Abstract
Gastroesophageal reflux disease (GERD) is quite prevalent worldwide, especially in the western hemisphere. The pathophysiology of GERD is complex, involving an incompetent esophagogastric junction (EGJ) as an anti-reflux barrier, as well as other co-morbid conditions such as gastroparesis, hiatal herniation or hyper acid secretion. Esophageal dysmotility is also frequently encountered in GERD, further contributing to the disease in the form of fragmented peristalsis, ineffective esophageal motility (IEM) or the more severe aperistalsis. The latter is quite common in systemic connective tissue disorders such as scleroderma. The main stay treatment of GERD is pharmacologic with proton pump inhibitors (PPI), with surgical fundoplication offered to patients who are not responsive to medications or would like to discontinue them for medical or other reasons. The presence of esophageal dysmotility that can worsen or create dysphagia can potentially influence the choice of fundoplication (partial or complete), or whether it is even possible. Most of the existing literature demonstrates that fundoplication may be safe in the setting of ineffective or weak peristalsis, and that post-operative dysphagia cannot be reliably predicted by pre-operative manometry parameters. In cases of complete aperistalsis (scleroderma-like esophagus), partial fundoplication can be offered in select patients who exhibit prominent reflux symptoms after a comprehensive multidisciplinary evaluation. Roux-en-Y gastric bypass is an alternative to fundoplication in patients with this extreme form of esophageal dysmotility, after careful consideration of the nutritional status.
Keywords: Manometry, dysphagia, reflux, esophagus, surgery
Introduction
The incidence of gastroesophageal reflux disease (GERD) continues to rise worldwide, with an estimated prevalence of 18–28% in North America and 9–26% in Europe (1). Pharmacological therapy with proton pump inhibitors (PPI) is the main stay treatment of this common disease, and accounts for the bulk of its high cost on society (2). Medications can lose efficacy over time and are associated with side effects such as small intestinal bacterial overgrowth, osteoporosis, and concerns have been recently raised regarding increased risk of cardiac events (3). Surgical management is reserved for patients with severe or uncontrolled symptoms despite medications, and those desiring to discontinue them because of side effects or other reasons. Historically, surgery consisted of a full or partial fundoplication and more recently, magnetic sphincteric augmentation (Linx®) demonstrated comparable short-term outcomes (4). The choice of surgery depends on many factors, including patient’ symptoms, esophageal function, and comorbid conditions. In fact, GERD is frequently associated with other disorders, such as gastroparesis, eosinophilic esophagitis and esophageal dysmotility. The latter can complicate the choice of fundoplication, from the fear of creating or worsening symptoms of dysphagia.
In this report, we review the pathophysiology of GERD with a particular focus on the contributing role of esophageal dysmotility. We also discuss the different surgical options for managing GERD in patients with baseline non-achalasia esophageal dysmotility syndromes.
Pathophysiology of GERD and esophageal dysmotility
The pathophysiology of GERD is multifactorial and complex, but revolves around an incompetent esophagogastric junction (EGJ) as an anti-reflux barrier, in the form of transient lower esophageal sphincter relaxations (TLESR) and/or a hypotensive EGJ (5). Furthermore, the presence of a hiatal hernia (HH) further impairs the anti-reflux barrier, contributing to reflux development. Additional non-esophageal pathologies can also promote GERD: for instance, xerostomia and impaired saliva production reduce the neutralization of the esophageal mucosa when acid gastro-esophageal reflux occurs. Delayed gastric emptying and acid hypersecretion conditions naturally lead to higher exposure to gastric acid at the EGJ as well. Finally, perception of reflux events can be affected by esophageal sensitivity (6).
The advent of high resolution manometry (HRM) has allowed a more sophisticated analysis of esophageal motor function in comparison to conventional manometry, and a better understanding of the EGJ, which comprises both the lower esophageal sphincter (LES) and crural diaphragm (7-9). Specifically, HRM characterizes esophageal peristalsis by the distal contractile integral (DCI), which measures the vigor of smooth muscle contraction taking into consideration the length, duration and amplitude of contraction. A DCI threshold of 450 mmHg/cm/s correlates with an average distal peristaltic amplitude of 30 mmHg, the original threshold defining ineffective swallows on conventional manometry (10).
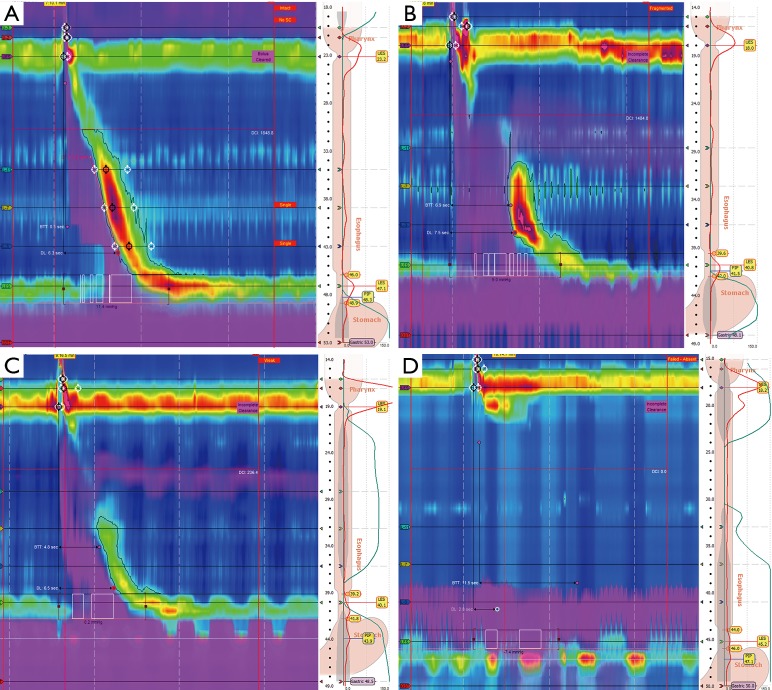
While intact peristalsis is the most common finding in patient with GERD, three main esophageal dysmotility patterns have been recently recognized (6). The most frequent abnormal pattern is a weak or absent second segment, which manifests as either fragmented peristalsis (>5 cm break with DCI >450 mmHg/cm/s in ≥50% sequences) or ineffective esophageal motility (IEM) where the DCI <450 mmHg/cm/s in ≥50% sequences. The most severe abnormality is absent contractility, characterized by a DCI <100 mmHg/cm/s in all sequences (Figure 1). This extreme form of aperistalsis, often described as a scleroderma-like esophagus, was found in 3.2% in a review of 1,081 patients who underwent HRM prior to anti-reflux surgery (11).
Figure 1.
High resolution manometry. (A) Normal esophageal motility; (B) fragmented peristalsis; (C) ineffective esophageal motility (IEM); (D) failed peristalsis.
Unsurprisingly, these hypomotility syndromes lead to ineffective clearance of the refluxate and prolong esophageal body acid exposure. In fact, Diener and colleagues demonstrated more reflux episodes, longer acid exposure and slower esophageal acid clearance in GERD patients with IEM compared to those with normal esophageal motility (12). In a review of 827 patients, Meneghetti et al. further showed that worsening of esophageal mucosal injury (in a spectrum from no esophagitis to Barrett’s esophagus) correlated with progressive deterioration of esophageal motor function with impairment of acid clearance and increased esophageal acid exposure (13). Whether esophageal dysmotility is a cause of magnification of the effect or reflux or a consequence of reflux itself remains unclear. It is believed that esophageal mucosal damage can lead to reduced esophageal compliance and an increased bolus progression resistance (14,15).
Systemic sclerosis and GERD
Systemic sclerosis is a rare multisystemic autoimmune connective tissue disorder characterized by fibrosis of the small arteries and excess deposition of collagen. The disease most commonly involves the skin, lungs, and gastrointestinal tract, particularly the esophagus, which is affected in up to 80% of patients (16). From a motor function standpoint, scleroderma esophagus is characterized by a combination of absent esophageal body contractility and a hypotensive LES, both of which are found in more than 50–60% of patients with the systemic disease (17). Scleroderma is also associated with gastric dysmotility and impaired saliva production, which further compromised bolus transit and reflux clearance contributing to GERD. As such, as many as 80% of patients develop heartburn and dysphagia within 2 years of their diagnosis (18). Complications of GERD are also common in scleroderma, including erosive esophagitis (up to 65%), peptic strictures (up to 30%), Barrett’s esophagus (up to 37%) (16). In this context, it is important to note that obstructive symptoms of dysphagia may not just be due to esophageal dysmotility, but also to peptic stricture or candida esophagitis (18). This notion is important in interpreting the incidence of dysphagia before and after fundoplication in the literature, not just in patients with scleroderma, but any patient with dysmotility syndrome undergoing anti-reflux surgery. In fact, a recent review of 269 patients without prior surgery did not show a good correlation between esophageal symptoms and HRM metrics (19).
Effects of fundoplication on esophageal motility
In order to better review the literature on the safety of fundoplication in the context of esophageal dysmotility syndromes, it is worthwhile to first examine the effects of anti-reflux surgery on esophageal motility. It is also important to distinguish between ineffective esophageal motility and complete aperistalsis when interpreting the literature, and take into consideration the nuances between conventional manometry and HRM. As discussed earlier, GERD is pathophysiologically related to inappropriate and unprovoked transient LES relaxations. Surgical fundoplication (± HH repair) is believed to change the mechanical properties and action of the EGJ that result in incomplete abolition of the high-pressure zone during LES relaxation and reduced triggering of transient sphincter relaxations (20,21). As we review here, the effect of fundoplication on the actual esophageal motility varies throughout the literature.
For instance, an early report by Stein and colleagues showed normalization of the LES pressure, increased contraction amplitude and reduced prevalence of low-amplitude contractions in 40 patients who underwent stationary manometry before and at a median of 30 months after Nissen (360 degrees) fundoplication (22). Despite adequate and durable reflux symptom control after either a Nissen or a 180-degree posterior fundoplication, Rydberg et al. did not report any significant change in esophageal motor function on repeat manometry 3 years after surgery (23). Similarly, Fibbe and colleagues randomized 200 patients into two groups: 100 patients with normal esophageal motility, and another 100 patients with motility disorders. In each group, patients were randomized to undergo either Nissen or Toupet (270 degrees) fundoplication (n=50 in each of the 4 arms). Esophageal motility disorders were defined as mean contraction amplitude less than 40 mmHg and/or failed primary peristalsis of 10 wet swallows in more than 40% on conventional manometry. At 4-month follow-up, repeat manometry showed no significant difference in esophageal motor function in 85% of patients. The only noticeable finding was an increase in the LES resting pressures post-operatively, and that was more significant in the Nissen group compared to the Toupet, even more so in patients with pre-operative esophageal dysmotility (24). In a series of 300 patients who underwent either a Nissen or a Toupet, Hunter and colleagues reported improvement in esophageal peristalsis in 47% of patients with baseline impaired motility at 1 year, with the esophageal body pressure increasing in 75% of those with low preoperative contraction amplitude (<60 mmHg) (25). On the other hand, worsening esophageal body pressure was seen in 10% of patients.
While all these studies are based on conventional manometry, Rerych et al. recently reported on 25 patients with GERD who underwent HRM before and 3–5 months after laparoscopic Nissen fundoplication (26). The authors found a significant increase in the mean and minimal basal EGJ pressure in the post-operative patients. Moreover, DCI was significantly higher post-operatively, and based on the DCI threshold of 450 mmHg/sec/cm a trend from ineffective to effective esophageal motility was also observed (P=0.07).
Clinical outcomes of fundoplication in patients with esophageal dysmotility
The early literature describing the outcomes of anti-reflux surgery in patients with esophageal dysmotility was mainly on scleroderma patients with complete aperistalsis, and reported mixed results (27-31). For instance, Mansour and Malone described their 12 years’ experience on 11 patients using a variety of anti-reflux procedures including Belsey-Mark IV, Collis-Nissen and Collis-Belsey. Despite early encouraging results in terms of reflux control, all patients demonstrated recurrence of esophagitis on endoscopic studies at a mean follow-up of 7.4 years (29). Orringer and co-workers initially reported reasonable control of GERD in scleroderma patients with a Collis-Belsey fundoplication (30). However, late recurrence of reflux symptoms occurred in 41% of these patients over 42 months, prompting the authors to favor a Collis-Nissen repair, which demonstrated a 25% recurrence at 22 months postoperatively (31). In a different study, a Hill repair was performed on 29 patients with symptomatic GERD, 73% of which had pre-operative non-stricture related dysphagia. The authors reported resolution of the dysphagia after surgery, despite persistence of the esophageal dysmotility on radionuclide esophageal transit studies that were performed on 20/29 patients post-operatively (32).
Fundoplication in patients with weak/ineffective esophageal peristalsis
With the advent of minimally invasive approaches and the refinement of esophageal functional studies, multiple publications attempted to elucidate the role of a tailored fundoplication in the management of esophageal reflux. In general, weak peristalsis was defined on conventional manometry as a distal esophageal contraction amplitude <30 or <40 mmHg. Reviewing their experience at UCSF, Patti et al. reported that 19% of patients with esophageal dysmotility (n=141) who underwent a partial (240 degrees) fundoplication had objective evidence of symptomatic reflux, compared to 4% symptomatic failure rate in the group of patients (n=94) who underwent a laparoscopic Nissen fundoplication; the incidence of post-operative dysphagia was similar between the 2 groups and the average duration of follow-up was 67 months; the author (33). Similarly, a multicenter retrospective review of 48 patients with severe esophageal dysmotility (contraction amplitude <30 mmHg and/or >70% non-peristaltic esophageal body contractions) appear to demonstrate the safety of laparoscopic Nissen fundoplication. Although early dysphagia occurred in 35 patients (73%), persistent dysphagia was found in two patients only (4.2%), including one patient with severe preoperative dysphagia which improved postoperatively; only one patient required a re-operative fundoplication (34). These results were comparable to another series by Tsereteli and co-workers, who found that the patients who experienced dysphagia post-Nissen were the same who had pre-operative dysphagia, whether they were in the IEM (n=21) or normal motility group (n=63). Of note, the mean follow-up time in this study was 6 months only (range, 1–60 months), and patients did not undergo any post-operative manometry testing (35).
Two randomized trials were also conducted to investigate the need for tailoring fundoplication. Booth et al. stratified 127 patients with established GERD into effective (n=75) and ineffective (n=52) esophageal motility groups, based on preoperative manometry. Patients in each group were randomized to either Nissen (n=64) or Toupet (n=63) fundoplication. Dysphagia of any degree (27% vs. 9%; P=0.018) and chest pain while eating (22% vs. 5%; P=0.018) were more prevalent at 1 year in the Nissen group, but there were no differences in postoperative symptoms between the effective and IEM groups. On post-operative manometry at 6 months (75 out of the 127 patients), there was also no clear pattern of transition from normal preoperative motility to IEM, or the other way around (36). Strate and colleagues also randomized 200 patients in a similar fashion based on conventional manometry.
Although dysphagia was seen more often in the total group of Nissen patients than in the total group of Toupet patients, there was no difference between the effective and IEM groups; furthermore, satisfaction with surgery was comparable between the latter 2 groups (83% vs. 87%, respectively). On manometry at 2 years, both Nissen and Toupet fundoplications significantly increased the postoperative LES intra-abdominal length, but a significantly increased LES pressure was only seen after Nissen (37).
These studies suggest that tailoring anti-reflux surgery in patients with ineffective or weak esophageal peristalsis may not be necessary, as motility disorders do not seem to correlate with postoperative dysphagia. Pre-operative dysphagia seems to be a better predictor of postoperative dysphagia than the motor pattern, and both partial and total fundoplication can result in similar symptomatic reflux improvement in that setting. As discussed earlier, the clear lack of correlation between dysphagia and esophageal functional studies has also been found in the more contemporary HRM, underlying the complexity of esophageal perception and symptom generation that may potentially involve factors other than circular muscle contraction (19,38).
Fundoplication in patients with esophageal aperistalsis
The literature describing the outcomes of fundoplication in patients with complete esophageal aperistalsis is more limited, and quite often patients are mixed along those with any degree of esophageal dysmotility. In fact, some authors consider the absence of esophageal contractility as a contra-indication to fundoplication from the fear of creating pseudo-achalasia (39). This being said, Armijo and colleagues recently reported a series of 51 patients with esophageal dysmotility on either conventional manometry or HRM: 9 patients had esophageal body motility and 42 severe hypomotility. These patients underwent a Toupet fundoplication with a hiatal hernia repair (31 patients had a HH >5 cm). At a mean follow-up of 25 months (1–7 years), the authors reported significant improvement in GI symptoms, including heartburn, regurgitation and use of PPI. Despite persistence of dysmotility on upper gastro-intestinal studies that were performed at 12 months, the long-term incidence of dysphagia was 26.7% compared to 58.8% pre-operatively (40). Watson and colleagues reported on 26 patients with an aperistaltic esophagus who underwent a laparoscopic fundoplication (4 Nissen, 22 Dor). Using a standardized symptom assessment questionnaire, good long-term outcomes were observed at 5–12 years’ follow-up (overall improvement of 93%), with 87% of patients eating a normal diet at 2 years. Only two patients underwent re-operative surgery (41).
We also recently published our own experience on 34 patients with GERD and esophageal hypomotility on HRM, 10 of which had systemic scleroderma (13 patients had scleroderma-like esophagus, 21 had ineffective peristalsis). Minimally invasive fundoplications included Toupet (30), Dor (2), and Nissen (2). Only one patient required surgical revision at 4 months postoperatively, while 41% of patients were asymptomatic and 56% had reduced symptoms at a mean follow-up of 36 weeks. Persistent dysphagia was noted in four patients (11.7%) and was successfully treated with endoscopic dilation (42). We are currently reviewing our more extensive experience in patients with reflux disease in the setting of esophageal dysmotility, including those with interstitial lung disease; we particularly plan on examining the long-term outcomes of fundoplication and confirm the safety of partial fundoplication in scleroderma-like esophagus. In our practice, patients with esophageal motility disorders and reflux disease are discussed in a multidisciplinary conference after comprehensive testing including HRM. In general, we favor a partial posterior (270 degrees) fundoplication (Toupet) in patients with ineffective motility or fragmented peristalsis. Patients with complete aperistalsis (scleroderma-like esophagus) and prominent reflux symptoms can selectively be offered a tailored partial posterior wrap (180–270 degrees) after thorough counseling.
Surgical alternatives to fundoplication in patients with GERD and severe esophageal dysmotility or aperistalsis
Surgical fundoplication is not the only surgical procedure described for the management of GERD in the setting of severe esophageal dysmotility. In fact, Roux-en-Y gastric bypass (RYGBP) is known to reduce acid reflux in the morbidly obese population, partially by achieving the greatest excess weight loss among all bariatric surgeries (43,44). Furthermore, the small gastric pouch has minimal acid content as the acid-producing mucosa of the fundus is excluded, and the roux limb prevents biliary reflux into the pouch and the esophagus.
For these reasons, RYGBP has been proposed as a viable surgical alternative for GERD patients with complete aperistalsis. For instance, Yan and colleagues recently reviewed a series of 14 patients with systemic sclerosis (scleroderma) and GERD who underwent 7 RYGBP and 7 fundoplications: 2 Nissen, 4 Toupet (including 2 concomitant Collis gastroplasties) and 1 Dor (45). Impaired esophageal motility on HRM was present in all RYGBP patients and 5 out of the 7 fundoplication patients. Eleven patients (five in RYGBP group and six in fundoplication group) had their GERD symptoms assessed during follow-up. All five RYGBP patients had symptom resolution or improvement, while only 50% (n=3) of patients reported partial improvement in the fundoplication group. An issue that has to be discussed herein is the nutritional impact and weight loss associated with RYGBP in non-obese patient with significant upper GI symptoms and generally limited oral intake to begin with. The mean body-mass index (BMI) of the RYGBP and fundoplication groups in this study went from 28 and 24 kg/m2 pre-operatively, to 24 and 25 respectively. It is also important to note that 4 RYGBP patients (57%) had concurrent feeding tube placement at the time of surgery, one of which required long-term tube feeding due to persistent dysphagia. Kent et al. also had reported their experience with GERD in scleroderma esophagus based on conventional manometry (46). Long-term follow up was available for 7 out of 8 patients who underwent RYGBP and 7 out of 10 patients who underwent a fundoplication (1 Toupet, 1 Collis Toupet, 5 Nissen, 3 Collis Nissen). Despite the small number of patients, the authors reported a statistically lower incidence of post-operative dysphagia, which was otherwise less severe in the RYGBP group. The GERD-HRQOL score was also significantly lower in that group, with a mean score of 4, compared to 15.6 in the fundoplication group. A score above 15 is generally accepted to indicate significant reflux. Comparable to the series by Yan et al., the authors performed a short Roux limb (<100 cm) to limit the malabsorption and weight loss except in 3 patients who a had a BMI >35 (mean pre-operative BMI was 32.3). The authors reported placement of a gastrostomy tube in the gastric remnant in all patients who underwent RYGBP however. Despite these encouraging results, RYGBP should be pursued with caution in patients with scleroderma, because of the associated small intestinal dysmotility and the possibility of bacterial overgrowth (16).
For complete thoroughness, other surgical alternatives are worthwhile mentioning. Biliary/duodenal diversion was described for complex/scleroderma esophagitis but is associated with high morbidity (47,48). Esophagectomy with either gastric or colon interposition has also been reported with variable success, especially in patients with prior surgical intervention and recurrent symptoms, albeit with significant morbidity too (45,49). In the series by Kent et al., 1 out of the 4 patients who underwent esophagectomy died and the other three had major complications (46).
Finally, magnetic sphincteric augmentation is generally contra-indicated in patients with GERD and known esophageal dysmotility (i.e., manometry showing effective swallows <70–80% and/or distal esophageal amplitude of <35 mmHg) (4).
Conclusions
Esophageal dysmotility is common in the setting of GERD, covering a spectrum of ineffective or fragmented peristalsis to the more extreme complete absence of contractility. The body of the literature points to the safety of fundoplication in cases of moderate hypomotility, and that more weight should be placed on pre-operative symptoms than just on manometry parameters. Both Toupet and Nissen fundoplications seem to achieve good clinical outcomes and reflux control without significant worsening of the obstructive symptoms. There is paucity of evidence regarding “tailoring of the wrap”, except in cases of aperistalsis. RYGBP can be cautiously considered in cases of scleroderma-like esophagus as an alternative to fundoplication, taking into account the nutritional status and pre-operative BMI.
Acknowledgments
Herit Vachhani, MD (gastroenterology fellow, Temple University Hospital).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.El-Serag HB, Sweet S, Winchester CC, et al. Update on the epidemiology of gastroesophageal reflux disease: a systematic review. Gut 2014;63:871-80. 10.1136/gutjnl-2012-304269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shaheen NJ, Hansen RA, Morgan DR, et al. The burden of gastrointestinal and liver diseases, 2006. Am J Gastroenterol 2006;101:2128-38. 10.1111/j.1572-0241.2006.00723.x [DOI] [PubMed] [Google Scholar]
- 3.Schnoll-Sussman F, Katz PO. Clinical implications of emerging data on the safety of proton pump inhibitors. Curr Treat Options Gastroenterol 2017;15:1-9. 10.1007/s11938-017-0115-5 [DOI] [PubMed] [Google Scholar]
- 4.Skubleny D, Switzer NJ, Dang J, et al. LINX® magnetic esophageal sphincter augmentation versus Nissen fundoplication for gastroesophageal reflux disease: a systematic review and meta-analysis. Surg Endosc 2017;31:3078-84. 10.1007/s00464-016-5370-3 [DOI] [PubMed] [Google Scholar]
- 5.Dodds WJ, Dent J, Hogan WJ, et al. Mechanisms of gastroesophageal reflux in patients with reflux esophagitis. N Engl J Med 1982;307:1547-52. 10.1056/NEJM198212163072503 [DOI] [PubMed] [Google Scholar]
- 6.Gyawali CP, Roman S, Bredenoord AJ, et al. Classification of esophageal motor findings in gastro-esophageal reflux disease: Conclusions from an international consensus group. Neurogastroenterol Motil 2017;29. doi: . Epub 2017 May 24. 10.1111/nmo.13104 [DOI] [PubMed] [Google Scholar]
- 7.Pandolfino JE, Kim H, Ghosh SK, et al. High-resolution manometry of the EGJ: an analysis of crural diaphragm function in GERD. Am J Gastroenterol 2007;102:1056-63. 10.1111/j.1572-0241.2007.01138.x [DOI] [PubMed] [Google Scholar]
- 8.Kahrilas PJ, Bredenoord AJ, Fox M, et al. International High Resolution Manometry Working Group The Chicago Classification of esophageal motility disorders, v3.0. Neurogastroenterol Motil 2015;27:160-74. 10.1111/nmo.12477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gyawali CP, Kahrilas PJ, Savarino E, et al. Modern diagnosis of GERD: the Lyon Consensus. Gut 2018;67:1351-62. 10.1136/gutjnl-2017-314722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xiao Y, Kahrilas PJ, Kwasny MJ, et al. High-resolution manometry correlates of ineffective esophageal motility. Am J Gastroenterol 2012;107:1647-54. 10.1038/ajg.2012.286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chan WW, Haroian LR, Gyawali CP. Value of preoperative esophageal function studies before laparoscopic antireflux surgery. Surg Endosc 2011;25:2943-9. 10.1007/s00464-011-1646-9 [DOI] [PubMed] [Google Scholar]
- 12.Diener U, Patti MG, Molena D, et al. Esophageal dysmotility and gastroesophageal reflux disease. J Gastrointest Surg 2001;5:260-5. 10.1016/S1091-255X(01)80046-9 [DOI] [PubMed] [Google Scholar]
- 13.Meneghetti AT, Tedesco P, Damani T, et al. Esophageal mucosal damage may promote dysmotility and worsen esophageal acid exposure. J Gastrointest Surg 2005;9:1313-7. 10.1016/j.gassur.2005.08.033 [DOI] [PubMed] [Google Scholar]
- 14.Bremner RM, DeMeester TR, Crookes PF, et al. The effect of symptoms and nonspecific motility abnormalities on outcomes of surgical therapy for gastroesophageal reflux disease. J Thorac Cardiovasc Surg 1994;107:1244-9. [PubMed] [Google Scholar]
- 15.Jiang LQ, Ye BX, Wang MF, et al. Acid exposure in patients with gastroesophageal reflux disease is associated with esophageal dysmotility. J Dig Dis 2019;20:73-7. 10.1111/1751-2980.12703 [DOI] [PubMed] [Google Scholar]
- 16.Denaxas K, Ladas SD, Karamanolis GP. Evaluation and management of esophageal manifestations in systemic sclerosis. Ann Gastroenterol 2018;31:165-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roman S, Hot A, Fabien N, et al. ; Réseau Sclérodermie des Hospices Civils de Lyon. Esophageal dysmotility associated with systemic sclerosis: a high-resolution manometry study. Dis Esophagus 2011;24:299-304. 10.1111/j.1442-2050.2010.01150.x [DOI] [PubMed] [Google Scholar]
- 18.Abu-Shakra M, Guillemin F, Lee P. Gastrointestinal manifestations of systemic sclerosis. Semin Arthritis Rheum 1994;24:29. 10.1016/0049-0172(94)90097-3 [DOI] [PubMed] [Google Scholar]
- 19.Xiao Y, Kahrilas PJ, Nicodème F, et al. Lack of correlation between HRM metrics and symptoms during the manometric protocol. Am J Gastroenterol 2014;109:521-6. 10.1038/ajg.2014.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ireland AC, Holloway RH, Toouli J, et al. Mechanisms underlying the antireflux action of fundoplication. Gut 1993;34:303-8. 10.1136/gut.34.3.303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lundell L, Abrahamsson H, Ruth M, et al. Lower esophageal sphincter characteristics and esophageal acid exposure following partial or 360 degrees fundoplication: results of a prospective, randomized, clinical study. World J Surg 1991;15:115-20. 10.1007/BF01658980 [DOI] [PubMed] [Google Scholar]
- 22.Stein HJ, Bremner RM, Jamieson J, et al. Effect of Nissen fundoplication on esophageal motor function. Arch Surg 1992;127:788-91. 10.1001/archsurg.1992.01420070040010 [DOI] [PubMed] [Google Scholar]
- 23.Rydberg L, Ruth M, Lundell L. Does oesophageal motor function improve with time after successful antireflux surgery? Results of a prospective, randomised clinical study. Gut 1997;41:82-6. 10.1136/gut.41.1.82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fibbe C, Layer P, Keller J, et al. Esophageal motility in reflux disease before and after fundoplication: a prospective, randomized, clinical, and manometric study. Gastroenterology 2001;121:5-14. 10.1053/gast.2001.25486 [DOI] [PubMed] [Google Scholar]
- 25.Hunter JG, Trus TL, Branum GD, et al. A physiologic approach to laparoscopic fundoplication for gastroesophageal reflux disease. Ann Surg 1996;223:673-85. 10.1097/00000658-199606000-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rerych K, Kurek J, Klimacka-Nawrot E, et al. High-resolution manometry in patients with gastroesophageal reflux disease before and after fundoplication. J Neurogastroenterol Motil 2017;23:55-63. 10.5056/jnm16062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Henderson RD, Pearson F. Surgical management of esophageal scleroderma. J Thorac Cardiovasc Surg 1973;66:686. [PubMed] [Google Scholar]
- 28.Poirier NC, Taillefer R, Topart P, et al. Antireflux operations in patients with scleroderma. Ann Thorac Surg 1994;58:66. 10.1016/0003-4975(94)91073-1 [DOI] [PubMed] [Google Scholar]
- 29.Mansour KA, Malone C. Surgery for scleroderma of the esophagus: a 12-year experience. Ann Thorac Surg 1988;46:513-4. 10.1016/S0003-4975(10)64687-5 [DOI] [PubMed] [Google Scholar]
- 30.Orringer MB, Dabich L, Zarafonetis CJD, et al. Gastroesophageal reflux in esophageal scleroderma: diagnosis and implications. Ann Thorac Surg 1976;22:120-30. 10.1016/S0003-4975(10)63972-0 [DOI] [PubMed] [Google Scholar]
- 31.Orringer MB, Orringer JS, Dabich L, et al. Combined Collis gastroplasty-fundoplication operations for scleroderma reflux esophagitis. Surgery 1981;90:624-30. [PubMed] [Google Scholar]
- 32.Russell CO, Pope CE, Gannan RM, et al. Does surgery correct esophageal motor dysfunction in gastro- esophageal reflux. Ann Surg 1981;194:290-6. 10.1097/00000658-198109000-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Patti MG, Robinson T, Galvani C, et al. Total fundoplication is superior to partial fundoplication even when esophageal peristalsis is weak. J Am Coll Surg 2004;198:863-9; discussion 869-70. 10.1016/j.jamcollsurg.2004.01.029 [DOI] [PubMed] [Google Scholar]
- 34.Novitsky YW, Wong J, Kercher KW, et al. Severely disordered esophageal peristalsis is not a contraindication to laparoscopic Nissen fundoplication. Surg Endosc 2007;21:950-4. 10.1007/s00464-006-9126-3 [DOI] [PubMed] [Google Scholar]
- 35.Tsereteli Z, Sporn E, Astudillo JA, et al. Laparoscopic Nissen fundoplication is a good option in patients with abnormal esophageal motility. Surg Endosc 2009;23:2292-5. 10.1007/s00464-008-0314-1 [DOI] [PubMed] [Google Scholar]
- 36.Booth MI, Stratford J, Jones L, et al. Randomized clinical trial of laparoscopic total (Nissen) versus posterior partial (Toupet) fundoplication for gastro-oesophageal reflux disease based on preoperative oesophageal manometry. Br J Surg 2008;95:57-63. 10.1002/bjs.6047 [DOI] [PubMed] [Google Scholar]
- 37.Strate U, Emmermann A, Fibbe C, et al. Laparoscopic fundoplication: Nissen versus Toupet two-year outcome of a prospective randomized study of 200 patients regarding preoperative esophageal motil- ity. Surg Endosc 2008;22:21-30. 10.1007/s00464-007-9546-8 [DOI] [PubMed] [Google Scholar]
- 38.Kapadia S, Osler T, Lee A, Borrazzo E. The role of preoperative high resolution manometry in predicting dysphagia after laparoscopic Nissen fundoplication. Surg Endosc 2018;32:2365-72. 10.1007/s00464-017-5932-z [DOI] [PubMed] [Google Scholar]
- 39.Yadlapati R, Hungness ES, Pandolfino JE. Complications of antireflux surgery. Am J Gastroenterol 2018;113:1137-47. 10.1038/s41395-018-0115-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Armijo PR, Hennings D, Leon M, et al. Surgical Management of Gastroesophageal reflux disease in patients with severe esophageal dysmotility. J Gastrointest Surg 2019;23:36-42. 10.1007/s11605-018-3968-6 [DOI] [PubMed] [Google Scholar]
- 41.Watson DI, Jamieson GG, Bessell JR, et al. Laparoscopic fundoplication in patients with an aperistaltic esophagus and gastroesophageal reflux. Dis Esophagus 2006;19:94-8. 10.1111/j.1442-2050.2006.00547.x [DOI] [PubMed] [Google Scholar]
- 42.Goldberg MB, Abbas AE, Smith MS, et al. Minimally invasive fundoplication is safe and effective in patients with severe esophageal hypomotility. Innovations (Phila) 2016;11:396-9. 10.1097/imi.0000000000000318 [DOI] [PubMed] [Google Scholar]
- 43.Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA 2004;292:1724-37. 10.1001/jama.292.14.1724 [DOI] [PubMed] [Google Scholar]
- 44.Madalosso CA., Gurski S, Callegari-Jacques RR, et al. The impact of gastric bypass on gastroesophageal reflux disease in patients with morbid obesity. Ann Surg 2010; 251:244-8. 10.1097/SLA.0b013e3181bdff20 [DOI] [PubMed] [Google Scholar]
- 45.Yan J, Strong AT, Sharma G, et al. Surgical management of gastroesophageal reflux disease in patients with systemic sclerosis. Surg Endosc 2018;32:3855-60. 10.1007/s00464-018-6115-2 [DOI] [PubMed] [Google Scholar]
- 46.Kent MS, Luketich JD, Irshad K, et al. Comparison of surgical approaches to recalcitrant gastroesophageal reflux disease in the patient with scleroderma. Ann Thorac Surg 2007;84:1710-5; discussion 1715-6. [DOI] [PubMed]
- 47.Peix JL, Maroun J, Tekinel O, et al. Treatment of sclerodermic esophagitis: value of duodenal diversion. Ann Chir 1993;47:302-6. [PubMed] [Google Scholar]
- 48.Fékété F, Kabbej M, Sauvenet A. Total duodenal diversion in the treatment of complex peptic esophagitis. Chirurgie 1996;121:326. [PubMed] [Google Scholar]
- 49.Orringer MB, Orringer J. Esophagectomy: definitive treatment for esophageal neuromotor dysfunction. Ann Thorac Surg 1982;34:237-48. 10.1016/S0003-4975(10)62492-7 [DOI] [PubMed] [Google Scholar]