Abstract
Background
Auditory verbal hallucinations (AVH) are a cardinal feature of schizophrenia, but they can also appear in otherwise healthy individuals. Imaging studies implicate language networks in the generation of AVH; however, it remains unclear if alterations reflect biologic substrates of AVH, irrespective of diagnostic status, age or illness-related factors. We applied multimodal imaging to identify AVH-specific pathology, evidenced by overlapping gray or white matter deficits between schizophrenia patients and healthy voice-hearers.
Methods
Diffusion-weighted and T1-weighted magnetic resonance images were acquired in 35 schizophrenia patients with AVH (SCZ-AVH), 32 healthy voice-hearers (H-AVH) and 40 age-and sex-matched controls without AVH. White matter fractional anisotropy (FA) and gray matter thickness (GMT) were computed for each region comprising ICBM-DTI and Desikan-Killiany atlases, respectively. Regions were tested for significant alterations affecting both SCZ-AVH and H-AVH groups, relative to controls.
Results
Compared to controls, the SCZ-AVH showed widespread FA and GMT reductions; but no significant differences emerged between H-AVH and control groups. While no overlapping pathology appeared in the overall study groups, younger (<40years) H-AVH and SCZ-AVH subjects displayed overlapping FA deficits across four regions (p<.05): the genu and splenium of the corpus callosum, as well as the anterior limbs of the internal capsule. Analyzing these regions with free-water imaging ascribed overlapping FA abnormalities to tissue-specific anisotropy changes.
Conclusions
We identified white matter pathology associated with the presence of AVH, independent of diagnostic status. However, commonalities were constrained to younger and more homogenous groups, after reducing pathologic variance associated with advancing age and chronicity effects.
Keywords: Diffusion MRI, cortical thickness, gray matter thickness, free-water, tissue-specific fractional anisotropy, symptom dimensions, age, chronicity, psychosis
Introduction
Auditory verbal hallucinations (AVH) are a cardinal symptom of schizophrenia, but can also occur in several other neuropsychiatric disorders, as well as in the healthy population (Daalman et al., 2012). Despite transdiagnostic similarities (Daalman et al., 2011), pathological substrates of AVH remain largely elusive, with contradictory findings as to specific brain regions and pathophysiological mechanisms involved.
Magnetic resonance imaging (MRI) studies comparing schizophrenia patients with and without AVH implicate volume and gray matter thickness (GMT) changes in predominantly language-related gray matter areas, such as temporal cortical regions, Broca’s area and prefrontal regions in the pathology of AVH (Kühn and Gallinat, 2012, Palaniyappan et al., 2012). These findings cohere with a hypothesis that the generation of AVHs are caused by disrupted corollary discharges (Mathalon and Ford, 2008). This hypothesis posits that the abnormal integration of activity in cortical areas responsible for speech production and auditory perception results in the inability to accurately perceive self-generated signals as arising from external/internal sources or intrinsic activity (Catani and ffytche, 2005, Stephan et al., 2009).
In addition to pathology affecting gray matter regions, functional disintegration may result from disrupted anatomical connectivity involving the brain’s white matter architecture (Whitford et al., 2010a, Whitford et al., 2011). Evidence for white matter pathology derives from diffusion MRI studies, reporting changes in diffusion tensor imaging (DTI) indices such as the fractional anisotropy (FA). However, little consensus exists regarding the location of abnormalities, with some studies reporting changes in circuits connecting speech areas (Catani et al., 2011, Ćurčić-Blake et al., 2015, Kubicki et al., 2011, Oestreich et al., 2016, Psomiades et al., 2016, Seok et al., 2007), and others in commissural fibers and subcortical-cortical connections (Hubl et al., 2004, Mulert et al., 2012, Xi et al., 2016, Zhang et al., 2018), which are outside language-linked circuitry. In addition, some studies report increased (Hubl et al., 2004, Mulert et al., 2012), and others, decreased FA (Ćurčić-Blake et al., 2015, Oestreich et al., 2016, Seok et al., 2007) in schizophrenia patients with AVH. Thus, it seems that white matter pathology is associated with AVH in schizophrenia patients, however the nature of microstructural deficits and specific tracts involved remains unclear.
We point to three primary reasons for heterogenous findings across prior DTI studies. First, schizophrenia is diagnostically characterized by complex clinical phenotypes, broadly categorized into positive, negative, and cognitive symptoms. Thus, previous observations of imaging marker abnormalities in groups of schizophrenia patients with AVH may not be specific to hallucinations, but rather relate to other symptom dimensions or illness-related factors (Ford et al., 2014). Second, age-related changes have been shown to substantially impact white and gray matter abnormalities in schizophrenia (Cropley et al., 2016, Di Biase et al., 2017, Di Biase et al., 2018, Jones et al., 2006, Schnack et al., 2016). As such, mixed findings in previous AVH studies may reflect heterogeneity in age-range and illness duration (which are intricately linked), where findings in older schizophrenia patients are confounded by more evolved pathologies associated with prolonged illness and cumulative medication effects (Ford et al., 2014). Analyzing younger cohorts with shorter illness durations could potentially mitigate these confounding effects to elucidate AVH-specific phenomena. Third, methodological limitations may contribute to the lack of consensus, such that traditional DTI indices may not be able to distinguish specific microstructural features and as such, caution is warranted when interpreting FA changes, which may reflect various, non-specific underlying biological mechanisms across the studies (O’Donnell and Pasternak, 2015). For example, recent studies suggest that reduced FA is mostly ascribed to excess extracellular free-water fractions in patients with a first-episode of psychosis (Lyall et al., 2017, Pasternak et al., 2012), whereas in chronic schizophrenia patients, reduced FA reflects cellular-specific white matter deficits (Oestreich et al., 2017, Pasternak et al., 2015). It follows that FA changes associated with AVH could either originate from extracellular or cellular mechanisms, which may reflect distinct pathological processes (Pasternak et al., 2016).
One way to address the first limitation, and isolate the contribution of AVH from the broader collection of schizophrenia sequela, is to identify shared neurobiological deficits across voice-hearers, including schizophrenia patients (SCZ-AVH) and healthy individuals experiencing AVH who are otherwise healthy (i.e. no clinical diagnosis) and are not treatment-seeking (H-AVH; Baumeister et al., 2017, Larøi, 2012). In an earlier study, focused on the arcuate fasciculus (AF), we found altered white matter microstructure in SCZ-AVH, but not in H-AVH, possibly suggesting that FA alterations of the AF reflect disease-specific processes (de Weijer et al., 2013). Here, we extend our examination to include both white and gray matter spanning the entire brain. To comprehensively characterize the structural neuropathology underlying AVH, multimodal imaging was used to probe overlapping abnormalities across both GMT and white matter microstructure (FA) between H-AVH and SCZ-AVH. As FA abnormalities may either reflect changes in extracellular free-water pools or cellular-specific changes within the white matter tissue, we applied free-water imaging to separately examine these distinct biological compartments and to provide insight into the microstructural underpinnings of any shared FA abnormalities. Moreover, to reduce patient heterogeneity associated with age-related effects and pinpoint AVH-specific pathology, all analyses were repeated on younger subjects with shorter illness durations and cumulative medication exposure.
Methods
Participants
This study was approved by the Human Ethics Committee of the University Medical Center, Utrecht. All participants provided written informed consent, consistent with the Helsinki Declaration of 1975, as revised in 2008. A total of 107 participants were included in this study: 35 patients with a schizophrenia-spectrum disorder (SCZ-AVH; mean age=35.89±10.41), 32 healthy voice-hearers (H-AVH; mean age=39.06 ±11.09) and 40 healthy controls (HC), without a history of AVH (mean age=38.42±13.25). Most subjects overlapped with subjects comprising the de Weijer et al. (2013) investigation (30/35 SCZ-AVH; 32/35 H-AVH; 35/40 controls). Key demographic and clinical characteristics are presented in Table 1.
Table 1.
Sample population and clinical characteristics: overall study population
| Controls | H-AVH | SCZ-AVH | |
|---|---|---|---|
| (n=40) | (n=32) | (n=35) | |
| Variables | M(SD) | M(SD) | M(SD) |
| Age | 38.42 (13.25) | 39.06 (11.09) | 35.89 (10.41) |
| Years of Education | 14.40 (2.69) | 13.81 (1.77) | 13.11 (2.17) |
| Illness Duration (years) | - | - | 17.00 (10.40) |
| SPQ (Total) | 7.95 (6.24) | 27.58 (13.05) | - |
| AVH (PSYRATS total)* | - | 16.69 (4.08) | 31.34 (5.38) |
| Distress (PSYRATS subscale)* | - | 19.44 (5.67) | 39.97 (7.47) |
| Frequency (PSYRATS subscale)* | - | 7.84 (1.39) | 9.24 (1.90) |
| Attribution (PSYRATS subscale)* | - | 3.30 (1.39) | 4.94 (1.55) |
| Loudness (PSYRATS subscale) | - | 1.55 (0.93) | 3.33 (1.80) |
| General psychopathology (PANSS total) | - | - | 57.48 (12.76) |
| n(%) | n(%) | n(%) | |
| Sex, Female | 21 (52.5) | 20 (62.5) | 17 (48.6) |
| Antipsychotics # | |||
| none | - | - | 7 (20.6) |
| typical | - | - | 6 (17.6) |
| atypical | - | - | 20 (58.8) |
| both | - | - | 1 (2.9) |
Significant difference between H-AVH and SCZ-AVH at the level of p<.05
Missing data for 1 patient
Detailed recruitment and sample characteristics are described elsewhere (Daalman et al., 2011). In brief, eligible SCZ-AVH patients were diagnosed with a schizophrenia-spectrum disorder according to the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV; First and Gibbon, 2004) and presented with AVHs. Diagnoses were confirmed with the Comprehensive Assessment of Symptoms and History (CASH; Andreasen et al., 1992) by an independent psychiatrist. The Positive and Negative Syndromes Scale (PANSS; Kay et al., 1987) was used to assess general psychopathology (PANSS total) and positive symptoms (PANSS positive subscale) in the patient group.
The H-AVH subjects were recruited via an online self-report questionnaire (www.verkenuwgeest.nl), which quantifies hallucination tendencies in healthy individuals (Larøi et al., 2004). Respondents endorsing voice-hearing items were selected for telephone screening by trained psychologists and invited to participate if they met the following criteria: (i) voices were distinct from thoughts; (ii) voices were experienced at least once a month, for over one year; (iii) no lifetime history of diagnosis or treatment for psychiatric disorders; (iv) no alcohol or drug abuse for at least 3 months; and (v) no lifetime history of a chronic somatic disorder (Daalman et al., 2011). Individuals fulfilling these criteria were invited to undergo a psychiatric interview. For both healthy groups (H-AVH and HC), the absence of current or history of diagnosable psychopathology, including substance abuse, was confirmed with CASH (Andreasen et al., 1992) and with the Structured Clinical Interview for Personality Disorders (First and Gibbon, 2004) by an independent psychiatrist.
For both AVH groups, detailed characteristics of hallucinations were assessed using the Psychotic Symptom Rating Scale (PSYRATS; Haddock et al., 1999). Four hallucination dimensions were measured with PSYRATS (Woodward et al., 2014): Distress (Distress/Negative Content/Control), Frequency (Frequency/Duration/Disruption), Attribution (Attribution of voices: Location and Origin), and Loudness (Loudness). All clinical ratings were performed on the scan day by trained interviewers.
Magnetic resonance imaging acquisition and pre-processing
All Diffusion MRI and T1-weighted scans were acquired between 2007 and 2010 at the University Medical Center, Utrecht, on a 1.5T Philips Achieva MR scanner using an eight-channel SENSE head-coil. No scanner upgrades were performed during the study lifetime. For diffusion MRI, thirty volumes of diffusion-weighted images (DWI) with non-colinear diffusion gradient directions, and a baseline (b0) volume were obtained with single-shot EPI-DTI imaging sequence. Acquisition parameters were as follows: b-value = 1000s/mm2, isotropic 2 × 2 × 2mm3 voxels, image matrix = 128 × 128, repetition time/echo time = 7035/68ms, FOV = 240mm. A second set of diffusion volumes were acquired with the same parameters as the first set, but with a reversed k-space readout (anterior direction), to facilitate EPI distortion correction in the pre-processing pipeline. The parameters for T1-weighted images were as follows: slice thickness = 1mm (without gap), field of view = 244 × 160 × 168 mm3, repetition time/echo time = 9.87/4.6ms, flip angle = 8°, data matrix size = 256 × 256.
Tract-based spatial Statistics (TBSS) pipeline
Diffusion MRI data were pre-processed with FMRIB Software Library (FSL; Jenkinson et al., 2012). This involved visual inspection for artifacts, during which eight scans were removed from the analysis (2 controls, 2 H-AVH and 4 SCZ-AVH) due to poor image quality (movement artifacts, ghosting, and signal drops). The remaining scans (sample population described in Table 1) underwent correction for EPI distortions by combining the phase flipped images; motion and gradient-induced eddy currents using affine registration to the first b0 image; brain masking performed with FSL’s Brain Extraction Tool (BET; Jenkinson et al., 2012) and subsequent manual editing in Slicer (https://www.slicer.org); computation of a FA volume by fitting a single-tensor to the DWI data (as previously done in de Weijer et al. (2013)), as well as computation of the free-water (FW) fraction and tissue-specific fractional anisotropy (FAT) volumes by applying free-water imaging (Pasternak et al., 2009). Free-water imaging applies a regularization framework to fit a two compartment (bi-tensor) model that separates the contribution of free-water from that of tissue-specific diffusivity (Pasternak et al., 2009). Free-water constitutes unrestricted extracellular water molecules found in CSF, blood, or the interstitial space. Reduced FA may reflect increased partial volume of free-water pools (e.g., in fibers adjacent to ventricles), rather than changes in the white matter microstructure. As such, disambiguating these distinct biological compartments (i.e., into FW and FAT) provides insight into the extracellular and microstructural underpinnings of FA deficits.
We used FSL’s tract-based spatial statistics (TBSS; Smith et al., 2006) and ENIGMA-DTI protocols (http://enigma.ini.usc.edu/protocols/dti-protocols/) as a data-driven approach, which preclude the need for a-priori hypotheses regarding tract/region-specific changes, in light of mixed findings across previous AVH studies. ENIGMA-DTI protocols were used to extract average diffusion (FA, FAT, FW) estimates from white matter regions of interest (ROIs; Jahanshad et al., 2013). In brief, FA maps were registered to the common ENIGMA-DTI target, derived from 400 adult brains (Jahanshad et al., 2013). Registration followed a two-step procedure performed with FSL (Jenkinson et al., 2012): first, an affine transformation was computed to match the FA volume to the template (Andersson et al., 2007); next, nonlinear normalization applied the deformation field that warps the original FA volume to the template, setting the previous affine transformation as the starting estimate (Andersson et al., 2010). The ENIGMA-DTI target was skeletonized with TBSS (Smith et al., 2006) and FA voxels were projected onto the skeleton mask. The FA-derived transformations and projection parameters were used to project FAT and FW maps onto the skeleton mask. Resulting skeletonized maps (FA, FAT, FW) were used to extract values from a subset of white matter regions comprising the ICBM-DTI white matter labels atlas (38 ROIs in total: 17 left and right lateralized respectively, as well as 4 midline structures; Supplementary Table 1; Mori et al., 2005). To define ROIs, diffusion metrics were averaged over skeleton-voxels traversing a given atlas region. Diffusion metrics were obtained from deep white matter structures (i.e., the skeleton) to minimize partial volume effects and from ROIs (rather than voxels), to reduce multiple comparisons and bolster statistical power, given the modest sample size.
Gray matter thickness estimation
T1-weighted images were processed using the FreeSurfer automated neuroanatomical segmentation software (version 5.3; Fischl and Dale, 2000) to generate a three-dimensional model of the cortical surface for each participant. Surface-based cortical reconstruction was performed to derive the white matter and pial surfaces, from which GMT estimates were derived (Dale et al., 1999, Fischl and Dale, 2000, Fischl et al., 2002, Fischl et al., 1999, Fischl et al., 2004). All images were visually inspected to verify the accuracy of reconstructions and identify errors, including skull-strip errors, gross segmentation errors and white/gray matter and pial surface inaccuracies. Mean GMT was extracted from each region comprising the Desikan-Killiany atlas (68 cortical regions in total: 34 left and right lateralized respectively; Desikan et al., 2006) using standard procedures for ROI extraction, provided by FreeSurfer. Cortical thickness was examined in ROIs (rather than voxels), to reduce multiple comparisons and bolster statistical power, given the modest sample size.
Statistical analyses
Between group differences and overlap
All statistical analyses were performed in MATLAB (version 2017b). Diffusion and GMT metrics were normally distributed, as assessed with One-sample Kolmogorov-Smirnov test and homogeneity of variances was checked by Levene’s test. The FA and GMT measures were initially compared between SCZ-AVH and HC groups at each white matter ROI by general linear models (GLM) to regress out variance associated with sex and age. The false discovery rate (FDR; Benjamini and Heller, 2007) was used to provide multiple comparisons error control across the regions (38 FA regions; 68 GMT regions). Regions showing a significant group effect after FDR correction were then compared by GLMs between H-AVH and HC groups. Regions surviving FDR correction were taken to reflect shared pathology between H-AVH and SCZ-AVH cohorts. To determine the nature of any overlapping FA deficits, subsidiary analyses examined FAT and FW diffusion metrics in regions with overlapping FA abnormalities between H-AVH and SCZ-AVH. Support for a two-step GLM procedure was based on our aim to identify pathology affecting both AVH groups relative to HCs, irrespective of differences occurring between AVH groups (e.g., significantly lower FA in SCZ-AVH compared to H-AVH).
All analyses were repeated on a subsample of younger subjects (<40 years of age) to minimize the effects of aging, cumulative medication exposure, illness chronicity or repeated AVH experiences. A threshold of 40 years of age was used to coincide with the median split—a robust statistical means to dichotomize data (Iacobucci et al., 2015), as well as the timing of peak white matter maturation, as shown through diffusion imaging lifespan studies (Cropley et al., 2016, Westlye et al., 2009). Refer to Table 2 for demographic and clinical characteristics of the younger subsample.
Table 2.
Sample population and clinical characteristics: younger subset
| Controls | H-AVH | SCZ-AVH | |
|---|---|---|---|
| (n=25) | (n=16) | (n=24) | |
| Variables | M(SD) | M(SD) | M(SD) |
| Age | 29.44 (5.24) | 30.00 (7.21) | 30.04 (4.52) |
| Years of Education* | 15.08 (2.04) | 13.75 (1.00) | 12.67 (1.94) |
| Illness Duration (years) | - | - | 10.13 (4.65) |
| SPQ (Total) | 7.28 (5.88) | 32.00 (13.53) | - |
| AVH (PSYRATS total)* | - | 16.94 (4.49) | 31.82 (5.49) |
| Distress (PSYRATS subscale)* | - | 20.06 (6.37) | 40.45 (7.58) |
| Frequency (PSYRATS subscale)* | - | 7.56 (1.26) | 9.43 (1.85) |
| Attribution (PSYRATS subscale)* | - | 2.88 (1.36) | 5.2381 (1.58) |
| Loudness (PSYRATS subscale) | - | 1.67 (1.03) | 3.35 (0.88) |
| General psychopathology (PANSS total) | - | - | 59.43 (13.58) |
| n(%) | n(%) | n(%) | |
| Sex, Female | 14 (56.0) | 9 (56.2) | 8 (33.3) |
| Antipsychotics # | |||
| none | - | - | 5 (21.7) |
| typical | - | - | 4 (17.4) |
| atypical | - | - | 13 (56.5) |
| both | - | - | 1 (4.3) |
Significant difference between H-AVH and SCZ-AVH at the level of p<.01
Missing data for 1 patient
Demographic and clinical characteristics
Analysis of variance (ANOVA) was used to test differences in age and number of years educated between the three study groups. Differences in clinical variables (PYSRATS Total and 4 subscales) between H-AVH and SCZ-AVH groups were analysed with independent t-tests. Partial correlations were used to test relationships between clinical variables (PYSRATS Total and 4 subscales) and diffusion metrics across the pooled H-AVH and SCZ-AVH groups, after controlling for group membership as a binary variable. To investigate potential substrates of hallucinatory phenomena, correlations were confined to regions with overlapping pathology between H-AVH and SCZ-AVH groups. The Bonferroni correction provided error control across the correlation analyses (5 symptom scores) and thus, an adjusted p-value of .01 was considered statistically significant.
Results
Demographic and clinical characteristics
There were no significant differences in age or sex between SCZ-AVH, H-AVH and HC groups (p>0.05). Compared to controls, H-AVH and SCZ-AVH were educated for fewer years (p<0.05; see Table 1). Hallucination severity differed between SCZ-AVH and H-AVH groups, with SCZ-AVH showing significantly higher total PSYRATS scores, as well as Distress, Frequency and Attribution subscale scores compared to the H-AVH group (p<0.05; see Table 1). The SCZ-AVH and H-AVH groups did not differ with respect to Loudness (PSYRATS subscale) scores (p>0.05). There was no relationship between hallucination severity (total PSYRATS scores, as well as Distress, Frequency and Attribution subscale scores) and age (p>0.05). Furthermore, hallucination severity did not differ between younger and older subgroups within SCZ-AVH and H-AVH cohorts respectively (Supplementary Table 2).
Microstructural changes in SCZ-AVH and H-AVH
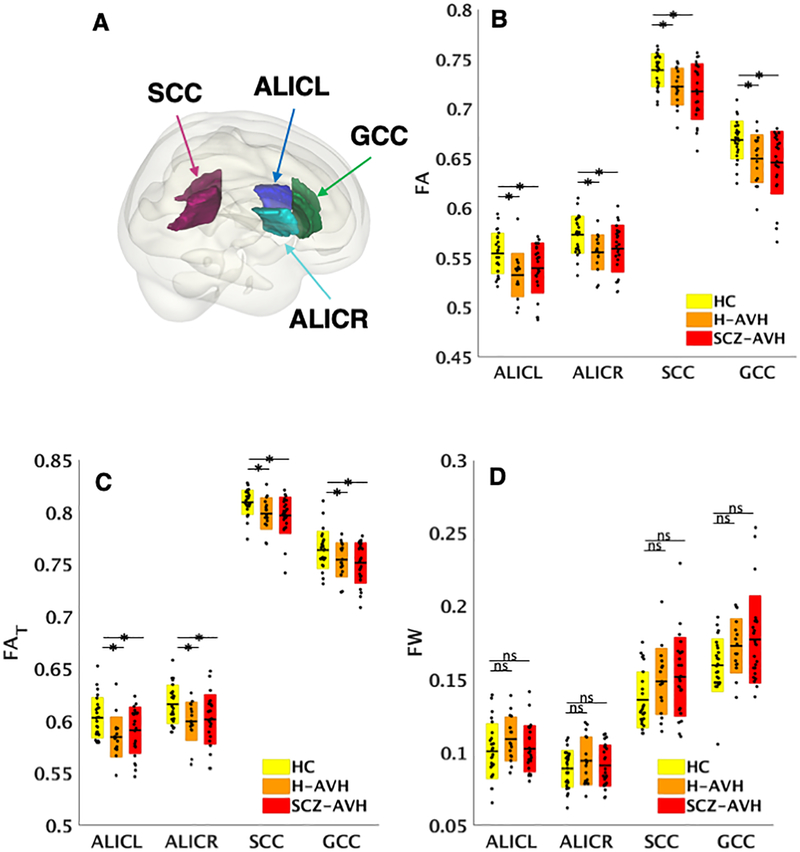
Compared to HCs, schizophrenia patients showed reduced FA in 34/38 ROIs (FDRp<0.05; Supplementary Table 3). Post-hoc analyses constrained to these regions did not detect any differences between H-AVH and HCs (FDRp>0.05). In the younger subgroups, fewer regions (24/38) showed reduced FA in SCZ-AVH patients relative to HCs (FDRp<0.05; Supplementary Table 4). Post-hoc comparisons constrained to these regions revealed lower FA in younger H-AVH compared to HCs in four regions (Figure 1A and 1B): (i) left (t=3.62, FDRp=.001); and (ii) right anterior limbs of the internal capsule (ALIC; t=3.22, FDRp=.003); (iii) splenium of the corpus callosum (SCC; t=2.87, FDRp=.007); and (iv) genu of the corpus callosum (GCC; t=2.85, FDRp=.007). Supplemental unconstrained comparisons (i.e., across all 38 white matter regions) did not reveal differences localized elsewhere in H-AVH subjects, suggesting that alterations were specifically confined to regions associated with schizophrenia pathology.
Figure 1. Overlapping deficits between younger healthy voice-hearers and schizophrenia patients.
(A) Surface rendering displays regions with overlapping FA and FAT pathology. (B) Boxplots represent regions with overlapping FA pathology for healthy controls (HC), healthy voice-hearers (H-AVH) and schizophrenia patient (SC-AVH) groups across. (C) Regions with overlapping FA reductions were investigated for (C) cellular-specific (FAT) and (D) free-water volume (FW) changes. Boxes represent standard deviations and dots reflect data points for each subject across the groups, after multiple comparison correction with the false discovery rate. Stars denote significant differences and ns, not significant. Overlapping pathology reflects regions where the H-AVH and SCZ-AVH patients displayed common significant differences from controls.
To investigate the nature of shared FA abnormalities, FAT and FW diffusion metrics were examined within these four white matter regions. Significantly lower FAT was found in SCZ-AVH and H-AVH relative to controls across these four regions (FDRp <0.05; Figure 1C), indicating shared cellular-specific white matter pathology between SCZ-AVH and H-AVH groups. No FW differences emerged between SCZ-AVH or H-AVH relative to healthy controls (FDRp>0.05; Figure 1D). The impact of sex was not significant across any region or diffusion metric (FDRp>0.05).
Gray matter thickness changes in SCZ-AVH and H-AVH
Compared to HCs, schizophrenia patients displayed reduced GMT spanning 55/68 ROIs (FDRp<0.05; Supplementary Table 5). Across these regions associated with schizophrenia pathology, no significant differences were found between H-AVH and controls. However, two regions displayed reduced GMT in H-AVH subjects before correcting for multiple comparisons, including the left bank of the superior temporal gyrus (t=2.00, p=.049, uncorrected) and the right fusiform gyrus (t=2.15, p=.035, uncorrected). In the younger study groups, 37/68 ROIs showed lower GMT in SCZ-AVH compared to HCs (FDRp<0.05; Supplementary Table 6). Within these regions, no significant GMT reductions were observed for younger H-AVH subjects compared to HCs (FDRp>0.05). Similar to the overall H-AVH group, younger H-AVH subjects displayed reduced GMT in three temporal regions before correcting for multiple comparisons: (i) the left bank of the superior temporal gyrus (t=2.48, p=.018, uncorrected); (ii) the right fusiform gyrus (t=2.26, p=.029, uncorrected) and; (iii) the left middle-temporal gyrus (t=2.08, p=.044, uncorrected). The impact of sex was not significant across any region (FDRp>0.05).
Clinical correlations with overlapping pathology in white matter microstructure
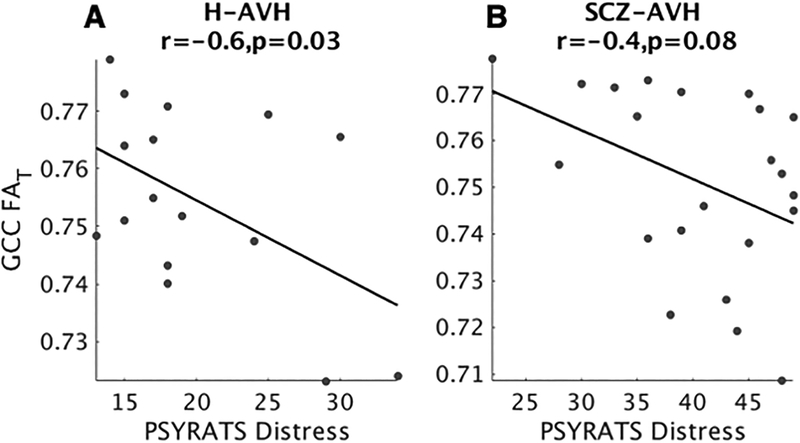
In the younger (<40 years) H-AVH and SCZ-AVH groups who displayed overlapping white matter pathology, there was a significant negative correlation between FAT in the GCC and the degree of distress associated with AVHs (r=−0.6, p=0.007, Bonferroni corrected). This relationship was significant in H-AVH subjects (r=−0.6, p=0.03; Figure 2A) and trended toward significance in SCZ-AVH patients (r=−0.4, p=0.08; Figure 2B). There was no relationship between FA and hallucination symptom severity (PSYRATS total or subscale scores). No overlapping deficits emerged across FW or GMT metrics, and were hence, omitted from symptom correlations.
Figure 2. Associations between FAT and hallucination distress.
Decreased FAT in the genu of the corpus callosum (GCC) was associated with increased hallucination distress in A) healthy voice-hearers (H-AVH) and B) schizophrenia patients (SCZ-AVH). Correlations controlled for age.
Discussion
We tested for overlapping abnormalities in regional white matter microstructure and gray matter thickness between schizophrenia patients and healthy voice-hearers, relative to age- and sex-matched healthy controls. While significant differences were not identified in healthy voice-hearers overall, younger AVH groups displayed overlapping microstructural deficits across several white matter regions, regardless of a clinical diagnostic status. These abnormalities were driven by cellular-specific white matter deficits, rather than changes in the extracellular free-water compartment and were correlated with the degree of distress associated with hallucinatory experiences. Schizophrenia patients also displayed globally reduced gray matter thickness, as well as more extensive white matter deficits than healthy voice-hearers, which could reflect more evolved AVH symptomatology or the compounding of other biological/psychological disease processes in schizophrenia.
Our findings indicate a crucial role for white matter deficits in AVH. Consistent with several previous studies (Hubl et al., 2004, Xi et al., 2016, Zhang et al., 2018), white matter deficits affected the corpus collosum and internal capsule, confirming involvement of widely distributed commissural, frontal, reciprocal thalamo-cortical and auditory-linked circuitry in the generation of AVH. However, different white matter tracts have been implicated across prior reports, including the superior longitudinal fasciculus (Ćurčić-Blake et al., 2015, Seok et al., 2007), inferior longitudinal fasciculus (Seok et al., 2007), inferior fronto-occipital fasciculus (Ćurčić-Blake et al., 2015, Oestreich et al., 2016), anterior thalamic radiation (Ćurčić-Blake et al., 2015) and arcuate fasciculus (the AF was not examined in the current study; Hubl et al., 2004, Psomiades et al., 2016). As nearly all previous studies examined AVH in the context of schizophrenia, abnormalities likely reflected a combination of disease processes and AVH-related phenomena. Indeed, we previously found decreased FA within the AF of schizophrenia patients, but not healthy voice-hearers (de Weijer et al., 2011). It is further notable that several previous studies specifically focused on various language-linked white matter fibers of interest (Ćurčić-Blake et al., 2015, de Weijer et al., 2011, Oestreich et al., 2016) and hence, may have missed abnormalities localized elsewhere.
Our findings, when considered together with previous work, suggest that white matter pathology underlying AVH involves pathways beyond language and auditory-linked circuits. The specific connections disrupted in younger AVH subjects could implicate auditory perception failures in AVH manifestation. Specifically, reduced interhemispheric (GCC) and thalamocortical (ALIC) information transfer to frontal regions could underlie attention and impulse control deficits, resulting in self-monitoring failures (Hugdahl et al., 2008). In addition, the splenium of the corpus callosum carries fibers to temporal, parietal and occipital lobes and thus, deficits in this structure could interrupt information flow between regions which are crucial for delivering and coordinating speech percepts (Friederici et al., 2007). These notions accord with a more sophisticated view of AVH as emerging from comprised structural connections between multiple extra-sensory, association and perception areas, as opposed to pathways specific to the sensory modality in question (Penfield and Perot, 1963, Stephan et al., 2009).
After determining the spatial extent of AVH-specific pathology, we next sought to examine the possible mechanisms underlying connectional deficits. To this end, we applied free-water imaging, allowing for greater interrogation of distinct biological compartments–not previously possible with conventional DTI metrics. Using this method, we could ascribe FA abnormalities to cellular-specific FAT changes, i.e., decreased anisotropy of water diffusion in the tissue, as opposed to an increase in extracellular volume. Although FAT cannot establish the exact tissue-specific pathology, taken together with repeated neuropathologic findings of altered oligodendrocyte morphology in schizophrenia patients (Uranova et al., 2011, Uranova et al., 2014), it is possible microstructural changes in AVH result from a myelin deficit. Indeed, myelin pathology has been proposed as the primary mechanism accounting for hallucinatory phenomena, by way of disrupting temporal coordination between spatially disparate neuronal populations, for example, through slowing of the conduction velocity and inducing synaptic changes (Whitford et al., 2010a). With regard to the corollary discharge system, conduction delays could slow ‘efference copies’ of neural transmission of motor commands, which are consequently received too late by the auditory cortex to accurately predict and supress the sensations of an impending action (Whitford et al., 2010a). Of relevance to our findings, thalamic pathway involvement in corollary discharge failures can occur by way of impairing the updating of sensory information, resulting in less coherent percepts (Bellebaum et al., 2005). Furthermore, the corollary discharge mechanism has been shown to rely on cross-hemispheric transfer for relaying corollary discharge signals (Colby et al., 2005) and integrating auditory percepts (Westerhausen and Hugdahl, 2008). Therefore, our findings may accord with accumulating evidence that AVH involves dysfunctional corollary discharge mechanisms, and in particular, suggest that tissue-specific white matter changes involving key corollary discharge circuits contribute to the pathophysiology underlying AVH.
Decreased FAT in the genu of the corpus callosum (GCC) was correlated to AVH-related distress (sum of PSYRATS items: negative content, distress and control), which was significant in healthy voice-hearers and showed a tendency toward significance in patients. This association was not detected with the more general FA metric, suggesting that cellular-specific white matter changes are more sensitive to AVH symptom dimensions. White matter of the GCC interconnects interhemispheric prefrontal regions involved in a wide range of somatosensory, attention and language processes (Edwards et al., 2014, Hinkley et al., 2012) and disruptions to this region was previously associated with stress (Jackowski et al., 2008), early psychosis (Di Biase et al., 2017) and positive symptoms in schizophrenia (Whitford et al., 2010b). Extending this work, our findings indicate that anterior callosal fibers may be vulnerable to AVH-induced distress. Notably, greater self-reported distress accompanying psychosis symptoms may increase risk for developing full-blown psychotic symptoms and transition to other mental health difficulties (Baumeister et al., 2017, Yung et al., 2005). Consistent with this finding, we observed significantly higher distress scores in patients compared to healthy voice-hearers. Interestingly, this was accompanied by comparable FAT reductions within the GCC across younger clinical and non-clinical AVH groups compared to controls, indicating that greater distress likely involves disruption to additional brain regions, as seen in patient groups. Given that self-reported distress increases risk for developing psychosis (Baumeister et al., 2017, Yung et al., 2005) and that GCC deficits transcend diagnostic status, white matter deficits involving the GCC in particular, may signify poor outcomes for younger individuals with AVH symptoms.
Overlapping pathology and symptom correlations in this study were notably constrained to younger AVH groups, as no differences emerged between healthy voice-hearers and controls in the overall study population. We reasoned that pathological variance could increase with age, stress of repeated AVH experiences, and general medication exposure. These confounds could result in pathology that is differentially localized in spatial extent or severity, precluding group-level findings. Indeed, we observed greater variance in average FA across the overall sample (SD=0.022) compared to the younger subset (SD=.019), in line with our expectation. Such inter-subject heterogeneity, combined with the subtle alterations that characterize these otherwise healthy individuals, may explain our null result in the overall healthy voice-hearing population. In contrast, we observed overlap across younger AVH subgroups, where homogenous pathology may relate more purely to AVH symptom constructs, rather than extraneous age, stress, or other chronicity effects. However, it is possible that similarities across younger subgroups might not relate to AVH as an isolated construct, but rather as part of a general vulnerability to psychosis or schizophrenia-spectrum disorders (Sommer et al., 2010). For example, it is conceivable that symptoms experienced by younger healthy voice-hearers worsen overtime or that other psychosis symptoms transpire, bringing about clinical risk for schizophrenia-spectrum disorders (Yung et al., 2005). However, given that most younger healthy voice-hearers missed the peak window of risk for psychosis onset (i.e., late adolescence to early adulthood; McGrath et al., 2016), and did not display other dimensions of psychopathology at the time of this study; it is reasonable to ascribe our observed white matter deficits specifically to dimensions of AVH, rather than psychosis risk more generally.
Insofar as we did not detect significant gray matter abnormalities in the overall or younger healthy voice-hearers, our findings do not support a central role for gray matter thickness alterations in AVH. However, it is noteworthy that the largest differences in healthy voice-hearers relative to controls, involved thinning of key brain regions for language processing and recognition, including the superior temporal gyrus, right fusiform gyrus and left middle-temporal gyrus. Reduced thickness and volume in extra-sensory auditory regions are consistently reported in association with AVH within a schizophrenia context (Allen et al., 2008, Palaniyappan et al., 2012) and are proposed to reflect neuropil loss, which could underlie dysfunctional cortical activations (van Swam et al., 2012). It may be speculated that our results did not survive multiple comparison correction because cortical thinning is mild in healthy voice-hearers. Notably, in addition to cortical thinning, the years of education and clinical presentation (i.e., AVH severity) of the healthy voice-hearers in this study exhibited intermediate properties of the patient and healthy control groups, in line with a quasi-dimensional view (Claridge and Beech, 1995) of AVH and other psychotic symptoms. However, it is alternatively possible that reduced thickness across these regions were due to chance and hence, nonessential to the pathophysiology underlying AVH. If gray matter abnormalities are indeed involved in the pathophysiology of AVH, it is our contention that they are spatially constrained to auditory association areas, relative to more widely distributed connectional deficits.
Limitations.
Our findings of cellular-specific white matter changes were limited to younger study groups, comprising relatively small samples. Furthermore, a portion of these healthy voice-hearers may develop clinical risk or frank psychosis, and hence, we cannot discount the possibility that the observed changes relate to psychosis risk or other illness dimensions. Key risk factors that mediate vulnerability to psychosis and AVH manifestation, including environmental (e.g. trauma exposure; Fusar-Poli et al., 2017) and genetic (e.g. family history of psychiatric illness; Agerbo et al., 2015) factors, were unavailable for examination by the current study. Therefore, assessment of white matter pathology in younger individuals with AVH in larger samples utilizing risk assessments and longitudinal designs is warranted, in order to map symptom-linked white matter trajectories. Finally, while we observed cellular-specific deficits in AVH, we cannot disentangle the specific mechanisms accounting for changes within the tissue compartment (e.g., demyelination and axonal degeneration), or the relationship to functional or corollary discharge mechanisms. Future multimodal studies that combine advanced white matter mapping with functional parameters will help to resolve the precise pathophysiological mechanisms, the relationship to corollary discharge failures, as well as the temporal course of functional versus anatomical connectivity alterations invoked by AVH experiences.
In summary, we found evidence for overlapping white matter abnormalities across younger schizophrenia patients and healthy voice-hearers. These commonalities were characterized by cellular-specific deficits in white matter microstructure, involving callosal, frontal and thalamo-cortical circuitry. However, the structural basis of AVH could not be straightforwardly ascribed to all AVH subjects, implying that advancing age and other illness-related contributions to brain structure possibly dilute associations with AVH. Future studies could address this complexity by longitudinally mapping the progression of white and gray matter abnormalities in individuals experiencing AVH.
Supplementary Material
Acknowledgements
We wish to gratefully acknowledge all participants for making this study possible.
Financial Support
This work was supported by the following National Institutes of Health (NIH) grants: R01MH108574, R01MH102377, R01MH074794, P41EB015902.
Footnotes
Conflict of interest
None
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committee on human experimentation with the Helsinki Declaration of 1975, as revised in 2008. The study protocol was approved by the ethics committee of each participating institution and all participants provided written informed consent, consistent with the Helsinki Declaration of 1975, as revised in 2008.
References
- Agerbo E, Sullivan PF, Vilhjalmsson BJ, Pedersen CB, Mors O, Børglum AD, Hougaard DM, Hollegaard MV, Meier S & Mattheisen M (2015). Polygenic risk score, parental socioeconomic status, family history of psychiatric disorders, and the risk for schizophrenia: a Danish population-based study and meta-analysis. JAMA Psychiatry 72, 635–641. [DOI] [PubMed] [Google Scholar]
- Allen P, Larøi F, McGuire PK & Aleman A (2008). The hallucinating brain: A review of structural and functional neuroimaging studies of hallucinations. Neuroscience & Biobehavioral Reviews 32, 175–191. [DOI] [PubMed] [Google Scholar]
- Andersson J, Jenkinson M & Smith S (2007) - Non-linear optimisation. FMRIB Technical Report. (http://www.fmrib.ox.ac.uk/analysis/techrep). Accessed 15 August 2018. [Google Scholar]
- Andersson J, Jenkinson M & Smith S (2010) - Non-linear registration, aka spatial normalisation. (http://www.fmrib.ox.ac.uk/analysis/techrep). Accessed 15 August 2018.
- Andreasen NC, Flaum M & Arndt S (1992). The Comprehensive Assessment of Symptoms and History (CASH): an instrument for assessing diagnosis and psychopathology. Archives of general psychiatry 49, 615–623. [DOI] [PubMed] [Google Scholar]
- Baumeister D, Sedgwick O, Howes O & Peters E (2017). Auditory verbal hallucinations and continuum models of psychosis: A systematic review of the healthy voice-hearer literature. Clinical Psychology Review 51, 125–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellebaum C, Daum I, Koch B, Schwarz M & Hoffmann K-P (2005). The role of the human thalamus in processing corollary discharge. Brain 128, 1139–1154. [DOI] [PubMed] [Google Scholar]
- Benjamini Y & Heller R (2007). False discovery rates for spatial signals. Journal of the American Statistical Association 102, 1272–1281. [Google Scholar]
- Catani M, Craig MC, Forkel SJ, Kanaan R, Picchioni M, Toulopoulou T, Shergill S, Williams S, Murphy DG & McGuire P (2011). Altered integrity of perisylvian language pathways in schizophrenia: relationship to auditory hallucinations. Biological Psychiatry 70, 1143–1150. [DOI] [PubMed] [Google Scholar]
- Catani M & ffytche DH (2005). The rises and falls of disconnection syndromes. Brain 128, 2224–2239. [DOI] [PubMed] [Google Scholar]
- Claridge G & Beech T (1995). Fully and quasi-dimensional constructions of schizotypy. In Schizotypal Personality (ed. Raine A, Lencz T and Mednick SA), pp. 192–216. Press Syndicate of the University of Cambridge: Melbourne. [Google Scholar]
- Colby CL, Berman RA, Heiser LM & Saunders RC (2005). Corollary discharge and spatial updating: when the brain is split, is space still unified? Progress in Brain Research 149, 187–205. [DOI] [PubMed] [Google Scholar]
- Cropley VL, Klauser P, Lenroot RK, Bruggemann J, Sundram S, Bousman C, Pereira A, Di Biase MA, Weickert TW, Weickert CS, Pantelis C & Zalesky A (2016). Accelerated gray and white matter deterioration with age in schizophrenia. American Journal of Psychiatry 174, 286–295. [DOI] [PubMed] [Google Scholar]
- Ćurčić-Blake B, Nanetti L, van der Meer L, Cerliani L, Renken R, Pijnenborg GH & Aleman A (2015). Not on speaking terms: hallucinations and structural network disconnectivity in schizophrenia. Brain Structure and Function 220, 407–418. [DOI] [PubMed] [Google Scholar]
- Daalman K, Boks M, Diederen K, de Weijer AD, Blom JD, Kahn RS & Sommer I (2011). The same or different? A phenomenological comparison of auditory verbal hallucinations in healthy and psychotic individuals. Journal of clinical Psychiatry 72, 320–325. [DOI] [PubMed] [Google Scholar]
- Daalman K, Diederen K, Derks EM, van Lutterveld R, Kahn RS & Sommer IE (2012). Childhood trauma and auditory verbal hallucinations. Psychological Medicine 42, 2475–2484. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B & Sereno MI (1999). Cortical surface-based analysis: I. Segmentation and surface reconstruction. NeuroImage 9, 179–194. [DOI] [PubMed] [Google Scholar]
- de Weijer A, Mandl R, Diederen K, Neggers S, Kahn R, Pol HH & Sommer I (2011). Microstructural alterations of the arcuate fasciculus in schizophrenia patients with frequent auditory verbal hallucinations. Schizophrenia Research 130, 68–77. [DOI] [PubMed] [Google Scholar]
- de Weijer AD, Neggers SF, Diederen K, Mandl RC, Kahn RS, Pol H, Hilleke E & Sommer IE (2013). Aberrations in the arcuate fasciculus are associated with auditory verbal hallucinations in psychotic and in non-psychotic individuals. Human Brain Mapping 34, 626–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP & Hyman BT (2006). An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage 31, 968–980. [DOI] [PubMed] [Google Scholar]
- Di Biase MA, Cropley VL, Baune BT, Olver J, Amminger GP, Phassouliotis C, Bousman C, McGorry PD, Everall I, Pantelis C & Zalesky A (2017). White matter connectivity disruptions in early and chronic schizophrenia. Psychological Medicine, 1–14. [DOI] [PubMed] [Google Scholar]
- Di Biase MA, Cropley VL, Cocchi L, Fornito A, Calamante F, Ganella EP, Pantelis C & Zalesky A (2018). Linking cortical and connectional pathology in schizophrenia. Schizophrenia Bulletin sby121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards TJ, Sherr EH, Barkovich AJ & Richards LJ (2014). Clinical, genetic and imaging findings identify new causes for corpus callosum development syndromes. Brain 137, 1579–1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB & Gibbon M (2004). The Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I) and the Structured Clinical Interview for DSM-IV Axis II Disorders (SCID-II). In Comprehensive Handbook of Psychological Assessment (ed. Hersen M, Haynes SN, Goldstein G, Heiby EM, Hilsenroth MJ, Beers SR, Segal DL and Thomas JC), pp. 134–143. John Wiley & Sons Inc: New Jersey. [Google Scholar]
- Fischl B & Dale AM (2000). Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proceedings of the National Academy of Sciences 97, 11050–11055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, Van Der Kouwe A, Killiany R, Kennedy D & Klaveness S (2002). Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron 33, 341–355. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI & Dale AM (1999). Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. NeuroImage 9, 195–207. [DOI] [PubMed] [Google Scholar]
- Fischl B, van der Kouwe A, Destrieux C, Halgren E, Ségonne F, Salat DH, Busa E, Seidman LJ, Goldstein J & Kennedy D (2004). Automatically parcellating the human cerebral cortex. Cerebral Cortex 14, 11–22. [DOI] [PubMed] [Google Scholar]
- Ford JM, Morris SE, Hoffman RE, Sommer I, Waters F, McCarthy-Jones S, Thoma RJ, Turner JA, Keedy SK & Badcock JC (2014). Studying hallucinations within the NIMH RDoC framework. Schizophrenia Bulletin 40, S295–S304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friederici AD, von Cramon DY & Kotz SA (2007). Role of the Corpus Callosum in Speech Comprehension: Interfacing Syntax and Prosody. Neuron 53, 135–145. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P, Tantardini M, De Simone S, Ramella-Cravaro V, Oliver D, Kingdon J, Kotlicka-Antczak M, Valmaggia L, Lee J, Millan MJ, Galderisi S, Balottin U, Ricca V & McGuire P (2017). Deconstructing vulnerability for psychosis: Meta-analysis of environmental risk factors for psychosis in subjects at ultra high-risk. European Psychiatry 40, 65–75. [DOI] [PubMed] [Google Scholar]
- Haddock G, McCarron J, Tarrier N & Faragher E (1999). Scales to measure dimensions of hallucinations and delusions: the psychotic symptom rating scales (PSYRATS). Psychological Medicine 29, 879–889. [DOI] [PubMed] [Google Scholar]
- Hinkley LBN, Marco EJ, Findlay AM, Honma S, Jeremy RJ, Strominger Z, Bukshpun P, Wakahiro M, Brown WS, Paul LK, Barkovich AJ, Mukherjee P, Nagarajan SS & Sherr EH (2012). The role of corpus callosum development in functional connectivity and cognitive processing. PLoS ONE 7, e39804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubl D, Koenig T, Strik W, Federspiel A, Kreis R, Boesch C, Maier SE, Schroth G, Lovblad K & Dierks T (2004). Pathways that make voices: white matter changes in auditory hallucinations. Archives of General Psychiatry 61, 658–668. [DOI] [PubMed] [Google Scholar]
- Hugdahl K, Løberg E-M, Specht K, Steen VM, van Wageningen H & Jørgensen HA (2008). Auditory hallucinations in schizophrenia: the role of cognitive, brain structural and genetic disturbances in the left temporal lobe. Frontiers in Human Neuroscience 1, 6–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacobucci D, Posavac SS, Kardes FR, Schneider MJ & Popovich DL (2015). The median split: Robust, refined, and revived. Journal of Consumer Psychology 25, 690–704. [Google Scholar]
- Jackowski AP, Douglas-Palumberi H, Jackowski M, Win L, Schultz RT, Staib LW, Krystal JH & Kaufman J (2008). Corpus callosum in maltreated children with posttraumatic stress disorder: a diffusion tensor imaging study. Psychiatry Research: Neuroimaging 162, 256–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahanshad N, Kochunov PV, Sprooten E, Mandl RC, Nichols TE, Almasy L, Blangero J, Brouwer RM, Curran JE, de Zubicaray GI, Duggirala R, Fox PT, Hong LE, Landman BA, Martin NG, McMahon KL, Medland SE, Mitchell BD, Olvera RL, Peterson CP, Starr JM, Sussmann JE, Toga AW, Wardlaw JM, Wright MJ, Hulshoff Pol HE, Bastin ME, McIntosh AM, Deary IJ, Thompson PM & Glahn DC (2013). Multi-site genetic analysis of diffusion images and voxelwise heritability analysis: a pilot project of the ENIGMA-DTI working group. NeuroImage 81, 455–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW & Smith SM (2012). Fsl. NeuroImage 62, 782–790. [DOI] [PubMed] [Google Scholar]
- Jones DK, Catani M, Pierpaoli C, Reeves SJ, Shergill SS, O’sullivan M, Golesworthy P, McGuire P, Horsfield MA & Simmons A (2006). Age effects on diffusion tensor magnetic resonance imaging tractography measures of frontal cortex connections in schizophrenia. Human Brain Mapping 27, 230–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay SR, Flszbein A & Opfer LA (1987). The Positive and Negative Syndrome Scale (PANSS) for Schizophrenia. Schizophrenia Bulletin 13, 261–276. [DOI] [PubMed] [Google Scholar]
- Kubicki M, Alvarado JL, Westin C-F, Tate DF, Markant D, Terry DP, Whitford TJ, De Siebenthal J, Bouix S, McCarley RW, Kikinis R & Shenton ME (2011). Stochastic Tractography Study of Inferior Frontal Gyrus Anatomical Connectivity in Schizophrenia. NeuroImage 55, 1657–1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn S & Gallinat J (2012). Quantitative Meta-Analysis on State and Trait Aspects of Auditory Verbal Hallucinations in Schizophrenia. Schizophrenia Bulletin 38, 779–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larøi F (2012). How do auditory verbal hallucinations in patients differ from those in non-patients? Frontiers in Human Neuroscience 6, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larøi F, Marczewski P & Van der Linden M (2004). Further evidence of the multi-dimensionality of hallucinatory predisposition: factor structure of a modified version of the Launay-Slade Hallucinations Scale in a normal sample. European Psychiatry 19, 15–20. [DOI] [PubMed] [Google Scholar]
- Lyall A, Pasternak O, Robinson D, Newell D, Trampush J, Gallego J, Fava M, Malhotra A, Karlsgodt K & Kubicki M (2017). Greater extracellular free-water in first-episode psychosis predicts better neurocognitive functioning. Molecular Psychiatry 23, 701–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathalon DH & Ford JM (2008). Corollary discharge dysfunction in schizophrenia: Evidence for an elemental deficit. Clinical EEG and Neuroscience 39, 82–86. [DOI] [PubMed] [Google Scholar]
- McGrath JJ, Saha S, Al-Hamzawi AO, Alonso J, Andrade L, Borges G, Bromet EJ, Oakley Browne M, Bruffaerts R, Caldas de Almeida JM, Fayyad J, Florescu S, de Girolamo G, Gureje O, Hu C, de Jonge P, Kovess-Masfety V, Lepine JP, Lim CCW, Navarro-Mateu F, Piazza M, Sampson N, Posada-Villa J, Kendler KS & Kessler RC (2016). Age of Onset and Lifetime Projected Risk of Psychotic Experiences: Cross-National Data From the World Mental Health Survey. Schizophrenia Bulletin 42, 933–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S, Wakana S, Van Zijl PC & Nagae-Poetscher L (2005). MRI atlas of human white matter. Elsevier: Amsterdam. [DOI] [PubMed] [Google Scholar]
- Mulert C, Kirsch V, Whitford TJ, Alvarado J, Pelavin P, McCarley RW, Kubicki M, Salisbury DF & Shenton ME (2012). Hearing voices: a role of interhemispheric auditory connectivity? The World Journal of Biological Psychiatry 13, 153–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell LJ & Pasternak O (2015). Does diffusion MRI tell us anything about the white matter? An overview of methods and pitfalls. Schizophrenia Research 161, 133–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oestreich LK, Lyall AE, Pasternak O, Kikinis Z, Newell DT, Savadjiev P, Bouix S, Shenton ME, Kubicki M, Whitford TJ & McCarthy-Jones S (2017). Characterizing white matter changes in chronic schizophrenia: A free-water imaging multi-site study. Schizophrenia Research 189, 153–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oestreich LK, McCarthy-Jones S & Whitford TJ (2016). Decreased integrity of the fronto-temporal fibers of the left inferior occipito-frontal fasciculus associated with auditory verbal hallucinations in schizophrenia. Brain Imaging and Behavior 10, 445–454. [DOI] [PubMed] [Google Scholar]
- Palaniyappan L, Balain V, Radua J & Liddle PF (2012). Structural correlates of auditory hallucinations in schizophrenia: a meta-analysis. Schizophrenia Research 137, 169–173. [DOI] [PubMed] [Google Scholar]
- Pasternak O, Kubicki M & Shenton ME (2016). In vivo imaging of neuroinflammation in schizophrenia. Schizophrenia Research 173, 200–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasternak O, Sochen N, Gur Y, Intrator N & Assaf Y (2009). Free water elimination and mapping from diffusion MRI. Magnetic Resonance in Medicine 62, 717–730. [DOI] [PubMed] [Google Scholar]
- Pasternak O, Westin C-F, Bouix S, Seidman LJ, Goldstein JM, Woo T-UW, Petryshen TL, Mesholam-Gately RI, McCarley RW, Kikinis R, Shenton ME & Kubicki M (2012). Excessive extracellular volume reveals a neurodegenerative pattern in schizophrenia onset. The Journal of Neuroscience 32, 17365–17372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasternak O, Westin CF, Dahlben B, Bouix S & Kubicki M (2015). The extent of diffusion MRI markers of neuroinflammation and white matter deterioration in chronic schizophrenia. Schizophrenia Research 161, 113–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penfield W & Perot P (1963). The brain’s record of auditory and visual experience: a final summary and discussion. Brain 86, 595–696. [DOI] [PubMed] [Google Scholar]
- Psomiades M, Fonteneau C, Mondino M, Luck D, Haesebaert F, Suaud-Chagny M-F & Brunelin J (2016). Integrity of the arcuate fasciculus in patients with schizophrenia with auditory verbal hallucinations: A DTI-tractography study. NeuroImage: Clinical 12, 970–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnack HG, van Haren NEM, Nieuwenhuis M, Hulshoff Pol HE, Cahn W & Kahn RS (2016). Accelerated Brain Aging in Schizophrenia: A Longitudinal Pattern Recognition Study. American Journal of Psychiatry 173, 607–616. [DOI] [PubMed] [Google Scholar]
- Seok J-H, Park H-J, Chun J-W, Lee S-K, Cho HS, Kwon JS & Kim J-J (2007). White matter abnormalities associated with auditory hallucinations in schizophrenia: A combined study of voxel-based analyses of diffusion tensor imaging and structural magnetic resonance imaging. Psychiatry Research: Neuroimaging 156, 93–104. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM & Behrens TE (2006). Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. NeuroImage 31, 1487–1505. [DOI] [PubMed] [Google Scholar]
- Sommer IE, Daalman K, Rietkerk T, Diederen KM, Bakker S, Wijkstra J & Boks MP (2010). Healthy individuals with auditory verbal hallucinations; who are they? Psychiatric assessments of a selected sample of 103 subjects. Schizophrenia Bulletin 36, 633–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan KE, Friston KJ & Frith D (2009). Dysconnection in Schizophrenia: From Abnormal Synaptic Plasticity to Failures of Self-monitoring. Schizophrenia Bulletin 35, 509–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uranova NA, Vikhreva OV, Rachmanova VI & Orlovskaya DD (2011). Ultrastructural alterations of myelinated fibers and oligodendrocytes in the prefrontal cortex in schizophrenia: a postmortem morphometric study. Schizophrenia Research and Treatment 2011, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uranova NA, Vikhreva OV, Rachmanova VI & Orlovskaya DD (2014). Microglial activation in white matter in schizophrenia: findings from a postmortem electron microscopic morphometric study. Neurology, Psychiatry and Brain Research 20, 25. [Google Scholar]
- van Swam C, Federspiel A, Hubl D, Wiest R, Boesch C, Vermathen P, Kreis R, Strik W & Dierks T (2012). Possible dysregulation of cortical plasticity in auditory verbal hallucinations–a cortical thickness study in schizophrenia. Journal of Psychiatric Research 46, 1015–1023. [DOI] [PubMed] [Google Scholar]
- Westerhausen R & Hugdahl K (2008). The corpus callosum in dichotic listening studies of hemispheric asymmetry: a review of clinical and experimental evidence. Neuroscience & Biobehavioral Reviews 32, 1044–1054. [DOI] [PubMed] [Google Scholar]
- Westlye LT, Walhovd KB, Dale AM, Bjørnerud A, Due-Tønnessen P, Engvig A, Grydeland H, Tamnes CK, Østby Y & Fjell AM (2009). Life-span changes of the human brain white matter: diffusion tensor imaging (DTI) and volumetry. Cerebral Cortex 20, 2055–2068. [DOI] [PubMed] [Google Scholar]
- Whitford TJ, Ford JM, Mathalon DH, Kubicki M & Shenton ME (2010a). Schizophrenia, myelination, and delayed corollary discharges: a hypothesis. Schizophrenia Bulletin 38, 486–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitford TJ, Kubicki M, Schneiderman JS, O’Donnell LJ, King R, Alvarado JL, Khan U, Markant D, Nestor PG, Niznikiewicz M, McCarley RW, Westin C-F & Shenton ME (2010b). Corpus callosum abnormalities and their association with psychotic symptoms in patients with schizophrenia. Biological Psychiatry 68, 70–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitford TJ, Mathalon DH, Shenton ME, Roach BJ, Bammer R, Adcock RA, Bouix S, Kubicki M, De Siebenthal J, Rausch AC, Schneiderman JS & Ford JM (2011). Electrophysiological and diffusion tensor imaging evidence of delayed corollary discharges in patients with schizophrenia. Psychological Medicine 41, 959–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward TS, Jung K, Hwang H, Yin J, Taylor L, Menon M, Peters E, Kuipers E, Waters F & Lecomte T (2014). Symptom dimensions of the psychotic symptom rating scales in psychosis: a multisite study. Schizophrenia Bulletin 40, S265–S274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi Y-B, Guo F, Li H, Chang X, Sun J-B, Zhu Y-Q, Liu W-M, Cui L-B, Chen G & Wang H-N (2016). The structural connectivity pathology of first-episode schizophrenia based on the cardinal symptom of auditory verbal hallucinations. Psychiatry Research: Neuroimaging 257, 25–30. [DOI] [PubMed] [Google Scholar]
- Yung AR, Buckby JA, Cotton SM, Cosgrave EM, Killackey EJ, Stanford C, Godfrey K & McGorry PD (2005). Psychotic-like experiences in nonpsychotic help-seekers: associations with distress, depression, and disability. Schizophrenia Bulletin 32, 352–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Gao J, Zhu F, Wang W, Fan Y, Ma Q, Ma X & Yang J (2018). Reduced white matter connectivity associated with auditory verbal hallucinations in first-episode and chronic schizophrenia: A diffusion tensor imaging study. Psychiatry Research: Neuroimaging 273, 63–70. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.