Abstract
During an average individual's lifespan, the human heart pumps nearly 200 million liters of blood delivered by approximately 3 billion heartbeats. Therefore, it is not surprising that native myocardium under this incredible demand is extraordinarily complex, both structurally and functionally. As a result, successful engineering of adult-mimetic functional cardiac tissues is likely to require utilization of highly specialized biomaterials representative of the native extracellular microenvironment. There is currently no single biomaterial that fully recapitulates the architecture or the biochemical and biomechanical properties of adult myocardium. However, significant effort has gone toward designing highly functional materials and tissue constructs that may one day provide a ready source of cardiac tissue grafts to address the overwhelming burden of cardiomyopathic disease. In the near term, biomaterial-based scaffolds are helping to generate in vitro systems for querying the mechanisms underlying human heart homeostasis and disease and discovering new, patient-specific therapeutics. When combined with advances in minimally-invasive cardiac delivery, ongoing efforts will likely lead to scalable cell and biomaterial technologies for use in clinical practice. In this review, we describe recent progress in the field of cardiac tissue engineering with particular emphasis on use of biomaterials for therapeutic tissue design and delivery.
Keywords: cardiac tissue engineering, biomaterials, hydrogel, nanomaterials, bioprinting
1. Introduction:
Cardiovascular disease (CVD) continues to yield profound morbidity and mortality across the globe despite more than a half-century of advances in preventive medicine. According to the most recent global statistics published in 2017, there were greater than 400 million CVD cases annually with nearly 18 million deaths (Roth et al., 2017). Regenerative medicine strategies offer promise for secondary management of CVD, especially in the setting of myocardial infarction (MI) and congestive heart failure (CHF) which continue to have an increasing prevalence worldwide (Sanchis-Gomar et al., 2016; Savarese and Lund, 2017). Unlike organ systems such as lung (Zacharias et al., 2018), liver (Wang et al., 2015), intestine (Beumer and Clevers, 2016), skeletal muscle (Relaix and Zammit, 2012) and skin (Blanpain and Fuchs, 2006), which have functional regenerative capacity via tissue-resident stem cells, the mammalian heart is virtually non-regenerative (Bergmann et al., 2015) as it does not possess a robust pool of progenitor cells able to generate new cardiomyocytes (Li, Y. et al., 2018; van Berlo et al., 2014). Growing evidence supports that cardiomyocytes can undergo limited self-renewal through innate processes of dedifferentiation and proliferation (Bergmann et al., 2015; Nakada et al., 2017; Wang, W.E. et al., 2017), with ongoing attempts to potentiate these effects after myocardial injury (Bassat et al., 2017; D'Uva et al., 2015; Leach et al., 2017; Mohamed et al., 2018); however, safety and efficacy of these strategies remain to be proven in large animals and humans. Given its inability to renew functional myocardium after acute, or chronic insults, the mammalian heart instead relies upon tissue remodeling via endogenous cellular components to adapt toward a new, and often maladaptive, physiologic state (Cohn et al., 2000). A potential approach to address this adverse cardiac remodeling is the implantation of regenerative exogenous stem or cardiac progenitor cells into the heart. Still, despite twenty years of clinical research, adult stem cell therapies for ischemic heart disease show only marginal benefits, primarily due to low cell engraftment rates and their inability to generate new functional muscle. Accordingly, there has been a high level of commitment in the research community towards developing functional engineered cardiac tissues (fECTs) for use in cell-based regenerative therapies (Jackman et al., 2015; Shadrin et al., 2017), cardiac disease modeling (Benam et al., 2015; Sadeghi et al., 2017; Turnbull et al., 2018; Wang et al., 2014) and discovery science (Liau et al., 2017; Mills et al., 2017). Recent advances in biomaterials for construction and delivery of fECTs (Capulli et al., 2016; Radisic and Christman, 2013; Reis et al., 2016; Weinberger et al., 2017) as well as for cell-free injection (Ungerleider and Christman, 2014) have become the foundation for the development of new bioengineering therapies for myocardial infarction and heart failure. In this review, we discuss various strategies for in vitro fabrication of fECTs and their applications in cardiac regenerative therapies in vivo, while focusing on biomaterial aspects of tissue design and delivery.
2. Cell Sources and Architectures for Cardiac Tissue Engineering
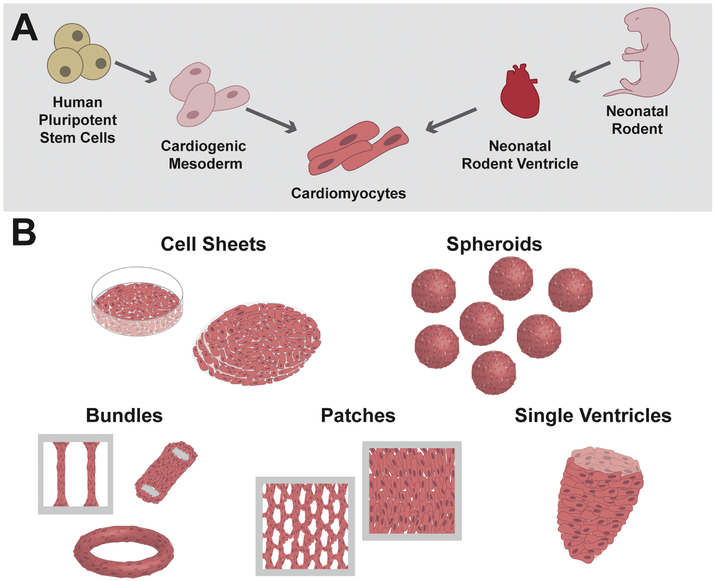
The main goals of cardiac tissue engineering are to develop high-fidelity, 3-dimensional (3D) cell culture systems to study heart function, dysfunction, and pharmacology in vitro as well as to provide a ready source of functional tissues for therapeutic transplantation. Before achieving these goals, proof-of-concept myocardial tissue engineering studies were needed to demonstrate that cardiomyocytes could be successfully cultured in a functional 3D network in vitro. The first such study, published in 1997 by Eschenhagen et al. demonstrated survival of embryonic chick cardiomyocytes in a collagen-I hydrogel matrix anchored against two Velcro-coated glass cylinders (Eschenhagen et al., 1997). In response to electric shocks, cultured chick cardiomyocytes in this 3D environment maintained force-generating capacity for up to 11 days. Together with early pioneering work by Vandenburgh et al., and the first engineering of fECTs with mammalian cells by Bursac et al., these studies laid the groundwork for the following two decades of dedicated striated muscle tissue engineering research (Bursac et al., 1999; Powell et al., 1999; Vandenburgh and Karlisch, 1989). As visualized in Figure 1, fECTs have diversified to encompass constructs fabricated from various cell sources and possess a multitude of 3D sizes and architectures necessary for specific in vitro and in vivo applications
Figure 1. Cell sources and architectures for engineered cardiac tissues.
A) Cardiomyocytes for engineering of fECTs can be differentiated from human pluripotent stem cells or isolated from neonatal rodent ventricles; B) A variety of fECT architectures have been developed, including cell sheets, spheroids, bundles, patches, and single ventricle analogs.
2.1. Neonatal Rodent Ventricular Myocytes
In cardiovascular research, transgenic mouse models provide a versatile, syngeneic platform for systematic studies of heart development and disease in vivo (Vandamme, 2014) while rat models are mostly used as a therapeutic testbed, and as a source of primary heart cells due to their larger hearts. Adult ventricular myocytes have been dissociated from both rats and mice using the Langendorff perfusion method, and have been used to study single-cell electrophysiology and contractile function (Louch et al., 2011; Wolska and Solaro, 1996). However, these cells exhibit low viability in long-term culture which makes them unsuitable for use in cardiac tissue engineering. Neonatal rat ventricular myocytes (NRVMs), on the other hand, can be isolated with high yield and purity with relatively simple enzymatic dissociation protocols and they exhibit long-term viability in culture making them an ideal cell source for tissue engineering (Figure 1A). Therefore, since the late 1990s NRVMs have been used to generate 3D fECTs in which cells have been shown to undergo progressive electrical and mechanical maturation with time in culture (Bursac et al., 1999; Bursac et al., 2003; Papadaki et al., 2001; Shimizu et al., 2002; Zimmermann et al., 2000; Zimmermann et al., 2002). Methods for engineering of fECTs from neonatal mouse ventricular myocytes (NMVMs) (de Lange et al., 2011) were developed more than ten years after those for NRVMs. While one obvious advantage of mouse over rat-based systems is the availability of transgenic mouse models, difficulties with achieving high purity and yield of dissociated NMVMs have been the main hurdle to wide-spread use of this model in cardiac research. Although transgenic rats are likely to become more ubiquitous in the future, methodologies for their generation have been technically challenging (Pradhan and Majumdar, 2016).
Collective cardiac tissue engineering efforts from multiple groups using rodent-sourced cardiomyocytes, whether primary or mouse pluripotent stem cell-derived, have led to development of 3D culture conditions that permit continued functional maturation of cells akin to postnatal cardiac development in vivo (Bian et al., 2014a; Christoforou et al., 2013; Didie et al., 2013; Kensah et al., 2013; Liau et al., 2011; Miki et al., 2012; Stohr et al., 2013). Recently, Jackman et al. have described small cylindrical fECTs that, after 2 weeks of culture, generate high contractile stresses (~60 mN/mm2) and conduction velocities (52.5 cm/s) approaching those of adult rat myocardium (Jackman et al., 2016). Use of dynamic culture (on a rocking platform) to enhance mass transport drove many of the functional improvements which were mediated via mTORC1 signaling rather than by mechanotransduction. Further structural and functional maturation beyond 2-week culture was achieved by low-rate (0.2 Hz) electrical pacing to maintain tissue contractions and the application of thyroid hormone (T3) (Jackman, C. et al., 2018). After a total of 5 weeks, resulting fECTs showed improved cardiomyocyte size, electromechanical function, expression of contractile proteins, and ultrastructural properties (t-tubules, M-lines, Z-disks, intercalated disks) that quantitatively matched those of similarly-aged native rat ventricular myocytes in vivo. In a large number of proof-of-concept studies starting in 1999, in vitro engineered rodent fECTs have been implanted in mouse and rat models of heart disease showing their ability to reverse pathological cardiac remodeling and improve contractile function, thus paving the way for the translational studies with human cells (Li et al., 1999).
2.2. Human Pluripotent Stem Cell-Derived Cardiomyocytes
Rodent fECTs have been critical for developing methods to improve cardiomyocyte maturation in vitro. Given that they achieve the most advanced functional maturation to date, rodent fECTs remain a valuable platform for in vitro studies of postnatal cardiac development. However, for preclinical research and therapeutic applications, human pluripotent stem cells (hPSCs) represent an ideal, and potentially unlimited source, of bona fide functional human cardiomyocytes (hPSC-CMs)(Figure 1A). Rapid advances in cardiac differentiation of hPSCs from early low-efficiency embryoid body-based protocols (Itskovitz-Eldor et al., 2000) to reporter-based CM selection (Anderson et al., 2007), and finally transgene-free directed differentiation procedures (Kempf et al., 2015; Lian et al., 2012), have unlocked the potential of producing large numbers of disease-specific, autologous cardiac-lineage cells for basic research and therapeutic indications (Jackman et al., 2015; Ogle et al., 2016; Oikonomopoulos et al., 2018). Building on extensive experience with rodent cardiomyocytes, robust human hPSC-CM differentiation protocols have enabled generation of the first human fECTs and evidence that CM maturation in a 3D environment is enhanced compared to that in standard 2D culture (Nunes et al., 2013; Zhang et al., 2013). Further progress has resulted in optimization of culture conditions to advance structural and functional maturation of 3D cultured hPSC-CMs (Jackman et al., 2016; Ronaldson-Bouchard et al., 2018) as well as the scale-up of engineered tissue size to clinically relevant dimensions without loss in magnitude or spatial homogeneity of functional output (Shadrin et al., 2017). Similar to rodent studies, survival and beneficial effects of human fECTs in rodent (Riegler et al., 2015; Shadrin et al., 2017; Weinberger et al., 2016; Wendel et al., 2015), and recently porcine (Gao et al., 2018; Kawamura et al., 2017), myocardial infarction models have been reported. However, the modest beneficial effects are most likely a result of mechanical effects and paracrine action rather than direct functional coupling between the fECT and host myocardium.
2.3. Non-Myocytes
By volume, cardiomyocytes represent the largest fraction of resident myocardial cells. Consensus estimates over the past several decades suggest 70-80% of myocardial volume is comprised of cardiomyocytes (Zhou and Pu, 2016). However, non-myocytes, inclusive of cardiac fibroblasts (CFs), endothelial cells (ECs), vascular smooth muscle cells (VSMCs) and immune system cells comprise the largest fraction of myocardium when measured by cell number. Given the importance of non-myocytes for homeostatic function and disease response in native myocardium, efforts to recapitulate native heterocellular environments in rodent and human fECTs are rapidly expanding. Zhang et al. generated hydrogel-based human fECTs consisting of varying ratios of hPSC-CMs (48-90%) to non-myocytes spontaneously arising from CM differentiation that included cells positive for markers of CFs, VSMCs, and occasionally ECs (Zhang et al., 2013). Interestingly, this group found that the speed of action potential propagation in fECTs linearly increased with CM purity, while generated contractile force showed a biphasic trend with highest force amplitudes occurring at a 70-80% CM fraction. Having a larger than 90% pure CM population was found to impede both tissue formation and contractile function, which likely resulted from the inadequate levels of hydrogel remodeling by insufficient numbers of non-myocytes. These results were further confirmed in a number of follow-up studies that employed either endogenously arising or exogenously added cardiac and dermal fibroblasts or other non-myocyte cell types (Giacomelli et al., 2017b; Li, Y. et al., 2017; Liau et al., 2017; Ronaldson-Bouchard et al., 2018).
2.4. Tissue Architectures
Application of tissue engineering technology for CVD targets is primed for explosive growth over the next decade. Part of the attractiveness of fECT strategies is the ability to fabricate diverse tissue structures that may be exploited for specific engineering and scientific goals (Jackman et al., 2015; Weinberger et al., 2017). These tissue architectures range from stackable cell sheets or cylindrical constructs with relatively small numbers of cells, to large tissue patches of clinically relevant dimensions and rodent-scale single ventricle organoids (Figure 1B). Several major tissue architectures regularly utilized by various groups are detailed below.
2.4.1. Cell Sheets
Traditional two-dimensional (2D) cell culture systems have been extensively used for studies of cardiomyocyte function, metabolism, and drug response. In 2003, the Okano Lab developed thermosensitive poly(N-isopropylacrylamide) (PNIPAAm)-coated cell culture surfaces that, upon cooling to room temperature, could induce the detachment of intact, free-floating 2D cell sheets (Shimizu et al., 2003). Multiple cell sheets can then be stacked into a thicker 3D tissue that can survive for extended times in culture and on infarcted rodent, porcine, canine, and human hearts (Cyranoski, 2018; Kawamura et al., 2017; Shimizu et al., 2009). The main advantage of this scaffold-free approach is that application of sutures during epicardial implantation is not needed since cell sheets spontaneously adhere to the heart surface. Furthermore, the lack of biomaterial scaffold decreases the chance for immune rejection inherent to transplantation of xenobiotic or non-autologous materials. Challenges to using cell-sheet technology primarily lie in mechanical fragility of sheets and the need for a large number of fibroblasts or other matrix secreting non-myocytes to induce successful sheet detachment. Clinical trials with cell sheets made from autologous skeletal myoblasts, and recently allogeneic hPSC-CMs are currently under way in Japan (Imamura et al., 2016; Yui, 2018).
2.4.2. Spheroids
Small, self-aggregating and scaffold-free tissue constructs termed “organoids” are powerful platforms for discovery biology. Contemporary reports detail specific organoids for most human tissue systems (Rossi et al., 2018). As a scientific tool, organoids have extraordinary scalability, given low requirement for cell input (low-thousands) and ease of formation, thus allowing high-throughput screening of heterocellular interactions and drug responses. Like many other cell types used in organoid systems, cardiomyocytes self-aggregate into spheroids and can be supplemented by other cardiac resident cells such as fibroblasts (Kofron et al., 2017), endothelial cells (Giacomelli et al., 2017a), and immune system cells. Recent work with human cardiac spheroids has been aimed at disease modeling (Campbell et al., 2018; Polonchuk et al., 2017). The major drawback of spheroids is the lack of functional architecture, afforded by more structurally advanced systems, that is amenable to direct functional measurement of isometric force generation and electrical conduction. Regardless, we expect to see a growing number of groups publishing chemical and genetic screening studies on spheroid-based fECTs in the near future. Importantly, recent studies with intramyocardial injection of cardiac spheroids in rodents have reported higher cell engraftment rates compared to traditional single cell injection, as well as electrical coupling with host cardiomyocytes, thus warranting future developments towards potential clinical use (Mattapally et al., 2018; Oltolina et al., 2015). Methods to spatially organize and electromechanically integrate spheroids into larger functional structures may further facilitate these efforts (Kim et al., 2018; Ong et al., 2017).
2.4.3. Bundles
Thin, cylindrical cardiac tissue constructs in the form of a cable, ribbon, or ring (here collectively termed “bundles”) remain a mainstay of fECT design with their main appeal being the pseudo-1D geometry that self-directs aligned, anisotropic organization of cells. Structural and functional anisotropy is a hallmark of mature cardiac tissues and is paramount to fast-velocity action potential propagation and strong directional contraction. Cardiac tissue bundles offer the most flexible platform for easy-to-interpret measurements of electrical and mechanical function (Black et al., 2009; Hansen et al., 2010; Hansen et al., 2018; Jackman, C. et al., 2018; Jackman et al., 2016; Nunes et al., 2013; Ronaldson-Bouchard et al., 2018; Schwan et al., 2016). Dependent on dimensions, small bundles have inherent scalability, like spheroids, for medium-throughput screening assays (Mannhardt et al., 2017; Nunes et al., 2013; Ronaldson-Bouchard et al., 2018). Bundles can also be multiplexed to create larger tissue constructs for potential in-vivo regenerative therapy applications, although robust electromechanical communication among stacked individual bundles remains to be demonstrated (Tiburcy et al., 2017; Weinberger et al., 2016; Zimmermann et al., 2006).
2.4.4. Patches
Future regenerative therapies with fECTs will likely require generation of 3D tissues with dimensions sufficient to cover the epicardial surface above an infarct region. Generation of thin multilayer fECTs, or patches, has thus been a major research area in therapeutic cardiac tissue engineering. In their simplest form, patches consist of multiple layers of randomly oriented and electromechanically integrated cardiomyocytes usually formed within hydrogels or porous polymer scaffolds (Bursac et al., 1999; Shadrin et al., 2017). More complex fabrication strategies enable improved patch anisotropy via generation of aligned fibrous scaffolds. Network patches contain microfabricated honeycomb structures that guide local cell alignment through spatially distributed cues while preserving the large surface area of the patch (Bian et al., 2014a; Bian et al., 2014b; Liau et al., 2011). Recently, Shadrin et al. generated hPSC-CM patches with a clinically-relevant surface area of 16cm2 and electromechanical properties (conduction velocity of approximately 30 cm/s and specific force of approximately 25 mN/mm2) (Shadrin et al., 2017) approaching metrics of adult human myocardium (Durrer et al., 1970; Hasenfuss et al., 1991; Mulieri et al., 1992). Survival and beneficial effects of NRVM and fECTs upon implantation in rodent and porcine hearts have been demonstrated in multiple studies, but major limitations remain with respect to formation of thicker (greater than 100 μm) tissues and robust electromechanical integration with host myocardium (Gao et al., 2018; Jackman, C.P. et al., 2018; Wendel et al., 2015).
2.4.5. Single Ventricles
In practice, the previously discussed fECT architectures fail to fully recapitulate the complex anatomy of the mammalian heart. Most of the constructs have served to model a subsection of myocardium, or to function as tissue grafts for a section of damaged myocardium, instead of functioning as complete replacement pumps for failing hearts. Several studies have described efforts to generate more realistic heart chamber geometries using hydrogel- and electrospinning-based methods. In an early study, “pouchlike” single ventricle constructs were slipped over the epicardial surface of a whole rodent heart as a ventricular-assist device, although the functional integration with host myocardium was limited (Yildirim et al., 2007). In independent studies, single ventricle constructs were generated by seeding NRVMs in a hydrogel around a modified Foley-catheter balloon (Lee et al., 2008). A similar method was recently used by the same group for generation of ventricle-like cardiac organoid chambers (hvCOC) made from hPSC-CMs with successful demonstration of pressure-volume loop recordings (Li, R.A. et al., 2018). A modified electrospinning method was also used to generate single ventricle human fECTs which the authors cultured in a specialized bioreactor that allowed for continuous hemodynamic monitoring (MacQueen et al., 2018). While interesting methodologically, these single ventricle engineering approaches appear rather difficult to scale up and deliver for human use, and they would also require large numbers of cardiomyocytes if used for higher throughput drug testing applications.
3. Biomaterial Scaffolds for Cardiac Tissue Engineering
Mammalian myocardium is a complex, heterocellular environment built upon, and residing within, a highly specialized extracellular matrix (ECM) (Nielsen et al., 2017; Rienks et al., 2014). The cardiac ECM is composed of structural (e.g. collagen I/III) and basement membrane (e.g. laminin/collagen IV) proteins along with non-structural glucosoaminoglycans and proteoglycans. Human cardiac ECM provides scaffolding for nearly 10 billion cells organized in a compact, heavily vascularized environment (Tirziu et al., 2010). Some estimates place cardiomyocyte density in human adults near 30,000 cells/mm3, all surrounded by a dense vascular network (~3,000 capillaries/mm3) necessary for successful nutrient exchange under high metabolic demand (Bergmann et al., 2015; Hudlicka et al., 1992). At a molecular level, cardiac ECM provides biochemical cues through extracellular protein binding sites along with reservoirs of secreted paracrine factors. From a tissue and organ standpoint, the cardiac ECM possesses advanced biophysical properties that provide both rigidity and elasticity in response to dynamic physiology while also facilitating coordinated transmission of electrical and mechanical signals throughout the myocardium.
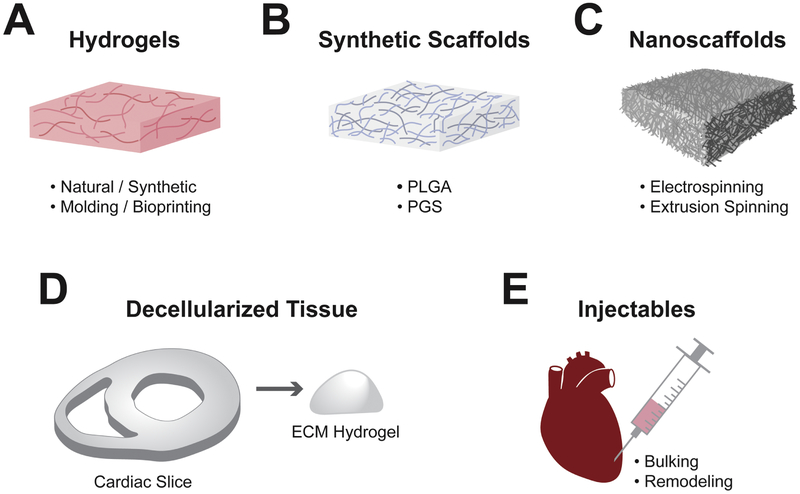
Design of biomaterials to recapitulate the native cardiac ECM architecture and composition through various stages of heart development would be an ideal approach to generate high fidelity, biomimetic fECTs (Williams et al., 2014). Unfortunately, no current technology can accurately replicate the complexity of the native ECM environment. Whilst cell sheet and spheroid methods can generate scaffold-free tissues, most studies utilize biomaterial scaffolds to spatially organize cardiomyocytes in a desired 3D geometry while also presenting important cues for their maturation. An ideal 3D scaffold should provide: 1) biomimetic diversity of binding sites to engage functionally relevant integrins in both cardiomyocytes and non-myocytes, 2) sufficient numbers of cell-binding sites to permit physiological tissue density, 3) capacity for significant remodeling of tissue structure to allow rapid cell spreading, alignment, and replacement of original with cell-secreted ECM, 4) appropriate biomechanical properties to enable continuous tissue contractions, 5) minimal immunogenicity when transplanted in vivo, and 6) scalability to allow generation and implantation of tissues of clinically relevant size. As described below, strategies to achieve these requirements have utilized natural and synthetic hydrogels, synthetic polymer scaffolds, decellularized native myocardial ECM, nanomaterial scaffolds, and 3D bioprinting. Table 1 lists many of the most commonly used biomaterials for cardiac tissue engineering while Figure 2 illustrates strategies for deployment of biomaterials in 3D architectures for research and potential therapeutics.
Table 1.
Common biomaterial substrates for functional cardiac tissue engineering.
| Scaffold biomaterial | Source | FCTE type supported |
Advantages | Disadvantages | Example references |
|---|---|---|---|---|---|
| Collagen I/B114Gelatin | Nature-derived | Bundle; Patch |
Native cardiac ECM protein; scalable | High Stiffness; slow gelation; batch-to-batch variability | (Eschenhagen et al., 1997; Lee et al., 2017; Nunes et al., 2013; Turnbull et al., 2014) |
| Fibrin | Nature-derived | Bundle; Patch |
Native protein; fast gelation; high biodegradability; high elasticity; scalable | Batch-to-batch variability; rapid biodegradability | (Hirt et al., 2014 Ronaldson-Bouchard et al., 2018; Shadrin et al., 2017; Yuan Ye et al., 2011; Zhang et al., 2013) |
| Matrigel: Collagen IV + laminin | Murine sarcoma | Bundle; Patch |
Basal lamina components; commercial product | Xenogencity; batch-to-batch variability; not a standalone product | (Cashman et al., 2016; Jackman, Li, and Bursac, 2018a; Jackman, Ganapathi, et al., 2018b; Shadrin et al., 2017) |
| Alginate | Brown algae | Injectable | Native cardiac ECM-like properties; Scalable; 1st clinical trials | Bio-inertness; difficulty to remodel | (Liberski et al., 2016; Mann et al., 2016; Rao et al., 2015) |
| Polyethylene Glycol (PEG) | Synthetic | Bundle; Injectable |
Low-immunogenicity; Readily modifiable; scalable | Bio-inertness; difficulty to remodel | (Jongpaiboonkit et al., 2008; Kraehenbuehl et al., 2009; Rane et al., 2011) |
| Hyaluronic Acid (HA) | Synthetic | Bundle | Readily modifiable; tunable stiffness; scalable | Bio-inertness; difficulty to remodel | (Young and Engler, 2011) |
| Poly(DL-lactic-co-glycolic) acid (PLGA) | Synthetic | Bundle | High porosity; clinically approved | High stiffness | (Bursac et al., 2007; Bursac et al., 1999) |
| Polyglycerol sebacate (PGS) | Synthetic | Bundle | Tunable mechanical properties | Fatigability; low porosity; long degradation time | (Engelmayr Jr. et al., 2008) |
| Polycaprolactone (PCL) | Synthetic | Single ventricle | Elastomeric; cell alignment | Scalability; 3D porosity; long degradation time | (MacQueen et al., 2018) |
| PEG-Fibrinogen | Hybrid | Bundle | Early hiPSC to CM differentiation control | Density of cell attachment sites | (Kerscher et al., 2016) |
| PLGA/PGA-laminin | Hybrid | Electro-spinning | Ease of processing; clinically approved | Scalability, 3D porosity, mechanical properties | (Papadaki et al., 2001; Yu et al., 2014) |
| Rodent ECM Decellularized | Nature-derived | Bundle; Whole heart |
Native cardiac ECM; high biocompatibility | Xenogenicity; scalability; reproducibility | (Lu et al., 2013; Ott et al., 2008) |
| Porcine ECM Decellularized | Nature-derived | Bundle; Whole heart |
Native cardiac ECM; human-scale tissue; 1st in clinical trial | Xenogenicity; scalability; reproducibility | (Freytes et al., 2014; Hodgson et al., 2017; Schwan et al., 2016; Spang and Christman, 2018; Wainwright et al., 2010) |
| Human ECM Decellularized | Nature-derived | Bundle; Whole heart |
Native human cardiac ECM; not | Availability; reproducibility | (Becker et al., 2017; Guyette et al., 2016; Sanchez et al., 2015) |
| Apple Decellularized | Nature-derived | Patch | Scalable | Xenogenicity; 3D porosity; unclear safety & efficacy | (Modulevsky et al., 2014) |
| Leaf Decellularized | Nature-derived | Patch | Preset vascular-like network; Scalable | Xenogenicity; 3D porosity; unclear safety & efficacy | (Gershlak et al., 2017) |
Becker et al. (2017); Bursac et al. (2007); Bursac et al. (1999); Cashman et al. (2016); Engelmayr Jr. et al. (2008); Eschenhagen et al. (1997); Freytes et al. (2014); Gershlak et al. (2017); Guyette et al. (2016); Hirt et al. (2014); Hodgson et al. (2017); Jackman et al. (2018a); Jackman et al. (2018b); Jongpaiboonkit et al. (2008); Kerscher et al. (2016); Kraehenbuehl et al. (2009); Lee et al. (2017); Liberski et al. (2016); Lu et al. (2013); MacQueen et al. (2018); Mann et al. (2016); Modulevsky et al. (2014); Nunes et al. (2013); Ott et al. (2008); Papadaki et al. (2001); Rane et al. (2011); Rao et al. (2015); Ronaldson-Bouchard et al. (2018); Sanchez et al. (2015); Schwan et al. (2016); Shadrin et al. (2017); Spang and Christman, 2018); Turnbull et al. (2014); Wainwright et al. (2010); Young and Engler (2011); Yu et al. (2014); Yuan Ye et al. (2011); Zhang et al. (2013).
Figure 2. Materials and techniques for engineering cardiac tissues.
Materials and techniques most frequently used for fECT engineering and therapy include: A) hydrogel scaffolds made from either natural or synthetic materials formed with 3D molding or bioprinting; B) fibrous scaffolds generated from synthetic materials including PLGA, PGS and others; C) nanofibrous scaffolds generated through electrospinning or extrusion spinning; D) decellularized tissues either intact or homogenized and crosslinked into a hydrogel; and E) injectable scaffolds to provide bulking and/or beneficial remodeling of the treated heart.
3.1. Hydrogels
Hydrogels are the most widely used biomaterials for cardiac tissue engineering due to their useful biochemical and biophysical properties. This includes the ability to generate numerous geometries in a highly scalable fashion. Successful deployment of hydrogels for fECTs generally requires a casting mold to achieve desired form although 3D bioprinting methods can also serve to provide spatial boundaries. In addition to the mold, anchoring supports within the hydrogel provide mechanical loading necessary for tissue remodeling along with support for cardiomyocyte growth, alignment and maturation. Hydrogels, however, are generally soft scaffolds with stiffnesses less than that of native myocardium. This can create handling difficulties for early engineered tissues which are susceptible to researcher-induced mechanical forces. Nevertheless, as the fECTs mature in culture, the original hydrogel is remodeled through deposition of ECM proteins from seeded cells and by cell-mediated compaction of the original hydrogel substrate. The optimal hydrogel substrate has been the subject of extensive study, but several natural protein products utilized regularly include collagen I, the blood clotting protein fibrin, and the collagen-IV/laminin composite Matrigel. Synthetic and carbohydrate-based hydrogels have also been explored for supporting fECT form and function although they often lack native cell-binding motifs. Accordingly, natural protein-based hydrogels continue to demonstrate the most mature and functional end-products. While conjugating multiple (natural and/or synthetic) hydrogels may further promote biomimetic properties of fECTs, no consensus on the optimal hydrogel milieu exists at this time.
3.1.1. Collagen I
Collagen I and collagen III are the most abundant structural proteins found in the ECM of mammalian myocardium (Takawale et al., 2015). Collagen I fibrils provide the majority of the tensile strength whereas addition of collagen III to collagen I has been found to increase tissue elasticity (Asgari et al., 2017). Thus, collagen I hydrogel has long been utilized for production of fECTs using cardiomyocytes from different species (Eschenhagen et al., 1997; Naito et al., 2006; Ruan et al., 2016; Tiburcy et al., 2017; Turnbull et al., 2014; Zhang et al., 2017). Nevertheless, collagen I scaffolds have several limitations for use in cardiac tissue engineering because 1) they are not easily remodeled to yield physiological cell density and connectivity, 2) they do not stimulate endogenous matrix secretion by cardiac fibroblasts, and 3) are stiffer than other hydrogel scaffolds. As a result, collagen I may not provide ideal conditions for cardiomyocyte maturation and macroscopic contractions. Furthermore, collagen I hydrogels have a long gelling time which can lead to settling of seeded cells during polymerization, yielding nonuniform cell density. A denatured form of collagen I, gelatin, has also been used for coating of 2D culture surfaces, but it requires chemical processing to form a 3D hydrogel that is compatible with cardiomyocyte culture. Specifically, gelatin methacrylol (GelMA) hydrogels have tunable biophysical and biochemical properties and have been increasingly utilized for generation of fECTs (Shin et al., 2016), however, functional properties of cardiomyocytes embedded inside GelMA hydrogels remain far inferior to those in collagen I and fibrin-based tissues.
3.1.2. Fibrin
Fibrin is the product of thrombin mediated cleavage of its pro-protein fibrinogen. Fibrin deposition is crucial to hemostasis in the initial clotting cascade and for the process of early wound healing (Laurens et al., 2006). Advantageous to cardiac tissue engineering, fibrin is extensively remodeled and degraded during wound healing which makes it a good candidate for achieving high cardiomyocyte spreading, density, and interconnectivity in fECTs. Compared to collagen I fibrils, fibrin fibrils have significantly lower tensile stiffness and higher elasticity, comparable to those of native myocardial ECM (Collet et al., 2005; Grassl et al., 2002; Yang et al., 2007). Furthermore, fibrin-based hydrogels stimulate collagen and other ECM secretion from cells within the hydrogel (Ross and Tranquillo, 2003) and their short gelation time can be tuned by both concentration of thrombin and temperature, allowing for uniform seeding of cells in 3D geometries. Given these benefits, along with the fact that it is an FDA approved material, fibrin scaffolds have gained wide adoption in the cardiac tissue engineering field (Hirt et al., 2014; Ronaldson-Bouchard et al., 2018; Schaaf et al., 2011; Shadrin et al., 2017; Yuan Ye et al., 2011; Zhang et al., 2013). Disadvantages to fibrin hydrogels as an fECT scaffold are significant batch-to-batch variability and a fast degradation rate, which can be overcome by inclusion of the anti-fibrinolytic agents aminocaproic acid and aprotinin (Khodabukus and Baar, 2009) in batch-dependent concentrations. Secondary cross-linking of fibrinogen with poly(ethylene glycol), PEG, to generate PEGylated fibrin permits tuning of scaffold porosity and mechanical properties and has been shown to also support the generation of mature fECTs (Kerscher et al., 2016).
3.1.3. Matrigel
While collagen I is the most abundant ECM protein in myocardium, cardiomyocytes directly interact with basal lamina proteins (e.g. laminin, collagen IV). This prompted supplementation of Matrigel (a commercially available basal lamina extract isolated from mouse Engelbreth–Holm–Swarm tumors) to collagen I and fibrin hydrogels at the time of tissue fabrication. Unlike collagen I and fibrin, Matrigel does not require enzymatic or pH modulation to induce gelation since a rise in temperature is sufficient to initiate this process. In addition to use in 3D fECTs (Cashman et al., 2016; Jackman, C. et al., 2018; Jackman et al., 2016; Jackman, C.P. et al., 2018; Zimmermann et al., 2002), Matrigel is widely utilized as a 2D cell-surface coating for culture of hPSCs and cardiomyocytes. A number of studies have shown requisite roles of Matrigel in achieving advanced structural and functional maturation of 3D-cultured cardiomyocytes. Naturally, the major drawback for use of Matrigel-containing fECTs in clinical applications is the origin of Matrigel from murine tumor cells. However, engineering Matrigel-free fECTs necessitates careful optimization of culture media and cell composition (Naito et al., 2006; Tiburcy et al., 2017). Encouragingly, recent studies have identified PEG-based synthetic hydrogels that could serve as an alternative to Matrigel for common cell culture applications (Nguyen et al., 2017).
3.2. Synthetic Polymer Scaffolds
Synthetic polymer scaffolds designed for cardiac tissue engineering are attractive given that there is nearly infinite potential for their modification to suit the biophysical and biochemical needs of fECTs. Synthetic polymers can be designed to take on different 3D geometries, serving as hydrogels in the case of PEG or PNIPAAm, or as fibrous mesh scaffolds if generated from poly(DL-lactic-co-glycolic acid) (PLGA) or poly(glycerol sebacate) (PGS). Successful implementation of synthetic polymers as fECT scaffold replacements or additives necessitates 1) high biocompatibility and porosity, 2) adequate biodegradability and biomechanical characteristics, and 3) low immunogenicity.
PEG is one such candidate given that it is FDA approved, readily modifiable, and has low immunogenicity. The inherently inert and non-remodeling nature of PEG requires incorporation of cell-attachment and/or matrix metalloproteinase (MMP)-sensitive peptide motifs to support cell attachment, remodeling, and formation of physiologically dense tissues. Specifically, conjugating RGD integrin-binding domains onto the PEG backbone can improve cardiomyocyte viability in 3D culture compared to unmodified substrate (Jongpaiboonkit et al., 2008), while biodegradability and stimulation of cardiogenic differentiation of PEG can be improved by adding MMP-sensitivity (Kraehenbuehl et al., 2011). Similarly, use of PEG to crosslink other hydrogels, including collagen, fibrin, hyaluronic acid (HA) (Young and Engler, 2011) and gelatin (Lee et al., 2017) enables additional control of material stiffness to yield pro-maturation benefits on cardiomyocytes.
In addition to hydrogels, synthetic polymer scaffolds with diverse 3D architectures, chemical composition, porosity, degradability, and mechanical properties have long been pursued as biomaterials for cardiac tissue engineering. Specifically, fibrous scaffolds made from PGA or PLGA, two FDA-approved materials, were early candidates for generation of fECTs given their high porosity, tunable biodegradation, and processability (Bursac et al., 2007; Bursac et al., 1999; Papadaki et al., 2001). Unfortunately, the high stiffness of PLGA is not compatible with the need for extensive structural remodeling and macroscopic contractions of fECTs, which can limit the ultimate maturation state of cardiomyocytes. PGS-based 3D scaffolds also generated considerable interest among cardiac tissue engineers given their bioresorbable nature, elastomeric properties, and ability to precisely control their mechanical properties (Engelmayr et al., 2008). When combined with laser etching to generate a honeycomb-like porous architecture, PGS scaffolds exhibited mechanical anisotropy resembling that of ventricular tissue, however, the compartmentalized nature of the scaffold and low porosity created significant impediments to electrical conduction throughout the tissue. Other synthetic elastomeric biomaterials including polyurethane (PU) (Alperin et al., 2005) and poly(octamethylene maleate (anhydride) citrate) (POMaC) (Zhang et al., 2016) have been tested as 3D scaffolds in cardiac tissue engineering, but they are limited by low porosity and low biodegradation as well as material fatigue induced by repeated cycles of stretch.
While tremendous versatility in the design of 3D synthetic scaffolds offers promise for the field of cardiac tissue engineering, achieving biomimetic cardiomyocyte density, anisotropic structural organization, and functional maturation using synthetic substrates comparable to their natural counterparts has not yet been achieved. To address deficiencies of individual scaffolding substrates, a logical leap is to multiplex synthetic and natural biomaterials to synergize advantageous characteristics such as mechanical stability and unique geometries of polymer scaffolds with the high density of bioactive sites and porosity of natural hydrogels. We expect to see a proliferation of studies exploring these combined strategies for successful engineering of robust, therapeutic fECTs.
3.3. Decellularized Scaffolds
De novo generation of 3D scaffolds which are fully mimetic of native cardiac ECM could be a nearly impossible task, regardless of the extensive efforts detailed above. This notion prompted the development of tissue and organ decellularization procedures to extract native myocardial ECM. Decellularized myocardial ECM can be applied directly as a nature-formed scaffold with intact 3D architecture, or as a cardiomyocyte-compatible hydrogel. Methods to decellularize whole hearts and other organs typically use enzymatic and/or detergent-based methods to remove the cells while retaining the most ECM components possible (Gilpin and Yang, 2017). A key quality measure is evaluation of retained DNA as a proxy for level of decellularization and potential for immunogenicity of the scaffold. Ott et al. provided the first proof-of-concept study by reseeding NRVMs onto decellularized whole rat hearts and demonstrating NRVM viability, inotropic response to phenylephrine, and rudimentary pump function after 4 weeks of bioreactor culture (Ott et al., 2008). Currently studied decellularized cardiac ECM scaffolds include those sourced from rodent, pig, and human hearts with the first clinical trial of myocardial injection of a porcine-sourced product currently underway (Spang and Christman, 2018). Noncardiac ECM products are already commercially available for a multitude of human indications (Badylak et al., 2009), which may speed future clinical use of decellularized heart ECM.
3.3.1. Animal
Given their large size and ready availability, porcine hearts are a great source of cardiac ECM. Significant work is underway to optimize porcine heart decellularization with current techniques demonstrating removal of more than 98% of DNA and immunogenic substances (Hodgson et al., 2017; Weymann et al., 2011). Generation of fECTs using porcine myocardial ECM as a solubilized hydrogel matrix or a decellularized 3D tissue scaffold holds promise for therapeutic and experimental applications. Nevertheless, the ability to preserve native ECM component stoichiometry, such as basal lamina versus structural protein fractions, with reproducibility between different donor hearts remains a significant challenge. Further demonstrating compatibility with human cardiomyocytes while maintaining biomechanical stability and remodeling properties is also required before cardiac ECM derivatives compete with state-of-the-art fECTs made of fibrin and collagen I hydrogels. Additionally, recellularization of the whole decellularized hearts with the goal of organ transplantation (Kitahara et al., 2016; Lu et al., 2013; Ott et al., 2008) is faced with a number of daunting challenges including the need for large cell numbers (upwards of 10 billion assuming no cell loss during recellularization), redistribution of specific cell types to their original positions, preservation of original viscoelastic properties, and significant risks of arrhythmias, coronary artery thrombosis, and immune rejection.
3.3.2. Human
Given that future use of animal-derived scaffolds may be limited by their xeno-immunogenicity, decellularized human cardiac ECM may be a more viable alternative for clinical use. While some progress with human ECM-based scaffolds has been made (Guyette et al., 2016; Sanchez et al., 2015), the function of the resulting fECTs has been very low with generated forces and pressure gradients in decellularized scaffolds and whole hearts amounting to only 100uN and 2.5mmHg, respectively (Guyette et al., 2016). Processing smaller human cardiac tissue samples, such as those from surgical biopsies, holds promise for generation of solubilized hydrogels for myocardial injection therapies (Becker et al., 2017). Overall, the limited supply of human-derived ECM components will likely constrain their use to tools for fECT-based discovery science rather than as scaffolds for wide-spread clinical use.
3.3.3. Plant
Supply and immunogenicity challenges for animal- and human-derived scaffolds have prompted research on plant-derived biomaterials for use in human tissue engineering (Fontana et al., 2017). Cellulose scaffolds such as those derived from decellularized apple hypanthium have supported long-term survival of cultured C2C12 cells (Modulevsky et al., 2014) and demonstrated transient immune response upon subcutaneous implantation that gradually disappeared by 8 weeks in vivo (Modulevsky et al., 2016). A similar concept was utilized to produce “pre-vascularized” network scaffolds made from decellularized spinach and parsley leaves (Gershlak et al., 2017). Seeding leaf-based scaffolds with human ECs and CMs generated 2D fECTs that maintained spontaneous contractility up to 21 days in culture. Along with other cellulose-based scaffolds (Entcheva et al., 2004; Wippermann et al., 2009), these novel materials await careful evaluation of their ability to support in vitro engineering and in vivo therapeutic efficacy of 3D fECTs while hopefully limiting immunogenicity.
3.4. Nanofibrous Scaffolds and 3D Bioprinting
While bulk cell seeding of hydrogels, synthetic polymer scaffolds, and decellularized heart ECM represents a more traditional approach to generation of fECTs, recent advances in nanomaterial technology may drive the design of more complex heterocellular microenvironments mimicking that of native myocardium. Specifically, increasingly sophisticated methods for electrospinning and 3D bioprinting offer the ability to use natural and/or synthetic biomaterials while controlling scaffold architecture and cellular composition in a spatially precise fashion. As a result, an increasing number of researchers have been developing new nanomaterial-based strategies to harness fundamental biochemical and biophysical cues from native cardiac ECM that would allow generation of more functional ECTs.
3.4.1. Nanofibrous Scaffolds
Nanofibrous scaffolds that faithfully recapitulate native ECM topography and bioactivity may present unique platforms for generation of functional myocardium as well as other cardiovascular tissues including heart valves, vascular outflow tracts, and blood vessels (Capulli et al., 2016). Electrospinning functions by extrusion of electrically-charged polymers through a small orifice to create nanofibers with precisely controlled dimensions and the ability to generate higher-order 3D geometries based on fiber collection methodologies (Bhardwaj and Kundu, 2010; Senel Ayaz et al., 2014). Most electrospinning studies have utilized synthetic polymers (PLGA, Polycaprolactone (PCL), etc.) and have generated aligned human fECTs with therapeutic potential (Li, J. et al., 2017). Recent methodological advances have permitted increased throughput along with incorporation of native proteins (e.g. gelatin, collagen) into spun nanofibers, thus enabling the formation of aligned patches and single ventricle fECTs made from NRVMs or hPSC-CMs (Badrossamay et al., 2010; Kharaziha et al., 2013; MacQueen et al., 2018). Nevertheless, current nanofiber scaffolding technologies are plagued by several limitations that must be overcome to enable generation of highly functional and therapeutically relevant fECTs including: 1) low porosity that precludes deep penetration of seeded cells, 2) inability to efficiently incorporate various cardiac ECM proteins, and 3) difficulty scaling-up current technologies for human applications.
3.4.2. 3D Bioprinting
Bioprinting is a very promising technology permitting versatile generation of large heterocellular fECTs with complex 3D architecture. Early work by Gaetani et al. utilized RGD-conjugated alginate or HA/alginate matrices with human cardiac progenitor cells (hCPCs) to 3D-print a six-layered, 2cm × 2cm fECT patch which retained high cell viability for 2 weeks in vitro (Gaetani et al., 2012) and 4 weeks implanted on infarcted rat hearts (Gaetani et al., 2015). Arguably the most exciting prospect of soft-tissue 3D bioprinting is the generation of thick perfusable fECTs. Kolesky et al. demonstrated this possibility by generating 1cm-thick, perfused osteogenic tissues printed from a cell-laden dual-crosslinked gelatin-fibrin matrix (Kolesky et al., 2016). Accordingly, a very recent study reported the construction of heterocellular (mouse iPSC-CMs + human ECs), 3D-bioprinted fECTs in a PEG-fibrinogen hydrogel which exhibited improved connectivity to host vasculature upon subcutaneous implantation in NOD-SCID mice (Maiullari et al., 2018). Overall, 3D bioprinting for the purpose of functional cardiac tissue engineering appears to be in early stages of development, awaiting the discovery of optimal bioinks and cell mixtures, but preliminary studies and fast-paced advances suggest enormous potential for creating the next-generation of therapeutic fECTs.
4. Therapeutic Use of Biomaterials without Cells
Intramyocardial or coronary artery injections of exogenous cells in the heart, from initial studies with skeletal myoblasts (Taylor et al., 1998) to more recent transplantations of large numbers of hPSC-CMs into non-human primates (Liu, Y.W. et al., 2018), have demonstrated the potential for cell-based myocardial recovery in humans (Garbern and Lee, 2013). Attempts to improve engraftment of transplanted cells by co-delivery of biomaterials have revealed that injected biomaterials alone, without cells, can also improve compromised cardiac function (Christman et al., 2004). It is now well-understood that injectable biomaterials exert multiple therapeutic benefits to post-MI hearts including: 1) amelioration of maladaptive remodeling induced by altered inflammatory response, 2) reduction of abnormal mechanical loading induced by tissue bulking, and 3) recruitment of endogenous regenerative cells (Reis et al., 2016).
Of the three therapeutic modalities, the most clinically advanced concept is tissue bulking (Table 2 lists recent clinical trial utilizing tissue bulking). The leading biomaterial candidate for this strategy is the inert seaweed-derived compound, alginate (Liberski et al., 2016). In the PRESERVATION I trial, a proprietary sodium alginate and calcium gluconate product was introduced via intracoronary perfusion 2-5 days after coronary artery stenting in patients with ST-elevation myocardial infarction (STEMI) (Rao et al., 2015). At 6 months post injection, alginate-treated and placebo saline-injected patients exhibited similar end-diastolic volumes (a direct measurement of LV chamber dilation) and no difference in mortality events, demonstrating clinical safety but not efficacy of the therapy (Rao et al., 2016). On the other hand, a smaller 12-month trial called AUGMENT-HF that performed intramyocardial injection of a proprietary calcium-alginate solution (Algisyl) vs. a saline control has demonstrated a small, yet significant, improvement in peak VO2 (an oxygen utilization measure) and 6-minute walk tests which are clinically relevant measures of functional capacity in patients with heart failure (Mann et al., 2016). The phase II trial of AUGMENT-HF is already planned with the targeted completion in 2020.
Table 2.
Recent clinical trials of cell-free biomaterials for heart disease.
| Trial Name | Clinicaltrials.gov Identifier | Substrate | Route of Administration | Patient numbers | Cardiac Injury | Results | Ref |
|---|---|---|---|---|---|---|---|
| PRESERVATION I | NCT01226563 | Sodium alginate + Calcium Gluconate | Intracoronary | 303 | Recent STEMI | Complete; No functional improvement; No safety concerns |
(Rao et al., 2015; Rao et al., 2016) |
| AUGMENT HF | NCT01311791 | Calcium Alginate | Intramyocardial | 58 | Congestive Heart Failure; EF < 35% | Complete; Mild improved peak VO2; Mildly improved 6-min walk test |
(Mann et al., 2016) |
| AUGMENT HF II | NCT03082508 | Calcium Alginate | Intramyocardial | > 200 planned | Congestive Heart Failure; EF < 35% | Not yet enrolling | N/A |
| VentriGel | NCT02305602 | Porcine ECM | Trans-endocardial | 15 | Remote STEMI | Closed; no published results | (Self-Naraghi et al., 2013) |
While clinical trials with tissue-bulking agents have shown mixed results, efforts to develop new injectable biomaterials with pleiotropic effects on the diseased heart are ongoing. Specifically, bioactivity appears to be an important biomaterial attribute for the induction of sustained functional benefits. This potentially explains why bio-inert materials, such as alginate or unmodified PEG (Rane et al., 2011), fail clinical efficacy studies. Hence, a soon to be completed Phase I safety study is testing transendocardial injection of a decellularized porcine heart hydrogel product (VentriGel) in 15 patients who have experienced recent STEMI and who have evidence of maladaptive left ventricular remodeling (Seif-Naraghi et al., 2013). As a lack of reproducibility of natural-derived products may hinder wide-spread clinical use, other groups are exploring the therapeutic potential of injectable synthetic hydrogels with built-in bioactive moieties or pro-regenerative gene products (Eckhouse et al., 2014; Purcell et al., 2014; Wang, L.L. et al., 2017). Based on the favorable clinical safety profile, we expect to witness rapid expansion of basic discovery, large animal, and first-in-man studies testing injectable synthetic biomaterials as cardiac therapeutics.
5. The Future of Cardiac Tissue Engineering
The exciting field of cardiac tissue engineering holds potential to greatly improve our understanding of congenital and acquired heart diseases as well as facilitate development of new regenerative therapies for MI and CHF. A simple PubMed search for “cardiac tissue engineering” produces more than 1000 articles published annually over the past three years. Further narrowing this search string by adding the term “biomaterials” produces over 200 annual articles, underlining the importance of technological advances in biomaterial science for the purpose of creating new, or improved, fECTs. Given its tremendous promise, we anticipate that biomaterial research for cardiac tissue engineering and therapeutics will continue to follow its current steep trajectory.
As evidenced by recently active clinical trials discussed in Section 4, the first successful therapies for heart disease based on tissue-engineering principles will likely rely on introduction of cell-free biomaterials as injectable scaffolds. Such scaffolds may serve to provide mechanical stability for acutely injured myocardium after MI, or by acting as a substrate to direct positive myocardial remodeling in the setting of chronic ischemic or infiltrative cardiomyopathies. Modifying bioactivity, mechanical, and degradation properties of injectable biomaterials will allow design of multi-faceted, spatiotemporally controlled therapies to precisely modify immune/inflammatory and regenerative signaling leading to positive clinical outcomes.
The next generation of injectable biomaterials will likely involve incorporation of cell-sourced products to improve their bioactivity. Early examples of this strategy have included use of secreted ECM modulatory proteins (MMPs, TIMP-3) or cardiac-specific miRNAs to improve functional outcomes in animal models of myocardial infarction (Purcell et al., 2014; Wang, L.L. et al., 2017). Moreover, it is evident that the moderate functional improvements seen across a multitude of stem cell-based clinical therapies can be attributed to paracrine action of the transplanted cells (Hodgkinson et al., 2016). Secreted exosomes are currently thought to be likely candidates for such observed therapeutic effects (Sahoo and Losordo, 2014) with the possibility that the exosome-mediated effects could be further augmented via biomaterial delivery through an intramuscular or epicardial route (Liu, B. et al., 2018). Similarly, injection of PLGA-based microparticles containing oxygen-releasing moieties (Fan et al., 2018) or fused with stem cell membrane fragments (Tang et al., 2017) offer additional biomaterial-based approaches to improve heart function after myocardial injury. While the exact therapeutic cargos, reproducibility, and longevity of these strategies remain unknown, their cell-free and likely non-immunogenic nature suggest future developments toward potential clinical applications.
Despite being one of the original goals of cardiac tissue engineering, the clinical use of biomimetic fECTs for epicardial transplantation remains the furthest from completion. While the first clinical trials with human iPSC derivatives for treatment of ischemic heart disease appear to be safe (Menasche et al., 2018), applications of hiPSC-derived fECTs in humans await development of reproducible methods to overcome several technical hurdles including: 1) invasive surgical procedures, 2) immature cardiomyocyte phenotype, 3) small tissue thickness, and 4) lack of electromechanical integration between the grafted fECT and host myocardium (Jackman, C.P. et al., 2018; Shadrin et al., 2017). Recent studies have demonstrated progress towards accelerated structural and functional maturation of hiPSC-CMs in vitro (Ronaldson-Bouchard et al., 2018; Shadrin et al., 2017), which is likely to alleviate arrhythmogenic risks observed in initial cell injection studies in non-human primates (Chong et al., 2014; Liu, Y.W. et al., 2018). However, it is unknown if highly mature cardiomyocytes will be the optimal cell source for achieving thick tissue grafts with the capacity to robustly survive and functionally integrate in vivo. Novel cell engineering and biomaterial strategies employed for fECT construction and delivery will likely play key roles toward improving functional integration as well as promoting long-term viability of engrafted fECTs (Montgomery et al., 2017; Nguyen et al., 2016, 2018; Zhang et al., 2016). True benefit for human disease will be measured in years, rather than weeks-to-months. In the meantime, fECTs will continue to serve as an important in vitro tool for studying human development, biology, and disease including genetic and pharmacological screens to discover and develop new therapeutics. Reproducibility among various research groups, including standardization of cell sources, handling procedures, and animal models of disease would further accelerate progress. As the field moves forward, one can easily envision synergy between biomaterial science and iPSC-based technologies as a powerful, scalable platform for future preclinical and clinical applications in cardiovascular science and medicine.
5. Conclusions
Materializing the promise of cardiac tissue engineering into viable clinical therapies is increasingly becoming a realistic goal. Recent clinical studies with injectable biomaterials are leading ongoing translational efforts, yet synergistic delivery of bioactive cell-based products with biomaterial scaffolds will likely be the next horizon in ameliorating human heart disease. Parallel advances in pluripotent stem cell technology to incorporate adult functionality and patient specificity into engineered cardiac tissues will also drive the field forward. The depth and breadth of efforts toward the goal of functional, human disease modeling and regenerative therapeutics will require coordinated efforts from the diverse pool of talented researchers involved in this exciting field. In this review, we have discussed the present understanding of cell sources, architectures, and biomaterial scaffolds used for creation of functional engineered cardiac tissues (fECTs). Despite significant progress toward generating adult-like fECTs, work remains to demonstrate complete maturation, functional integration, longevity, and human scalability of such constructs. We envision that multiplexing natural and synthetic biomaterials deployed by innovative technologies, such as 3D bioprinting, will further unlock the capacity to create heterocellular, biomimetic fECTs for disease modeling, drug development, and therapeutic applications. Given current progress, we may one day be able to both build and rebuild a heart.
6. Acknowledgments
The authors thank L. Cushman for assistance with figures and Dr. A. Khodabukus for manuscript review. This work was supported by NIH grants U01HL134764, R01HL126524, R01HL132389, UG3TR002142, and Foundation Leducq grant to NB; NIH grants T32HL007101-41 and LRP (2018-2020) to JEP; and NSF Graduate Research Fellowship (2017-2020) to AH. We apologize that the limited length of this manuscript does not allow for citing all significant contributions to this burgeoning field of research.
Abbreviations:
- (CM)
cardiomyocyte
- (CVD)
cardiovascular disease
- (CHF)
congestive heart failure
- (EC)
endothelial cell
- (fECT)
functional engineered cardiac tissue
- (ECM)
extracellular matrix
- (hPSC)
human pluripotent stem cell
- (LV)
left ventricular
- (MMP)
matrix metalloproteinase
- (MI)
myocardial infarction
- (NMVM)
neonatal mouse ventricular myocyte
- (NRVM)
neonatal rat ventricular myocyte
- (PCL)
polycaprolactone
- (PLGA)
poly-DL-lactic-co-glycolic acid
- (PEG)
polyethylene glycol
- (PGS)
polyglycerol sebacate
- (PNIPAAm)
poly(N-isopropylacrylamide)
- (POMaC)
poly(octamethylene maleate (anhydride) citrate)
- (PU)
polyurethane
- (STEMI)
ST-segment elevation myocardial infarction
- (VSMC)
vascular smooth muscle cell
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
7. References:
- Alperin C, Zandstra PW, Woodhouse KA, 2005. Polyurethane films seeded with embryonic stem cell-derived cardiomyocytes for use in cardiac tissue engineering applications. Biomaterials 26(35), 7377–7386. [DOI] [PubMed] [Google Scholar]
- Anderson D, Self T, Mellor IR, Goh G, Hill SJ, Denning C, 2007. Transgenic enrichment of cardiomyocytes from human embryonic stem cells. Mol Ther 15(11), 2027–2036. [DOI] [PubMed] [Google Scholar]
- Asgari M, Latifi N, Heris HK, Vali H, Mongeau L, 2017. In vitro fibrillogenesis of tropocollagen type III in collagen type I affects its relative fibrillar topology and mechanics. Sci Rep 7(1), 1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badrossamay MR, McIlwee HA, Goss JA, Parker KK, 2010. Nanofiber assembly by rotary jetspinning. Nano Lett 10(6), 2257–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badylak SF, Freytes DO, Gilbert TW, 2009. Extracellular matrix as a biological scaffold material: Structure and function. Acta Biomater 5(1), 1–13. [DOI] [PubMed] [Google Scholar]
- Bassat E, Mutlak YE, Genzelinakh A, Shadrin IY, Baruch Umansky K, Yifa O, Kain D, Rajchman D, Leach J, Riabov Bassat D, Udi Y, Sarig R, Sagi I, Martin JF, Bursac N, Cohen S, Tzahor E, 2017. The extracellular matrix protein agrin promotes heart regeneration in mice. Nature 547(7662), 179–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker M, Maring JA, Oberwallner B, Kappler B, Klein O, Falk V, Stamm C, 2017. Processing of Human Cardiac Tissue Toward Extracellular Matrix Self-assembling Hydrogel for In Vitro and In Vivo Applications. J Vis Exp(130). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benam KH, Dauth S, Hassell B, Herland A, Jain A, Jang KJ, Karalis K, Kim HJ, MacQueen L, Mahmoodian R, Musah S, Torisawa YS, van der Meer AD, Villenave R, Yadid M, Parker KK, Ingber DE, 2015. Engineered in vitro disease models. Annu Rev Pathol 10, 195–262. [DOI] [PubMed] [Google Scholar]
- Bergmann O, Zdunek S, Felker A, Salehpour M, Alkass K, Bernard S, Sjostrom SL, Szewczykowska M, Jackowska T, Dos Remedios C, Malm T, Andra M, Jashari R, Nyengaard JR, Possnert G, Jovinge S, Druid H, Frisen J, 2015. Dynamics of Cell Generation and Turnover in the Human Heart. Cell 161(7), 1566–1575. [DOI] [PubMed] [Google Scholar]
- Beumer J, Clevers H, 2016. Regulation and plasticity of intestinal stem cells during homeostasis and regeneration. Development 143(20), 3639–3649. [DOI] [PubMed] [Google Scholar]
- Bhardwaj N, Kundu SC, 2010. Electrospinning: a fascinating fiber fabrication technique. Biotechnol Adv 28(3), 325–347. [DOI] [PubMed] [Google Scholar]
- Bian W, Badie N, Himel H.D.t., Bursac N, 2014a. Robust T-tubulation and maturation of cardiomyocytes using tissue-engineered epicardial mimetics. Biomaterials 35(12), 3819–3828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian W, Jackman CP, Bursac N, 2014b. Controlling the structural and functional anisotropy of engineered cardiac tissues. Biofabrication 6(2), 024109–024109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black LD 3rd, Meyers JD, Weinbaum JS, Shvelidze YA, Tranquillo RT, 2009. Cell-induced alignment augments twitch force in fibrin gel-based engineered myocardium via gap junction modification. Tissue Eng Part A 15(10), 3099–3108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanpain C, Fuchs E, 2006. Epidermal stem cells of the skin. Annu Rev Cell Dev Biol 22, 339–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bursac N, Loo Y, Leong K, Tung L, 2007. Novel anisotropic engineered cardiac tissues: studies of electrical propagation. Biochem Biophys Res Commun 361(4), 847–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bursac N, Papadaki M, Cohen RJ, Schoen FJ, Eisenberg SR, Carrier R, Vunjak-Novakovic G, Freed LE, 1999. Cardiac muscle tissue engineering: toward an in vitro model for electrophysiological studies. Am J Physiol 277(2), H433–444. [DOI] [PubMed] [Google Scholar]
- Bursac N, Papadaki M, White JA, Eisenberg SR, Vunjak-Novakovic G, Freed LE, 2003. Cultivation in rotating bioreactors promotes maintenance of cardiac myocyte electrophysiology and molecular properties. Tissue Eng 9(6), 1243–1253. [DOI] [PubMed] [Google Scholar]
- Campbell M, Chabria M, Figtree GA, Polonchuk L, Gentile C, 2018. Stem Cell-Derived Cardiac Spheroids as 3D In Vitro Models of the Human Heart Microenvironment. Methods Mol Biol. [DOI] [PubMed] [Google Scholar]
- Capulli AK, MacQueen LA, Sheehy SP, Parker KK, 2016. Fibrous scaffolds for building hearts and heart parts. Adv Drug Deliv Rev 96, 83–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cashman TJ, Josowitz R, Johnson BV, Gelb BD, Costa KD, 2016. Human Engineered Cardiac Tissues Created Using Induced Pluripotent Stem Cells Reveal Functional Characteristics of BRAF-Mediated Hypertrophic Cardiomyopathy. PLoS One 11(1), e0146697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong JJ, Yang X, Don CW, Minami E, Liu YW, Weyers JJ, Mahoney WM, Van Biber B, Cook SM, Palpant NJ, Gantz JA, Fugate JA, Muskheli V, Gough GM, Vogel KW, Astley CA, Hotchkiss CE, Baldessari A, Pabon L, Reinecke H, Gill EA, Nelson V, Kiem HP, Laflamme MA, Murry CE, 2014. Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature 510(7504), 273–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christman KL, Fok HH, Sievers RE, Fang Q, Lee RJ, 2004. Fibrin glue alone and skeletal myoblasts in a fibrin scaffold preserve cardiac function after myocardial infarction. Tissue Eng 10(3-4), 403–409. [DOI] [PubMed] [Google Scholar]
- Christoforou N, Liau B, Chakraborty S, Chellapan M, Bursac N, Leong KW, 2013. Induced pluripotent stem cell-derived cardiac progenitors differentiate to cardiomyocytes and form biosynthetic tissues. PLoS One 8(6), e65963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn JN, Ferrari R, Sharpe N, 2000. Cardiac remodeling--concepts and clinical implications: a consensus paper from an international forum on cardiac remodeling. Behalf of an International Forum on Cardiac Remodeling. J Am Coll Cardiol 35(3), 569–582. [DOI] [PubMed] [Google Scholar]
- Collet JP, Shuman H, Ledger RE, Lee S, Weisel JW, 2005. The elasticity of an individual fibrin fiber in a clot. Proc Natl Acad Sci U S A 102(26), 9133–9137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyranoski D, 2018. 'Reprogrammed' stem cells approved to mend human hearts for the first time. Nature 557(7707), 619–620. [DOI] [PubMed] [Google Scholar]
- D'Uva G, Aharonov A, Lauriola M, Kain D, Yahalom-Ronen Y, Carvalho S, Weisinger K, Bassat E, Rajchman D, Yifa O, Lysenko M, Konfino T, Hegesh J, Brenner O, Neeman M, Yarden Y, Leor J, Sarig R, Harvey RP, Tzahor E, 2015. ERBB2 triggers mammalian heart regeneration by promoting cardiomyocyte dedifferentiation and proliferation. Nat Cell Biol 17(5), 627–638. [DOI] [PubMed] [Google Scholar]
- de Lange WJ, Hegge LF, Grimes AC, Tong CW, Brost TM, Moss RL, Ralphe JC, 2011. Neonatal mouse-derived engineered cardiac tissue: a novel model system for studying genetic heart disease. Circ Res 109(1), 8–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Didie M, Christalla P, Rubart M, Muppala V, Doker S, Unsold B, El-Armouche A, Rau T, Eschenhagen T, Schwoerer AP, Ehmke H, Schumacher U, Fuchs S, Lange C, Becker A, Tao W, Scherschel JA, Soonpaa MH, Yang T, Lin Q, Zenke M, Han DW, Scholer HR, Rudolph C, Steinemann D, Schlegelberger B, Kattman S, Witty A, Keller G, Field LJ, Zimmermann WH, 2013. Parthenogenetic stem cells for tissue-engineered heart repair. J Clin Invest 123(3), 1285–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durrer D, van Dam RT, Freud GE, Janse MJ, Meijler FL, Arzbaecher RC, 1970. Total excitation of the isolated human heart. Circulation 41(6), 899–912. [DOI] [PubMed] [Google Scholar]
- Eckhouse SR, Purcell BP, McGarvey JR, Lobb D, Logdon CB, Doviak H, O'Neill JW, Shuman JA, Novack CP, Zellars KN, Pettaway S, Black RA, Khakoo A, Lee T, Mukherjee R, Gorman JH, Gorman RC, Burdick JA, Spinale FG, 2014. Local hydrogel release of recombinant TIMP-3 attenuates adverse left ventricular remodeling after experimental myocardial infarction. Sci Transl Med 6(223), 223ra221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelmayr GC Jr., Cheng M, Bettinger CJ, Borenstein JT, Langer R, Freed LE, 2008. Accordionlike honeycombs for tissue engineering of cardiac anisotropy. Nat Mater 7(12), 1003–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entcheva E, Bien H, Yin L, Chung CY, Farrell M, Kostov Y, 2004. Functional cardiac cell constructs on cellulose-based scaffolding. Biomaterials 25(26), 5753–5762. [DOI] [PubMed] [Google Scholar]
- Eschenhagen T, Fink C, Remmers U, Scholz H, Wattchow J, Weil J, Zimmermann W, Dohmen HH, Schafer H, Bishopric N, Wakatsuki T, Elson EL, 1997. Three-dimensional reconstitution of embryonic cardiomyocytes in a collagen matrix: a new heart muscle model system. FASEB J 11(8), 683–694. [DOI] [PubMed] [Google Scholar]
- Fan Z, Xu Z, Niu H, Gao N, Guan Y, Li C, Dang Y, Cui X, Liu XL, Duan Y, Li H, Zhou X, Lin PH, Ma J, Guan J, 2018. An Injectable Oxygen Release System to Augment Cell Survival and Promote Cardiac Repair Following Myocardial Infarction. Sci Rep 8(1), 1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana G, Gershlak J, Adamski M, Lee JS, Matsumoto S, Le HD, Binder B, Wirth J, Gaudette G, Murphy WL, 2017. Biofunctionalized Plants as Diverse Biomaterials for Human Cell Culture. Adv Healthc Mater 6(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaetani R, Doevendans PA, Metz CH, Alblas J, Messina E, Giacomello A, Sluijter JP, 2012. Cardiac tissue engineering using tissue printing technology and human cardiac progenitor cells. Biomaterials 33(6), 1782–1790. [DOI] [PubMed] [Google Scholar]
- Gaetani R, Feyen DA, Verhage V, Slaats R, Messina E, Christman KL, Giacomello A, Doevendans PA, Sluijter JP, 2015. Epicardial application of cardiac progenitor cells in a 3D-printed gelatin/hyaluronic acid patch preserves cardiac function after myocardial infarction. Biomaterials 61, 339–348. [DOI] [PubMed] [Google Scholar]
- Gao L, Gregorich ZR, Zhu W, Mattapally S, Oduk Y, Lou X, Kannappan R, Borovjagin AV, Walcott GP, Pollard AE, Fast VG, Hu X, Lloyd SG, Ge Y, Zhang J, 2018. Large Cardiac Muscle Patches Engineered From Human Induced-Pluripotent Stem Cell-Derived Cardiac Cells Improve Recovery From Myocardial Infarction in Swine. Circulation 137(16), 1712–1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbern JC, Lee RT, 2013. Cardiac stem cell therapy and the promise of heart regeneration. Cell Stem Cell 12(6), 689–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershlak JR, Hernandez S, Fontana G, Perreault LR, Hansen KJ, Larson SA, Binder BY, Dolivo DM, Yang T, Dominko T, Rolle MW, Weathers PJ, Medina-Bolivar F, Cramer CL, Murphy WL, Gaudette GR, 2017. Crossing kingdoms: Using decellularized plants as perfusable tissue engineering scaffolds. Biomaterials 125, 13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacomelli E, Bellin M, Orlova VV, Mummery CL, 2017a. Co-Differentiation of Human Pluripotent Stem Cells-Derived Cardiomyocytes and Endothelial Cells from Cardiac Mesoderm Provides a Three-Dimensional Model of Cardiac Microtissue. Curr Protoc Hum Genet 95, 21 29 21–21 29 22. [DOI] [PubMed] [Google Scholar]
- Giacomelli E, Bellin M, Sala L, van Meer BJ, Tertoolen LG, Orlova VV, Mummery CL, 2017b. Three-dimensional cardiac microtissues composed of cardiomyocytes and endothelial cells codifferentiated from human pluripotent stem cells. Development 144(6), 1008–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin A, Yang Y, 2017. Decellularization Strategies for Regenerative Medicine: From Processing Techniques to Applications. Biomed Res Int 2017, 9831534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassl ED, Oegema TR, Tranquillo RT, 2002. Fibrin as an alternative biopolymer to type-I collagen for the fabrication of a media equivalent. J Biomed Mater Res 60(4), 607–612. [DOI] [PubMed] [Google Scholar]
- Guyette JP, Charest JM, Mills RW, Jank BJ, Moser PT, Gilpin SE, Gershlak JR, Okamoto T, Gonzalez G, Milan DJ, Gaudette GR, Ott HC, 2016. Bioengineering Human Myocardium on Native Extracellular Matrix. Circ Res 118(1), 56–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen A, Eder A, Bonstrup M, Flato M, Mewe M, Schaaf S, Aksehirlioglu B, Schwoerer AP, Uebeler J, Eschenhagen T, 2010. Development of a drug screening platform based on engineered heart tissue. Circ Res 107(1), 35–44. [DOI] [PubMed] [Google Scholar]
- Hansen KJ, Laflamme MA, Gaudette GR, 2018. Development of a Contractile Cardiac Fiber From Pluripotent Stem Cell Derived Cardiomyocytes. Front Cardiovasc Med 5, 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasenfuss G, Mulieri LA, Blanchard EM, Holubarsch C, Leavitt BJ, Ittleman F, Alpert NR, 1991. Energetics of isometric force development in control and volume-overload human myocardium. Comparison with animal species. Circ Res 68(3), 836–846. [DOI] [PubMed] [Google Scholar]
- Hirt MN, Boeddinghaus J, Mitchell A, Schaaf S, Bornchen C, Muller C, Schulz H, Hubner N, Stenzig J, Stoehr A, Neuber C, Eder A, Luther PK, Hansen A, Eschenhagen T, 2014. Functional improvement and maturation of rat and human engineered heart tissue by chronic electrical stimulation. J Mol Cell Cardiol 74, 151–161. [DOI] [PubMed] [Google Scholar]
- Hodgkinson CP, Bareja A, Gomez JA, Dzau VJ, 2016. Emerging Concepts in Paracrine Mechanisms in Regenerative Cardiovascular Medicine and Biology. Circ Res 118(1), 95–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgson MJ, Knutson CC, Momtahan N, Cook AD, 2017. Extracellular Matrix from Whole Porcine Heart Decellularization for Cardiac Tissue Engineering. Methods Mol Biol. [DOI] [PubMed] [Google Scholar]
- Hudlicka O, Brown M, Egginton S, 1992. Angiogenesis in skeletal and cardiac muscle. Physiol Rev 72(2), 369–417. [DOI] [PubMed] [Google Scholar]
- Imamura T, Kinugawa K, Sakata Y, Miyagawa S, Sawa Y, Yamazaki K, Ono M, 2016. Improved clinical course of autologous skeletal myoblast sheet (TCD-51073) transplantation when compared to a propensity score-matched cardiac resynchronization therapy population. J Artif Organs 19(1), 80–86. [DOI] [PubMed] [Google Scholar]
- Itskovitz-Eldor J, Schuldiner M, Karsenti D, Eden A, Yanuka O, Amit M, Soreq H, Benvenisty N, 2000. Differentiation of human embryonic stem cells into embryoid bodies compromising the three embryonic germ layers. Mol Med 6(2), 88–95. [PMC free article] [PubMed] [Google Scholar]
- Jackman C, Li H, Bursac N, 2018. Long-term contractile activity and thyroid hormone supplementation produce engineered rat myocardium with adult-like structure and function. Acta Biomater 78, 98–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackman CP, Carlson AL, Bursac N, 2016. Dynamic culture yields engineered myocardium with near-adult functional output. Biomaterials 111, 66–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackman CP, Ganapathi AM, Asfour H, Qian Y, Allen BW, Li Y, Bursac N, 2018. Engineered cardiac tissue patch maintains structural and electrical properties after epicardial implantation. Biomaterials 159, 48–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackman CP, Shadrin IY, Carlson AL, Bursac N, 2015. Human Cardiac Tissue Engineering: From Pluripotent Stem Cells to Heart Repair. Curr Opin Chem Eng 7, 57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jongpaiboonkit L, King WJ, Lyons GE, Paguirigan AL, Warrick JW, Beebe DJ, Murphy WL, 2008. An adaptable hydrogel array format for 3-dimensional cell culture and analysis. Biomaterials 29(23), 3346–3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura M, Miyagawa S, Fukushima S, Saito A, Miki K, Funakoshi S, Yoshida Y, Yamanaka S, Shimizu T, Okano T, Daimon T, Toda K, Sawa Y, 2017. Enhanced Therapeutic Effects of Human iPS Cell Derived-Cardiomyocyte by Combined Cell-Sheets with Omental Flap Technique in Porcine Ischemic Cardiomyopathy Model. Sci Rep 7(1), 8824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempf H, Kropp C, Olmer R, Martin U, Zweigerdt R, 2015. Cardiac differentiation of human pluripotent stem cells in scalable suspension culture. Nat Protoc 10(9), 1345–1361. [DOI] [PubMed] [Google Scholar]
- Kensah G, Roa Lara A, Dahlmann J, Zweigerdt R, Schwanke K, Hegermann J, Skvorc D, Gawol A, Azizian A, Wagner S, Maier LS, Krause A, Drager G, Ochs M, Haverich A, Gruh I, Martin U, 2013. Murine and human pluripotent stem cell-derived cardiac bodies form contractile myocardial tissue in vitro. Eur Heart J 34(15), 1134–1146. [DOI] [PubMed] [Google Scholar]
- Kerscher P, Turnbull IC, Hodge AJ, Kim J, Seliktar D, Easley CJ, Costa KD, Lipke EA, 2016. Direct hydrogel encapsulation of pluripotent stem cells enables ontomimetic differentiation and growth of engineered human heart tissues. Biomaterials 83, 383–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharaziha M, Nikkhah M, Shin SR, Annabi N, Masoumi N, Gaharwar AK, Camci-Unal G, Khademhosseini A, 2013. PGS:Gelatin nanofibrous scaffolds with tunable mechanical and structural properties for engineering cardiac tissues. Biomaterials 34(27), 6355–6366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khodabukus A, Baar K, 2009. Regulating fibrinolysis to engineer skeletal muscle from the C2C12 cell line. Tissue Eng Part C Methods 15(3), 501–511. [DOI] [PubMed] [Google Scholar]
- Kim TY, Kofron CM, King ME, Markes AR, Okundaye AO, Qu Z, Mende U, Choi BR, 2018. Directed fusion of cardiac spheroids into larger heterocellular microtissues enables investigation of cardiac action potential propagation via cardiac fibroblasts. PLoS One 13(5), e0196714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitahara H, Yagi H, Tajima K, Okamoto K, Yoshitake A, Aeba R, Kudo M, Kashima I, Kawaguchi S, Hirano A, Kasai M, Akamatsu Y, Oka H, Kitagawa Y, Shimizu H, 2016. Heterotopic transplantation of a decellularized and recellularized whole porcine heart. Interact Cardiovasc Thorac Surg 22(5), 571–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kofron CM, Kim TY, King ME, Xie A, Feng F, Park E, Qu Z, Choi BR, Mende U, 2017. Gq-activated fibroblasts induce cardiomyocyte action potential prolongation and automaticity in a three-dimensional microtissue environment. Am J Physiol Heart Circ Physiol 313(4), H810–H827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolesky DB, Homan KA, Skylar-Scott MA, Lewis JA, 2016. Three-dimensional bioprinting of thick vascularized tissues. Proc Natl Acad Sci U S A 113(12), 3179–3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraehenbuehl TP, Langer R, Ferreira LS, 2011. Three-dimensional biomaterials for the study of human pluripotent stem cells. Nat Methods 8(9), 731–736. [DOI] [PubMed] [Google Scholar]
- Laurens N, Koolwijk P, de Maat MP, 2006. Fibrin structure and wound healing. J Thromb Haemost 4(5), 932–939. [DOI] [PubMed] [Google Scholar]
- Leach JP, Heallen T, Zhang M, Rahmani M, Morikawa Y, Hill MC, Segura A, Willerson JT, Martin JF, 2017. Hippo pathway deficiency reverses systolic heart failure after infarction. Nature 550(7675), 260–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee EJ, Kim DE, Azeloglu EU, Costa KD, 2008. Engineered cardiac organoid chambers: toward a functional biological model ventricle. Tissue Eng Part A 14(2), 215–225. [DOI] [PubMed] [Google Scholar]
- Lee S, Serpooshan V, Tong X, Venkatraman S, Lee M, Lee J, Chirikian O, Wu JC, Wu SM, Yang F, 2017. Contractile force generation by 3D hiPSC-derived cardiac tissues is enhanced by rapid establishment of cellular interconnection in matrix with muscle-mimicking stiffness. Biomaterials 131, 111–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Minami I, Shiozaki M, Yu L, Yajima S, Miyagawa S, Shiba Y, Morone N, Fukushima S, Yoshioka M, Li S, Qiao J, Li X, Wang L, Kotera H, Nakatsuji N, Sawa Y, Chen Y, Liu L, 2017. Human Pluripotent Stem Cell-Derived Cardiac Tissue-like Constructs for Repairing the Infarcted Myocardium. Stem Cell Reports 9(5), 1546–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li RA, Keung W, Cashman TJ, Backeris PC, Johnson BV, Bardot ES, Wong AOT, Chan PKW, Chan CWY, Costa KD, 2018. Bioengineering an electro-mechanically functional miniature ventricular heart chamber from human pluripotent stem cells. Biomaterials 163, 116–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li RK, Jia ZQ, Weisel RD, Mickle DA, Choi A, Yau TM, 1999. Survival and function of bioengineered cardiac grafts. Circulation 100(19 Suppl), II63–69. [DOI] [PubMed] [Google Scholar]
- Li Y, Asfour H, Bursac N, 2017. Age-dependent functional crosstalk between cardiac fibroblasts and cardiomyocytes in a 3D engineered cardiac tissue. Acta Biomater 55, 120–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, He L, Huang X, Bhaloo SI, Zhao H, Zhang S, Pu W, Tian X, Li Y, Liu Q, Yu W, Zhang L, Liu X, Liu K, Tang J, Zhang H, Cai D, Ralf AH, Xu Q, Lui KO, Zhou B, 2018. Genetic Lineage Tracing of Nonmyocyte Population by Dual Recombinases. Circulation 138(8), 793–805. [DOI] [PubMed] [Google Scholar]
- Lian X, Hsiao C, Wilson G, Zhu K, Hazeltine LB, Azarin SM, Raval KK, Zhang J, Kamp TJ, Palecek SP, 2012. Robust cardiomyocyte differentiation from human pluripotent stem cells via temporal modulation of canonical Wnt signaling. Proc Natl Acad Sci U S A 109(27), E1848–1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liau B, Christoforou N, Leong KW, Bursac N, 2011. Pluripotent stem cell-derived cardiac tissue patch with advanced structure and function. Biomaterials 32(35), 9180–9187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liau B, Jackman CP, Li Y, Bursac N, 2017. Developmental stage-dependent effects of cardiac fibroblasts on function of stem cell-derived engineered cardiac tissues. Sci Rep 7, 42290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberski A, Latif N, Raynaud C, Bollensdorff C, Yacoub M, 2016. Alginate for cardiac regeneration: From seaweed to clinical trials. Glob Cardiol Sci Pract 2016(1), e201604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Lee BW, Nakanishi K, Villasante A, Williamson R, Metz J, Kim J, Kanai M, Bi L, Brown K, Di Paolo G, Homma S, Sims PA, Topkara VK, Vunjak-Novakovic G, 2018. Cardiac recovery via extended cell-free delivery of extracellular vesicles secreted by cardiomyocytes derived from induced pluripotent stem cells. Nat Biomed Eng 2(5), 293–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YW, Chen B, Yang X, Fugate JA, Kalucki FA, Futakuchi-Tsuchida A, Couture L, Vogel KW, Astley CA, Baldessari A, Ogle J, Don CW, Steinberg ZL, Seslar SP, Tuck SA, Tsuchida H, Naumova AV, Dupras SK, Lyu MS, Lee J, Hailey DW, Reinecke H, Pabon L, Fryer BH, MacLellan WR, Thies RS, Murry CE, 2018. Human embryonic stem cell-derived cardiomyocytes restore function in infarcted hearts of non-human primates. Nat Biotechnol 36(7), 597–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louch WE, Sheehan KA, Wolska BM, 2011. Methods in cardiomyocyte isolation, culture, and gene transfer. J Mol Cell Cardiol 51(3), 288–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu TY, Lin B, Kim J, Sullivan M, Tobita K, Salama G, Yang L, 2013. Repopulation of decellularized mouse heart with human induced pluripotent stem cell-derived cardiovascular progenitor cells. Nat Commun 4, 2307. [DOI] [PubMed] [Google Scholar]
- MacQueen LA, Sheehy SP, Chantre CO, Zimmerman JF, Pasqualini FS, Liu X, Goss JA, Campbell PH, Gonzalez GM, Park S-J, Capulli AK, Ferrier JP, Kosar TF, Mahadevan L, Pu WT, Parker KK, 2018. A tissue-engineered scale model of the heart ventricle. Nature Biomedical Engineering. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiullari F, Costantini M, Milan M, Pace V, Chirivi M, Maiullari S, Rainer A, Baci D, Marei HE, Seliktar D, Gargioli C, Bearzi C, Rizzi R, 2018. A multi-cellular 3D bioprinting approach for vascularized heart tissue engineering based on HUVECs and iPSC-derived cardiomyocytes. Sci Rep 8(1), 13532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann DL, Lee RJ, Coats AJ, Neagoe G, Dragomir D, Pusineri E, Piredda M, Bettari L, Kirwan BA, Dowling R, Volterrani M, Solomon SD, Sabbah HN, Hinson A, Anker SD, 2016. One-year follow-up results from AUGMENT-HF: a multicentre randomized controlled clinical trial of the efficacy of left ventricular augmentation with Algisyl in the treatment of heart failure. Eur J Heart Fail 18(3), 314–325. [DOI] [PubMed] [Google Scholar]
- Mannhardt I, Saleem U, Benzin A, Schulze T, Klampe B, Eschenhagen T, Hansen A, 2017. Automated Contraction Analysis of Human Engineered Heart Tissue for Cardiac Drug Safety Screening. J Vis Exp(122). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattapally S, Zhu W, Fast VG, Gao L, Worley C, Kannappan R, Borovjagin AV, Zhang J, 2018. Spheroids of cardiomyocytes derived from human-induced pluripotent stem cells improve recovery from myocardial injury in mice. Am J Physiol Heart Circ Physiol 315(2), H327–H339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menasche P, Vanneaux V, Hagege A, Bel A, Cholley B, Parouchev A, Cacciapuoti I, Al-Daccak R, Benhamouda N, Blons H, Agbulut O, Tosca L, Trouvin JH, Fabreguettes JR, Bellamy V, Charron D, Tartour E, Tachdjian G, Desnos M, Larghero J, 2018. Transplantation of Human Embryonic Stem Cell-Derived Cardiovascular Progenitors for Severe Ischemic Left Ventricular Dysfunction. J Am Coll Cardiol 71(4), 429–438. [DOI] [PubMed] [Google Scholar]
- Miki K, Uenaka H, Saito A, Miyagawa S, Sakaguchi T, Higuchi T, Shimizu T, Okano T, Yamanaka S, Sawa Y, 2012. Bioengineered myocardium derived from induced pluripotent stem cells improves cardiac function and attenuates cardiac remodeling following chronic myocardial infarction in rats. Stem Cells Transl Med 1(5), 430–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills RJ, Titmarsh DM, Koenig X, Parker BL, Ryall JG, Quaife-Ryan GA, Voges HK, Hodson MP, Ferguson C, Drowley L, Plowright AT, Needham EJ, Wang QD, Gregorevic P, Xin M, Thomas WG, Parton RG, Nielsen LK, Launikonis BS, James DE, Elliott DA, Porrello ER, Hudson JE, 2017. Functional screening in human cardiac organoids reveals a metabolic mechanism for cardiomyocyte cell cycle arrest. Proc Natl Acad Sci U S A 114(40), E8372–E8381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modulevsky DJ, Cuerrier CM, Pelling AE, 2016. Biocompatibility of Subcutaneously Implanted Plant- Derived Cellulose Biomaterials. PLoS One 11(6), e0157894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modulevsky DJ, Lefebvre C, Haase K, Al-Rekabi Z, Pelling AE, 2014. Apple derived cellulose scaffolds for 3D mammalian cell culture. PLoS One 9(5), e97835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed TMA, Ang YS, Radzinsky E, Zhou P, Huang Y, Elfenbein A, Foley A, Magnitsky S, Srivastava D, 2018. Regulation of Cell Cycle to Stimulate Adult Cardiomyocyte Proliferation and Cardiac Regeneration. Cell 173(1), 104–116 e112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery M, Ahadian S, Davenport Huyer L, Lo Rito M, Civitarese RA, Vanderlaan RD, Wu J, Reis LA, Momen A, Akbari S, Pahnke A, Li RK, Caldarone CA, Radisic M, 2017. Flexible shape-memory scaffold for minimally invasive delivery of functional tissues. Nat Mater 16(10), 1038–1046. [DOI] [PubMed] [Google Scholar]
- Mulieri LA, Hasenfuss G, Leavitt B, Allen PD, Alpert NR, 1992. Altered myocardial force-frequency relation in human heart failure. Circulation 85(5), 1743–1750. [DOI] [PubMed] [Google Scholar]
- Naito H, Melnychenko I, Didie M, Schneiderbanger K, Schubert P, Rosenkranz S, Eschenhagen T, Zimmermann WH, 2006. Optimizing engineered heart tissue for therapeutic applications as surrogate heart muscle. Circulation 114(1 Suppl), I72–78. [DOI] [PubMed] [Google Scholar]
- Nakada Y, Canseco DC, Thet S, Abdisalaam S, Asaithamby A, Santos CX, Shah AM, Zhang H, Faber JE, Kinter MT, Szweda LI, Xing C, Hu Z, Deberardinis RJ, Schiattarella G, Hill JA, Oz O, Lu Z, Zhang CC, Kimura W, Sadek HA, 2017. Hypoxia induces heart regeneration in adult mice. Nature 541(7636), 222–227. [DOI] [PubMed] [Google Scholar]
- Nguyen EH, Daly WT, Le NNT, Farnoodian M, Belair DG, Schwartz MP, Lebakken CS, Ananiev GE, Saghiri MA, Knudsen TB, Sheibani N, Murphy WL, 2017. Versatile synthetic alternatives to Matrigel for vascular toxicity screening and stem cell expansion. Nat Biomed Eng 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen HX, Kirkton RD, Bursac N, 2016. Engineering prokaryotic channels for control of mammalian tissue excitability. Nat Commun 7, 13132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen HX, Kirkton RD, Bursac N, 2018. Generation and customization of biosynthetic excitable tissues for electrophysiological studies and cell-based therapies. Nat Protoc 13(5), 927–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen SH, Mouton AJ, DeLeon-Pennell KY, Genovese F, Karsdal M, Lindsey ML, 2017. Understanding cardiac extracellular matrix remodeling to develop biomarkers of myocardial infarction outcomes. Matrix Biol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes SS, Miklas JW, Liu J, Aschar-Sobbi R, Xiao Y, Zhang B, Jiang J, Masse S, Gagliardi M, Hsieh A, Thavandiran N, Laflamme MA, Nanthakumar K, Gross GJ, Backx PH, Keller G, Radisic M, 2013. Biowire: a platform for maturation of human pluripotent stem cell-derived cardiomyocytes. Nat Methods 10(8), 781–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogle BM, Bursac N, Domian I, Huang NF, Menasche P, Murry CE, Pruitt B, Radisic M, Wu JC, Wu SM, Zhang J, Zimmermann WH, Vunjak-Novakovic G, 2016. Distilling complexity to advance cardiac tissue engineering. Sci Transl Med 8(342), 342ps313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oikonomopoulos A, Kitani T, Wu JC, 2018. Pluripotent Stem Cell-Derived Cardiomyocytes as a Platform for Cell Therapy Applications: Progress and Hurdles for Clinical Translation. Mol Ther 26(7), 1624–1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oltolina F, Zamperone A, Colangelo D, Gregoletto L, Reano S, Pietronave S, Merlin S, Talmon M, Novelli E, Diena M, Nicoletti C, Musaro A, Filigheddu N, Follenzi A, Prat M, 2015. Human Cardiac Progenitor Spheroids Exhibit Enhanced Engraftment Potential. PLoS One 10(9), e0137999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong CS, Fukunishi T, Zhang H, Huang CY, Nashed A, Blazeski A, DiSilvestre D, Vricella L, Conte J, Tung L, Tomaselli GF, Hibino N, 2017. Biomaterial-Free Three-Dimensional Bioprinting of Cardiac Tissue using Human Induced Pluripotent Stem Cell Derived Cardiomyocytes. Sci Rep 7(1), 4566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott HC, Matthiesen TS, Goh SK, Black LD, Kren SM, Netoff TI, Taylor DA, 2008. Perfusion-decellularized matrix: using nature's platform to engineer a bioartificial heart. Nat Med 14(2), 213–221. [DOI] [PubMed] [Google Scholar]
- Papadaki M, Bursac N, Langer R, Merok J, Vunjak-Novakovic G, Freed LE, 2001. Tissue engineering of functional cardiac muscle: molecular, structural, and electrophysiological studies. Am J Physiol Heart Circ Physiol 280(1), H168–178. [DOI] [PubMed] [Google Scholar]
- Polonchuk L, Chabria M, Badi L, Hoflack JC, Figtree G, Davies MJ, Gentile C, 2017. Cardiac spheroids as promising in vitro models to study the human heart microenvironment. Sci Rep 7(1), 7005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell C, Shansky J, Del Tatto M, Forman DE, Hennessey J, Sullivan K, Zielinski BA, Vandenburgh HH, 1999. Tissue-engineered human bioartificial muscles expressing a foreign recombinant protein for gene therapy. Hum Gene Ther 10(4), 565–577. [DOI] [PubMed] [Google Scholar]
- Pradhan BS, Majumdar SS, 2016. An Efficient Method for Generation of Transgenic Rats Avoiding Embryo Manipulation. Mol Ther Nucleic Acids 5, e293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell BP, Lobb D, Charati MB, Dorsey SM, Wade RJ, Zellars KN, Doviak H, Pettaway S, Logdon CB, Shuman JA, Freels PD, Gorman JH 3rd, Gorman RC, Spinale FG, Burdick JA, 2014. Injectable and bioresponsive hydrogels for on-demand matrix metalloproteinase inhibition. Nat Mater 13(6), 653–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radisic M, Christman KL, 2013. Materials science and tissue engineering: repairing the heart. Mayo Clin Proc 88(8), 884–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rane AA, Chuang JS, Shah A, Hu DP, Dalton ND, Gu Y, Peterson KL, Omens JH, Christman KL, 2011. Increased infarct wall thickness by a bio-inert material is insufficient to prevent negative left ventricular remodeling after myocardial infarction. PLoS One 6(6), e21571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao SV, Zeymer U, Douglas PS, Al-Khalidi H, Liu J, Gibson CM, Harrison RW, Joseph DS, Heyrman R, Krucoff MW, 2015. A randomized, double-blind, placebo-controlled trial to evaluate the safety and effectiveness of intracoronary application of a novel bioabsorbable cardiac matrix for the prevention of ventricular remodeling after large ST-segment elevation myocardial infarction: Rationale and design of the PRESERVATION I trial. Am Heart J 170(5), 929–937. [DOI] [PubMed] [Google Scholar]
- Rao SV, Zeymer U, Douglas PS, Al-Khalidi H, White JA, Liu J, Levy H, Guetta V, Gibson CM, Tanguay JF, Vermeersch P, Roncalli J, Kasprzak JD, Henry TD, Frey N, Kracoff O, Traverse JH, Chew DP, Lopez-Sendon J, Heyrman R, Krucoff MW, 2016. Bioabsorbable Intracoronary Matrix for Prevention of Ventricular Remodeling After Myocardial Infarction. J Am Coll Cardiol 68(7), 715–723. [DOI] [PubMed] [Google Scholar]
- Reis LA, Chiu LL, Feric N, Fu L, Radisic M, 2016. Biomaterials in myocardial tissue engineering. J Tissue Eng Regen Med 10(1), 11–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Relaix F, Zammit PS, 2012. Satellite cells are essential for skeletal muscle regeneration: the cell on the edge returns centre stage. Development 139(16), 2845–2856. [DOI] [PubMed] [Google Scholar]
- Riegler J, Tiburcy M, Ebert A, Tzatzalos E, Raaz U, Abilez OJ, Shen Q, Kooreman NG, Neofytou E, Chen VC, Wang M, Meyer T, Tsao PS, Connolly AJ, Couture LA, Gold JD, Zimmermann WH, Wu JC, 2015. Human Engineered Heart Muscles Engraft and Survive Long Term in a Rodent Myocardial Infarction Model. Circ Res 117(8), 720–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rienks M, Papageorgiou AP, Frangogiannis NG, Heymans S, 2014. Myocardial extracellular matrix: an ever-changing and diverse entity. Circ Res 114(5), 872–888. [DOI] [PubMed] [Google Scholar]
- Ronaldson-Bouchard K, Ma SP, Yeager K, Chen T, Song L, Sirabella D, Morikawa K, Teles D, Yazawa M, Vunjak-Novakovic G, 2018. Advanced maturation of human cardiac tissue grown from pluripotent stem cells. Nature 556(7700), 239–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross JJ, Tranquillo RT, 2003. ECM gene expression correlates with in vitro tissue growth and development in fibrin gel remodeled by neonatal smooth muscle cells. Matrix Biol 22(6), 477–490. [DOI] [PubMed] [Google Scholar]
- Rossi G, Manfrin A, Lutolf MP, 2018. Progress and potential in organoid research. Nat Rev Genet 19(11), 671–687. [DOI] [PubMed] [Google Scholar]
- Roth GA, Johnson C, Abajobir A, Abd-Allah F, Abera SF, Abyu G, Ahmed M, Aksut B, Alam T, Alam K, Alla F, Alvis-Guzman N, Amrock S, Ansari H, Arnlov J, Asayesh H, Atey TM, Avila-Burgos L, Awasthi A, Banerjee A, Barac A, Barnighausen T, Barregard L, Bedi N, Belay Ketema E, Bennett D, Berhe G, Bhutta Z, Bitew S, Carapetis J, Carrero JJ, Malta DC, Castaneda-Orjuela CA, Castillo-Rivas J, Catala-Lopez F, Choi JY, Christensen H, Cirillo M, Cooper L Jr., Criqui M, Cundiff D, Damasceno A, Dandona L, Dandona R, Davletov K, Dharmaratne S, Dorairaj P, Dubey M, Ehrenkranz R, El Sayed Zaki M, Faraon EJA, Esteghamati A, Farid T, Farvid M, Feigin V, Ding EL, Fowkes G, Gebrehiwot T, Gillum R, Gold A, Gona P, Gupta R, Habtewold TD, Hafezi-Nejad N, Hailu T, Hailu GB, Hankey G, Hassen HY, Abate KH, Havmoeller R, Hay SI, Horino M, Hotez PJ, Jacobsen K, James S, Javanbakht M, Jeemon P, John D, Jonas J, Kalkonde Y, Karimkhani C, Kasaeian A, Khader Y, Khan A, Khang YH, Khera S, Khoja AT, Khubchandani J, Kim D, Kolte D, Kosen S, Krohn KJ, Kumar GA, Kwan GF, Lal DK, Larsson A, Linn S, Lopez A, Lotufo PA, El Razek HMA, Malekzadeh R, Mazidi M, Meier T, Meles KG, Mensah G, Meretoja A, Mezgebe H, Miller T, Mirrakhimov E, Mohammed S, Moran AE, Musa KI, Narula J, Neal B, Ngalesoni F, Nguyen G, Obermeyer CM, Owolabi M, Patton G, Pedro J, Qato D, Qorbani M, Rahimi K, Rai RK, Rawaf S, Ribeiro A, Safiri S, Salomon JA, Santos I, Santric Milicevic M, Sartorius B, Schutte A, Sepanlou S, Shaikh MA, Shin MJ, Shishehbor M, Shore H, Silva DAS, Sobngwi E, Stranges S, Swaminathan S, Tabares-Seisdedos R, Tadele Atnafu N, Tesfay F, Thakur JS, Thrift A, Topor-Madry R, Truelsen T, Tyrovolas S, Ukwaja KN, Uthman O, Vasankari T, Vlassov V, Vollset SE, Wakayo T, Watkins D, Weintraub R, Werdecker A, Westerman R, Wiysonge CS, Wolfe C, Workicho A, Xu G, Yano Y, Yip P, Yonemoto N, Younis M, Yu C, Vos T, Naghavi M, Murray C, 2017. Global, Regional, and National Burden of Cardiovascular Diseases for 10 Causes, 1990 to 2015. J Am Coll Cardiol 70(1), 1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan JL, Tulloch NL, Razumova MV, Saiget M, Muskheli V, Pabon L, Reinecke H, Regnier M, Murry CE, 2016. Mechanical Stress Conditioning and Electrical Stimulation Promote Contractility and Force Maturation of Induced Pluripotent Stem Cell-Derived Human Cardiac Tissue. Circulation 134(20), 1557–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadeghi AH, Shin SR, Deddens JC, Fratta G, Mandla S, Yazdi IK, Prakash G, Antona S, Demarchi D, Buijsrogge MP, Sluijter JPG, Hjortnaes J, Khademhosseini A, 2017. Engineered 3D Cardiac Fibrotic Tissue to Study Fibrotic Remodeling. Adv Healthc Mater 6(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahoo S, Losordo DW, 2014. Exosomes and cardiac repair after myocardial infarction. Circ Res 114(2), 333–344. [DOI] [PubMed] [Google Scholar]
- Sanchez PL, Fernandez-Santos ME, Costanza S, Climent AM, Moscoso I, Gonzalez-Nicolas MA, Sanz-Ruiz R, Rodriguez H, Kren SM, Garrido G, Escalante JL, Bermejo J, Elizaga J, Menarguez J, Yotti R, Perez del Villar C, Espinosa MA, Guillem MS, Willerson JT, Bernad A, Matesanz R, Taylor DA, Fernandez-Aviles F, 2015. Acellular human heart matrix: A critical step toward whole heart grafts. Biomaterials 61, 279–289. [DOI] [PubMed] [Google Scholar]
- Sanchis-Gomar F, Perez-Quilis C, Leischik R, Lucia A, 2016. Epidemiology of coronary heart disease and acute coronary syndrome. Ann Transl Med 4(13), 256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savarese G, Lund LH, 2017. Global Public Health Burden of Heart Failure. Card Fail Rev 3(1), 7–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaaf S, Shibamiya A, Mewe M, Eder A, Stohr A, Hirt MN, Rau T, Zimmermann WH, Conradi L, Eschenhagen T, Hansen A, 2011. Human engineered heart tissue as a versatile tool in basic research and preclinical toxicology. PLoS One 6(10), e26397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwan J, Kwaczala AT, Ryan TJ, Bartulos O, Ren Y, Sewanan LR, Morris AH, Jacoby DL, Qyang Y, Campbell SG, 2016. Anisotropic engineered heart tissue made from laser-cut decellularized myocardium. Sci Rep 6, 32068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seif-Naraghi SB, Singelyn JM, Salvatore MA, Osborn KG, Wang JJ, Sampat U, Kwan OL, Strachan GM, Wong J, Schup-Magoffin PJ, Braden RL, Bartels K, DeQuach JA, Preul M, Kinsey AM, DeMaria AN, Dib N, Christman KL, 2013. Safety and efficacy of an injectable extracellular matrix hydrogel for treating myocardial infarction. Sci Transl Med 5(173), 173ra125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senel Ayaz HG, Perets A, Ayaz H, Gilroy KD, Govindaraj M, Brookstein D, Lelkes PI, 2014. Textile-templated electrospun anisotropic scaffolds for regenerative cardiac tissue engineering. Biomaterials 35(30), 8540–8552. [DOI] [PubMed] [Google Scholar]
- Shadrin IY, Allen BW, Qian Y, Jackman CP, Carlson AL, Juhas ME, Bursac N, 2017. Cardiopatch platform enables maturation and scale-up of human pluripotent stem cell-derived engineered heart tissues. Nat Commun 8(1), 1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu T, Sekine H, Yamato M, Okano T, 2009. Cell sheet-based myocardial tissue engineering: new hope for damaged heart rescue. Curr Pharm Des 15(24), 2807–2814. [DOI] [PubMed] [Google Scholar]
- Shimizu T, Yamato M, Isoi Y, Akutsu T, Setomaru T, Abe K, Kikuchi A, Umezu M, Okano T, 2002. Fabrication of pulsatile cardiac tissue grafts using a novel 3-dimensional cell sheet manipulation technique and temperature-responsive cell culture surfaces. Circ Res 90(3), e40. [DOI] [PubMed] [Google Scholar]
- Shimizu T, Yamato M, Kikuchi A, Okano T, 2003. Cell sheet engineering for myocardial tissue reconstruction. Biomaterials 24(13), 2309–2316. [DOI] [PubMed] [Google Scholar]
- Shin SR, Zihlmann C, Akbari M, Assawes P, Cheung L, Zhang K, Manoharan V, Zhang YS, Yuksekkaya M, Wan KT, Nikkhah M, Dokmeci MR, Tang XS, Khademhosseini A, 2016. Reduced Graphene Oxide-GelMA Hybrid Hydrogels as Scaffolds for Cardiac Tissue Engineering. Small 12(27), 3677–3689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spang MT, Christman KL, 2018. Extracellular matrix hydrogel therapies: In vivo applications and development. Acta Biomater 68, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stohr A, Friedrich FW, Flenner F, Geertz B, Eder A, Schaaf S, Hirt MN, Uebeler J, Schlossarek S, Carrier L, Hansen A, Eschenhagen T, 2013. Contractile abnormalities and altered drug response in engineered heart tissue from Mybpc3-targeted knock-in mice. J Mol Cell Cardiol 63, 189–198. [DOI] [PubMed] [Google Scholar]
- Takawale A, Sakamuri SS, Kassiri Z, 2015. Extracellular matrix communication and turnover in cardiac physiology and pathology. Compr Physiol 5(2), 687–719. [DOI] [PubMed] [Google Scholar]
- Tang J, Shen D, Caranasos TG, Wang Z, Vandergriff AC, Allen TA, Hensley MT, Dinh PU, Cores J, Li TS, Zhang J, Kan Q, Cheng K, 2017. Therapeutic microparticles functionalized with biomimetic cardiac stem cell membranes and secretome. Nat Commun 8, 13724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor DA, Atkins BZ, Hungspreugs P, Jones TR, Reedy MC, Hutcheson KA, Glower DD, Kraus WE, 1998. Regenerating functional myocardium: improved performance after skeletal myoblast transplantation. Nat Med 4(8), 929–933. [DOI] [PubMed] [Google Scholar]
- Tiburcy M, Hudson JE, Balfanz P, Schlick S, Meyer T, Chang Liao ML, Levent E, Raad F, Zeidler S, Wingender E, Riegler J, Wang M, Gold JD, Kehat I, Wettwer E, Ravens U, Dierickx P, van Laake LW, Goumans MJ, Khadjeh S, Toischer K, Hasenfuss G, Couture LA, Unger A, Linke WA, Araki T, Neel B, Keller G, Gepstein L, Wu JC, Zimmermann WH, 2017. Defined Engineered Human Myocardium With Advanced Maturation for Applications in Heart Failure Modeling and Repair. Circulation 135(19), 1832–1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirziu D, Giordano FJ, Simons M, 2010. Cell communications in the heart. Circulation 122(9), 928–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbull IC, Karakikes I, Serrao GW, Backeris P, Lee JJ, Xie C, Senyei G, Gordon RE, Li RA, Akar FG, Hajjar RJ, Hulot JS, Costa KD, 2014. Advancing functional engineered cardiac tissues toward a preclinical model of human myocardium. FASEB J 28(2), 644–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbull IC, Mayourian J, Murphy JF, Stillitano F, Ceholski DK, Costa KD, 2018. Cardiac Tissue Engineering Models of Inherited and Acquired Cardiomyopathies. Methods Mol Biol 1816, 145–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungerleider JL, Christman KL, 2014. Concise review: injectable biomaterials for the treatment of myocardial infarction and peripheral artery disease: translational challenges and progress. Stem Cells Transl Med 3(9), 1090–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Berlo JH, Kanisicak O, Maillet M, Vagnozzi RJ, Karch J, Lin SC, Middleton RC, Marban E, Molkentin JD, 2014. c-kit+ cells minimally contribute cardiomyocytes to the heart. Nature 509(7500), 337–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandamme TF, 2014. Use of rodents as models of human diseases. J Pharm Bioallied Sci 6(1), 2–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenburgh HH, Karlisch P, 1989. Longitudinal growth of skeletal myotubes in vitro in a new horizontal mechanical cell stimulator. In Vitro Cell Dev Biol 25(7), 607–616. [DOI] [PubMed] [Google Scholar]
- Wang B, Zhao L, Fish M, Logan CY, Nusse R, 2015. Self-renewing diploid Axin2(+) cells fuel homeostatic renewal of the liver. Nature 524(7564), 180–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, McCain ML, Yang L, He A, Pasqualini FS, Agarwal A, Yuan H, Jiang D, Zhang D, Zangi L, Geva J, Roberts AE, Ma Q, Ding J, Chen J, Wang DZ, Li K, Wang J, Wanders RJ, Kulik W, Vaz FM, Laflamme MA, Murry CE, Chien KR, Kelley RI, Church GM, Parker KK, Pu WT, 2014. Modeling the mitochondrial cardiomyopathy of Barth syndrome with induced pluripotent stem cell and heart-on-chip technologies. Nat Med 20(6), 616–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LL, Liu Y, Chung JJ, Wang T, Gaffey AC, Lu M, Cavanaugh CA, Zhou S, Kanade R, Atluri P, Morrisey EE, Burdick JA, 2017. Local and sustained miRNA delivery from an injectable hydrogel promotes cardiomyocyte proliferation and functional regeneration after ischemic injury. Nat Biomed Eng 1, 983–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang WE, Li L, Xia X, Fu W, Liao Q, Lan C, Yang D, Chen H, Yue R, Zeng C, Zhou L, Zhou B, Duan DD, Chen X, Houser SR, Zeng C, 2017. Dedifferentiation, Proliferation, and Redifferentiation of Adult Mammalian Cardiomyocytes After Ischemic Injury. Circulation 136(9), 834–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger F, Breckwoldt K, Pecha S, Kelly A, Geertz B, Starbatty J, Yorgan T, Cheng KH, Lessmann K, Stolen T, Scherrer-Crosbie M, Smith G, Reichenspurner H, Hansen A, Eschenhagen T, 2016. Cardiac repair in guinea pigs with human engineered heart tissue from induced pluripotent stem cells. Sci Transl Med 8(363), 363ra148. [DOI] [PubMed] [Google Scholar]
- Weinberger F, Mannhardt I, Eschenhagen T, 2017. Engineering Cardiac Muscle Tissue: A Maturating Field of Research. Circ Res 120(9), 1487–1500. [DOI] [PubMed] [Google Scholar]
- Wendel JS, Ye L, Tao R, Zhang J, Zhang J, Kamp TJ, Tranquillo RT, 2015. Functional Effects of a Tissue-Engineered Cardiac Patch From Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes in a Rat Infarct Model. Stem Cells Transl Med 4(11), 1324–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weymann A, Loganathan S, Takahashi H, Schies C, Claus B, Hirschberg K, Soos P, Korkmaz S, Schmack B, Karck M, Szabo G, 2011. Development and evaluation of a perfusion decellularization porcine heart model--generation of 3-dimensional myocardial neoscaffolds. Circ J 75(4), 852–860. [DOI] [PubMed] [Google Scholar]
- Williams C, Quinn KP, Georgakoudi I, Black LD 3rd, 2014. Young developmental age cardiac extracellular matrix promotes the expansion of neonatal cardiomyocytes in vitro. Acta Biomater 10(1), 194–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wippermann J, Schumann D, Klemm D, Kosmehl H, Salehi-Gelani S, Wahlers T, 2009. Preliminary results of small arterial substitute performed with a new cylindrical biomaterial composed of bacterial cellulose. Eur J Vasc Endovasc Surg 37(5), 592–596. [DOI] [PubMed] [Google Scholar]
- Wolska BM, Solaro RJ, 1996. Method for isolation of adult mouse cardiac myocytes for studies of contraction and microfluorimetry. Am J Physiol 271(3 Pt 2), H1250–1255. [DOI] [PubMed] [Google Scholar]
- Yang L, van der Werf KO, Koopman BF, Subramaniam V, Bennink ML, Dijkstra PJ, Feijen J, 2007. Micromechanical bending of single collagen fibrils using atomic force microscopy. J Biomed Mater Res A 82(1), 160–168. [DOI] [PubMed] [Google Scholar]
- Yildirim Y, Naito H, Didie M, Karikkineth BC, Biermann D, Eschenhagen T, Zimmermann WH, 2007. Development of a biological ventricular assist device: preliminary data from a small animal model. Circulation 116(11 Suppl), I16–23. [DOI] [PubMed] [Google Scholar]
- Young JL, Engler AJ, 2011. Hydrogels with time-dependent material properties enhance cardiomyocyte differentiation in vitro. Biomaterials 32(4), 1002–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Ye K, Sullivan KE, Black LD, 2011. Encapsulation of cardiomyocytes in a fibrin hydrogel for cardiac tissue engineering. J Vis Exp(55). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yui Y, 2018. Concerns on a new therapy for severe heart failure using cell sheets with skeletal muscle or myocardial cells from iPS cells in Japan. NPJ Regen Med 3, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacharias WJ, Frank DB, Zepp JA, Morley MP, Alkhaleel FA, Kong J, Zhou S, Cantu E, Morrisey EE, 2018. Regeneration of the lung alveolus by an evolutionarily conserved epithelial progenitor. Nature 555(7695), 251–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Montgomery M, Chamberlain MD, Ogawa S, Korolj A, Pahnke A, Wells LA, Masse S, Kim J, Reis L, Momen A, Nunes SS, Wheeler AR, Nanthakumar K, Keller G, Sefton MV, Radisic M, 2016. Biodegradable scaffold with built-in vasculature for organ-on-a-chip engineering and direct surgical anastomosis. Nat Mater 15(6), 669–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Shadrin IY, Lam J, Xian HQ, Snodgrass HR, Bursac N, 2013. Tissue-engineered cardiac patch for advanced functional maturation of human ESC-derived cardiomyocytes. Biomaterials 34(23), 5813–5820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Kong CW, Tong MH, Chooi WH, Huang N, Li RA, Chan BP, 2017. Maturation of human embryonic stem cell-derived cardiomyocytes (hESC-CMs) in 3D collagen matrix: Effects of niche cell supplementation and mechanical stimulation. Acta Biomater 49, 204–217. [DOI] [PubMed] [Google Scholar]
- Zhou P, Pu WT, 2016. Recounting Cardiac Cellular Composition. Circ Res 118(3), 368–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann WH, Fink C, Kralisch D, Remmers U, Weil J, Eschenhagen T, 2000. Three-dimensional engineered heart tissue from neonatal rat cardiac myocytes. Biotechnol Bioeng 68(1), 106–114. [PubMed] [Google Scholar]
- Zimmermann WH, Melnychenko I, Wasmeier G, Didie M, Naito H, Nixdorff U, Hess A, Budinsky L, Brune K, Michaelis B, Dhein S, Schwoerer A, Ehmke H, Eschenhagen T, 2006. Engineered heart tissue grafts improve systolic and diastolic function in infarcted rat hearts. Nat Med 12(4), 452–458. [DOI] [PubMed] [Google Scholar]
- Zimmermann WH, Schneiderbanger K, Schubert P, Didie M, Munzel F, Heubach JF, Kostin S, Neuhuber WL, Eschenhagen T, 2002. Tissue engineering of a differentiated cardiac muscle construct. Circ Res 90(2), 223–230. [DOI] [PubMed] [Google Scholar]