Abstract
Objective:
Asthma poses an increased risk for serious pneumococcal disease, but little is known about the influence of asthma status on the 23-valent serotype-specific pneumococcal antibody response. We examined differences in antibody titers between pre- and post-vaccination with 23-valent pneumococcal polysaccharide vaccine (PPSV-23) in relation to asthma status.
Methods:
Asthma status was retrospectively ascertained by the Predetermined Asthma Criteria in an existing vaccine cohort through comprehensive medical record review. Twenty-three serotype-specific pneumococcal antibody titers measured at baseline and 4–6 weeks post-vaccination were analyzed. Vaccine responses to PPSV-23 were calculated from pre- to post-vaccine titers for each of the serotypes.
Results:
Of the 64 eligible and enrolled subjects, 18 (28%) had asthma. Controls (i.e., subjects without asthma) demonstrated a statistically significant fold change response compared to their baseline for all serotypes, while those with asthma did not mount a significant response to serotypes 7F, 22F, and 23F. The overall vaccine response as measured by fold change over baseline was lower in subjects with asthma than controls.
Conclusions:
Poorer humoral immune responses to PPSV-23 as measured by fold change were more likely to be observed in subjects with asthma compared to controls. We recommend the consideration of asthma status when interpreting vaccine response for immune competence workup through larger studies. Further studies are warranted to replicate these findings.
Introduction
Immune response to 23-valent pneumococcal polysaccharide vaccine (PPSV-23) is routinely employed to assess immune competence for evaluation of immune deficiencies (1). While the guidelines exist regarding the administration and interpretation of the testing, there are many aspects to this assessment that make a comprehensive application to all populations difficult (1). For example, the pattern of response to the various serotypes shows individual differences and may depend on multiple factors. Thus, a global overview of the immune response is challenging to ascertain. Additionally, asthma is associated with an increased risk of infections caused by Streptococcus pneumoniae infections (2). For example, there is mounting evidence suggesting that subjects with asthma are at a significantly increased risk of invasive pneumococcal disease, and the population-attributable risk percentage for asthma is 11% to 17% (2–4). In this regard, the assessment of vaccine response in a background of asthma not only has implications related to interpretation, but it also helps clinicians have a better understanding of the mechanisms underlying the increased propensity to pneumococcal infections in subjects with asthma.
Studies evaluating pneumococcal titers have demonstrated the role of concurrent comorbidities in immune responses to pneumococcus. A recent study consisting of 16 subjects with asthma and 14 controls demonstrated that 44% of the subjects with asthma had 12 or more positive serotype-specific polysaccharide antibodies, whereas 86% of control subjects had 12 or more positive serotype-specific polysaccharide antibodies (5). These results suggest that subjects with asthma may show pneumococcal antibody responses distinct from those of controls, although there are studies reporting otherwise (6). Therefore, the consideration of the comorbid status may be a necessary caveat for interpretation of the results. However, limitations such as a cross-sectional design, lack of baseline antibody titers or reliance on a priori designated cut-offs (e.g. an adequate “protective” response to each pneumococcal serotype defined as a titer equal to or greater than 1.3 μg/mL antibody titers or as conversion of 70% of the serotypes tested with at least a 2-fold increase in the titers (1)) may hamper such conclusions to be made (5–7). Additionally, dichotomizing continuous variables [e.g. >1.3 μg/mL vs. ≤1.3 μg/mL] facilitates analyses but may do so at the expense of loss of information such as pre-vaccination titer or magnitude of antibody responses (6, 8).
In order to study the influence of asthma status on immune responses to PPSV-23 in adults, we conducted a wide supervised and unsupervised analysis in a vaccine cohort and determined differences in antibody titers between pre- and post-vaccination with 23-valent pneumococcal polysaccharide vaccine (PPSV-23) and fold change of each of the serotypes in relation to asthma status. The results of this exploratory study provide an insight into the relationship between asthma status and pneumococcal vaccine response.
Methods
Study design
This was a retrospective analysis of vaccine response in a convenience sample of subjects who were enrolled in a previous prospective vaccine cohort study for the evaluation of pneumococcal vaccine response. The vaccine cohort study was approved by the institutional review boards at the Mayo Clinic, MN and Olmsted Medical Center, MN.
Subjects
Vaccine response data was obtained from residents of Olmsted County, Minnesota, who were recruited for a vaccine cohort study. These subjects were individuals who were self-defined as ‘healthy’ and met the following inclusion/exclusion criteria. Subjects were excluded if they: (1) had previous or current diagnosis of an immunodeficiency (primary and secondary); (2) had previous or current diagnosis of a rheumatological disorder (Rheumatoid arthritis, Lupus, Sjögren, and vasculitis), cancer (chronic lymphocytic leukemia, non-Hodgkin lymphoma, and B-cell malignancy), diabetes, active infection (pneumonia, otitis media, HIV, and EBV), renal disease (nephrotic syndrome and protein losing enteropathy), or other chronic diseases (multiple sclerosis, etc); (3) had current or previous use (within the last 6 months) of systemic/inhaled corticosteroids, captopril, fenclofenac, sulfasalazine, or other immunosuppressive agents (cyclosporin, methotrexate, and mycophenolic acid), anti-convulsants (phenytoin and carbamazepine), gold, d-penicillamine, or anti-malarials (quinine, chloroquine, and hydroxychloroquine); (4) had previous pneumococcal vaccination; or (5) were pregnant. Enrollment was delayed two weeks if subjects had signs and/or symptoms suggestive of upper respiratory infections or viral illness.
Plasma 23 Serotype-Specific Pneumococcal Antibody Measurement
Twenty-three serotype-specific pneumococcal antibody titers (IgG) were measured using a fluorescent, multiplex bead-based immunoassay at Mayo Clinic Clinical Immunology Laboratory at baseline (before administration of PPSV-23) and 4–6 weeks post-vaccination (9, 10). Changes in the post-vaccination titers over pre-vaccine levels were calculated as fold-changes for each of the serotypes.
Ascertainment of Asthma
We retrospectively applied asthma ascertainment criteria on the enrolled subjects. Those subjects who fulfilled the Predetermined Asthma Criteria (PAC) in Table 1 were considered to have asthma, and those who did not meet the criteria were categorized as control subjects. These criteria for asthma have been widely used in asthma research in the past (3, 5, 11–15) and were found to have high reliability (16, 17). For example, both epidemiological (3, 14, 15, 18) and laboratory studies (19–21) using the PAC have consistently demonstrated the associations of asthma status (as either exposure or outcome) with environmental factors, genetic factors, and/or the risk of serious infections (i.e., pneumococcal diseases). Apart from the advantages basing asthma status on the PAC as an asthma criterion (e.g., 1] minimizing ascertainment bias for asthma status by using International Classification of Diseases [ICD] codes or self-report and 2] availability of index date of asthma better defining temporality), our greatest rationale for using the PAC in the present study is its consistency and coherence of our earlier work based on the PAC which showed increased risk of pneumococcal diseases and impaired humoral immune responses associated with asthma status defined by the PAC (3, 5, 11, 12, 18–21). We have included both definite and probable asthma according to the criteria because most cases of probable asthma (85%) become definite over time (17, 22). For the laboratory studies such as this, we included those who had asthma diagnosis by clinicians in addition to meeting the PAC in order to make sure they have true asthma (19–21).
Table 1.
Ascertainment criteria used to define asthma in the cohort
Patients were considered to have definite asthma if a physician made a diagnosis of asthma and/or if each of the following 3 conditions were present. Patients were considered to have probable asthma if the first 2 of the following 3 conditions were present:
|
Patients were excluded from the study if any of these conditions were present:
|
FVC, forced vital capacity; and FEV1, forced expiratory volume in one second.
Bipartite Network Visualization
The continuous data on baseline titers for each of pneumococcal serotypes was used for the generation of the network graph. This is one of only a few suitable unsupervised agnostic data analyses taking into account baseline titers, individual serotypes, and individual fold changes of antibody responses. Data was scaled using a min-max algorithm (feature scaling) using the formula,
where is the scaled value for pneumococcal antibody titers, x is the original value, min(x) is the minimum value for that variable across all subjects, and max(x) is the maximum value for that variable across all subjects. This standardizes the vaccine response of each serotype and preserves the proportionality of response for each serotype from 0 to 1. The data was then converted into a format suitable for use in Gephi (www.Gephi.org), an open-source software for graph visualization and network analysis (23, 24). A bipartite graph was generated, following which force directed algorithm was applied to reveal inherent patterns in data since these algorithms allow for strongly connected nodes to come closer together and weakly connected nodes to move away from each other in the graph based on mathematical relationships.
The results of bipartite network (the term bipartite refers to a network with two nodes, i.e., subjects and serotype) were expressed in a graphical format consisting of three main network basics: nodes, edges (i.e. lines connecting between nodes), and measures of location such as centrality (which pertain to the relative spatial properties of the network). The network in our study is formed by a set of nodes connected in pairs by edges and edges can connect only nodes from different sets of nodes (i.e., edges connect only subjects and serotype-specific titers; edges neither connect subjects to subjects nor serotypes to serotypes). The edges reflect the relation between subjects and their serotype-specific titers at baseline (the shorter the edges, the stronger relation between subjects and serotype-specific titers) and also graphically result in clusters of related entities with the resultant measure of centrality. We used weighted degree centrality (WDC) as a centrality measure since it is a relative value derived from the total number and magnitude of connections to a node. Using baseline titers in a continuous variable format as a basis for graphical analysis, we modeled fold increase response in each subject across all serotypes in relation to asthma. Cumulative fold-changes for all serotypes and subjects were calculated and scaled by a factor of 0.1 and 0.5, respectively for graphical overlay in the network. Node size in our analysis represents fold-change from pre-vaccination to post-vaccination values with larger nodes depicting higher fold-changes and vice versa. Asthma status was then overlaid on the subject’s nodes to assess location in the graph.
Statistical Analysis
Characteristics of subjects with and without asthma were compared using the Wilcoxon rank-sum test to examine statistical differences for continuous variables between groups and the Chi-square/Fisher’s exact tests were used for categorical variables where appropriate. For each serotype, the Wilcoxon signed-rank test was used for paired analysis of pre and post vaccination. The Benjamini-Hochberg method was used for multiple testing corrections. All statistical tests were two-sided and statistical significance was determined at a P-value <0.05. Analyses were performed by using JMP 10 (SAS Institute Inc., Cary, NC, USA).
Results
Characteristics of study subjects
The study enrolled 64 eligible subjects; of whom, 31 (48%) were male, 58 (91%) Caucasian, the mean age (±SD) 43.1 years (±13.8), and 18 (28%) had a history of asthma. The demographic and clinical characteristics of the subjects with and without asthma are presented in Table 2. There were no differences in the clinical variables except that the incidence of other atopic diseases was higher in the subjects with asthma than in control subjects.
Table 2.
Characteristics of the participants
| Variable | Subjects with asthma (n=18, 28%) |
Subjects without asthma (n=46, 72%) | P- value* |
|---|---|---|---|
| Age at baseline, | 0.43 | ||
| Median (IQR**) | 41.5 (28.8–51.0) | 43.5 (32.5–57.0) | |
| Sex | 0.69 | ||
| Female, n (%) | 10/18 (56) | 23/46 (50) | |
| Race/Ethnicity | 1.00 | ||
| White, n (%) | 16/18 (89) | 42/46 (91) | |
| Non-white, n (%) | 2/18 (11) | 4/46 (9) | |
| Years of Education*** | 0.80 | ||
| Median (IQR) | 16 (13.5–16.5) | 16 (14.0–17.0) | |
| Any atopic condition (atopic dermatitis and/or allergic rhinitis), n (%) | |||
| 16/18 (89) | 21/44 (48) | <0.01 |
P value was calculated by χ2 test for categorical variables or Wilcoxon Rank Sum test for continuous variables between groups.
IQR, Inter-quartile range between 25th and 75th percentile.
Missing education information for two subjects without asthma.
The baseline (pre-vaccine) and post-vaccine pneumococcal antibody titers and asthma status
Subjects with asthma tended to demonstrate higher baseline titers in certain serotypes (Table 3). However, none of these serotype comparisons met the significance threshold (P≤ 0.05) after adjustment for multiple comparisons (Benjamini-Hochberg). The numbers of serotype titers greater than the threshold of 1.3μg/mL were not significantly different between subjects with and without asthma at baseline (17.5 [12 – 20.5] vs. 14.5 [8 – 20]). For post-vaccination titers, the subjects with asthma had a trend of higher median values in certain serotypes (Table 4). Again, none of the serotype comparisons met the significance threshold after adjustment for multiple comparisons (Benjamini-Hochberg).
Table 3.
Comparison of serotype specific pre-vaccine (baseline) titers between subjects with and without asthma
| Subjects with asthma | Subjects without asthma | ||||
|---|---|---|---|---|---|
| Serotype | Median (μg/mL) |
IQR | Median (μg/mL) |
IQR | P-valueU |
| 1 | 2.65 | 1.07–9.20 | 1.25 | 0.15–3.40 | 0.029 |
| 2 | 2.25 | 0.90–5.40 | 1.15 | 0.30–2.70 | 0.052 |
| 3 | 2.60 | 0.50–5.20 | 1.20 | 0.70–2.10 | 0.067 |
| 4 | 0.35 | 0.17–1.12 | 0.20 | 0.05–0.75 | 0.223 |
| 5 | 4.65 | 3.50–11.10 | 3.75 | 1.67–9.50 | 0.167 |
| 6B | 5.95 | 1.30–17.07 | 2.80 | 1.40–5.47 | 0.162 |
| 7F | 9.65 | 5.52–24.15 | 4.95 | 1.47–11.47 | 0.013 |
| 8 | 5.60 | 2.50–13.77 | 1.45 | 0.77–4.45 | 0.004 |
| 9 | 5.80 | 2.75–14.07 | 1.90 | 0.80–6.00 | 0.008 |
| 9V | 2.65 | 1.17–7.42 | 2.00 | 0.67–6.00 | 0.160 |
| 10A | 8.20 | 4.52–24.52 | 2.90 | 1.05–11.02 | 0.021 |
| 11A | 1.25 | 0.45–2.82 | 0.90 | 0.50–2.32 | 0.488 |
| 12F | 0.85 | 0.20–4.37 | 0.30 | 0.10–1.02 | 0.037 |
| 14 | 3.20 | 1.37–12.22 | 2.00 | 0.60–7.20 | 0.422 |
| 15B | 1.35 | 0.70–2.92 | 0.90 | 0.30–2.15 | 0.159 |
| 17F | 8.15 | 4.15–34.50 | 7.05 | 1.50–14.15 | 0.180 |
| 18C | 0.65 | 0.17–1.85 | 1.10 | 0.30–1.82 | 0.418 |
| 19A | 6.35 | 1.47–16.15 | 3.65 | 1.40–9.67 | 0.234 |
| 19F | 3.15 | 1.65–12.07 | 3.75 | 1.55–11.17 | 0.916 |
| 20 | 2.30 | 1.07–5.85 | 1.30 | 0.47–3.70 | 0.053 |
| 22F | 19.10 | 8.87–56.87 | 6.95 | 2.90–23.17 | 0.021 |
| 23F | 12.85 | 4.97–37.52 | 6.80 | 2.00–15.60 | 0.105 |
| 33F | 2.10 | 0.87–12.82 | 1.25 | 0.60–2.90 | 0.066 |
Unadjusted.
IQR: Inter-quartile range between 25th and 75th percentile.
Table 4.
Comparison of serotype specific post-vaccine titers between subjects with and without asthma
| Subjects with asthma | Subjects without asthma | ||||
|---|---|---|---|---|---|
| Serotype | Median (μg/mL) |
IQR | Median (μg/mL) |
IQR | P-valueU |
| 1 | 9.65 | 3.77–47.85 | 6.30 | 2.90–31.42 | 0.496 |
| 2 | 7.15 | 4.00–24.55 | 2.70 | 1.50–13.15 | 0.064 |
| 3 | 5.20 | 2.97–10.75 | 5.85 | 2.07–9.95 | 0.708 |
| 4 | 2.70 | 1.10–7.20 | 1.55 | 0.50–4.42 | 0.193 |
| 5 | 13.75 | 8.00–28.15 | 6.45 | 2.97–23.05 | 0.103 |
| 6B | 23.50 | 3.77–69.90 | 9.60 | 3.10–28.22 | 0.122 |
| 7F | 11.50 | 5.82–26.70 | 5.15 | 3.42–16.25 | 0.031 |
| 8 | 14.85 | 6.72–35.80 | 7.00 | 3.75–20.05 | 0.052 |
| 9 | 15.05 | 5.87–25.25 | 13.65 | 5.15–26.22 | 0.697 |
| 9V | 7.85 | 2.87–17.10 | 4.95 | 2.22–19.67 | 0.627 |
| 10A | 17.15 | 5.20–80.92 | 7.45 | 2.07–24.77 | 0.019 |
| 11A | 4.10 | 2.67–15.90 | 5.05 | 1.60–18.32 | 0.952 |
| 12F | 1.45 | 0.77–10.25 | 0.95 | 0.30–2.85 | 0.113 |
| 14 | 13.6 | 4.07–49.65 | 14.50 | 2.97–47.70 | 0.904 |
| 15B | 7.30 | 3.65–20.12 | 11.10 | 3.97–32.50 | 0.455 |
| 17F | 23.90 | 10.07–67.0 | 9.75 | 4.72–29.97 | 0.070 |
| 18C | 6.30 | 2.12–20.20 | 8.40 | 1.80–19.27 | 0.805 |
| 19A | 24.55 | 5.42–176.35 | 19.45 | 4.17–76.52 | 0.420 |
| 19F | 11.0 | 3.92–27.62 | 12.85 | 4.05–30.27 | 0.799 |
| 20 | 6.85 | 2.9–17.77 | 4.40 | 1.65–19.87 | 0.317 |
| 22F | 21.8 | 10.42–68.20 | 11.10 | 3.65–31.35 | 0.041 |
| 23F | 12.1 | 4.5–61.7 | 10.80 | 2.87–32.25 | 0.390 |
| 33F | 9.35 | 3.27–24.22 | 7.20 | 1.95–20.82 | 0.535 |
Unadjusted.
IQR: Inter-quartile range between 25th and 75th percentile.
Fold-change response to pneumococcal antibody titers and asthma status
The change in the titers of pneumococcal serotypes expressed as a ratio of post-vaccination titers over pre-vaccination titers was calculated (Table 5). Fold change response to serotype 9 and 23F was higher in control subjects than those with asthma, but comparisons did not meet the significance threshold after adjustment for multiple comparisons (Benjamini-Hochberg). Median fold-change was lower in the subjects with asthma compared to control subjects in 18 of 23 serotypes and the subjects with asthma did not mount a significant response to serotypes 7F, 22F, or 23F.
Table 5.
Comparison of fold changes in response to PPV-23 vaccine between asthmatics and non-asthmatics
| Subjects with asthma | Subjects without asthma | ||||
|---|---|---|---|---|---|
| Serotype | Median | IQR | Median | IQR | P-valueU |
| 1 | 4.48*** | 1.75–9.67 | 5.81*** | 3.22–20.65 | 0.147 |
| 2 | 2.15** | 1.14–25.98 | 4.41*** | 1.76–7.62 | 0.335 |
| 3 | 2.03*** | 1.45–4.69 | 3.90*** | 1.65–10.75 | 0.153 |
| 4 | 7.71*** | 2.70–13.43 | 6.50*** | 2.00–50.93 | 0.748 |
| 5 | 1.87** | 1.09–3.99 | 1.64*** | 1.17–3.47 | 0.822 |
| 6B | 3.82*** | 1.20–6.18 | 2.39*** | 1.23–7.30 | 0.632 |
| 7F | 1.08 | 0.98–1.32 | 1.25*** | 0.97–1.85 | 0.201 |
| 8 | 1.76*** | 1.40–4.44 | 4.25*** | 1.39–9.36 | 0.167 |
| 9 | 1.58** | 1.16–3.17 | 4.10*** | 1.46–9.34 | 0.016 |
| 9V | 2.02*** | 1.29–3.02 | 2.95*** | 1.44–6.40 | 0.169 |
| 10A | 1.24** | 0.99–2.72 | 1.26*** | 1.08–3.23 | 0.725 |
| 11A | 3.93*** | 1.62–8.39 | 4.45*** | 2.66–10.72 | 0.463 |
| 12F | 1.43** | 1.00–3.79 | 2.12*** | 1.15–4.75 | 0.353 |
| 14 | 2.51*** | 1.35–7.83 | 2.57*** | 1.10–10.71 | 0.681 |
| 15B | 6.12*** | 2.52–12.99 | 10.06*** | 3.50–32.59 | 0.143 |
| 17F | 2.10* | 1.15–3.48 | 2.05*** | 1.31–6.62 | 0.464 |
| 18C | 10.82*** | 6.17–17.93 | 8.85*** | 3.75–20.20 | 0.424 |
| 19A | 3.21*** | 1.18–14.4 | 4.46*** | 1.59–14.18 | 0.560 |
| 19F | 1.81*** | 1.39–8.17 | 2.47*** | 1.31–7.72 | 1.0 |
| 20 | 2.53** | 1.23–4.74 | 3.05*** | 1.16–11.06 | 0.259 |
| 22F | 1.07 | 1.0–1.32 | 1.16* | 0.98–1.63 | 0.424 |
| 23F | 1.0 | 0.94–1.17 | 1.21** | 0.99–2.51 | 0.023 |
| 33F | 2.03*** | 1.01–8.16 | 4.13*** | 1.97–11.42 | 0.067 |
Superscript asterisk denotes statistical significance of fold change (i.e. pre-vaccine to post vaccine with a group using paired Wilcoxon Signed-Rank Test).
P<0.05,
P<0.01,
P<0.001
IQR: Inter-quartile range between 25th and 75th percentile.
P-value in the last column is comparison between groups (non-asthmatics vs. asthmatics).
Unadjusted.
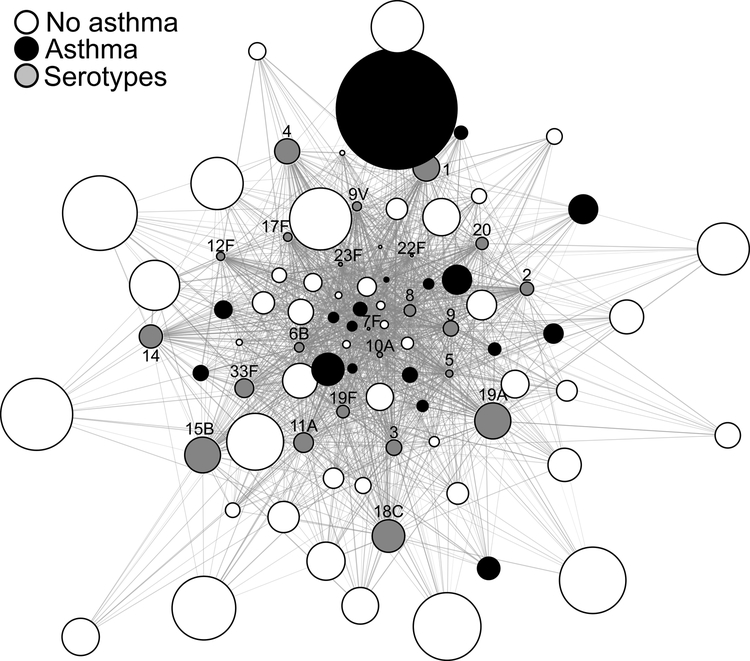
A global unsupervised overview of baseline titers and fold-changes by network visualization
This bipartite network graph is a mathematical representation of a global overview of patterns among individual patients, serotypes, and fold-changes of pneumococcal antibody responses taking into account the baseline data. The topology of the network demonstrated a core-periphery relationship of the nodes. The nodes in the center depict the strongly connected nodes. For example, the nodes representing the subjects in the center were those with higher baseline titers compared to those in the periphery, which were weakly connected nodes, and thus had lower baseline titers. The size of nodes which depicts fold-changes shows that serotype nodes located in the center are smaller (e.g. 7F or 10A), thus portraying smaller fold-changes, compared to the nodes located in the periphery, portraying higher fold changes (e.g. 15B or 19A). Asthma status was then assigned to subjects in the network using conditional overlay (black nodes = fulfilled asthma ascertainment criteria [i.e. asthma], and white nodes = did not fulfill asthma ascertainment criteria, Figure 1).The majority of the control subjects’ nodes (white) were larger and located in the periphery. Overall, maximum fold change (post/pre vaccine) was observed for serotypes nodes depicted by the greatest node sizes (15B, 18C, and 19A), which were closer to the white nodes (control subjects). Thus, this method allowed us to simultaneously examine the relationship of pre-vaccination titers of all 23 serotypes for all 64 subjects, including the vaccine response as fold changes (thus incorporating the post-vaccine response as well) and their asthma status in the network for integrated patterns of pneumococcal antibody responses.
Figure 1.
Network graph depicting simultaneously the relationships between basal titers of all 23 serotypes (grey nodes) for all subjects, the vaccine response as fold change (node sizes) for summative patterns of pneumococcal antibody responses in control subjects (white) to those with asthma (black).
In addition to the visual inspection, we further wished to confirm the network pattern using traditional analytic approaches. Central position in the network is defined by high WDC reflecting pre-vaccine titers. In the highest quartile of WDC, there were proportionally more subjects with asthma (43.7%, 7/16); thus, confirming their position in the center of the network. Meanwhile, the lowest quartile (Q1) of WDC included 12.5% of the subjects with asthma (2/16) compared to controls, thereby confirming their peripheral position. Additionally, the median node size (reflecting the cumulative fold change) in control subjects was 90.7 (interquartile range [IQR] 53.2 to 147.1) compared to those with asthma (50.4 [IQR 36.6 to 85.8], P=0.03), corroborating the larger node sizes (i.e., fold change) in the control subjects taking into account the WDC or pre-vaccine titers.
Discussion
The aim of this exploratory study was to determine the vaccine response patterns to PPSV-23 in relation to asthma status among an existing adult vaccine cohort. We found that while individual serotype titers did not differ by asthma status; on a continuous scale, cumulatively poor responses to PPSV-23 vaccine as measured by fold change from baseline were likely to be observed in the subjects with asthma compared to control subjects after taking into account pre-vaccine titers.
The small fold change in post-vaccine titers in the subjects with asthma may reflect higher baseline antibody titers to pneumococcus, which, on their own, were not significant to discriminate the subjects with asthma from the control subjects. The effect of baseline titers on pneumococcal vaccine response has been previously documented (25). Also, high colonization rates of pneumococcus have been reported in subjects with asthma (26), and may subsequently influence the baseline titers. In the network analysis of the current study subjects, more subjects with asthma were centrally distributed, supporting the finding of higher baseline antibody titers to pneumococcus in those with asthma. The conceptual and analytic framework for bipartite networks has been described in the literature (27–30). The basis for network graph is that networks in biological or social domains are not mathematically random, but follow organizing rules based on data and are reflected in their topological patterns that distinguish them from randomly linked networks (28). In the presented network, the more centrally located nodes indicate that they have higher WDC and thus have higher baseline antibody titers. In our network analysis, this is how we took into account the effect of baseline titers (i.e., titers before vaccination) in assessing the relationship between pneumococcal antibody response patterns and asthma status. The edges (i.e., lines) represent closeness or relatedness between two entities of interest which are mathematically similar to Euclidean distance measuring a distance between two physical points (i.e., the shorter the edges, the closer the points or entities). Taken together, our global network analyses results suggest that poor responders to PPSV-23 vaccine as measured by fold change from baseline titers were more likely to be observed in the subjects with asthma compared to control subjects taking into account pre-vaccine titers.
Previous studies have examined pneumococcal responses in children with asthma (31) and atopic children (32). For example, Lee et al (31) reported that children with asthma showed suboptimal antibody responses to PPSV-23, compared to controls. Furthermore, Arkwright et al (32) demonstrated that patients with atopic dermatitis had a poorer antibody response to pneumococcal vaccine, compared to those without such condition. These data potentially suggest that suboptimal responses might be an unrecognized characteristic of asthma (i.e., potential endotypes) in a subgroup of subjects with asthma. Corresponding with previous studies, our analysis in adults also indicates poor immune responses to pneumococcal vaccine in the subjects with asthma, but in the context of elevated baseline titers.
Differential baseline titers and supposed colonization patterns between subjects with and without asthma might suggest differential susceptibility and immune functions between the two groups with regard to pneumococci. Immune mechanisms underlying suboptimal antibody response to PPSV-23 in the subjects with asthma are unknown. Pneumococcal polysaccharide is a T-cell−independent type II (TI-2) antigen. Recently, it has been demonstrated that neutrophils in the marginal zone lymphoid follicles produce cytokines that drive isotype class-switching of marginal zone B cells to produce IgG antibodies against polysaccharide antigens without T cell help (33). Despite the fact that antibody responses to TI-2 antigens are regarded as T-cell independent, T-B cell interactions may still be required to produce optimal responses to pneumococcal polysaccharide antigens (34, 35). In this respect, asthma has a Th2-predominant immune environment, which has been reported to suppress humoral immune responses to pneumococci in humans and mice (11, 36). The literature and our study findings invite a question as to the possibility of interaction of asthma status, pneumococcal infection or colonization, humoral immune responses, and their clinical implications. Future large scale prospective studies are needed to address these questions.
Greatest changes (i.e., antibody responses) across all the subjects were observed for serotype 19A, 15B, 18C, 4, and 1, which represent the top quartile of serotypes for cumulative fold changes. On the other hand, the fold change was least for 23F, 22F, and 7 serotypes, suggesting that there exists a reduced responsiveness to these serotypes in our study population. Additionally, only the subjects with asthma did not mount a statistically significant response to serotypes 7F, 22F, and 23F, likely due to their high baseline titers while all control subjects did. While this finding may suggest the prevailing colonization and herd immunity, the nature of this finding needs to be studied by addressing the following questions: whether the differential antibody responses among various serotypes suggest intrinsic differential immunogenicity of serotypes (37); and whether asthma status or pneumococcal epidemiology influences these questions, given the large proportion of subjects affected by asthma in the US.
This unsupervised part of the study is also an attempt to analyze the pneumococcal vaccine response data without a priori assigned arbitrary cut-offs (2-fold, 4-fold, 1.3 μg/mL, 70% or more of pneumococcal serotypes, etc.) as the current clinical recommendations are not designed to incorporate multiple aspects of this complex assay at the same time (e.g. number of serotypes, baseline values, cut-offs for positivity for individual serotypes, cut-offs for protectivity, and fold changes for optimal response) (1, 7, 25, 38–40). This analysis simultaneously takes into account many of these variables in an integrated manner which utilizes a high level overview as a supplement to the current analysis method of pneumococcal vaccine response. This analytic approach might be a useful tool in the field of asthma and vaccine research in the future.
Nevertheless, the present study has limitations. First, the study may lack sufficient power to differentiate individual serotype differences between subjects with and without asthma due to a relatively small number of subjects; therefore, a reliable conclusion may be difficult to be made. Recognizing the lack of sufficient power, the differences were visualized in the global network. We speculate that these findings need to be replicated in a prospective study with a large sample size. Second, we used a convenience sample of subjects enrolled in a previous prospective vaccine cohort study; therefore, the current study may not represent the general population. Although we speculate that our study subjects may be reasonable for the aim of this exploratory study, the study findings need to be replicated in future large general population-based studies with random sampling method. Third, information on pulmonary function was not available on all subjects since the asthma ascertainment was made retrospectively based on the predefined criteria as shown in Table 1. Fourth, even though a fold change was the robust discriminating factor of asthma status in this analysis, this in itself may not inform of underlying mechanisms since it was likely dependent on collective factors influencing the baseline values of vaccine titers and immune responses. Fifth, no data on immunological markers (e.g., B cell functions or Th1 and Th2 cytokines) or colonization patterns were measured. Finally, we excluded those who had used systemic or inhaled corticosteroids, thus limiting the asthma severity to mild or intermittent. How these findings would relate to those with severe asthma warrants further studies.
This study has the following strengths. First, serotype-specific antibody responses to PPSV-23 vaccines were measured before and after vaccination in a US cohort in order to ascertain fold increases in each serotype-specific antibody. Second, we used conventional analytical approaches and supplemented with a global network approach based on the raw vaccine response data (without arbitrary cut-offs or a priori categorization) in order to explore the relationship between fold changes in vaccine response and asthma status. Networks have been increasingly used to analyze global patterns in data including cytokines, drugs-targets, symptoms, microbiome data, etc. and to offer a parallel view of the experimental data which supplements coventional analysis (41–45). Third, for the unsupervised analysis, we did not use a priori cut-off points (1, 5, 7, 11, 25, 38, 40), and based the current analysis on quantitative data alone in order to generate the network with the final overlay of asthma status. Thus, the present findings have a few important clinical implications: (1) clinicians should be aware that the subjects with asthma may not demonstrate a robust response to pneumococcal vaccine, (2) such inability to mount a robust PPSV-23 vaccine response might be an unrecognized feature of asthma, and (3) these findings provide evidence that the inclusion of asthma status might affect interpretation of immune competence assessment utilizing PPSV-23 vaccine.
Conclusion/key findings
Poorer responses to PPSV-23 as measured by a fold increase were more likely to be observed in the subjects with asthma. Our study results deserve further investigation with a large sample size to identify the clinical and immunological characteristics of subjects with asthma with poor immune responses to pneumococcal or other vaccines.
Acknowledgments
We would like to thank the staff of the Pediatric Asthma Epidemiology Research Unit for research support, the Antibody Immunology staff for generating the pneumococcal antibody data and the individuals involved in critical appraisal of the manuscript.
Funding
The authors have nothing to disclose that pose a conflict of interest. Funding Source: This work was supported by the National Institute of Allergy and Infectious Diseases (R21 AI101277), the National Heart, Lung, and Blood Institute (R01 HL126667) and the Scholarly Clinician Award from the Mayo Foundation (to YJJ). This study was approved by the institutional review boards at the Mayo Clinic and the Olmsted Medical Center.
Footnotes
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the article. Part of this analysis was presented at the ERS meeting in 2015.
References
- 1.Orange JS, Ballow M, Stiehm ER, Ballas ZK, Chinen J, De La Morena M, et al. Use and interpretation of diagnostic vaccination in primary immunodeficiency: a working group report of the Basic and Clinical Immunology Interest Section of the American Academy of Allergy, Asthma & Immunology. J Allergy Clin Immunol. 2012;130(3 Suppl):S1–24. [DOI] [PubMed] [Google Scholar]
- 2.Talbot TR, Hartert TV, Mitchel E, Halasa NB, Arbogast PG, Poehling KA, et al. Asthma as a risk factor for invasive pneumococcal disease. N Engl J Med. 2005;352(20):2082–90. [DOI] [PubMed] [Google Scholar]
- 3.Juhn YJ, Kita H, Yawn BP, Boyce TG, Yoo KH, McGree ME, et al. Increased risk of serious pneumococcal disease in patients with asthma. J Allergy Clin Immunol. 2008;122(4):719–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Juhn YJ. Risks for infection in patients with asthma (or other atopic conditions): is asthma more than a chronic airway disease? J Allergy Clin Immunol. 2014;134(2):247–57; quiz 58–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Podjasek JC, Jung JA, Kita H, Park MA, Juhn YJ. The FACT score in predicting pneumococcal antibody levels in asthmatics. J Asthma. 2015;52(4):370–5. [DOI] [PubMed] [Google Scholar]
- 6.Quezada A, Maggi L, Norambuena X, Inostroza J, Quevedo F. Response to pneumococcal polysaccharide vaccine in children with asthma, and children with recurrent respiratory infections, and healthy children. Allergol Immunopathol (Madr). 2016;44(4):376–81. [DOI] [PubMed] [Google Scholar]
- 7.Sorensen RU, Leiva LE, Javier FC 3rd Sacerdote DM, Bradford N, Butler B, et al. Influence of age on the response to Streptococcus pneumoniae vaccine in patients with recurrent infections and normal immunoglobulin concentrations. J Allergy Clin Immunol. 1998;102(2):215–21. [DOI] [PubMed] [Google Scholar]
- 8.Royston P, Altman DG, Sauerbrei W. Dichotomizing continuous predictors in multiple regression: a bad idea. Stat Med. 2006;25(1):127–41. [DOI] [PubMed] [Google Scholar]
- 9.Andrade DC, Borges IC, Laitinen H, Ekstrom N, Adrian PV, Meinke A, et al. A fluorescent multiplexed bead-based immunoassay (FMIA) for quantitation of IgG against Streptococcus pneumoniae, Haemophilus influenzae and Moraxella catarrhalis protein antigens. J Immunol Methods. 2014;405:130–43. [DOI] [PubMed] [Google Scholar]
- 10.Andrade DC, Borges IC, Adrian PV, Meinke A, Barral A, Ruuskanen O, et al. Effect of Pneumococcal Conjugate Vaccine on the Natural Antibodies and Antibody Responses Against Protein Antigens From Streptococcus pneumoniae, Haemophilus influenzae and Moraxella catarrhalis in Children With Community-acquired Pneumonia. Pediatr Infect Dis J 2016;35(6):683–9. [DOI] [PubMed] [Google Scholar]
- 11.Jung JA, Kita H, Dhillon R, Jacobson RM, Nahm MH, Park M, et al. Influence of asthma status on serotype-specific pneumococcal antibody levels. Postgrad Med. 2010;122(5):116–24. [DOI] [PubMed] [Google Scholar]
- 12.Ryoo E, Kumar R, Kita H, Juhn YJ. Serum 25-hydroxyvitamin D concentrations and waning pneumococcal antibody titers among individuals with atopy. Allergy Asthma Proc. 2013;34(4):370–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Juhn YJ, Weaver A, Katusic S, Yunginger J. Mode of delivery at birth and development of asthma: a population-based cohort study. J Allergy Clin Immunol. 2005;116(3):510–6. [DOI] [PubMed] [Google Scholar]
- 14.Silverstein MD, Reed CE, O’Connell EJ, Melton LJ 3rd O’Fallon WM, Yunginger JW. Long-term survival of a cohort of community residents with asthma. N Engl J Med. 1994;331(23):1537–41. [DOI] [PubMed] [Google Scholar]
- 15.Wi CI, Sohn S, Rolfes MC, Seabright A, Ryu E, Voge G, et al. Application of a Natural Language Processing Algorithm to Asthma Ascertainment. An Automated Chart Review. Am J Respir Crit Care Med. 2017;196(4):430–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beard CM, Yunginger JW, Reed CE, O’Connell EJ, Silverstein MD. Interobserver variability in medical record review: an epidemiological study of asthma. J Clin Epidemiol. 1992;45(9):1013–20. [DOI] [PubMed] [Google Scholar]
- 17.Yunginger JW, Reed CE, O’Connell EJ, Melton LJ 3rd O’Fallon WM, Silverstein MD. A community-based study of the epidemiology of asthma. Incidence rates, 1964–1983. Am Rev Respir Dis. 1992;146(4):888–94. [DOI] [PubMed] [Google Scholar]
- 18.Dhillon RK, Yawn BP, Yoo KH, Boyce TG, Jacobson RM, McGree ME, et al. Impact of Asthma on the Severity of Serious Pneumococcal Disease. Epidemiology (Sunnyvale). 2011;Suppl 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoo KH, Jacobson RM, Poland GA, Weaver A, Lee L, Chang T, et al. Asthma status and waning of measles antibody concentrations after measles immunization. Pediatr Infect Dis J. 2014;33(10):1016–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hanchard NA, Jacobson RM, Poland GA, Juhn YJ. An assessment of the association between childhood asthma and HLA DRB1*03 using extended haplotype analysis. Tissue Antigens. 2010;76(6):491–4. [DOI] [PubMed] [Google Scholar]
- 21.Juhn YJ, Kita H, Lee LA, Smith RW, Bagniewski SM, Weaver AL, et al. Childhood asthma and human leukocyte antigen type. Tissue Antigens. 2007;69(1):38–46. [DOI] [PubMed] [Google Scholar]
- 22.Juhn YJ, Kita H, Lee LA, Swanson RJ, Smith R, Bagniewski SM, et al. Childhood asthma and measles vaccine response. Ann Allergy Asthma Immunol. 2006;97(4):469–76. [DOI] [PubMed] [Google Scholar]
- 23.Jacomy M, Venturini T, Heymann S, Bastian M. ForceAtlas2, a continuous graph layout algorithm for handy network visualization designed for the Gephi software. PLoS One. 2014;9(6):e98679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.aJM Bastian M. Gephi: an open source software for exploring and manipulating networks. In International AAAI Conference on Weblogs and Social Media 2009:361–2. [Google Scholar]
- 25.Hare ND, Smith BJ, Ballas ZK. Antibody response to pneumococcal vaccination as a function of preimmunization titer. J Allergy Clin Immunol. 2009;123(1):195–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jounio U, Juvonen R, Bloigu A, Silvennoinen-Kassinen S, Kaijalainen T, Kauma H, et al. Pneumococcal carriage is more common in asthmatic than in non-asthmatic young men. Clin Respir J. 2010;4(4):222–9. [DOI] [PubMed] [Google Scholar]
- 27.Pillai RR, Divekar R, Brasier A, Bhavnani S, Calhoun WJ. Strategies for molecular classification of asthma using bipartite network analysis of cytokine expression. Current allergy and asthma reports. 2012;12(5):388–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barabasi AL, Gulbahce N, Loscalzo J. Network medicine: a network-based approach to human disease. Nat Rev Genet. 2011;12(1):56–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hidalgo CA, Blumm N, Barabasi AL, Christakis NA. A dynamic network approach for the study of human phenotypes. PLoS Comput Biol. 2009;5(4):e1000353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Loscalzo J, Kohane I, Barabasi AL. Human disease classification in the postgenomic era: a complex systems approach to human pathobiology. Mol Syst Biol. 2007;3:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee HJ, Kang JH, Henrichsen J, Konradsen HB, Jang SH, Shin HY, et al. Immunogenicity and safety of a 23-valent pneumococcal polysaccharide vaccine in healthy children and in children at increased risk of pneumococcal infection. Vaccine. 1995;13(16):1533–8. [DOI] [PubMed] [Google Scholar]
- 32.Arkwright PD, Patel L, Moran A, Haeney MR, Ewing CI, David TJ. Atopic eczema is associated with delayed maturation of the antibody response to pneumococcal vaccine. Clin Exp Immunol. 2000;122(1):16–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Puga I, Cols M, Barra CM, He B, Cassis L, Gentile M, et al. B cell-helper neutrophils stimulate the diversification and production of immunoglobulin in the marginal zone of the spleen. Nat Immunol. 2012;13(2):170–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Griffioen AW, Toebes EA, Rijkers GT, Claas FH, Datema G, Zegers BJ. The amplifier role of T cells in the human in vitro B cell response to type 4 pneumococcal polysaccharide. Immunol Lett. 1992;32(3):265–72. [DOI] [PubMed] [Google Scholar]
- 35.Khan AQ, Lees A, Snapper CM. Differential regulation of IgG anti-capsular polysaccharide and antiprotein responses to intact Streptococcus pneumoniae in the presence of cognate CD4+ T cell help. J Immunol. 2004;172(1):532–9. [DOI] [PubMed] [Google Scholar]
- 36.Khan AQ, Shen Y, Wu ZQ, Wynn TA, Snapper CM. Endogenous pro- and anti-inflammatory cytokines differentially regulate an in vivo humoral response to Streptococcus pneumoniae. Infect Immun. 2002;70(2):749–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Borrow R, Stanford E, Waight P, Helbert M, Balmer P, Warrington R, et al. Serotype-specific immune unresponsiveness to pneumococcal conjugate vaccine following invasive pneumococcal disease. Infection and immunity. 2008;76(11):5305–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Akamatsu T, Inui N, Kusagaya H, Nakamura Y, Suda T, Chida K. Evaluation of antibody levels over 3 years after 23-valent pneumococcal polysaccharide vaccination in patients with pulmonary diseases receiving steroids and immunosuppressive agents. Clin Biochem. 2015;48(3):125–9. [DOI] [PubMed] [Google Scholar]
- 39.Kamchaisatian W, Wanwatsuntikul W, Sleasman JW, Tangsinmankong N. Validation of current joint American Academy of Allergy, Asthma & Immunology and American College of Allergy, Asthma and Immunology guidelines for antibody response to the 23-valent pneumococcal vaccine using a population of HIV-infected children. J Allergy Clin Immunol. 2006;118(6):1336–41. [DOI] [PubMed] [Google Scholar]
- 40.Bonilla FA, Khan DA, Ballas ZK, Chinen J, Frank MM, Hsu JT, et al. Practice parameter for the diagnosis and management of primary immunodeficiency. J Allergy Clin Immunol. 2015;136(5):1186–205 e1–78. [DOI] [PubMed] [Google Scholar]
- 41.Divekar RD, Samant S, Rank MA, Hagan J, Lal D, O’Brien EK, et al. Immunological profiling in chronic rhinosinusitis with nasal polyps reveals distinct VEGF and GM-CSF signatures during symptomatic exacerbations. Clin Exp Allergy. 2015;45(4):767–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yildirim MA, Goh KI, Cusick ME, Barabasi AL, Vidal M. Drug-target network. Nat Biotechnol. 2007;25(10):1119–26. [DOI] [PubMed] [Google Scholar]
- 43.Lal D, Rounds AB, Rank MA, Divekar R. Clinical and 22-item Sino-Nasal Outcome Test symptom patterns in primary headache disorder patients presenting to otolaryngologists with “sinus” headaches, pain or pressure. Int Forum Allergy Rhinol. 2015;5(5):408–16. [DOI] [PubMed] [Google Scholar]
- 44.Muegge BD, Kuczynski J, Knights D, Clemente JC, Gonzalez A, Fontana L, et al. Diet drives convergence in gut microbiome functions across mammalian phylogeny and within humans. Science. 2011;332(6032):970–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hornig M, Montoya JG, Klimas NG, Levine S, Felsenstein D, Bateman L, et al. Distinct plasma immune signatures in ME/CFS are present early in the course of illness. Sci Adv. 2015;1(1). [DOI] [PMC free article] [PubMed] [Google Scholar]