Abstract
BACKGROUND
Muscimol is a gamma-aminobutyric acid receptor agonist that selectively and temporarily inhibits neurons. Local bolus injection of muscimol has been used experimentally to inhibit neuronal populations within discrete anatomical structures and discern their physiological function.
OBJECTIVE
To determine the safety and behavioral effects of convection-enhanced delivery of muscimol into the bilateral subthalamic nuclei (STN) of nonhuman primate rhesus macaques (NHPs).
METHODS
Six awake NHPs underwent co-infusion of gadolinium-diethylenetriamine pentaacetic acid (Gd-DTPA), a surrogate magnetic resonance imaging (MRI) tracer, with increasing concentrations of muscimol for behavioral and histological assessment. Three other NHPs were co-infused with Gd-DTPA and 3H-muscimol into the STN to determine muscimol distribution by MRI and autoradiography. Two NHPs underwent microcatheter implantation without muscimol infusion for control comparison.
RESULTS
MRI revealed selective and complete perfusion of the bilateral STN in animals infused with Gd-DTPA and muscimol. No abnormal movements occurred at 0.125 mM. Muscimol doses between 0.25 and 4.4 mM resulted in transient, dose-dependent hyperkinesia. Muscimol (8.8 mM) resulted in severe bilateral dyskinesias, ballistic movements, and sedation. An 88.8 mM dose produced unresponsiveness in 1 animal. Infusion-related pathological abnormities or toxicity was not present on histological examination. MRI distribution of co-infused Gd-DTPA was similar to autoradiographic distribution of 3H-muscimol (Vd; R = 0.94). Mean Vd of infused animals was 37.9 mm3 ± 11.7 mm3 and mean Vd: Vi 7.6 ± 2.3.
CONCLUSION
Bilateral convection-enhanced delivery of muscimol into the primate STN resulted in dose-related hyperkinetic movements that resolved after stopping the infusion. Muscimol was not toxic to brain tissue. Gd-DTPA accurately tracked muscimol distribution.
Keywords: Convection, Muscimol, Parkinson's disease, Primates, Subthalamus
ABBREVIATIONS
- CED
convection-enhanced delivery
- DBS
deep brain stimulation
- GABA
γ-aminobutyric acid
- Gd-DTPA
gadolinium-diethylenetriamine pentaacetic acid
- GPi
globus pallidus interna
- H&E
hematoxylin & eosin
- MPTP
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- MRI
magnetic resonance imaging
- MTD
maximum tolerable dose
- NHP
nonhuman primate
- PD
Parkinson's disease
- SNc
substantia nigra pars compacta
- STN
subthalamic nuclei
Muscimol is a psychoactive compound naturally produced by the Amanita mushroom family that directly competes with γ-aminobutyric acid (GABA) binding to the GABA-A receptor.1 Muscimol is not degraded by GABA transaminases and has 10-fold greater affinity than GABA for the GABA-A receptor in vitro.2,3 Based on its properties, muscimol potently and selectively inhibits neural activity. Early studies using small intraoperative microinjections of muscimol into the deep brain nuclei (subthalamic nuclei [STN] and globus pallidus interna [GPi]) of Parkinson's disease (PD) patients before performing thermal lesioning or deep brain stimulation (DBS) demonstrated the potent transient relief of PD symptoms.4-6 While these studies showed the effect of unilateral microinjections of muscimol into the STN or GPi of PD patients, they did not assess the optimal dosing for clinical effect, the real-time distribution of muscimol (with clinical correlation), or the impact of bilateral treatment. The goals of this investigation were to (1) determine the maximum tolerated dose of muscimol delivered into the STN using convection-enhanced delivery (CED), (2) evaluate the accuracy of tracking muscimol distribution with real-time imaging of the surrogate tracer (gadolinium-diethylenetriamine pentaacetic acid; Gd-DTPA), and (3) document the behavioral effects of bilateral muscimol treatment in nonhuman primates (NHP).
METHODS
Ethical Statement
Animal use was approved by the Institutional Animal Care and Use Committee, conducted in accordance with ARRIVE guidelines,7 and complied with the Guide for the Care and Use of Laboratory Animals.8
Study Groups and Design
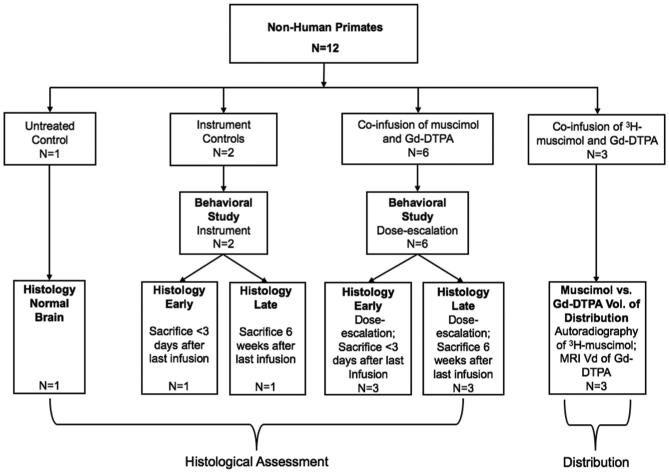
Twelve adult NHP rhesus monkeys were studied (4 male, 8 female), weighing between 4.7 and 10.6 kg (mean ± standard deviation, 6.9 ± 2.0 kg). All NHPs in this study were housed in individual cages with wood-fiber bedding, a 12-h light/dark cycle, and free access to environmental enrichment. The fewest animals (12) needed to test our hypothesis were used. Animals were monitored for signs of discomfort or distress. Eight animals were chair-trained for 3 to 4 wk before microcatheter implantation and behavioral assessment during muscimol infusion. The study design is outlined in Figure 1 and discussed below.
FIGURE 1.
Schematic illustrating the study design. Twelve nonhuman primates (NHP) were divided into a control group, a <72 h dose-escalation study group (sacrificed within 72 h after final infusion), a 6-wk dose-escalation study group (sacrificed 6 wk after final infusion), and an autoradiography study group.
Surgical Procedure
Details of the surgical procedure to implant the infusion cannula have been described.9-11 Briefly, each animal was sedated with intramuscular Ketamine (3 mg/kg) and Dexdormitor (0.1 mg/kg). Intramuscular Antisedan (0.1 mg/kg) was administered prior to placing the NHP on general anesthesia, which was maintained with isoflurane. Preoperative T1- and T2-weighted sequences were acquired with 1 mm spacing on a Philips 3-Tesla magnetic resonance imaging (MRI) scanner. The animal was positioned in a stereotactic head frame (Model 9-YSTI-35, Crist Instrument Co Inc, Hagerstown, Maryland). The stereotactic coordinates for the bilateral STN were identified from the preoperative coronal and sagittal T1 MR images. Bilateral 5 mm burr holes were placed in the calvarium over the identified underlying STN target sites. A multiported guide pedestal was affixed to the calvarium over each burr hole. MRI was used to identify the proper hole in the guide pedestal with a trajectory passing into the STN. A 1-piece fused silica stepped cannula was inserted through each guide pedestal until the cannula tips rested in the bilateral STN. The fused silica step cannula had a 28-gauge distal segment projecting 3 mm beyond its 45 mm 22-gauge proximal segment. The bilateral infusion cannulae were removed 5 min after the CED infusion was completed. All infusions were 5 μL in volume and delivered at a rate of 0.5 μL/min over 10 min.
Behavioral Assessment During Convective Co-infusion of Muscimol and Gd-DTPA
The clinical effects of escalating doses of muscimol were observed through detailed behavioral and neurologic assessments. Six NHPs underwent 5 μL co-infusions of Gd-DTPA (5 mM) and muscimol at serially increasing muscimol concentrations (0.0625, 0.125, 0.25, 0.5, 1.1, 2.2, 4.4, and 8.8 mM). Two animals received a maximal dose of 88.8 mM to discover the lethal dose. One animal (animal no. 1) was sacrificed during the dose-escalation study and did not undergo MRI assessment of muscimol distribution. Infusions occurred every 3 to 5 d until reaching the maximum tolerable dose (MTD). A scoring system was devised (reaction score), ranging from 0 to 4, for grading post-infusion hyperkinetic movements. Scores of 0, 1, 2, 3, and 4 represented normal, mild dyskinesia, moderate dyskinesia, severe dyskinesia, and alternating severe dyskinesia and somnolence, respectively. Behavior in the infusion chair was assessed for 30 min after infusion. Animals were monitored in their cages every 30 min thereafter until their behavior returned to normal.
After the final infusion, 3 animals were sacrificed within 72 h and 3 were sacrificed 6 wk later. Upon sacrifice, brains were extracted, fixed in 4% paraformaldehyde, paraffin-embedded, cut into 20 micron-thick sections, and mounted on glass slides. Hematoxylin and eosin (H&E) stained slides were evaluated for evidence of infusion-related toxicity by a neuropathologist.
Histological and Behavioral Effects of Microcatheter Placement
To isolate the effects of catheter placement, 2 animals underwent stereotactic placement of the infusion catheter without infusion. These NHPs underwent the same neurological and behavioral assessments as those co-infused with Gd-DTPA and muscimol. Animals were sacrificed 3 d and 6 wk, respectively, after catheter placement.
Fluoro-Jade C Staining
Fluoro-Jade C, a histological stain detecting degenerating neurons with ultra-high resolution and contrast,12 analyzed for the effect of microcatheter placement on neuronal degeneration in treated and control animals.
Distribution Assessment Study: Convective Co-infusion of 3H-muscimol and Gd-DTPA
After the MTD was determined from the dose-escalation study, 3 NHPs were co-infused with 5 μL of 4.4 mM (MTD) 3H-muscimol (PerkinElmer, Waltham, Massachusetts) and Gd-DTPA (Berlex, Wayne, New Jersey) delivered at a rate of 0.5 μL/min. The 3H-muscimol and Gd-DTPA solution for co-infusion was prepared as previously described.13 Infusions and MRI were performed as described above. Immediately after infusion, the animals were euthanized with a lethal dose of phenobarbital. Their brains were removed and sectioned, taking 2 adjacent 20 μm-thick sections every 200 μm. One section of each pair was stained with routine hematoxylin and eosin. The other section was selected for autoradiography and organized in a cassette according to its anteroposterior location in the STN and adjacent brain. The cassette slides were covered with imaging plates (BAS-TR2025 plates; Fujifilm Medical Systems, Stamford, Connecticut), which were exposed for 2 wk and read by a BAS-5000 Bio-Imaging Analyzer (Fujifilm Medical Systems).
Volumes of distribution of muscimol in brain by autoradiography (VdAR) were calculated from the dimensions of the radioactive tracer signal in 3 planes using the formula for the volume of an ellipsoid (length × width × height × π/6).14 VdMRI of Gd-DTPA was measured on T1-weighted MRI using threshold-based 3D segmentation analysis and OsiriX software (Pixmeo, Geneva, Switzerland), setting a MR segmentation threshold of 2 standard deviations above the surrounding noninfused region.15 VdAR and VdMRI were compared using statistical analysis software (JMP 13.0, SAS Institute Inc, Cary, North Carolina). A P value less than .05 was considered statistically significant.
RESULTS
Behavioral Assessment During Convective Co-infusion of Muscimol and Gd-DTPA
Involuntary movements were not observed during infusions of 0.0625 or 0.125 mM muscimol. At a dose of 2.2 mM, animals exhibited mild dyskinesia (reaction score 1) within 1 h after infusion, with occasional random involuntary movements that subsided within 2 to 3 h of infusion completion. A more pronounced, nearly continuous hyperkinesia (reaction score 2) was observed in animals infused with 4.4 mM, which resolved spontaneously within 3 h. One NHP infused with this concentration initially exhibited dyskinesia in 1 limb beginning 10 min after infusion completion. Within 20 to 30 min, the single limb dyskinesia progressed to involve all 4 extremities (reaction score 3), but did not prevent the animal from grasping offered food. Dyskinesia subsided completely by 3 h post-infusion. Higher concentrations (8.8 mM) of muscimol produced bouts of severe bilateral dyskinesia and bilateral ballismus interspersed with periods of somnolence lasting almost 6 h post-infusion (reaction score 4). One of the animals infused with 88.8 mM of muscimol became unconscious and hypothermic, and was euthanized, while the other, though also becoming unconscious and hypothermic, responded to external warming and survived without permanent side effects. The post-infusion behaviors of all animals in the dose-escalation study are described in Table 1, and their reaction scores are plotted in Figure 2.
TABLE 1.
Behavioral Responses After Varying Concentrations of Muscimol Infusions
| Animal | Experimental | Dose | VdMRI (mm3) | VdMRI (mm3) | ||
|---|---|---|---|---|---|---|
| no. | group | (mM) | Reaction | (right STN) | (left STN) | Histology |
| 1 | Early | |||||
| 8.8 | Alternating severe dyskinesia and somnolence | a | a | Predominantly normal, scattered neurons undergoing axonal regeneration | ||
| 88.8 | Anesthetic coma requiring sacrifice | |||||
| 2 | Early | |||||
| 0.0625 | Normal | Predominantly normal, scattered neurons undergoing axonal regeneration | ||||
| 0.125 | Normal | 44.35 | 28.61 | |||
| 0.25 | Normal | |||||
| 0.5 | Normal | |||||
| 1.1 | Normal | |||||
| 2.2 | Mild dyskinesia | |||||
| 4.4 | Moderate dyskinesia | |||||
| 8.8 | Alternating severe dyskinesia & somnolence | |||||
| 3 | Early | |||||
| 0.125 | Normal | 43.64 | 35.44 | Normal | ||
| 0.25 | Normal | |||||
| 0.5 | Normal | |||||
| 1.1 | Mild dyskinesia | |||||
| 2.2 | Mild dyskinesia | |||||
| 4.4 | Moderate dyskinesia | |||||
| 4 | Late | |||||
| 0.125 | Normal | 26.40 | 21.65 | Normal | ||
| 0.25 | Normal | |||||
| 0.5 | Normal | |||||
| 1.1 | Mild dyskinesia | |||||
| 2.2 | Mild dyskinesia | |||||
| 4.4 | Severe dyskinesia | |||||
| 8.8 | Alternating severe dyskinesia and somnolence | |||||
| 88.8 | Anesthetic coma; survived without permanent side effects | |||||
| 5 | Late | |||||
| 0.0625 | Normal | Normal | ||||
| 0.125 | Normal | 49.25 | 41.73 | |||
| 0.25 | Mild dyskinesia | |||||
| 0.5 | Mild dyskinesia | |||||
| 1.1 | Mild dyskinesia | |||||
| 2.2 | Mild dyskinesia | |||||
| 4.4 | Moderate dyskinesia | |||||
| 8.8 | Alternating severe dyskinesia and somnolence | |||||
| 6 | Late | |||||
| 0.125 | Normal | 61.42 | 42.93 | Normal | ||
| 0.25 | Normal | |||||
| 0.5 | Normal | |||||
| 1.1 | Mild dyskinesia | |||||
| 2.2 | Mild dyskinesia | |||||
| 4.4 | Moderate dyskinesia |
aDistribution data from animal no. 1 was not obtained due to sacrifice of the animal before real-time MR-imaging of Gd-DTPA distribution.
FIGURE 2.
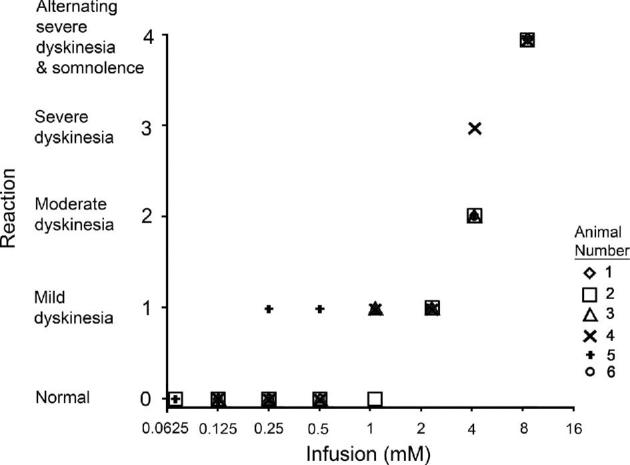
Behavioral reaction scores at different concentrations of muscimol. Reaction scores between 0 and 4 were plotted against varying concentrations of muscimol. Each plotted shape represents the score of a single NHP. Two animals received 88.8 mM muscimol: 1 became unconscious, hypothermic, and was euthanized; the other also became unconscious and hypothermic but responded to external warming and survived without permanent side effects.
Early and Late Histology
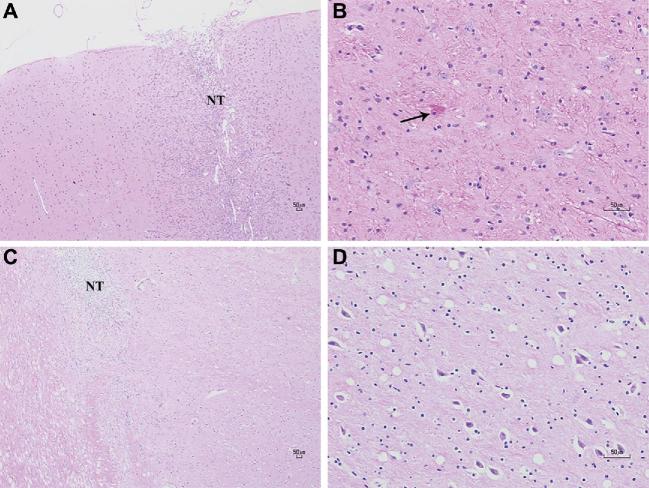
The 6 animals in the dose-escalation study underwent histological evaluation <72 h (n = 3) and 6 wk (n = 3) after their last infusion. In H&E-stained histological sections of all animals, the needle tract was surrounded by lymphocytic infiltrate from the cortical surface to the parenchyma (Figures 3A and 3B), like that of instrument controls. In sections of the STN at the early time point, a few neurons exhibited central chromatolysis, indicating reversible axonal regeneration with characteristically hypereosinophilic cell bodies and peripherally displaced nuclei (Figure 3C). These histopathological abnormalities in the STN were not seen at the late time point, suggesting that early neuronal changes induced by muscimol were transient and reversible (Figure 3D).
FIGURE 3.
Assessments for acute and chronic histopathological change on H&E. A, Lymphocytic infiltrates are seen encompassing the needle tract (NT) at the cortical surface of an animal, sacrificed within 72 h after final infusion–magnification ×40. B, There was evidence of central chromatolysis (black arrow) in some neurons in the STN—magnification ×200. C, The needle tract (NT) of an animal sacrificed 6 wk after final infusion, again surrounded by many lymphocytes—magnification ×40. D, A closer view of the STN did not reveal any histological abnormalities—magnification ×200.
Fluoro-Jade C Staining
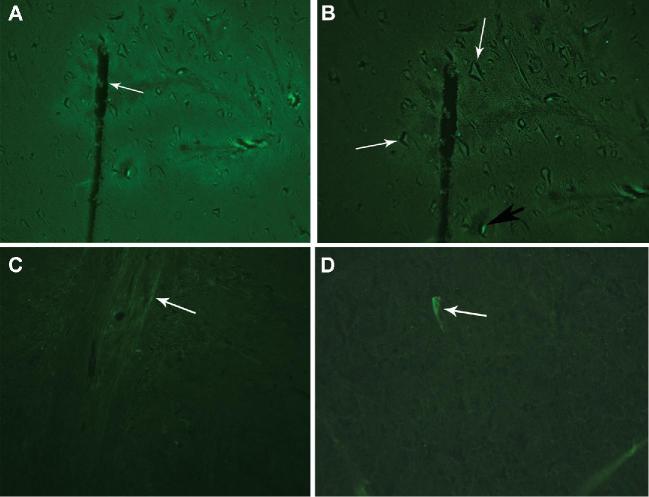
In control NHPs, neuronal degeneration by Fluoro-Jade staining was not visible at ×100 magnification (Figure 4A), but appeared near the needle tract at ×200 magnification (Figure 4B). In treated NHPs, no degenerating neurons were seen at ×100 magnification (Figure 4C), but one degenerating neuron was seen at ×400 magnification (Figure 4D).
FIGURE 4.
Fluoro-Jade C staining to show early neuronal degeneration. A, Fluro-Jade C stain imaging in the control animal (no muscimol infusion) demonstrated that the insertion of the microcatheter (white arrow) alone did not cause neuronal degeneration observable at ×100 magnification. B, Healthy, nondegenerating neurons can be seen near the tip of the microcatheter (white arrows) in the control animal (no muscimol infusion). One degenerating neuron may be present near the needle tract (black arrow)—magnification ×200. C, Fluoro-Jade C stain from a treatment NHP in the dose-escalation behavioral study who received a dose of muscimol with concentration of 4.4 mM. The needle tract (white arrow) is negative for neuron degeneration at ×100 magnification. D, One degenerating neuron (white arrow) was seen with the Fluoro-Jade C stain in a treatment animal receiving a dose of muscimol with concentration of 4.4 mM magnification ×400.
Volumes of Distribution on Autoradiograms and MRI
After determining the MTD with escalating muscimol doses, 3 NHPs underwent co-infusions of 3H-muscimol (4.4 mM) and Gd-DTPA (5 mM) over 10 min into the bilateral STN. Gd-DTPA had similar distribution to muscimol, based on the VdAR and VdMRI of each compound. Specifically, the percentage difference between VdAR and VdMRI ranged between 6.7 and 19.6%, which was not statistically significant (P = .37; Table 2). The distribution of muscimol on autoradiographic images and Gd-DTPA on T1-weighted MR images appeared similar when viewed side by side (Figure 5). Consistent with previous work in other brain structures,13 these findings showed that the distribution of muscimol in the NHP brain was tracked accurately on MRI by Gd-DTPA.
TABLE 2.
Volumes of Distribution in the Autoradiography Study
| Animal no. | STN | Vd (mm3) on autoradiography | Vd (mm3) on MRI | % difference |
|---|---|---|---|---|
| 8 | Right | 22.5 | 26.9 | 19.6 |
| Left | 28.9 | 32.9 | 13.8 | |
| 9 | Right | 27.8 | 24.5 | –11.9 |
| Left | 24.3 | 26.5 | 9.1 | |
| 10 | Right | 55.3 | 51.6 | –6.7 |
| Left | 42.2 | 49.1 | 16.4 |
Vd = volume of distribution.
FIGURE 5.
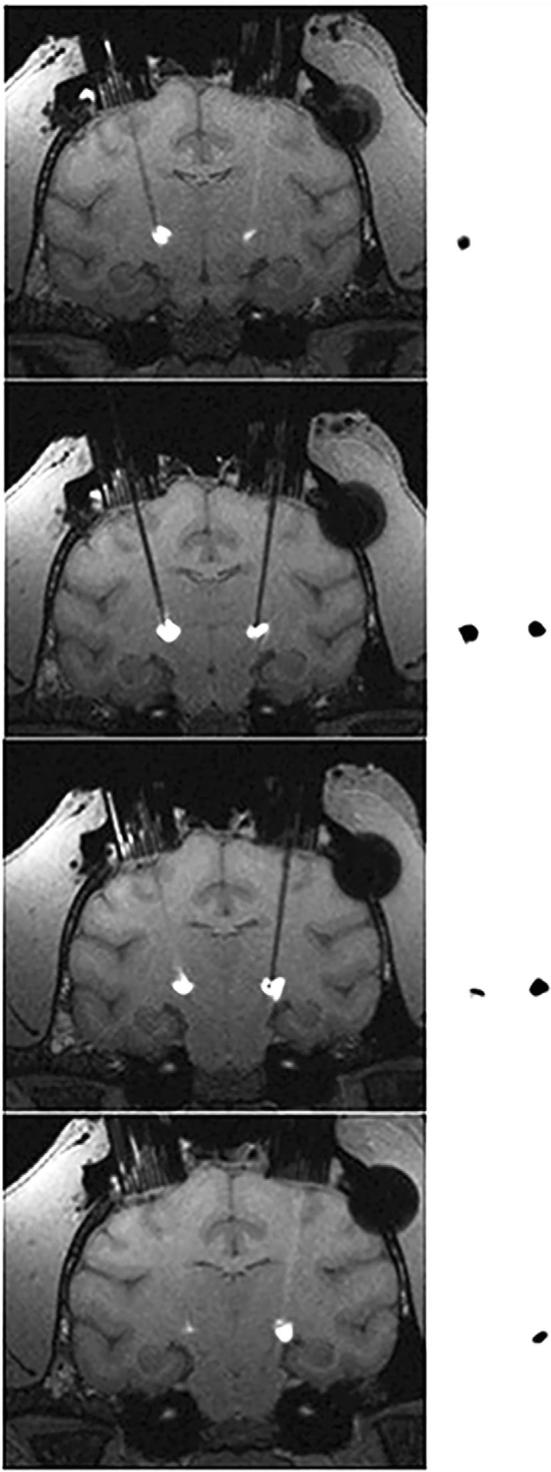
Comparison between T1-weighted MRI and autoradiograms. Representative serial coronal slices of T1-weighted MR images post-contrast were compared to corresponding autoradiographic sections (20 μm) from an NHP who underwent co-infusion of 3H-muscimol and Gd-DTPA. Anatomically registered MRI-autoradiogram pairs are separated by 1 mm.
Histological Confirmation of 3H-muscimol Into the Subthalamus
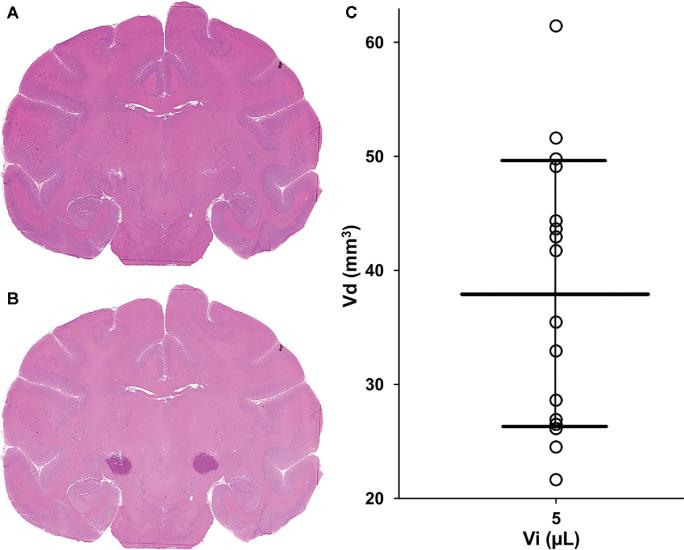
H&E-stained sections adjacent to sections used for the autoradiography studies confirmed accurate delivery of muscimol into the STN. Infusion of 3H-muscimol into the STN was confirmed histologically by superimposing basal ganglia structures identified on H&E-stained slides with adjacent autoradiograms (Figurse 6A and 6B). Some radioactive tracer signal extended into the internal capsule lateral to the STN.
FIGURE 6.
Volumes of distribution of muscimol. A, H&E-stained coronal section of the brain was overlaid with an adjacent (20 μm) coronal autoradiographic image (not shown), demonstrating B, radioactive tracer encompassing the entirety of the STN. Volumes of distribution on MRI (VdMRI) from the dose-escalation study were plotted against the infusate volume of 5 μL for these animals. The mean volume of distribution was 37.9 mm3 ± 11.7 mm3. Distribution data from 16 STN infusions (8 animals) are shown. C, Distribution data from another animal that underwent muscimol infusion were not obtained due to sacrifice of the animal before real-time MRI of Gd-DTPA distribution.
Determination of Vd/Vi Ratios
The mean VdMRI was 37.9 mm3 ± 11.7 mm3, and the mean ratio of volume of distribution to volume of infusate (VdMRI/Vi) was 7.6 ± 2.3. VDAR and VdMRI values varied between animals even though all animals received the same volume of infusion (Vi) of 5 μL (Figure 6C).
DISCUSSION
In this study, bilateral muscimol infusions, in concentrations of 2.2 to 4.4 mM, into the STN of awake, nonparkinsonian NHPs caused dose-dependent, hyperkinetic movements. Higher muscimol doses of 8.8 mM produced severe bilateral hyperkinesia, hemiballismus, and interspersed periods of somnolence that spontaneously remitted 3 to 6 h after infusion. The highest muscimol dose of 88.8 mM produced obtundation consistent with overdose. Increasing muscimol doses resulted in pronounced hyperkinetic movements and dyskinesias, but at different doses in some animals. For example, mild dyskinesia was noted in 4 of 5 animals receiving the 1.1 mM dose, whereas 1 animal (no. 2) did not experience dyskinesia until the muscimol dose reached 2.2 mM (Table 1, Figure 2). The volume of distribution (21.6-61.4 mm3) of muscimol and Gd-DTPA also varied among animals, even though the infused volume was the same, 5 μL, in all animals (Table 1), suggesting that the clinical response was more dependent on the concentration reaching the STN than the infused volume itself. Although animal no. 2 had a CED volume of distribution of 44.35 mm3, in the center of the volume of distribution range, muscimol can escape the STN by convection through white matter tracts around the STN or simple diffusion. These processes could have reduced the STN muscimol concentration, preventing dyskinesias at the 1.1 mM dose in animal no. 2.16
Muscimol did not produce early or late neurotoxicity by histological examination of all infused tissues. Minimal, early axonal changes, like those seen in the instrument controls, resolved by 6 wk and likely arose from catheter placement. Additionally, consistent with earlier work,13 we showed that Gd-DTPA accurately tracks muscimol distribution in the deep brain parenchyma of NHPs. We overlaid autoradiograms of 3H-muscimol on stained histological sections and confirmed that the distribution of muscimol encompassed the STN.
Muscimol infusions into the human brain were first performed by Penn et al,6 who injected 2.5 μL of 8.8 mM muscimol unilaterally into the GPi of a 54-yr-old male with PD before creating a pallidotomy with thermal lesioning. Notably, 20 min after infusion, the patient's baseline arm tremor significantly increased and cog-wheel rigidity completely abated. Pallidotomy abolished his tremor. The authors suggested that the reduction of cogwheel rigidity produced by local muscimol infusion could be used to confirm GPi localization during pallidotomy to treat PD. The low dose and unilateral infusion of muscimol were not associated with major side effects, such as central nervous system (CNS) depression. Later, Pahapill et al5 studied unilateral muscimol infusions into the ventral thalamic nuclei of 6 patients with medically intractable essential tremor in the operating room immediately before thalamotomy or thalamic deep brain stimulation (DBS) electrode placement. The authors observed reversible tremor suppression with 8.8 mM muscimol in volumes of 1 to 5 μL, without affecting speech or voluntary movements. In a later study, the same group reported the effects of unilateral muscimol infusions into the STN of 2 patients with PD.4 Following microinjections of 8.8 mM muscimol in volumes of 5 to 10 μL, both patients had dramatic reduction of contralateral resting tremor and reduced microelectrode-measured neuronal oscillatory activity near the infusion site. Taken together, these studies (Table 3) suggest that doses up to 10 μL of 8.8 mM muscimol could be safely infused unilaterally into deep parenchymal structures (GPi and STN) of the brain in human subjects.
TABLE 3.
A Summary of Muscimol Infusion Into the Basal Ganglia of Human Patients.
The VdMRI/Vi of 7.6 ± 2.3 calculated in the present study was similar to the 8.2 ± 1.3 value previously reported in a study of muscimol infusion into the hippocampus of NHPs.17 Assuming the human striatum is roughly 6 times larger than the NHP striatum18 and a volume of 120 mm3 for the human STN,6 the volume of the NHP STN would be approximately 20 mm3. In this study, the mean volume of distribution was 37.9 mm3 (±11.7 mm3), which encompassed the entire estimated volume of the NHP. Considering the volume difference between the human STN and the NHP STN and the aforementioned human studies,19 higher concentrations and greater volumes of muscimol might be tolerated in human patients.
Microinjections of muscimol have been used previously to investigate STN involvement in neuronal pathways in animal models. Injections of muscimol into the NHP STN demonstrated the fundamental role of the STN in transmitting stimulation of the primary somatosensory and motor cortices into excitation of the globus pallidus, as part of the cortico-subthalamo-pallidal pathway.20 Likewise, STN inhibition with muscimol in NHPs reduced burst firing of neurons in the substantia nigra pars compacta (SNc) and altered dopamine release in the striatum,21 demonstrating that the STN also projects efferents onto the SNc. Furthermore, the NHP STN was shown to be morphologically and synaptically similar to the human STN, suggesting that studying STN inhibition with muscimol in the NHP model was translatable and valid.22 Muscimol has not been used to treat PD since its first testing in humans more than 20 yr ago.6 The practical problems of chronic infusion, the fact that muscimol cannot be patented, and that clinical testing would cost millions of dollars to pass FDA regulations are some of the problems that have slowed down development. Also, DBS treats many PD symptoms that are refractory to medical management. The safety results of muscimol in NHPs are encouraging but full good laboratory practice23 studies of muscimol toxicity are necessary if muscimol is to be given more than once in human studies.
In our study, we found that higher concentrations of muscimol (8.8 mM) resulted in alternating severe dyskinesia and somnolence, and ballismus, which persisted for up to 6 h after infusion. Hemiballismus after injury to the contralateral STN is well established in both humans and NHPs.24-26 Furthermore, contralateral dyskinesias after muscimol infusions into the STN were previously reported in NHPs. Nambu et al20 found that 1 μL infusions of 8.8 mM muscimol into the STN of normal NHPs resulted in contralateral hemiballismus in 3 of 8 animals. They hypothesized that inhibition of the STN with muscimol removed STN inhibition of the thalamus and created abnormal discharges from the globus pallidus.20 Karachi et al27 found that 1.5 μL of 8.8 mM muscimol into the unilateral STN of normal NHPs produced contralateral hemiballismus that persisted for 1 h and spontaneously resolved. Likewise, Baron et al28 found that infusions of 1 μL of 0.88 mM muscimol into the STN of parkinsonian 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) NHPs resulted in transient and very mild, choreiform dyskinesias of the contralateral lower limb in some but not all animals. Microinjections of muscimol have been previously utilized to delineate the anatomical and functional boundaries of the STN, including a somatosensory region,29 suggesting targeted infusion of muscimol into the somatosensory region of the STN would produce dyskinetic movements at lower concentrations. Baron et al28 found that dyskinesias occurred when muscimol was infused into the more lateral and dorsal areas of the STN. These findings were not reproducible across 2 animals. The STN may be more sensitive to muscimol inhibition in the MPTP NHP model of PD than in normal NHPs. The significance of this possibility in human patients is unclear. Regardless, our data from bilateral muscimol infusions confirmed previous findings of hyperkinetic movements induced by unilateral muscimol infusions into the STN of normal primates. Dyskinesia from muscimol infusions into the nonparkinsonian STN appears to represent a normal physiological response to STN inhibition. These dyskinetic movements, which are seen in humans with STN infarctions, were not observed in earlier studies of PD patients infused with 8.8 mM muscimol into the STN.
CONCLUSION
In this study, we found that muscimol convected into the bilateral STN of awake, nonparkinsonian NHPs resulted in transient, dose-dependent hyperkinesia and somnolence. There was no acute or long-term toxicity from the infusions. There were no observed toxic effects of adding Gd-DTPA to the infused muscimol solution. Gd-DTPA was visible on MRI scans and a satisfactory surrogate tracer for muscimol distribution.
Disclosures
This research was supported by the Intramural Research Program of the National Institute of Neurological Disorders and Stroke at the National Institutes of Health. The authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
Acknowledgments
The authors wish to thank Sam Antonio, Cindy Prevost, and Renee Hill for their technical assistance.
Notes
Preliminary data contained in this study was presented as an oral presentation at the 2015 Annual Scientific Meeting of the American Association of Neurological Surgeons in Washington, District of Columbia, from May 2-6, 2015.
COMMENT
Over the years many investigators have envisioned the delivery of medications directly into localized areas the brain to treat a multitude of different neurological diseases from Parkinson's disease to other movement disorders and psychiatric conditions. Unfortunately, progress has very been slow. This is not because the concept is wrong but because the practical problems to be overcome in delivering medications have been so great. In this article muscimol, an all-purpose GABA A agonist, is shown to distribute into brain parenchyma of nonhuman primates in the region of STN and cause the expected dyskinesia. Dose-response data were obtained with simultaneous infusion of gadolinium the area of perfusion. The limited pathological specimens are normal although not taken under GLP conditions. This is all encouraging and means that muscimol could potentially be used in human neurological diseases in which selective delivery would be potentially valuable. The inhibition it produces is much more localized and specific than electrical stimulation so it would potentially have limited and constrained effects on gray matter neurons. This might decrease unwanted complications due to white matter tracts activation. It might however be clinically less effective for the same reasons; the most important part of stimulation might be activation of such tracts. A limitation of infusion is much slower kinetics. The response to drug infusion builds up gradually and is equally slow to dissipate. This is because of the low flow of CSF through the brain parenchyma that determines washout time. DBS may be turned on and off quickly and is even be able to respond to rapidly changing local field potentials. Which technique would be best clinically has yet to be determined.
Over the 25 years since the original testing of muscimol in Parkinson's disease, no drug or pump company has been willing to invest enough in development to provide the information the needed data for safe human experimentation. To do so mean would required devoting a great deal of time, energy, and money, 5–10 years and 50 to a hundred million dollars. It is easy to see why a company would elect to put its money elsewhere. Fortunately, a few laboratories are willing to continue the work in this important field until a more enticing situation for developing selective delivery of drugs opens up. Meanwhile, the old dream of having a chemode for treating brain disease will have to wait.
Richard D. Penn
Chicago, Illinois
REFERENCES
- 1. Johnston GA. Muscimol as an ionotropic GABA receptor agonist. Neurochem Res. 2014;39(10):1942–1947. [DOI] [PubMed] [Google Scholar]
- 2. Ebert B, Frolund B, Diemer NH, Krogsgaard-Larsen P. Equilibrium binding characteristics of [3H]thiomuscimol. Neurochem Int. 1999;34(5):427–434. [DOI] [PubMed] [Google Scholar]
- 3. Enna SJ, Collins JF, Snyder SH. Stereospecificity and structure-activity requirements of GABA receptor binding in rat brain. Brain Res. 1977;124(1):185–190. [DOI] [PubMed] [Google Scholar]
- 4. Levy R, Lang AE, Dostrovsky JO et al.. Lidocaine and muscimol microinjections in subthalamic nucleus reverse Parkinsonian symptoms. Brain. 2001;124(10):2105–2118. [DOI] [PubMed] [Google Scholar]
- 5. Pahapill PA, Levy R, Dostrovsky JO et al.. Tremor arrest with thalamic microinjections of muscimol in patients with essential tremor. Ann Neurol. 1999;46(2):249–252. [DOI] [PubMed] [Google Scholar]
- 6. Penn RD, Kroin JS, Reinkensmeyer A, Corcos DM. Injection of GABA-agonist into globus pallidus in patient with Parkinson's disease. Lancet North Am Ed. 1998;351(9099):340–341. [DOI] [PubMed] [Google Scholar]
- 7. Kilkenny C, Browne W, Cuthill IC et al.. Animal research: reporting in vivo experiments–the ARRIVE guidelines. J Cereb Blood Flow Metab. 2011;31(4):991–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. National Research Council Institute for Laboratory Animal R. Guide for the Care and Use of Laboratory Animals. Washington, DC: National Academies Press (US)Copyright 1996 by the National Academy of Sciences. All rights reserved; 1996. [Google Scholar]
- 9. Ksendzovsky A, Walbridge S, Saunders RC, Asthagiri AR, Heiss JD, Lonser RR. Convection-enhanced delivery of M13 bacteriophage to the brain. J Neurosurg. 2012;117(2):197–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Iyer RR, Butman JA, Walbridge S, Gai ND, Heiss JD, Lonser RR. Tracking accuracy of T2- and diffusion-weighted magnetic resonance imaging for infusate distribution by convection-enhanced delivery. J Neurosurg. 2011;115(3):474–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Asthagiri AR, Walbridge S, Heiss JD, Lonser RR. Effect of concentration on the accuracy of convective imaging distribution of a gadolinium-based surrogate tracer. J Neurosurg. 2011;115(3):467–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schmued LC, Stowers CC, Scallet AC, Xu L. Fluoro-Jade C results in ultra high resolution and contrast labeling of degenerating neurons. Brain Res. 2005;1035(1):24–31. [DOI] [PubMed] [Google Scholar]
- 13. Heiss JD, Walbridge S, Asthagiri AR, Lonser RR. Image-guided convection-enhanced delivery of muscimol to the primate brain. J Neurosurg. 2010;112(4):790–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. De Salles AA, Brekhus SD, De Souza EC et al.. Early postoperative appearance of radiofrequency lesions on magnetic resonance imaging. Neurosurgery. 1995;36(5):932–937. [DOI] [PubMed] [Google Scholar]
- 15. Nguyen TT, Pannu YS, Sung C et al.. Convective distribution of macromolecules in the primate brain demonstrated using computerized tomography and magnetic resonance imaging. J Neurosurg. 2003;98(3):584–590. [DOI] [PubMed] [Google Scholar]
- 16. Bobo RH, Laske DW, Akbasak A, Morrison PF, Dedrick RL, Oldfield EH. Convection-enhanced delivery of macromolecules in the brain. Proc Natl Acad Sci USA. 1994;91(6):2076–2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Heiss JD, Walbridge S, Morrison P et al.. Local distribution and toxicity of prolonged hippocampal infusion of muscimol. J Neurosurg. 2005;103(6):1035–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yin D, Valles FE, Fiandaca MS et al.. Striatal volume differences between non-human and human primates. J Neurosci Methods. 2009;176(2):200–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Massey LA, Miranda MA, Zrinzo L et al.. High resolution MR anatomy of the subthalamic nucleus: Imaging at 9.4T with histological validation. Neuroimage. 2012;59(3):2035–2044. [DOI] [PubMed] [Google Scholar]
- 20. Nambu A, Tokuno H, Hamada I et al.. Excitatory cortical inputs to pallidal neurons via the subthalamic nucleus in the monkey. J Neurophysiol. 2000;84(1):289–300. [DOI] [PubMed] [Google Scholar]
- 21. Shimo Y, Wichmann T. Neuronal activity in the subthalamic nucleus modulates the release of dopamine in the monkey striatum. Eur J Neurosci. 2009;29(1):104–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yelnik J, Percheron G. Subthalamic neurons in primates: a quantitative and comparative analysis. Neuroscience. 1979;4(11):1717–1743. [DOI] [PubMed] [Google Scholar]
- 23. FDA. Good Laboratory Practice for Nonclinical Laboratory Studies: Food & Drug Administration - Department of Health & Human Services; 2017. [Google Scholar]
- 24. Carpenter MB, Whittier JR, Mettler FA. Analysis of choreoid hyperkinesia in the rhesus monkey. Surgical and pharmacological analysis of hyperkinesia resulting from lesions in the subthalamic nucleus ol luys. J Comp Neurol. 1950;92(3):293–331. [DOI] [PubMed] [Google Scholar]
- 25. Lee MS, Marsden CD. Movement disorders following lesions of the thalamus or subthalamic region. Mov Disord. 1994;9(5):493–507. [DOI] [PubMed] [Google Scholar]
- 26. Whittier JR, Mettler FA. Studies on the subthalamus of the rhesus monkey. II. Hyperkinesia and other physiologic effects of subthalamic lesions, with special reference to the subthalamic nucleus of Luys. J Comp Neurol. 1949;90(3):319–372. [DOI] [PubMed] [Google Scholar]
- 27. Karachi C, Grabli D, Baup N et al.. Dysfunction of the subthalamic nucleus induces behavioral and movement disorders in monkeys. Mov Disord. 2009;24(8):1183–1192. [DOI] [PubMed] [Google Scholar]
- 28. Baron MS, Wichmann T, Ma D, DeLong MR. Effects of transient focal inactivation of the basal ganglia in parkinsonian primates. J Neurosci. 2002;22(2):592–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Buot A, Yelnik J. Functional anatomy of the basal ganglia: limbic aspects. Rev Neurol (Paris). 2012;168(8-9):569–575. [DOI] [PubMed] [Google Scholar]