Abstract
Pectin, a natural polysaccharide, has gained increasing attention due to not only its biomaterial properties but also its biomedical activities. One of the abundant sources of pectin is mangosteen (Garcinia mangostana L.) rind. In this study, we characterized the pectin from Indonesian mangosteen rind extract and evaluated its antioxidant activity. Pectin was extracted in acid condition and evaluated its physicochemical properties by fourier transform infrared (FTIR), powder X-ray diffractometer (PXRD), water content, ash content, equivalent weight, methoxyl level and of galacturonic acid content. Furthermore, the antioxidant activity of pectin was also observed by 2,2-diphenyl-1-picrylhydrazyl (DPPH) method. Pectin was successfully extracted from dry weight of Indonesian mangosten rind with yield about 1,16 ± 0,17%, fine powder, brownish and odorless. FTIR and PXRD results showed that pectin from mangosteen rind extract was amorphous and similar characteristic with a commercial pectin. The chemical properties of pectin such as water content, ash content, equivalent weight, methoxyl level and of galacturonic acid level were 9.85 ± 0.12%, 3.91 ± 0.17%, 6330.76 ± 220.43 g/mol, 2.86 ± 0.05% and 75.98 ± 0.88%, respectively. In addition, pectin showed an antioxidant activity with the IC50 about 161.94 ± 31.57 ppm. These results suggest that pectin from Indonesian mangosteen rind has the potential properties as biopolymers for biomedical applications with a low-methylated pectin and a moderate antioxidant activity.
Keywords: Materials science, Mangosteen, Biopolymer, Antioxidant, Polysaccharide, Pectin, Biomaterials, Sol-gel, Polymers, Materials characterization, Natural product
1. Introduction
Mangosteen (Garcinia mangostana L.) is one of the tropical fruits that has been widely used as a traditional medicine in Southeast Asia. Indonesia, the Southeast Asia country with the largest mangosteen producer in the world, has abundant mangosteen waste which can be used as pharmaceutical raw material [1, 2]. The important part of mangosteen fruits is rind which contains various bioactive compounds, such as phenolic acid, tannin, xanthone, anthocyanins [3, 4, 5, 6, 7], and pectin [8].
Pectin, a heterogeneous group of polysaccharides, mainly contains 1,4-linked-α-D-galacturonic acid and presents in plant cell walls [9]. Recent studies proved that pectin has various biological properties, such as antioxidants, antitumor, and anti-inflammatory activities [10]. In addition, because of pectin properties in forming a stable gel with simple gelling mechanism, the use of pectin as a matrix for biomedical applications has increased in the fields of drug delivery, tissue engineering, and wound dressing [11, 12, 13].
The outstanding quality of pectin has a more than 65% of galacturonic acid content [14]. Pectin from mangosteen rind has the highest galacturonic acid content (73,16%), meanwhile, the galacturonic acid content of pectin from lime and mango peels are 72.5% and 56.67%, respectively [15]. These properties lead pectin from mangosteen rind has a high potential to be extracted and investigated for the antioxidant activity.
Apparently, no studies discussed about the characterization of pectin along with its antioxidant activity from Indonesian mangosteen rind. Therefore, in this study, we characterized physicochemical properties of pectin from Indonesian mangosteen rind and evaluated its antioxidant activity using DPPH method to be developed for biomedical applications.
2. Materials and methods
2.1. Materials
Mangosteen fruits were obtained from farmers in Puspahiang (Tasikmalaya, West Java, Indonesia, latitude: -7.422070, longitude: 108.044540). The fruit was harvested in 100 days after anthesis. A commercial pectin as standard was bought from Cargill (Jakarta, Indonesia). Phenolphthalein indicators were obtained from Asia Chemical (Jakarta, Indonesia). 2,2-diphenyl-1-picrylhydrazyl (DPPH) and ascorbic acid were acquired from Sigma Aldrich (Jakarta, Indonesia). All reagents were procured from Emsure (Jakarta, Indonesia).
2.2. Extraction of pectin from mangosteen rind
The rind of mangosteen fruits were dried at 60 °C and milled to 250–500 μm size. 50 g of mangosteen powder was added to 250 mL of distilled water, and acidified with H2SO4 at pH 2 in 90 °C for 120 min. The filtrate was then coagulated with 96 % ethanol (ratio 1:1) for 10 h, then, the precipitate was separated by cloth filters. Afterward, the precipitation was washed with ethanol and acetone to remove organic solvents. The pectin was dried in oven at 40 °C for 6 h until constant weight and the pectin yield was calculated. The percentage of yield was determined from the weight ratio of pectin produced and dry raw material [16].
2.3. Scanning electron microscope analysis
Pectins were put on aluminum stubs and coated with gold-palladium for 10 seconds, and analyzed by SEM (SU3500 Hitachi, Tokyo, Japan) [17].
2.4. Fourier-transform infrared spectroscopy analysis
Pectins (2 mg) were mixed with 300 mg of dry KBr crystals and printed with a rotary vacuum pump. The pellet of KBr was scanned by FTIR (IR Prestige-21 Shimadzu, Tokyo, Japan) in the range of 400–4000 cm−1 [16].
2.5. X-ray diffractometric analysis
The X-ray diffraction (XRD) patterns were acquired by an X-ray diffractometer (Bruker, Yokohama, Japan). Briefly, pectins were irradiated with filtered Cu–K (α) at a voltage of 40.0 kV and a current of 40.0 mA. The scanning level was 2°/min at a 2θ diffraction angle which ranged from 5° to 60° [18].
2.6. Determination of moisture and ash contents
A total of 0.5 g of pectin samples were dried in oven (Memmert UN55, Shanghai, China) at 100 °C for 4 h until constant weight. To determine ash content, porcelain crucible was spread in the furnace at a temperature of 600 °C then cooled in a desiccator and weighed as the weight of the container. As much as 0.5 g of pectin was weighed and put in the silicate crucible, then, added to the furnace with a temperature of 600 °C for 4 h until constant weight [19].
2.7. Determination of equivalent weight, methoxyl level, and galacturonic acid level
The equivalent weight was measured by weighing 0.25 g of pectin in a tube and moistened with 2.5 mL ethanol. One gram of NaCl, carbon free distillation water (100 mL), and six drops of phenolphthalein indicator were added. The mixture was then stirred quickly and the titration was done slowly until the indicator color changed to pink (pH 7.5). The neutralized solution was used to measure methoxyl. The determination of methoxyl content was carried out by adding 12.5 mL of 0.25 N NaOH to a neutral solution, then shaken correctly and left for 30 min at room temperature in a closed Erlenmeyer. Afterward, 12.5 mL of 0.25 N HCl and the phenolphthalein indicator were added and titrated with 0.1 N NaOH titrant until the solution turned to pink [19]. Galacturonic level (%AUA) was calculated from the volume of NaOH obtained from the determination of BE (equivalent weight) and methoxyl content as shown in Eqs. (1), (2), and (3) [20].
| (1) |
| (2) |
| (3) |
2.8. Antioxidant activity of pectin
Ascorbic acid was used as the reference for antioxidant activity of pectin. 2 mL of pectin solutions in ethanol (500 μg/mL) were reacted with a DPPH solution (40 μg/mL) in 5 mL of volumetric flask and then incubated for 30 min. The absorbance was measured at 400–600 nm by UV-visible spectrophotometer (Analytic Jena Specord 200, Germany). The antioxidant activity was carried out with the variation of concentration of 2, 4, 8, 16, and 32 μg/mL for ascorbic acid and 500, 1000, 1500, 2000, 2500 μg/mL for pectin. In this case, ethanol was used as blank reagent. The IC50 level was calculated from the linear regression curve and Eq. (4) below [21]:
| % inhibition = 1 – (Absorbance sample/Absorbance blank DPPH) × 100% | (4) |
3. Results
3.1. Characterization of pectin from mangosteen rind extract
To obtain mangosteen powder, we successfully extracted pectin from mangosteen rind with a fine powder, a brownish color and odorless (Fig. 1). The yield of extraction was 1,16 ± 0,17%.
Fig. 1.
Macroscopic image of pectin from mangosteen rind extract.
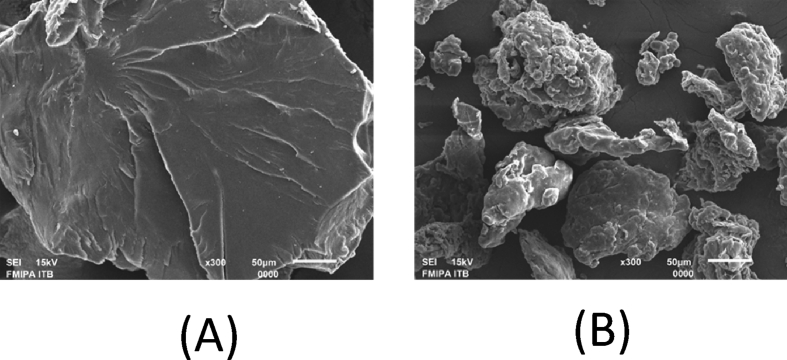
We next investigated the morphology of the pectin by using SEM. Based on the results in Fig. 2 show that the pectin from mangosteen peel extract had the smaller in size than the standard.
Fig. 2.
SEM images of pectins (A) standard pectin (B) extracted pectin.
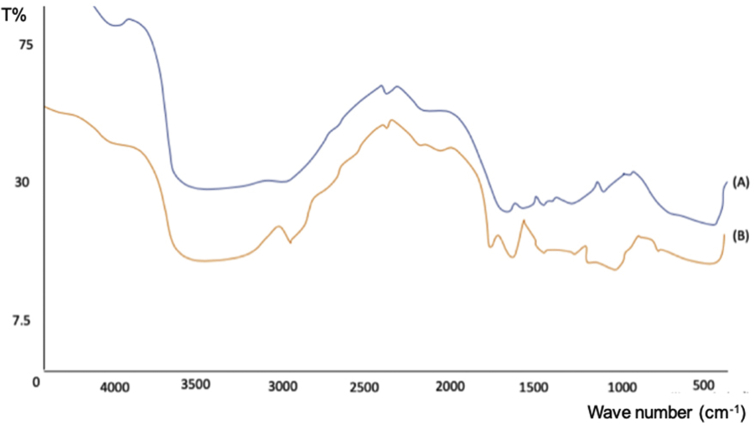
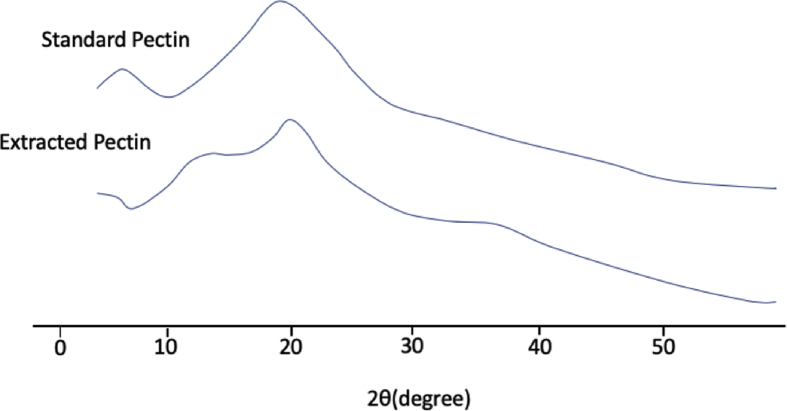
To confirm the functional group of pectin from mangosteen rind extract and standard pectin, pectin was analyzed by FTIR. The result in Fig. 3 describes that extracted pectin and standard pectin had similar FTIR spectrums. PXRD analysis was performed to investigate the crystallinity of the pectins. Fig. 4 describes that the X-ray diffraction patterns of extracted pectin and standard pectin were amorphous.
Fig. 3.
FTIR spectra of pectins (A) standard pectin (B) extracted pectin.
Fig. 4.
PXRD patterns of pectins (A) standard pectin (B) extracted pectin.
To observe the purity of pectins, we next analyzed moisture content, ash content, equivalent wight, methoxyl level, and galacturonic acid level as shown in Table 1.
Table 1.
Purity of pectin from mangosteen rind extract.
| Parameter | Result |
|---|---|
| Moisture content (%) | 9.85 ± 0.12% |
| Ash content (%) | 3.91 ± 0.17% |
| Equivalent weight (g/mol) | 6330.76 ± 220.43 g/mol |
| Methoxyl level (%) | 2.86 ± 0.05% |
| Galacturonic acid level (%) | 75.98 ± 0.88% |
3.2. Antioxidant activity of pectin
In this examination, DPPH solution was stable for 30 min at the maximum wavelength at 516 nm. The antioxidant activity result as shown in Table 2. The ascorbic acid had the IC50 value of 3.39 ± 0.20 and 161.93 ± 31.57 for pectin from mangosteen rind extract.
Table 2.
IC50 values of ascorbic acid and extracted pectin.
| Ascorbic Acid |
Pectin from mangosteen rind extract |
||||
|---|---|---|---|---|---|
| Concentration (ppm) | % Inhibition | IC50 | Concentration (ppm) | % Inhibition | IC50 |
| 2 | 33.30 | 3.39 ± 0.20 | 500 | 74.15 | 161.93 ± 31.57 |
| 4 | 53.39 | 1000 | 86.09 | ||
| 8 | 82.38 | 1500 | 86.88 | ||
| 16 | 92.70 | 2000 | 91.49 | ||
| 32 | 96.53 | 2500 | 95.17 | ||
4. Discussion
4.1. Characterization of pectin from mangosteen rind extract
To obtain the optimum yield of pectin, the extraction of pectin was done by heating at low pH. This condition increased the hydrolysis of protopectin to pectin [21]. The pectin yield from mangosteen rind extract was 1,16 ± 0,17%. The yield and physicochemical properties of pectin depend on the sources and the extraction process [22].
The physicochemical properties of pectin from Indonesian mangosteen rind were observed to compare with a commercial pectin standard. Firstly, to observe the morphology and particle size of the pectin, we next analyzed pectin by SEM. Based on Fig. 2, the surface of control was smoother than that of pectin from mangosteen rind extract. In addition, extracted pectin had a surface with relatively smaller particles compared to standard pectin. The standard pectin is a marketable pectin which has been modified by the addition of sugar, therefore, the sugar may affects its morphology. Interestingly, Malviya and Kulkarni identified pectin from mango peel extract and it had similar morphology with pectin from Indonesian mangosteen rind [23].
We next investigated the chemical structure of pectin by FTIR analysis (Fig. 3). The major peaks of hydroxyl group were appeared at 3402.43 cm−1 and 3367.71 cm−1 for pectin and standard pectin, respectively. The aliphatic C–H groups and C=O carbonyl group were shown in 2927.94 cm−1 and 1747.51 cm−1 for pectin, and 2939.52 cm−1 and 1639.49 cm−1 for standard pectin. The peak at 1442.7 cm−1 describes the presence of a CH methyl group for both of extracted pectin and standard pectin. In addition, 1161.15 cm−1 and 1153.43 cm−1 indicate the presence of C–O bonds in alcohols, esters, and carboxylic acids. These results suggest that FTIR spectrums of extracted pectin was similar with pectin standard. The result of X-ray diffraction analysis corroborated the FTIR study which indicated amorphous system in both extracted pectin and standard pectin (Fig. 4).
4.2. Purity of pectin
The quality of pectin is necessary to be investigated by observing its purity. The purity of pectin was analyzed by calculating the moisture content, ash content, equivalent weight, methoxyl level and galacturonic acid level. The result of chemical properties of pectin from mangosteen rind is shown in Table 1. The moisture and ash content of pectin were 9.85 ± 0.12% and 3.91 ± 0.17%, respectively. The moisture content is closely related to the water content in mangosteen rind sources. Mai and Dui successfully extracted pectin from vietnamese mangosteen with moisture content of 4.64% [8]. The ash content represents the purity of the pectin. The lower the ash content, the higher the purity of the pectin [20]. In addition, the ash content in pectin extracted from dragon fruits were varied from 7 to 10% depend on extraction time [16]. These results suggest that pectin from Indonesian mangosteen rind has a high moisture content and a high purity of pectin.
Equivalent weight is a measurement of the free galacturonic acid content in the pectin. Pure pectin acid, a pectate, is composed entirely of polyalgalactic acid which is free of methyl ester groups [19]. The equivalent weight of pectin depends on the type of plant, quality of raw material, method of extraction and extraction process. Pectin extracted from the Indonesian mangosteen rind had a great equivalent weight, which was 6330.76 ± 220.43 g/mol.
Methoxyl and galacturonic acid content describe the quality of pectin. Their level in pectin can affect the structure and texture of the pectin gel formed [24]. The methoxyl level and galacturonic acid contents of pectin were 2.86 ± 0.05% and 75.98 ± 0.88%, respectively. These results were in acceptable range as stated in Indonesian Pharmacopeia and Handbook of Pharmaceutical Excipient (<6.7% for methoxyl level and ≤74% for galacturonic acid) [25,26] In addition, these result suggest that the methoxyl level of pectin from Indonesian mangosteen rind extract is low-methylated pectin [27]. Low-methoxy pectin is gelled with calcium ions and independent on the presence of acid or high solids content [25]. On the other hand, the galacturonic acid level is quite good in quality.
4.3. Antioxidant activity of pectin
Antioxidant effect of pectin is important for biomedical application. Therefore, we determined the antioxidant activity of pectin by DPPH method. The compound 1,1-diphenyl-2-pikril-hidrazil (DPPH) is a free radical molecule that is stable with the delocalization of electrons around its molecules, therefore the DPPH solution is purple. When reacting with antioxidant compounds, DPPH will be reduced and change to yellow due to the formation of DPPH-H. DPPH acts as a hydrogen donor for antioxidant compounds [28]. Pectin has been found to have antioxidant activity depending on the structure. It predict that hydroxyl groups of polysaccharides such as pectin can show good antioxidant activity when the viscosity is not too high [29]. The result of antioxidant activity in Table 2 show that pectin from Indonesian mangosteen rind extract was moderate antioxidant with IC50 value was 161.93 ± 31.57 ppm [30].
5. Conclusion
In conclusion, pectin was successfully extracted from Indonesian mangosteen rind and had a distinctive characteristic. It was amorphous form and classified as low-methylated pectin. The antioxidant activity of this pectin was moderate antioxidant compare to ascorbic acid. These results suggest that pectin from Indonesian mangosteen has a good quality to be developed as biomaterial for biomedical application.
Declarations
Author contribution statement
Nasrul Wathoni: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Chu Yuan Shan & Wong Yi Shan: Performed the experiments; Analyzed and interpreted the data.
Tina Rostinawati, Raden Bayu Indradi, Rimadani Pratiwi & Muchtaridi Muchtaridi: Analyzed and interpreted the data; Wrote the paper.
Funding statement
This work was supported by Academic Leadership Grants (ALG) 2019, Universitas Padjadjaran (1373b/UN6.O/LT/2019).
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Pedraza-Chaverri J., Cárdenas-Rodríguez N., Orozco-Ibarra M., Pérez-Rojas J.M. Medicinal properties of mangosteen (Garcinia mangostana) Food Chem. Toxicol. 2008;46:3227–3239. doi: 10.1016/j.fct.2008.07.024. [DOI] [PubMed] [Google Scholar]
- 2.Mansyah E., Muas I., S M.J.A., Affandi A. The research for supporting sustainable mangosteen (Garcinia mangostana L.) production. Int. J. Adv. Sci. Eng. Inf. Technol. 2016;3:16. [Google Scholar]
- 3.Suttirak W., Manurakchinakorn S. In vitro antioxidant properties of mangosteen peel extract. J. Food Sci. Technol. 2014;51:3546–3558. doi: 10.1007/s13197-012-0887-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zadernowski R., Czaplicki S., Naczk M. Phenolic acid profiles of mangosteen fruits (Garcinia mangostana) Food Chem. 2009;112:685–689. [Google Scholar]
- 5.Pothitirat W., Chomnawang M.T., Supabphol R., Gritsanapan W. Comparison of bioactive compounds content, free radical scavenging and anti-acne inducing bacteria activities of extracts from the mangosteen fruit rind at two stages of maturity. Fitoterapia. 2009;80:442–447. doi: 10.1016/j.fitote.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 6.Zarena A.S., Sankar U. Screening of xanthone from mangosteen (Garcinia mangostana L.) peels and their effect on cytochrome c reductase and phosphomolybdenum activity. J. Nat. Prod. 2009;2:23–30. [Google Scholar]
- 7.Palapol Y., Ketsa S., Stevenson D., Cooney J.M., Allan A.C., Ferguson I.B. Colour development and quality of mangosteen (Garcinia mangostana L.) fruit during ripening and after harvest. Postharvest Biol. Technol. 2009;51:349–353. [Google Scholar]
- 8.Mai D.S., Duy T. Pectin extraction from the fresh rind of Vietnamese mangosteen (Garcinia mangostana) Acta Hortic. 2015;1088:553–556. [Google Scholar]
- 9.Christiaens S., Van Buggenhout S., Houben K., Jamsazzadeh Kermani Z., Moelants K.R.N., Ngouémazong E.D., Van Loey A., Hendrickx M.E.G. Process–structure–function relations of pectin in food. Crit. Rev. Food Sci. Nutr. 2016;56:1021–1042. doi: 10.1080/10408398.2012.753029. [DOI] [PubMed] [Google Scholar]
- 10.Ho G.T.T., Ahmed A., Zou Y.F., Aslaksen T., Wangensteen H., Barsett H. Structure-activity relationship of immunomodulating pectins from elderberries. Carbohydr. Polym. 2015;125:241–248. doi: 10.1016/j.carbpol.2015.02.057. [DOI] [PubMed] [Google Scholar]
- 11.Liu L.S., Fishman M.L., Hicks K.B. Pectin in controlled drug delivery - a review. Cellulose. 2007;14:15–24. [Google Scholar]
- 12.Mishra R.K., Datt M., Pal K., Banthia A.K. Preparation and characterization of amidated pectin based hydrogels for drug delivery system. J. Mater. Sci. Mater. Med. 2008;19:2275–2280. doi: 10.1007/s10856-007-3310-4. [DOI] [PubMed] [Google Scholar]
- 13.Munarin F., Guerreiro S.G., Grellier M.A., Tanzi M.C., Barbosa M.A., Petrini P., Granja P.L. Pectin-based injectable biomaterials for bone tissue engineering. Biomacromolecules. 2011;12:568–577. doi: 10.1021/bm101110x. [DOI] [PubMed] [Google Scholar]
- 14.Chakraborty A., Ray S. Development of a process for the extraction of pectin from citrus fruit wastes viz. Lime peel, spent guava extract, apple pomace etc. J. Food Saf. 2011;13:391–397. Internet. FoodHACCP.Com Publ. [Google Scholar]
- 15.Madhav A., Pushpalatha P. Characterization of pectin extracted from different fruit wastes. J. Trop. Agric. 2002;40:53–55. [Google Scholar]
- 16.Abang Zaidel N., Md Rashid J., Hamidon N.H., Md Salleh L., Mohd Kassim A.S. Extraction and characterization of pectin from dragon fruit (Hylocereus polyrhizus) peels. Chem. Eng. Trans. 2017;56:805–810. [Google Scholar]
- 17.Wathoni N., Motoyama K., Higashi T., Okajima M., Kaneko T., Arima H. Physically crosslinked-sacran hydrogel films for wound dressing application. Int. J. Biol. Macromol. 2016;89:465–470. doi: 10.1016/j.ijbiomac.2016.05.006. [DOI] [PubMed] [Google Scholar]
- 18.Wathoni N., Hasanah A.N., Mohammed A.F.A., Pratiwi E.D., Mahmudah R. Accelerated wound healing ability of sacran hydrogel film by keratinocyte growth factor in alloxan-induced diabetic mice. Int. J. Appl. Pharm. 2018;10:57–61. [Google Scholar]
- 19.Ranganna Shamanna. Ranganna - Google Books, Tata McGraw-Hill Publ. Co. Ltd.; New Delhi: 1986. Handbook of Analysis and Quality Control for Fruit and Vegetable Products - S; pp. 594–647. [Google Scholar]
- 20.Ismail N.S.M., Ramli N., Hani N.M., Meon Z. Extraction and characterization of pectin from dragon fruit (Hylocereus polyrhizus) using various extraction conditions. Sains Malays. 2012;41:41–45. [Google Scholar]
- 21.Urias-Orona V., Huerta-Oros J., Carvajal-Millán E., Lizardi-Mendoza J., Rascón-Chu A., Gardea A.A. Component analysis and free radicals scavenging activity of cicer arietinum L. husk pectin. Molecules. 2010;15:6948–6955. doi: 10.3390/molecules15106948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kumar A., Chauhan G.S. Extraction and characterization of pectin from apple pomace and its evaluation as lipase (steapsin) inhibitor. Carbohydr. Polym. 2010;82:454–459. [Google Scholar]
- 23.Malviya R., Kulkarni G.T. Extraction and characterization of mango peel pectin as pharmaceutical excipient. Polim. Med. 2012;42:185–190. [PubMed] [Google Scholar]
- 24.Constenla D., Lozano J.E. Kinetic model of pectin demethylation. Lat. Am. Appl. Res. 2003:91–95. [Google Scholar]
- 25.Rowe R.C., Sheskey P.J., Quinn M.E. Sixth. Pharmaceutical Press; Chicago: 2009. Handbook of Pharmaceutical Excipients; p. 478. [Google Scholar]
- 26.Dirjen Bina Kefarmasian Dan Alat Kesehatan, Farmakope Indonesia. V, Ministry of Health; Republic of Indonesia, Jakarta: 2014. pp. 1004–1005. [Google Scholar]
- 27.Birch G. Food chemicals codex. Food Chem. 2003;59:179–180. [Google Scholar]
- 28.Catteau J.-P., Vergoten G., Bernier J.-L., Zoete V., Bailly F., Vezin H. 4-Mercaptoimidazoles derived from the naturally occurring antioxidant ovothiols 2. Computational and experimental approach of the radical scavenging mechanism. Free Radic. Res. 2006;32:525–533. doi: 10.1080/10715760000300531. [DOI] [PubMed] [Google Scholar]
- 29.Ro J., Kim Y., Kim H., Jang S.B., Lee H.J., Chakma S., Jeong J.H., Lee J. Anti-oxidative activity of pectin and its stabilizing effect on retinyl palmitate. Korean J. Physiol. Pharmacol. 2013;17:197–201. doi: 10.4196/kjpp.2013.17.3.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wallock L.M., Milbury P.E., Blumberg J.B., Stephensen C.B., Ames B.N., Jacob R.A., Aiello G.M. Moderate antioxidant supplementation has No effect on biomarkers of oxidant damage in Healthy men with low fruit and vegetable intakes. J. Nutr. 2018;133:740–743. doi: 10.1093/jn/133.3.740. [DOI] [PubMed] [Google Scholar]