Abstract
We report two cases of keratitis caused by a rare fungus Myrothecium species. Clinical presentation, identification, and management were studied. Both fungi were grown from corneal scraping and identified as M. verrucaria and M. gramineum based on the microbiological techniques and DNA sequencing analysis. Both patients were treated with topical natamycin and or voriconazole/econazole. In the first patient, there was total healing of the with scar formation, however, the prognosis was poor in the second patient.
Keywords: Keratitis, Myrothecium, Polymerase chain reaction, Internal transcribed spacer, DNA sequencing
1. Introduction
Mycotic keratitis is one of the major causes of ophthalmic morbidity and visual loss globally. The etiologic agent responsible for mycotic keratitis varies regionally and the incidence is more common in tropical climates [1]. In South India, Fusarium spp. and Aspergillus spp. are the most common causative agent of mycotic keratitis [2]. However, different uncommon fungi causing mycotic keratitis has been reported from different parts of the India [1]. Myrothecium species are saprophytic fungi in the family Stachybotryaceae mainly found in the soil or as weak plant pathogens. More than 30 species have been reported worldwide [4]. The first report of plant disease was caused on tomato by this genus in America. Myrothecium spp. are pathogens of leaf spot of mulberry, chilli seed, and also cause diseases on the leaves of different type of vegetables. Myrothecium verrucaria was reported as a week pathogen in the seeds of rice and soybean [3]. The prime importance for the identification of this species because of medical and public health concerns, as the two major species like M. verrucaria and M. roridans produces the macrocyclic trichothecene mycotoxin which is a potent mycotoxin. Since, Myrotheciumspp. has the ability to grow on walls of houses and produce mycotoxins that are similar to Stachybotrys toxins; they can cause a variety of adverse health effects like inhibition of protein synthesis, immune suppression, and impairment of alveolar macrophage function [5]. The genus Myrothecium is a potential secondary metabolites producer, with more than 30 compounds from M. verrucaria [6]. There is one report of Myrothecium causing keratitis [7]. We report two cases of keratitis caused by M. verrucaria and M. gramineum and discuss the presentation, identification of the fungus and the management.
2. Cases
2.1. Case 1
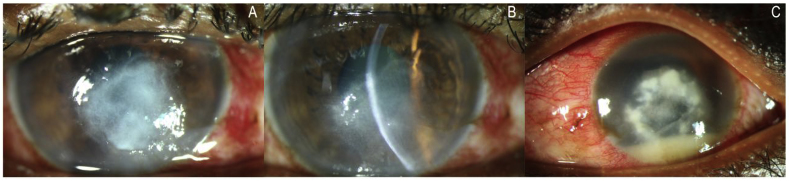
A 68-year-old male presented with 10 days (Day+10) history of acute intermittent pain, redness and irritation in his left eye. His initial visual acuity (VA) was hand motion (HM). He did not recall any history of ocular trauma or fall of foreign body. He did not have any history of systemic infections. There was a diffuse conjunctival congestion and the cornea had a 4 × 5 mm of ulcer upto 30% of depth into the corneal stroma (Fig. 1A). Corneal scraping was done to rule out the causative pathogens. The patient was treated with antifungal agents’ natamycin (5%) and econazole (2%) hourly for 7 days. At the end of one week the ulcer was totally healed with scar formation (Fig. 1B).
Fig. 1.
(A) Case 1 – slit-lamp microscopic image showed 4 × 5 mm of corneal ulcer up to 30% of depth into the corneal stroma (B) Case 1 healed ulcer with scar formation (C) Case 2 – slit-lamp microscopic image showed 5 × 5 mm epithelial defect, 4 × 4 mm full thickness stromal infiltrate with feathery edges and central endoexudates.
2.2. Case 2
A 65-year-old male presented with 10 days (Day+10) history of pain, redness, watering in his right eye. His initial visual acuity was light perception (LP+). He did not recall any history of ocular trauma or fall of foreign body. He had a systemic complication of asthma since 5 months. Conjunctival congestion was noted and the cornea had a 5 × 5 mm epithelial defect, 4 × 4 mm full thickness stromal infiltrate with feathery edges and central endoexudates and 3mm hypopyon (Fig. 1C). Corneal scraping was done to rule out the causative microorganisms. The patient was treated with antifungal agents’ natamycin (5%) and voricanazole (1%) hourly for 7 days. However this patient had a poor prognosis and had to have a corneal transplant.
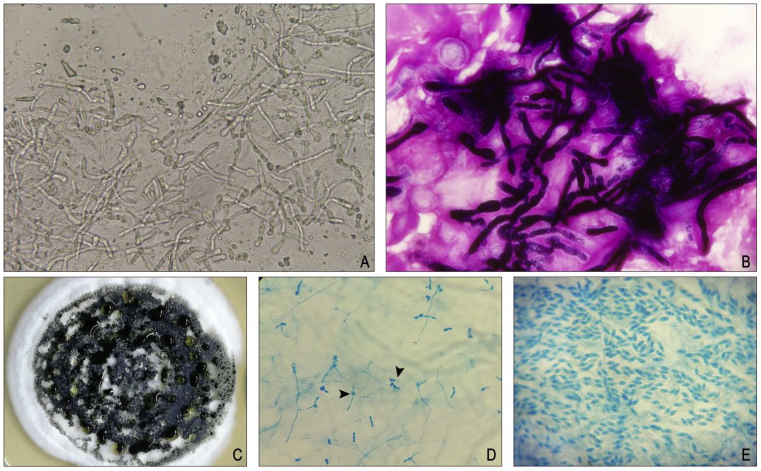
Corneal scraping material was obtained from both the patients and was subjected microbiological evaluation which included smear and culture methods. The 10% potassium hydroxide (KOH) wet mount and gram staining showed the presence of septate hyphae fungal filaments (Fig. 2A–B) The colony morphology of the fungus grown in potato dextrose agar showed creamy-white, floccose-dense colonies with irregular mycelia growth was observed after 7 days of incubation in which abundant distinct black sporodochia were formed in a concentric pattern after 12 days of incubation at 25 °C (Fig. 2C). Fungal spore morphology was identified by lactophenol cotton blue (LP) mount stain. The LP stain image showed germinating spores arising from the conidiophores (Fig. 2D) and the conidia were ellipsoidal or cylindrical in shape with round ends, smooth protuberant at top and truncated at bottom (Fig. 2E).
Fig. 2.
(A) 10% potassium hydroxide (KOH) wet mount showed the presence of septate fungal hyphae filaments seen (x40) (B) Gram stain image showed the thick cell wall, a few septate hyphae seen (x100 with oil immersion) (C) Growth of Myrothecium verrucaria on potato dextrose agar showed the morphology of abundant distinct black sporodochia in concentric pattern after 12 days of incubation at 25 °C (D) Lactophenol cotton blue mount image showed the presence of germinating spores (x40) (E) Conidia were ellipsoidal or cylindrical in shape with round ends, smooth protuberant at top and truncated at bottom (x40). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
The morphological identification of Myrothecium species is quite difficult due to following reasons (i) spore morphology cannot differentiate very accurately (ii) the genus probably comprises species complex. This prompted the use of polymerase chain reaction (PCR) followed by DNA sequencing analysis. DNA extraction was done from the both fungal mycelium growth was seen in the potato dextrose agar plates by using the Phenol-Chloroform method. PCR targeting the internal transcribed spacer (ITS) region 1 and 4 and the protocol and primer used were as previously published [8]. DNA sequencing was done using an ABI 3130 genetic analyzer (PE Applied Biosystems, Foster City, CA, USA). NCBI-BLAST analysis of ITS sequence of two strains showed each 100% similarity with Myrothecium verrucaria (Albifimbria verrucaria) and Myrothecium gramenium. We deposited the sequences in the NCBI GenBank database with the accession numbers of MK720180 and MK720190 respectively.
3. Discussion
Myrothecium species is ubiquitous and worldwide in its ecological distribution. The genera Myrothecium belongs to the order Hypocreales and Stachybotryaceae family. It is recognized as a weak plant pathogen and also found in the soil. It is also known to cause leaf spot disease in various vegetative plants of leaves and seeds [3]. Since, the fungus has the ability to produce the mycotoxins and also found in indoor air; contaminant must be considered as a potential health hazard [9]. However, no study has evaluated the risk to humans. In literature, there is no clinical information about the Myrothecium species causing keratitis and a single centre study reports available without any clinical information. Due to lack of sporulation and slow growth nature of the Myrothecium species is quite difficult in the identification based on morphological features and genus having species complex. Thus create a diagnostic challenge for the identification of this species. Previous studies demonstrate the species identification based on the phylogenetic tree analysis of ATP6, EF1-α, ITS, LSU, RPB1 and SSU sequences retrieved from the NCBI database, which shows the genus to be polyphyletic in Stachybotryaceae family. Morphology of Myrothecium species cannot virtually reflect phylogenetic relationship in the genus, because of the few characters available to differentiate species. Studies found that ITS sequence data can act as a primary barcode for Myrothecium species because its sequence data can accurately identify the 73% of taxa studied across kingdom fungi and has a high sequence and PCR success rate [10]. ITS sequence data has been very much helpful for molecular systematics at the species level and even within the species [11]. In our case reports, we have identified the fungus was M. gramineum and M. verrucaria based on ITS sequence analysis.
A presumptive diagnosis of mycotic keratitis requires empirical therapy. Current treatment and clinical management of such cases include topical antifungal medication and keratectomy surgery. Natamycin or Voriconazole is the drug of choice for filamentous fungus and amphotericin B for yeast infection and surgical intervention may require for non-healing ulcer or perforated cases. In the first case presented, M. gramineum responded to initial treatment with 5% natamycin and 2% econazole topical eye drops and the second case presented, M. verrucaria was not responded to the initial treatment with 5% natamycin and 1% voriconazole topical eye drops. Final follow up of this patient ulcer got thinning and perforated and then planned for keratoplasty surgery during his next visit. In both the case reports, the patients did not recall any history of ocular trauma. However, the chance of a trivial ocular trauma cannot be ruled out. Since, Myrothecium spp. is a saprophytic nature the infection may be associated with ecological factors. The treatment of this fungus is a major challenge for clinicians without knowing the clinical features and virulent nature of the organisms.
We report two cases of mycotic keratitis caused by an unusual pathogenic species, M. gramineum, and M. verrucaria. Although we describe the identification of this fungus upto species level by conventional microbiological techniques as well as molecular-based analysis of ITS regions. This is the first case reports we presented with clinical features, identification, and management. These cases generate awareness among ophthalmologist and diagnostic laboratories regarding the prompt identification and management of Myrothecium species associated keratomycosis.
Conflict of interest
There are none.
Acknowledgements
There are none.
References
- 1.Shah A., Sachdev A., Coggon D., Hossain P. Geographic variations in microbial keratitis: an analysis of the peer-reviewed literature. Br. J. Ophthalmol. 2011;95:762–767. doi: 10.1136/bjo.2009.169607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lalitha P., Prajna N.V., Manoharan G., Srinivasan M., Mascarenhas J., Das M. Trends in bacterial and fungal keratitis in South India, 2002-2012. Br. J. Ophthalmol. 2015;99:192–194. doi: 10.1136/bjophthalmol-2014-305000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen Y., Ran S.F., Dai D.Q., Wang Y. Mycosphere essays 2. Myrothecium. Mycosphere. 2016;7:64–80. [Google Scholar]
- 4.Watanable T. second ed. CRC Press; Boca Raton: Florida: 1994. Pictorial Atlas of Soil and Seed Fungi. Morphologies of Cultured Fungi and Key to Species. [Google Scholar]
- 5.Yang C.S., Johanning E. Airborne fungi and mycotoxins. In: Hurst C.J., Knudsen G.R., McInerney M.J., Stetzenbach L.D., Walter M.V., editors. Manual of Environmental Microbiology. ASM Press; Washington DC: 1997. 651–600. [Google Scholar]
- 6.Buckingham J. first ed. Chapman & Hall; London: 1994. Dictionary of Natural Products. [Google Scholar]
- 7.Refojo N., Minervini P., Hevia A.I., Abrantes R.A., Fernández J., Apestey N. Keratitis caused by moulds in santa lucía ophthalmology hospital in Buenos aires, Argentina. Rev. Iberoam. De. Micol. 2016;33:1–6. doi: 10.1016/j.riam.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 8.White T.J., Burns T., Lee S., Taylor J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis M.A., Gelfand D.H., Sninsky J.J., White T.J., editors. PCR Protocols, a Guide to Methods and Applications. Academic Press; London: 1990. 315-2. [Google Scholar]
- 9.Levetin E., Shaughnessy R. Myrothecium: a new indoor contaminant? Aerobiologia. 1997;13 227-4. [Google Scholar]
- 10.Nilsson R.H., Hyde K.D., Pawłowska J., Ryberg M., Tedersoo L., Aas A.B. Improving ITS sequence data for identification of plant pathogenic fungi. Fungal Divers. 2014;67:11–19. [Google Scholar]
- 11.Wang Y., Hyde K.D., McKenzie E.H.C., Jiang Y.L., Li D.W., Zhao D.G. Overview of Stachybotrys(Memnoniella) and current species status. Fungal Divers. 2015;71 17-3. [Google Scholar]