Abstract
In this present study, phytochemical screening, anti-ulcer assay, anti-diarrhea assay, anti-inflammatory assay, analgesic assay, lipase activity assay, amylase activity assay and the anti-bacterial activity of Eucalyptus camaladulensis Dehnh leaf extracted with methanol and 50% ethanol was analyzed for biological significance. Physical characterization of the non-volatile component revealed the higher yield of 16.92% in 50% ethanol expediting the use of 50% ethanol as a better alternative. Further use of crude extract revealed 33.89% (IC50 = 1.44 mg/ml) of α-amylase inhibition by methanol extract and 33.87% (IC50 = 3.21 mg/ml) lipase inhibition by 50% ethanol extract. Furthermore, 44.44% protective ratio towards ulcer was observed with the methanol extract, whereas 54.58% anti-inflammatory activity was shown by the 50% ethanol extract. The effectiveness of the extract was further enhanced by the presence of 62.54% motility and best analgesic property at 180 min of the exposure of the extract orally. The antioxidant activity of crude methanol extract revealed an IC50 value 601.8 μg/ml whereas, ethanol extract showed 1279.58 μg/ml in DPPH assay. Result revealed several health benefits of E. camaldulensis Dehnh leaf.
Keywords: Eucalyptus camaladulensis Dehnh, Pharmacological activity, Lipase activity, Amylase, Anti-ulcer, Anti-diarrhea, Anti-inflammatory, Analgesic activity
Graphical abstract
1. Introduction
Eucalyptus camaldulensis Dehnh also known as “red gum” is native to Australian mainland.1 In Nepal it is commonly known as Masala tree and widespread in the Siwalik region of Nepal. It belongs to the family Myrtacease and planted in many different countries worldwide. It has been reported that around 800 species of Eucalyptus distribution worldwide.2 In Nepal Eucalyptus grandis (E. grandis), Eucalyptus globulus (E. globulus) and Eucalyptus camaladulensis Dehnh (E. camaladulensis Dehnh) were successfully grown at different altitude.3 E. camaldulensis is the medium-sized tree, growing up to 30 m tall and are highly adaptive to an extreme condition like drought. The member of this genus have been reported to have a diverse secondary metabolites possessing different biological activities.4
Natural human antioxidant defense are not always sufficient to maintain the equilibrium between ROS generation and degradation.5 Excessive generation of ROS is associated with cancer, cardiovascular diseases and inflammatory diseases.6 Plants are huge source of bioactive secondary metabolites such as glycosides, saponins, flavonoids, steroids, tannins, alkaloids and terpenes, which act as strong antioxidant to protect body from oxidative damage.7 These secondary metabolites have shown several biological activities such as antibacterial, antioxidant, anti-inflammatory, antiulcer, analgesic and also used for the treatment of diabetes. The peptic ulcer is one of the common gastrointestinal diseases, which are characterized by the presence of mucosal damage caused by infection with Helicobacter pylori. Further, induced by factors like stress, smoking, and alcohol consumption as well as caused by the adverse effect of drugs.8,9 Plants rich in tannins are used for the treatment of gastric ulcers as tannins react with tissue proteins to form a protective layer over injured epithelial tissues of the stomach and promote healing process.10 Inflammation is an immune response of the body against pathogens, evoking inflammatory cells like macrophages, neutrophils, monocytes, dendritic and mast cells to form ‘inflammatory microenvironment' at the site of infection or wound to restore cellular or organ repair process.11 Excessive activation of pro-inflammatory cytokines like Interleukin-6(IL-6) and Tumour Necrosis Factor (TNF-α) may cause inflammatory disorders like rheumatoid arthritis, asthma, and atherosclerosis caused by the production of free radical species.12 Non-steroidal and anti-inflammatory drugs (NSAIDs) are currently is practice for prevention of inflammation. However, it is not effective against different types of inflammations and also caused the adverse side-effects leading to ulcers.
Several Eucalyptus spp have shown antibacterial, analgesic and anti-inflammatory activities.13,14 Furthermore, Diarrhea is still a leading cause of death in many developing countries, associated with fecal urgency and incontinence result from an imbalance between the absorptive and secretory mechanisms in the intestinal tract.15 Different studies have reported the pharmacological importance of plant extracts for treatment of diarrhea, inflammatory diseases, antiulcer and analgesic activities.16 Even though the traditional use of E. camaladulensis Dehnh for medicinal purposes has been known in Nepal community, the present study aims to evaluate the phytochemical, pharmacological and enzymatic inhibition properties of the leaf extract.
2. Materials and methods
2.1. Chemicals used
The enzymes α-amylase, lipase and 2,2-diphenyl-1-picrylhydrazyl (DPPH) were purchased from Sigma Aldrich, USA. All other chemicals were of highest purity and of analytical grades purchased from Thermo Fischer scientific, India.
2.2. Collection of plant materials
The plants were collected from Janakpur Zone, Nepal, and the headquarters of Mahottari District Latitude: 26° 38′ 49.60” N Longitude: 85° 48′ 2.92” E. Mahottari district of Nepal located 216 km South East to capital is the tropical region of the country with the warm climate. The plant materials were identified by National Herbarium and Plant Laboratory (KATH) and deposited at Department of Plant Resources (DPR), Government of Nepal (Voucher specimen 05/2017). Plants were collected in the polythene bags during morning hours and placed in the ice box for preservation and prevention of contamination. The leaves were air dried in the shade at room temperature and were protected from direct sunlight.
2.3. Extraction of metabolites
The 50% ethanol and methanol were used for the extraction of metabolites from E. camaladulensis Dehnh. 100 g shade dried leaves were weighed. 1000 ml of methanol and 50% ethanol was added as extracting solvents with ratio 1:10 in separate mantel fitted with the condenser in the top for 8 h cycle of reflux. The solvent was filtered through the Whatman filter paper. Rotatory evaporator (HS-2005V) was used to concentrate the extracts (semi dry). The extract was prepared for analysis latter by dissolving it in distilled water. Where as in case of phytochemical analysis the extracts (20 ml) was separated and placed in liquid form for analysis.
2.4. Mouse and rat model used
Swiss albino mice (20–40 g) and Wistar Rat (150–200 g) was obtained from the Natural Products Research Laboratory (NPRL), Department of Plant Resources (DPR), Government of Nepal. All the experiments were performed as per the guideline of NHRC. They were housed in environmentally controlled conditions (23 °C ± 2 °C, 12-h light-dark cycle), with free access to a standard diet and water. They were kept in standard mice cage. Mice were kept in fasting for 12 h prior to the experiments but allowed access to water.
2.5. Phytochemical screening and antioxidant activity
The phytochemical analysis of the alkaloids, flavonoids, phenolic content, saponin, quinone, sterols, cardiac glycoside, tannin, terpenoids and reducing sugar was performed following the standard protocol.17 The antioxidant activity was measured with DPPH (2, 2-diphenyl 1-picryl hydrazyl) reducing assay as described by Kim et al.18 The solution was prepared in methanol at different concentrations ranging from 200 to 1000 μg/ml. The freshly prepared methanol solution comprising DPPH radicals (0.004% (w/v), 2.7 ml) were assorted with various concentration of extract (0.3 ml). The mixture was vigorously shaken and left for 60 min in the dark. The range of reduction of the DPPH radical was measured at 517 nm. As a reference standard, ascorbic acid was used and DPPH solution without extract was used as the control. The extract concentration resulting in 50% decrease in DPPH concentration known via absorbance (IC50) was calculated from the graph of the absorbance against extract concentration using Origin pro 2015.
2.6. Amylase inhibition activity
Screening of E. camaladulensis Dehnh extract for amylase inhibitors was done via starch-iodine test.19 The reaction mixture composed of 0.2 M potassium phosphate buffer solution (pH 6.9), 1% (w/v) starch solution phosphate buffer saline (pH 7). Different concentrations of extract (100–500 μg/ml) were added followed by the enzymatic digestion with 100 μl amylase (13 U/ml) solution. Hydrolysis reaction was allowed to proceed for exactly 10 min at 37 °C. Starch solution 2 ml was added and incubated at 37 °C for another 10 min followed by stop solution (HCL 0.1 N, 2 ml). Further iodine solution (5 mM I2 and 5 mM KI, 500 μl) was added to the reaction mixtures and absorbance was taken at 620 nm. The control reaction representing 100% enzyme activity did not contain any plant extract. Yellow color indicates the absence of starch; dark-blue color indicated the presence of starch; while a brownish color indicated partially degraded starch in the reaction mixture. In the presence of inhibitors from the extracts, the starch added to the enzyme assay mixture is not degraded and gives a dark blue color complex whereas no color complex is developed in the absence of the inhibitor, indicating that starch is completely hydrolyzed by α-amylase.
2.7. Lipase inhibition activity
The assay for lipase activity using triglyceride as the substrate was performed following the standard protocol.20 The enzyme solutions were prepared immediately before use by suspending crude porcine PL type II Sigma type-II, 0.71 mg/ml as protein in McIlvaine buffer and mixed using a stirrer for 15 min. The eucalyptus extract (200–1000 μg/ml) and 1 ml of 50% tetrahydrofuran (THF) solution were mixed in test tube, 200 μl of the solution of porcine pancreatic lipase was then added and incubated at 37 °C for 15 min with shaking and 2 ml substrate was placed in the test tube and incubated for 24 h at 37 °C. The reaction was stopped by adding 95% ethanol (3 ml), and the released fatty acids followed by 2 drops of indicator. Thymolphthalein indicator was titrated by 0.05 M NaOH. The blank was measured using 50% THF without eucalyptus extract whereas the standard used was a mixture of distilled water, buffer, and enzyme only.
2.8. Antibacterial activity
The agar overlay technique was used to determine the antibacterial activity as described by Hocett et al. (2017) with slight modification.21 Bacterial strains were grown overnight on LB broth at 37 °C. The top layer of agar plate was overlain with the soft agar consisting of 0.75% agar and 5 × 107 CFU of the test microorganism. The disc size of 6 mm were dipped into the plant extract (5–0.625 mg/ml) and placed into the agar plate. They were cultured and incubated for 24 h at 37 °C. Bacterial growth inhibition was measured and compared with standard antibiotics.
2.9. Acute oral toxicity
The methanol and 50% ethanol crude extract was used to determine the acute oral toxicity test. The acute oral toxicity was measured as described by Ahmed et al.22 This test allowed us to determine the LD50 value of given extract. The test was performed in which animals were dosed at a concentration of 300–2000 mg/kg, one at a time. Each group consists of 6 test animals (mice weighing 25–30 g m). The test substances were administered in a constant volume (not exceeding 1 ml/100 g m body weight) over the range of doses to be tested by varying the concentration of dosing preparation. 50% ethanol extract and methanol extract with three different doses were given to 6 mice in each group.
2.10. Anti-ulcer activity
The anti-ulcer activity was measured following the standard protocol.23 The mice were divided into four groups each consisting of six mice with varying weights as given below.
Group-I: Normal/control animals received 2 ml/kg distilled water, per body weight orally
Group-II: 1 ml/kg ulcer inducer, ranitidine 50 mg/kg was used as the standard
Group-III: 1 ml/kg ulcer inducer, 300 mg/kg 50% ethanol extract
Group-IV: 1 ml/kg ulcer inducer, 300 mg/kg methanol extract
The weights of individual mice were noted. The mice were kept in fasting for 12 h prior to the test. The test mice groups were fed with extract and standard drug. One hour later ulcer inducer (60% ethanol, 37% HCl) in the ratio of (8:2) was given. The mice were subjected to chloroform anaesthesia after half an hour and sacrificed by cervical dislocation. The stomach of mice was isolated, washed gently under clean flowing water and cut open along the greater curvature. The macroscopic evaluation of stomachs were examined by a magnifier lens to assess the formation of ulcers. The pH, presence and absence of swelling was noted.
The number of ulcers was counted. Ulcer scores were recorded using the following classification category scale:
Red color: 0.5 point
Red spot: 1 point
Hemorrhagic streak: 1.5 point
3-5 Ulcer: 2 point
>5 Ulcer: 3 points
The percentage protection index was calculated.
| Mean ulcer index = Mean ulcer score/sample number |
2.11. Anti-inflammation activity
Anti-inflammatory activity of the plant extract was measured using the standard protocol.24 The rats were kept in fasting for 24 h. The rats weighing 150–200 g were divided into 4 groups each consisting of six rats. The different groups of rats were fed with standard drugs and extract. After 1 h, 0.1 ml of 1% w/v Carrageenan suspension was injected subcutaneously into the planar surface of the right hind paw. The paw volume was measured using a digital plethysmometer before injection, after injection i.e. in interval of 60 min for 3 h after carrageenan injection. The change in volume was determined.
Group I: 2 ml/kg distilled water
Group II: Positive control treated with dose 5 mg/kg
Group III: 300 mg/kg 50% ethanol extract
Group IV: 300 mg/kg methanol extract
Inflammation inhibition percentage was determined by the following equation:
2.12. Analgesic activity
Tail flick methods in rat model were used to test the analgesic activity of crude extract.25 The experiment was carried out by measuring tail withdrawal time from hot water. In total 24 mice were randomly divided into four groups (I-IV) of 6 mice per group and fasted for 12 h. Mouse tail (3–5 cm) was dipped in the water bath containing warm water maintained at temperature 50 ± 1 °C. Pain reaction time (PRT) taken by the mouse to flick the tail was recorded from 0 to 180 min duration.
Group I: 2 ml/kg distilled water
Group II: Positive control treated with 54 mg/kg of aspirin
Group III: 800 mg/kg 50% ethanol extract
Group IV: 800 mg/kg methanol extract
2.13. Anti-diarrheal activity
Gastrointestinal transit was measured using the charcoal propulsion test.26 These animals were divided into 4 groups each containing 6 mice. Thirty minutes following the treatments, each mouse was orally given 0.3 ml of a charcoal meal (5% activated charcoal suspended in aqueous solution). The animals were sacrificed with the chloroform anaesthesia. Mice were dissected 30 min later by cervical dislocation, and the intestines were removed from the pylorus through the ileocecal junction.
Group-I: Normal/control mice received 2 ml/kg distilled water
Group-II: 100 mg/kg loperamide was used as the standard
Group-III: 500 mg/kg 50% ethanol extract
Group-IV: 500 mg/kg methanol extract
The extent of charcoal propulsion in the small intestine was measured distance traveled by the charcoal (from the pylorus to the most distal part of the small intestine) and expressed as follows:
3. Result
3.1. Phytochemical screening and antioxidant activity
The extract obtained from the E. camaladulensis Dehnh varied in the yield and other physical parameters. The yield of the extract was high in 50% ethanol (16.92%) compared to that of methanol (14.20%). Other physical characters are summarized in Table 1. The phytochemical analysis of the crude extract shows the presence of glycoside, flavonoids, steroids, and result were summarized in Table 2. DPPH free radical scavenging activity was used to study the antioxidant properties of plant extract. The result showed higher radical quenching capability of methanol extract compared to the 50% ethanol extract with the IC50 value of 601.80 μg/ml compared to 1279.58 μg/ml.
Table 1.
Physical characteristics of the methanol and 50% ethanol extract of E. camaladulensis Dehnh.
| S.N | Extract | Color | Aroma | Yield (%) |
|---|---|---|---|---|
| 1. | 50% ethanol | Brownish green | Herbal | 16.92 |
| 2. | Methanol | Dark green | Herbal | 14.20 |
Table 2.
Phytochemical analysis of methanol and 50% ethanol extract of E. camaladulensis Dehnh.
| Phytonutrients | Methanol extract | 50% ethanol Extract |
|---|---|---|
| Carbohydrate | + | + |
| Protein | – | – |
| Starch | – | – |
| Saponin | – | + |
| Phenol | – | + |
| Flavonoids | + | + |
| Glycosides | + | + |
| Terpenoids | + | + |
| Cardiac Glycoside | + | + |
| Phytosterols | + | + |
| Diterpenes | + | + |
| Reducing Sugar | – | – |
| Tannin | – | – |
| Alkaloids | – | – |
Where, (+) is present and (−) indicates not detected.
3.2. Enzymatic assay
Enzymatic inhibition assay of α-amylase and lipase with crude methanol and 50% ethanol extract of E. camaladulensis Dehnh was performed as described in material and methods. Despite using crude extract, 33.89% (IC50 = 1.44 mg/ml) and 14.72% (IC50 = 8.86 mg/ml) of α-amylase inhibition was observed in methanol and 50% ethanol extract, respectively. The descriptive analysis of five different concentration of extract using one way ANOVA shows significant at 95% confidence interval analyzed. Similarly, 33.87% (IC50 = 3.2 mg/ml) and 33.09% (IC50 = 4.32 mg/ml) inhibition of lipase was obtained with 50% ethanol and methanol extract. The mean values are seen to be significant in 95% confidence interval analyzed via one way ANOVA in SPSS software.
3.3. Antibacterial activity
Four different concentrations (5–0.625) mg/ml of 50% ethanol and methanol extract were used to evaluate antibacterial activities of the crude extract against human pathogen using agar plate diffusion methods (Table 3). Both extracts showed the range of inhibition against a gram-positive and negative microorganism. It was observed that 50% ethanol extract at a concentration of 2.5 mg/ml showed the zone of inhibition against four human pathogens, whereas no inhibition was observed at 0.625 mg/ml. In addition, 1.25 mg/ml 50% ethanol and methanol extract revealed the inhibition of Pseudomonas aeruginosa ATCC 27853, whereas no growth inhibition was observed at this concentration with Escherichia coli ATCC 25922, Klebsiella pneumonia ATCC 700603 and Staphylococcus aureus ATCC 25923. Similarly, 2.5 mg/ml of methanol extract was found to be effective against all tested microorganism. Compared to the 50% ethanol extract, methanol extract revealed good antibacterial activities.
Table 3.
Antibacterial activity of E. camaladulensis Dehnh leaf extract.
| Solvent | Concentration of the extract | Diameter of zone of growth inhibition (mm) |
|||
|---|---|---|---|---|---|
| Staphylococcus aureus ATCC 25923 | Klebsiella pneumonia ATCC 700603 | E. coli ATCC 5922 | Pseudomonas aeruginosa ATCC 27853 | ||
| Methanol | 5 mg/ml | 11 | 12 | 15 | 15 |
| 2.5 mg/ml | 11 | 11 | 11 | 12 | |
| 1.25 mg/ml | ND | 10 | 9 | 11 | |
| 0.75 mg/ml | ND | ND | ND | ND | |
| 50%. ethanol | 5 mg/ml | 11 | 10 | 11 | 13 |
| 2.5 mg/ml | 9 | 9 | 10 | 11 | |
| 1.25 mg/ml | ND | ND | ND | 10 | |
| 0.75 mg/ml | ND | ND | ND | ND | |
Where ND is not detected.
3.4. Pharmacological assay
Furthermore, in-vivo pharmacological activities of E. camaladulensis Dehnh crude 50% ethanol and methanol extract were measured as described in material and methods. The E. camaladulensis Dehnh extract can be considered non-lethal up to a dose of 2000 mg/kg with 1 death less than half the total sampled population. This clearly indicates that leaf extract is considered to be safe for use. The anti-ulcer activity was performed in-vivo in the mouse model and the percentage protective ratio was calculated (Fig. 1). The standard drug ranitidine was used as a reference, the percent protective ratio of 44.44% and 41.67% was observed in methanol and 50% ethanol crude extract respectively (Table 4). Further, the anti-inflammation activity was analyzed via the carrageenan-induced paw edema. The 50% ethanol extract showed the higher protective ratio (54.58%) compared to that of methanol (37.64%) extract (Table 5). Furthermore, the anti-diarrheal efficacy of the extract was analyzed via the activated charcoal for the abdominal spasm. The methanol and 50% ethanol extract showed the preventive index 62.54% and 60.36%, respectively (Table 6). The result showed significances in one way ANOVA analysis at 95% confidence level.
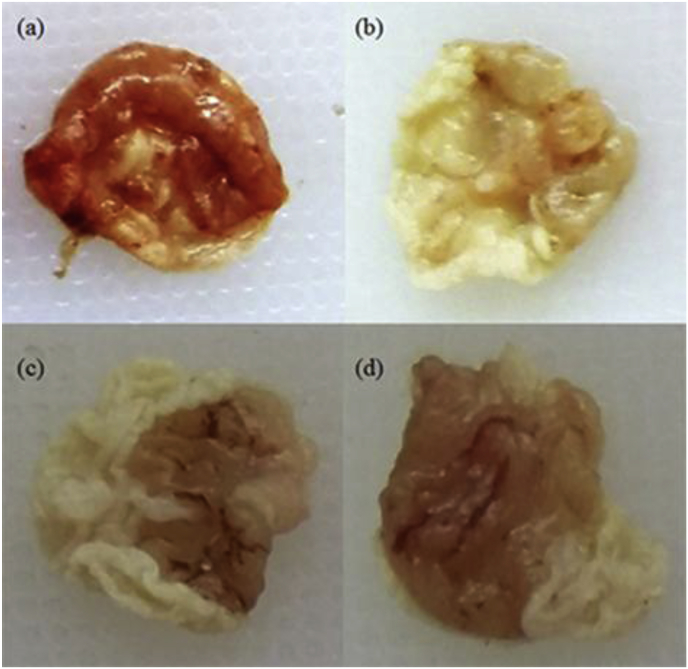
Fig. 1.
Gross appearance of gastric mucosa after various treatments. (a) Negative control: Ulcer inducer only, the maximum damage was observed as mucosal damage, spot ulcer, hemorrhagic streaks and bleeding ulcer. (b) Positive control: ranitidine treated, very few mucosal damage was observed along with all other parameters (c) Methanol extract treated (0.2 mL/20 g m), marked ulcer with mucosal damage, hemorrhagic streaks, and spot ulcer. Moderately reduced gastric ulcer as compared to the negative control. (d) Ethanol extract treated (0.2 mL/20 g m), marked ulcer with mucosal damage, hemorrhagic streaks, and spot ulcer. Moderately reduced gastric ulcer as compared to the negative control and less efficient than methanol extract.
Table 4.
Effect of various extract of E. camaladulensis Dehnh on pH and ulcer score in ulcer induced mice.
| Treatment (mg/kg) | pH | Ulcer score | Ulcer index | Percentage ulcer inhibition |
|---|---|---|---|---|
| Control | 3.67 ± 0.25 | 6.0 ± 2.44 | 1 ± 0.40 | – |
| Ranitidine-treated | 5.41 ± 0.37 | 1.0 ± 1.54 | 0.139 ± 0.23 | 86.1 |
| 50% ethanol | 4.67 ± 1.03 | 3.33 ± 1.25 | 0.56 ± 0.20 | 44.4 |
| Methanol | 4.58 ± 1.06 | 3.5 ± 2.44 | 0.583 ± 0.40 | 41.67 |
Data were analyzed using ANOVA and expressed as mean ± S.D (n = 6) followed by Dunnett's test and difference between means were regarded significant at (P < 0.05).
Table 5.
Effect of various extract of E. camaladulensis Dehnh on carrageenan-induced rat paw edema.
| Group | Treatment (mg/Kg) | Mean increase in paw volume (ml) |
% decrease in volume | |||
|---|---|---|---|---|---|---|
| 0 h | 1 h | 2 h | 3 h | |||
| 1 | Control | 1.15 ± 0.08 | 1.67 ± 0.14 | 1.88 ± 0.14 | 1.96 ± 0.15 | – |
| 2 | Aspirine | 1.12 ± 0.16 | 1.27 ± 0.16 | 1.40 ± 0.17 | 1.35 ± 0.11 | 68.08 |
| 3 | 50% ethanol | 1.19 ± 0.06 | 1.39 ± 0.22 | 1.59 ± 0.20 | 1.52 ± 0.14 | 54.58 |
| 4 | Methanol | 1.13 ± 0.18 | 1.44 ± 0.24 | 1.50 ± 0.14 | 1.69 ± 0.26 | 37.64 |
Data were analyzed using ANOVA and expressed as mean ± S.D (n = 6) followed by Dunnett's test and the difference between means was regarded significant at (P < 0.05). Here 0 h represent the volume of paw taken before the injection of carrageenan in rat paw whereas 1 h, 2 h and 3 h represents the volume of paw taken after 1 h, 2 h and 3 h of carrageen injection in planar surface of right hind paw.
Table 6.
An anti-spasmodic activity of E. camaladulensis Dehnh 50% ethanol and methanol extract.
| Test sample | Average distance traveled by charcoal (cm ± S.D) | % Mobility | % Inhibition |
|---|---|---|---|
| Blank | 16 ± 0.63 | 78.13 | 21.86 |
| Loperamide | 5.07 ± 1.34 | 22.48 | 77.51 |
| 50% ethanol extract | 14.08 ± 1.18 | 60.36 | 39.63 |
| Methanol extract | 14.91 ± 0.53 | 62.54 | 37.45 |
Data were analyzed using ANOVA and expressed as mean ± S.D (n = 6) followed by and difference between means was regarded significant at (P < 0.05).
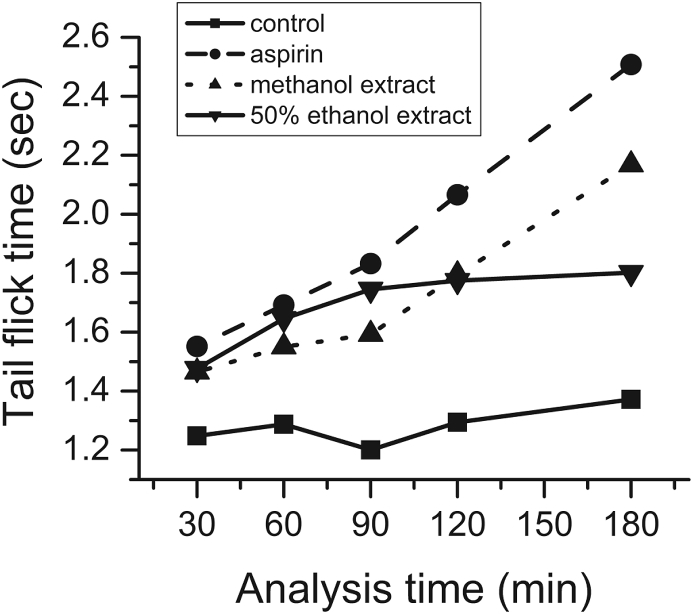
The analgesic activity was analyzed via tail flick method. Highest effect of the extract was seen in 180 min with the methanol extract whereas 50% ethanol extract showed the dominant activity in 60 and 90 min with the turn over time in around 90 min concluding the fact 50% ethanol extract may be full of an analgesic component which acts quickly in the sensory receptors with the increase in time their effectiveness may decrease whereas the methanol contains more robust component altering the activity of the sensory receptors. Detailed activity with respect to time is presented in Fig. 2.
Fig. 2.
Analgesic activity of methanol and 50% ethanol extract of E. camaladulensis Dehnh compared with aspirin as standard expressed in mean as per time of exposure plotted via Origin. The crude data are tested through one way ANOVA and found to be significant at 95% confidence level in SPSS.
4. Discussion
The result of present study signifies 50% ethanol extract of E. camaladulensis Dehnh showed greater extraction efficacy in comparison to methanol extract with greater yield and less hazardous effects. The phytochemical screening analysis revealed the presence of flavonoids, sterols, glycosides, terpenoids, and diterpenes. Saponin was detected only in 50% ethanol extract. The presence of these phytochemicals is in good agreement with potent antioxidant and antimicrobial activity of the plant extracts.
The methanol extract of E. camaladulensis Dehnh exerted greater antioxidant and antimicrobial activity in comparison to 50% ethanol extract. The presence of semi-polar compounds in methanol extract rather than the presence of highly polar compounds in 50% ethanol extract might have led to these results. The significant antioxidant activity shown by the plant extracts reveals the importance of E. camaladulensis Dehnh for developing an anti-aging cosmetic product, antioxidant medical product, and herbal products. Methanol extract and 50% ethanol extract demonstrated concentration-dependent inhibition of gram-positive and gram-negative bacteria. 50% ethanol extract did not show inhibition against any microorganism below 1.25 mg/ml concentration whereas methanol extract inhibited the growth of K. pneumoniae and E. coli up to 0.625 mg/ml.
α-Amylase hydrolyzes complex polysaccharides to simple oligosaccharides and disaccharides which are then hydrolyzed by α-glycosidase to monosaccharide. These monosaccharides are absorbed into the hepatic portal vein through small intestines, thus increasing postprandial glucose levels. Amylase inhibitors prevent dietary starch from being absorbed by the body lowering postprandial glucose levels. This property finds benefits to insulin resistance and glycemic index control in people with diabetes.27,28 Our study of E. camaladulensis Dehnh extracts exhibited low amylase inhibition activity. This result might be because of the use of crude plant extract and can be improved through purification. Digestion and absorption of dietary lipids by pancreatic lipase, a major source of excess calorie intake, serve as a potent target for the development of anti-obesity agents. The crude 50% ethanol and methanol extract of E. camaladulensis Dehnh examined in the present study revealed relatively lower enzyme inhibition activities which may be due to the use of crude extract resulting in multiple synergistic effects.29,30
The oral toxicity test carried out in mice model at four different concentrations from 300 mg/kg to 2000 mg/kg showed no significant death or signs of toxicity. At the highest concentration, only one death was observed which is statistically insignificant revealing that the extracts are well tolerated and test doses were safe for the animal.
50% ethanol extract of E. camaladulensis Dehnh exhibited better anti-ulcer property in comparison to its methanol extract. The efficacy of the extract in decreasing ulcer formation in the stomach is measured by its ability to reduce gastric volume, free and total acidity, spot formation, redness and increase in pH.31,32 In 50% ethanol extract treated mice group pH reached 4.67 ± 1.03 with 44.44% ulcer inhibition whereas in methanol extract treated mice group pH was slightly lower at 4.58 ± 1.06 with 41.67% ulcer inhibition. However, it has been reported that the protective effect of E. camaladulensis Dehnh extract as necrotization on gastric mucosa can be caused by ethanol or may be protection against 5-lipooxygenase or leukotriene pathway by stimulating prostaglandin synthesis involved in cytoprotection.33,34
The anti-inflammation activity analysis was carried out in carrageenan-induced edema in right paw of rats. As demonstrated by the result, 50% extract treated rat group showed maximum inhibition of inflammation, with the protective ratio of 54.58%, significantly comparable to standard drug aspirin. Methanol extract, however, showed inhibition of inflammation with the protective ratio of only 37.64%. On the other hand, methanol extract was found to act more quickly against inflammation than 50% ethanol extract. The increase in paw volume was lower in methanol extract treated mice after 2 h (1.50 ± 0.144 ml water displacement) of injecting the inducer compared to 50% ethanol extract 2 h later (1.59 ± 0.204 water displacement). Since the phytochemical analysis revealed higher amounts of phenolic and flavonoids in 50% ethanol extract. The anti-inflammatory actions of flavonoids in-vitro or in cellular models involve inhibition of synthesis and activities of different pro-inflammatory mediators such as eicosanoids, cytokines, adhesion molecules and C-reactive proteins.35 The higher activity of 50% ethanol extract than methanol extract can be owed to higher polarity of the 50% ethanol than that of later and could possibly have extracted polyphenolic compounds in greater concentration. Flavonoids in inflamed tissue have also been shown to inhibit cyclooxygenase, preventing the formation of prostaglandins, which stimulates pain receptors in the brain.34
Both methanol (62.54% mobility) and 50% ethanol extract (60.36% mobility) of E. camaladulensis Dehnh decreased the propulsive movement in the charcoal meal study, though the decrease in movement of charcoal was found to be less than standard drug, loperamide (22.48%) in comparison to control (78.13%). The decrease in mobility of charcoal might be due to spasmolytic and anti-enter polling property by which the extracts produced relief in diarrhea. The tannin present in plant extracts might be responsible for the anti-diarrhoeal activity which can be directly related to spasmolytic activity.
Tail-flick model was performed as thermal pain model to study the central mechanism of analgesic activity. The methanol and 50% ethanol extracts of E. camaladulensis Dehnh exhibited significant analgesic activity by increasing the reaction time of the rats to heat compared to control (D/W treated rats) at all-time points, except at 180 min. Highest effect of the methanol extract was seen in 180 min whereas 50% ethanol extract showed the dominant activity in 30 and 180 min. The results were comparable with that of aspirin. The methanol extract exhibited significant analgesic activity in the tail-flick model (P < 0.05) by increasing the reaction time of the rats to 1.9 s at 60 min after treatment in comparison to control (1 s). Similarly, 50% ethanol extract exhibited analgesic activity in a tail-flick model by increasing the reaction time to 1.45 s at 60 min after treatment. Analgesic property of the plant extracts could be attributed to the presence of flavonoids and tannins.
The present study reports the presence of different phytochemicals in extracts of E. camaladulensis Dehnh. Both 50% ethanol extract and methanol extract of the plant exhibited anti-oxidant, anti-bacterial, anti-ulcer, anti-inflammatory, analgesic and anti-diarrheal properties. The fractionation and purification of the plant extract to identify and isolate specific active compounds responsible for different properties of a plant should be investigated in future. This opens the prospect for utilizing the plant for development of new therapeutics while supporting the traditional use of plants for purposes already mentioned.
Ethics approval and consent to participate
All procedures for maintenance and sacrifice (care and use) of animals were carried out according to the Nepal Health Research Council guideline and were approved by Ethical committee of Department of Plant Resource Government of Nepal.
Conflicts of interest
The author declare no conflict of interest.
Funding
This study was self-funded.
Acknowledgement
We would like to acknowledge Natural Product Research Laboratory (NPRL), Department of Plant Resources, Ministry Of Forest and Soil Conservation, Government of Nepal for providing facility to conduct the animal model experiments. We also would like to acknowledge Research assistant Ms. Triza Maharjan, Ms. Jyoti Lama and Ms. Sony Manandhar for technical support. Further, we also like to acknowledge Mrs. Rosa Ranjit, Senior Scientist, Nepal Academy and Science and Technology (NAST) for providing the bacterial strains for antibacterial activity.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
References
- 1.Grattapaglia D., Vaillancourt R.E., Shepherd M. Progress in Myrtaceae genetics and genomics: Eucalyptus as the pivotal genus. Tree Genet Genomes. 2012;8:463–508. [Google Scholar]
- 2.Dhakad A.K., Pandey V.V., Beg S., Rawat J.M., Singh A. Biological, medicinal and toxicological significance of Eucalyptus leaf essential oil: a review. J Sci Food Agric. 2018;98(3):833–848. doi: 10.1002/jsfa.8600. [DOI] [PubMed] [Google Scholar]
- 3.Mulyaningsih S., Sporer F., Zimmermann S., Reichling J., Wink M. Synergistic properties of the terpenoids aromadendrene and 1,8-cineole from the essential oil of Eucalyptus globulus against antibiotic-susceptible and antibiotic-resistant pathogens. Phytomedicine. 2010;17(13):1061–1066. doi: 10.1016/j.phymed.2010.06.018. [DOI] [PubMed] [Google Scholar]
- 4.Singab A.N., Ayoub N., Alsayed E., Martiskainen O., Sinkkonen J., Pihlaja K. Phenolic constituents of Eucalyptus camaldulensis Dehnh, with potential antioxidant and cytotoxic activities. Record Nat Prod. 2011;5:271–280. [Google Scholar]
- 5.Birben E., Sahiner U.M., Sackesen C., Erzurum S., Kalayci O. Oxidative stress and antioxidant defense. World Allergy Organ J. 2012;5(1):9–19. doi: 10.1097/WOX.0b013e3182439613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pham-Huy L.A., He H., Pham-Huy C. Free radicals, antioxidants in disease and health. Int J Biomed Sci. 2008;4(2):89–96. [PMC free article] [PubMed] [Google Scholar]
- 7.Hussain M.S., Rahman M.A., Fareed S., Ansari S., Ahmad I. Mohd. Saeed. Current approaches toward production of secondary plant metabolites. J Pharm BioAllied Sci. 2012;4(1):10. doi: 10.4103/0975-7406.92725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Viana A.F.S.C., Fernandes H.B., Silva F.V. Gastroprotective activity of Cenostigma macrophyllum Tul. var. acuminata Teles Freire leaves on experimental ulcer models. J Ethnopharmacol. 2013;150(1):316–323. doi: 10.1016/j.jep.2013.08.047. [DOI] [PubMed] [Google Scholar]
- 9.Arun M., Asha V.V. Gastroprotective effect of Dodonaea viscosa on various experimental ulcer models. J Ethnopharmacol. 2008;118(3):460–465. doi: 10.1016/j.jep.2008.05.026. [DOI] [PubMed] [Google Scholar]
- 10.Khennouf Seddik, Benabdallah Hassiba, Gharzouli Kamel. 2003. Effect of Tannins from Quercus suber and Quercus Coccifera Leaves on Ethanol-induced Gastric Lesions in Mice. [DOI] [PubMed] [Google Scholar]
- 11.Lewis D.A. Anti-inflammatory drugs from plant and marine sources. Agents Actions Suppl. 1989;27:3–373. [PubMed] [Google Scholar]
- 12.Silva J., Abebe W., Sousa S.M., Duarte V.G., Machado M.I.L., Matos F.J.A. Analgesic and anti-inflammatory effects of essential oils of Eucalyptus. J Ethnopharmacol. 2003;89(2–3):277–283. doi: 10.1016/j.jep.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 13.Qabaha K., Ras S.A., Abbadi J., Al-Rimawi F. Anti-inflammatory activity of eucalyptus spp. and pistascia lentiscus leaf extracts. African J Tradit Complement Altern Med AJTCAM. 2016;13(5):1–6. doi: 10.21010/ajtcam.v13i5.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sebei K., Sakouhi F., Herchi W., Khouja M.L., Boukhchina S. Chemical composition and antibacterial activities of seven Eucalyptus species essential oils leaves. Biol Res. 2015;48(1):7. doi: 10.1186/0717-6287-48-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chitme H.R., Chandra M., Kaushik S. Studies on anti-diarrhoeal activity of Calotropis gigantea R.Br. in experimental animals. J Pharm Pharmaceut Sci. 2004;7(1):70–75. [PubMed] [Google Scholar]
- 16.Rahman M.K., Barua S., Islam M.F. Studies on the anti-diarrheal properties of leaf extract of Desmodium puchellum. Asian Pac J Trop Biomed. 2013;3(8) doi: 10.1016/S2221-1691(13)60129-X. :639–643-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yadav R., Agarwala M. Phytochemical analysis of some medicinal plants. J Phytol. 2011;3(12):10–14. [Google Scholar]
- 18.Kim J.-S. Radical scavenging capacity and antioxidant activity of the E vitamer fraction in rice bran. J Food Sci. 2005;70(3):C208–C213. doi: 10.1111/j.1365-2621.2005.tb07127.x. [DOI] [Google Scholar]
- 19.Imam H., Mahbub N.U., Khan M.F., Hana H.K., Sarker M.M.R. Alpha amylase enzyme inhibitory and anti-inflammatory effect of Lawsonia inermis. Pakistan J Biol Sci PJBS. 2013;16(23):1796–1800. doi: 10.3923/pjbs.2013.1796.1800. [DOI] [PubMed] [Google Scholar]
- 20.Beisson F., Tiss A., Rivière C., Verger R. Methods for lipase detection and assay: a critical review. Eur J Lipid Sci Technol. 2000:133–153. [Google Scholar]
- 21.Hockett K.L., Baltrus D.A. Use of the soft-agar overlay technique to screen for bacterially produced inhibitory compounds. JoVE. 2017;(119) doi: 10.3791/55064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ahmed M. Acute toxicity (lethal dose 50 calculation) of herbal drug somina in rats and mice. Pharmacol Pharm. 2015;6(6):185–189. [Google Scholar]
- 23.Paguigan ND, Castillo DHB, Chichioco-Hernandez CL. Anti-ulcer activity of leguminosae plants. Arq Gastroenterol. 51(1):64–67. [DOI] [PubMed]
- 24.Paul S., Saha D. Anti-inflammatory and analgesic activity of stem bark of moringa oleifera.' pharmacologyonline. Asian J Res Pharm Sci. 2012;2(2):95–97. [Google Scholar]
- 25.Shoaib M., Shah I., Ali N., Shah W.A. A mechanistic approach to anti-nociceptive potential of Artemisia macrocephala Jacquem. BMC Compl Alternative Med. 2016;16(1):141. doi: 10.1186/s12906-016-1114-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pladio LP, Villaseñor I. Anti-spasmodic constituents from drimys piperita hook F. Leaves. Philipp J Sci. http://philjournalsci.dost.gov.ph/19-past-issue-vol-133-no-1-2004/277-anti-spasmodic-constituents-from-drimys-piperita-hook-f-leaves.
- 27.Mohamed E.A.H., Siddiqui M.J.A., Ang L.F. Potent α-glucosidase and α-amylase inhibitory activities of standardized 50% ethanolic extracts and sinensetin from Orthosiphon stamineus Benth as anti-diabetic mechanism. BMC Compl Alternative Med. 2012;12(1):1193. doi: 10.1186/1472-6882-12-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rahimzadeh M., Jahanshahi S., Moein S., Moein M.R. Evaluation of alpha- amylase inhibition by Urtica dioica and Juglans regia extracts. Mashhad Univ Med Sci. 2014;17(6):465–469. [PMC free article] [PubMed] [Google Scholar]
- 29.Lunagariya N.A., Patel N.K., Jagtap S.C., Bhutani K.K. Inhibitors of pancreatic lipase: state of the art and clinical perspectives. EXCLI J. 2014;13:897–921. [PMC free article] [PubMed] [Google Scholar]
- 30.Davenport H.W. Gastric mucosal hemorrhage in dogs: effects of acid, aspirin, and alcohol. Gastroenterology. 1969;56(563):439–449. [PubMed] [Google Scholar]
- 31.Serafini M., Peluso I., Raguzzini A. Flavonoids as anti-inflammatory agents. Proc Nutr Soc. 2010;69(3):273–278. doi: 10.1017/S002966511000162X. [DOI] [PubMed] [Google Scholar]
- 32.Alibabaei zahra, Pilehvarian A., Shirani M. Effect of Euphorbia helioscopia on acetic acid-induced abdominal constrictions in Balb/c mice. J Shahrekord Univ Med Sci. 2010;11(4):9–14. [Google Scholar]
- 33.Ramezani M., Amin G., Jalili E. Antinociceptive and anti-inflammatory effects of hydroalcoholic extract of Vitex agnus castus fruit in mice. J Shahrekord Univ Med Sci. 2010;11(4):46–51. [Google Scholar]
- 34.Alcaraz M.J., Hoult J.R. Actions of flavonoids and the novel anti-inflammatory flavone, hypolaetin-8-glucoside, on prostaglandin biosynthesis and inactivation. Biochem Pharmacol. 1985;34(14):2477–2482. doi: 10.1016/0006-2952(85)90529-5. [DOI] [PubMed] [Google Scholar]
- 35.Sharma P., Vidyasagar G., Singh S., Ghule S., Kumar B. Antidiarrhoeal activity of leaf extract of celosia argentea in experimentally induced diarrhoea in rats. J Adv Pharm Technol Res. 2010;1(1):41–48. [PMC free article] [PubMed] [Google Scholar]