Abstract
Nowadays, plants have been considered as powerful agents for treatment of disorders regarding to their traditional use. Plants have a special role in the treatment of various diseases in Ayurveda (Indian Traditional Medicine). Diabetes with their devastating outcomes has been discussed in Ayurveda as well. In the present study, a marketed polyherbal products (DBC & DMV), retrieved from Ayurveda, was purchased from market and its pharmacognostic standardization were performed. Quality control test for the Ayurveda tablets were performed as per Indian Pharmacopoeia. In addition to the dissolution studies for the poly herbal Ayurveda marketed formulations were assessed based on the phenolic content. Fingerprinting of phytochemical constituents of DBC & DMV was performed using spectroscopical (like IR and UV) and chromatographic techniques like HPTLC and TLC. The results showed that DBC & DMV was successfully passed quality control tests. Moreover, DBC & DMV exhibited different pharmacognostic behavior of all herbs present in the product. In addition, TLC, IR and HPTLC fingerprinting of DBC & DMV demonstrated the presence of several phenolic constituents corresponding to the poly herbs. Regarding to the role of phenolic compounds in diabetic process, DBC & DMV could be an appropriate candidate for diabetic with respect to its traditional use in Ayurveda formulation. Moreover, HPTLC fingerprinting could be utilized as an applicable method for quality control of the prepared formulation.
Keywords: HPTLC fingerprinting, Ayurveda formulations, Dissolution studies, Standardisation, Spectroscopical evaluation
Graphical abstract
1. Introduction
India can emerge as the major country and play the lead role in production of standardized, therapeutically effective Ayurveda formulations. India needs to explore the medicinally important plants. This can be achieved only if the herbal products are evaluated and analyzed using sophisticated modern techniques of standardization. Poly herbal tablet formulation 1 (DBC) and Poly herbal tablet formulation 2 (DMV) are an Ayurveda Proprietary Drugs from Himalaya Herbal Healthcare Products, India and Patanjali Ayurved Limited, India respectively. DBC and DMV are the combination of 34 and 22 polyherbal materials respectively, and controls and manage Diabetes mellitus effectively. Chemical and instrumental analysis is routinely used for analyzing single herbal ingredient drugs for the purpose of standardization.1 A single herbal drug extract was standardized on the basis of its active principles. As per literature review, only very few chemical or analytical methods are available for polyherbal drug standardization.2 So there is a need to develop a novel scheme for the standardization of the finished Ayurveda product, made up of more then one polyherbal material. Standardization of the Triphala mixture of Emblica officinalis, Terminalia chebula and T. belerica in equal proportions has been reported by the HPLC method by using the RP 18 column with an acidic mobile phase. A complete extraction of phenolic compounds was also studied, which enabled the efficient separation of total phenol compounds, that is, gallic acid, tannic acid, syringic acid and epicatechin along with ascorbic acid, within a 20 min analysis. Validation of the method was also performed in order to demonstrate its selectivity, linearity, precision, accuracy and robustness.3 Asokan4 conducted pharmacognostical and Phyto-Chemical Standardization for Tila Kwatha which is a Polyherbal Formulation. There is no method for dissolution studies for polyherbal formulations due to the presence of poly constituents. Our aim is to develop the dissolution studies for DBC and DMV based on the presence of phenolic compounds. In this present study, the scheme for the standardization of Poly herbal formulations were developed, which will give answers for almost all the requirements for polyherbal medicine standardization. Our main objectives are to standardize the herbal formulations (DBC and DMV) based on pharmacognostic evaluation and Quality control evaluation and to identify and estimate of phenolic compound in both formulations using different chromatographic and spectroscopic technique and their Comparative Evaluation includes dissolution studies.
2. Materials and methods
A packet of 120 tablets of DBC and DMV has been taken from Local Market. DBC is a Mixture of the Following 34 Polyherbal Materials; Guggul (Commiphora wightii) (purified) 30 mg; Shilajeet (purified) 30 mg; Meshashringi (Gymnema sylvestre) 30 mg; Pitasara (Pterocarpus marsupium) 20 mg; Yashti-madhu (Glycyrrhiza glabra) 20 mg; Saptarangi (Casearia esculenta) 20 mg; Jambu (Eugenia jambolana Syn. Syzygium cumini) 20 mg; Shatavari (Asparagus racemosus) 20 mg; Punarnava (Boerhaavia diffusa) 20 mg; Mundatika (Sphaeranthus indicus) 10 mg; Guduchi (Tinospora cordifolia) 10 mg; Kairata (Swertia chirata Syn. S. chirayita) 10 mg; Gokshura (Tribulus terrestris) 10 mg; Bhumyaamlaki (Phyllanthus amarus) 10 mg; Gumbhari (Gmelina arborea) 10 mg; Karpasi (Gossypium herbaceum) 10 mg; Daru haridra (Berberis aristata) 5 mg; Kumari (Aloe vera Syn. A. barbadensis) 5 mg; Triphala 3 mg; Sushavi (Momordica charantia) 20 mg; Maricha (Piper nigrum) 10 mg; Vishnu priya (Ocimum sanctum Syn. O. tenuiflorum) 10 mg; Atibala (Abutilon indicum) 10 mg; Haridra (Curcuma longa) 10 mg; Jungli palak (Rumex maritimus) 5 mg; Vidangadi lauham 27 mg; Vang bhasma 5 mg; Abhrak bhasma 10 mg; Praval bhasma 10 mg; Akik pishti 5 mg; Shingraf 5 mg; Yashad bhasma 5 mg; Trikatu 5 mg.
DMV is a Mixture of the Following 22 Poly herbal Materials: Tinospora cardifolia 15 mg; Salacia chinesis15 mg; Azadiracta indica 26 mg; Kairata (swerita chirata) 21 mg; Holarrhenna antidysenterica 35 mg; Gymnema sylvestre 21 mg; Witharia somnifera 21 mg; Gokshura (Tribulus terrestis) 15 mg; Terminalla belerica 15 mg; Emblica officinalis 15 mg; Aegle mamelos 15 mg; Curcuma zedoaria 15 mg; Ficus bengalensis A.15 mg; Adatoda vasica 21 mg; Haldi (curcuma longa) 16 mg; Acacia Arabica 21 mg; Strychnos nux-vomica 7 mg; Centratherum anthelminticum 21 mg; Picrorhiza Kurroa 42 mg; Syzgium cuminii 42 mg; Trigonella Foenum-graecum 21 mg; Asphaltum 50 mg.
2.1. Organoleptic evaluation
Organoleptic evaluation refers to evaluation of formulation by color, odor, taste, texture etc. The organoleptic characters of the samples were carried out based on the method described by.5
2.2. Quality control test for tablet formulations
The general appearance involved measurement of size, shape, color presence (or) absence of powder taste and surface texture were observed. Standard physical tests for the marketed Ayurveda formulation tablets were performed and average values calculated. Mass variation was determined by weighing 20 tablets individually, and the average mass and percent variation of each tablet was calculated. Hardness was determined by taking 6 tablets from each formulation using a Monsanto hardness tester (Electrolab Pvt. Ltd. India) and the average pressure (kg cm−2) applied to crush the tablet was determined. Friability was determined by first weighing 20 tablets after dusting and then placing them in a Roche Friabilator, which was rotated for 4 min at 25 rpm. After dusting, the total remaining mass of the tablets was recorded and the percent friability calculated. Thickness was determined by Digital Vernier Calipers and expressed in mm. 21 Disintegration test was determined by one tablet was introduced into each tube of basket rack assembly of disintegration apparatus and cylindrical discs are placed on the top of tablets. The apparatus was operated by using water as an impression liquid at 37 ± 2 °C. Disintegration time for both formulations was noted.
2.3. Pharmacognostical evaluation
Tablets were powdered using Mortar and pestle. For microscopical study, fine powdered tablets were taken and stained with phoroglucinol and HCl. Physico-chemical studies like total ash, water soluble ash, acid insoluble ash, sulphated ash, water and alcohol soluble extract, loss on drying at 105 °C, and extractive values by soxhlet extraction method were carried out as per the WHO guide lines.6One mg of powdered drugs of each formulation was exposed to ultraviolet light at wavelength of 254 nm and 366 nm and in daylight while wet after being treated with different reagents.7
2.4. Extraction
The extracts of DBC and DMV were prepared by soxhlation with ethanol and water. The shade dried whole plant powder was packed in thimble kept in the soxhlet apparatus and extraction was allowed to run separately using the ethanol and water. Finally, the Marc was dried. Ethanol and aqueous extract were concentrated by evaporating the solvent and the obtained extracts were weighed. The physical characteristics and percentage yield of various extracts were reported. The dried extracts of all solvent were in desiccator prior to analysis.
2.5. Phytochemical screening
All the extracts were subjected to preliminary phytochemical screening for the detection of various chemical constituents. The presence or absence of different phytoconstituents viz. carbohydrates, proteins and amino acids, glycosides, saponins, alkaloids, phenolic contents and tannins were detected by usual prescribed methods.8,9
2.6. Preliminary thin layer chromatography
Qualitative determination of phyto constituents like phenolic content, tannins and flavonoids were determined by thin layer chromatography technique. Two extracts were dissolved their respective solvents and spotted on TLC plates (silica gel GF plates). The plates were developed in Toluene-Acetone-Formic acid (4.5: 4.5: 1) for the determination of phenolic compound; n-Butanol–Glacial acetic acid–water (4:1:5) for the determination of tannins; Toluene-ethyl acetate-Glacial acetic acid (30:40:5) for the determination of flavonoids. After developing the plate, they were dried and the resolution of components of extracts was studied by locating various spots on chromatogram using Folin CIO-calteu reagent and sodium carbonate solution for Phenolic content; UV Light for tannins; and mixture of 1% FeCl3 and 1% Potassium ferric cyanide for flavonoids. Measure and record the distance of each spot from the point of its application and calculate the Rf. Value.10,11
2.7. Total phenolic contents
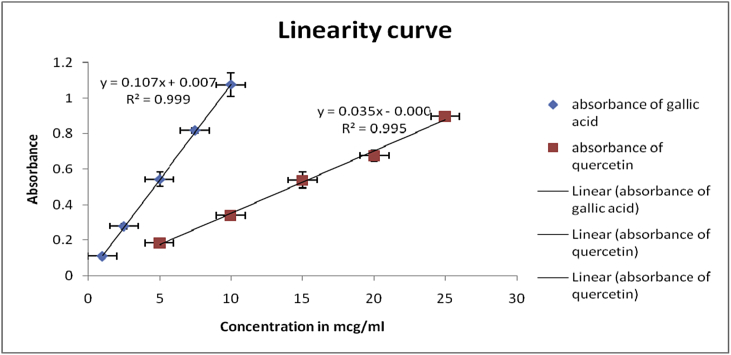
Total phenolic content was analyzed spectrophotometrically by a modified Folin- CIO calteu colorimetric method. 18, 22 0.125 ml of all the extracts (1:10 g/ml) was taken in each test tube. 1.5 ml of water and 0.125 ml of Folin-CIO calteu reagent were added and allowed to stand for 6 min. 1.25 ml of 7% sodium carbonate and 3 ml of water were added in to each mixture then allowed to stand for 90 min at room temperature. After the color formation, the absorbance was measured at 550 nm using Labindia UV- Visible spectrophotometer. Gallic acid was used to prepare a standard curve (1–10 μg/ml; y = 0.1071x+0.007829; r2 = 0.9987 ± 0.0016; y is the absorbance; x is the solution concentration). The results were expressed as milligrams of Gallic acid equivalents (GAE) per gram of powdered crude drug.
2.8. Total flavonoid contents
Total flavonoid contents of the DBC & DMV extracts were determined according to the described method using quercetin as a reference compound. Total flavanoids were determined by the following method. One mL of an extract in methanol (10 g/L) was mixed with 1 ml aliuminium trichloride in ethanol (20 g/L) individually and diluted with ethanol to 25 mL. The absorption of 400 nm was read after 40 min at 20 °C. Blank samples were prepared from 1 ml plant extract and 1 drop acetic acid, and diluted to 25 mL. The quercetin calibration curve was prepared in ethanolic solutions with same procedure. The different concentrations of quercetin were prepared with ethanolic solutions. The total content of flavonoid in plant extracts in quercetin equivalents was calculated. Quercetin was used to prepare a standard curve (5–25 μg/ml; y = 0.03524x+0.000093; r2 = 0.9941 ± 0.0071; y is the absorbance; x is the solution concentration). The results were expressed as milligrams of quercetin equivalents (QE) per gram of powdered crude drug. Linearity curve of gallic acid and quercetin were present in Fig. 1.
Fig. 1.
Linearity data for Gallic acid and quercetin.
2.9. FT-IR study
Infrared spectrum was taken (FT-IR, Spectrum RX 1, Perkin Elmer Ltd, Switzerland) by scanning the sample in potassium bromide discs. The samples of both formulationsand standards were scanned individually to find the common bands of the vibrational spectra of standards of phenolic compounds and formulations for ensuring the presence of phenolic group.
2.10. HPTLC analysis
HPTLC Analysis of marketed Ayurveda formulations (DBC & DMV) for Phenolic Profile was analyzed by the following. The water and ethanol extracts were dissolved in 1 ml of appropriate solvents and centrifuged at 3000 rpm for 5min. The same procedure was followed for the reference standards such as Quercetin, Rutin, Gallic acid and Kaempferol. These solutions were used as a test solution for HPTLC analysis. 2 μl of the above test solutions were loaded as 5 mm band length in the 5 × 10 cm Silica gel 60F254 TLC plate using a Hamilton syringe and Camag Linomat 5 instruments. The samples loaded plate was kept in TLC twin trough developing chamber (after saturated with Solvent vapor) with the mobile phase of Toluene-Acetone-Formic acid (4.5: 4.5: 1) and the plate was developed in the same mobile phase up to 90 mm. The developed plate was dried by hot air to evaporate solvents from the plate. The plate was kept in Photo-documentation chamber (Camag Reprostar 3) and captured the images at White light, UV 254 nm and UV366 nm. Before derivatization, the plate was fixed on scanner stage (Camag TLC Scanner 3) and scanning was done at UV 254 nm. The Peak table, Peak display and Peak densitogram were noted. Then the developed plate was sprayed with 20% Sodium carbonate solution sprayed and brief dried followed by Folin CIO-calteu reagent and dried at 100 °C in Hot air oven. The plate was photo-documented at Day light using Photo-documentation (Camag Reprostar 3) chamber.10,11
2.11. Dissolution studies based on the phenolic content
Release of Phenolic content in marketed Ayurveda formulations was determined using a Dissolution Apparatus Type II of USP (Paddle) at 50 rpm. The dissolution was studied using 900 mL of 5.8 pH phosphate buffer solution. The temperature was maintained at 37 ± 0.5 °C. The sample (5 mL) was withdrawn at different time intervals, i.e., 5, 15, 30, 45, 60 and 90 min, filtered through Whatman filter paper (Auroco Pvt Ltd, Thailand) and replaced by an equal volume of dissolution medium. Filtrate was placed in 50 ml volumetric flask individually. Then 10 ml of water and 1.25 ml of Folin-CIO calteu reagent were added and allowed to stand for 6 min. 12.5 ml of 7% sodium carbonate was added in to each mixture then made upto the mark with water. The mixtures individually were allowed to stand for 90 min at room temperature. After the color formation, the absorbance was measured at 550 nm using Elico UV- Visible spectrophotometer. Gallic acid was used to prepare a standard curve (1–10 μg/ml; y = 0.29146x-0.33607; r2 = 0.997695 ± 0.0015; y is the absorbance; x is the concentration). The percentage of phenolic content release was calculated.
3. Results and discussion
3.1. Quality control test
DBC was green on inner pale brown in color and DMV was grayish brown in color. DBC and DMV have slightly bitter in taste, characteristic odor and smooth, soft in touch and round in shape. Diameter & width of DBC and DMV were 11 mm & 0.3 mm and 10 mm & 0.3 mm respectively. In a weight variation test, mass values of the DBC tablets were between 0.45 and 0.51 gm; for DMV, is between 0.52 and 0.56 gm. The average percentage deviation of both tablet formulations was found to be within the limit, and hence all formulations passed the test for uniformity of weight as per official requirements. The hardness of DBC and DMV tablets were 2.33 ± 0.2581 kg/cm2 and 5.33 ± 0.2581 kg/cm2. Hardness studies indicated the strength of tablets. In general, tablets should be sufficiently hard (standard range from 4 to 8 kg/cm2) to resist breaking during normal handling and yet soft enough to disintegrate properly after swallowing. A force of minimum 4 Kg is considered as minimum requirement for a satisfactory tablet. For DBC tablets the hardness is not satisfactory, it may be because of less binder usage, punching pressure difference and less compressive force may affect the hardness.12The percentage friability of DBC and DMV tablets were0.843 and 0.752%w/w respectively. Conventional compressed tablets that lose less than 1% of their weight are generally considered acceptable. In the present study, the percentage friability for both formulations was below 1%, indicating that their friability was within the prescribed limits. Deterioration time of the poly herbal formulations depends upon the amount of water present in plant material. If the water content is high, the formulations can be easily deteriorated due to fungus. The percentage of loss on drying at 105 °C in DBC and DMV tablets were found to be 0.01 ± 0.005 and 0.02 ± 0.008%w/w respectively. Values of the hardness test and percent friability indicate good handling properties of the Ayurveda marketed tablets. Disintegration time for DBC was between 19min 22 s and 20min 50 s; for DMV was between 44min 55 s and 45min 35sec. The standard set for this experiment is to have the tablet disintegrate not more than half an hour in water medium. Tablets of DMV were shown more than 44min. But this result did not really imitate how the preparation would disintegrate in human body. Multiple parameters to really imitate our body system upon drug intake were not provided. DBC and DMV were given successful results.13
3.2. Pharmacognostic evaluation
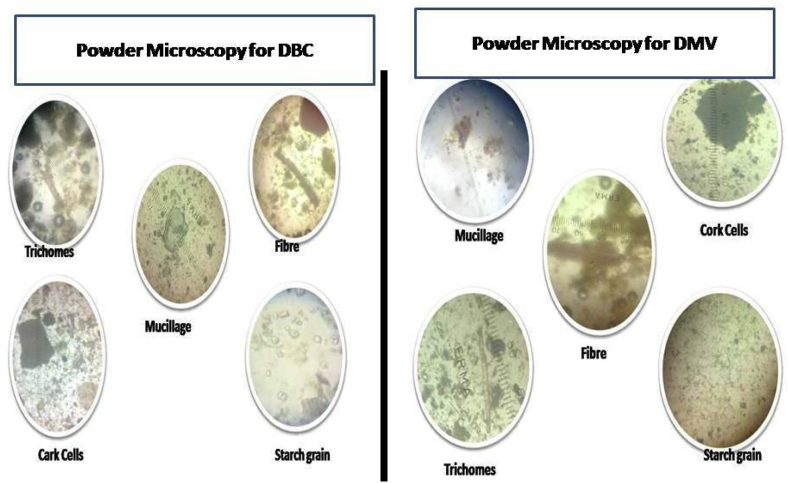
The powdered microscopy of the whole plant reveals the presence of the following, Cork cells was found to be thin walled & polygonally arranged. Unicellular trichomes, lignified phloem fibres and circular to oval shaped mucilage were observed. Starch grains present was circular to oval in shape. The results of quantitative microscopy and Physio-chemical parameters of whole powder of DBC and DMV were presented in Table 1. Fig. 2 expressed the powder microscopical report of DBC and DMV. Total ash value of plant material indicated the amount of minerals and earthy materials present in the plant material. Analytical results showed total ash value of DBC and DMV 13 and 17.5%w/w respectively. Ash values are used to determine quality and purity of crude drug. It indicates presence of various impurities like carbonate, oxalate and silicate. The water soluble ash is used to estimate the amount of inorganic compound present in drugs. The acid insoluble ash consist mainly silica and indicates contamination with earthy material. Moisture content of drugs should be at minimal levels to discourage the growth of bacteria, yeast or fungi during storage. Water-soluble extractive value indicated the presence of sugar, acids and inorganic compounds. The alcohol soluble extractive values indicated the presence of polar constituents like phenols, alkaloids, steroids, glycosides, flavonoids and n-hexane (hot) extractive values indicate the non-polar secondary metabolites present in the formulations.14 The extractive values of DMV in water were found to be higher than alcohol extractive values. But in DBC alcohol soluble extract value were higher than water soluble extractive values. In fluorescence analysis the powder samples were exposed to ultraviolet light at wavelength of 254 nm and 366 nm and day light after being treated with different reagents. Fluorescence analysis results shows whether any fluorescent ingredients are present or not. It will act as a tool to detect adulterants and substituent and will help in maintaining the quality, reproducibility and efficacy of natural drugs.
Table 1.
Phytochemical analysis data.
| Quantitative Parameter | DBC | DMV |
|---|---|---|
| Phloem fibers (width & length) | 12.5μ &146.875μ | 12.5μ & 125μ |
| Starch grains (width & length) | 9.375μ & 9.375 μ | 3.125 μ & 3.125 μ |
| Mucilage (width & length) | 31.25 μ & 50 μ | 31.25 μ & 31.25 μ |
| Cork cells (width & length) | 46.875 μ & 53.125 μ | 50 μ & 62.5 μ |
| Trichomes (width & length) | 6.25μ & 65.625μ | 3.125μ & 84.375μ |
| Total ash value | 13%w/w | 17.5%w/w |
| Acid insoluble ash | 0.5%w/w | 0.5%w/w |
| Water soluble ash | 4.5%w/w | 5%w/w |
| Sulphated ash | 2.47%w/w | 2.5%w/w |
| Moisture content | 0.013%w/w | 0.02%w/w |
| Alcohol soluble extract value | 27.4%w/w | 23.75%w/w |
| Water soluble extract value | 24.5%w/w | 31.45%w/w |
Fig. 2.
Powder microscopical image.
3.3. Extraction and phyto chemical screening
After extraction with ethanol and water solvents, the residues were dried and measured. The percentage yield obtained was 22.9% and 28.8%w/w for alcohol and water extract of DBC respectively; 21.5% and 55.45%w/w for alcohol and water extract of DMV respectively. The brown residues were for alcohol and water extract of DBC and DMV respectively. All the extracts were sticky in nature. The extractions of any crude drug with a particular solvent yield a solution containing different phytoconstituents. The compositions of these phytoconstituents depend upon the nature of the drug and the solvent used. It also gives an indication whether the crude drug is exhausted or not.15 Phytochemical screening of the ethanolic and water extracts of DBC and DMV were given general ideas regarding the nature of chemical constituents present in the drug.16 Preliminary phyto chemical studies confirmed the presence of carbohydrates, flavanoids, saponins, alkaloids, phenols and tannins in ethanolic and water extracts of DBC and DMV. The reports of phyto chemical analysis were reported that all four extracts doesn't consist glycosides and phytosterols.
3.4. Preliminary thin layer chromatography
Preliminary TLC separation and identification of phenolic compounds, flavonoids, tannins and saponins in alcoholic and water extracts of DBC and DMV were performed using the chromatographic system with Silicagel GF as stationary phase and the corresponding mobile phase mentioned above. Gallic acid, Tannic acid, Quercetin and liquorice used as a standard for phenolic compounds, tannins, flavonoids and saponins respectively. Spots of standards were easy to detect and compare with samples spots. After using visualizing agents, the spots color was dominantly appeared for above mentioned constituents. Rf values of Gallic acid, Tannic acid, Quercetin and liquorice are 0.57, 0.88, 0.66 and 0.96 respectively for corresponding mobile phase mentioned earlier. The TLC analysis in various solvent systems for each solvent type revealed the presence of spots. Each spot is presumably due to a pure natural product or phytochemical. Each also has a specific Rf value. One of the Rf values of sample spot was coincided with Rf values of standards used. Alcoholic extract of DBC and DMV were shown the presence of Gallic acid, tannic acid, quercetin and saponins, but in water extracts of both formulations, except quercetin, remaining three constituents were present. These results are preliminary studies to confirm the presence of phyto constituents.
3.5. Total phenolic content and total flavonoid content
The milligrams of Gallic acid equivalents (GAE) per gram of ethanol and aqueous extract of DBC were found to be 4.503 ± 0.41 and 2.201 ± 0.107 respectively. The milligrams of Gallic acid equivalents (GAE) per gram of ethanol and aqueous extract of DMV were found to be 5.21 ± 0.093 and 4.22 ± 0.385 respectively. The ethanol extract of DMV product contained maximum total phenolic content (5.21 mg GAE/g) than other extract. The milligrams of quercetin equivalents (QE) per gram of ethanol and aqueous extract of DBC were found to be 1.91 ± 0.134 and 1.67 ± 0.147 respectively. The milligrams of quercetin equivalents (QE) per gram of ethanol and aqueous extract of DMV were found to be 1.77 ± 0.93 and 2.4 ± 0.534 respectively. The water extract of DMV product contained maximum total phenolic content (2.4 mg QE/g) than other extract. Total phenolic content and flavonoid content estimation of the Alcoholic and water extracts of DBC and DMV were evaluated and reported in Fig. 3.18 reported that the usage of Folin-CIO calteu reagent also was measured based on the color measurement which was non-specific on phenol. Perhaps there were other components that can react with the reagent such as ascorbic acid. Besides, various phenolic compounds have different response to this assay. However, the measurement of color changes after 2 h storage could be used to determine the existence of phenol in samples. This may due to the antioxidant properties of plant extract that react as reducing agent which known as redox action.
Fig. 3.
Total phenolic content and flavonoid content.
3.6. FTIR studies for phenolic compounds
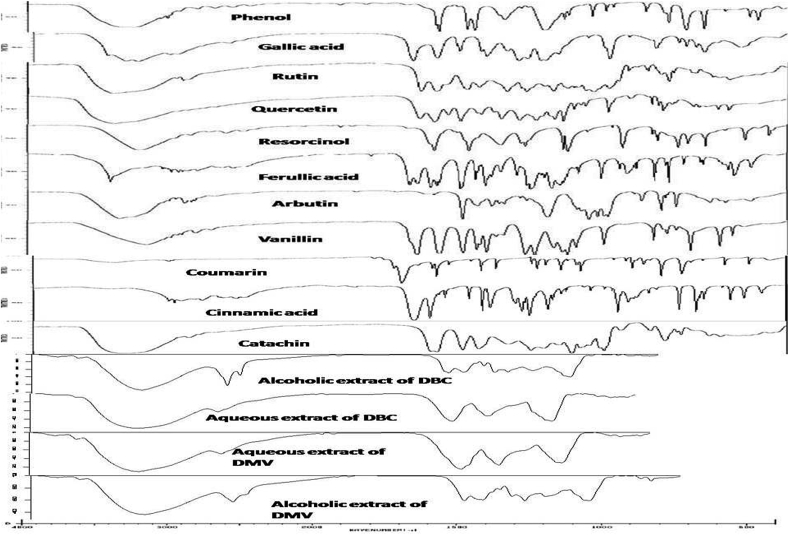
IR spectra of Standards like phenol, gallic acid, rutin, quercetin, resorcinol, ferulic acid, arbutin, vanillin, coumarin, cinnamic acid, and catachin were used for comparison studies. IR spectra of all extracts of both tablets were compared with Standard IR spectra using KBr Disc method. Overall results of FTIR spectra of alcoholic and water extracts of two different marketed formulations were shown that the presence of alcoholic group (at 3390/cm) and ethylenic group (at ∼1620/cm for C=C). It was compared with standards and the reports suggested that presence of phenolic groups in the extracts of Alcoholic and water extracts of DBC and DMV. IR spectrum of alcoholic extract of DBC products was shown the presence of carboxylic acid (at 2854/cm for COOH and at 1708/cm for C=O). From the results, the alcoholic extract of DBC product may consist cinnamic acid or ferulic acid or gallic acid etc., Fig. 4 expressed the overlap image of FTIR spectra of standard phenolic content and Extracts of DBC and DMV.
Fig. 4.
Overlap FTIR profile.
3.7. HPTLC analysis for phenolic compounds
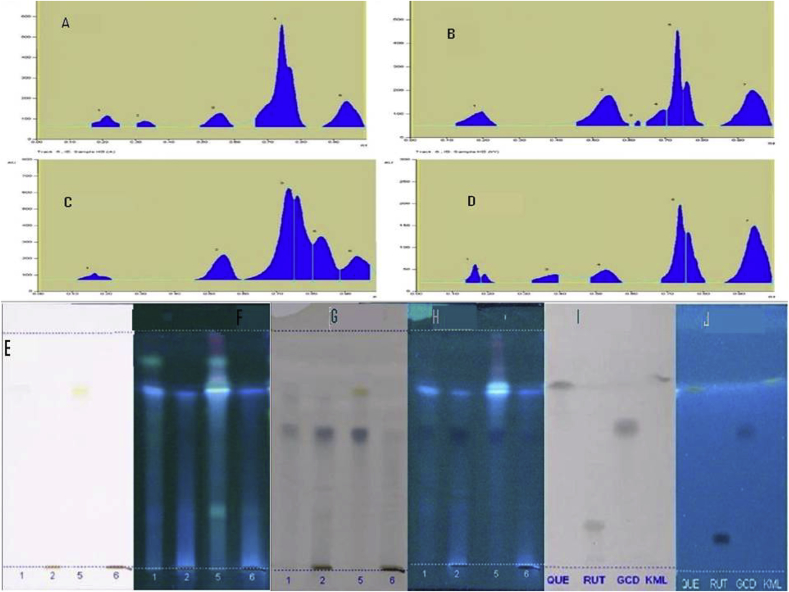
HPTLC coupled with CAMAG TLC SCANNER 3 was employed to separate, identify and quantify phenolic compounds in the alcoholic and aqueous extract of DBC and DMV. Blue colored zone at Day light mode present in the given standard and sample tracks observed in the chromatogram after derivatization, which may be the presence of Phenolics in the given samples. The concentrations were determined by calculating the spot areas which are proportional to the amount of analyte in a peak and presented. In ethanol extract of DMV product totally 5 spots were appeared among those two were found to be phenols which is similar to the Rf value of gallic acid and kaempferol. In aqueous extract of DMV product, totally 7 spots were appeared among those three were found to be phenols and among three, two spots were coincided with the Rf value of gallic acid and kaempferol. In aqueous extract of DBC product totally 7 spots were appeared among those one was found to be phenols which is similar to the Rf value of gallic acid. In ethanol extract of DBC product, totally 6 spots were appeared among those two were found to be phenols which is similar to the Rf value of gallic acid and kaempferol. Table 2 was expressed the results of HPTLC for the determination of phenol in DBC and DMV compare with Standards. Fig. 5 shows the Peak densitogram display and HPTLC chromatogram of DBC and DMV ethanol and aqueous extracts respectively. HPTLC fingerprinting of DBC and DMV demonstrated the presence of several phenolic compounds corresponding to the plant extracts. Moreover, characteristic peaks were observed in DBC & DMV profile, so HPTLC fingerprint could be used as an applicable method for quality control of the prepared formulation.
Table 2.
HPTLC Data profile of DBC, DMV and Standard phenolic compounds.
| Sample/Standard | No of spots found | No of spots identified as phenol | Rf values of identified spots | Peak area of identified spots | Identified compounds | |
|---|---|---|---|---|---|---|
| Alcoholic extract | DBC | 6 | 2 | 0.54 & 0.73 | 7176.8 & 21693.4 | Gallic acid & Kaempferol derivatives |
| DMV | 5 | 2 | 0.56 & 0.74 | 3084.4 & 21883.6 | Gallic acid & Kaempferol derivatives | |
| Aqueous extract | DBC | 7 | 1 | 0.53 | 1380.8 | Gallic acid derivatives |
| DMV | 7 | 2 | 0.55 & 0.73 | 7469.7 & 7461.5 | Gallic acid & Kaempferol derivatives | |
| Standards | Quercetin | 1 | 1 | 0.68 | 11711.0 | Quercetin |
| Rutin | 1 | 1 | 0.12 | 8282.3 | Rutin | |
| Gallic acid | 1 | 1 | 0.51 | 19467.8 | Gallic acid | |
| Kaempferol | 1 | 1 | 0.71 | 8957.7 | Kaempferol | |
Fig. 5.
HPTLC Profile; A & B are Peak densitogram of alcoholic extract and aqueous extract of DMV; C & D are Peak densitogram of alcoholic extract and aqueous extract of DBC; E & F are sample chromatogram under visible light and UV light (before derivatization); G & H are sample chromatogram under visible light and UV light (after derivatization); I & J are standard chromatogram under visible light and UV light (after derivatization); 1 & 2 denotes the alcoholic extract and aqueous extract of DMV; 5 & 6 denotes the alcoholic extract and aqueous extract of DBC; QUE- Quercetin; RUT-Rutin; GCD-Gallic acid; KML- Kaempferol.
3.8. Dissolution studies for tablet formulations
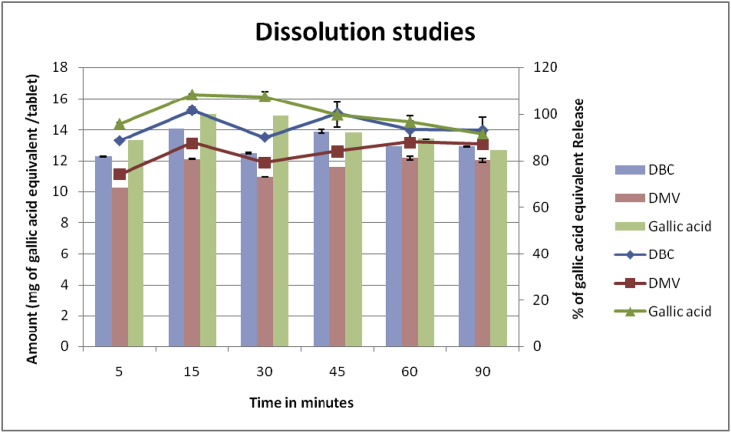
Dissolution studies were performed for DBC and DMV tablets based on the presence of phenolic compounds. Both tablets and pure gallic acid were placed in dissolution apparatus and the results were analyzed and calculated. The results were reported in milligrams of Gallic acid equivalents (GAE) per tablet in Table 3. Dissolution studies data of Amount (mg of gallic acid equivalent/tablet), % of gallic acid equivalent Release and Log% drug undissolved were evaluated. Maximum Percentage of gallic acid equivalent release was 93.78% and 81.07% for DBC and DMV respectively. Maximum gallic acid equivalent release was achieved at 15 min s for DBC; and at 60 min for DMV. Pure gallic acid was reached 100% gallic acid equivalent release at 15 min s.12 Fig. 6 expressed the Amount (mg of gallic acid/tablet) equivalent and % of gallic acid equivalent Release in DBC and DMV.
Table 3.
Dissolution studies for DBC and DMV.
| time | 5min | 15 min | 30min | 45min | 60min | 90min | |
|---|---|---|---|---|---|---|---|
| Amount (mg of gallic acid/tablet)equivalent | DBC | 13.31 ± 0.1 | 15.25 ± 0.02 | 13.52 ± 0.3 | 15.07 ± 0.8 | 14.02 ± 0.07 | 13.99 ± 0.06 |
| DMV | 11.12 ± 0.1 | 13.12 ± 0.2 | 11.89 ± 0.1 | 12.6 ± 0.1 | 13.19 ± 0.9 | 13.06 ± 0.8 | |
| Gallic acid | 14.39 ± 0.03 | 16.27 ± 0.01 | 16.15 ± 0.01 | 14.98 ± 0.01 | 14.51 ± 0.01 | 13.74 ± 0.01 | |
| % of gallic acid equivalent Release | DBC | 81.83 ± 0.1 | 93.78 ± 0.02 | 83.15 ± 0.3 | 92.65 ± 0.8 | 86.19 ± 0.02 | 86 ± 0.06 |
| DMV | 68.35 ± 0.1 | 80.69 ± 0.2 | 73.09 ± 0.1 | 77.46 ± 0.1 | 81.07 ± 0.9 | 80.31 ± 0.8 | |
| Gallic acid | 88.47 ± 0.03 | 100 ± 0.014 | 99.29 ± 0.01 | 92.08 ± 0.01 | 89.23 ± 0.01 | 84.48 ± 0.01 | |
| Log % drug undissolved | DBC | 1.25 ± 0.1 | 0.79 ± 0.02 | 1.22 ± 0.3 | 0.86 ± 0.8 | 1.14 ± 0.02 | 1.146 ± 0.06 |
| DMV | 1.5 ± 0.1 | 1.28 ± 0.2 | 1.42 ± 0.1 | 1.35 ± 0.1 | 1.27 ± 0.9 | 1.29 ± 0.8 | |
| Gallic acid | 1.06 ± 0.03 | 0 ± 0.014 | −0.14 ± 0.01 | 0.89 ± 0.01 | 1.03 ± 0.01 | 1.19 ± 0.01 |
Mean ± Standard deviation; n = 6.
Fig. 6.
Dissolution data.
TLC method is the most basic method of confirming the presence of phenolic compound. Results of Rf value of TLC and HPTLC expressed the presence of phenolic compound in the ethanol and aqueous extract of DBC and DMV. From the results of TLC and UV, The levels of these components in the various solvent extract of the DBC and DMV and also showed differences.17 found that rosemary methanol extract had higher phenolic contents than its water extract. Different levels reported in these studies may be attributed to the procedures and standards used to express as total phenolic contents used by individual groups of investigator. Based on the report of TLC, UV, IR and HPTLC, ethanol and water extracts contains remarkable levels of phenols. HPTLC analyses showed that these phenolic compounds belong for the most part to flavonoids and derivatives of phenol carboxylic acid. Both DBC and DMV contains a rich complex of biologically active compounds of phenolic nature. Diabetes mellitus is a metabolic disorder may be due to enhanced cellular oxidative stress and reduced antioxidant activity.19,20 Polyphenols and flavonoids are natural antidiabetic agents, which interferes the production of free radicals, reduce oxidative stress and inhibit digestive enzyme, thus lowering postprandial glucose. All analytical reports suggests that DBC and DMV, rich in flavonoids and phenolics have potential to contribute to the management of diabetes.
4. Conclusion
In this investigation, standardization of DBC (from Himalaya Herbal Healthcare Products, India) and DMV (from Patanjali Ayurved Limited, India)was performed based on pharmacognostic evaluation and Quality control evaluation. Identification and estimation of phenolic content in both formulations were done using different chromatographic and spectroscopic technique. Results of analytical reports suggested that DBC and DMV consists rich amount of phenolic content. Even, water and ethanol could extract the highest concentration of polyphenols from the marketed Ayurveda formulation. DBC and DMV may have a good pharmacological potency due to the presence of polyphenols. Regarding to the role of phenolic compounds in Diabetes, DBC & DMV could be an appropriate candidate for reducing the blood sugar level with respect to its traditional use in Ayurveda.
Conflicts of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this paper.
Acknowledgments
The authors wish to thank The Management and Dr.K.Ravishankar, Director, Aditya Pharmacy College for their valuable support.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
References
- 1.Sharma A., Lal K., Handa S.S. Herbal drug standardization: HPLC Determination of vasicine polyherbal formulation. Int J Pharmacog. 1992;30:205. [Google Scholar]
- 2.Dateo G.P., Long L., Jr. Gymnemic acid, the antisaccharine principle of Gymnema sylvestre: studies on isolation and heterogeneity of gymnemic acid A1. J Agric Food Chem. 1973;21:899–903. doi: 10.1021/jf60189a030. [DOI] [PubMed] [Google Scholar]
- 3.Singh D.P., Govindarajan R., Rawat A.K. High-performance liquid chromatography as a tool for the chemical standardization of Triphala: an Ayurveda formulation. Phytochem Anal. 2008;19:164–168. doi: 10.1002/pca.1032. [DOI] [PubMed] [Google Scholar]
- 4.Vasudevan Asokan, LaxmiPriya Dei, Harisha C.R., Shukla V.J. Pharmacognostical and phyto-chemical standardization of Tila Kwatha: a polyherbal formulation. Int. J. Pharmaceut. Bio. Arch. 2012;3(6):1410–1414. [Google Scholar]
- 5.Siddiqui A., Hakim M.A. Central council for research in unani medicine; New Delhi: 1995. Format for the Pharmacopoeial Analytical Standards of Compound Formulation, Workshop on Standardization of Unani Drugs. (appendix) [Google Scholar]
- 6.WHO . World Health Organisation; Geneva: 1998. Quality Control Methods for Medicinal Plant Materials. [Google Scholar]
- 7.Khandelwal K.R. ninth ed. Nirali Prakashan; New Delhi: 2002. Practical Pharmacognosy Techniques & Experiments. [Google Scholar]
- 8.Harborne J.B. second ed. Chapman & Hall; London: 1973. Phytochemical Methods. [Google Scholar]
- 9.Kokate C.K. fourth ed. VallabhPrakashan; New Delhi: 1997. Practical Pharmacognosy. [Google Scholar]
- 10.Wagner H., Bladt S. Springer publications; New York: 2004. Plant Drug Analysis, a Thin Layer Chromatography Atlas. [Google Scholar]
- 11.Stahl Egon. Springer publications; New York: 2007. Thin Layer Chromatography, a Laboratory Handbook. [Google Scholar]
- 12.Anand Kishore K., Amareshwar P. Quality evaluation and comparative study on tablet formulations of different pharmaceutical companies. J. Curr. Chem.Pharmaceut. Sci. 2012;2(1):24–31. [Google Scholar]
- 13.Uddin Md Sahab, Mamun Abdullah Al, Asaduzzaman Tanjuma Tasnuand Md. In-process and finished products quality control tests for pharmaceutical tablets according to Pharmacopoeias. J Chem Pharmaceut Res. 2015;7(9):180–185. [Google Scholar]
- 14.Fennema Owen R. third ed. Marcel Dekker, Inc; New York: 1996. Food Chemistry. [Google Scholar]
- 15.Tatiya A., Surana S., Bhavsar S., Patil D., Patil Y. Pharmacognostic and preliminary phytochemical investigation of Eulophia herbacea Lindl. Tubers (Orchidaceae) Asian Pac J Trop Disease. 2012;2(Suppl 1):S50–S55. [Google Scholar]
- 16.Rehman Shaheed Ur, Choe Kevin, Yoo Hye Hyun. Review on a traditional herbal medicine, eurycoma longifolia jack (Tongkat Ali): its traditional uses. Chem. Evi. Based Pharmacol. Toxicol. Mol. 2016;21(3):331. doi: 10.3390/molecules21030331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perez M.B., Galderon N.L., Croci C.A. Radiation induced enhancement of antioxidant activity in extracts of rosemary (Rosmarinus officinalis L.) Food Chem. 2007;104:585–592. [Google Scholar]
- 18.Shahidi F., Naczk M. Technomic Publishing Co. Inc.; Lancester: 1995. Methods of Analysis and Quantification of Phenolic Compounds. Food Phenolic: Sources, Chemistry, Effect and Applications. PA. [Google Scholar]
- 19.Sudha P., Zinjarde S., Bhargava S., Kumar A. Potent α-amylase inhibitory activity of Indian Ayurveda medicinal plants. BMC Compl Alternative Med. 2011;11(5):1–10. doi: 10.1186/1472-6882-11-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kamtekar Samidha, Keer Vrushali, Patil Vijaya. Estimation of phenolic content, flavonoid content, antioxidant and alpha amylase inhibitory activity of marketed poly herbal formulation. J Appl Pharmaceut Sci. 2014;4(09):061–065. [Google Scholar]
- 21.Lachman Leon, Lieberman Herbert A., Kanig Joseph L. Lea & Febiger; Philadelphia: 1986. The Theory and Practice Of Industrial Pharmacy. [Google Scholar]
- 22.Singleton V.L., Orthofer R., Lamuela Raventos R.M. Analysis of total phenols & other oxidation substrates & antioxidants by means of Folin–Ciocalteu reagent. Methods Enzymol. 1999;299:152–178. [Google Scholar]