Abstract
Malignant melanoma is an aggressive cancer with a high metastatic potential. Among the multiple sites of metastatic disease, the lung is one of the most frequently involved sites. Typically, pulmonary metastases from malignant melanoma occur as solid nodules. Rarely, pulmonary involvement in metastatic melanoma occurs as subsolid nodules.
The present article describes an unusual case of a patient with malignant melanoma that developed two synchronous pulmonary metastases with two different densities on CT images (one solid and the other subsolid) and different morphological patterns on histologic images. The radiologic-pathologic correlation of these two patterns of presentation was also reported.
Keywords: Malignant melanoma, Pulmonary metastases, Solid nodule, Subsolid nodule, Nonsolid nodule, Histology
1. Introduction
Malignant melanoma is an aggressive cancer with high metastatic potential. From the primary site of origin, malignant melanoma can exhibit early and rapid spreading through the lymphatic system and blood vessels and can metastasize to multiple organs and structures (i.e., the lymph nodes, lung, liver, brain and bone) [1].
Among the multiple sites of metastatic disease, the lung is one of the most frequently involved sites [2]. The literature reports that approximately 18% of melanoma patients develop lung metastases during follow-up [3]. Typically, pulmonary metastases from malignant melanoma occur as a single or multiple solid nodules on computed tomography (CT) images [3,4]. Rarely, pulmonary involvement in metastatic melanoma occurs as a single or multiple subsolid nodules [[3], [4], [5], [6], [7], [8]].
The present article describes an unusual case of a patient with malignant melanoma that developed two synchronous pulmonary metastases during follow-up with two different morphological patterns on CT and histologic images. On CT images, one pulmonary metastasis exhibited a typical solid nodular pattern, whereas in the other metastasis, a nonsolid nodular pattern was observed. The radiologic-pathologic correlation of these two different patterns of presentation was also reported.
2. Case report
A nonsmoker 73-year-old white European male was referred to our radiology department to undergo a chest X-ray as part of a routine follow-up for a previous diagnosis of cutaneous melanoma of the trunk. The surgical removal of the melanoma with the excision of the sentinel lymph node occurred two years earlier (pT3aN0; Breslow thickness, 2.9 mm; mitotic rate, 34 mitosis/mm2; no skin ulceration).
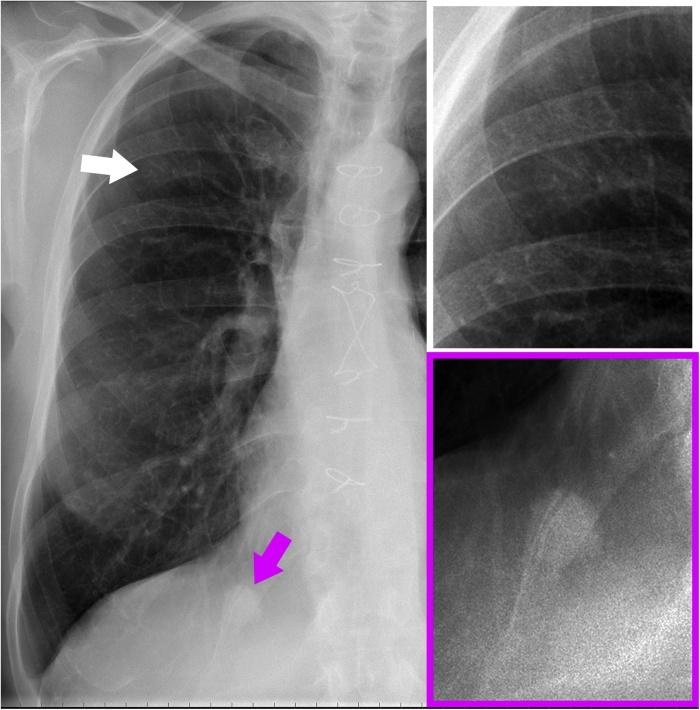
The chest radiograph revealed two pulmonary opacities located in the upper and lower lobes of the right lung (Fig. 1). Based on the medical history of the patient (previous melanoma and nonsmoker), our provisional diagnosis was pulmonary metastases. Thus, a total-body multidetector CT (MDCT) scan was performed five days later. The MDCT imaging was performed with a second generation dual-source CT scanner (Somatom Definition Flash, Siemens, Forchheim, Germany) with the following parameter: collimation, 128 x 0.6 mm; pitch value, 0.6; rotation time, 0.5 s, tube voltage, 120 kVp; tube current-time product, 240 mAs with automatic dose modulation. The MDCT images were acquired in single-source mode before and after contrast medium administration (120 ml of a 370 mg/mL iodinate contrast agent) at a flow rate of 3.5 mL/sec. The abdomen was scanned in precontrast, late arterial and portal venous phases using the bolus-tracking technique. The rest of the body was scanned only in the venous phase.
Fig. 1.
Posteroanterior chest X-ray (left) and magnifications of the upper (white box) and lower (purple box) lobes of the right lung show the two pulmonary opacities (white and purple arrows). Note that the opacity of the upper lobe (white arrow and white box) is fainter than that of the lower lobe (purple arrow and purple box) (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article).
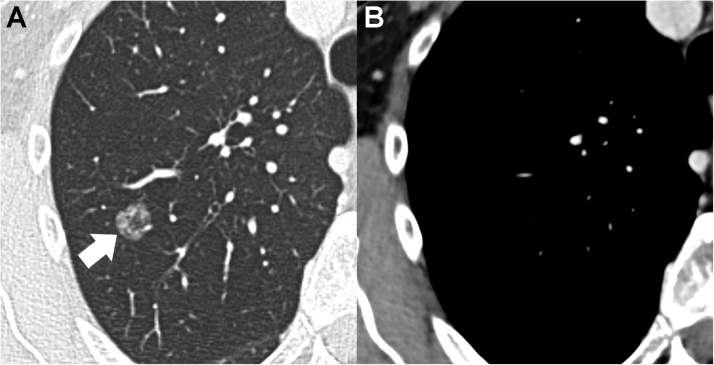
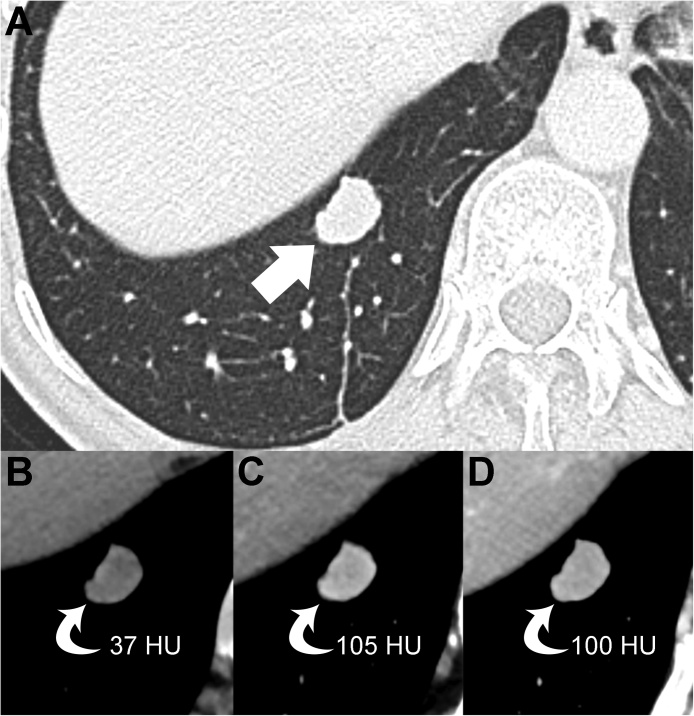
The MDCT scan confirmed that there were two pulmonary nodules in the right lung. The nodule in the right upper lobe had a subsolid consistency (nonsolid nodule) and a maximum diameter of 15 mm (Fig. 2). The nodule located in the right lower lobe had a solid consistency (solid nodule), a well-defined margin and a maximum diameter of 19 mm (Fig. 3). The solid nodule also exhibited a significant contrast-enhancement (Fig. 3).
Fig. 2.
Axial CT image with lung window setting (A) displays the nonsolid nodule in the right upper lobe (arrow). There is no evidence of the pulmonary nodule on axial CT image with mediastinal window setting (B).
Fig. 3.
Axial CT image with lung window setting (A) displays the solid nodule in the right lower lobe (arrow). Precontrast (B), late arterial phase (C), and portal venous phase (D) images of the upper abdomen show the contrast-enhancement of the pulmonary nodule (curved arrows).
No other lesions were found in the rest of the body; in particular, nonpathological hilar and mediastinal lymph nodes were observed.
Despite the nonsolid density of the nodule in the right upper lobe, our provisional diagnosis remained pulmonary metastases.
Based on the side (right), the number of nodules (two) and the absence of other metastatic lesions, surgical resection was considered a viable diagnostic and therapeutic option. Ten days later, the patient underwent wedge resections of the pulmonary nodules with lymph node sampling.
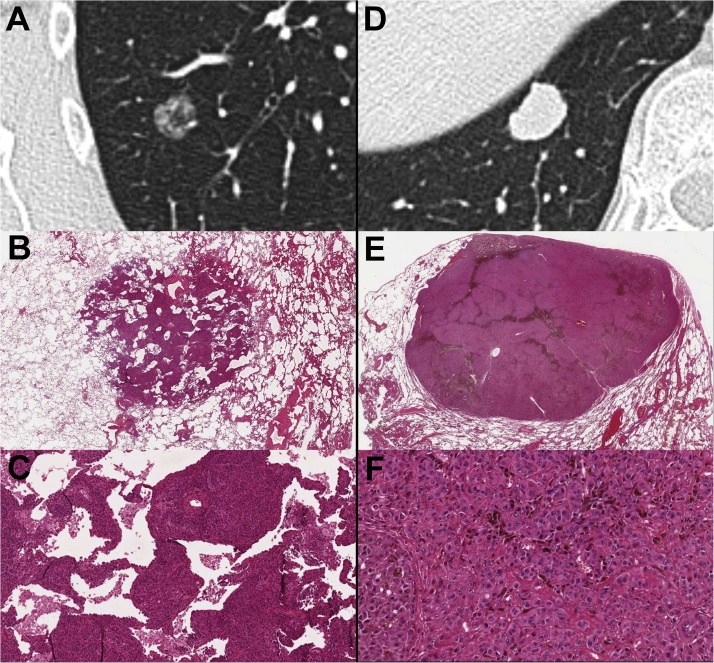
Histological examination confirmed our provisional diagnosis of pulmonary metastases from melanoma. However, these two pulmonary nodules also had a different morphological pattern of presentation on histologic images (Fig. 4).
Fig. 4.
Cropped axial CT images with the lung window setting showing the nonsolid (A) and solid (D) pulmonary nodules. (B, C) Histological hematoxylin and eosin (H-E) images of the nonsolid pulmonary nodule at the actual size (B) and at 10x (C) showing the neoplastic cell growth along the alveolar septa with the partial effacement of the alveolar architecture, which reflected the nonsolid appearance of this nodule on CT images. Note that some alveolar spaces are still recognizable. (E, F) Histological H-E images of the solid pulmonary nodule at the actual size (E) and at 20x (F) display the complete effacement of the alveolar architecture, which reflected the solid appearance of this nodule on CT images. Both pulmonary nodules were made by epithelioid malignant cells that were consistent with melanoma cells (C, F).
In the subsolid nodule, the neoplastic cells exhibited infiltrative growth that mainly occurred along the alveolar septa, with the partial sparing of the alveolar architecture (Fig. 4B and C). This histologic pattern of spread reflected the subsolid (nonsolid) appearance of the metastasis on CT images.
In the solid nodule, the neoplastic cells replaced the alveolar architecture with the complete effacement of the alveolar spaces (Fig. 4E and F). No lymph node metastases were found.
3. Discussion
The metastatic dissemination of cutaneous melanoma may be observed on the skin, in subcutaneous tissues, in locoregional lymph nodes or in distant organs or structures.
The lungs are the most common sites for distant metastases [2], and the prognosis in metastatic patients is very poor, with a 5-year survival rate of less than 10% [9].
Pulmonary metastases from malignant melanoma may exhibit different morphologic patterns [4]. The solid nodular pattern is usually found in pulmonary metastatic disease [3,4]. Conversely, the subsolid nodular pattern is rarely observed [3,4]. However, the literature reports that the subsolid appearance of pulmonary metastases is most commonly found in melanoma patients [[5], [6], [7], [8]].
The subsolid nodular pattern may be a manifestation of malignant or benign diseases [10,11]. Primary lung malignancies presenting as subsolid nodules usually represent in situ, minimally invasive or invasive adenocarcinoma with predominant lepidic growth [12]. Rarely, primitive pulmonary lymphomas may also exhibit a subsolid nodular pattern [13].
Subsolid pulmonary metastases from malignant melanoma typically exhibit a faster growth rate than primitive lung cancer [[5], [6], [7], [8]].
To the best of our knowledge, this article reports the first case of the occurrence of two synchronous pulmonary metastases with two different morphological patterns in a melanoma patient.
In a literature search of the PubMed database for publications from the past 15 years, we found only four case reports in which the occurrence of subsolid pulmonary metastases from melanoma was observed (Table 1).
Table 1.
Pulmonary metastases from melanoma exhibit a subsolid nodular pattern.
Other unusual CT patterns of pulmonary involvement from malignant melanoma include diffuse ground-glass opacities, “crazy-paving” patterns, the “halo sign”, endobronchial lesions, and cavitary nodules [4,[14], [15], [16]].
In our cases, the different morphological pattern of the two pulmonary metastases on CT images (Fig. 2, and 3) was clearly caused by the different growth patterns of the neoplastic cells that were observed on histologic images (Fig. 4). In the subsolid nodule, the neoplastic cells spread along the alveolar septa, with the partial effacement of the alveolar architecture (Fig. 4B and C). Conversely, in the solid nodule, the local spread of the neoplastic cells led to the complete effacement of the alveolar spaces (Fig. 4E, and F).
However, in both pulmonary metastases, the neoplastic cells had the same histologic characteristics (epithelioid malignant cells) (Fig. 4C and F). Therefore, it could be assumed that the two metastases were at different stages of development. In other words, the subsolid appearance of the metastasis in the upper lobe (Fig. 2), which was characterized by the partial effacement of the alveolar architecture, may reflect an early stage of cancer growth. Conversely, the solid appearance of the metastasis in the lower lobe (Fig. 3), which was characterized by the complete effacement of the alveolar architecture, may reflect a later stage of cancer growth.
4. Conclusion
The present article describes an unusual case of a patient with malignant melanoma that developed two synchronous pulmonary metastases during follow-up with two different morphological patterns on CT and histologic images. Although a subsolid nodular pattern is rare in pulmonary metastases from extrapulmonary malignancies, this CT pattern is most commonly found in melanoma patients. Therefore, lung metastases should be included in the differential diagnosis of a subsolid pulmonary nodule that is incidentally detected during the follow-up of a patient with previous melanoma. In addition, the radiologic-pathologic correlation observed in this case suggests that the subsolid nodular pattern observed in pulmonary metastases from melanoma may reflect an early stage of neoplastic growth in the lungs.
Declaration of Competing Interest
The authors declare no financial or other conflict of interest.
References
- 1.Braeuer R.R., Watson I.R., Wu C.J., Mobley A.K., Kamiya T., Shoshan E., Bar-Eli M. Why is melanoma so metastatic? Pigment Cell Melanoma Res. 2014;27:19–36. doi: 10.1111/pcmr.12172. [DOI] [PubMed] [Google Scholar]
- 2.Younes R., Abrao F.C., Gross J. Pulmonary metastasectomy for malignant melanoma: prognostic factors for long-term survival. Melanoma Res. 2013;23:307–311. doi: 10.1097/CMR.0b013e3283632cbe. [DOI] [PubMed] [Google Scholar]
- 3.Soliman M., Petrella T., Tyrrell P., Wright F., Look Hong N.J., Lu H., Zezos P., Jimenez-Juan L., Oikonomou A. The clinical significance of indeterminate pulmonary nodules in melanoma patients at baseline and during follow-up chest CT. Eur. J. Radiol. Open. 2019;6:85–90. doi: 10.1016/j.ejro.2019.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miyake M., Tateishi U., Maeda T., Kusumoto M., Satake M., Arai Y., Sugimura K. Pulmonary involvement of malignant melanoma: thin-section CT findings with pathologic correlation. Radiat. Med. 2005;23:497–503. [PubMed] [Google Scholar]
- 5.Okita R., Yamashita M., Nakata M., Teramoto N., Bessho A., Mogami H. Multiple ground-glass opacity in metastasis of malignant melanoma diagnosed by lung biopsy. Ann. Thorac. Surg. 2005;79:e1–2. doi: 10.1016/j.athoracsur.2004.03.096. [DOI] [PubMed] [Google Scholar]
- 6.Kang M.J., Kim M.A., Park C.M., Lee C.H., Goo J.M., Lee H.J. Ground-glass nodules found in two patients with malignant melanomas: different growth rate and different histology. Clin. Imaging. 2010;34:396–399. doi: 10.1016/j.clinimag.2009.10.036. [DOI] [PubMed] [Google Scholar]
- 7.Mizuuchi H., Suda K., Kitahara H., Shimamatsu S., Kohno M., Okamoto T., Maehara Y. Solitary pulmonary metastasis from malignant melanoma of the bulbar conjunctiva presenting as a pulmonary ground glass nodule: report of a case. Thorac. Cancer. 2015;6:97–100. doi: 10.1111/1759-7714.12124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dalpiaz G., Asioli S., Fanti S., Rea G., Marchiori E. Rapidly growing pulmonary ground-glass nodule caused by metastatic melanoma lacking uptake on 18 F-FDG PET-CT. J. Bras. Pneumol. 2018;44:171–172. doi: 10.1590/S1806-37562017000000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tas F. 2012. Metastatic Behavior in Melanoma : Timing, Pattern, Survival, and Influencing Factors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kobayashi Y., Mitsudomi T. Management of ground-glass opacities : should all pulmonary lesions with ground-glass opacity be surgically resected? Transl. Lung Cancer Res. 2013;2:354–363. doi: 10.3978/j.issn.2218-6751.2013.09.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Borghesi A., Michelini S., Bertagna F., Scrimieri A., Pezzotti S., Maroldi R. European Journal of Radiology Open Hilly or mountainous surface: a new CT feature to predict the behavior of pure ground glass nodules? Eur. J. Radiol. Open. 2018;5:177–182. doi: 10.1016/j.ejro.2018.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Borghesi A., Farina D., Michelini S., Ferrari M., Benetti D., Fisogni S., Tironi A., Maroldi R. Pulmonary adenocarcinomas presenting as ground-glass opacities on multidetector CT: three-dimensional computer-assisted analysis of growth pattern and doubling time. Diagn. Interv. Radiol. 2016;22:525–533. doi: 10.5152/dir.2016.16110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Albano D., Borghesi A., Bosio G., Bertoli M., Maroldi R., Giubbini R., Bertagna F. Pulmonary mucosa-associated lymphoid tissue lymphoma:18F-FDG PET/CT and CT findings in 28 patients. Br. J. Radiol. 2017;90 doi: 10.1259/bjr.20170311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dalpiaz G., Kawamukai K., Parisi A.M., La Torre L., Forcella D., Leuzzi G. Ground-glass opacity of the lung in a patient with melanoma: “The radiological seed of doubt”. Rev. Esp. Med. Nucl. Imagen Mol. 2015;34:390–392. doi: 10.1016/j.remn.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 15.Kalchiem-Dekel O., Maimon N., Shack A.R., Smoliakov A., Shaco-Levy R., Fruchter O., Fox B.D., Kramer M.R., Galante O. An unusual presentation of malignant melanoma metastatic to the lungs and bronchi: bilateral ground-glass opacities with a “Crazy Paving” component. Am. J. Respir. Crit. Care Med. 2015;191:954–955. doi: 10.1164/rccm.201411-1954IM. [DOI] [PubMed] [Google Scholar]
- 16.Alves Júnior S.F., Zanetti G., Marchiori E. CT halo sign in pulmonary metastases from a melanoma. QJM. 2019;112:133–134. doi: 10.1093/qjmed/hcy190. [DOI] [PubMed] [Google Scholar]