Abstract
This study intended to investigate the therapeutic effect of ethanolic extract of Tylophora villosa leaves (E2TL) against paracetamol (PC)-induced hepatotoxicity (PCIH) in mice (Mus musculus). PCIH were generated using daily 250 mg/kg body weight (bw) PC administration by gavage for seven days, and then daily 27.5; 55.0; 82.5; 110.0; or 220.0 mg/kg bw E2TL were treated by gavage for seven or fourteen days. Meanwhile, the controls were given solvent only in the same manner. Mortality, blood glucose, and condition (color, weight, volume) of the livers were observed on day 15 (D15). Serum glutamate pyruvate transaminase (SGPT) and serum glutamate oxaloacetate transaminase (SG0T) were examined on D15, D22, and D30, and then malondialdehyde (MDA) was determined on D15. Results of this study revealed that on D15, the dosage of 110.0 mg/kg bw E2TL most effectively decreased MDA due to PCIH, from 6.78 ± 1.70 μmol/L to 3.45 ± 0.43 μmol/L, approaching the control condition (2.45 ± 0.05 μmol/L). PC administration was really toxic dosage and caused 13.3 % mortality. Blood glucose, weight, and volume of the liver decreased as the effect of PC administration, and then 220.0 mg/kg bw E2TL treatment could recover the condition as well as the controls. Color of the liver indicated a similar recovery by E2TL treatment. SGPT and SG0T increased significantly by PC administration, and this PCIH facts could be recovered gradually near the controls according to the dosages (55.0; 110.0; or 220.0 mg/kg bw) and duration (seven or fourteen days) of E2TL treatment. It could be concluded that E2TL showed therapeutic effect against PCIH in M. musculus.
Keywords: Tylophoravillosa, Paracetamol-induced hepatotoxicity, Therapeutic effects, M. musculus
Graphical abstract
1. Introduction
Hepatotoxicity is a prevalent problem in Sumatra, Indonesia. Drug-induced hepatotoxicity (DIH) is a complication that occurs in the human liver which impacts the function of the liver caused by drugs. It is very likely to occur in the liver because the organ is the central of drugs metabolism. The disease is related to the way the toxin of a drug reacts to the body so that DIH is the main reason of drug withdrawal from markets.1 The cause of DIH is non-steroidal anti-inflammatory drugs (NSAIDs), such as paracetamol (PC). PC is a commonly used as analgesic and antipyretic drug.2 It is safe at therapeutic levels, but at high doses it can lead to undesirable side effects such as PC-induced hepatotoxicity (PCIH3;). In PCIH the amount of reactive oxygen species (ROS) will increase in mitochondria and impact the emergence of oxidative stress (OS). OS causes damage to cell membranes by morphological and biochemical changes, followed by the malfunctioning cells and it is ended by hepatocyte death that leads to apoptosis and necrosis of liver cells. Morphological changes that is preceded by the OS induces lipid peroxidation and malondialdehyde (MDA) as the final product.4, 5 The presence of hepatocellular injury can also be detected by measuring the level of two hepatic enzymes, namely serum glutamate pyruvate transaminase (SGPT) and serum glutamate oxaloacetate transaminase (SGOT).6, 7
Developing new drugs requires concept and methodology of natural products and traditional medicine through study of relationship between natural products, traditional medicines, and modern medicines.8 The use of herbal medicines for treating various diseases, including liver disease, is currently very popular.9 A number of reports are available which have shown hepatoprotective effects of natural plant products against toxic substances including PC.10, 11 Natural antioxidant products are increasingly being used to treat various pathological liver injuries because of the role of oxidative stress in their pathogenesis. Aqueous extract of Auricularia polytricha (AEAP), for example, has been used as food or medicine due to its antioxidant activity. PC increased significantly the SGPT and SGOT; furthermore, this PC-induced hepatotoxicity could be attenuated significantly by AEAP administration. These facts indicate that the AEAP has significant protective effect against PCIH due to its potent antioxidant activity.12 It was reported that flavonoids which play a role as antioxidants in biological systems are natural phenolic compounds present in fruit and vegetable species.13 The Flavonoids have many useful effects that include their being antioxidants and having anti-bacterial, anti-cancer, anti-mutagenic, and anti-inflammatory properties.14, 15
Residents of Lubuklinggau, South Sumatra traditionally use Nangka Kuning (Tylophora villosa Blume; http://www.theplantlist.org/tpl/record/tro-2602757) as drug to treat diseases associated with impaired function of the liver (http://mogasehat.blogspot.co id/2013/12/drug-natural-liver-disease-or-hepatitis.html). Since it has been widely accepted that T. villosa is an ethnomedicinal plant, traditionally known for curing several ailments such as liver diseases, the plant is not only taken from nature, but it has also been planted in the garden as an ornamental plant. This fact is an interesting challenge for researchers to immediately start collecting scientific data on this candidate medicinal plant. It was reported that leaves of T. villosa contain alkaloids, flavonoids, and triterpenoids and this is the reason why this plant can be used as an ethnomedicinal plant. Meanwhile, identification results using spectroscopy and 1H-NMR spectroscopy indicated that ethanolic extract of T. villosa leaves (E2TL) contains the compounds of tylophorine (C24H27NO4). Furthermore results of toxicity tests using Artemia salina Leach by the same researchers showed that E2TL has a LC50 at 124 ppm.16 There are still some things that should be examined from E2TL. Based on these considerations we believe that preliminary studies on therapeutic effect of E2TL against PCIH in mice (Mus musculus) should be performed.
2. Materials and methods
2.1. Preparation of crude extract
Leaf sample of T. villosa (http://www.theplantlist.org/tpl/record/tro-2602757; plant identification was verified with the help of Research of Biology, Indonesian Institute of Sciences; http://lipi.go.id/) as research plant materials were collected from the surroundings of Lubuklinggau, South Sumatra. Selected leaves located at the base of the stem were then washed and cut into small pieces. T. villosa fresh leaves were wind-dried for 7 days to get 250 g of dried leaves which then were crushed into fine powder. The leaf powder was macerated in 200 mL ethanol (80%) for 24 h at room temperature, and the resulting filtrate was concentrated by rotary evaporator17 so that the concentrated extract, the ethanolic extract of T. villosa leaves (E2TL), was ready to be used as test material of this research.18
2.2. Phytochemical tests
Phytochemical tests were conducted on E2TL. The crude extract samples were tested to determine their contents of terpenoids, flavonoids, steroids, alkaloids, saponins, and tannins using the standard procedures.19
2.3. Tested chemicals
Paracetamol (PC) in the form of Paracetamol Afi Farma 500 mg Tablet was purchased from PT AFI FARMA (https://www.goapotik.com/paracetamol-afi-farma-500-mg-tablet.html), Kediri, Indonesia.
2.4. Dosages of investigation
It has been reported that PC lethal dosage in mice is 400 mg/kg body weight (bw; https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4917664/), and PC toxicity were generated in this research using daily 250 mg/kg bw PC administration by gavage for seven days. T. villosa leaf is traditionally used as a herbal medicine for liver dysfunction at a dosage of 15 g per day for an adult weighing 50 kg,20 so an adult weighing 70 kg is recommended to consume 21 g. Since the conservation factor from 70 kg human to 20 g mice is 0.0026,21 the dosage for mice 0.0026 × 21 g is 55 mg. Based on the calculations, this study used five doses, namely 27.5; 55.0; 82.5; 110.0; or 220.0 mg/kg bw E2TL were treated by gavage for seven or fourteen days.
2.5. Experimental animals
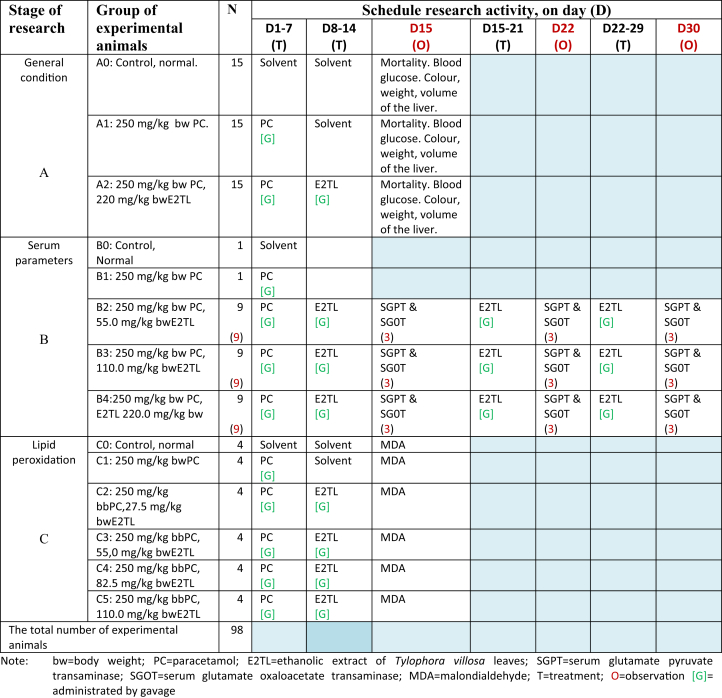
Swiss Webster mice (Mus musculus) were used as experimental animals with regard to local regulations established by the Institutional Animal Care and Use Committee (IACUC) of Bengkulu University (Komisi Bioetika Universitas Bengkulu). This study paid attention to the ethical use of animals including aspects of the humane treatment of animals, in accordance with the principle of 5F (Freedom), namely; (a) free from hunger and thirst, (b) free from discomfort, (c) free from pain, injury and disease, (d) free from fear and the long-term stress, (e) freely express behavior naturally, given the space and appropriate facilities.22 The animals were reared in a room at 23–27 °C, and 83 % humidity. Both food and water were given ad libitum.23, 24 Total number of male M. musculus aged 6–8 weeks with 25–35 g body weight (bw18;) used in PC detoxification on the liver of M. musculus by E2TL administration was 98 male animals.25 The male animals were used in this research to avoid individual variation derived from Mouse Estrous Cycle. This research was conducted in three stages of research (A, B and C; Table 1).
Table 1.
Experimental design to investigate therapeutic effect of ethanolic extract of Tylophoravillosa leaves (E2TL) against PC-induced hepatotoxicity (PCIH) of mice (Mus musculus). Both control and treated animals obtained ad libitum standard food and drink.
2.6. General condition (A)
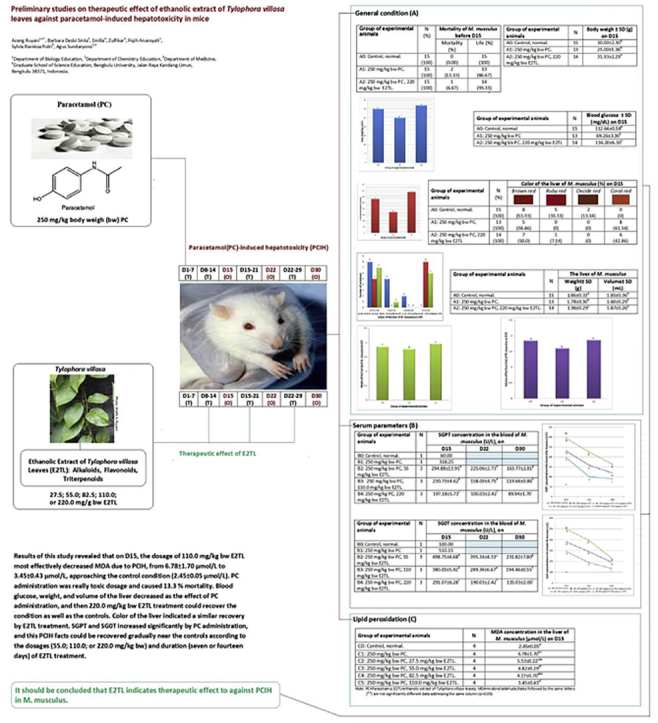
The forty-five experimental animals were divided into three groups (A0, A1, and A2), each consisting of fifteen male M. musculus. On day 1–7 (D1-7), 250 mg/kg bw PC was administered daily by gavage on A1 group. On D1-7, 250 mg/kg bw PC was administered daily by gavage, and then on D 8-14 220 mg/kg bw E2TL was given daily by gavage in A2 group. A0 group as the controls of investigation were administered solvent only in the same manner. Mortality of M. musculus was observed before D15, blood samples were collected on D15 from the tails for blood glucose determination using the Glucose test (multiCarein; http://www.biosys.it/en/pr_multicarein.html), and then both control and treated animals were sacrificed by cervical dislocation (CD) on D15 (Table 1). The animals were dissected immediately, and then their liver was separated from other organs to determine its color, weight, and volume.
2.7. Serum parameters (B)
The twenty-nine experimental animals were divided into five groups (B0, B1, B2, B3, and B4), B0 and B1, each consisting of one, while B2, B3, and B4, each consisting of nine male M. musculus. On D1-7, 250 mg/kg bw PC was administered daily by gavage in B1 group. On D1-7, 250 mg/kg bw PC was administered daily by gavage, and then on D 8-29, 55.0 mg/kg bw E2TL was given daily by gavage on B2 group. On D1-7, 250 mg/kg bw PC was administered daily by gavage, and then On D 8-29, 110.0 mg/kg bw E2TL was given daily by gavage in B3 group. On D1-7, 250 mg/kg bw PC was administered daily by gavage, and then On D8-29, 220.0 mg/kg bw E2TL was given daily by gavage in B4 group. B0 group as the controls of investigation were administered solvent only in the same manner. M. musculus were sacrificed by CD on D15, D22, or D30 (Table 1). The animals were dissected immediately, and blood samples were collected from the hearts. Briefly, the blood was allowed to stand for sometime followed by centrifugation at 2400 rpm for 20 min. Clear supernatant so obtained was designated as serum. Various serum parameters, namely serum glutamate oxaloacetate transaminase (SGOT) and Serum glutamate pyruvate transaminase (SGPT), were measured on Autoanalyzer (Erba Mannheim XL-640) using kits (Erba Mannheim XL System Packs26;).
2.8. Lipid peroxidation (C)
The twenty-four experimental animals were divided into six groups (C0, C1, C2, C3, C4, and C5), each consisting of four males M. musculus. On D1-7, 250 mg/kg bw PC was administered daily by gavage in C1 group. On D1-7, 250 mg/kg bw PC was administered daily by gavage, and then on D 8-14 27.5 mg/kg bw E2TL was given daily by gavage in C2 group. On D1-7, 250 mg/kg bw PC was administered daily by gavage, and then on D 8-14, 55.0 mg/kg bw E2TL was given daily by gavage in C3 group. On D1-7, 250 mg/kg bw PC was administered daily by gavage, and then on D 8-14, 82.5 mg/kg bw E2TL was given daily by gavage in C4 group. On D1-7, 250 mg/kg bw PC was administered daily by gavage, and then on D 8-14, 110.0 mg/kg bw E2TL was given daily by gavage in C5 group. C0 group as the controls of investigation were administered solvent only in the same manner. M. musculus were sacrificed by CD on D15 (Table 1). The animals were dissected immediately, and then their liver was separated from other organs to determine the MDA. The generation of MDA was determined as the indicator of lipid peroxidation using a Bioxytech MDA-586 Kit (Oxis Research, Portland, OR, USA). Briefly, the supernatants of liver homogenate were mixed with probucol, N-methyl-2-phenylindole, and hydrochloric acid to produce a stable dye. After centrifugation, the absorbance of the clear supernatant was measured using a spectrometry at 586 nm. The concentration of MDA was plotted against a standard curve and expressed in n mole per milligram of wet tissue.27
3. Results
3.1. Phytochemicals
Phytochemical test results in this study showed that E2TL contained alkaloids, flavonoids, and triterpenoids (Table 2), and the result was the same as it had been done before.16 These reproducible results were found because the E2TL were produced through the same protocol.
Table 2.
Results of different phytochemicals present in ethanolic extract of Tylophora villosa leaves (E2TL).
| No. | Phytochemical test | Results |
|---|---|---|
| 1 | Alkaloids | Positive (+) |
| 2 | Flavonoids | Positive (+) |
| 3 | Saponins | Negative (-) |
| 4 | Triterpenoids | Positive (+) |
| 5 | Steroids | Negative (-) |
3.2. The liver of M. musculus
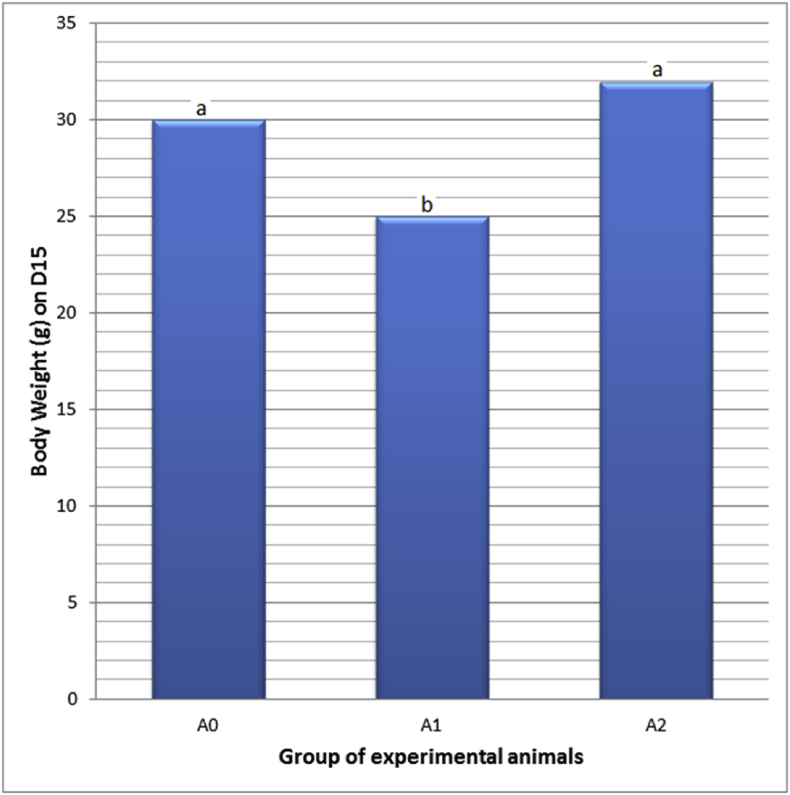
A condition of PC toxicity was generated by 250 mg/kg bw PC daily administration for seven days (D1-7), and the administration caused the death of two mice (13.33%). Meanwhile, the same PC administration which was followed by 220 mg/kg bw E2TL daily treatment during seven days (D8-14) could reduce mortality into one mouse (6.67%; Table 3). The facts showed that the PC administration stimulated the condition of PC toxicity in mice, and then level of the toxicity could be decreased by E2TL treatment. In general it revealed that E2TL indicates potential therapeutic effect against PC toxicity. The PC administration decreased body weight (25.00 ± 1.36 g), lower than the controls' (30.00 ± 2.30 g). Meanwhile, when the PC administration was followed by treatments with E2TL for seven days, the body weight increased again (32.00 ± 2.29 g), approaching the control's (Table 4). This fact is further evidence that E2TL is able to restore weight loss due to the PC administration towards the normal conditions (Fig. 1).
Table 3.
Mortality of M. musculus before day 15 (D15), which previously, on D1-7, received daily 250 mg/kg bw PC (A1), on D1-7 received daily 250 mg/kg bw PC and on D8-14 were given daily 220 mg/kg bw E2TL (A2), while the controls received only the solvent in the same manner (A0).
| Group of experimental animals | N (%) |
Mortality of M. musculus before D15 |
|
|---|---|---|---|
| Mortality (%) | Life (%) | ||
| A0: Control, normal. | 15 (100) |
0 (0.00) |
15 (100) |
| A1: 250 mg/kg bw PC. | 15 (100) |
2 (13.33) |
13 (86.67) |
| A2: 250 mg/kg bw PC, 220 mg/kg bw E2TL. | 15 (100) |
1 (6.67) |
14 (93.33) |
Note: PC=Paracetamo; E2TL = ethanolic extract of Tylophora villosa leaves; = 2.14; There are significant differences in mortality on A0, A1, and A2 (p < 0.05).
Table 4.
Body weight of M. musculus on day 15 (D15), which previously, on D1-7, received daily 250 mg/kg bw PC (A1), on D1-7 received daily 250 mg/kg bw PC and on D 8-14 were given daily 220 mg/kg bw E2TL (A2), while the controls received only the solvent in the same manner (A0).
| Group of experimental animals | N | Body weigh ± SD (g) on D15 |
|---|---|---|
| A0: Control, normal. | 15 | 30.00 ± 2.30a |
| A1: 250 mg/kg bw PC. | 13 | 25.00 ± 1.36b |
| A2: 250 mg/kg bw PC, 220 mg/kg bw E2TL. | 14 | 31.93 ± 2.29a |
Note: PC=Paracetamo; E2TL = ethanolic extract of Tylophora villosa leaves; Rates followed by the same letters (a, b) are not significantly different data addressing the same column (p < 0.05).
Fig. 1.
Body weight of M. musculus on day 15 (D15), which previously, on D1-7, received daily 250 mg/kg bw PC (A1), on D1-7 received daily 250 mg/kg bw PC and on D 8-14 were given daily 220 mg/kg bw E2TL (A2), while the controls received only the solvent in the same manner (A0). Rates followed by the same letters (a, b) are not significantly different data addressing the same column (p < 0.05).
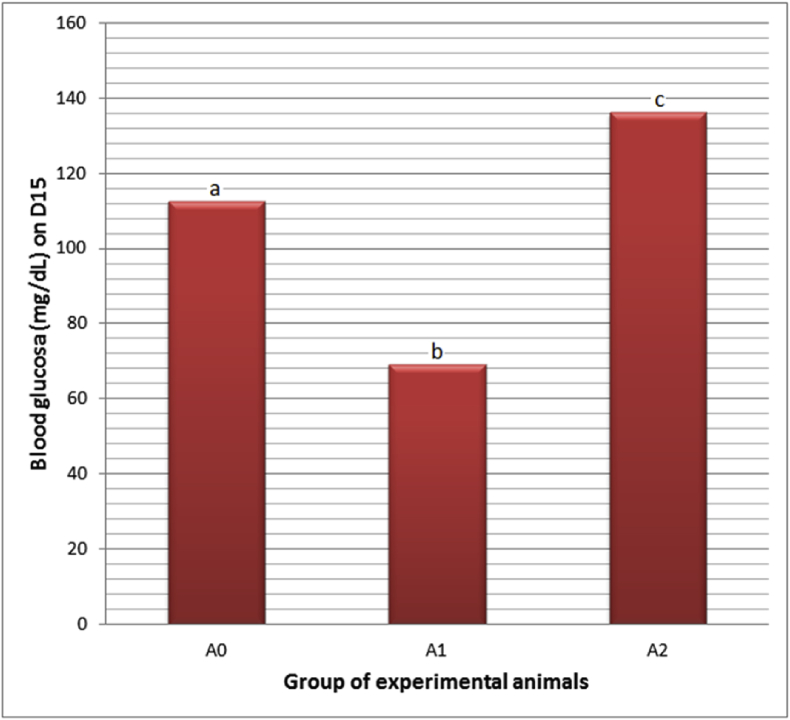
The PC administration reduced blood glucose (70.00 ± 3.30 mg/dL), lower than the controls' (120.00 ± 0.58 mg/dL). Meanwhile, when the PC administration was followed by E2TL treatments for seven days, they raised blood glucose (130.00 ± 6.30 mg/dL), close to control (Table 5). These facts revealed that E2TL was able to recover a decrease in blood glucose due to PC administration to the normal circumstances (Fig. 2).
Table 5.
Blood glucose levels of M. musculus on day 15 (D15), which previosly, on D1-7, received daily 250 mg/kg bw PC (A1), on D1-7 received daily 250 mg/kg bw PC and on D 8-14 were given daily 220 mg/kg bw E2TL (A2), while the controls received only the solvent in the same manner (A0).
| Group of experimental animals | N | Blood glucosa ± SD (mg/dL) on D15 |
|---|---|---|
| A0: Control, normal | 15 | 112.66 ± 0.58a |
| A1: 250 mg/kg bw PC | 13 | 69.26 ± 3.30b |
| A2: 250 mg/kg bb PC, 220 mg/kg bw E2TL | 14 | 136.20 ± 6.30c |
Note: PC=Paracetamo; E2TL = ethanolic extract of Tylophora villosa leaves; α = 0.05 Rates followed by the same letters (a, b) are not significantly different data addressing the same column (p < 0.05).
Fig. 2.
Blood glucose levels of M. musculus on day 15 (D15), which previously, on D1-7, received daily 250 mg/kg bw PC (A1), on D1-7 received daily 250 mg/kg bw PC and on D 8-14 were given daily 220 mg/kg bw E2TL (A2), while the controls received only the solvent in the same manner (A0). Rates followed by the same letters (a, b) are not significantly different data addressing the same column (p < 0.05).
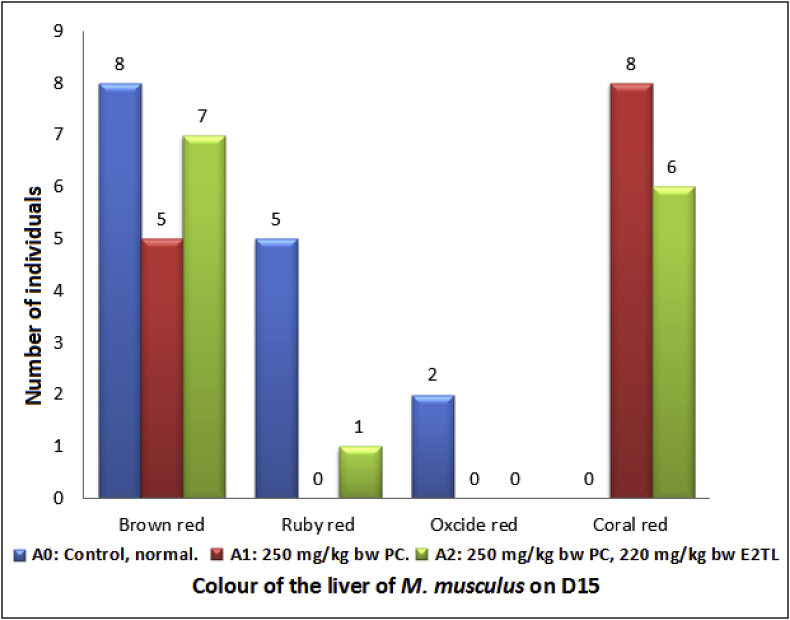
The PC administration also changed a percentage of brown red heart (5; 38.46%; A1), which was lower than the control's (8; 53.33%; A0). Meanwhile, when the PC administration was followed by E2TL treatments for seven days, they could increase the percentage of brown red heart (7; 50.0%; A2), approaching the control condition (Table 6). These facts showed that E2TL was able to recover the percentage of brown red heart as additional information due to PCIH to the normal circumstances (Fig. 3).
Table 6.
Colour of the M. musculus liver on day 15 (D15), which previously, on D1-7, received daily 250 mg/kg bw PC (A1), on D1-7 received daily 250 mg/kg bw PC and on D 8-14 were given daily 220 mg/kg bw E2TL (A2), while the controls received only the solvent in the same manner (A0).
| Group of experimental animals | N (%) |
Colour of the liver of M. musculus (%) on D15 |
|||
|---|---|---|---|---|---|
|
Brown red |
Ruby red |
Oxcide red |
Coral red |
||
 |
 |
 |
 |
||
| A0: Control, normal. | 15 (100) |
8 (53.33) |
5 (33.33) |
2 (13.34) |
0 (0) |
| A1: 250 mg/kg bw PC. | 13 (100) |
5 (38.46) |
0 (0) |
0 (0) |
8 (61.54) |
| A2: 250 mg/kg bw PC, 220 mg/kg bw E2TL | 14 (100) |
7 (50.0) |
1 (7.14) |
0 (0) |
6 (42.86) |
Note: PC=Paracetamo; E2TL = ethanolic extract of Tylophora villosa leaves. = 18.65; There are significant differences in color of the liver on A0, A1, and A2 (p < 0.05).
Fig. 3.
Colour of the M. musculus liver on day 15 (D15), which earlier on D1-7 received daily 250 mg/kg bwPC (A1), on D1-7 received daily 250 mg/kg bw PC and on D 8-14 were given daily 220 mg/kg bw E2TL (A2), while the controls received only the solvent in the same manner (A0). Χ2 = 18.65; There are significant differences in color of the liver on A0, A1, and A2 (p < 0.05).
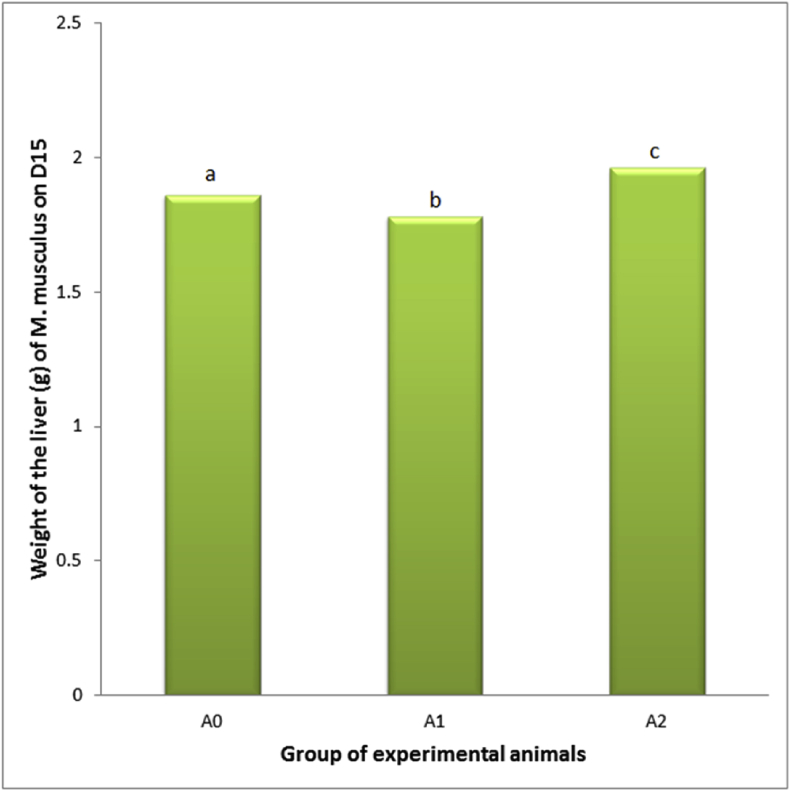
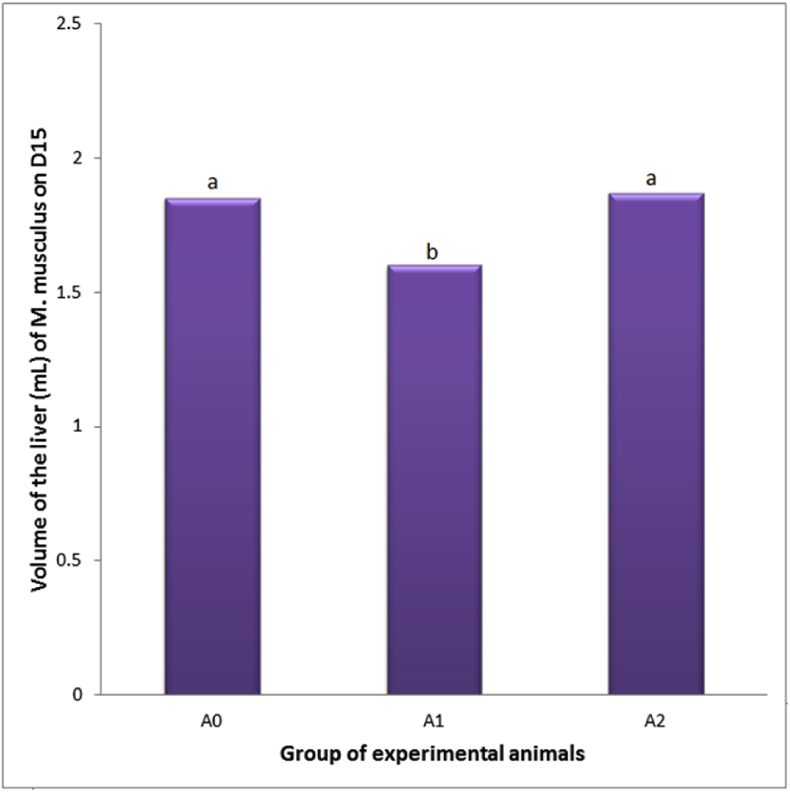
This research revealed that the PC administration decreased weight and volume of the liver (1.78 ± 0.30 g and 1.60 ± 0.29 mL, compared the control which was 1.86 ± 0.33 g and 1.85 ± 0.36 mL). Mmeanwhile, when the PC administration was followed by E2TL treatment for seven days, the weight and volume of the liver increased (1.96 ± 0.29 g and 1.87 ± 0.26 mL), approaching the control condition (Table 7). These facts indicated that E2TL was able to recover the weight and volume of liver in PCIH to the normal circumstances (Fig. 4, Fig. 5).
Table 7.
Weight and volume of the liver of M. musculus on day 15 (D15), which previously, on D1-7, received daily 250 mg/kg bw PC (A1), on D1-7 received daily 250 mg/kg bw PC and on D 8-14 were given daily 220 mg/kg bw E2TL (A2), while the controls received only the solvent in the same manner (A0).
| Group of experimental animals | N | The liver of M. musculus |
|
|---|---|---|---|
| Weight± SD (g) | Volume± SD (mL) | ||
| A0: Control, normal. | 15 | 1.86 ± 0.33a | 1.85 ± 0.36a |
| A1: 250 mg/kg bw PC. | 13 | 1.78 ± 0.30b | 1.60 ± 0.29b |
| A2: 250 mg/kg bw PC, 220 mg/kg bw E2TL. | 14 | 1.96 ± 0.29c | 1.87 ± 0.26a |
Note: PC=Paracetamo; E2TL = ethanolic extract of Tylophora villosa leaves. Rates followed by the same letters (a, b) are not significantly different data addressing the same column (p > 0.05).
Fig. 4.
Weight of the liver of M. musculus on day 15 (D15), which previously, on D1-7, received daily 250 mg/kg bw PC (A1), on D1-7 received daily 250 mg/kg bw PC and on D 8-14 were given daily 220 mg/kg bw E2TL (A2), while the controls received only the solvent in the same manner (A0). Rates followed by the same letters (a, b) are not significantly different data addressing the same column (p < 0.05).
Fig. 5.
Volume of the liver of M. musculus on day 15 (D15), which previosly, on D1-7, received daily 250 mg/kg bw PC (A1), on D1-7 received daily 250 mg/kg bw PC and on D 8-14 were given daily 220 mg/kg bw E2TL (A2), while the controls received only the solvent in the same manner (A0). Rates followed by the same letters (a, b) are not significantly different data addressing the same column (p < 0.05).
3.3. SGPT and SGOT
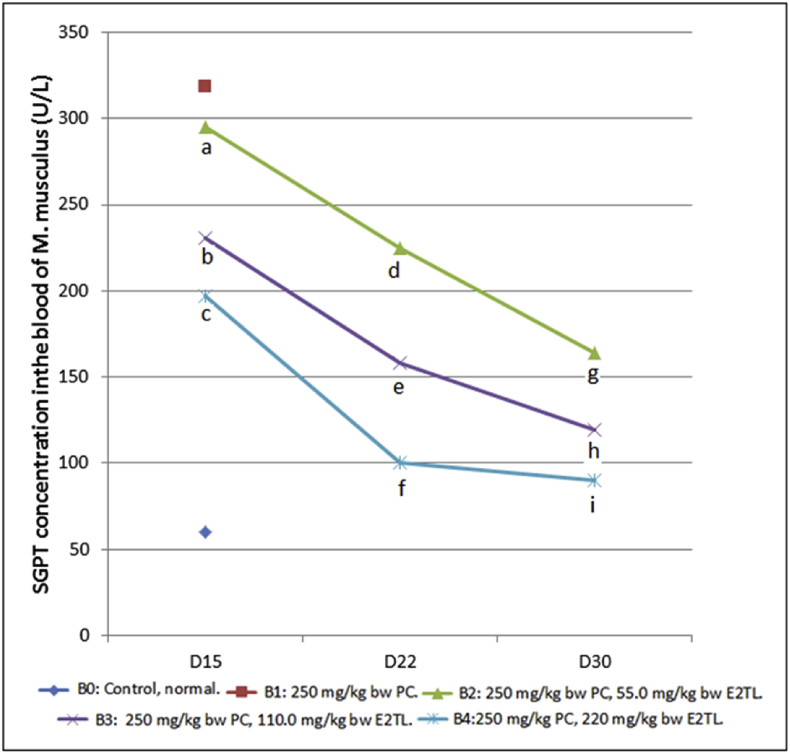
Serum parameter observation revealed the daily 250 mg/kg bw PC administration for seven day (D1-7) which was followed by daily 55.0 E2TL treatments (B2) for seven (D8-14), fourteen (D8-21), twenty-one (D8-29) days decreased SGPT levels to 294.88 ± 13.91 (D15), 225.06 ± 2.73 (D22), 163.77 ± 2.81 (D30) U/L respectively, lower than 318.25 U/L (B1; Positive control). Furthermore, the decline of SGPT levels also occurred in line with two dosages (110; 220 mg/kg bw) E2TL treatment and three time periods (D8-14; D8-21; D8-29) treatment. The highest dosage (220 mg/kg bw) and the longest period of time (D8-29) of E2TL treatment revealed the lowest levels of SGPT, 89.94 ± 1.70 U/L, approaching the value of 60.00 U/L (B0; Control; Table 8). This SGPT observation revealed that E2TL had therapeutic effect against PCIH in retaining SGPT level of M. musculus (Fig. 6).
Table 8.
SGPT levels of M. musculus on day 15, 22, and 30 (D15, D22, and D30) which previously, on D 1-7, received daily 250 mg/kg bw PC (B1), on D1-7 received daily 250 mg/kg bw PC and on D8-29 were given daily 55 (B2), 110 (B3), and 220 (B4) mg/kg bw E2TL respectively, while the controls received only the solvent in the same manner (B0).
| Group of experimental animals | N | SGPT concentration in the blood of M. musculus (U/L), on |
||
|---|---|---|---|---|
| D15 | D22 | D30 | ||
| B0: Control, normal. | 1 | 60.00 | ||
| B1: 250 mg/kg bw PC. | 1 | 318.25 | ||
| B2: 250 mg/kg bw PC, 55 mg/kg bw E2TL. | 3 | 294.88 ± 13.91a | 225.06 ± 2.73d | 163.77 ± 2.81g |
| B3: 250 mg/kg bw PC, 110.0 mg/kg bw E2TL. | 3 | 230.73 ± 4.62b | 158.03 ± 4.75e | 119.66 ± 3.80h |
| B4: 250 mg/kg PC, 220 mg/kg bw E2TL. | 3 | 197.18 ± 5.73c | 100.03 ± 2.42f | 89.94 ± 1.70i |
Note: PC=Paracetamo; E2TL = ethanolic extract of Tylophora villosa leaves; SGPT = serum glutamate pyruvate transaminase; Rates followed by the same letters (a,b) are not significantly different data addressing the same column and the same line (p < 0.05).
Fig. 6.
SGPT levels of M. musculus on day 15, 22, and 30 (D15, D22, and D30) which previously, on D 1-7, received daily 250 mg/kg bw PC (B1), on D1-7 received daily 250 mg/kg bw PC and on D8-21 were given daily 55 (B2), 110 (B3), and 220 (B4) mg/kg bw E2TL respectively, while the controls received only the solvent in the same manner (B0). Rates followed by the same letters (a, b) are not significantly different data addressing the same column and the same line (p < 0.05).
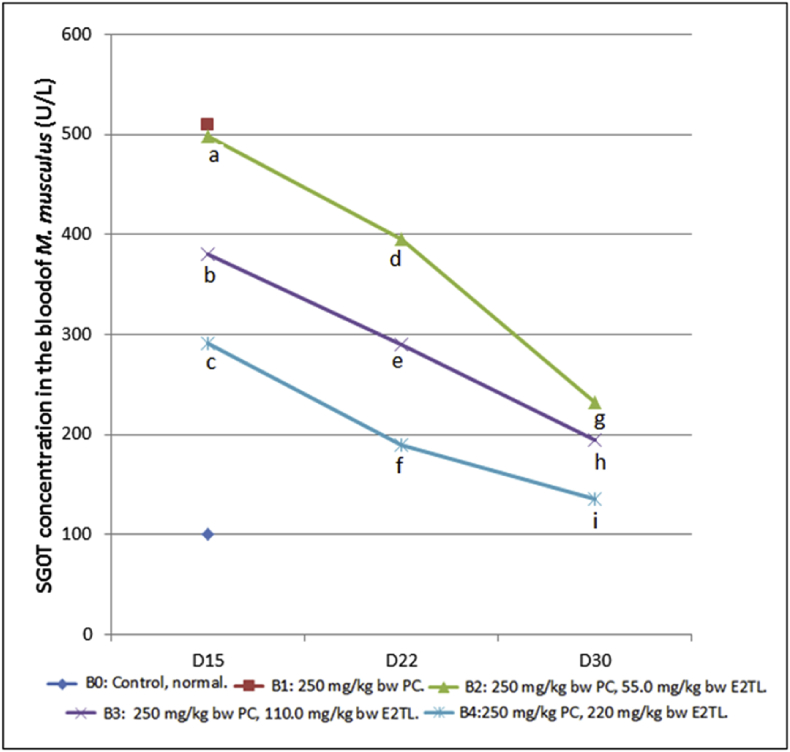
Other serum parameter observation revealed that the daily 250 mg/kg bw PC administration for (D1-7) which was followed by daily 55.0 E2TL treatments (B2) for seven (D8-14), fourteen (D8-21), twenty-one (D8-29) days decreased SGOT levels to 498.75 ± 4.68 (D15), 395.34 ± 4.33 (D22), 231.82 ± 7.80 (D30) U/L respectively lower than 510.15 U/L (B1; Positive control). Furthermore, the decline of SGOT levels also occurred in line with two dosages (110; 220 mg/kg bw) E2TL treatment and three time periods (D8-14; D8-21; D8-29) treatment. In the highest dosage (220 mg/kg bw) and the longest period time (D8-29) of E2TL treatment revealed the lowest levels of SGOT, 135.03 ± 2.00 U/L, approaching the value of 100.00 U/L (B0; Control; Table 9). This SGOT observation revealed that E2TL had therapeutic effect against PCIH in recovering SGOT level of M. musculus (Fig. 7).
Table 9.
SGOT levels of M. musculus on day 15, 22, and 30 (D15, D22, and D30) which previously, on D 1-7, received daily 250 mg/kg bw PC (B1), on D1-7 received daily 250 mg/kg bw PC and on D8-29 were given daily 55 (B2), 110 (B3), and 220 (B4) mg/kgbw E2TL respectively, while the controls received only the solvent in the same manner (B0).
| Group of experimental animals | N | SG0T concentration in the blood of M. musculus (U/L), on |
||
|---|---|---|---|---|
| D15 | D22 | D30 | ||
| B0: Control, normal. | 1 | 100.00 | ||
| B1: 250 mg/kg bw PC | 1 | 510.15 | ||
| B2: 250 mg/kg bw PC, 55 mg/kg bw E2TL. | 3 | 498.75 ± 4.68a | 395.34 ± 4.33d | 231.82 ± 7.80g |
| B3: 250 mg/kg bw PC, 110 mg/kg bw E2TL. | 3 | 380.03 ± 5.42b | 289.34 ± 6.67e | 194.46 ± 0.55h |
| B4: 250 mg/kg bw PC, 220 mg/kg bw E2TL. | 3 | 291.07 ± 6.28c | 190.03 ± 2.42f | 135.03 ± 2.00i |
Note: PC=Paracetamo; E2TL = ethanolic extract of Tylophora villosa leaves; SGOT = serum glutamate oxaloacetate transaminase. Rates followed by the same letters (a,b) are not significantly different data addressing the same column and the same line (p < 0.05).
Fig. 7.
SGOT levels of M. musculus on day 15, 22, and 30 (D15, D22, and D30) which previously, on D 1-7, received daily 250 mg/kg bw PC (B1), on D1-7 received daily 250 mg/kg bw PC and on D8-21 were given daily 55 (B2), 110 (B3), and 220 (B4) mg/kg bw E2TL respectively, while the controls received only the solvent in the same manner (B0). Rates followed by the same letters (a, b) are not significantly different data addressing the same column and the same line (p < 0.05).
3.4. MDA
A level of MDA in the liver of mice treated daily by 250 mg/kg bw PC for seven days (6.78 ± 1.70 mol/L; C1) was significantly higher than the controls (2.45 ± 0.05 mol/L; C0), however when PC toxicity conditions was given daily therapy at 27.5 mg/kg bw E2TL for seven days the levels of MDA slightly decreased (5.53 ± 0.22 μmol/L; C2). Therapy with daily dosage of 55.0, 82.5, and 110.0 mg/kg bwE2TL for seven days into the toxicity PC condition (6.78 ± 1.70 μmol/L; C1) appeared to reduce the level of MDA 4.82 ± 0.29, 4.17 ± 0.70, and 3.45 ± 0.43 μmol/L respectively. Furthermore it revealed that therapy with the dosage of 110.0 mg/kg bw E2TL for seven days into the toxicity PC condition was able to recover the levels of MDA (4.17 ± 0.7 μmol/L; C4), similar to the control condition (2.45 ± 0.05 μmol/L; C0; Table 10). The facts showed that E2TL had therapeutic effect against PCIH by inhibition of lipid peroxidation.
Table 10.
MDA levels of M. musculus liver on day 15 (D15), which previously, on D1-7, received daily 250 mg/kg bw PC (C1), on D1-7 received daily 250 mg/kg bw PC, and on D8-14 were given daily 27.5 (C2), 55.0 (C3),82.5 (C4), and 110 mg/kg bw E2TL respectively, while the controls only received the solvent in the same manner (C0).
| Group of experimental animals | N | MDA concentration in the liver of M. musculus (μmol/L) on D15 |
|---|---|---|
| C0: Control, normal. | 4 | 2.45 ± 0.05a |
| C1: 250 mg/kg bw PC. | 4 | 6.78 ± 1.70bc |
| C2: 250 mg/kg bw PC, 27.5 mg/kg bw E2TL. | 4 | 5.53 ± 0.22cde |
| C3: 250 mg/kg bw PC, 55.0 mg/kg bw E2TL. | 4 | 4.82 ± 0.29df |
| C4: 250 mg/kg bw PC, 82.5 mg/kg bw E2TL. | 4 | 4.17 ± 0.70ade |
| C5: 250 mg/kg bw PC, 110.0 mg/kg bw E2TL. | 4 | 3.45 ± 0.43af |
Note: PC=Paracetamo; E2TL = ethanolic extract of Tylophora villosa leaves; MDA = malondialdehyde; Rates followed by the same letters (a, b) are not significantly different data addressing the same column (p < 0.05).
4. Discussions
Lubuklinggau community use T. villosa as the traditional drug to treat diseases associated with impaired function of the liver. Some testimonies explained that T. villosa is the medicinal plant for hepatitis, so this claim needs to be tested scientifically. Meanwhile, Lubuklinggau communities also commonly use PC for their health purposes. PCIH remains the key factor limiting the clinical application of PC, and herbs are the important sources for isolation of compounds preventing PCIH.28 This study tried to gather data to verify whether this traditional practice can be classified into empirical and rational phenomena or just a psychological suggestion in the culture of society. PCIH in several group of experimental animals were treated by E2TL, and then this therapeutic phenomenon was analyzed starting from the levels of morphology to biochemical and enzymatic aspects as presented below.
M. musculus has a lethal dosage of PC of 400 mg/kg bw, while the sublethal dosage is 200 mg/kg bw.29 At therapeutic dosages, PC will be eliminated mainly by conjugation with glucuronide or sulfate in hepatocytes. The remaining PC is metabolized by the cytochrome P450 system and converted into a highly toxic intermediate, N-acetyl-p-benzoquinone imine (NAPQI). NAPQI is depleted by conjugation with glutathione (GSH) and the complex is excreted mainly into bile.30 However at toxic dosage of PC, excessive NAPQI depletes the cellular storage of GSH and binds to cellular proteins covalently, resulting in mitochondrial dysfunction and DNA damage, which leads to hepatocyte necrosis and cell death.31, 32 This study used the dosage of 250 mg/kg bw PC for seven days which caused two mortality (13.33%). This PC toxicity condition could be decreased by daily 220 mg/kg bw E2TL treatment for seven days which caused only one mortality (6.67%; Table 3). This fact indicated that E2TL was generally able to decrease the PC toxicity condition presumably because E2TL as a natural material is overcast alkaloids (matrine33;), and flavonoids (quercetin34; Table 2). It has been widely recognized that weight loss is usual in line with the decrease of blood glucose).35 This phenomenon was similar with results of the study which indicated that in PC toxicity conditions caused weight reduction (Table 4) along with a decrease of blood glucose (Table 5). Both decrease of body weight (Fig. 1) and blood glucose (Fig. 2) as the effects of PC administration could be recovered by E2TL treatments as well as the control. Results of macroscopic observation of the liver of mice after treatment using PC for seven days revealed some changes in color (Table 5), weight (Table 6), and volume (Table 7) of the liver which were significantly different from those of the control. The occurrence of liver macroscopic changes was a clue for PCIH. The PCIH were subsequently turned out to be restored with daily 220 mg/kg bw E2TL treatment for seven days so that the color (Fig. 3), weight (Fig. 4), and volume (Fig. 5) approached the control's. A similar phenomenon was shown by the study of the patoprotective activity of methanol extract of M. malabathricum leaves (MEMM) against PCIH).36 E2TL and MEMM showed antioxidant activity due to both natural materials that contain flavonoids.
It is well known that the existence of hepatocellular injury can also be determined by measuring the level of two hepatic enzymes; SGPT and SGOT.6, 7 This research indicated the same phenomenon of SGPT and SG0T. The daily 250 mg/kg bw PC administration for seven days (D1-7) which was followed by daily 55.0 E2TL treatments (B2) for seven (D8-14), fourteen (D8-21), twenty-one (D8-29) days decreased SGPT and SGOT levels to lower levels than the positive control (B1) respectively. Furthermore, the decline of SGPT and SGOT levels also occurred in line with two dosages (110; 220 mg/kg bw) E2TL treatment and three time periods (D8-14; D8-21; D8-29) treatment. The highest dosage (220 mg/kg bw) and the longest period time (D8-29) of E2TL treatment resulted in the lowest levels of SGPT and SGOT, 89.94 ± 1.70 135.03 ± 2.00 U/L approaching the value of 60.00, 100.00 U/L (B0; control; Table 8, Table 9) respectively. These observations showed that E2TL had therapeutic effect against PCIH in recovering SGPT and SGOT level of M. musculus (Fig. 6, Fig. 7). This therapeutic effects of E2TL was thought to have come from three phytochemical contents (alkaloids, flavonoids, and riterpenoids; Table 2). Some earlier reports stated that six derivatives of flavonoids such as baicalin,37 luteolin,38 galangin,39 rutin, quercetin, hyperforin34 are antioxidant which are able to recover back PCIH to normal conditions. This fact can be a preliminary indication that E2TL has the opportunity to be developed as a new herbal medicine.
The amount of ROS in mitochondria can be increased by excessive PC administration, and impacts on the emergence of OS. Furthermore, OS causes cell membranes damage by morphological and biochemical changes followed by the malfunctioning cells and it is ended by hepatocyte death that leads to necrosis and apoptosis of liver cells. MDA is the final product of morphological changes that is preceded by the OS induces lipid peroxidation.4, 5 This research revealed that the level of MDA in the liver of mice treated daily by 250 mg/kg bw PC for seven days (C1) was significantly higher than the controls' (C0). However, when PC toxicity conditions was given daily therapy 27.5 mg/kg bw E2TL for seven days the levels of MDA decreased slightly (C2). Therapy with daily dosage of 55.0, 82.5, and 110.0 mg/kg bw E2TL for seven days into the PCIH (C1) appeared to reduce the level of MDA. Furthermore, it revealed that therapy with the dosage of 110.0 mg/kg bw E2TL for seven days into the PCIH was able to recover the levels of MDA (C4), similar to the control condition (C0; Table 10). The facts showed that E2TL had therapeutic in PCIH by inhibition of lipid peroxidation. This therapeutic effect of E2TL was thought to have come from three phytochemical contents (alkaloids, flavonoids, and triterpenoids; Table 2). Some earlier reports stated that two derivatives of flavonoids such as genistein40 and naringenin41 are antioxidant which were able to recover back PCIH to normal conditions. These facts can be additional preliminary indication that E2TL has the opportunity to be developed as the new herbal medicine.
5. Conclusion
The present experimental findings revealed that phytoconstituents of E2TL had therapeutic effect against PCIH of M. musculus by inhibition of radicals and lipid peroxidation.
Conflict of interest
We wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
Authors' contributions
AR conceived of the study, and participated in its design and coordination and helped to draft the manuscript. BDS participated in the preparing of experimental animals and serum parameters analysis. EM and ZF participated in the study of PC detoxification on blood and liver. FA and SRP carried out determination of lipid peroxidation. AS carried out extract preparation and phytochemical test. All authors read and approved the final manuscript.
Acknowledgement
This research was supported by Graduate School of Science Education and Department of Medicine, Bengkulu University, Jalan Raya Kandang Limun, Bengkulu 38371, Indonesia. We express our gratitude to Dr. Rosane Medriati for her valuable recommendations to conduct the research. We also gratefully acknowledge Nurus Sa'adiyah for her statistical analysis, Shyfa F. Ruyani for her illustration preparations, and Dr. Wiryono for his grammatical editing of this manuscript.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
References
- 1.Bayupurnama P. In: Buku Ajar Ilmu Penyakit Dalam Jilid I. Imbas Obat Hepatotoksisitas, Dalam Sudoyo AW., Setiyohadi B., Alwi I., Simadibrata M., Setiati S., editors. Pusat Penerbitan Departemen Ilmu Penyakit Dalam FKUI; Jakarta: 2009. pp. 708–712. [Google Scholar]
- 2.Kanno S.I., Tomizawa A., Yomogida S. Detecting mRNA predictors of acetaminophen-induced in mouse blood using quantitative real-time PCR. Biol Pharm Bull. 2016;39:440–445. doi: 10.1248/bpb.b15-00734. [DOI] [PubMed] [Google Scholar]
- 3.Ahmad S.T., Arjumand W., Nafees S. Hesperidin alleviates acetaminophen induced toxicity in Wistar ratsby abrogation of oxidative stress, apoptosis and inflammation. Toxicol Lett. 2012;208:149–161. doi: 10.1016/j.toxlet.2011.10.023. [DOI] [PubMed] [Google Scholar]
- 4.Ayala A., Muñoz M.F., Argüelles S. Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid Med Cell Longev. 2014:31. doi: 10.1155/2014/360438. Volume, Article ID 360438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.El-Shafey M.M., Abd-Allah G.M., Mohamadin A.M., Harisa G.I., Mariee A.D. Quercetin protects against acetaminophen-induced hepatorenal toxicity by reducing reactive oxygen and nitrogen species. Pathophysiology. 2015;22:49–55. doi: 10.1016/j.pathophys.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 6.Mata-Marin J.A., Gaytan-Martinez J., Grados-Chavarria B.H. Correlation between HIV viral load and amino-transferases as liver damage markers in HIV infected naïve patients: a concordance cross-sectional study. Virol J. 2009;6:181. doi: 10.1186/1743-422X-6-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crum-Cianflone N., Dilay A., Collins G. Nonalcoholic fatty liver disease among HIV-infected persons. J Acquir Immune Defic Syndr. 2009;50(5):464–473. doi: 10.1097/QAI.0b013e318198a88a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yuan H., Ma Q., Ye L., Piao G. The traditional medicine and modern medicine from natural products molecules. 2016;21:559. doi: 10.3390/molecules21050559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gupta M., Majumder U.K., Thamilselvan V. Potential hepatoprotective effect and antioxidant role of methanol extract of Oldenlandia umbellate in carbon tetrachloride induced hepatotoxicity in Wistar rats. Iran J Pharmacol Ther. 2007;6:5–9. [Google Scholar]
- 10.Akther N., Shawl A.S., Sultana S., Chandan B.K., Akhter M. Hepatoprotective activity of Marrubium vulgare against paracetamol induced toxicity. J Pharm Res. 2013;7:565–570. [Google Scholar]
- 11.Subramanian M., Balakrishnan S., Chinnaiyan S.K., Sekar V.K., Chandu A.N. Hepatoprotective effect of leaves of Morinda tinctoria Roxb. against paracetamol induced liver damage in rats. Drug Invent Today. 2013;5:223–228. [Google Scholar]
- 12.Chellappan D.K., Ganasen S., Batumalai S. The protective action of the aqueous extract of Auricularia polytricha in paracetamol induced hepatotoxicity in rats. Recent Pat Drug Deliv Formul. 2016;10(1):72–76. doi: 10.2174/1872211309666151030110015. [DOI] [PubMed] [Google Scholar]
- 13.Hamid Z.A., Budin S.B., Jie N.W. Nephroprotective effects of Zingiber zerumbet Smith ethyl acetate extractagainst paracetamol-induced nephrotoxicity and oxidative stress in rats. J Zhejiang Univ Sci B. 2012;13:176–185. doi: 10.1631/jzus.B1100133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Topal F., Topal M., Gocer H. Antioxidant activity of taxifolin: an activity-structure relationship. J Enzym Inhib Med Chem. 2016;31:674–683. doi: 10.3109/14756366.2015.1057723. [DOI] [PubMed] [Google Scholar]
- 15.Ekinci-Akdemir F.N., Gulcin I., Alwasel S.A. Comparative study on the antioxidant effects of hesperidin and ellagic acid against skeletal muscle ischemia/reperfusion injury. J Enzym Inhib Med Chem. 2016:31,114–118. doi: 10.1080/14756366.2016.1220378. [DOI] [PubMed] [Google Scholar]
- 16.Prishellya N., Sundaryono A., Ruyani A., Zamzaili . Program Pascasarjana Pendidikan IPA, Fakultas Keguruan Dan Ilmu Pendidikan. Universitas Bengkulu; Indonesia: 2012. Isolasi senyawa aktif daun Tylophora villosa Blume dan uji toksisitas terhadap Artemia salia Leach sebagai sumber belajar kimia bahan alam. [Google Scholar]
- 17.Sopi R.B., Khan M.F.H. Bronchodilatory effect of ethanolic extract of the leaves of Nyctanthes arbortristis. Pharmacogn Res. 2013;5(3):169–172. doi: 10.4103/0974-8490.112422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ruyani A., Sundaryono A., Rozi Z.F., Samitra D., Grasinta E. Potential assessment of leaf ethanolic extract honje (Etlingera hemisphaerica) in regulating glucose and triglycerides on mice (Mus musculus) Int J Sci. 2014;3(1):70–76. [Google Scholar]
- 19.Wadood A., Ghufran M., Jamal S.B. Phytochemical analysis of medicinal plants occurring in local area of mardan. Biochem Anal Biochem. 2013;2:144. [Google Scholar]
- 20.Setiawan . Penebar Swadaya; Jakarta: 2006. Ramuan Tradisional Untuk Pengobatan Hepatitis. [Google Scholar]
- 21.Kusumawati D. Gajah Mada University Press; Yogyakarta: 2004. Bersahabat Dengan Hewan Coba. Fakultas Kedokteran Hewan. [Google Scholar]
- 22.Ridwan E. Etika pemanfaatan hewan percobaan dalam penelitian Kesehatan. J Indon Med Assoc. 2013;63(3):112–116. [Google Scholar]
- 23.Ruyani A., Sudarwati S., Sutasurya L.A., Sumarsono S.H., Kim D.J., Chung J.H. A teratoproteomics analysis: heat shock protein 70 is up-regulated in mouse forelimb bud by methoxyacetic acid treatment. Birth Defects Res A Clin Mol Teratol. 2005;73(7):517–521. doi: 10.1002/bdra.20146. http://www.ncbi.nlm.nih.gov/pubmed/15959878 [DOI] [PubMed] [Google Scholar]
- 24.Ruyani A., Karyadi B., Kadir A., Fitri D., Tanjung R.Y., Puspa Y. Alteration of ossification rate on fetal humerus and femur swiss Webster mice (Mus muculus) as the teratogenic effects of Dioscorea hispida Dennst. J Kedokt Dan Farm Med. 2011;37:596–603. [Google Scholar]
- 25.Federer W.T. Oxford and IBH Publ. Co; New Delhi, Ramsey SC, Galeano: 1967. Experimental Design, Theory and Application. [Google Scholar]
- 26.Kumar M., Kaur P., Chandel M., Singh A.P., Jain A., Satwinderjeet Kaur S. Antioxidant and hepatoprotective potential of Lawsonia inermis L. leaves against 2-acetylaminofluorene induced hepatic damage in male Wistar rats. BMC Complement Altern Med. 2017;17:56. doi: 10.1186/s12906-017-1567-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liao C.-C., Day Y.-J., Lee H.-C., Liou J.-T., Chou A.-H., Liu F.-C. Baicalin attenuates IL-17-mediated acetaminophen-induced liver injury in a mouse model. PLoS ONE. 2016;11(11) doi: 10.1371/journal.pone.0166856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang H., Jiang T., Li P., Mao Q. The protection of glycyrrhetinic acid (GA) towards acetaminophen (APAP)-induced toxicity partially through fatty acids metabolic pathway. Afr Health Sci. 2015;15(3):1023–1027. doi: 10.4314/ahs.v15i3.42. Sep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barman P.K., Mukherjee R., Prusty B.K., Suklabaidya S., Senapati S., Ravindran B. Chitohexaose protects against acetaminophen-induced hepatotoxicity in mice. Cell Death Dis. 2016;7 doi: 10.1038/cddis.2016.131. May 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bunchorntavakul C., Reddy K.R. Acetaminophen-related hepatotoxicity. Clin Liver Dis. 2013;17:587–607. doi: 10.1016/j.cld.2013.07.005. PMID: 24099020. [DOI] [PubMed] [Google Scholar]
- 31.Hilson J.A., Roberts D.W., James L.P. Mechanisms of acetaminophen-induced liver necrosis. Handb Exp Pharmacol. 2010;196:369–405. doi: 10.1007/978-3-642-00663-0_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McGill M.R., Sharpe M.R., Williams C.D., Taha M., Curry S.C., Jaeschke H. The mechanism underlying acetaminophen-induced hepatotoxicity in humans and mice involves mitochondrial damage and nuclear DNA fragmentation. J Clin Invest. 2012;122:1574–1583. doi: 10.1172/JCI59755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wan X.Y., Luo M., Li X.D., He P. Hepatoprotective and anti-hepatocarcinogenic effects of glycyrrhizin and matrine. Chem Biol Interact. 2009;181(1):15–19. doi: 10.1016/j.cbi.2009.04.013. Epub May 6. Sep 14. [DOI] [PubMed] [Google Scholar]
- 34.Hohmann M.S., Cardoso R.D., Fattori V. Hypericum perforatum reduces paracetamol-induced hepatotoxicity and lethality in mice by modulating inflammation and oxidative stress. Phytother Res. 2015;29(7):1097–1101. doi: 10.1002/ptr.5350. Jul, Epub 2015 Apr 8. [DOI] [PubMed] [Google Scholar]
- 35.Stanford J., Kaiser M., Ablah E., Dong F., Paull-Forney B., Early J. The effect of weight loss on fasting blood sugars and hemoglobin A1c in overweight and obese diabetics and non-diabetics. J Diabetes Mellitus. 2012;2:126–130. [Google Scholar]
- 36.Mamat, SS., Kamarolzaman, MFF., Yahya, F., et al. Methanol extract of Melastoma malabathricum leaves exerted antioxidant and liver protective activity in rats. BMC Complement Altern Med 13:326. http://www.biomedcentral.com/1472-6882/13/326. [DOI] [PMC free article] [PubMed]
- 37.Liao C.C., Day Y.J., Lee H.C., Liou J.T., Chou A.H., Liu F.C.E.R.K. Signaling pathway plays a key role in baicalin protection against acetaminophen-induced liver injury. Am J Chin Med. 2017;45(1):105–121. doi: 10.1142/S0192415X17500082. Epub 2017 Jan 13. [DOI] [PubMed] [Google Scholar]
- 38.Shanmugam S., Thangaraj P., Lima B.D. Effects of luteolin and quercetin 3-β-d-glucoside identified from Passiflora subpeltata leaves against acetaminophen induced hepatotoxicity in rats. Biomed Pharmacother. 2016;83:1278–1285. doi: 10.1016/j.biopha.2016.08.044. Epub 2016 Aug 25. Oct. [DOI] [PubMed] [Google Scholar]
- 39.Tsai M.S., Chien C.C., Lin T.H. Galangin prevents acute hepatorenal toxicity in novel propacetamol-induced acetaminophen-overdosed mice. J Med Food. 2015;18(11):1187–1197. doi: 10.1089/jmf.2014.3328. Nov; Epub 2015 Jun 4. [DOI] [PubMed] [Google Scholar]
- 40.Fan Y.J., Rong Y., Li P.F. Genistein protection against acetaminophen-induced liver injury via its potential impact on the activation of UDP-glucuronosyl transferase and antioxidant enzymes. Food Chem Toxicol. 2013;55:172–181. doi: 10.1016/j.fct.2013.01.003. Epub 2013 Jan 16. May. [DOI] [PubMed] [Google Scholar]
- 41.Lv Y., Zhang B., Xing G., Wang F., Hu Z. Protective effect of naringenin against acetaminophen-induced acute liver injury in metallothionein (MT)-null mice. Food Funct. 2013 Feb;4(2):297–302. doi: 10.1039/c2fo30213f. [DOI] [PubMed] [Google Scholar]