazole product 28 was obtained in 72% yield using this strategy to minimize hydroxide/water addition.
Scheme 5.
Origin of Hydroxide/Alkoxide Selectivity in the Davis–Beirut Reaction
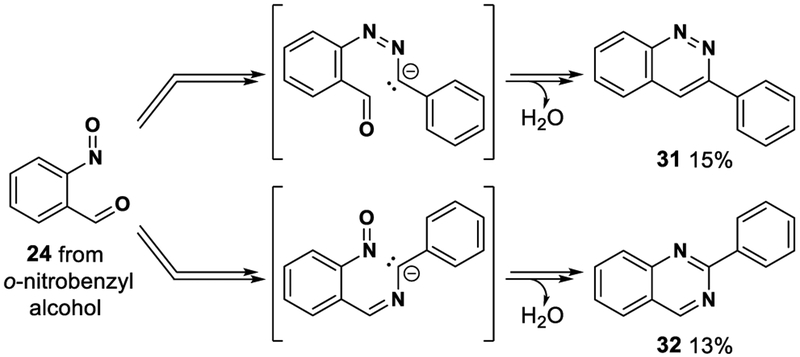
With these new mechanistic insights, we aimed to exploit the idea that o-nitrobenzyl alcohol 29 could be used to generate nitrosobenzaldehyde 24 in situ (Scheme 6). Amine reaction with 24 generates nitroso imine 2, and by using isopropanol as the reaction solvent, 2H-indazole formation was effectively shut down due to slow addition of isopropanol/isopropoxide to the nitroso imine intermediate. Consequently, this allows nitrosobenzaldehyde to cycle between nitroso imine 2, nitroso hemiaminal 23, and nitrosobenzaldehyde 24 until it reaches a thermodynamic sink–that is, cyclization to give indazolone 30. On the basis of this idea, two sets of reaction conditions were developed for this chemistry: one mediated by base25 and heat and the other mediated by light (Scheme 6).26 High heat (100 °C) was required when the reaction was performed under basic conditions, and the use of allyl, propargyl, or benzyl amines did not deliver the expected indazolones due to C–H bond acidity issues (resulting in cinnoline 31 and quinazoline 32 side-product formation; Scheme 7). Using UV light from a readily available and affordable light source27 alleviates this problem of C–H bond acidity since there is no strong base present. However, aryl halides were no longer tolerated due to light-mediated scission of the carbon–halogen bonds. In contrast to the base-mediated method, the light-mediated reaction could be conducted at just 30 °C in aqueous phosphate-buffered saline (PBS) solution, a biologically relevant solvent system. But, again consistent with the original Davis–Beirut reaction, using aniline unfortunately resulted in minimal heterocycle formation.
Scheme 6.
Base versus UV-Mediated Indazolone Synthesis
Scheme 7.
Competing C–C and C–N Bond-Forming Reactions
While the initially proposed mechanistic model for synthesizing 3-alkoxy 2H-indazoles via the Davis–Beirut reaction was deceptively straightforward (Scheme 8, pathway B), recent advances have demonstrated a willingness of the key nitroso imine intermediate to engage alternative reaction pathways A and C. Only productive reaction pathways involving nitroso imine 2 are shown in Scheme 8, with less productive pathways omitted for clarity. For example, the pathway where the amine reacts with the nitroso imine at the nitroso first to generate the corresponding azo compound is not shown. Also, the conversion of o-nitrobenzyl alcohol to o-nitroso benzaldehyde is abridged and the possibility of amine addition to the nitroso imine is excluded. Indeed, the synthetic utility of these alternative reaction pathways are currently limited, but, with additional optimization efforts, these pathways may yet prove to be valuable. The network of reactions detailed in Scheme 8 illustrates the rich and diverse reactions of the nitroso group, which has allowed us to extend the reach of the Davis–Beirut reaction to multiple classes of 6–5-fused N–N bond-containing heterocycles.
Scheme 8.
Chemistry of o-Nitrobenzyl Compounds
Recently, pathway A for synthesizing 2H-indazole N-oxide (33; Scheme 9A) was optimized under basic conditions in DMSO.28 The synthesis of these N-oxide heterocycles has significant implications on the mechanisms of both the Davis–Beirut reaction and the Cadogan cyclization—a well-established method for preparing 2H-indazoles from nitro imines through a reductive cyclization mechanism.10a Phosphorus reagents are typically employed for the Cadogan cyclization, and it is thought that the nitro group is completely deoxygenated to a nitrene which then undergoes cyclization.10a Typically, the Cadogan cyclization is carried out under harsh conditions with high heat and excess phosphorus. At process scale, this reaction relies on an azide rather than phosphorus reduction of a nitro group to generate the nitrene intermediate.29 With the realization that the Davis–Beirut reaction and Cadogan cyclization both share nitroso imine 2 as a reactive intermediate10a,13 and the consideration that the Davis–Beirut reaction does not require nitrene for N–N bond heterocyclization, we wondered whether the nitrene pathway was the only operational mechanism in the Cadogan process. Indeed, N-hydroxy products that are inconsistent with a nitrene mechanism have been observed in the related Sundberg reaction,10b which has been studied computationally.30
Scheme 9.
2H-Indazole N-Oxide Synthesis
When we carried out reactions of nitroso imine 2 under nonreductive and minimally deoxygenating conditions, 2H-indazole N-oxides were synthesized (Scheme 9). Unsurprisingly, 2H-indazole N-oxides derived from anilines were not successfully synthesized. Treatment of the synthesized N-oxide heterocycle 34 with either zinc/HCl or MeOH/KOH smoothly delivered the corresponding Cadogan or Davis–Beirut products, respectively (Scheme 9B). Taken together, these results suggest that, in addition to nitroso imine 2, 2H-indazole N-oxide 33 is also a common reactive intermediate in both the Cadogan and Davis–Beirut reactions. Interestingly, the nitroso imine → 2H-indazole N-oxide → 2H-indazole sequence is formally a Cadogan cyclization carried out at room temperature using chemistry discovered through the Davis–Beirut reaction. While it is currently impossible to rule out nitrene formation in the Cadogan reaction, the mechanism for 2H-indazole N-oxide formation was studied computationally using several levels of theory and found to be kinetically feasible, as well as exergonic; the formation of these N-oxides is competitive with regards to phosphorus-mediated 2H-indazole synthesis.
Since our discovery that the Davis–Beirut reaction was rooted in an anthranil synthesis claim,13 it is only fitting that anthranils were serendipitously synthesized when we treated the benzimidazole derivative of o-nitrobenzyl compounds 35 with KOH (Scheme 10).31 We selected 35 as a Davis–Beirut substrate in an attempt to use the benzimidazole’s nucleophilic nitrogen under basic conditions to trap the nitroso to generate 6–6–5–6 fused benzo[4,5]imidazo[1,2-b]cinnolin-12(5H)-one; anthranil 36 was obtained instead. The key for enabling this chemistry was replacement of the benzyl amine or alcohol moiety with C2 of benzimidazole, which shuts down the deprotection mechanism (pathway C in Scheme 8). The mechanism of this unexpected anthranil-forming reaction (Scheme 11) was studied computationally and found to be a cascade of reactions involving a 6π electrocyclization, ring-fragmentation, [1,5]-sigmatropic shift, and concerted proton transfer/electrocyclization, followed by aromatization. The synthesis of these N–O bond-containing heterocycles highlights the potential usefulness of the intermediates shown in Scheme 9 for methodology development research. Indeed, there is evidence of a nitroso intermediate in the anthranil synthesis, as demonstrated by the formation of dimer side-product 37—the result of condensation between the anion of starting material 35 and the nitroso intermediate derived from 35.
Scheme 10.
Anthranils from o-Nitrobenzyl Chemistry via a Cascade of π-Electron-Mediated Reactions
Scheme 11.
Proposed Mechanism of Anthranil Formation
SUMMARY AND PERSPECTIVE
This Account provided an overview of the unfolding of the Davis–Beirut reaction, a reliable method for accessing multiple classes of substituted 2H-indazoles in good yields. This reaction affords the opportunity to exploit an in situ generated nitroso intermediate in a multitude of different reactions to access several classes of heterocycles (Scheme 12). The fact that many of these important heterocycle products can then be converted into a second or third class of heterocycles is very consistent with our group’s interest in heterocycle-to-heterocycle synthetic strategies.32 Indeed, the synthesis of simple or fused indazolones, 3-alkoxy 2H-indazoles, 2H-indazole N-oxides, and 2H-indazoles have been demonstrated.
Scheme 12. Reactions Enabled by Nitroso Groupa.
aConditions: (a) Y = NR, KOH, H2O, MeOH, 60 °C; (b) Y = O, KOH, H2O, 100 °C, primary amine; (c) Y = N(CH2)3OH, KOH, iPrOH, MeOH, reflux; (d) Y = N(CH2)3STr, DCM, TFA, SiEt3H, rt, then KOH, H2O, MeOH, reflux; (e) Y = NR, KOH, DMSO, H2O; (f) AcOH, reflux; (g) KI, DMF, 170 °C; (h) Na2WO4·H2O, 30% H2O2(aq), H2O, EtOAc, rt; (i) Zn0, 1 M HCl(aq), MeOH, rt; and (j) KOH, H2O, MeOH, 60 °C.
That said, the chemistries of the nitro and nitroso groups are not yet fully defined. Many of the advances reviewed here are directly a consequence of efforts to unfold the mechanistic details operative in the Davis–Beirut reaction. Although it originally seemed unlikely that such a reliable method of delivering 2H-indazoles could be interrupted or modified, being open-minded about the original mechanistic model culminated in the discovery of many new modes of reactivity. Our current efforts are focused on the direct synthesis of nonfused N-aryl indazoles and indazolones, as their chemistry remains challenging. The high reactivity of the nitroso intermediates provides many opportunities for discovery, future methodology development, mechanistic investigations, and contributions to chemical biology and medicinal chemistry.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the creative efforts of all postdoctoral researchers, graduate students, and undergraduate students, as well as financial support from funding agencies (National Science Foundation and National Institutes of Health [most recently DK072517 and DK067003] named in refs 1g, 14–24, 26, 28, and 31) and royalties from the Beirut Reaction (M.J.H.). J.S.Z. is supported by the UC Davis Tara K. Telford CF Fund, a UC Davis Dissertation Year Fellowship, and the R. Bryan Miller Graduate Fellowship.
Biography
Jie S. Zhu obtained his M.S. from California State University Long Beach under the guidance of Prof. Young-Seok Shon and Ph.D. from University of California Davis under the guidance of Prof. Mark J. Kurth. He will conduct postdoctoral research at Stanford University with Prof. Justin Du Bois.
Makhluf J. Haddadin obtained his M.S. from the American University of Beirut under the guidance of Prof. Costas H. Issodorides and Ph.D. from University of Colorado Boulder under the guidance of Prof. Alfred Hassner. He conducted postdoctoral research at Harvard University with Prof. Louis Fieser and then was appointed to the American University of Beirut faculty, where he is currently a Professor of chemistry.
Mark J. Kurth obtained his Ph.D. from University of Minnesota under the guidance of Prof. Thomas R. Hoye. He conducted postdoctoral research at the University of Geneva with Prof. Wolfgang Oppolzer and then was appointed to the University of California Davis faculty, where he is currently a Distinguished Professor of chemistry.
Footnotes
The authors declare no competing financial interest.
DEDICATION
The authors dedicate this Account of the Davis–Beirut reaction to Prof. George S. Zweifel (UC Davis), a wonderful colleague and gracious mentor, on the occasion of his 93rd birthday.
REFERENCES
- (1).(a) Epstein JB; Silverman S Jr.; Paggiarino DA; Crockett S; Schubert MM; Senzer NN; Lockhart PB; Gallagher MJ; Peterson DE; Leveque FG Benzydamine HCl for prophylaxis of radiation-induced oral mucositis. Cancer 2001, 92, 875–885. [DOI] [PubMed] [Google Scholar]; (b) Cappelli A; Nannicini C; Gallelli A; Giuliani G; Valenti S; Mohr GLP; Anzini M; Mennuni L; Ferrari F; Caselli G; Giordani A; Peris W; Makovec F; Giorgi G; Vomero S Design, Synthesis, and Biological Evaluation of AT1 Angiotensin II Receptor Antagonists Based on the Pyrazolo[3,4-b]pyridine and Related Heteroaromatic Bicyclic Systems. J. Med. Chem 2008, 51, 2137–2146. [DOI] [PubMed] [Google Scholar]; (c) Jones P; Altamura S; Boueres J; Ferrigno F; Fonsi M; Giomini C; Lamartina S; Monteagudo E; Ontoria JM; Orsale MV; Palumbi MC; Pesci S; Roscilli G; Scarpelli R; Schultz-Fademrecht C; Toniatti C; Rowley M Discovery of 2-{4-[(3S)-Piperidin-3-yl]phenyl}-2H-indazole-7-carboxamide (MK-4827): A Novel Oral Poly(ADP-ribose)polymerase (PARP) Inhibitor Efficacious in BRCA-1 and –2 Mutant Tumors. J. Med. Chem 2009, 52, 7170–7185. [DOI] [PubMed] [Google Scholar]; (d) Harris PA; Boloor A; Cheung M; Kumar R; Crosby RM; Davis-Ward RG; Epperly AH; Hinkle KW; Hunter RN; Johnson JH; Knick VB; Laudeman CP; Luttrell DK; M ook RA; Nolte RT; Rudolph SK; Szewczyk JR; Truesdale AT; Veal JM; Wang L; Stafford JA Discovery of 5-[[4-[(2,3-Dimethyl-2H-indazol-6-yl)methylamino]-2-pyrimidinyl]- amino]-2-methyl-benzenesulfonamide (Pazopanib), a Novel and Potent Vascular Endothelial Growth Factor Receptor Inhibitor. J. Med. Chem 2008, 51, 4632–4640. [DOI] [PubMed] [Google Scholar]; (e) Pan Z; Scheerens H; Li SJ; Schultz BE; Sprengeler PA; Burrill LC; Mendonca RV; Sweeney MD; Scott KCK; Grothaus PG; Jeffery DA; Spoerke JM; Honigberg LA; Young PR; Dalrymple SA; Palmer JT Discovery of Selective Irreversible Inhibitors for Bruton’s Tyrosine Kinase. ChemMedChem 2007, 2, 58–61. [DOI] [PubMed] [Google Scholar]; (f) Folkes AJ; Ahmadi K; Alderton WK; Alix S; Baker SJ; Box G; Chuckowree IS; Clarke PA; Depledge P; Eccles SA; Friedman LS; Hayes A; Hancox TC; Kugendradas A; Lensun L; Moore P; Olivero AG; Pang J; Patel S; Pergl-Wilson GH; Raynaud FI; Robson A; Saghir N; Salphati L; Sohal S; Ultsch MH; Valenti M; Wallweber HJA; Wan NC; Wiesmann C; Workman P; Zhyvoloup A; Zvelebil MJ; Shuttleworth SJ The Identification of 2-(1H-Indazol-4-yl)-6-(4-methanesulfonyl-piperazin-1-ylmethyl)-4-morpholin-4-yl-thieno[3,2-d]pyrimidine (GDC-0941) as a Potent, Selective, Orally Bioavailable Inhibitor of Class I PI3 Kinase for the Treatment of Cancer. J. Med. Chem 2008, 51 , 5522–5532. [DOI] [PubMed] [Google Scholar]; (g) Roth A; Ott S; Farber KM; Palazzo TA; Conrad WE; Haddadin MJ; Tantillo DJ; Cross CE; Eiserich JP; Kurth MJ Inhibition of myeloperoxidase: Evaluation of 2H-indazoles and 1H-indazolones. Bioorg. Med. Chem 2014, 22, 6422–6429. [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Yan SB; Peek VL; Ajamie R; Buchanan SG; Graff JR; Heidler SA; Hui Y-H; Huss KL; Konicek BW; Manro JR; Shih C; Stewart JA; Stewart TR; Stout SL; Uhlik MT; Um SL; Wang Y; Wu W; Yan L; Yang WJ; Zhong B; Walgren RA LY2801653 is an orally bioavailable multi-kinase inhibitor with potent activity against MET, MST1R, and other oncoproteins, and displays anti-tumor activities in mouse xenograft models. Invest. New Drugs 2013, 31, 833–844. [DOI] [PMC free article] [PubMed] [Google Scholar]; (i) Hu-Lowe DD; Zou HY; Grazzini ML; Hallin ME; Wickman GR; Amundson K; Chen JH; Rewolinski DA; Yamazaki S; Wu EY; McTigue MA; Murray BW; Kania RS; O ‘Connor P; Shalinsky DR ; Bender SL. Nonclinical Antiangiogenesis and Antitumor Activities of Axitinib (AG-013736), an Oral, Potent, and Selective Inhibitor of Vascular Endothelial Growth Factor Receptor Tyrosine Kinases 1, 2, 3. Clin. Cancer Res 2008, 14, 7272. [DOI] [PubMed] [Google Scholar]
- (2).Patani GA; LaVoie EJ Bioisosterism: a rational approach in drug design. Chem. Rev 1996, 96, 3147–3176. [DOI] [PubMed] [Google Scholar]
- (3).(a) Humphrey GR ; Kuethe JT Practical methodologies for the synthesis of indoles. Chem. Rev 2006, 106, 2875–2911. [DOI] [PubMed] [Google Scholar]; (b) Kochanowska-Karamyan AJ; Hamann MT Marine indole alkaloids: potential new drug leads for the control of depression and anxiety. Chem. Rev 2010, 110, 4489–4497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Keri RS; Hiremathad A; Budagumpi S; Nagaraja BM Comprehensive Review in Current Developments of Benzimidazole-Based Medicinal Chemistry. Chem. Biol. Drug Des 2015, 86, 19–65. [DOI] [PubMed] [Google Scholar]
- (5).Karrouchi K; Radi S; Ramli Y; Taoufik J; Mabkhot Y; Al-Aizari F; et al. Synthesis and pharmacological activities of pyrazole derivatives: A review. Molecules 2018, 23, 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Halland N; Nazaré M; R’kyek O; Alonso J; Urmann M; Lindenschmidt AA General and Mild Palladium-Catalyzed Domino Reaction for the Synthesis of 2H-Indazoles. Angew. Chem., Int. Ed 2009, 48, 6879–6882. [DOI] [PubMed] [Google Scholar]
- (7).(a) Qian Y; Bolin D; Conde-Knape K; Gillespie P; Hayden S; Huang K-S; Olivier AR; Sato T; Xiang Q; Yun W; Zhang X Design and synthesis of 2-N-substituted indazolone derivatives as non-carboxylic acid glycogen synthase activators. Bioorg. Med. Chem. Lett 2013, 23, 2936–2940. [DOI] [PubMed] [Google Scholar]; (b) Vega MC; Rolón M; Montero-Torres A; Fonseca-Berzal C; Escario JA; Gómez-Barrio A; Gálvez J; Marrero-Ponce Y; Arán VJ Synthesis, biological evaluation and chemometric analysis of indazole derivatives. 1,2-Disubstituted 5-nitroindazolinones, new prototypes of antichagasic drug. Eur. J. Med. Chem 2012, 58, 214–227. [DOI] [PubMed] [Google Scholar]; (c) Wang W-J; Chen J-H; Chen Z-C; Zeng Y-F; Zhang X-J; Yan M; Chan AS A Convenient Synthesis of 1-Aryl-and 2-Aryl-Substituted Indazolones via Intramolecular C-N Coupling Promoted by KOt-Bu. Synthesis 2016, 48, 3551–3558. [Google Scholar]; (d) Welsch SJ; Kalinski C; Umkehrer M; Ross G; Kolb J; Burdack C; Wessjohann LA Palladium and copper catalyzed cyclizations of hydrazine derived Ugi products: facile synthesis of substituted indazolones and hydroxytriazafluorendiones. Tetrahedron Lett 2012, 53, 2298–2301. [Google Scholar]; (e) Wheeler RC; Baxter E; Campbell IB; Macdonald SJ. A General, One-Step Synthesis of Substituted Indazoles using a Flow Reactor. Org. Process Res. Dev 2011, 15, 565–569. [Google Scholar]; (f) Jin T; Yamamoto Y An Efficient, Facile, and General Synthesis of 1H-Indazoles by 1,3-Dipolar Cycloaddition of Arynes with Diazomethane Derivatives. Angew. Chem., Int. Ed 2007, 46, 3323–3325. [DOI] [PubMed] [Google Scholar]; (g) Shamsabadi A; Chudasama VA facile route to 1H- and 2H-indazoles from readily accessible acyl hydrazides by exploiting a novel aryne-based molecular rearrangement. Chem. Commun 2018, 54, 11180–11183. [DOI] [PubMed] [Google Scholar]; (h) Thomé I; Besson C; Kleine T; Bolm C Base-Catalyzed Synthesis of Substituted Indazoles under Mild, Transition-Metal-Free Conditions. Angew. Chem., Int. Ed 2013, 52, 7509–7513. [DOI] [PubMed] [Google Scholar]
- (8).(a) Niemeier JK; Kjell DP Hydrazine and Aqueous Hydrazine Solutions: Evaluating Safety in Chemical Processes. Org. Process Res. Dev 2013, 17, 1580–1590. [Google Scholar]; (b) Wang Z; Richter SM; Gandarilla J; Kruger AW; Rozema MJ Safe Scale-Up of a Hydrazine Condensation by the Addition of a Base. Org. Process Res. Dev 2013, 17, 1603–1610. [Google Scholar]
- (9).(a) Correa A; Tellitu I; Dominguez E; SanMartin R Esther Dominguez, Raul San Martin Novel Alternative for the N-N Bond Formation through a PIFA-Mediated Oxidative Cyclization and Its Application to the Synthesis of Indazol-3-ones. J. Org. Chem 2006, 71, 3501–3505. [DOI] [PubMed] [Google Scholar]; (b) Lin W; Hu M-H; Feng X; Cao C-P; Huang Z-B; Shi D-Q An efficient and convenient synthesis of heterocycle-fused indazoles via the N-N bond forming reaction of nitroarenes induced by low-valent titanium reagent. Tetrahedron 2013, 69, 6721 – 6726. [Google Scholar]; (c) Yu D-G; Suri M; Glorius F RhIII/CuII-Cocatalyzed Synthesis of 1H-Indazoles through C-H Amidation and N-N Bond Formation. J. Am. Chem. Soc 2013, 135, 8802–8805. [DOI] [PubMed] [Google Scholar]
- (10).(a) Cadogan JIG; Cameron-Wood M; Mackie RK; Searle RJG 896. The reactivity of organophosphorus compounds. Part XIX. Reduction of nitro-compounds by triethyl phosphite: a convenient new route to carbazoles, indoles, indazoles, triazoles, and related compounds. J. Chem. Soc 1965, 4831–4837. [Google Scholar]; (b) Sundberg R Deoxygenation of nitro groups by trivalent phosphorus. Indoles from o-nitrostyrenes. J. Org. Chem 1965, 30, 3604–3610. [Google Scholar]
- (11).(a) Genung NE; Wei L; Aspnes GE Regioselective Synthesis of 2H-Indazoles Using a Mild, One-Pot Condensation-Cadogan Reductive Cyclization. Org. Lett 2014, 16, 3114–3117. [DOI] [PubMed] [Google Scholar]; (b) Nykaza TV; Harrison TS; Ghosh A; Putnik RA; Radosevich ATA Biphilic Phosphetane Catalyzes N-N Bond-Forming Cadogan Heterocyclization via PIII/PV-O Redox Cycling. J. Am. Chem. Soc 2017, 139, 6839–6842. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Schoene J; Bel Abed H; Schmieder P; Christmann M; Nazaré;, M. A General One-Pot Synthesis of 2H-Indazoles Using an Organophosphorus-Silane System. Chem.- Eur. J 2018, 24, 9090–9100. [DOI] [PubMed] [Google Scholar]
- (12).(a) Boduszek B; Halama A; Zoń J The Cleavage of 1-Amino—2′-Nitrobenzylphosphonates in a Basic Medium. Tetrahedron 1997, 53, 11399–11410. [Google Scholar]; (b) Chen L-J; Burka LT Formation of o-nitrosobenzaldehyde from hydrolysis of o-nitrobenzyl tosylate. Evidence of intramolecular nucleophilic interaction. Tetrahedron Lett 1998, 39, 5351–5354. [Google Scholar]
- (13).Kurth MJ; Olmstead MM; Haddadin MJ Claimed 2,1-Benzisoxazoles Are Indazalones. J. Org. Chem 2005, 70, 1060–1062. [DOI] [PubMed] [Google Scholar]
- (14).Mills AD; Nazer MZ; Haddadin MJ; Kurth MJ N,N-Bond-Forming Heterocyclization: Synthesis of 3-Alkoxy-2H-indazoles. J. Org. Chem 2006, 71, 2687–2689. [DOI] [PubMed] [Google Scholar]
- (15).Mills AD; Maloney P; Hassanein E; Haddadin MJ; Kurth MJ Synthesis of a Library of 2-Alkyl-3-alkyloxy-2H-indazole-6-carboxamides. J. Comb. Chem 2007, 9, 171–177. [DOI] [PubMed] [Google Scholar]
- (16).Avila B; Solano DM; Haddadin MJ; Kurth MJ Facile Syntheses of Novel Benzo- 1,3-dioxolo-, Benzothiazolo-, Pyrido-, and Quinolino-fused 5H-Benzo[d]-pyrazolo[5,1-b][1,3]-oxazines and 1H-Pyrazoles. Org. Lett 2011, 13, 1060–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).(a) Avila B; El-Dakdouki MH; Nazer MZ; Harrison JG; Tantillo DJ; Haddadin MJ; Kurth MJ Acid and base catalyzed Davis-Beirut reaction: experimental and theoretical mechanistic studies and synthesis of novel 3-amino-2H-indazoles. Tetrahedron Lett 2012, 53, 6475–6478. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Butler JD; Solano DM; Robins LI; Haddadin MJ; Kurth MJA Facile Synthesis of New 5H-Indazolo[3,2-b]benzo[d]-1,3-oxazines via One-Pot Intramolecular Bis-heterocyclizations. J. Org. Chem 2008, 73, 234–240. [DOI] [PubMed] [Google Scholar]
- (18).Farber KM; Haddadin MJ; Kurth MJ Davis-Beirut Reaction: Route to Thiazolo-, Thiazino-, and Thiazepino-2H-indazoles. J. Org. Chem 2014, 79, 6939–6945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Oakdale JS; Solano DM; Fettinger JC; Haddadin MJ; Kurth MJ An Oxazolo[3,2-b]indazole Route to 1H-Indazolones. Org. Lett 2009, 11, 2760–2763. [DOI] [PubMed] [Google Scholar]
- (20).Donald MB; Conrad WE; Oakdale JS; Butler JD; Haddadin MJ; Kurth MJ Nucleophilic Substitution of Oxazino-/ Oxazolino-/Benzoxazin [3,2-b]indazoles: An Effective Route to 1H-Indazolones. Org. Lett 2010, 12, 2524–2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Conrad WE; Rodriguez KX; Nguyen HH; Fettinger JC; Haddadin MJ; Kurth MJ A One-Pot-Three-Step Route to Triazolotriazepinoindazolones from Oxazolino-2H-indazoles. Org. Lett 2012, 14, 3870–3873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Conrad WE; Fukazawa R; Haddadin MJ; Kurth MJ The Davis-Beirut Reaction: N1,N2-Disubstituted-1H-Indazolones via 1,6-Electrophilic Addition to 3-Alkoxy-2H-Indazoles. Org. Lett 2011, 13, 3138–3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Zhu JS; Duong MR; Teuthorn AP; Lu JY; Son J-H; Haddadin MJ; Kurth MJ Davis-Beirut Reaction: Alkoxide versus Hydroxide Addition to the Key o-Nitrosoimine Intermediate. Org. Lett 2018, 20, 1308–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).(a) Il’ichev YV; Schwörer MA; Wirz J Photochemical reaction mechanisms of 2-nitrobenzyl compounds: methyl ethers and caged ATP. J. Am. Chem. Soc 2004, 126, 4581–4595. [DOI] [PubMed] [Google Scholar]; (b) Klán P; Šolomek T; Bochet CG; Blanc AL; Givens R; Rubina M; Popik V; Kostikov A; Wirz J Photoremovable protecting groups in chemistry and biology: reaction mechanisms and efficacy. Chem. Rev 2013, 113, 119. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Zhao H; Sterner ES; Coughlin EB; Theato P o-Nitrobenzyl alcohol derivatives: opportunities in polymer and materials science. Macromolecules 2012, 45, 1723–1736. [Google Scholar]
- (25).Zhu JS; Kraemer N; Shatskikh ME; Li CJ; Son J-H; Haddadin MJ; Tantillo DJ; Kurth MJ N-N Bond Formation between Primary Amines and Nitrosos: Direct Synthesis of 2-Substituted Indazolones with Mechanistic Insights. Org. Lett 2018, 20, 4736–4739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Zhu JS; Kraemer N; Li CJ; Haddadin MJ; Kurth MJ Photochemical Preparation of 1,2-Dihydro-3H-indazol-3-ones in Aqueous Solvent at Room Temperature. J. Org. Chem 2018, 83, 15493–15498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Aung T; Liberko CA Bringing Photochemistry to the Masses: A Simple, Effective, and Inexpensive Photoreactor, Right Out of the Box. J. Chem. Educ 2014, 91, 939–942. [Google Scholar]
- (28).Zhu JS; Li CJ; Tsui KY; Kraemer N; Son J-H; Haddadin MJ; Tantillo DJ; Kurth MJ Accessing Multiple Classes of 2H-Indazoles: Mechanistic Implications for the Cadogan and Davis-Beirut Reactions. J.Am. Chem. Soc 2019, 141, 6247–6253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Chung CK; Bulger PG; Kosjek B; Belyk KM; Rivera N; Scott ME; Humphrey GR; Limanto J; Bachert DC; Emerson KM Process Development of C-N Cross-Coupling and Enantioselective Biocatalytic Reactions for the Asymmetric Synthesis of Niraparib. Org. Process Res. Dev 2014, 18, 215–227. [Google Scholar]
- (30).Davies IW; Guner VA; Houk K Theoretical evidence for oxygenated intermediates in the reductive cyclization of nitrobenzenes. Org. Lett 2004, 6, 743–746. [DOI] [PubMed] [Google Scholar]
- (31).Zhu JS; Son J-H; Teuthorn AP; Haddadin MJ; Kurth MJ; Tantillo DJ Diverting Reactive Intermediates Toward Unusual Chemistry: Unexpected Anthranil Products from Davis-Beirut Reaction. J. Org. Chem 2017, 82, 10875–10882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Coffman KC; Palazzo TA; Hartley TP; Fettinger JC; Tantillo DJ; Kurth MJ Heterocycle-Heterocycle Strategies: (2-Nitrophenyl)isoxazole Precursors to 4-Aminoquinolines, 1H-Indoles, and Quinolin-4(1H)-ones. Org. Lett 2013, 15, 2062–2065. [DOI] [PMC free article] [PubMed] [Google Scholar]