Graphical abstract
Abbreviations: AVS, Anti-Venom Serum; RVV, Russell's viper venom; RV, Russell’s Viper; VTH, Veterinary Teaching Hospital; PT, Prothrombine Time; aPTT, Activated Partial Thromboplastine Time; CT, Clotting Time; ARF, Acute Renal Failure; AST, Aspartate aminotransferase; ALT, Alanine aminotransferase; (TP), Total protein; (BUN), Blood Urea Nitrogen; (UO), Urine output; (BP), Blood pressure
Keywords: Daboia russelii, Dogs, Anti-Venom serum, Prothrombine time, Activated partial thromboplastine time, Clotting time
Highlights
-
•
This is the first detailed study of Russell's viper (RV) envenoming of dogs (n = 65).
-
•
The common clinical presentations were pain, cardiovascular toxicities, heamatotoxicities and respiratory abnormalities.
-
•
The major toxic effects of venom were acute renal failure and mortality of the patients.
-
•
The minimum number of Anti-venom vials to initiate the treatment for D.russelii envenoming in dogs was determined as 06 vials.
Abstract
Introduction
Russell’s viper envenoming in dogs is a significant problem in Sri Lanka. The current study focused on investigating clinical profile, laboratory findings of three selected tests and to develop a treatment strategy with Indian polyvalent Anti-Venom Serum (AVS). It was also intended to report adverse effects and complications caused by both Russell's viper venom (RVV) and AVS in Russell’s Viper (RV) envenomed dogs.
Objective
To evaluate and report the clinical manifestations, to find out the minimum effective vials of AVS and to record AVS induced adverse reactions of RV envenoming in dogs.
Materials and methods
A prospective study was conducted on Russell's viper bitten dogs (n = 65) admitted to the Veterinary Teaching Hospital (VTH) in Sri Lanka. Indian polyvalent AVS was used to treat all the envenomed dogs. The number of vials of AVS that was administered to a patient was decided upon by a second degree polynomial model with a number of vials of AVS in the X axis verses Prothrombine Time (PT), Activated Partial Thromboplastine Time (aPTT) and Clotting Time (CT) in the Y axis respectively.
Results
Varying degrees of pain were exhibited by all the victim dogs. Mild swelling and necrosis at the site of bite was seen in 54% (n = 35) and 37% (n = 24) of dogs respectively. Prolonged values of, PT, aPTT and CT were seen from all the RV envenomed dogs. The mean leukocyte count in these dogs was 39.79 × 103/μL (normal range; 4–20 × 103/μL) (IQR:29.05 × 103/μL-45.92 × 103/μL). Statistical analysis showed that the initial vials of 7 AVS would be the minimum required vials. Therefore, a range of 6–15 AVS vials in total were administered to these dogs and in 7.6% (n = 5) of dogs, the results of PT, aPTT and CT became normal with 6 AVS vials at 32–97 minutes. Acute Renal Failure (ARF) was detected from 29% (n = 19) of dogs as a complication.
Conclusions
Systemic clinical signs of haemorrhagic lesions, cardio respiratory toxicities were common in Russell's viper envenomed dogs. Initially 6 vials of AVS must be administered. AVS induced reactions were reported commonly. Russell's viper envenoming was found to be lethal in dogs.
1. Introduction
Experiments on venoms of Indian cobra and Russell’s viper were first described by a British herpetologist Patric Russell (1781–1791). Sri Lankan viper species is scientifically named as Daboia russelii and the species is found to be distributed all over the country except in the high mountain ranges [1]. Young snakes are very bright and are venomous from birth. It is the deadliest venomous viper in Sri Lanka [1,2]. The high morbidity and mortality due to snakebites in dogs in Sri Lanka result from Russell's viper (Daboia russelii), cobra (Naja naja), and hump nosed viper (Genus Hypnale) [3]. The proteome profile of Sri Lankan Russell’s vipers is identical, where composition of the venom is dominated by the neurotoxic basic phospholipases A2 (>30% of total protein abundance) and several hemotoxic or coagulopathic protein families (approximately 50% in total) and is correlated with the functional and toxinological characterizations of the venom, and reflects the pathophysiological effects of envenoming [4]. Local and systemic clinical manifestation in Russell’s viper envenoming has been studied in vitro and in vivo. However, records regarding envenoming in man are abundant. Pain, swelling, blister formation and hemorrhagic necrosis are the symptoms generally seen following a snake bite in man [2,5,6]. Local effects due to D.russelii envenoming are usually insignificant compared with bites of others in the viperidae family [7]. Cause of death by Russell’s viper envenoming is due to intra-cerebral and subarachnoid hemorrhage; which is a characteristic vasculotoxic effect of D.russelii venom [7]. Russell’s viper venom induces the formation of microthrombi which are deposited in blood vessels of the kidneys and the other organs and lead to death or organ failure [8]. The most prominent clinical features of RV envenoming are coagulopathy followed by neurotoxicity and nephrotoxicity and in contrast, the cardiac effects are minute [9]. Neurotoxicity and neuromuscular paralysis is often seen in Russell’s viper envenoming in Sri Lanka and in South India [10]. The venom of Russell’s viper is rich in nephrotoxins, which causes ARF as a major complication [11,12] The myotoxic action of phospholipase A2 on muscles can be seen histologically as fragmentation of muscle fibers, separation of muscle fibers by oedema, dilated capillaries, myonecrosis and mononuclear cell infiltration [13]. It is obvious that two types of Phospholipases A2 (PLA2s) with weak myotoxic properties cause mild and non-life threatening myotoxicity in Sri Lankan Russell's viper envenoming [14]. In order to observe and monitor cardiotoxic effects [15], RVV was injected intravenously to different animals and resulted in hypotension, bradycardia, low pulse pressure, and subsequently death. Cardiotoxicity is commonly caused by snake venom toxins of NKCT1 and NN-32 [16]. Clinical pictures of signs and symptoms and treatment options of D.russelii envenoming in human are well recognized, though records on animal bites are scarce. As the specific treatment for snake envenoming, antivenoms are administered intravenously. Dosing strategies were established on the basis of neutralizing capacity of each milliliter (ml) of antivenom and concentration of particular venom in mg, (dry weight) neutralized by each antivenom [17]. When administered intravenously, therapeutic success depends in their ability to alleviate the progress of toxic effects induced by snake venom components [18]. Therefore, this study was carried out with a view to document the local and systemic effect of D.russelii envenoming in dogs.
2. Materials and methods
2.1. Study setting and data collection
A prospective study was conducted on Russell’s viper (D.russelii) bitten dogs (n = 65) admitted to the VTH University of Peradeniya with the complaint of the, between January 2012 and December 2014. Information regarding the incident was gathered in detail from the owner of the dog. Characteristic features of the offending snakes were collected from dog owners. Signalment of patients (breed, sex, age, and body weight) was recorded on each dog. History related to bite which include the time of bite, abnormalities noted, first aid attempts, and time of admission were recorded. Since dogs have variable fur patterns, colours and thick skin, site/s of the fang marks were noted with the help of owners’ information.
2.2. Snake identification
Morphological features of dead or live specimens brought by the dog owners were done using identification keys in De Silva, 1980. Pictures of the offending snake were taken by the owners (Using mobile phones and other electronic devices). In addition, whenever the snake specimen was not available for identification, preserved specimens and photographs of Russell's vipers were shown to the dog owner for the tentative establishment of the identity of the snake.
2.3. General Clinical Examination (GCE) and recording of clinical manifestations of patients
The patients were examined in the Intensive care unit (ICU), to minimize disturbances and allowed to calming down of the victim. Complete general clinical examination was performed adopting adequate physical and psychological restraint methods on conscious patients. When a ferocious patient was presented, the owners were instructed to calm the patient by psychological means of restraint and the examination procedures were carried out. Predictable local and systemic effects due to envenoming and their progression were documented according to studies conducted by Refs. [2,19,3], on envenoming. Expected local clinical manifestations of envenoming due to Russell’s viper bites included inflammation (swelling, pyrexia, and erythema), bruising, blistering, and local necrosis at the site detected by fang marks, pain, haemorrhages from inter-digital spaces and gingival regions, lymphadenopathy, and infected wounds at the site of the bite. Cardiac arrhythmias, bradycardia, hypotension, bleeding and clotting disorders, pallor, melena, hematochesia, haematuria, neurotoxic signs such as pupillary dilatation and hypersalivation, drowsiness and lethargy signs of respiratory system (dyspnoea, wheezing, crackles), and renal system, were considered as specific systemic signs of envenoming. Nausea and vomiting were considered as nonspecific systemic clinical manifestation.
2.4. Laboratory investigations and treatments
Treatment plans were determined consequent to laboratory findings, clinical signs and symptoms of the patients. Termination of treatment was decided upon the combination of results of laboratory investigations and clinical manifestation of the patient.
2.4.1. Collection of samples for laboratory investigations
Two intravenous catheters (22 G, 24 G, 20 G or 18 G) were placed in separate veins to get simultaneous venous access for continuous administration of AVS and blood sampling for continuous testing. Blood samples were collected using one catheter while therapeutic agents were administered through the other. Parallel infusions were administered in certain instances with the aid of the two IV catheters. Indian polyvalent AVS (VINS Bioproducts) was used to treat the patients in this study. Prior to AVS therapy, auxiliary treatments were started for all the patients to prevent and minimize possible complications likely to be caused by IgG and other factors that constitute in AVS. Blood samples were collected by venipuncture (cephalic vein, saphenous vein or sometimes jugular vein) from all the patients (n = 65) before initiation of treatment and 2.5 mL of blood was placed in a plastic tube containing EDTA 1.5–2 mg/mL as the anticoagulant to perform Full Blood Count (FBC), liver and renal profiles. These tests were performed in order to assess patient’s condition based on FBC, alanine aminotransferase (ALT), aspartate aminotransferase (AST), Total protein (TP), Albumin, Blood Urea Nitrogen (BUN) and Creatinine values. FBC, Renal and liver profiles were repeated 24 h after the initiation of the specific treatment taking the clinical manifestations of the patients into consideration.
2.4.2. Performing coagulation tests (PT, aPTT, and CT)
A volume of 0.9 mL of blood was collected into 3.2% tri-sodium citrate at a ratio of 1:9 mL to perform PT and aPTT. About 1 mL of blood was placed in a Khan tube to measure the CT. Collection of blood and testing of PT, aPTT, and CT were performed prior to administration of AVS. Repeated measurements of these three parameters were done after each AVS vial i.e. every 5 min for the initial 5 vials. These tests were used to assess and to determine the adequacy of antibodies to neutralize the venom toxins. After the 5th dose of AVS, further AVS administration was decided upon the test results of coagulation panel and clinical signs and symptoms of the patient.
2.4.3. Auxiliary treatments
In order to prevent immunogenic reactions due to AVS, adrenaline (Adrivit 0.1% W/V, 1 mL, Healthcare Pvt. Ltd, India; 0.01 to 0.02 mg/kg, s.c., stat.) was given to agonist α and β receptors to suppress the initiation of hypersensitivities. Administration of antihistamine, chlorpheniramine maleate (Allervit 1% W/V, 1 mL, Healthcare Pvt. Ltd, India; 0.4 mg/kg, IV, q.i.d.) was done for the initial 24 h. Parallel infusion of hydrocortisone (Hydrocortisone Sodium Succinate for injection BP 100 mg, AMN Life Science Pvt Ltd, India; 10 mg/kg, IV, q.i.d.) up to the maximum dose of 1000 mg was administered throughout the continuation of AVS therapy starting from 5 min prior to AVS. Then IV hydrocortisone at the rate of 10 mg/kg IV q.i.d. was continued in the initial 24 h. In order to prevent secondary bacterial infections of the patients due to bite wounds and immunocompromised state with envenoming, Cloxacillin (Cloxacillin Sodium for injection BP 250 mg, Vysali Pharmaceuticals Limited, India; 20 mg/Kg, IV, b.i.d.) and Metronidazole (Metronidazole Intravenous Infusion BP 500 mg/100 mL, Claris Life Sciences Limited, India; 20 mg/Kg, IV, b.i.d.) were initiated and continued up to the completion or termination of treatment of the patient.
2.5. Initiation, continuation of AVS and laboratory investigations
Each vial (Anti Snake Venom Serum I.P. 10, (I.V mL/ Lyophilized™), VINS Bioproduct Ltd., Hyderabad, India) with the ability to neutralize 6 mg of venom antigen was dissolved in 10 mL of normal saline. Then to 10 mL of the dissolved AVS a further 10 mL normal saline was added. After that the drug was administered at the rate of 2 mL/minute (AVS). Hence, each vial was given over a period of 5 min. Initiation of AVS treatment was done with 5 vials continuously. A total of 5 vials of AVS (1 vial dissolved in 10 mL of normal saline) were then added to 50 mL of normal saline which was initially administered continuously to all patients. Immediately after administration of each vial blood samples were collected to perform coagulation tests. Total volume was given intravenously over a duration of 25 min. Immediately after the infusion, blood samples were collected in a citrated tube and in a Kahn tube in order to perform PT, aPTT, and CT. After administration of the 5th vial of AVS, continuation of further treatment was decided upon by the laboratory results and clinical signs and symptoms of the patient. This took approximately 20 min after the 5th AVS vial. However, values for PT, aPTT, and CT were obtained after administration of each additional AVS vial in order to further administrate AVS. Therefore, there was a 20 min gap between AVS vials after the 5th vial. Since snake envenoming is considered as a medical emergency, all the vital parameters were measured with the initiation of treatment and the patient was kept at the ICU for 24 h after admission initially. The vital parameters: RR, HR, PR and quality, and blood pressure (BP) were recorded.
2.6. Management of AVS induced adverse reactions
Any clinical signs observed during and after administration of AVS was considered as adverse reactions to AVS. Therefore, monitoring of patients for the development of adverse reactions was initiated immediately after AVS administration. The expected clinical manifestations were: hypersalivation, fever, restlessness, itching, dyspnea, hypotension, cardiac arrhythmia, tachycardia and signs of laryngeal edema. In general, patients observed with signs of hypersensitivity, were treated with adrenaline (Adrivit 0.1% W/V, 1 mL, Healthcare Pvt. Ltd, India; 0.01 to 0.02 mg/kg, sc.s.o.s.), chlorpheniramine maleate (Allervit 1% W/V, 1 mL, Healthcare Pvt. Ltd, India; 0.4 mg/kg, IV, q.i.d.) and hydrocortisone (Hydrocortisone Sodium Succinate for injection BP 100 mg, AMN Life Science Pvt Ltd, India; 10 mg/kg, IV, q.i.d.) to minimize the activation of immune mediators (histamine, prostaglandin, leukotrin, cytokines etc.). Atropine sulphate (Atrover 0.6 mg/mL ampule, Veve Human Care Laboratories, India; 0.02 mg/kg, IV, stat.) was administered to avert hypersalivation. Any type of cardiovascular manifestations (bradyarrhythmias and hypotension) were corrected by administration of inotropes intravenously with Dopamine (Sterile dopamine concentrate BP, Ciron Drugs & Pharmaceuticals Pvt. Ltd. Nagarrabhat Nagar, Ground Floor, Jogeshwari (West) Mumbai, 400 102, India; 5 mcg/kg/min) and adrenaline (Adrivit 0.1% W/V, 1 mL, Healthcare Pvt. Ltd, India; 0.01 to 0.02 mg/kg, stat.). Patients that showed signs of laryngeal oedema were sedated using diazepam (Calmvita 5 mg/mL, Healthcare Pvt Ltd, India; 0.5 mg/kg, IV, stat.) and anesthetized (light anestehesia) using Propofol Hydrochloride (Anesia™ 1% Injection for Intravenous Infusion 50 mL /Vial, Claris Lifesciences Limited, Ahmedabad, India; 4 mg/kg, IV, stat.) to undergo endotracheal intubation in order to facilitate breathing. Subsequently, continuous oxygen therapy was initiated just after intubation for each patient.
2.7. Assessment of kidney function and management of acute renal failure
In order to assess renal function, urinalysis followed by BUN and creatinine in blood were investigated on admission. The same tests were repeated after 24 h. Urine output (UO) was measured from the time of admission and continued until termination of treatment of the patient. Continuous assessment of envenomed patients that were encountered with Russell’s viper envenoming and impending insufficient renal functions was diagnosed with the aid of clinical signs and laboratory investigation of BUN, creatinine, and urine analysis together with UO. Patients that developed anuria or oliguria were treated with frusemide (Furosemide 20 mg/2 mL Solution For Injection, Steril-Gene Life Sciences (P) Ltd, India; 2 mg/kg IV, 4 mg/kg or 6 mg/kg IV), administered at standard intervals (at least half an hour was maintained between frusemide doses) until urine production was observed. Once a given patient started to respond to treatment then frusemide (Furosemide 20 mg/2 mL Solution For Injection, Steril-Gene Life Sciences (Pvt) Ltd, India; 3 mg/kg, IV b.i.d.) was continued until the urine production reversed to the normal range (UO > 66%).
2.8. Elevated serum concentrations of hepatic enzymes and related components
Patients that had elevated ALT, AST at admission were detected as the patients prone to possible hepatic impairment; consequently, some of the treatment measures were changed and management plans were altered. Such patients were treated with metronidazole (Metronidazole Intravenous Infusion BP 500 mg/100 mL, Claris Life Sciences Limited, India; 7.5 mg/kg, IV, b.i.d.) in addition to routine auxiliary treatments. Continuation of the altered treatment plan was done until those elevated serum enzyme levels returned to normal.
2.9. Statistical analysis
Data management and descriptive statistics were done using Microsoft Excel (Microsoft, Redmond WA, USA) and MINITAB 16 software (Minitab Inc.; State College PA, USA).
3. Results
There were 65 dogs which included 34 males and 31 females in this study. Their median age was 3.5 years (Interquartile range: IQR: 2–7 years). Bites were clustered during 0600–1200, n = 22 (34%) and 1800–2400, n = 19 (29%). The patients were presented to the VTH with a median of 3.0 h (IQR: 1.62–10.00 h) after the bite incident and n = 15 (23%) of victims had been subjected to some form of first aid measures. Fang marks were observed in all the dogs and multiple fang marks were observed in n = 29 (45%) dogs while fang marks on the head region were seen in n = 31 (48%) dogs.
3.1. Clinical manifestations observed in envenomed patients
The frequencies of each clinical manifestation (local and systemic) seen in patients envenomed by D.russelii at the admission are shown in Table 1. All the patients presented, appeared to be suffering from varying degrees of pain. Mild swelling on site of bite was noticed only in n = 35 (54%) of the patients.
Table 1.
Clinical manifestations observed in dogs (n = 65) with D. russelii envenoming reported to VTH during January 2012 to December 2014.
| Clinical signs and symptoms of envenoming | Number of patients | Percentage (%) |
|---|---|---|
| Pain | 65 | 100 |
| Swelling at the site of the bite | 35 | 54 |
| Local necrosis at the site of fang marks and infected wound |
24 | 37 |
| Cardiac arrhythmias | 54 | 84 |
| Hypotension | 08 | 12 |
| Dyspnoea, wheezing, crackles | 34 | 52 |
| Haematuria | 16 | 25 |
| Petechiation on skin | 15 | 23 |
| Hematemesis, | 3 | 5 |
| Melena, | 3 | 5 |
| Haemorrhages from inter digital spaces | 4 | 6 |
| Bleeding from the bite site | 7 | 11 |
| Bleeding from sclera | 1 | 1.5 |
| Urinary incontinence | 2 | 3 |
| Pupillary dilatation | 7 | 11 |
| Hypersalivation | 17 | 26 |
| Pyrexia | 9 | 14 |
| Vomiting | 7 | 10 |
Cardiovascular toxicities of cardiac arrhythmias, bradycardia and hypotension were detected in 84% (n = 54) of them. Heamatotoxicities were seen as bleeding from site of bite, spontaneous bleeding from injection site, hematemesis, heamatochesia, petechia, heamorrhages from interdigital spaces, and sclera in 84% (n = 54) of dogs. Respiratory abnormalities of dyspnea, wheezing, and crackles were seen in 52% (n = 34,) of them. Heamaturia, pyuria, and proteinuria were seen in 40% (n = 26) of dogs on admission.
3.2. Hemorrhagic lesions of envenoming
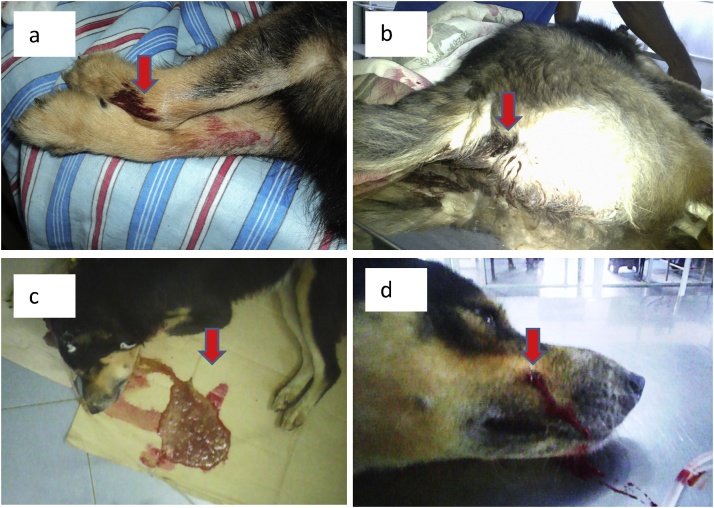
Hemorrhagic lesions caused by the venom of D.russelii were observed in various organ systems and the lesions are shown in Fig. 1.
Fig. 1.
Hemorrhagic lesions caused by venom of D.russelii in dogs. (a - Bleeding from injection sites, b - Melena, c - Hematemesis, d - Bleeding from bite site).
3.3. Full blood count
Blood samples of all 65 dogs studied showed leukocytosis (Mean = 39.79 × 103/μL, IQR = 16.87 × 103/μL). Neutrophilia suggestive of a toxemic insult, was detected in the differential count in all patients. There were 2 (3%) critically anemic patients (PCV < 10%) with thrombocytopenia and leukocytosis. The other hematological parameters that were changed in RV envenomed patents are shown in Table 2.
Table 2.
Hematological changes observed on admission in 65 dogs reported to the VTH with history of RV envenoming during January 2012 to December 2014.
| Condition | Number | Percentage (%) |
|---|---|---|
| Critical anemia (Hct<10%) | 2 | 3 |
| Severe anemia (Hct<20,>10%) | 12 | 18 |
| Mild anemia (Hct<26,>20%) | 11 | 17 |
| Elevated MCHC | 10 | 15 |
| Thrombocytopenia | 25 | 38 |
In addition, there were 38% dogs with thrombocytopenia and mild to critical anemia.
3.4. Determination of lowest effective vials using changes of PT, aPTT and CT with AVS vials
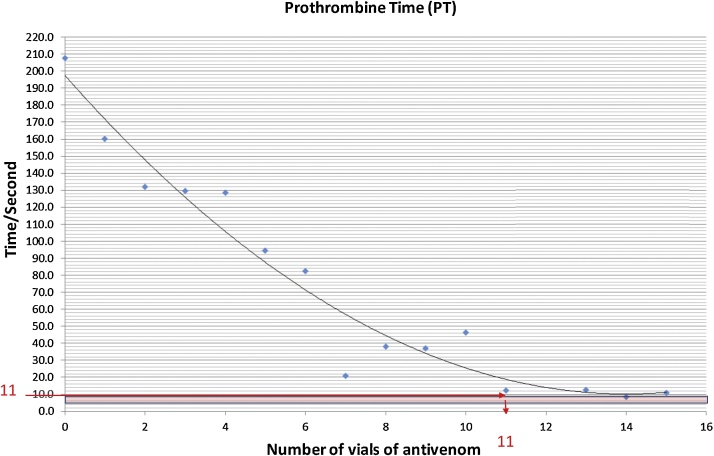
The relationship between the PT and the number of vials of AVS is depicted in Fig. 2. The reduction in PT with the vials of AVS could also be best explained by a second degree polynomial. More than 94% of the observed variation could be explained by the fitted model (R2 = 0.94772. As the graph shows, the relationship between the PT and vials of AVS was negative. The minimum number of AVS vials required to achieve near to normal value of PT was calculated from the predicted equation (y = 0.9256x2 - 26.494x + 197.27). Accordingly, in order to obtain near to the normal value of PT, a minimum of 11 vials of AVS was required.
Fig. 2.
Changes of PT, according to vials of AVS.
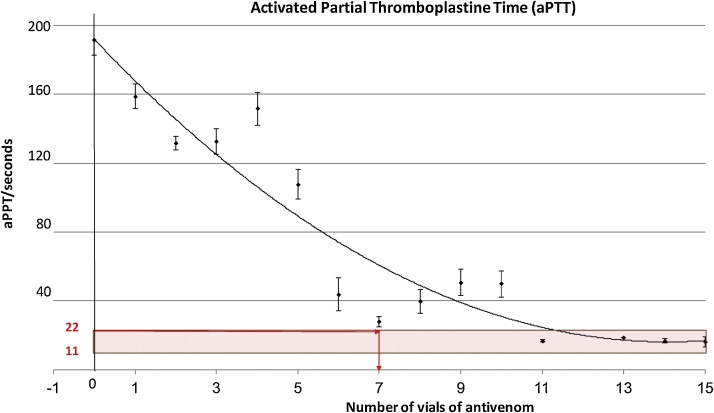
The aPTT of patients was prolonged with deficiencies (<30%) of any one of factors VIII (including Von Willebrand’s factor), IX, X, XI or XII, a deficiency of fibrinogen, factor I (<0.5 g/l), and inhibition by anticoagulants [20]. The relationship between aPTT and the number of vials of AVS is shown in Fig. 3. More than 89% of the observed variation could be explained by the fitted model (R2 = 0.899). The equation (y = 0.8867x2 - 24.969x + 191.86) for Fig. 3 was used to determine the lowest number of AVS vials that were needed to administer to achieve the normal range of the aPPT.
Fig. 3.
Changes in aPTT by doses of AVS in 65 dogs reported to VTH after envenoming.
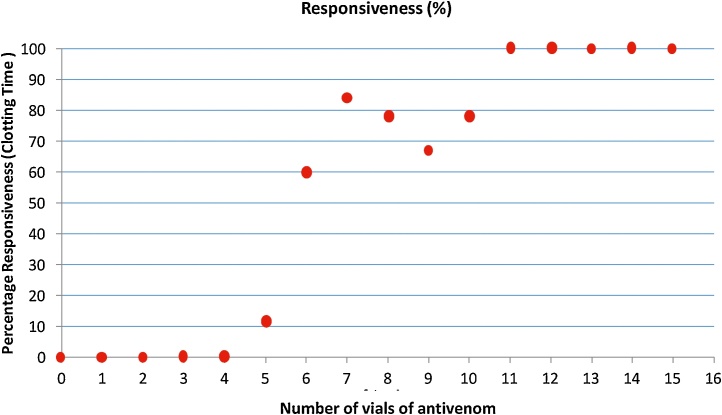
Percentage responsiveness, determined by CT, was plotted against the number of vials of the AVS (Fig. 4). All patients showed prolonged CT on admission. Patients were administered with AVS, and the responsiveness to AVS therapy was determined by restoration of normal values of CT. In the preliminary study, AVS treatment was initiated with one vial, and repeated administration of a single vial of AVS at a recommended time was continued until CT values returned to normal.
Fig. 4.
vials of anti-venom (AVS) verses clotting time (CT).
No animal responded to AVS vials of up to 4 (Fig. 4). When the dose increased to 5 vials of AVS, a responsiveness of 10% was observed. Therefore, during the subsequent study, initial treatment was commenced with 5 vials of AVS continuously. There was a remarkable increase in response rate upon administration of 6 vials and the responsiveness was more than 80% with 7 vials as shown in the Fig. 5.
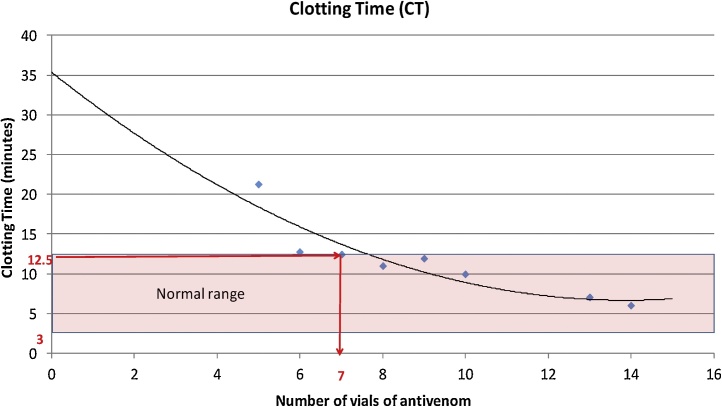
Fig. 5.
Changes in clotting time (CT) by vials of AVS in 65 dogs reported to the VTH after administration of AVS.
The relationship between CT and the number of vials of AVS is described in Fig. 5. Reduction in CT with the vials of AVS could be best explained by a second degree polynomial. More than 85% of the observed variation could be explained by the fitted model (R2 = 0.8567). As the graph shows, an inverse relationship was found between the CT and vials of AVS. The minimum number of AVS vials required to achieve visible CT was calculated from the predicted equation (y = 0.1488x2 - 4.1301x + 35.321). Accordingly, in order to obtain observable CT at normal range, a minimum of 7 vials of AVS was required. In this study a minimum of 6 AVS vials and a maximum of 15 AVS vials had to be administered, and 7.6% (n = 5) dogs started to show normal ranges of selected tests with 6 vials of AVS. However, they developed complications related to envenoming. In this study majority of envenomed dogs (26.2%) exhibited restoring normal values of PT, aPTT, and CT when they were treated with 10 AVS.
3.5. Observation of adverse reactions
A total of 28 (43%) of Russell’s viper bite envenomed patients had clinical signs of adverse reactions to AVS. Minimum time period taken to show adverse reaction was three hours of post AVS administration. Hypersalivation, fever, restlessness, itching, dyspnea, tachypnea, wheezing, stridor, crackles, hypotension, bradycardia, tachycardia, and signs of laryngeal edema were the clinical signs observed as adverse reactions (Table 3). In order to correct hypotension, administration of inotropes intravenously (adrenaline and dopamine as described in 2.6) were initiated soon after detection of low blood pressure. Three (5%) patients with hypotension had undetectable Blood pressure (BP) which persisted for a maximum period of 45 min. Time lapse to restore normal BP (80–120 mmHg) was observed with treatment. The minimum time period taken to correct hypotension with the help of inotropes was 3.25 h.
Table 3.
Adverse reactions of AVS in 28 dogs reported to VTH after RV envenoming.
| Clinical signs of adverse reactions | Number of patients | Percentage (%) |
|---|---|---|
| Respiratory manifestations | ||
| Dyspnoea, wheezing, crackles, stridor | 11 | 17 |
| Laryngeal oedema | 3 | 5 |
| Cardiovascular manifestations | ||
| Bradycardia (<80/beats/minute) | 11 | 17 |
| Tachycardia (>160/beats/minute) | 7 | 11 |
| Detectable hypotension (30-70 mmHg) | 7 | 11 |
| Undetectable hypotension | 3 | 5 |
| Restlessness | 10 | 15 |
| Hypersalivation | 8 | 12 |
| Itching | 5 | 8 |
| Fever | 5 | 8 |
3.6. Renal toxicity
3.6.1. Urinalysis of the patients
Urine samples collected from Russell’s viper bite patients at admission, demonstrated a range of abnormalities. Gross hematuria was observed in 14% of patients. Dipstick (URIPATH-Plasmatic laboratories-UK) reading of urine samples indicated hematuria (hemolysis to 250 RBC/μL), pyuria (25 to 500 WBC/μL) and proteinuria (trace to 300 mg/dL). Spectrophotometer reading indicated isosthenuria, or hyposthenuria. Microscopic (Accu-Scope 3000 series, USA) examination of sediment under the magnification of X400, showed granular and cellular cast. The variations observed in urinalysis on admission of patients are depicted in Table 4.
Table 4.
Urine abnormalities observed in patients with Russell’s viper envenoming.
| Character of urine | Number of patients | Percentage (%) |
|---|---|---|
| Haematuria (haemolyis -250;RBC/μL) | 28 | 43 |
| Pyuria (25-500/μL) | 28 | 43 |
| Proteinuria (trace-300 mg;WBC/dL) | 27 | 41 |
| Granular casts and cellular casts (2-3 or >3 /field ×10 × 40 power) | 26 | 40 |
| Isosthenuria | 24 | 37 |
3.6.2. Development of acute renal failure
BUN and creatinine levels were within normal ranges in all the samples that were collected at the time of admission. However, 19 (29%) RV bite envenomed patients developed signs of acute renal failure later, indicating oliguria or anuria, (reduction of UO) high concentrations of BUN levels (>28 mg/dL), and elevated serum creatinine levels (>1.5 mg/dL). Clinical parameters and laboratory finding in patients with ARF are indicated in Table 5.
Table 5.
Clinical parameters and laboratory findings in patients with ARF.
| Character | Range | Median |
|---|---|---|
| Time lapse from bite to admission (Hours) | 0.5-15 | 4.2 |
| Time taken to develop ARF from admission (Hours) | 8-72 | 23.7 |
| Time taken for recovery from the development of ARF (Hours) | 12-60 | 39.3 |
| BUN (mg/dL) | 64.67-318.13 | 213.12 |
| Creatinine (mg/dL) | 2.56-10.39 | 5.11 |
3.6.3. Development of hepatotoxicity
Only 2 (3%) patients out of 65 had elevated ALT (Median = 152.5 (U/L) and AST levels (Median = 124 U/L) in samples collected at the time of admission and samples collected 24 h later. However, TP and albumin levels in the samples on admission and 24 h later were within normal ranges even in subsequent samples. Elevated serum ALT and AST levels gradually reduced with the treatment measures that were adopted. Patients with elevated hepatic enzymes of ALT and AST recovered in 96 h and 120 h of hospitalization period, respectively.
3.7. Deaths reported due to Russell’s viper envenoming
A total of 8 (12%) deaths were reported during the study period due to RV envenoming regardless of the specific and palliative treatment measures. Out of the 8 patients 2 patients were succumbed due to possible AVS reactions while 4 patients died due to ARF and 2 were showing toxic insult of RVV at the time of death. Patients that died of AVS reactions were clinically manifesting wheezing, crackles, dyspnea, tachypnea and stridor. Whereas, ARF patients were azotemic, and/or oliguric at the time of death and patients with toxic insult were presented with melena, hematemesis, hematuria, pyuria, hypotension, wheezing and crackles. Patients who died due to toxic insult had signs of cardiotoxicty indicating undetectable BP and died in 5th and 12th hour of admission.
4. Discussion
The reason for a substantially high number of cases during the period of study may be due to irritable behavioral nature of the dogs and the tendency to repeated attacks by RV [02]. Both dogs and RV naturally tend to fight each other rather than trying to escape, which is in contrast to man-snake encounters. In addition, both male and female dogs (34:31) are equally prone to be victimized whereas in humans the males are more vulnerable due to occupational predisposition [9]. However, adult dogs are more prone (IQR 2–7 years) to D. russelii envenoming with the case fatality of 12%. The highest proportion of death due to RV bites is reported in adult human (97%) and young males at the age of 4th decades are more prone to die in Sri Lanka [2,5]. Venom of RV consist of cytotoxins [21] which possibly are the reason to experience varying degree of pain by the victim. A study about snake envenoming in hospitals of Anuradhapura district in Sri Lanka reported that 73% of cases were admitted due to RV bites and all the fatalities were due to RV envenoming [22] which indicated very effective bites by this snake. Mild swelling on bitten area (n = 35; 54%) and local necrosis (n = 24; 37%) were observed in victims in this study. In contrast, a few (<10%) showed hemorrhagic blisters with severe necrosis in man [2]. The vasculotoxic effect of the RVV on the capillary endothelium lead to exudation of plasma or blood; so that, swelling of the site of bite and skin discoloration would be seen as an extravasation of blood into the subcutaneous tissue [23]. Clinical manifestations of cardiovascular toxicities were observed in n = 54 (84%) of dogs. PLA2s and cytotoxins are the major components in the RVV causing cardiotoxicity following envenoming [20]. Constituents of this venom lead to cardiovascular toxicity, including hypotension. Release of bradykinine induced by the RVV caused hypotension and circulatory collapse [24]. This could be the reason for hypotension of dog patients. However, clinical signs of cardiovascular toxicity is a rare manifestation in man (3–12 %) following RV envenoming [2].
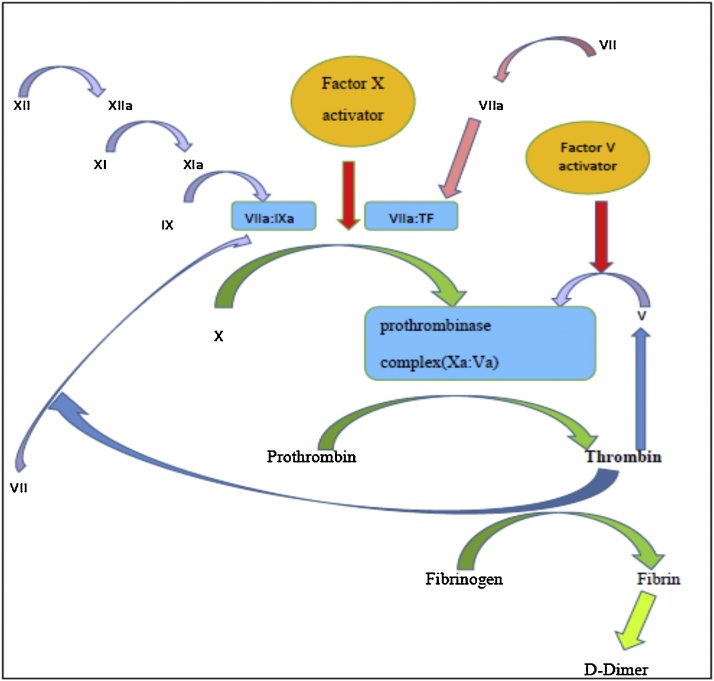
Heamorrhagic lesions were seen in 54 (84%) dogs, which is almost similar to man (77%) [25]. PLA2s, which is a basic protein called “direct lytic factor” causing hemolysis is found only in elapid venoms [21]. In addition to causing hemolysis, it directs hydrolysis of red cell membrane and/or indirectly by producing strongly hemolytic lysolecithin from plasma lecithin [22]. The major toxic compounds present in the RVV lead to degradation of fibrinogen, Factor V, VIII and X by exhibiting an association between pre-antivenom venom concentration and severity of coagulopathy [26]. Therefore, bleeding from the site of bite, petecheation, melena, hematemesis, spontaneous bleeding from injection sites, bleeding from interdigital spaces and hemorrhages from sclera were observed as clinical manifestations of hematotoxicites in these dogs. All the dogs admitted due to RV envenoming had elevated clotting profiles of PT, aPTT, and CT. In the past, an association between the venom concentration and the severity of coagulopathy, measured using INR and aPTT has been documented [26]. Furthermore, (n = 25, 38%) dogs in this study showed thrombocytopenia, yet another indication of heamatotoxicity. Venom of RV contains activators for factor V and X leading to formation of prothrombinase complex (VaXa) (Fig. 6). The complex then activates the entire clotting cascade by converting prothrombin to thrombin; which leads to consumption of fibrinogen, factor VIII and further consumption of factor V by indicating prolonged aPTT [26]. Therefore, anaemia, thrombocytopenia, coagulopathy could be seen in a majority of dog patients with RV envenoming. However, studies on dynamics of platelets when exposed to RV envenoming are still scarce.
Fig. 6.
Diagrammatic representation of mode of action of Russell’s viper factor V and factor X on clotting pathway [26].
Dyspnea and wheezing crackles were seen as signs of toxicities of respiratory system in 34, (52%) of dogs. These effects could mainly be due to vasculotoxic effects of RVV. Therefore, any sign of impending respiratory failure must be managed aggressively with endotracheal intubation in order to facilitate respiration and to prevent possibilities of aspiration. Close monitoring of vital signs of patient’s oxygen saturation and activities of the cardiovascular system using pulse oximetry is important to re-establish impending critical situations. Sedation or mild anesthesia is needed to facilitate endotracheal intubation in such dogs.
This study observed 11% of dogs with neurotoxicity which is also a rare manifestation in man [26]. Neurotoxins act as an acetylcholine receptor antagonist; bind to the acetylcholine receptors that may lead to continuous parasympathetic stimulation which causes hypersalivation and other neurological signs. Currently the extracted immunoglobulin or immunoglobulin fragments F (ab) 2 from serum are used instead of crude serum [27,28]. Indian polyspecific anti-venom consists of F (ab) 2 antibody, and the half-life lies between 80–100 hours [29]. In this study, early responsiveness for AVS therapy was observed by restoration of CT, and more than 80% of the dogs showed restoration of coagulopathy by detecting observable clot when 7 vials of AVS was administered (Fig. 4). Further, aPTT reached near-normal levels when 7 vials of AVS were administered (Fig. 3), while 11 vials had to be administered for PT results to become normal (Fig. 2). However, n = 5 (7.6%) of the patients recovered with 6 vials of AVS. CT is much more beneficial to assess both toxicity and responsiveness for AVS therapy, since early responsiveness is better observed using this test. Another advantage of CT is that it is a simple, easy to perform, cost effective and is a bed side test.
In this study, envenoming patients exhibited clinical manifestations of venom induced consumption coagulopathy such as hematuria, petechiations on skin, hematemesis, melena, haemorrhages from inter digital spaces bleeding from the bite site, bleeding from sclera, and bleeding from venipuncture site. By means of AVS treatments, the coagulation abnormalities returned to normal range in less than 2 h. This can be due to the production of depleted clotting factor by liver which may be faster than in human. Usually it takes 12–36 hours for regeneration of depleted coagulation factors [30]. However, D. russelii envenomed dogs in Sri Lanka have long been treated on the basis of clinical manifestations. Moreover, there are no scientific documents or recommendations at present. Kidney and liver are the main organs that are exposed to serious insult due to venom and toxins in experimental animals [21]. As the clinical manifestation of nephrotoxicity in RVV; ARF was observed in 19 (29%) of patients [2]. Furthermore, formation of microthrombin blood is induced by RVV which is deposited in blood vessels leading to interference with glomerular filtration.
Hence the ultimate clinical manifestation would be anuria or oliguria in the patients leading to death or organ failure [8]. There were 8 deaths in this study and 4 of them died with evidence of ARF. However, the death of those patients cannot be attributed solely to ARF. Other factors such as coagulation disorders, cardiogenic shock and respiratory abnormalities caused by venom toxins may also have contributed. In the present study n = 19 (29%) of dogs developed ARF with oliguria/anuria with azotemia. Analysis of their urine on admission revealed hematuria, granular or cellular casts 2–3 or more/field under the power of ×10 × 40. Therefore, urinalysis of RV envenomed dogs helped in early diagnosis of ARF. The current study proved that 4 patients with hypotension had developed ARF. Of the 8 dogs that died, 5 had hypotension together with other complications of coagulopathy and some evidence of ARF.
Venom toxins of RV cause renal tubular degeneration and necrosis. The tubules as a result, slough off and interfere with the urine output leading to oliguria / anuria. These signs of ARF however, can be cured with appropriate management measures. Hypotension induced by venom leads to hypoxic trauma to renal vasculature. Hypovolemic hypoxia on renal tissues causes degeneration and necrosis. It interferes with the integrity of the basement membrane and irreversible damage to renal tubules. On the other hand, hypotension also can lead to hypoxia. Though hepatotoxicity following RV envenoming was noted in 2 (3%) of dogs, it was corrected with symptomatic and supportive care. Leukocytosis was observed on all the RV envenomed dog victims which has also been observed in man [10,22]. However, the evidence on causes for leukocytosis following such snakebite envenoming is scarce [31]. Anaphylactoid and anaphylaxis reactions for AVS are seen in around 80% of patients due to Fc fragments resulting in papain digestion and IgG aggregates and due to mast cell activation [32].
5. Conclusion
In this study, haemorrhagic clinical signs, cardio respiratory toxicities, and generalized pain was detected as the principle clinical outcome of the RV envenoming in the dogs. Initial dose of anti-venom treatment must be started from 6 vials. Anti-venom induced reactions were commonly reported. Envenoming of dogs from RV may end up with death in dogs. Currently available AVS causes considerable percentage of adverse reactions in dogs. This emphasize current needs of species specific anti-venom to treat such patients. Adverse reaction like acute lung injury must be handled with appropriate setting with sufficient facilities. Since the condition is managed under light anesthesia in order to facilitate respiration by means of intubation, care must be taken to safeguard the life of the patient (safe anesthetics, Oxygen, inotropes, trained staff etc.). Dogs that are highly excited must be taken to a peaceful environment in order to calm them down; in addition, the action would also minimize the venom spread within the body of the victim. Therefore, assessment and stabilization along with supportive care are critical in managing these patients.
Ethics approval
Ethical clearance was obtained from the Ethical Review Committee of the Faculty of Veterinary Medicine and Animal Science, University of Peradeniya, Sri Lanka, which is on par with the international standards of ethics on animal experimentations. All experimental procedures and animal care had been approved by the Faculty Ethics Committee, Faculty of Veterinary Medicine and Animal Science, University of Peradeniya, Sri Lanka.
Consent for publication
We certify this manuscript has not been published elsewhere and is not submitted to another Journal. All authors have approved the manuscript and agreed with submission to Toxicology reports.
Availability of data and material
The datasets and materials are contained during the in this study are available if necessary.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors’ contributions
RBA, IDS conceived the study, concept, and design and conducted the laboratory experiments; analyzed and interpreted experimental results. IDS, AD, IBG and DDN contributed to the proposal for study design and supervision of the study, interpretation of data, drafting of the proposal. ADP, RBA, contributed with data analysis, critical revision of the manuscript and manuscript preparation. CM supported carrying out laboratory experiments. All authors read and approved the final manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Dog owners who brought their pets to the VTH are highly appreciated. The authors wish to thank the technical support given by the staff of the Department of Veterinary Clinical Sciences, Faculty of Veterinary Medicine and Animal Science, University of Peradeniya., Sri Lanka.
References
- 1.Keyler D.E., Gawarammana I., Gutierrez J.M., Sellahewa K.H., McWhorter K., Malleappah R. Antivenom for snakebite envenoming in Sri Lanka: the need for geographically specific antivenom and improved efficacy. Toxicon. 2013;69:90–97. doi: 10.1016/j.toxicon.2013.01.022. [DOI] [PubMed] [Google Scholar]
- 2.Kularatne S.A.M. 2013. Handbook on the Management of Snake Bite in Sri Lanka.https://www.nhbs.com/snakes-snakebite-and-envenoming-in-sri-lanka-book [Google Scholar]
- 3.Adhikari R.B., Dangolla A., Gawarammana I.B., De Silva D.D.N., Premarathna A.D., Silva I.D. Epidemiology of snakebite in dogs in Sri Lanka. Toxicol. Commun. 2018;2.1:107–112. [Google Scholar]
- 4.Tan N.H., Fung Hong S.Y., Tan K.Y., Yap M.K.K., Gnanathasan C.A., Tan C.H. Functional venomics of the Sri Lankan Russell’s viper (Daboia russelii) and its toxinological correlations. J. Proteomics. 2015;128:403–423. doi: 10.1016/j.jprot.2015.08.017. [DOI] [PubMed] [Google Scholar]
- 5.De Silva A., Ranasinghe L. Epidemiology of snakebite in Sri Lanka. Ceylon Med. J. 1983;28:144–154. https://www.ncbi.nlm.nih.gov/pubmed/6386199 [PubMed] [Google Scholar]
- 6.Mehta S.R., Sashindran V.K. Clinical features and management of snake bite. Med. J. Armed Forces India. 2011;58(3):247–249. doi: 10.1016/S0377-1237(02)80140-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mukherjee A.K., Ghosal S.K., Maity C.R. Some biochemical properties of Russell’s viper (Daboia russelli) venom from Eastern India: correlation with clinico-pathological manifestation in Russell’s viper bite. Toxicon. 2000;38(2):163–175. doi: 10.1016/s0041-0101(99)00125-7. [DOI] [PubMed] [Google Scholar]
- 8.Kohli H.S., Sakhuja V. Snake bites and acute renal failure. Saudi J. Kidney Dis. Transplant. 2003;14.2:165. [PubMed] [Google Scholar]
- 9.Kularatne S.A.M. Epidemiology and clinical picture of the Russell’s viper (Daboia russelii russelii) bites in Anuradhapura, Sri Lanka: a prospective study of 336 patients. Southeast-Asian Journal of Tropical Medicine and Public Health. 2003;34:855–862. [PubMed] [Google Scholar]
- 10.Ariaratnam C.A., Sjostrom L., Raziek Z., Kularatne S.A., Arachchi R.W., Sheriff M.H., Theakston R.D., Warrell D.A. An open, randomized comparative trial of two antivenoms for the treatment of envenoming by SriLankan Russell’s viper (Daboia russelii russelii) Trans. R. Soc. Trop. Med. Hyg. 2001;95:74–80. doi: 10.1016/s0035-9203(01)90339-6. [DOI] [PubMed] [Google Scholar]
- 11.Gunathilake M., Jayakody R.L., Angunawela P. A. De Tissera. Direct nephrotoxic effects produced by venoms of Sri Lankan cobra, Russell’s viper and hump nosed viper. Ceylon J. Med. Sci. 2003;46:61–66. [Google Scholar]
- 12.Oluyombo R., Okunola O.O., Olakulehin O.A. Snakebite nephrotoxicity: a case report and review of the literature. Trop. J. Med. Res. 2017;20.1:91. [Google Scholar]
- 13.Carla C.N.M., de Queiroz M.R., Fonseca K.C., de Morais N.C.G., Filho A.G., Beletti M.E., Stanziola L., de Oliveira F. Histological and ultrastructural analyses of muscle damage induced by a myotoxin isolated from Bothrops alternatus snake venom. Protein Pept. Lett. 2013;20.2:192–199. doi: 10.2174/092986613804725352. [DOI] [PubMed] [Google Scholar]
- 14.Silva A., Johnston C., Kuruppu Kneisz S., Maduwage K., Kleifeld O., Smith A.L., Siribaddana S., Buckley N.A., Hodgson W.C., Isbister G.K. Clinical and pharmacological investigation of myotoxicity in sri lankan russell’s viper (Daboia russelii) envenoming. PLoS Negl. Trop. Dis. 2016;2,10:12. doi: 10.1371/journal.pntd.0005172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goonawardene I.P. Cardiotoxicity of Russell,s viper venom;an overview. J. Ceylon Coll. Physicians. 1996;29:57–60. [Google Scholar]
- 16.Saha P.P., Bhowmik T., Dasgupta A.K., Gomes A. In vivo and in vitro toxicity of nanogol d conjugated snake venom protein toxin GNP-NKCT1. Toxicol. Rep. 2014;1:74–84. doi: 10.1016/j.toxrep.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mana K., Ghosh R., Gantait K., Saha K., Parua P., Chatterjee U., Sarkhel S. Incidence and treatment of snakebites in West Bengal, India. Toxicol. Rep. 2019;6:239–243. doi: 10.1016/j.toxrep.2019.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leon G., Herrera M., Segura A., Villalta M., Vargas M., Gutierrez J.M. Pathogenic mechanisms underlying adverse reactions induced by intravenous administration of snake antivenoms. Toxicon. 2013;15:63–76. doi: 10.1016/j.toxicon.2013.09.010. [DOI] [PubMed] [Google Scholar]
- 19.Gobikrushanth M., Abeygunawardena H.E., Bandara A.M.R., Silva I.D. 2011. 63rd Annual Scientific Sessions of the Sri Lanka Veterinary Association, Abstract of Scientific Papers.https://www.slva.org/data/files/downloads/proceedings_slva_annual_sessions.pdf p.22. [Google Scholar]
- 20.Bush B.M. Blackwell Scientific Publications; Oxford: 1991. Interpretation of Laboratory Results for Small Animal Clinicians. [Google Scholar]
- 21.Shelke R.R., Sathish S., Gowda T.V. Isolation and characterization of a novel postsynaptic/cytotoxic neurotoxinfrom Daboia russelii russelii venom. J. Pept. Res. 2002;59:257–263. doi: 10.1034/j.1399-3011.2002.02969.x. [DOI] [PubMed] [Google Scholar]
- 22.Silva A., Pilapitiya S., Siribaddana S. Acute Myocardial Infarction following a possible direct intravenous bite of Russell’s viper (Daboia russelli) BMC Res. Notes. 2012;5,1:500. doi: 10.1186/1756-0500-5-500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ohsaka A. Hemorrhagic, necrotizing and edema forming effects of snake venoms. In:snake venoms. In: Lee C.Y., editor. Vol. 52. Berlin;Springer-verlag; 1979. p. 480. (Handbook of Experimental Pharmacology). [Google Scholar]
- 24.Ganesh A., Balaji M.V., Udhayakumar N.P., Subramanian T.M. Acute renal failure in snake envenomation: a large prospective study. Saudi J. Kidney Dis. Transplant. 2008;19:404–410. [PubMed] [Google Scholar]
- 25.Kularatne S.A., Gawarammana I.B., Kumarasiri P.V. Safety and efficacy of subcutaneous adrenaline as treatment for anaphylactic reactions to polyvalent antivenom. Ceylon Med. J. 2003;48:123–128. doi: 10.4038/cmj.v48i4.3339. [DOI] [PubMed] [Google Scholar]
- 26.Isbister G.K., Maduwage K., Scorgie F.E., Shahmy S., Mohamed F., Abeysinghe C., Harendra K., OLeary M.A., Gnanathasan C.A., Lisa F.L. Venom concentrations and clotting factor levels in a prospective cohort of Russell’s viper bites with coagulopathy. PLoS Negl. Trop. Dis. 2015;9(8) doi: 10.1371/journal.pntd.0003968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.2016. WHO Guidelines for the Production Control and Regulation of Snake Antivenom Immunoglobulins.https://www.who.int/biologicals/BS2300_ANTIVENOMS_WHO_Guidelines.pdf [DOI] [PubMed] [Google Scholar]
- 28.Teeling J.L., French R.R., Cragg M.S., van den Brakel J., Pluyter M., Huang H., Chan C., Parren P.W., Hack C.E., Dechant M., Valerius T., van de Winkel J.G., Glennie M.J. Characterization of new human CD20 monoclonal antibodies with potent cytolytic activity against non-Hodgkin lymphomas. Blood. 2004;104.6:1793–1800. doi: 10.1182/blood-2004-01-0039. [DOI] [PubMed] [Google Scholar]
- 29.Isbister G.K., Maduwage K., Saiao A., Buckley N.A., Jayamanne S.F., Seyed S., Mohamed F., Chathuranga U., Mendes A., Abeysinghe C., Karunathilake H., Gawarammana I., Lalloo D.G., de Silva H.J. Population pharmacokinetics of an indian F(ab’)2 snake antivenom in patients with Russell’s viper (Daboia russelii) bites. PLoS Negl. Trop. Dis. 2015;9(7) doi: 10.1371/journal.pntd.0003873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baranidharan G.R., Nambi A.P., Kavitha S., Thirunavukkarasu P.S., Sridhar P., Hamsa Yamini H., Muthuvel M. Anticoagulant rodenticide toxicity and secondary haemostatic disorder in a dog. International Journal of Veterinary Science. 2014;3(1):37–39. http://www.ijvets.com/pdf-files/Volume-3-no-1-2014/37-39.pdf [Google Scholar]
- 31.Silva A., Gunawardena P., Weilgama D., Maduwage K., Gawarammana I. Comparative in-vivo toxicity of venoms from South Asian hump-nosed pit vipers (Viperidae: crotalinae: hypnale) BMC Res. Notes. 2012;5:471. doi: 10.1186/1756-0500-5-471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gawarammana I.B., Kularatne S.A., Dissanayake W.P., Kumarasiri R.P., Senanayake N., Ariyasena H. Parallel infusion of hydrocortisone +/- chlorpheniramine bolus injection to prevent acute adverse reactions to antivenom for snakebites: a randomized, double-blind, placebo-controlled study. Med. J. Aust. 2004;180:20–23. doi: 10.5694/j.1326-5377.2004.tb05768.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets and materials are contained during the in this study are available if necessary.