Abstract
This study aimed to identify the mechanism behind idiopathic sudden sensorineural hearing loss (ISSNHL) in patients with tinnitus by investigating aberrant activity in areas of the brain and functional connectivity. High-density electroencephalography (EEG) was used to investigate central nervous changes in 25 ISSNHL subjects and 27 healthy controls. ISSNHL subjects had significantly reduced activity in the left frontal lobe at the alpha 2 frequency band compared with controls. Linear lagged connectivity and lagged coherence analysis showed significantly reduced functional connectivity between the temporal gyrus and supramarginal gyrus at the gamma 2 frequency band in the ISSNHL group. Additionally, a significantly reduced functional connectivity was found between the central cingulate gyrus and frontal lobe under lagged phase synchronization analysis. These results strongly indicate inhibition of brain area activity and change in functional connectivity in ISSNHL with tinnitus patients.
Keywords: tinnitus, ISSNHL, EEG, source localization, functional connectivity
Introduction
Sudden sensorineural hearing loss (SSNHL) defines rapid onset hearing loss with reduction in hearing of no less than 30 dB in at least three consecutive audiometric frequencies within 3 days (Stachler et al., 2012). SSNHL is typically accompanied by sudden onset of tinnitus, vertigo and ear fullness (Koç and Sanisoğlu, 2003). The incidence of SSNHL ranges from 3.9 to 27.5 people per 100,000/year (Nosrati-Zarenoe et al., 2010). The etiology of more than two thirds of SSNHL is unclear and considered idiopathic (Chau et al., 2010). Clinicians employ multiple treatment options to cure idiopathic SSNHL (ISSNHL), including oral and transtympanic steroid therapy (Kuhn et al., 2011). However, the therapeutic effectiveness between ISSNHL patients is highly variable. Unfortunately, some ISSNHL subjects do not improve even with high quality treatment, and indeed there may be some adverse effects (Kuhn et al., 2011). The therapeutic effectiveness of ISSNHL treatments may be improved were a better understanding of the mechanism behind ISSNHL available.
Tinnitus, a common and troublesome symptom of ISSNHL, is the perception of sound that lacks any external or internal sound source (Kang et al., 2017). The incidence of tinnitus in ISSNHL patients is between 80 and 95% (Rah et al., 2015; Nogueira-Neto et al., 2016). Tinnitus brings a series of adverse effects to ISSNHL patients, including insomnia and depression (Chen et al., 2013). Additionally, tinnitus symptoms often continue in ISSNHL patients with tinnitus even after treatment, indicating that these patients may have aberrant changes in the brain. Therefore, it is advisable that clinicians pay more attention to the treatment of tinnitus when acting to cure ISSNHL patients.
To treat ISSNHL and tinnitus subjects effectively requires an in-depth investigation into the underlying mechanism of ISSNHL. Current research shows abnormal brain area activity and network connectivity in ISSNHL patients. For example, Micarelli et al. (2017) showed that ISSNHL subjects had hypermetabolism in the right superior and medial frontal gyrus, and a relative reduction in fluorodeoxyglucose uptake in the right middle temporal, precentral and postcentral gyrus, left posterior cingulate cortex, left lingual, superior, middle temporal and middle frontal gyrus and left insula. A study by Xu et al. (2016) showed that SSNHL subjects had some increased activities between auditory and non-auditory networks, including the default mode network (DMN) and limbic system. Other studies have explored the intrinsic mechanism of chronic tinnitus with hearing impairment. It is generally believed that the generation and development of tinnitus is not only related to peripheral hearing impairment, but also correlated with abnormal neural activity (Chen et al., 2014, 2015a, 2016, 2017). A functional imaging study by Lanting et al. (2009) suggested that tinnitus patients had hyperactivity in the auditory system. Tinnitus subjects also show some aberrant neural activity in non-auditory regions, including the frontal gyrus, limbic system, cerebellum and insula (Chen et al., 2017). A number of studies have identified abnormal brain networks in tinnitus including the auditory network (Leaver et al., 2016), DMN (Leaver et al., 2016), dorsal attention network (Schmidt et al., 2013), ventral attention network (Burton et al., 2012) and visual system (Chen et al., 2015b). However, there are few studies exploring the difference of brain area and network connectivity between ISSNHL patients and healthy subjects. Therefore, our research aimed to investigate the underlying mechanism of ISSNHL and tinnitus based on resting-state electroencephalography (EEG).
Approximately 85% of tinnitus patients have an uninterrupted perception of tinnitus sound (Schecklmann et al., 2014). Resting state brain function measurement is therefore suitable for exploring the neural mechanism of tinnitus and ISSNHL. EEG investigates instantaneous electric activity of the brain by recording electrical potentials with electrodes placed on the scalp. It is a technique that is non-invasive and has high temporal resolution (Ingber and Nunez, 2011). At present, a number of methods can be used to extract useful information from multichannel EEG signals, including spectrum analysis, source localization and neural network function analysis. These analytical methods are useful to explore topological property alterations of the brain.
It is still unknown as to whether neural activity changes occur in ISSNHL patients with tinnitus. Thus, our study aim was to gain brain region activity and functional connectivity of central networks in ISSNHL and tinnitus patients based on resting-state EEG. Moreover, we explored the change in balance between excitation and inhibition of the whole brain in these patients.
Materials and Methods
Participants
ISSNHL patients with tinnitus were recruited from the Ear, Nose and Throat clinic, Sun Yat-sen Memorial hospital, Guangzhou, China. Selection criteria for ISSNHL subjects with tinnitus were as follows:
-
(a)
Patients had no less than 30 dB sensorineural hearing impairment associated with tinnitus in more than three consecutive audiometric frequencies that had onset within 3 days.
-
(b)
Patients had sought clinical help due to hearing impairment and tinnitus.
-
(c)
Subjects with middle ear surgery, pulsatile tinnitus, Ménière’s disease, autoimmune hearing loss, acoustic neurinoma, central nervous system disorders, head trauma, depression or insomnia were excluded.
Data was collected before patients received any therapeutic intervention within 30 days. In this study, 25 ISSNHL subjects with tinnitus (9 males and 16 females; age mean = 46.16 years, SD = 13.15 years) and 27 healthy subjects (10 males and 17 females; age mean = 41.48 years, SD = 13.53 years) were included. All participants were told about the background and purpose of the study. A written consent form was signed by all subjects. This research was approved by the Institution Review Board of the Sun Yat-sen Memorial Hospital at Sun Yat-sen University of China.
Audiological Examinations
All participants underwent otoscopy and pure tone audiometry. Subjects were asked to sit in an acoustically shielded room for the pure tone audiometry test. Hearing thresholds of the subjects were then measured. Air conduction thresholds at 0.125, 0.25, 0.5, 1, 2, 4, and 8 kHz were measured on both ears. Bone conduction thresholds at 0.25, 0.5, 1, 2, and 4 kHz were also determined. Mean hearing threshold was calculated from hearing thresholds at 0.5, 1, 2, and 4 kHz (British Society of Audiology Recommendation, 1988; Dispenza et al., 2011; Bing et al., 2018).
Tinnitus Specific Assessments
Information about the duration and laterality of tinnitus was collected. A tinnitus pitch matching test was then performed (Teismann et al., 2011). An approximate pitch match was determined using the frequencies 0.125, 0.25, 0.5, 1, 2, 3, 4, 6, and 8 kHz. Patients were asked to compare the matching pitch and perceived tinnitus pitch; the matching pitch was adjusted based on the feedback from the subject. A more accurate matching pitch was obtained by adjusting the frequency in half-octave steps. Narrow band noise was used if a matching pure tone could not be found. After finding the best matching pitch, the initial level was set to 5 dB above the audiometric threshold to find an approximation of the tinnitus loudness. Loudness was adjusted in 1 dB steps until the patient expressed a loudness match. Tinnitus loudness was computed by subtracting the presented sound intensity level with the auditory threshold at that frequency. Finally, tinnitus severity was evaluated using a Tinnitus Handicap Inventory (THI). The THI used an increasing handicap scale from 0 to 100 to assess the level of handicap (Newman et al., 1996). Patients were asked to fill in the THI before the experiment commenced.
Data Collection
Electroencephalography signals were gained using a standard procedure. EEG signals were recorded using an EGI 128 channel system. Before the experiment, participants were informed of the aim of this study to ensure they were in a relaxed state. Participants sat in an acoustic and electrically shielded room. Their eyes were open and they were asked to view a computer screen, to blink as little as possible, not to move their head and to keep awake. A mesh electrode cap was positioned with a careful positioning of the center electrode Vref. Impedances of all electrodes were kept at no more than 50 kΩ. The recording system synchronized the EEG data, and the recording lasted for 7 min.
Preprocessing of EEG Data
Recordings were analyzed in MATLAB for R2013a using EEGLAB for v13.0.0. The whole process was a seven-step instruction as follows. (1) The sampling rate was reduced to 500 Hz. (2) ERPLAB filter was used for sag filtering to remove 50 Hz power frequency interference and the data was band-pass filtered (0.01 Hz high-pass and 100 Hz low-pass). (3) The average reference of all electrodes was used. (4) EEG data was removed with a wide range of drift periods. If the electrode signal was unstable, linear interpolation was performed on the electrode. (5) Independent component analysis algorithm (ICA) was applied to remove independent components related to artifacts. (6) The EEG data was segmented (divided into 2 s). If the amplitude of a segment at any one electrode exceeded 75 μV, then the segment would be removed. (7) Average Fourier cross-spectral matrices were computed for frequency bands, including delta (0.5–3.5 Hz), theta (4–7.5 Hz), alpha 1 (8–10 Hz), alpha 2 (10–12 Hz), beta 1 (13–18 Hz), beta 2 (18.5–21 Hz), beta 3 (21.5–30 Hz), gamma 1 (30.5–44 Hz), and gamma 2 (55–100 Hz).
Data Analysis
Source Localization
Standardized low-resolution brain electromagnetic tomography (sLORETA) was applied to analyze the activation of brain regions in each group and understand the activation distribution of central brain regions in ISSNHL patients with tinnitus in the nine frequency bands mentioned above. After performing a common mean reference transformation (Pascual-Marqui, 2002), the sLORETA algorithm was applied. Electrical neuronal activity was calculated by sLORETA as current density (A.m–2). The LORETA-Key software was used to analyze the solution space applied in the research and correlated lead field matrix. The software revisited realistic electrode coordinates (Jurcak et al., 2007) and the lead field (Fuchs et al., 2002) applying the boundary element on the MMI-152 (Montreal Neurological Institute, Canada). According to probabilities returned by the Demon Atlas, the neocortical MNI-152 volume in 6239 voxels was divided and labeled by the sLORETA-Key anatomical template (Lancaster et al., 2000). The co-registration used the correct translation from the MNI-152 space into the Talairach and Tournoux space.
Functional Connectivity Analysis
Cross-talking between areas contributing to the source activity can be used to interpret the lagged phase coherence between two brain areas (Congedo et al., 2010). When two brain areas oscillate coherently with a phase lag, cross-talk can be interpreted as information sharing by axonal transmission. Precisely, the data were decomposed in a limited number of cosine and sine waves at the Fourier frequencies by using the discrete Fourier transform. The work of Pascual-Marqui (2007a, b) showed the significant threshold for a given lagged phase coherence value based on asymptotic results. We used sLORETA to extract current density for regions of interest with time series. Power in all 6,239 voxels was normalized to a power of 1 and log transformed at each time point. Therefore, region of interest values reflect the log transformed fraction of total power across all voxels for specific frequencies. As shown in Table 1, regions of interest were determined according to previous studies and the result of source localization (Raichle, 2011).
TABLE 1.
The regions of interest.
| Brain region | X | Y | Z |
| Posterior cingulate | 0 | –52 | 27 |
| Medial prefrontal | –1 | 54 | 27 |
| Left lateral parietal | –46 | –66 | 30 |
| Right lateral parietal | 49 | –63 | 33 |
| Left inferior temporal | –61 | –24 | –9 |
| Right inferior temporal | 0 | –12 | 9 |
| Medial dorsal thalamus | –25 | –81 | –33 |
| Right posterior cerebellum | 25 | –81 | –33 |
| Left frontal eye field | –29 | –9 | 54 |
| Right frontal eye field | 29 | –9 | 54 |
| Left posterior intraparietal sulcus | –26 | –66 | 48 |
| Right posterior intraparietal sulcus | 26 | –66 | 48 |
| Left anterior intraparietal sulcus | –44 | –39 | 45 |
| Right anterior intraparietal sulcus | 41 | –39 | 45 |
| Left middle temporal | –50 | –66 | –6 |
| Right middle temporal | 53 | –63 | –6 |
| Left Visual system | –7 | 83 | 2 |
| Right Visual system | 7 | 83 | 2 |
| Left Auditory system | –62 | –30 | 12 |
| Right Auditory system | 59 | –27 | 15 |
Statistical Analysis
To identify potential differences in resting-state ongoing cortical oscillatory activity between ISSNHL subjects and normal controls, non-parametric statistical analysis of LORETA-KEY images (statistical non-parametric mapping; SnPM) were applied to every contrast using LORETA-KEY’s built-in voxel-wise randomization tests (5000 permutations). We employed at statistic for independent groups with a threshold of P < 0.01 (corrected for multiple comparison). A relatively strict threshold for statistical significance was applied as the number of subjects was relatively small. A correction for multiple comparisons in SnPM using 5000 random permutations has been shown to yield similar results with those acquired from a statistical parametric mapping approach using a general linear model with multiple comparisons corrections.
We compared lagged connectivity differences between two groups and used the t-statistics for independent groups with a threshold of P < 0.05. Additionally, LORETA-KEY’s built-in voxel-wise randomization tests were used to correct for multiple comparisons for all the voxels included in the region of interest for connectivity analysis (5000 permutations).
Results
Source Localization
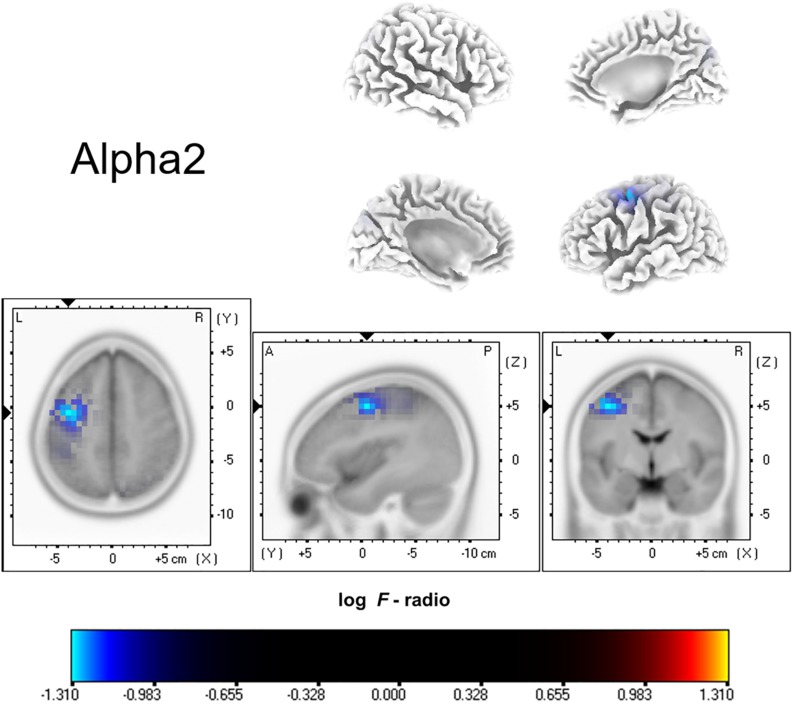
As shown in Figure 1, compared with healthy controls, ISSNHL subjects demonstrated significantly reduced electric activity in the left frontal lobe (BA6; P < 0.05) alpha 2 frequency band. In other frequency bands, no significant difference was found between the two groups. Moreover, there was no significant difference in other brain areas.
FIGURE 1.
Standardized low-resolution brain electromagnetic tomography. Compared with the healthy group, the ISSNHL group had significantly decreased activity in the left frontal lobe for the alpha 2 frequency band. Labels L, R, A and P represent left, right, anterior and posterior, respectively. Labels S and I represent superior and inferior.
Functional Connectivity
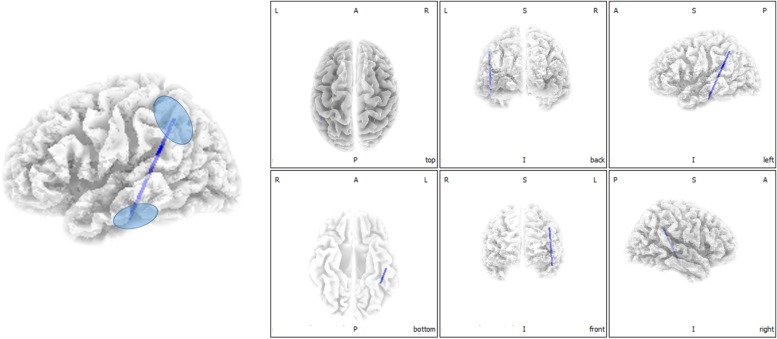
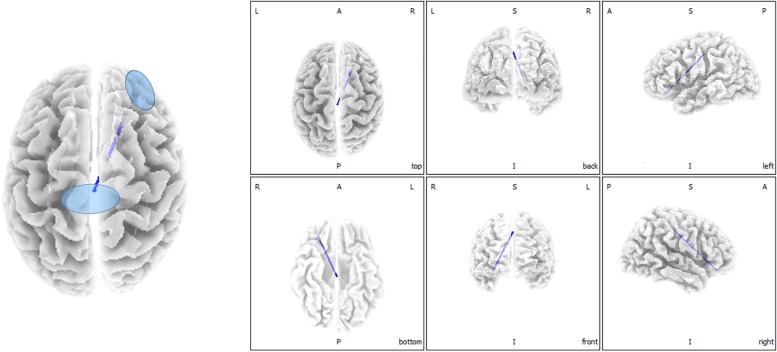
Functional connectivity analysis suggested significant differences between ISSNHL subjects and controls in the gamma 2 frequency band. There was significantly decreased functional connectivity between the temporal gyrus (BA20) and supramarginal gyrus (BA40) in the gamma 2 frequency band in the linear lagged connectivity and lagged coherence analysis (Figure 2). Additionally, there was a significantly reduced functional connectivity between the central cingulate gyrus (BA23) and right frontal lobe (BA10) for the gamma 2 frequency band under lagged phase analysis (Figure 3). With other frequency bands there was no significant difference between the two groups.
FIGURE 2.
Decreased functional connectivity between temporal gyrus and supramarginal gyrus for the gamma 2 frequency band in the linear lagged connectivity and lagged coherence analysis for ISSNHL patients with tinnitus. Labels L, R, A and P represent the left, right, anterior and posterior, respectively.
FIGURE 3.
Decreased functional connectivity between central cingulate gyrus and right frontal lobe for gamma 2 frequency band in the lagged phase analysis for ISSNHL patients with tinnitus. Labels L, R, A and P represent the left, right, anterior and posterior, respectively.
Discussion
This study explored changes in central nervous activities in ISSNHL with tinnitus patients when compared with healthy subjects. Overall, in the alpha 2 frequency band, the neural activity of ISSNHL patients with tinnitus was abnormally decreased in the left frontal lobe (BA6). Connections between the temporal gyrus (BA20) and supramarginal gyrus (BA40) as well as between central cingulate gyrus (BA23) and right frontal lobe (BA10) were weakened in the gamma 2 frequency band. This suggests that ISSNHL patients with tinnitus had aberrant brain area activity and functional connectivity.
Source localization analysis found that ISSNHL patients had decreased activity in the left frontal lobe (BA6) in the alpha 2 frequency band, showing similarity to the study by Micarelli et al. (2017), where ISSNHL subjects had a significant decrease of glucose metabolism in the middle frontal gyrus. It is known that the frontal lobe is one of the key areas of the DMN. Under normal circumstances, the regions of the DMN have a raised level of neuronal activity at rest (van den Heuvel and Hulshoff Pol, 2010). Activity and connectivity of the DMN are associated with the core processes of cognition, such as the integration of cognition and processing of emotion (Greicius et al., 2003), monitoring the environment (Gusnard et al., 2001) and mind-wandering (Mason et al., 2007). A study by Zhang et al. (2016) showed that long-term unilateral ISSNHL contributed to alterations of DMN, and that these alterations might influence cognitive abilities in these subjects. In addition, tinnitus as a symptom involving a perceived phantom sound, might lead to dysfunction of DMN (Chen et al., 2017). Our finding of decreased activation of the left frontal lobe might suppose that the DMN was suppressed in resting state, which consequently may activate other brain networks. It might be the reason for patients with attention and emotion issues.
Linear lagged connectivity and lagged coherence showed a weakened connection between the temporal gyrus (BA20) and supramarginal gyrus (BA40) in the gamma 2 frequency band. Previous studies by Fan et al. (2015) showed that unilateral ISSNHL patients had a significant decrease in contralateral auditory gyrus gray matter. A study by Micarelli et al. (2017) suggested a hypometabolism in the contralateral auditory gyrus in ISSNHL patients. The damage to the hair cells in ISSNHL patients causes a lack of auditory signal in the auditory network (van der Loo et al., 2009). These current results are consistent with our previous EEG microstate study (Cai et al., 2019) where there was a reduction of transition from microstate D to A in ISSNHL subjects with tinnitus that correlated with tinnitus scores. Microstate A is related to activation in the bilateral superior and middle temporal gyrus regions that are correlated with phonological processing. The supramarginal gyrus is a part of a dorsal attention network which relates to microstate D. These results indicated that generation of tinnitus in ISSNHL patients may be due to disruption of auditory and non-auditory networks.
Lagged phase synchronization showed that the connection between the central cingulate gyrus (BA23) and right frontal lobe (BA10) was decreased. Both areas are part of the DMN. The DMN is suggested to be particularly active at rest, when no tasks are performed (Mantini et al., 2007). The posterior cingulate node seems to be particularly vital, because it appears to be directly correlated with other nodes of the network and putatively acting as a mediator of intrinsic connectivity across these areas (Rosazza and Minati, 2011). This is likely because it is one of the most intensively interconnected areas in the whole brain (Cavanna and Trimble, 2006). In a task-based scenario, the network deactivated (Raichle and Snyder, 2007). Conversely, a significant increase in fluorodeoxyglucose uptake was seen in the right superior and medial frontal gyrus in ISSNHL patients (Micarelli et al., 2017). This might be a result of heterogeneity between studies. Therefore, more research is needed to understand this difference. In addition, some functional magnetic resonance imaging (fMRI) studies on tinnitus patients found aberrant functional connectivity within the DMN (Burton et al., 2012; Schmidt et al., 2013). The suppression of tinnitus would lead to a differential response pattern among the network nodes. This might result in the inhibiting effect of DMN.
In summary, the findings of our study identify specific inhibition of brain areas and functional connectivity in ISSNHL with tinnitus subjects. The inhibition of the DMN implies that ISSNHL patients have suppressed activity at resting state and are more active in involving attention or emotional behavior when compared with healthy controls. The reduced connection between the auditory system and dorsal attention network suggests decreased attention to auditory information. The brain activity is in a state of inhibition for ISSNHL patients with tinnitus.
Some limitations in this study need to be realized. ISSNHL is a heterogeneous disease. ISSNHL subjects had differences in hearing loss, laterality or accompanying symptoms (van der Loo et al., 2009). So, it was difficult to eliminate the heterogeneous factors completely even using strict inclusion and exclusion criteria. Without specific subgroups of ISSNHL with tinnitus patients, the results of our study need to be read with caution. In addition, the small sample size in the present study is unable to rule out heterogeneous factors. Therefore, we need to explain the results carefully. A further study with larger sample sizes and more subgroup comparisons is needed to investigate the underlying mechanisms in ISSNHL with tinnitus.
Conclusion
In this study, it was found that the central system of ISSNHL with tinnitus patients had been changed when compared with healthy controls. By using resting-state EEG and analyzing source localization and functional connectivity, we found that ISSNHL and tinnitus patients were abnormally weakened in the left frontal lobe (BA6) in the alpha 2 frequency band. The connection between the temporal gyrus (BA20) and supramarginal gyrus (BA40) is weakened in the Gamma 2 frequency band as well as the connection between the central cingulate gyrus (BA 23) and right frontal lobe (BA 10). These findings could help to make sense of the central pathogenesis in ISSNHL patients.
Data Availability
There is no other available data. Nonetheless, the data that support the results of the research can be obtained by emailing zhengyiq@mail.sysu.edu.cn.
Ethics Statement
The studies involving human participants were reviewed and approved by Institutional Review Board of the Sun Yat-sen Memorial Hospital at Sun Yat-sen University of China. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
The concept and design of this research come from YxC and JL. The experiments were performed by YhC, WC, and WL. CD, GC, SC, and ML analyzed the data. FZ and YZ drafted this manuscript. All authors participated in preparing this manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Dr. Christopher Wigham for his contribution to proofreading.
Footnotes
Funding. This study was funded by the National Natural Science Foundation of China (Grant Nos. 81600808, 81570935, and 31400874), Natural Science Foundation of Guangdong Province (Grant No. 2016A030313318), High-level University Construction of Guangzhou Medical University of China (Grant No. B185004187), and Guangzhou Science and Technology Programme livelihood projects (2014Y2-00511).
References
- Bing D., Ying J., Miao J., Lan L., Wang D., Zhao L., et al. (2018). Predicting the hearing outcome in sudden sensorineural hearing loss via machine learning models. Clin. Otolaryngol. 43 868–874. 10.1111/coa.13068 [DOI] [PubMed] [Google Scholar]
- British Society of Audiology Recommendation (1988). Descriptors for pure-tone audiograms. Br. J. Audiol. 22:123 10.3109/03005368809077807 [DOI] [Google Scholar]
- Burton H., Wineland A., Bhattacharya M., Nicklaus J., Garcia K. S., Piccirillo J. F. (2012). Altered networks in bothersome tinnitus: a functional connectivity study. BMC Neurosci. 13:3. 10.1186/1471-2202-13-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Y., Chen S., Chen Y., Li J., Wang C., Zhao F., et al. (2019). Altered resting-state eeg microstate in idiopathic sudden sensorineural hearing loss patients with tinnitus. Front. Neurosci. 13:443. 10.3389/fnins.2019.00443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanna A. E., Trimble M. R. (2006). The precuneus: a review of its functional anatomy and behavioural correlates. Brain 129 564–583. 10.1093/brain/awl004 [DOI] [PubMed] [Google Scholar]
- Chau J. K., Lin J. R. J., Atashband S., Irvine R. A., Westerberg B. D. (2010). Systematic review of the evidence for the etiology of adult sudden sensorineural hearing loss. Laryngoscope 120:1011. 10.1002/lary.20873 [DOI] [PubMed] [Google Scholar]
- Chen J., Liang J., Ou J., Cai W. (2013). Mental health in adults with sudden sensorineural hearing loss: an assessment of depressive symptoms and its correlates. J. Psychosom. Res. 75 72–74. 10.1016/j.jpsychores.2013.03.006 [DOI] [PubMed] [Google Scholar]
- Chen Y. C., Feng Y., Xu J. J., Mao C. N., Xia W., Ren J., et al. (2016). Disrupted brain functional network architecture in chronic tinnitus patients. Front. Aging Neurosci. 8:174. 10.3389/fnagi.2016.00174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y. C., Li X., Liu L., Wang J., Lu C. Q., Yang M., et al. (2015a). Tinnitus and hyperacusis involve hyperactivity and enhanced connectivity in auditory-limbic-arousal-cerebellar network. Elife 4:e06576. 10.7554/eLife.06576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y. C., Zhang J., Li X. W., Xia W., Feng X., Qian C., et al. (2015b). Altered intra-and interregional synchronization in resting-state cerebral networks associated with chronic tinnitus. Neural. Plast. 2015:475382. 10.1155/2015/475382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y. C., Wang F., Wang J., Bo F., Xia W., Gu J. P., et al. (2017). Resting-state brain abnormalities in chronic subjective tinnitus: a meta-analysis. Front. Hum. Neurosci. 11:22. 10.3389/fnhum.2017.00022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y. C., Zhang J., Li X. W., Xia W., Feng X., Gao B., et al. (2014). Aberrant spontaneous brain activity in chronic tinnitus patients revealed by resting-state functional MRI. Neuroimage Clin. 6 222–228. 10.1016/j.nicl.2014.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Congedo M., John R. E., De Ridder D., Prichep L., Isenhart R. (2010). On the “dependence” of “independent” group EEG sources; an EEG study on two large databases. Brain Topogr. 23:134. 10.1007/s10548-009-0113-6 [DOI] [PubMed] [Google Scholar]
- Dispenza F., Amodio E., De Stefano A., Gallina S., Marchese D., Mathur N., et al. (2011). Treatment of sudden sensorineural hearing loss with transtympanic injection of steroids as single therapy: a randomized clinical study. Eur. Arch. Otorhinol. 268 1273–1278. 10.1007/s00405-011-1523-0 [DOI] [PubMed] [Google Scholar]
- Fan W., Zhang W., Li J., Zhao X., Mella G., Lei P., et al. (2015). Altered Contralateral auditory cortical morphology in unilateral sudden sensorineural hearing loss. Otol. Neurotol. 36 1622–1627. 10.1097/MAO.0000000000000892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs M., Kastner J., Wagner M., Hawes S., Ebersole J. S. (2002). A standardized boundary element method volume conductor model. Clin. Neurophysiol. 113 702–712. 10.1016/s1388-2457(02)00030-5 [DOI] [PubMed] [Google Scholar]
- Greicius M. D., Krasnow B., Reiss A. L., Menon V. (2003). Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc. Natl. Acad. Sci. U. S. A. 100 253–258. 10.1073/pnas.0135058100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard D. A., Akbudak E., Shulman G. L., Raichle M. E. (2001). Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proc. Natl. Acad. Sci. U. S. A. 98 4259–4264. 10.1073/pnas.071043098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingber L., Nunez P. L. (2011). Neocortical dynamics at multiple scales: EEG standing waves, statistical mechanics, and physical analogs. Math. Biosci. 229 160–173. 10.1016/j.mbs.2010.12.003 [DOI] [PubMed] [Google Scholar]
- Jurcak V., Tsuzuki D., Dan I. (2007). 2007. 10/20, 10/10 and 10/5 systems revisited: their validity as relative head-surface-based positioning systems. NeuroImage 34 1600–1611. 10.1016/j.neuroimage.2006.09.024 [DOI] [PubMed] [Google Scholar]
- Kang W. S., Yang C. J., Shim M., Song C. I., Kim T. S., Lim H. W., et al. (2017). Prognostic factors for recovery from sudden sensorineural hearing loss: a retrospective study. J. Audiol. Otol. 21 9–15. 10.7874/jao.2017.21.1.9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koç A., Sanisoðlu O. (2003). Sudden sensorineural hearing loss: literature survey on recent studies. J. Otolaryngol. 32:308. 10.2310/7070.2003.11288 [DOI] [PubMed] [Google Scholar]
- Kuhn M., Heman-Ackah S. E., Shaikh J. A., Roehm P. C. (2011). Sudden sensorineural hearing loss: a review of diagnosis. Treatment, and Prognosis. Trends Amplif. 15 91–105. 10.1177/1084713811408349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster J. L., Woldorff M. G., Parsons L. M., Liotti M., Freitas C. S., Rainey L., et al. (2000). Automated talairach atlas labels for functional brain mapping. Hum. Brain Mapp. 10 120–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanting C. P., de Kleine E., van Dijk P. (2009). Neural activity underlying tinnitus generation: results from PET and fMRI. Hear. Res. 255 1–13. 10.1016/j.heares.2009.06.009 [DOI] [PubMed] [Google Scholar]
- Leaver A. M., Turesky T. K., Seydell-Greenwald A., Morgan S., Kim H. J., Rauschecker J. P. (2016). Intrinsic network activity in tinnitus investigated using functional MRI. Hum. Brain Mapp. 37 2717–2735. 10.1002/hbm.23204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantini D., Perrucci M. G., Gratta C. D., Romani G. L., Corbetta M. (2007). Electrophysiological signatures of resting state networks in the human brain. Proc. Natl. Acad. Sci. U. S. A. 104 13170–13175. 10.1073/pnas.0700668104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason M. F., Norton M. I., John D. V. H., Wegner D. M., Grafton S. T., Macrae C. N. (2007). Wandering minds: the default network and stimulus-independent thought. Science 315 393–395. 10.1126/science.1131295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micarelli A., Chiaravalloti A., Viziano A., Danieli R., Schillaci O., Alessandrini M. (2017). Early cortical metabolic rearrangement related to clinical data in idiopathic sudden sensorineural hearing loss. Hear. Res. 350 91–99. 10.1016/j.heares.2017.04.011 [DOI] [PubMed] [Google Scholar]
- Newman C. W., Jacobson G. P., Spitzer J. B. (1996). Development of the tinnitus handicap inventory. Arch. Otolaryngol Head Neck Surg. 122:143. [DOI] [PubMed] [Google Scholar]
- Nogueira-Neto F. B., Gallardo F. P., Suzuki F. A., Penido Nde O. (2016). Prognostic and evolutive factors of tinnitus triggered by sudden sensorineural hearing loss. Otol. Neurotol. 37 627–633. 10.1097/MAO.0000000000001049 [DOI] [PubMed] [Google Scholar]
- Nosrati-Zarenoe R., Hansson M., Hultcrantz E. (2010). Assessment of diagnostic approaches to idiopathic sudden sensorineural hearing loss and their influence on treatment and outcome. Acta Otolaryngol. 130 384–391. 10.3109/00016480903161541 [DOI] [PubMed] [Google Scholar]
- Pascual-Marqui R. D. (2002). Standardized low-resolution brain electromagnetic tomography (sLORETA): technical details. Methods Find. Exp. Clin. Pharmacol. 24(Suppl. D), 5–12. [PubMed] [Google Scholar]
- Pascual-Marqui R. D. (2007a). Discrete, 3D distributed, linear imaging methods of electric neuronal activity. Part 1: exact, zero error localization. arXiv [Google Scholar]
- Pascual-Marqui R. D. (2007b). Instantaneous and lagged measurements of linear and nonlinear dependence between groups of multivariate time series: frequency decomposition. arXiv [Google Scholar]
- Rah Y. C., Park K. T., Yi Y. J., Seok J., Kang S. I., Kim Y. H. (2015). Successful treatment of sudden sensorineural hearing loss assures improvement of accompanying tinnitus. Laryngoscope 125 1433–1437. 10.1002/lary.25074 [DOI] [PubMed] [Google Scholar]
- Raichle M. E. (2011). The restless brain. Brain Connect. 1 3–12. 10.1089/brain.2011.0019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle M. E., Snyder A. Z. (2007). A default mode of brain function: a brief history of an evolving idea. Neuroimage 37 1083–1090. 10.1016/j.neuroimage.2007.02.041 [DOI] [PubMed] [Google Scholar]
- Rosazza C., Minati L. (2011). Resting-state brain networks: literature review and clinical applications. Neurol. Sci. 32 773–785. 10.1007/s10072-011-0636-y [DOI] [PubMed] [Google Scholar]
- Schecklmann M., Landgrebe M., Langguth B. Tri Database Study Group (2014). Phenotypic characteristics of hyperacusis in tinnitus. PLoS One 9:e86944. 10.1371/journal.pone.0086944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt S. A., Akrofi K., Carpenter-Thompson J. R., Husain F. T. (2013). Default mode, dorsal attention and auditory resting state networks exhibit differential functional connectivity in tinnitus and hearing loss. PLoS One 8:e76488. 10.1371/journal.pone.0076488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stachler R. J., Chandrasekhar S. S., Archer S. M., Rosenfeld R. M., Schwartz S. R., Barrs D. M., et al. (2012). Clinical practice guideline: sudden hearing loss. Otolaryngol. Head Neck Surg. 146 S1–S35. 10.1177/0194599812436449 [DOI] [PubMed] [Google Scholar]
- Teismann H., Okamoto H., Pantev C. (2011). Short and intense tailor-made notched music training against tinnitus: the tinnitus frequency matters. PLoS One 6:e24685. 10.1371/journal.pone.002468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel M. P., Hulshoff Pol H. E. (2010). Exploring the brain network: a review on resting-state fMRI functional connectivity. Eur. Neuropsychopharm. 20 519–534. 10.1016/j.euroneuro.2010.03.008 [DOI] [PubMed] [Google Scholar]
- van der Loo E., Gais S., Congedo M., Vanneste S., Plazier M., Menovsky T., et al. (2009). Tinnitus intensity dependent gamma oscillations of the contralateral auditory cortex. Plos One 4:e7396. 10.1371/journal.pone.0007396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H., Fan W., Zhao X., Li J., Zhang W., Lei P., et al. (2016). Disrupted functional brain connectome in unilateral sudden sensorineural hearing loss. Hear. Res. 335 138–148. 10.1016/j.heares.2016.02.016 [DOI] [PubMed] [Google Scholar]
- Zhang G. Y., Yang M., Liu B., Huang Z. C., Li J., Chen J. Y., et al. (2016). Changes of the directional brain networks related with brain plasticity in patients with long-term unilateral sensorineural hearing loss. Neuroscience 313 149–161. 10.1016/j.neuroscience.2015.11.042 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
There is no other available data. Nonetheless, the data that support the results of the research can be obtained by emailing zhengyiq@mail.sysu.edu.cn.