Abstract
This paper provided spectroscopic data that is relevant with research article entitled “Synthesis and structural characterization of 6-(N-methyl-pyridin-2-ylcarbamoyl)-pyridine-2-carboxylic acid methyl ester Isomer” (Kadir et al., 2017) [1]. From the reported study, four new ligand of monoamide isomers were successfully synthesized using acyl chloride methods. The monoamide compounds namely 6-(3-methyl-pyridin-2-ylcarbamoyl)-pyridine-2-carboxylic acid methyl ester (L1), 6-(4-methyl-pyridin-2-ylcarbamoyl)-pyridine-2-carboxylic acid methyl ester (L2), 6-(5-methyl-pyridin-2-ylcarbamoyl)-pyridine-2-carboxylic acid methyl ester (L3) and 6-(6-methyl-pyridin-2-ylcarbamoyl)-pyridine-2-carboxylic acid methyl ester (L4) were fully characterized by Fourier Transform Infrared (FTIR), 1H Nuclear Magnetic Resonance (1H NMR) and 13C Nuclear Magnetic Resonance (13C NMR), Ultraviolet Visible (UV–Vis) and elemental analyzer (CHNS).
Keywords: Monoamide, Ligand, Acyl chloride, Isomers
Specification table
| Subject area | Chemistry |
| More specific subject area | Synthetic chemistry, spectroscopy |
| Type of data | FTIR spectra, NMR spectra, UV spectra, graph, table |
| How data was acquired | CHNS Analyzer Flashea 1112 series, FTIR Perkin Elmer Spectrum 100 and the spectra was recorded in range of 4000–400 cm−1 utilizing potassium bromide (KBr) pellet, Spectrophotometer Shimadzu UV-1800, Bruker Avance II 400 spectrometer was used to record the 1H and 13C Nuclear Magnetic Resonance |
| Data format | JPEG, Tiff (Raw) |
| Experimental factors | Product was isolated using column chromatography and obtained as pale yellow precipitate. For NMR and UV Vis analysis, sample was dissolved in suitable solvent. |
| Experimental features | All chemicals used were commercially available and used as received without purification. |
| Data source location | Universiti Malaysia Terengganu |
| Data accessibility | Data is included with this article |
| Related research article | M.A. Kadir*,N. Mansor, M.U. Osman, Synthesis and Structural Characterization of 6-(N-methyl-pyridin-2-ylcarbamoyl)-pyridine-2-carboxylic acid methyl ester Isomer, Sains Malaysiana, (2017), 46(5), 725 – 731. |
Value of the data
|
1. Data
Four new compounds namely 6-(3-methyl-pyridin-2-ylcarbamoyl)-pyridine-2-carboxylic acid methyl ester (L1), 6-(4-methyl-pyridin-2-ylcarbamoyl)-pyridine-2-carboxylic acid methyl ester (L2), 6-(5-methyl-pyridin-2-ylcarbamoyl)-pyridine-2-carboxylic acid methyl ester (L3) and 6-(6-methyl-pyridin-2-ylcarbamoyl)-pyridine-2-carboxylic acid methyl ester (L4) were synthesized from reaction between 6-(methoxycarbonyl)pyridine-2-carboxylic acid and aminomethylpyridine in dichloromethane [1]. These compounds were varied by different placements of methyl substituents at ortho, meta and para. Acyl chloride method was selected to enhance the nucleophilicity of aminopyridin in the reaction [2], [3].
2. Experimental design, materials, and methods
A suspension of 6-(methoxycarbonyl)pyridine-2-carboxylic acid (0.5 g, 2.0 mol), thionyl chloride (0.5 mL) and dried DMF (1 μL) was refluxed in dichloromethane (100 mL). After an hour, the dichloromethane was removed using rotary evaporator to remove the solvent. The obtaining white solid (1.67 g, 3.5 mol) was redissolved in dichloromethane (40 mL) before added with 2-amino-3-methyl pyridine (1.567 g, 3.5 mol). The mixture was continued to reflux for another 24 h. After the reaction was completed, the solvent was removed using rotary evaporator. Then, the residue was dissolved in dichloromethane and washed with sodium hydrogen bicarbonate. The residue was dried over magnesium sulfate before being removed by rotavap. The residue was further purified by column chromatography on silica gel eluting with 8:2 ethyl acetate: dichloromethane to give product as pale yellow precipitate of 6-(3-methyl-pyridin-2-ylcarbamoyl)-pyridine-2-carboxylic acid methyl ester (L1). Compound L1 was obtained as yellow precipitate. The rest of the compounds (L2-L4) were prepared using similar methods described for L1, by replacing 2-amino-3-methyl pyridine with 2-amino-4-methyl pyridine, 2-amino-5-methyl pyridine and 2-amino-6-methyl pyridine, respectively (see Table 1, Table 2, Table 3, Table 4, Table 5).
Table 1.
The FTIR spectra data for all four monoamide ligands, L1, L2, L3 and L4.
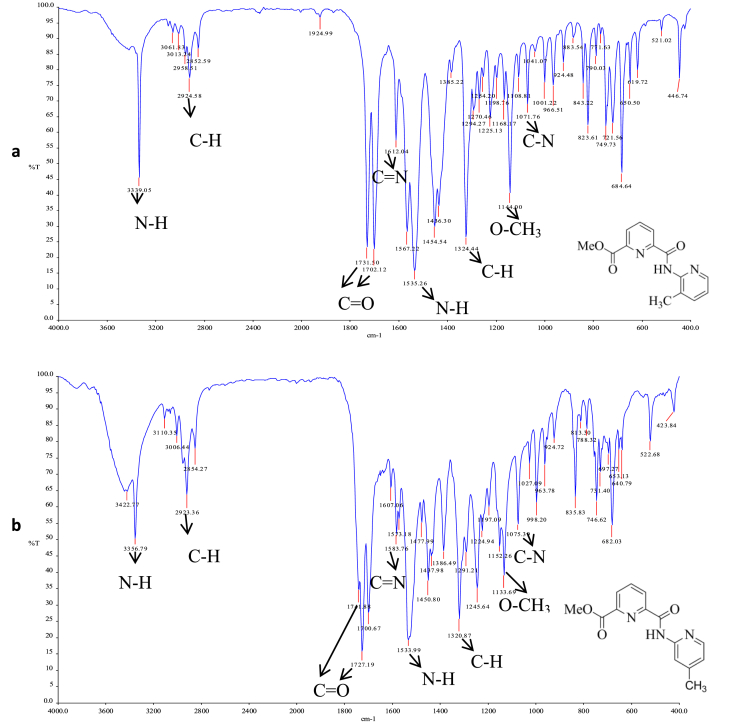
| Vibrational modes | L1 (cm−1) | L2 (cm−1) | L3 (cm−1) | L4 (cm−1) |
|---|---|---|---|---|
| ν(CH3) | 2925 | 2923 | 2962 | 2920 |
| ν(N–H str) | 3339 | 3357 | 3350 | 3358 |
| ν(C O) | 1732, 1702 | 1742, 1727 | 1731, 1702 | 1725, 1699 |
| ν(N–H bend) | 1567, 1535 | 1533 | 1533 | 1525 |
| ν(CH3 bend) | 1324 | 1321 | 1322 | 1320 |
| ν(O–CH3) str | 1144 | 1133 | 1133 | 1133 |
| ν(C–N) | 1071 | 1075 | 1076 | 1076 |
| ν(C N) | 1613 | 1583 | 1583 | 1583 |
Table 2.
1H NMR (a) L1, (b) L2, (c) L3, (d) L4.
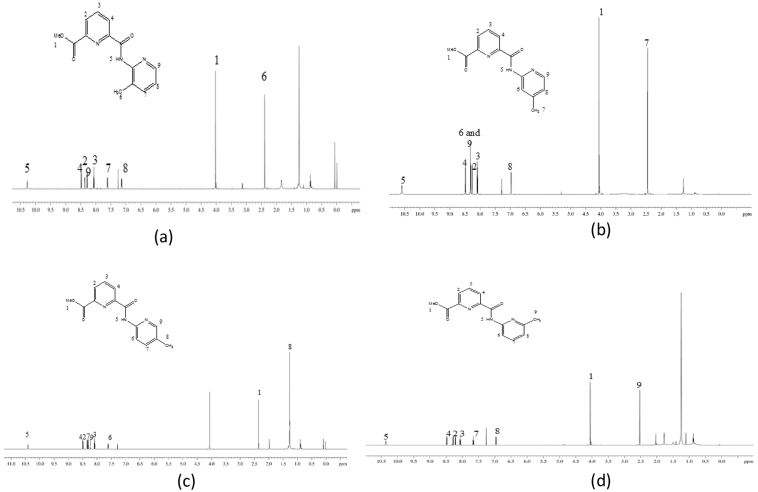
| Compound | 1H NMR (δ, ppm) | |
|---|---|---|
| L1 | 2.39 | 3H, s, δ (Py-CH3) |
| 4.02 | 3H, s, δ (O–CH3) | |
| 7.15 | 1H, d, δ (py-H) | |
| 7.61 | 1H, t, 7 Hz, δ (py-H) | |
| 8.06 | 1H, d, 7.7 Hz, δ (py-H) | |
| 8.29 | 1H, d, 7.7 Hz, δ (py-H) | |
| 8.37 | 1H, d, 4.9 Hz, δ ((py-H) | |
| 8.48 | 1H, d, 7.7 Hz, δ (py-H) | |
| 10.28 | 1H, s, δ ((N–H) | |
| L2 | 2.45 | 3H, s, δ (Py-CH3) |
| 4.06 | 3H, s, δ (O–CH3) | |
| 6.97 | 1H, d, 4.9 Hz, δ (py-H) | |
| 8.09 | 1H, t, 7.7 Hz, δ (py-H) | |
| 8.26 | 1H, d, 4.9 Hz, δ (py-H) | |
| 8.31 | 2H, d, 8.4 Hz, δ (py-H) | |
| 8.49 | 1H, d, 7.7 Hz, δ ((py-H) | |
| 10.28 | 1H, s, δ ((N–H) | |
| L3 | 2.36 | 3H, s, δ (Py-CH3) |
| 4.06 | 3H, s, δ (O–CH3) | |
| 7.61 | 1H, d, 7.7 Hz, δ (py-H) | |
| 8.08 | 1H, t, 7.7 Hz, δ (py-H) | |
| 8.23 | 1H, s, δ (py-H) | |
| 8.31 | 1H, d, 7.7 Hz, δ (py-H) | |
| 8.34 | 1H, d, 8.4 Hz, δ ((py-H) | |
| 8.49 | 1H, d, 7.7 Hz, δ (py-H) | |
| 10.41 | 1H, s, δ ((N–H) | |
| L4 | 2.53 | 3H, s, δ (Py-CH3) |
| 4.06 | 3H, s, δ (O–CH3) | |
| 6.96 | 1H, d, 7.7 Hz, δ (py-H) | |
| 7.66 | 1H, t, 7.7 Hz, δ (py-H) | |
| 8.07 | 1H, d, 7.7 Hz, δ (py-H) | |
| 8.21 | 1H, d, 8.4 Hz, δ (py-H) | |
| 8.28 | 1H, d, 7.7 Hz, δ ((py-H) | |
| 8.48 | 1H, d, 7.7 Hz, δ (py-H) | |
| 10.36 | 1H, s, δ ((N–H) |
Table 3.
13C NMR of (a) L1, (b) L2, (c) L3, (d) L4.
| Compound | 13C NMR (δ, ppm) | |
|---|---|---|
| L1 | 18.07 | (py-CH3) |
| 52.95 | (py-OCH3) | |
| 121.67 | (py-C) | |
| 125.75 | (py-C) | |
| 127.68 | (py-C) | |
| 127.70 | (py-C) | |
| 138.81 | (py-C) | |
| 139.74 | (py-C) | |
| 146.32 | (py-C) | |
| 146.60 | (py-C) | |
| 149.13 | (py-C) | |
| 149.79 | (py-C) | |
| 161.27 | (C O) | |
| 164.86 | (C O) | |
| L2 | 21.55 | (py-CH3) |
| 53.03 | (py-OCH3) | |
| 114.97 | (py-C) | |
| 121.38 | (py-C) | |
| 125.64 | (py-C) | |
| 127.85 | (py-C) | |
| 138.82 | (py-C) | |
| 146.97 | (py-C) | |
| 147.07 | (py-C) | |
| 149.49 | (py-C) | |
| 150.62 | (py-C) | |
| 150.68 | (py-C) | |
| 161.91 | (C O) | |
| 164.95 | (C O) | |
| L3 | 17.93 | (py-CH3) |
| 53.00 | (py-OCH3) | |
| 113.77 | (py-C) | |
| 125.59 | (py-C) | |
| 127.73 | (py-C) | |
| 129.59 | (py-C) | |
| 138.76 | (py-C) | |
| 138.93 | (py-C) | |
| 146.92 | (py-C) | |
| 148.13 | (py-C) | |
| 148.79 | (py-C) | |
| 149.70 | (py-C) | |
| 161.70 | (C O) | |
| 164.99 | (C O) | |
| L4 | 24.13 | (py-CH3) |
| 53.01 | (py-OCH3) | |
| 111.13 | (py-C) | |
| 119.70 | (py-C) | |
| 125.64 | (py-C) | |
| 127.74 | (py-C) | |
| 138.59 | (py-C) | |
| 138.76 | (py-C) | |
| 146.91 | (py-C) | |
| 149.76 | (py-C) | |
| 150.25 | (py-C) | |
| 157.28 | (py-C) | |
| 161.83 | (C O) | |
| 164.96 | (C O) |
Table 4.
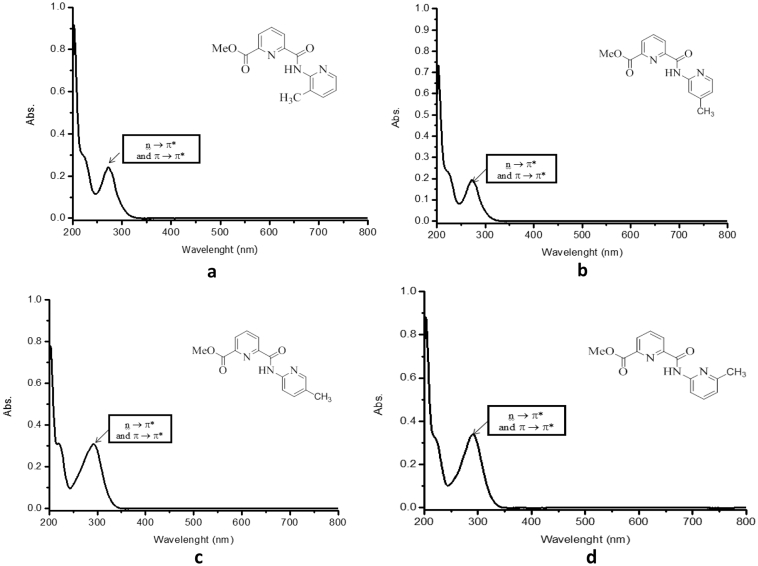
The result of UV–Vis spectroscopy for L1-L4.
| Compound | Chromophores | Transition | λmax (nm) | ε, L mol−1 cm−1 |
|---|---|---|---|---|
| L1 | Pyridine, C O | n → π*, π → π* | 273 | 2.73 × 107 |
| L2 | Pyridine, C O | n → π*, π → π* | 273 | 2.73 × 107 |
| L3 | Pyridine, C O | n → π*, π → π* | 293 | 2.93 × 107 |
| L4 | Pyridine, C O | n → π*, π → π* | 291 | 2.91 × 107 |
Table 5.
The elemental analysis data of L1-L4.
| Percentage of element | |||
|---|---|---|---|
| Compound | %C | %H | %N |
| L1 | 62.6 | 5.1 | 15.0 |
| L2 | 58.0 | 5.0 | 14.6 |
| L3 | 61.4 | 4.7 | 15.7 |
| L4 | 61.7 | 4.8 | 15.2 |
The methyl ester derivatives were characterized by using combination of spectroscopic techniques such as FTIR, 1H NMR and 13C NMR, UV–Vis. The spectroscopic data was supported [4], [5] and are depicted in Fig. 1, Fig. 2, Fig. 3 and Fig. 4, respectively.
Fig. 1.
The FTIR spectrum for (a) L1, (b) L2, (c) L3 and (d) L4.
Fig. 2.
1H NMR (a) L1, (b) L2, (c) L3, (d) L4 in DMSO-d6.
Fig. 3.
13C NMR of (a) L1, (b) L2, (c) L3, (d) L4 in DMSO-d6.
Fig. 4.
UV spectra of (a)L1, (b)L2, (c)L3, (d)L4 in methanol solution.
Acknowledgments
Authors greatly acknowledge the scientific and financial support from Universiti Malaysia Terengganu and Ministry of Higher Education Malaysia.
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Kadir M.A., Mansor N., Osman M.U. Synthesis and structural characterization of 6-(N-methyl-pyridin-2-ylcarbamoyl)-pyridine-2-carboxylic acid methyl ester isomers. Sains Malays. 2017;46:725–731. doi: 10.17576/jsm-2017-4605-07. [DOI] [Google Scholar]
- 2.Zhang L., Wang X.J., Wang J., Grinberg N., Krishnamurthy D.K., Senanayake C.H. An improved method of amide synthesis using acyl chlorides. Tetrahedron Lett. 2009;50:2964–2966. doi: 10.1016/j.tetlet.2009.03.220. [DOI] [Google Scholar]
- 3.Leggio A., Belsito E.L., Gioia L.D., Leotta V., Romio E., Siciliano C., Liguori A. Silver Acetate-assisted formation of amide from acyl chlorides. Tetrahedron Lett. 2015;56:199–202. [Google Scholar]
- 4.Ochędzan-Siodłak W., Bihun-Kisiel A., Siodłak D., Poliwoda A., Dziuk B. 2-(1,3-oxazolin-2-yl)pyridine and 2,6-bis(1,3-oxazolin-2-yl)pyridine. Data in Brief. 2018;21:449–465. doi: 10.1016/j.dib.2018.09.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Todorova S., Atanassova M., Kurteva V. Data on the synthesis and characterization of two novel polydentate ligands possessing unsymmetrical NH–urea fragment. Data in Brief, 2018;20:933–939. doi: 10.1016/j.dib.2018.08.136. [DOI] [PMC free article] [PubMed] [Google Scholar]