Abstract
Background:
This study examines relationships between neonatal white and gray matter microstructure and neurodevelopment in very preterm infants (VPT; ≤30 weeks gestation) with high-grade brain injury (BI).
Methods:
Term-equivalent diffusion tensor magnetic resonance imaging (DTI) data were obtained in 32 VPT infants with high-grade BI spanning grade III/IV intraventricular hemorrhage, post-hemorrhagic hydrocephalus (PHH), and cystic periventricular leukomalacia (BI group); 69 VPT infants without high-grade injury (VPT group); and 55 term-born infants. The Bayley-III assessed neurodevelopmental outcomes at age 2-years.
Results:
BI infants had lower fractional anisotropy (FA) in the posterior limb of the internal capsule (PLIC), cingulum, and corpus callosum; and higher mean diffusivity (MD) in the optic radiations and cingulum than VPT infants. PHH was associated with higher MD in the optic radiations and left PLIC; and higher FA in the right caudate. For BI infants, higher MD in the right optic radiation and lower FA in the right cingulum, PLIC, and corpus callosum were related to motor impairments.
Conclusions:
BI infants demonstrated altered white and gray matter microstructure in regions affected by injury in a manner dependent upon injury type. PHH infants demonstrated the greatest impairments. Aberrant white matter microstructure was related to motor impairment in BI infants.
Introduction
Premature birth remains a major public health problem in the United States, with rates ranging from 9.6-10.4% over the last decade (1). Due to advances in antenatal and neonatal care, greater numbers of preterm infants born at earlier gestational ages are surviving to hospital discharge. Despite these improvements, rates of common forms of perinatal brain injury, including intraventricular hemorrhage (IVH), post-hemorrhagic hydrocephalus (PHH), and cystic periventricular leukomalacia (cPVL), remain high among preterm survivors (2,3). Neurological and neurodevelopmental outcomes for preterm infants with these common forms of brain injury remain among the worst in newborn medicine, with impairment rates as high as 85% across cognitive, language, and motor domains (3–5).
Prior work utilizing conventional MRI has shown that perinatal brain injury alters cerebral white and gray matter development (6). Diffusion tensor imaging (DTI), which measures the three-dimensional movements of water molecules to evaluate microstructural tissue properties, has been successfully used to delineate the effects of both prematurity and perinatal brain injury on the microstructural development of white and gray matter in very preterm infants (VPT; born ≤32 weeks gestation) (7–10). Alterations in mean diffusivity (MD) and fractional anisotropy (FA) in the optic radiations, corpus callosum, and cingulum bundle have been reported in VPT infants with brain hemorrhage and/or ventricular enlargement (11–16). However, most prior studies have included only small numbers (6–15%) of preterm infants with high-grade brain injury (7,11,12,14,15,17). These investigations have also focused on individual forms of brain injury in isolation to other common types, precluding the ability to examine microstructrual abnormalities uniquely associated with IVH, PHH, and cPVL (5,11,12). Further, the extant literature has centered on white matter development, with few studies including DTI measures of subcortical gray matter microstructural development (7,9). Thus, the manner and extent that high-grade brain injury alters both white and gray matter microstructure remains undefined, highlighting the need for a prospective study of a selectively recruited cohort of infants with BI.
Findings from longitudinal studies utilizing DTI suggest that alterations in neonatal white matter microstructure may underlie the high rates of neurodevelopmental impairment in VPT infants. Aberrant measures of MD and/or FA in the cingulum, corpus callosum, posterior limb of the internal capsule (PLIC), and optic radiations have been linked to poorer cognitive, language, motor, and socio-emotional abilities in preterm children from early childhood through school-age (10,12,17). However, there is a paucity of studies focusing on associations between white and gray matter microstructure and neurodevelopmental impairments among VPT infants with high-grade brain injury. One study of VPT infants examined the extent that microstructural development of subcortical gray matter from birth to term-equivalent age in infants with high-grade injury (spanning severe white matter lesions, IVH, and ventriculomegaly) was associated with neurodevelopmental impairments (7). This investigation found that slower increases in FA of the caudate, lentiform nuclei, and thalamus from birth to term-equivalent age were associated with poorer motor and cognitive outcomes at age 18 months, an association independent of the effects of white matter lesions and IVH. However, Chau and colleagues examined the caudate, lentiform nuclei, and thalamus as a single region of interest, precluding the study of region-specific associations between brain injury and neurodevelopmental outcomes.
This prospective study examines associations between high-grade brain injury on white and gray matter microstructural development by comparing VPT infants with Grades III/IV IVH, PHH and cPVL to both VPT infants without brain injury and term-born control infants across DTI region of interest (ROI). We also examine the extent that the independent and joint effects of brain injury and altered white and gray matter microstructure increase risks of adverse neurodevelopmental outcomes at age 2-years. We hypothesized that: 1) white and gray matter regions in proximity to areas most prominently affected by injury (corpus callosum, cingulum bundles, optic radiations, thalamus, lentiform nucleus) would show the greatest microstructural alterations; 2) microstructural alterations related to brain injury would be distinct and more severe than those resulting from prematurity alone, with differences based upon injury type; and 3) MD and FA in white and gray matter regions at term-equivalent age would be associated with neurodevelopmental outcomes of VPT infants with brain injury at age 2-years.
Methods
Sample
This prospective, longitudinal study utilized data from three infant cohorts (Table 1). The first group consisted of 32 VPT infants (≤30 weeks gestation) with common patterns of high-grade perinatal brain injury known to place infants at increased risk for adverse neurodevelopmental outcomes. These infants were prospectively recruited from a large regional Level-III Neonatal Intensive Care Unit (NICU) and selected for study enrollment based upon evidence of common forms of brain injury, including Papile grade III/IV IVH (18), PHH (IVH requiring subsequent neurosurgical treatment for hydrocephalus based upon frontal-occipital ratio ≥0.55 on US or MRI, progressive increase in occipitofrontal circumference, splaying of the sagittal suture ≥2 mm in the mid-parietal region, and palpation of the anterior fontanel above the level of the surrounding bone) (19), or cystic white matter lesions, on cranial ultrasound within the first month of life. Given their comparable high-risk neurodevelopmental profile (3–5), VPT infants with grade III/IV IVH (n=15), PHH (n=10), and cPVL (n=7) were analyzed together as a single Brain Injury (BI) group. The vast majority of BI infants (13/15 grade III/IV IVH infants, 10/10 PHH infants, and 4/7 cPVL infants) had bilateral patterns of high-grade injury across left and right brain hemispheres.
Table 1.
Infant Clinical and Social Background Characteristics of the Sample
| Variable | FT (n = 55) | VPT (n = 69) | BI (n = 32) | p |
|---|---|---|---|---|
| Gestational age at birth (weeks), m (SD) | 39.00 (1.3) | 26.72 (1.7) | 25.72 (2.1) | <.001*** |
| Birthweight (g) , m (SD) | 3236.84 (469.9) | 944.44 (254.3) | 915.38 (250.3) | <.001*** |
| Multiple birth, % (n) | - | 31.9 (22) | 9.4 (3) | .02 |
| Male, % (n) | 49.1 (27) | 40.6 (28) | 59.4 (19) | .20 |
| African American, % (n) | 74.5 (41) | 39.1 (27) | 37.5 (12) | <.001*** |
| Intra-uterine growth restriction, % (n) | - | 7.2 (5) | 3.1 (1) | .42 |
| Did not receive antenatal steroids, % (n) | - | 5.8 (4) | 21.9 (7) | .02* |
| Received postnatal steroids, % (n) | - | 13.0 (9) | 12.5 (4) | .94 |
| Oxygen at 36 weeks, % (n) | - | 49.3 (34) | 75.0 (24) | .02* |
| Patent ductus arteriosus, % (n) | - | 40.6 (28) | 28.1 (9) | .23 |
| Retinopathy of prematurity, % (n) | - | 15.9 (11) | 25.0 (8) | .28 |
| Necrotizing enterocolitis , % (n) | - | 5.8 (4) | 28.1 (9) | .002** |
| Confirmed Sepsis, % (n) | - | 26.1 (18) | 46.9 (15) | .04* |
| Infant medical propensity score, m (SD) | 0.0 (0.0) | 0.25 (0.14) | 0.47 (0.28) | <.001*** |
| High-Grade Brain Injury, % (n) | ||||
| IVH Grade III/IV | - | - | 46.9 (15) | |
| IVH Grade III/IV with surgical treatment | - | - | ||
| (PHH) | 31.3 (10) | |||
| cPVL | - | - | 21.9 (7) | - |
| High-Grade Brain Injury Laterality, % (n) | ||||
| Bilateral | - | - | 84.3 (27) | |
| Left hemisphere | - | - | 12.5 (4) | |
| Right hemisphere | - | - | 3.1 (1) | - |
| Postmenstrual age at scan, m (SD) | 39.04 (1.2) | 37.67 (1.5) | 38.41 (1.6) | <.001*** |
Note. Factors that contributed to the medical risk propensity score: oxygen at 36 weeks, patent ductus arteriosus, necrotizing enterocolitis, and did not receive antenatal steroids.
IVH, intraventricular hemorrhage; PHH, post-hemorrhagic hydrocephalus; cPVL, cystic periventricular leukomalacia.
p ≤ 0.05;
p ≤ 0.01;
p ≤ 0.001
The second group included 69 VPT infants recruited from the same Level-III NICU as the BI group. These VPT infants did not have high-grade injury either on cranial ultrasound or term equivalent MRI. The third group included 55 full-term (FT) singleton control infants (38–42 weeks gestation) who were recruited from an adjoining maternity hospital. Exclusion criteria across all groups included parent unable to give informed consent, chromosomal abnormalities, and/or proven congenital infections (e.g., cytomegalovirus, toxoplasma, rubella). Additional exclusion criteria for the FT group included acidosis (pH <7.20) on umbilical cord or arterial blood gas assessed during the first hour of life and evidence of in utero illicit substance exposure. Parents provided informed written consent for all infants. All procedures were approved by the Washington University Human Research Protection Office.
Imaging protocol
BI and VPT infants underwent an MRI scan at/near term equivalent age (35–43 weeks postmenstrual age) based upon clinical status. FT infants were scanned within the first four days of life. All infants were prepared and transported to the scanner using institutional neonatal imaging guidelines. Infants were imaged without sedation during natural sleep or while resting quietly. For all infants, images were acquired using a 3T Siemens TIM Trio system (Erlangen, Germany) using an infant-specific, quadrature head coil (Advanced Imaging Research, Cleveland, OH, USA). DTI data were collected using a diffusion-weighted sequence [TR 13300 ms, TE 112 ms, 1266 Hz/Px bandwidth, 128 mm field of view (FoV), 1.2×1.2×1.2 mm3 voxels, 48 b-directions with amplitudes ranging from 0 to 1200 s/mm2].
Diffusion processing
The diffusion signal attenuation curve was modeled as a monoexponential function plus a constant. Diffusion parameters were estimated using Bayesian Probability Theory. Maps of FA, MD, radial diffusivity (RD), and axial diffusivity (AD) were generated. ROIs were manually placed in white and gray matter regions in native space using FA, RD, and color maps to identify anatomical structures by single raters. ROI placement was reviewed by a pediatric neuroradiologist (JSS). A second rater placed ROIs on an overlapping subset of MRI subjects (10%) to ensure reproducibility of ROI placement. Overall mean inter-rater reliability was 0.91 for MD and 0.88 for FA (range: 0.70–0.99) (10). Mean inter-rater reliability was also high and similar across each of the study groups: 0.92 for MD and 0.88 for FA (range: 0.70–0.97) in the FT group, 0.88 for MD and 0.87 for FA (range: 0.70–0.99) in the VPT group, and 0.93 for MD and 0.89 for FA (range: 0.70–0.99) across the BI groups. Bilateral ROIs were placed in the anterior limb of the internal capsule (ALIC), PLIC, optic radiations, cingulum, centrum semiovale, frontal lobe, corpus callosum, caudate nucleus, lentiform nucleus, and thalamus (Figure 1). Tracings for each region were placed on three contiguous axial slices in order to minimize through-slice partial volume averaging. ROIs were sampled using ANALYZE 10.0 software (AnalyzeDirect, Biomedical Imaging Resource at Mayo Clinic, Rochester, MN) in combination with laboratory-written scripts (11, 12).
Figure 1.
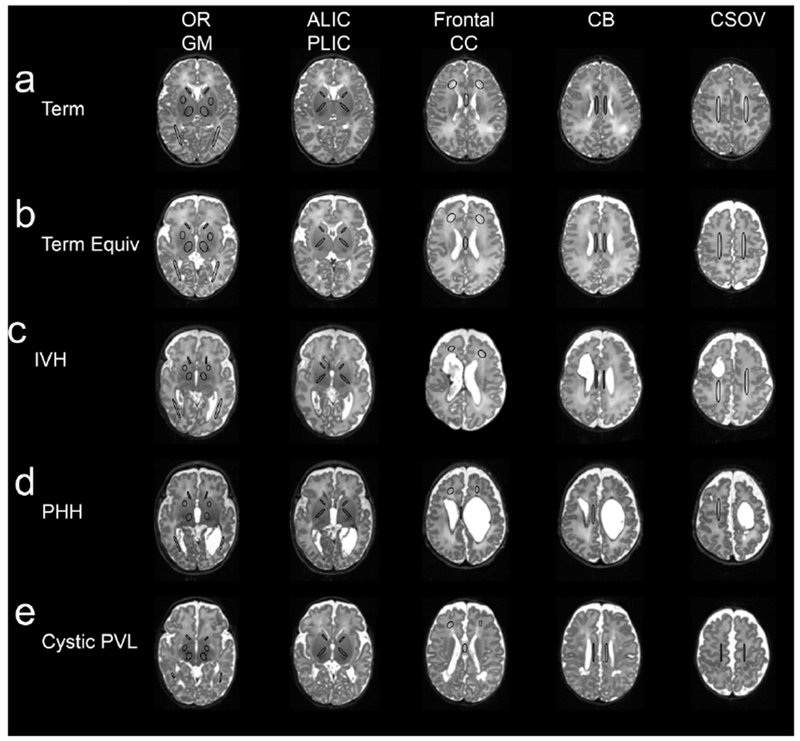
Region of Interest Placed on MRI Scans at Term-Equivalent Postmenstrual Age. Figure 1 demonstrates overall placement of regions-of-interest (ROIs) placed on the MRI scans of full-term infants (a), very preterm infants with no high-grade brain injury (b), and very preterm infants with high-grade intraventricular hemorrhage (c), post-hemorrhagic hydrocephalus (d), and cystic periventricular leukomalacia (e). ROIs, denoted by black circles, were placed in the optic radiations (OR), subcortical gray matter (GM), anterior (ALIC) and posterior (PLIC) limbs of the internal capsule, frontal white matter, corpus callosum (CC), cingulum bundle (CB), and centrum semiovale (CSOV).
Clinical variables
Infant clinical information was prospectively collected from infant medical records. A medical risk propensity score was calculated using a forwards conditional logistic regression procedure to weigh medical factors for VPT and BI infants. Based upon prior literature (9,10,17), ten medical factors were included as independent variables: intrauterine growth restriction (>2SD below the mean), oxygen therapy at 36 weeks, maternal antenatal steroids not administered, postnatal steroids administered, necrotizing enterocolitis, patent ductus arteriosus requiring medical treatment with indomethacin or ibuprofen, retinopathy of prematurity, culture-positive sepsis, >3 SD decrease in weight-for-height/length standard deviation score from birth to term-equivalent MRI, and >75th percentile for length of total parenteral nutrition. Factors that significantly contributed to the propensity score for VPT and BI infants included oxygen therapy at 36 weeks (p=.01), patent ductus arteriosus (p=.03), necrotizing enterocolitis (p=.02), and not receiving maternal antenatal steroids (p=.04).
Neurodevelopmental outcomes at corrected-age 2-years
Neurodevelopmental outcomes were assessed at corrected-age 2-years by a psychometrician blind to children’s birth group and injury status using the Bayley Scales of Infant and Toddler Development, 3rd Edition (Bayley-III) (20). The Bayley-III provides standardized cognitive, language, and motor composite scores (m=100, SD=15), with higher scores indicating better neurodevelopmental functioning (20). Delay was defined using a standardized composite score <85 (21). Bayley-III data was obtained for 27 BI (84%) and 59 VPT (86%) infants. Reasons for missing Bayley-III data included withdrew from study (VPT=2), unable to contact family (BI=1, VPT=7), and failed to attend scheduled testing (BI=4, VPT=1).
Statistical analysis
Microstructural alterations in white and gray matter regions of interest:
Statistical analyses were performed using SPSS version 25 (IBM Corporation, Armonk, NY). Associations between preterm birth and brain injury on white and gray matter ROIs were examined using general linear models. Group (BI, VPT, FT) was used as the independent variable and DTI measures as the dependent variables. Postmenstrual age (PMA) at scan, race, and medical risk propensity score were included as covariate factors in all DTI analyses. Models were fitted separately for white (i.e., ALIC, PLIC, optic radiations, cingulum, centrum semiovale, frontal lobe, and corpus callosum) and gray (i.e., caudate, thalamus, and lentiform nucleus) matter regions across diffusion measures (i.e., FA, MD, AD, and RD). Adjusted means and 95% confidence intervals (CI) are reported. As left and right hemispheric differences were found across ROIs using paired t-tests, DTI measures for each hemisphere were analyzed separately. To examine the extent that shared variance among preterm twins (n=22) and triplets (n=3) might be contributing to DTI findings, additional analysis was performed by excluding one sibling in each set of twins and two siblings in the single set of triplets and repeating all analyses. As the results of this analysis were virtually identical to the results of the whole group analysis, findings for all infants are reported. Next, an additional set of analyses were performed to delineate the effect of injury type (i.e., grade III/IV IVH, PHH, and cPVL) on white and gray matter microstructure among BI infants. In accordance with recommended statistical practices (22), general linear models adjusted for continuous covariate factors were performed with embedded Bonferroni correction to adjust confidence intervals and corresponding p-values of estimated marginal means for multiple comparisons.
Associations between white and gray matter microstructure and outcomes at age 2-years:
Statistical analyses were performed using SPSS version 25 (IBM Corporation, Armonk, NY). BI and VPT between-group differences on Bayley-III composite scores were examined using independent samples t-tests for continuous variables and chi-square tests for categorical variables. Bayley-III scores then were adjusted for medical risk and race based upon higher medical risk propensity scores in BI infants (p=.04) and African American children performing less well on Bayley-III Language tasks (p=.05), using analysis of covariance for continuous variables and logistic regression for categorical variables. Sex was also considered as an additional covariate, although this factor was not different between BI and VPT infants (p=.08) nor associated with Bayley-III outcomes (p≥.29). In line with other researchers (23,24), Bayley-III results were not adjusted for multiple comparisons on the basis that this analysis was intended to be hypothesis generating, focusing on the overall pattern and magnitude of group differences rather than individual p-values. Next, associations between high-grade brain injury and altered white and gray matter microstructure on Bayley-III scores were examined using linear mixed effect-models. Diffusion ROIs and Bayley-III scales were selected for this analysis based upon significant post-hoc comparisons between BI and VPT infants. In this analysis, group (VPT=1, BI=2), race, PMA, infant medical propensity score, ROI, and a group by ROI interaction term were entered as fixed factors. Mother-and subject-within-mother was entered as a random factor with random intercept to take into account family clustering among preterm twins and triplets.
Results
Microstructural alterations in white matter regions of interest
Table 2 provides a summary of MD and FA across white matter ROIs for BI, VPT, and full-term infants. In terms of specific BI and VPT between-group differences, BI infants had reduced FA in the left (p=.02) and right (p=.003) PLIC, left (p=.01) and right (p<001) cingulum bundle, and the corpus callosum (p<.001). In terms of MD, BI infants had higher MD in the left (p=.03) and right (p=.01) optic radiation, and left (p=.02) and right (p=.03) cingulum bundles compared to VPT infants. To delineate whether alterations in MD were due to differences in impaired axonal development and/or myelination, AD and RD values were generated for the optic radiations and cingulum bundles (Supplemental Table S1, online). Results of this analysis indicated that BI infants had higher RD values across the left (p=.02) and right (p=.002) optic radiations and left (p=.004) and right (p=.002) cingulum bundles than VPT infants. Additionally, BI infants had higher AD values in the left (p=.007) and right (p=.008) optic radiations.
Table 2.
Comparison of diffusion parameters in white matter regions between FT, VPT, and BI infants
| Parameter | Regiona | Estimated marginal mean (95% confidence intervals) b | VPT v. BIc | ||
|---|---|---|---|---|---|
| FT (n = 55) | VPT (n = 69) | BI (n = 32) | |||
| FA | L ALIC*** | 0.33 (.31–.35) | 0.28 (.27–.30)*** | 0.27 (.24–.29)*** | |
| R ALIC** | 0.34 (.32–.36) | 0.30 (.28–.31)* | 0.27 (.25–.30) ** | ||
| L PLIC*** | 0.47 (.46–.49) | 0.46 (.45–.47) | 0.43 (.41–.45)** | * | |
| R PLIC *** | 0.48 (.46–.50) | 0.46 (.46–.47) | 0.42 (.40–.44)** | ** | |
| L OPRA | 0.32 (.30–.34) | 0.31 (.30–.33) | 0.30 (.28–.32) | ||
| R OPRA | 0.32 (.30–.33) | 0.32 (.31–.33) | 0.31 (.29–.33) | ||
| L CNBD** | 0.31 (.30–.33) | 0.30 (.30–.31) | 0.28 (,26–.29)* | * | |
| R CNBD *** | 0.30 (.28–.31) | 0.30 (.29–.30) | 0.26 (.24–.28)* | *** | |
| L CSOV *** | 0.23 (.21–.24) | 0.15 (.14–.17)*** | 0.16 (.14–.18)*** | ||
| R CSOV*** | 0.21 (.20–.23) | 0.15 (.14–.16)*** | 0.17 (.15–.19)** | ||
| L FRNT | 0.14 (.13–.16) | 0.14 (.13–.14) | 0.12 (.11–.14) | ||
| R FRNT | 0.14 (.13–.16) | 0.13 (.13–.14) | 0.13 (.11–.14) | ||
| CC *** | 0.51 (.48–.54) | 0.44 (.42–.46)** | 0.36 (.32–.40)*** | *** | |
| MD | L ALIC | 1.37 (1.34–1.40) | 1.37 (1.35–1.39) | 1.38 (1.34–1.41) | |
| R ALIC | 1.37 (1.35–1.40) | 1.36 (1.35–1.38) | 1.35 (1.32–1.39) | ||
| L PLIC | 1.25 (1.23–1.28) | 1.22 (1.20–1.24) | 1.23 (1.21–1.26) | ||
| R PLIC * | 1.23 (1.21–1.26) | 1.20 (1.19–1.22) | 1.23 (1.21–1.26) | ||
| L OPRA** | 1.50 (1.45–1.54) | 1.57 (1.53–1.60) | 1.65 (1.59–1.70)** | * | |
| R OPRA** | 1.50 (1.45–1.54) | 1.54 (1.51–1.57) | 1.62 (1.57–1.67)** | ** | |
| L CNBD* | 1.42 (1.39–1.45) | 1.45 (1.43–1.47) | 1.49 (1.46–1.53)* | * | |
| R CNBD ** | 1.42 (1.39–1.44) | 1.46 (1.44–1.48) | 1.51 (1.48–1.55)*** | * | |
| L CSOV*** | 1.44 (1.39–1.49) | 1.70 (1.67–1.74)*** | 1.61 (1.55–1.68) *** | * | |
| R CSOV*** | 1.43 (1.38–1.48) | 1.71 (1.67–1.74)*** | 1.61 (1.55–1.67)*** | ** | |
| L FRNT | 1.66 (1.62–1.71) | 1.69 (1.66–1.73) | 1.71 (1.66–1.76) | ||
| R FRNT | 1.67 (1.62–1.72) | 1.70 (1.67–1.74) | 1.71 (1.65–1.76) | ||
| CC | 1.60 (1.49–1.71) | 1.69 (1.62–1.77) | 1.79 (1.65–1.93) | ||
Note. Models adjusted for post-menstrual age at scan, medical risk propensity score and race. General linear models performed with embedded Bonferroni correction. ALIC, anterior limb of the internal capsule; PLIC, posterior limb of the internal capsule; OPRA, optic radiations; CNBD, cingulum; CSOV, centrum semiovale; FRNT, frontal lobe; CC, corpus callosum. L, left; R, right.
F statistic in analysis of variance,
pairwise comparisons with FT infants;
pairwise comparisons between VPT and BI infants.
p ≤ 0.05;
p ≤ 0.01;
p ≤ 0.001
In contrast to the pattern of group differences found across the optic radiations and corpus callosum, VPT infants had higher MD in the right (p=.02) and left (p=.01) centrum semiovale than BI infants. This result was attributable to VPT infants having higher AD values in the right (p=.001) and left (p=.001) centrum semiovale, as well as higher RD in the right centrum semiovale (p=.04).
Microstructural alterations in gray matter regions of interest
As shown in Table 3, BI infants had lower FA in the right and left lentiform nucleus compared to full-term infants (p<.05). However, in contrast to the research hypothesis, there were no pair-wise differences in MD or FA across subcortical gray matter ROIs between BI and VPT infants (p>.05, Table 3).
Table 3.
Comparison of diffusion parameters in gray matter regions between FT, VPT, and BI infants
| Parameter | Region a | Estimated marginal mean (95% confidence intervals) b | VPT v. BIc | ||
|---|---|---|---|---|---|
| FT (n = 55) | VPT (n = 69) | BI (n = 32) | |||
| FA | L CAUD | 0.09 (.08–.09) | 0.08 (.08–.09) | 0.09 (.08–.10) | |
| R CAUD | 0.08 (.08–.09) | 0.09 (.08–.09) | 0.09 (.09–.10) | ||
| L THAL | 0.15 (.14–.16) | 0.16 (.15–.16) | 0.16 (.15–.17) | ||
| R THAL | 0.17 (.16–.18) | 0.16 (.16–.17) | 0.15 (.14–.17) | ||
| L LENT** | 0.13 (.12–.13) | 0.12 (.11–.12) | 0.11 (.10–.11)** | ||
| R LENT* | 0.13 (.12–.13) | 0.12 (.11–.12) | 0.10 (.10–.12) * | ||
| MD | L CAUD | 1.35 (1.33–1.38) | 1.37 (1.36–1.39) | 1.37 (1.34–1.40) | |
| R CAUD | 1.35 (1.33–1.37) | 1.37 (1.35–1.38) | 1.36 (1.33–1.38) | ||
| L THAL | 1.24 (1.22–1.27) | 1.21 (1.20–1.23) | 1.22 (1.19–1.25) | ||
| R THAL | 1.25 (1.23–1.28) | 1.21 (1.20–1.23) | 1.22 (1.20–1.25) | ||
| L LENT | 1.29 (1.26–1.31) | 1.30 (1.28–1.31) | 1.30 (1.27–1.32) | ||
| R LENT | 1.29 (1.27–1.31) | 1.29 (1.28–1.31) | 1.30 (1.27–1.32) | ||
Note. Models adjusted for post-menstrual age at scan, medical risk propensity score and race. General linear models performed with embedded Bonferroni correction. CAUD, caudate; THAL, thalamus, LENT, lentiform nucleus. L, left; R, right.
F statistic in analysis of variance,
pairwise comparisons with FT infants;
pairwise comparisons between VPT and BI infants.
p ≤ 0.05;
p ≤ 0.01;
p ≤ 0.001
Associations between injury type and DTI measures within the BI group
To delineate the extent to which specific types of brain injury are associated with regional alterations in white and gray matter microstructure, the BI group (N=32) was categorized into high-grade IVH (n=15), PHH (n=10), and cPVL (n=7) subgroups and compared across DTI measures (Supplemental Table S2, online). For white matter ROIs, between-group differences were found in MD of the right ALIC (p=.02), left PLIC (p=.02), and right (p<.001) and left (p=.008) optic radiations. Group differences across these specific ROIs were attributable to PHH infants having the highest MD values, particularly in comparison to IVH infants (p≤.02) (Figure 2). In contrast to MD findings, there were no between-groups differences regarding injury subtype and FA of white matter ROIs.
Figure 2.
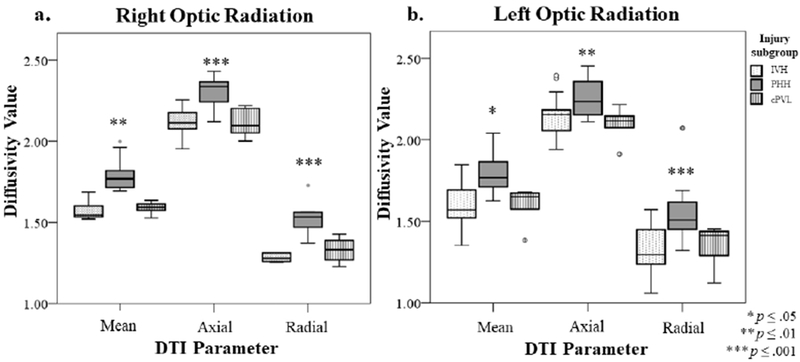
Diffusivity Values in the Right (a) and Left (b) Optic Radiation by Injury Subgroup (n=32). Figure 2 shows the mean, axial, and radial diffusivity values for BI infants with high-grade IVH, PHH, and cPVL in the right (a) and left (b) optic radiation at term-equivalent post-menstrual age. Higher mean, axial, and radial diffusivity was associated with the PHH group in both the right and left optic radiation, whereas differences were less pronounced between IVH and cPVL groups.
For gray matter ROIs, there was a significant between-groups difference in FA for the right caudate (p=.04), with this finding attributable to IVH infants having lower FA values than the PHH infants (p=.04). There was also evidence of an association between type of brain injury and altered MD in the right thalamus (p=.04), driven by higher AD in PHH infants compared to both IVH (p=.01) and cPVL (p=.01) infants.
Neurodevelopmental outcomes at corrected-age 2-years
Table 4 shows the mean Bayley-III cognitive, language and motor composite scores and rates of delay for BI and VPT infants at corrected age 2-years. Unadjusted results indicated that BI infants obtained lower cognitive (d=.49, p=.03), language (d=.56, p=.02), and motor (d=1.07, p<.001) scores compared to VPT infants. BI infants also had higher rates of language (OR 2.53, p=.05) and motor (OR 8.44, p=.05) delay. Importantly, lower motor composite scores (p<.001) and higher rates of motor delay (p<.001) persisted for the BI group compared to the VPT group after covariate adjustment for medical propensity scores and race.
Table 4.
Mean Bayley-III scores and rates of delay in VPT and BI infants at age 2 years
| m (95% C.I) | VPT (n = 59) | BI (n = 27) | Unadjusted p | Adjusted pa | d/OR |
|---|---|---|---|---|---|
| Cognitive ability | 86.61 (83.75 – 89.47) | 80.96 (76.65 – 85.26) | .03* | .16 | 0.49 |
| Cognitive delay, % | 30.5 | 50.0 | .09 | .18 | 2.28 |
| Language ability | 89.81 (86.51 – 93.10) | 82.85 (77.97 – 87.73) | .02* | .11 | 0.56 |
| Language delay, % | 31.6 | 53.8 | .05* | .11 | 2.53 |
| Motor ability | 85.59 (82.40 – 88.77) | 72.22 (67.55 – 76.89) | <.001*** | .001*** | 1.07 |
| Motor delay,% | 29.3 | 77.8 | <.001*** | .001*** | 8.44 |
Note. Delay defined as standard score <85.
Adjusted for medical risk propensity score and race
p ≤ 0.05;
p ≤ 0.01;
p ≤ 0.001
Supplementary analysis performed within the BI group showed that there was a significant association between type of brain injury and motor outcomes at age 2-years (p=.02), but not for cognitive (p=.06) or language (p=.08) outcomes. Specifically, PHH infants (m=68.33, SD=11.8, p=.05) and cPVL infants (m=62.20, SD=15.1, p=.01) demonstrated poorer motor abilities compared to IVH infants (m =78.77, SD =10.1). While cPVL infants obtained motor composite scores approximately 6 points lower than PHH infants, this difference was not significant (p=.35). As expected, rates of motor delay were high across PHH (77.8%), IVH (69.2%), and cPVL (100%) infants (p=.37).
Associations between DTI measures and neurodevelopmental outcomes
Based upon the pattern of between-group differences in Tables 2 and 3, white matter microstructure in the bilateral PLIC, cingulum bundles, central semiovale, optic radiations and corpus callosum were examined in relation to motor outcomes at age 2-years for BI and VPT infants. As shown in Table 5, there were significant main effects of white matter microstructure in relation to Bayley-III motor scores. Specifically, higher MD in the right optic radiation (p=.02) and lower FA in the right PLIC (p=.006), right cingulum bundle (p=.04), and the corpus callosum (p=.05) were associated with lower motor scores at age 2-years. Additionally, the significant interaction terms for the left (p=.05) and right (p=.01) PLIC and right cingulum bundle (p=.03) indicated that the association between altered neonatal white matter microstructure and subsequent motor problems was stronger in the BI group than in the VPT group, suggesting that associations between aberrant neonatal white matter microstructure and subsequent motor problems was attributable to the BI group.
Table 5.
Associations between Neonatal Diffusion Measures and Bayley-III Motor Scores at Age 2 Years in VPT (n = 69) and BI Infants (n = 32)
| Parameter | Left ROI | Right ROI | |||||
|---|---|---|---|---|---|---|---|
| Region | Estimate | SE | p | Estimate | SE | p | |
| FA | - | - | - | ||||
| CC (Single ROI) | Brain injury | 31.75 | 13.8 | .03* | - | - | - |
| CC | 60.19 | 30.3 | .05* | - | - | - | |
| Brain injury by CC | –63.03 | 35.5 | .08 | - | - | - | |
| PLIC | Brain injury | 70.73 | 29.7 | .02* | 58.65 | 17.41 | .002** |
| PLIC | 107.00 | 61.84 | .09 | 87.88 | 29.9 | .006** | |
| Brain injury by PLIC | –136.36 | 68.8 | .05* | –112.13 | 40.7 | .01** | |
| CNBD | Brain injury | 36.21 | 22.6 | .11 | 57.87 | 22.52 | .01** |
| CNBD | 60.98 | 72.0 | .40 | 159.13 | 74.5 | .04* | |
| Brain injury by CNBD | –97.33 | 79.5 | .23 | –185.12 | 84.0 | .03* | |
| MD | |||||||
| OPRA | Brain injury | –31.91 | 32.5 | .33 | –53.77 | 35.7 | .14 |
| OPRA | –27.04 | 16.3 | .10 | –38.71 | 16.8 | .02* | |
| Brain injury by OPRA | 26.89 | 19.8 | .18 | 39.75 | 22.0 | .08 | |
| CNBD | Brain injury | –24.12 | 59.9 | .69 | 21.21 | 65.5 | .75 |
| CNBD | –4.70 | 32.4 | .89 | 3.18 | 38.7 | .94 | |
| Brain injury by CNBD | 22.39 | 40.5 | .58 | −8.11 | 43.8 | .85 | |
| CSOV | Brain injury | −11.19 | 37.1 | .76 | −2.48 | 31.3 | .94 |
| CSOV | 1.53 | 20.4 | .94 | 6.70 | 16.33 | .68 | |
| Brain injury by CSOV | 13.46 | 22.56 | .55 | 6.58 | 18.9 | .73 | |
Note. Models adjusted for post-menstrual age at scan, medical risk propensity score, race, and sibling correlation.
CC, corpus callosum; PLIC, posterior limb of the internal capsule; CNBD, cingulum bundle; OPRA, optic radiations; CSOV, centrum semiovale.
p ≤ 0.05;
p ≤ 0.01;
p ≤ 0.001
Discussion
This study examined neonatal alterations in white and gray matter microstructure among preterm infants with high-grade brain injury, and the extent that these factors increased risks of subsequent neurodevelopmental impairments at age 2-years (Please see Table 6 for a summary of key findings). At term-equivalent age, BI infants had lower FA and higher MD across white and gray matter regions compared to VPT infants. Alterations were pronounced in regions anatomically approximate to the ventricles. Within the BI group, infants with PHH had higher MD in the bilateral optic radiations, right ALIC, left PLIC, and right thalamus; whereas infants with cPVL had lower FA in the right caudate. Neonatal microstructural alterations in the optic radiations, PLIC, cingulum bundle, and corpus callosum were related to poorer motor outcomes among BI infants at age 2-years.
Table 6.
Summary of Major Findings Relevant to Very Preterm Infants with Perinatal High-Grade Brain Injury (BI)
| Study Aim | Major Findings Relevant to BI Infants |
|---|---|
| To investigate the effect of BI on neonatal white and gray matter microstructure between very preterm infants with and without BI | - BI is associated with lower fractional anisotropy in posterior limb of the internal capsule, cingulum bundle, and corpus callosum - BI is associated with higher mean diffusivity in the optic radiations and cingulum bundle - Findings highlight vulnerability of white matter tracts closest to the ventricles |
| To compare neurodevelopmental outcomes at age 2 years between very preterm infants with and without BI | - BI is associated with poorer cognitive, language, and motor abilities - Motor problems in BI infants persist after covariate adjustment for medical and social risk factors, indicating robust associations - BI is associated with a two- to three-fold increase in risk for motor impairment, representing a major area of concern |
| To elucidate the effects of BI and aberrant white and gray matter microstructure on neurodevelopmental outcomes | - Aberrant microstructure in the cingulum bundle, corpus callosum, posterior limb of the internal capsule, and optic radiations and is associated with poorer motor abilities in BI infants - Altered neonatal white matter microstructure in the context of BI is an important neurobiological mechanism for subsequent motor impairments |
Our results in the BI group align with prior DTI studies (14,17,25), with injured infants demonstrating both lower FA in the bilateral cingulum bundles, PLIC, and corpus callosum, and higher MD in the bilateral cingulum bundles and optic radiations compared to VPT infants. Prior work has shown that the right hemisphere may be more vulnerable to white matter microstructural aberrations than the left hemisphere in VPT children (12), potentially due to hemispheric differences in rates of myelination (26). Our findings with regards to white matter microstructural alterations across both right and left hemispheres potentially reflects the characteristics of the BI group, such that the majority of BI infants demonstrated bilateral patterns of injury. Although the precise neurobiological mechanism is unclear, lower FA potentially reflects less restricted water diffusion and/or higher tissue water content in white matter tracts with immature or impaired myelination (27). In contrast, higher MD reflects microstructural anomalies in white matter rising from increased edema or reduced cellularity as a consequence of brain injury (28). Further investigation of radial and axial diffusivity values suggested that for BI infants, the cingulum bundle and optic radiations demonstrated impaired myelination, whereas the optic radiations additionally demonstrated axonal damage (29). In combination, these findings suggest that these major white matter tracts are uniquely vulnerable to both forms of injury, which is notable given both their immediate proximity to the lateral ventricles and the fact that these structures myelinate relatively early in typical neonatal brain development (9,30).
This is the first study to compare the effects of high-grade IVH, PHH, and cPVL on regional measures of white and gray matter microstructure. We identified within-group differences in the bilateral optic radiations, right ALIC, and left PLIC. Prior investigations have also linked congenital hydrocephalus to microstructural aberrations in the optic radiations (11,31). Our results suggest that the white matter microstructure in this region is impacted by the combined effects of hemorrhage and impaired CSF resorption that develop as a complication of severe IVH. As the optic radiations myelinate early in the neonatal period (32) and are in proximity to regions directly affected by ventricular enlargement, these tracts may be uniquely vulnerable to the inflammation, deformation, transependymal flow, and/or edema occurring in PHH (33). In contrast to our hypotheses, we did not find that IVH, PHH, or cPVL were differentially associated with microstructural alterations in the cingulum bundle or corpus callosum. While other studies have shown that IVH alters the macrostructural development of the corpus callosum (12,14) and that IVH and hydrocephalus may differentially impact anterior or posterior regions of the corpus callosum (12,13), we focused on white matter microstructure and comparisons between types of brain injury. Collectively, these findings suggest that DTI is sensitive to the underlying microstructural neuroanatomical abnormalities in white matter associated with common forms of preterm brain injury, including cPVL and PHH.
This is the first study to also examine the effects of high-grade brain injury on the microstructural properties of the thalamus, caudate, and lentiform nuclei as separate subcortical gray matter structures (7). Although BI infants had altered gray matter in the lentiform nuclei compared to FT control infants, we did not find evidence for differences in gray matter microstructure in the thalamus, caudate, or lentiform nuclei between BI and VPT infants, which was in contrast to our hypothesis. However, within the BI group, there was evidence to suggest that PHH infants had higher MD in the right thalamus, with additional differences in AD relative to cPVL and IVH infants. Prior clinical-case studies have linked hydrocephalus to DTI alterations in the thalamus (34). Thus, in preterm infants with PHH, higher thalamic MD and AD may be indicative of reduced tissue density and neuronal loss (35). Moreover, these findings might also suggest that local pressure effects or impaired reabsorption of CSF or edema caused by PHH is impacting the diffusion values in the thalamus due to proximity of the ROI to the enlarged ventricle (36).
As expected, BI infants demonstrated poorer cognitive, language, and motor outcomes compared to VPT infants at age 2-years, with findings for motor outcomes persisting after covariate adjustment. Additional analysis within the BI group indicated that infants with PHH and cPVL were at greatest risk for motor impairments. Previous studies suggest that neonatal neurological complications spanning IVH, ventricular dilatation, and PVL are strongly implicated in the development of motor impairments among VPT infants (13,37). Prior work has similarly linked brain injury and alterations in white matter microstructure with neurodevelopmental outcomes in preterm children and children with congenital hydrocephalus (7,8,11,13). Specifically, we found that higher MD in the optic radiations as well as lower FA in the cingulum bundle, corpus callosum, and PLIC was associated with motor outcomes at age 2-years, and that these associations are pronounced among BI infants. The findings in the corpus callosum are consistent with those from investigations of congenital hydrocephalus (13). In a previous study (14), significant relationships were identified between FA values in the corpus callosum and language, motor, and adaptive skills of children and adolescents prior to surgery and again 3 and 12 months post-operatively. DTI alterations in the PLIC have also been associated with poor motor function in preterm infants with periventricular hemorrhagic infarction (38). Further, the cingulum has been previously implicated in motor control processes due its connectivity with motor areas (39), with longitudinal studies of VPT infants also linking altered FA in the cingulum with visuo-motor skills at age 2-years (8). As the optic radiations have not readily been implicated in motor function in preterm infants (8,17), the link identified in our investigation may be a secondary association stemming from the widespread deleterious effects of brain injury (40).
Strengths of this study include longitudinal study design, selective recruitment of VPT infants with perinatal brain injury, inclusion of a regionally representative FT cohort, and assessment of neurodevelopmental outcomes using standardized measures at age 2-years. However, findings should be interpreted in light of study limitations. First, although this study included a larger proportion of VPT infants with brain injury than previous studies, the sample size of the BI group is modest. It is therefore possible that we were unable to detect small effect sizes, particularly pertaining to between-group differences for the PHH, IVH, and cPVL groups. Second, due to extensive injury in some BI infants who were unable to have a corpus callosum placed in a standard location, our reported associations between BI and the microstructural integrity of corpus callosum in relation to motor outcomes may have been slightly underestimated. Additionally, as data loss for the corpus callosum was confined to the PHH group, we were unable to quantify the effect of PHH on the microstructure of this specific region. Third, assessment at age 2-years may be considered relatively early in development, with subtle cognitive and language impairments not yet readily discernable. Longer-term follow-up and larger samples of BI infants will be vital to understand how these children grow into their impairments as the developmental demands placed on children increase by early school age. Future studies using probabilistic tractography may be useful to further elucidate the effects of brain injury on fiber organization in tracts identified by the current study as important for motor development.
Conclusions
Preterm infants with BI showed altered FA and MD across multiple white matter regions, including the corpus callosum, cingulum, and the optic radiations. The effect of brain injury was most pronounced in regions anatomically approximate to the ventricles. Thus, regional proximity to the area of direct injury is an important factor for white matter microstructural development. Within the BI group, infants with PHH infants had altered MD in regions including the optic radiations and right thalamus, whereas infants with IVH or cPVL had lower FA in the right caudate. When taken together, these findings suggest that DTI is sensitive to the regionally-specific microstructural abnormalities associated with both lesions in periventricular white matter and hemorrhage leading to impairments in cerebrospinal fluid resorption. Finally, among BI infants, lower FA in the right cingulum bundle, right PLIC, and corpus callosum as well as higher MD in the right optic radiation were associated with motor impairments at age 2-years. Thus, neonatal white matter microstructural alterations may be an important neurobiological mechanism related to subsequent motor impairments in high-risk VPT infants with brain injury.
Supplementary Material
Article Impact Answers.
Preterm infants with high-grade brain injury show altered microstructural organization across the corpus callosum, cingulum, posterior limb of the internal capsule, and the optic radiations.
Regional proximity to the area of direct injury is an important factor for white matter microstructural development.
Infants with post-hemorrhagic hydrocephalus show altered microstructure in the optic radiations and thalamus, suggesting that DTI is sensitive to the regionally-specific microstructural abnormalities associated with both lesions in periventricular white matter and hemorrhage leading to impaired cerebrospinal fluid resorption.
In very preterm infants with high-grade brain injury, altered microstructure in the corpus callosum, cingulum, posterior limb of the internal capsule, and the optic radiations was associated with subsequent motor impairments at age 2-years.
Neonatal white matter microstructural alterations are an important neurobiological mechanism related to subsequent motor impairments in high-risk VPT infants with brain injury.
Acknowledgments:
We thank Karen Lukas, Anthony Barton, and Jessica Perkins for study coordination; the IDDRC at Washington University for assistance with data collection; and the children and their families for participating in the study.
Financial Support: This work was supported by the National Institutes of Health [grant numbers K02 NS089852 to C.D.S., K23 MH105179 to C.E.R., TL1 TR002344 to R.H.H., P30 NS098577, R01 HD061619, R01 HD057098, U54 HD087011]; Child Neurology Foundation [to C.D.S.]; Cerebral Palsy International Research Foundation [to C.D.S.]; The Dana Foundation [to C.D.S.]; March of Dimes [to C.D.S. and D.D.L.]; and The Doris Duke Charitable Foundation [to C.E.R]. The funding sources had no involvement in study design, data analysis, writing the report, or the decision to submit the article for publication.
Disclosure Statement: Dr. Limbrick receives research funds and/or research equipment for unrelated projects from Medtronic, Inc. and Microbot Medical, Inc. Dr. Limbrick has received philanthropic equipment contributions for humanitarian relief work from Karl Storz, Inc. and Aesculap, Inc. The authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
References
- 1.National Center for Health Statistics. March of Dimes, final natality data. [Internet]. PeriStats. 2017. [cited 2017 Oct 29]. Available from: www.marchofdimes.org/peristats
- 2.Christian EA, Jin DL, Attenello F, et al. Trends in hospitalization of preterm infants with intraventricular hemorrhage and hydrocephalus in the United States, 2000-2010. Journal of Neurosurgery: Pediatrics. 2016. March;17(3):260–9. [DOI] [PubMed] [Google Scholar]
- 3.Srinivasakumar P, Limbrick D, Munro R, et al. Posthemorrhagic Ventricular Dilatation-Impact on Early Neurodevelopmental Outcome. American Journal of Perinatology. 2013. August 16;30(03):207–14. [DOI] [PubMed] [Google Scholar]
- 4.Al Rifai MT, Al Tawil KI. The Neurological Outcome of Isolated PVL and Severe IVH in Preterm Infants: Is It Fair to Compare? Pediatric Neurology. 2015. November;53(5):427–33. [DOI] [PubMed] [Google Scholar]
- 5.Wang S, Fan G, Xu K, Wang C. Potential of diffusion tensor MR imaging in the assessment of cognitive impairments in children with periventricular leukomalacia born preterm. European Journal of Radiology. 2013. January;82(1):158–64. [DOI] [PubMed] [Google Scholar]
- 6.Khwaja O, Volpe JJ. Pathogenesis of cerebral white matter injury of prematurity. Archives of Disease in Childhood - Fetal and Neonatal Edition. 2007. July 18;93(2):F153–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chau V, Synnes A, Grunau RE, Poskitt KJ, Brant R, Miller SP. Abnormal brain maturation in preterm neonates associated with adverse developmental outcomes. Neurology. 2013. December 10;81(24):2082–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Counsell SJ, Edwards AD, Chew ATM, et al. Specific relations between neurodevelopmental abilities and white matter microstructure in children born preterm. Brain. 2008. December;131(12):3201–8. [DOI] [PubMed] [Google Scholar]
- 9.Smyser TA, Smyser CD, Rogers CE, Gillespie SK, Inder TE, Neil JJ. Cortical Gray and Adjacent White Matter Demonstrate Synchronous Maturation in Very Preterm Infants. Cerebral Cortex. 2015. July 24;1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rogers CE, Smyser T, Smyser CD, Shimony J, Inder TE, Neil JJ. Regional white matter development in very preterm infants: perinatal predictors and early developmental outcomes. Pediatric Research. 2016. January;79(1–1):87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Drobyshevsky A, Bregman J, Storey P, et al. Serial diffusion tensor imaging detects white matter changes that correlate with motor outcome in premature infants. Developmental Neuroscience. 2007;29(4–5):289–301. [DOI] [PubMed] [Google Scholar]
- 12.Thompson DK, Lee KJ, Egan GF, et al. Regional white matter microstructure in very preterm infants: Predictors and 7 year outcomes. Cortex. 2014;52(1):60–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mangano FT, Altaye M, McKinstry RC, et al. Diffusion tensor imaging study of pediatric patients with congenital hydrocephalus: 1-year postsurgical outcomes. Journal of Neurosurgery: Pediatrics. 2016. September;18(3):306–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ou X, Glasier CM, Ramakrishnaiah RH, et al. Impaired white matter development in extremely low-birth-weight infants with previous brain hemorrhage. American Journal of Neuroradiology. 2014. October;35(10):1983–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Myall NJ, Yeom KW, Yeatman JD, Gaman-Bean S, Feldman HM. Case Series: Fractional Anisotropy Along the Trajectory of Selected White Matter Tracts in Adolescents Born Preterm With Ventricular Dilation. Journal of Child Neurology. 2012. June;28(6):774–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brouwer MJ, De Vries LS, Kersbergen KJ, et al. Effects of Posthemorrhagic Ventricular Dilatation in the Preterm Infant on Brain Volumes and White Matter Diffusion Variables at Term-Equivalent Age. Journal of Pediatrics. 2016;168:41–9. [DOI] [PubMed] [Google Scholar]
- 17.Thompson DK, Inder TE, Faggian N, et al. Corpus callosum alterations in very preterm infants: Perinatal correlates and 2 year neurodevelopmental outcomes. NeuroImage. 2012;59(4):3571–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: A study of infants with birth weights less than 1,500 gm. The Journal of Pediatrics. 1978. April;92(4):529–34. [DOI] [PubMed] [Google Scholar]
- 19.Wellons JC, Shannon CN, Holubkov R, et al. Shunting outcomes in posthemorrhagic hydrocephalus: results of a Hydrocephalus Clinical Research Network prospective cohort study. Journal of Neurosurgery: Pediatrics. 2017. April 28;20(1):19–29. [DOI] [PubMed] [Google Scholar]
- 20.Bayley N Bayley Scales of Infant and Toddler Development. 3rd ed. San Antonio, TX: Pearson Education, Inc.; 2006. [Google Scholar]
- 21.Johnson S, Moore T, Marlow N. Using the Bayley-III to assess neurodevelopmental delay: which cut-off should be used? Pediatric research. 2014. May 3;75(5):670–4. [DOI] [PubMed] [Google Scholar]
- 22.Field A Discovering Statistics Using SPSS. Third Edition. London, United Kingdom: Sage Publications LTD; 2009. [Google Scholar]
- 23.Murray AL, Scratch SE, Thompson DK, et al. Neonatal brain pathology predicts adverse attention and processing speed outcomes in very preterm and/or very low birth weight children. Neuropsychology. 2014;28(4):552–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anderson PJ, Treyvaud K, Neil JJ, et al. Associations of Newborn Brain Magnetic Resonance Imaging with Long-Term Neurodevelopmental Impairments in Very Preterm Children. The Journal of Pediatrics. 2017;187:58–65.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Malavolti AM, Chau V, Brown-Lum M, et al. Association between corpus callosum development on magnetic resonance imaging and diffusion tensor imaging, and neurodevelopmental outcome in neonates bom very preterm. Developmental Medicine and Child Neurology. 2017. April;59(4):433–40. [DOI] [PubMed] [Google Scholar]
- 26.Deoni SCL, Mercure E, Blasi A, et al. Mapping Infant Brain Myelination with Magnetic Resonance Imaging. Journal of Neuroscience. 2011. January 12;31(2):784–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rose SE, Hatzigeorgiou X, Strudwick MW, Durbridge G, Davies PSW, Colditz PB. Altered white matter diffusion anisotropy in normal and preterm infants at term-equivalent age. Magnetic Resonance in Medicine. 2008. October;60(4):761–7. [DOI] [PubMed] [Google Scholar]
- 28.O’Donnell LJ, Westin C-F. An Introduction to Diffusion Tensor Image Analysis. Neurosurgery Clinics of North America. 2011. April;22(2): 185–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thompson DK, Thai D, Kelly CE, et al. Alterations in the optic radiations of very preterm children—Perinatal predictors and relationships with visual outcomes. Neuroimage: Clinical. 2014;4:145–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Drobyshevsky A, Song S-K, Gamkrelidze G, et al. Developmental Changes in Diffusion Anisotropy Coincide with Immature Oligodendrocyte Progression and Maturation of Compound Action Potential. Journal of Neuroscience. 2005. June 22;25(25):5988–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rajagopal A, Shimony JS, McKinstry RC, et al. White matter microstructural abnormality in children with hydrocephalus detected by probabilistic diffusion tractography. American Journal of Neuroradiology. 2013. December;34(12):2379–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Knight MJ, Smith-Collins A, Newell S, Denbow M, Kauppinen RA. Cerebral White Matter Maturation Patterns in Preterm Infants: An MRI T2 Relaxation Anisotropy and Diffusion Tensor Imaging Study. Journal of Neuroimaging. 2018. January;28(l):86–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Uluğ AM, Truong TN, Filippi CG, et al. Diffusion imaging in obstructive hydrocephalus. American Journal of Neuroradiology. 2003;24(6): 1171–6. [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao C, Li Y, Cao W, et al. Diffusion tensor imaging detects early brain microstructure changes before and after ventriculoperitoneal shunt in children with high intracranial pressure hydrocephalus. Medicine. 2016. October;95(42):e5063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takeuchi H, Taki Y, Sekiguchi A, et al. Mean diffusivity of basal ganglia and thalamus specifically associated with motivational states among mood states. Brain structure & function. 2017. March 30;222(2): 1027–37. [DOI] [PubMed] [Google Scholar]
- 36.Akbari SHA, Limbrick DD, McKinstry RC, et al. Periventricular hyperintensity in children with hydrocephalus. Pediatric Radiology. 2015. July;45(8):1189–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marret S, Marchand-Martin L, Picaud J-C, et al. Brain Injury in Very Preterm Children and Neurosensory and Cognitive Disabilities during Childhood: The EPIPAGE Cohort Study. Wang K, editor. PLoS ONE. 2013. May 2;8(5):e62683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roze E, Benders MJ, Kersbergen KJ, et al. Neonatal DTI early after birth predicts motor outcome in preterm infants with periventricular hemorrhagic infarction. Pediatric Research. 2015. September;78(3):298–303. [DOI] [PubMed] [Google Scholar]
- 39.Bubb EJ, Metzler-Baddeley C, Aggleton JP. The cingulum bundle: Anatomy, function, and dysfunction. Neuroscience and biobehavioral reviews. 2018. September;92:104–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yuan W, McKinstry RC, Shimony JS, et al. Diffusion tensor imaging properties and neurobehavioral outcomes in children with hydrocephalus. AJNR Am J Neuroradiol. 2013. February;34(2):439–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


