Abstract
One of the most important tools used to evaluate kidney function in the context of chronic kidney disease or other renal function related pathologies is the exploration of glomerular filtration rate (GFR). Iohexol is up to this moment a good candidate molecule for the GFR assessment since it exhibits minimum protein binding rates and minimum extra-renal clearance, being neither secreted nor reabsorbed at the tubular level. This study proposes and evaluates a new LC-MS/MS method for the iohexol determination from capillary blood, prelevated using volumetric absorbative microsampling (VAMS) systems. As an alternative to VAMS, a brand new HemaPEN® device for micro-prelevation was also tested. A new high throughput sample preparation protocol adapted for iohexol quantification from whole blood VAMS samples was developed. The medium term stability study of iohexol in dried whole blood VAMS samples that was conducted showed a good stability of this molecule for up to 12 days. By collecting only 10 μL of blood, iohexol can be analyzed from dried whole blood VAMS samples for concentration ranges between 1 and 250 μg/mL. Due to the analyte stability in VAMS for up to 12 days, this approach might be successfully applied for GFR assessment for clinical cases allowing minimum invasiveness and even delayed analysis.
Keywords: Microsampling, Iohexol, VAMS, LC-MS/MS, HemaPEN
Graphical abstract
Highlights
-
•
Successful VAMS-based bioanalytical method for iohexol analysis in whole human blood.
-
•
Proven medium term stability of iohexol in VAMS samples.
-
•
HemaPens as a promising alternative for iohexol analysis using only 2.74 μL of blood.
1. Introduction
Assessing kidney function in some physiological and pathological conditions is of utmost importance. For this purpose, one of the best physiological measures is the evaluation of glomerular filtration rate (GFR). Moreover, GFR is an important tool for the monitoring, and diagnosis of chronic kidney disease (CKD). The ideal marker for measuring GFR, appearing endogenously in plasma at a constant rate, is freely filtered by the glomerulus without renal absorption or secretion and without any extra-renal elimination.
The gold standard for the measurement of GFR is the renal clearance of inulin, a fructose-derived compound. However, the procedure is expensive and time consuming. Isotopic methods such as 99Tc-diethylenetriaminepentaacetic acid (DTPA) and 51Cr-ethylenediaminetetra-acetic acid (EDTA) clearance are known but are costly and involve special handling [1]. Contrast agents such as iohexol (IOH) and iothalamate (IOTH) are the most used for the GFR evaluation since they exhibit features of an ideal marker, while being safe and easily available [2].
Iohexol (IOH), 5-[N-(2,3-dihydroxypropyl)acetamido]-2,4,6-triiodo-N,N′-bis(2,3dihydroxypropyl) isophthalamide, is a non-ionic, low molecular weight iodinated molecule. It exhibits endo-exo stereoisomerism, relative to the substituted aromatic ring, the ratio of the two isomers being constant. The molecule is often used as contrast agent for computed tomography, catheter-based angiography or other types of intervention. Concerning its pharmacokinetic properties, IOH is freely distributed, its protein binding being as low as 1.5 %. Extra-renal clearance of IOH is very low (not more than 5 %) and the compound is neither secreted nor reabsorbed at the tubular level. As IOH has a low osmolality and is not nephrotoxic, it is generally considered as safe. No severe adverse effect or no anaphylactic reaction has been reported worldwide [3]. Several analytical methods were developed for the quantification of IOH in different biological matrices such as plasma, serum, whole blood when using dried blood spots (DBS) [4,5] systems and lastly, urine. The most encountered analytical techniques are liquid chromatography with ultraviolet detection (LC-UV) [6,7] or coupled to tandem mass spectrometry (LC-MS/MS) [8]. Other techniques such as capillary electrophoresis and X-ray fluorescence are also reported in the literature [9,10].
IOH clearance is often established using plasma or serum samples. For the GFR evaluation, repeated sample (usually blood) tests at different time increments are proposed. This involves that the patient has to be subjected to sample collection procedures through venipuncture or cannula. Moreover, for pediatric cases where repeated blood sampling can be traumatizing, the approach of collecting blood samples from infant or newborn heel using blood micro-prelevation systems may represent a suitable alternative. Microsampling devices systems such as DBS or volumetric absorptive microsampling (VAMS) systems which can collect only small amounts (few microliters) of capillary blood by a simple finger prick can reduce the invasiveness of the classical blood collection procedures while increasing the patient’s compliance [11]. DBS has been used successfully for GFR calculations using IOH [12]. Good results in terms of the agreement between GFR values calculated using blood spots and GFRs based on venous classical sampling techniques were obtained [13,14]. VAMS consists of a porous hydrophilic tip, capable of collecting small and reproducible amounts of blood (10, 20 or 30 μL). The hydrophilic tip is found at the end of a plastic handler that can be attached to automated sample processing systems. VAMS is successfully used today for pharmacokinetic and therapeutic drug monitoring studies both in animal and human experiments [15,16]. VAMS devices were created for their ability of remote blood sampling, easy transportation of collected dried blood samples without using special transportation equipment such as cold packs or dried ice, assuring analyte stability. Moreover, VAMS devices turned out to overcome the DBS inherited issues concerning the hematocrit-induced biases for the extraction yields and reproducibility [17]. Of course, each analyte exhibits its own characteristics including the stability in a complex analytical matrix such as dried blood, which may jeopardize the utility of the remote sampling-late analysis concept. A wide spectrum of analytes has been successfully determined from VAMS-based samples. For pharmacokinetic studies, estetrol from whole blood VAMS samples obtained from mice was efficiently assessed [18]. Itraconazole was analyzed from VAMS as low soluble molecule model [19]. For therapeutic drug monitoring, the first-generation anti-epileptic drugs and hydrochloroquine were determined using VAMS samples [20,21]. Even asenapine enantiomers have been quantified from VAMS samples using an LC-Diode array detector platform [22]. For pharmacokinetic studies, therapeutic drug monitoring or in the context of glomerular filtration rate assessment, multiple samples have to be collected at different time points and further analyzed. This involves accurate assessment of the analyte stability for short- to long-term stability in different storage conditions.
An alternative to VAMS or DBS is a new state of the art microsampling device called HemaPEN. This new micro-blood collection system integrates four end-to-end capillaries for blood collection coupled to a dispensing platform represented by DBS format filters. The filter paper substrate types are either Whatmann 903 or D226™ Perkin Elmer, both being approved by the Food and Drug Administration (FDA) for DBS-like experiments. Each HemaPEN integrates four filters while each filter is capable of storing an accurate volume of 2.74 μL of blood, thus assuring up to four replicates for each collected sample. Due to its pen-like design, this new device is ergonomic and easy to use, assuring sample integrity and preventing any contamination when compared to classical DBS [23,24].
The aim of this study was to fully develop a sample preparation protocol assisted by UHPLC-MS/MS for the analysis of IOH from human whole blood collected with VAMS systems as an alternative to already reported DBS-based methods. This approach might considerably improve patient compliance and overall analysis while assuring analyte stability. The overall analytical methodology fulfilled FDA validation criteria, which demonstrates the reliability of the analytical approach. Moreover, some preliminary investigations were carried out on HemaPEN devices which might represent an alternative to VAMS for the analysis of IOH from human whole blood.
2. Materials and methods
2.1. Chemicals, standards and reagents
Methanol (MeOH), acetonitrile (ACN), isopropanol (IPA) and water (H2O) were all of UHPLC-MS grade and were obtained from Biosolve (Valkenswaard, Netherlands). Formic acid (FA) 99% UHPLC-MS grade was acquired from Biosolve (Valkenswaard, Netherlands) and ammonia 25% was obtained from Merck (Darmstadt, Germany). IOH analytical standard and ioversol (IOV) United States Pharmacopoeia (USP) reference standard used as internal standard were both acquired from Sigma Aldrich-Merck (Saint Louis, MO, USA and Rockville, MD, USA). VAMS devices were obtained from Neoteryx (Torrance, CA, USA). HemaPEN devices containing D226™ Perkin Elmer substrate filter papers were obtained from Trajan Scientific and Medical (Ringwood, Victoria, Australia). Antioxidants (AOX) such as citric acid and ascorbic acid were bought from Merck (Darmstadt, Germany) and dithiothreitol (DTT) from Sigma Aldrich-Merck (St-Louis, MI, USA).
2.2. Stock solutions
IOH stock solutions were prepared in MeOH to reach two concentration levels (2.04 and 5.00 mg/mL). IOV was dissolved in MeOH in order to reach a stock solution with a concentration level of 4.00 mg/mL. Stock solutions were aliquoted in amber polypropylene Eppendorf® tubes and stored at -80 °C until further analysis. Tubes with potassium K3-EDTA with 0.5 mL volume capacity were used for human capillary blood collection. All dilutions were made using Axygen Maximum Recovery® pipette tips.
2.3. UHPLC-ESI-MS/MS method development
An Agilent Technologies 1290 Series UHPLC system hyphenated with an Agilent Technologies 6495 Series triple quadrupole mass spectrometer (QQQ) equipped with iFunnel technology (Agilent Technologies, Waldbronn, Germany) was used as analytical platform. Chromatographic separation was performed using a Phenomenex Kinetex biphenyl chromatographic column (50 mm × 2.10 mm) packed with 1.7 μm particles and thermostated at 50 °C. Analytes were eluted using a binary chromatographic pump that delivered the mobile phase under a gradient elution mode. The mobile phase, made up of two solvents: solvent A – H2O/FA (100/0.1; v/v) and solvent B – MeOH/FA (100/0.1; v/v), was delivered under a flow of 0.5 mL/min. After injecting 0.5 μL of sample, the gradient started at 98% solvent A (2% solvent B) and immediately linearly ramped for 1 min at 55% solvent B. Afterwards a further linear increase at 75% solvent B, from 1 min to 4 min occurred. Next the gradient sharply ramped to 98% solvent B, and maintained at this percentage for 2 min. Finally the gradient returned at 2% solvent B for column reequilibration for 2 min. Thus the total run time took 8 min. Four minutes after each injection, the interior of the needle was rinsed using a wash containing ACN/MeOH/IPA/H2O (1/1/1/1; v/v/v/v) in bypass mode. The exterior of the needle was washed using a solvent composed of IPA/MeOH/H2O (4/4/2; v/v/v).
MS parameters were determined by injecting a solution containing IOH and IOV at a concentration level of 100 ng/mL in selected ion monitoring mode and afterwards in multiple reaction monitoring (MRM) mode. The analytes were ionized using an electrospray ionization source in positive mode (ESI+). The ionization source ran under the following parameters: applied capillary voltage: 3000 V (+); gas flow: 11 L/min; gas temperature: 180 °C; nebulizer: 60 psi; sheath gas heater: 400 °C; sheath gas flow: 10 L/min. Ion funnel parameters were selected as follows: positive high pressure radio frequency: 190 V; positive low pressure radio frequency: 100 V. Two MRM transitions for IOH and one for IOV were selected to scan and detect both analytes: IOH – 821.9 (+) m/z→803.7 (+) m/z; 821.9 (+) m/z→603.0 (+) m/z, and IOV – 807.9 (+) m/z→589.0 (+) m/z.
2.4. Sample preparation protocol
In order to analyze IOH from VAMS samples, an extraction protocol for IOH was developed. Samples were subjected to matrix removal procedures and final resuspension into an optimal solvent. IOV was used as internal standard. IOV is also a hydrophilic, polar, iodinated molecule used as contrast agent. The chemical structure of IOV is very similar to that of IOH, which makes this molecule a good candidate as internal standard. Indeed isotope labeled derivative for IOH in the form of deuterated analogue is commercially available. Nevertheless, the concentration level chosen for the internal standard (micrograms per milliliter) involves using high amounts of isotope-labeled internal standard, which increases the overall cost per analysis since deuterated IOH is significantly more expensive than IOV.
Capillary blood collected on K3-EDTA spiked with IOH was used in order to develop the sample preparation protocol. After a gentle mixing by vortex, the spiked blood was further transferred into a LoBind Eppendorf tube. Whole blood was then sampled using VAMS devices by putting into contact with the edge of the VAMS tip with the surface of the blood, without fully immersing the VAMS tip. The VAMS tip was held into contact for 2 s or 3 s so that the blood could have been absorbed by capillarity into the VAMS. Extra care was paid not to touch the walls of the Eppendorf tube. For extra 2 s or 3 s the tip was held in place, and then removed from the tube, moved and dispensed in a conditioning rack with the help of the VAMS handler. VAMS samples were dried for 2 h in the darkness at 22 ± 1 °C under a static air current suspended and without touching any surfaces.
After 2 h, the dried VAMS was individually submerged in the prefilled wells of an OSTRO® (Waters, Dublin, Ireland) sample preparation plate. The wells were previously filled in with 200 μL extraction solvent containing IOV as internal standard at a concentration level of 4 μg/mL. The VAMS was incubated for 5 min with the extraction solvent after which extraction process was continued. The extraction of the dried VAMS took place using a ThermoMixer C (Eppendorf, Hamburg, Germany) set at 450 rpm and 22 °C for 20 min. The extractions were performed protected from light. The samples were passed through the OSTRO phospholipids removal plate for 5 min. To each extracted sample, 10 μL of a solution containing a mixture of AOX (citric acid, ascorbic acid, DTT - 100 mg/mL each) was added to a final concentration of 5 mg/mL. The samples were then subjected to evaporation at 30 °C up to dryness using a vacuum concentrator (LabConco, Kansas City, MO, USA). Finally, dried samples were resuspended for 15 min using 50 μL of water/MeOH/FA (80/20/0.1; v/v/v) into Agilent micro well plates (Agilent Technologies, Waldbronn, Germany).
2.5. Extraction solution screening
The extraction rates for IOH from dried VAMS were evaluated by screening different types of extraction solutions containing at least one organic modifier (ACN or MeOH) and water in different ratios. Moreover, solvents containing an acidic or basic additive (0.1% FA or 0.1% ammonia) were tested. IOH was spiked in whole blood in order to reach 1 μg/mL concentration. Each extraction solvent was tested in duplicate. In order to select the best extraction solvent, process efficiency was computed. Process efficiency was calculated as a function of the analytical signal in terms of peak areas obtained from extracted VAMS and a calibration curve in neat medium covering different percentages of process efficiency ratios from 10 % to 100 %. On the other hand, the solvents that induced hemolysis or any coloration of the extraction solvent after phospholipids removal were rejected and the subsequent samples were not injected into the LC-MS/MS system.
2.6. short- to medium-term stability
A short- to medium-term stability study of IOH in VAMS was conducted in order to highlight any analyte degradation all along the storage time segment. Whole blood samples were spiked with IOH at two different concentration levels: 1 μg/mL (low level) and 50 μg/mL (high level). Different time points were set as follows: 2, 6, 22, 48, 144, and 288 h. The samples were stored at room temperature at 22 ± 1 °C in the dark under a static-normal air current. Three VAMSs per concentration level and per time point were analyzed and each extracted VAMS was injected twice. For each time point, IOH stability was calculated relative to the amount of IOH found in the samples stored for the lowest drying time, i.e. 2 h. After 12 days (288 h) all samples were analyzed at the same time.
Moreover, the stability of the dried extracts into the freezer at -80 °C for 12 days was assessed. For this purpose, three VAMSs for each concentration level were dried for 2 h, extracted, filtered through Ostro plate and vacuum dried in order to remove the solvent. The dried extracts were stored at –80 °C for 12 days after which they were analyzed. IOH stability was computed relative to the amount of IOH found for the 2 h dried VAMS.
2.7. Matrix effect
Matrix effects were evaluated at four IOH concentration levels, i.e. 1, 50, 250 and 500 μg/mL in whole blood. Three types of parameters were calculated according to Matuszewski et al. [25] approach, namely matrix effect (ME), process efficiency (PE) and extraction recovery (ER). Neat standard solutions (A) using water/MeOH/FA (80/20/0.1; v/v/v) as solvent, at the aforementioned concentration levels, were prepared. Post-extraction spiked matrices (B) were prepared by adding to the extracted blank blood sample solutions containing IOH in water/MeOH/FA (80/20/0.1; v/v/v) at the appropriate concentrations. Pre-extraction spiked samples (C) were prepared by extracting IOH spiked blood VAMS and reconstituting the extracts in water/MeOH/FA (80/20/0.1; v/v/v). Each type of sample was prepared in duplicate. Following LC-MS/MS analysis integrated averaged peak areas were used to calculate: ME = B/A*100; ER = C/B*100 and PE = C/A*100 [25].
2.8. HemaPEN studies
HemaPEN experiments were also conducted in order to determine concentration-response relationship (domain comprised between 1 and 250 μg/mL IOH spiked in whole blood) and intra-device-repeatability assessment for six different concentration levels. Extraction recovery rates studies for IOH at three different concentration levels, 1, 10 and 100 μg/mL, were performed. Sample preparation protocol was the same as for VAMS using the same extraction solvent. Filters extractions were accomplished using 500 μL Agilent Technologies well plates and Eppendorf ThermoMixer after which extracted samples were transferred to Ostro plates where they were passed through, evaporated and resuspended in water/MeOH/FA (80/20/0.1; v/v/v).
2.9. Method validation
The entire analytical process was subjected to validation [26]. Selectivity, limit of detection (LOD), lower limit of quantification (LLOQ), carryover, calibration curve, linearity, trueness, repeatability, intermediate precision, overall accuracy and analyte stability were tested according to FDA’s bioanalytical method validation guidance. All parameters were computed using e-noval 4.1 software application (Pharmalex-Arlenda, Belgium).
Selectivity was tested on blanks, unspiked samples provided from different sources of blood matrix. The presence of interferences was assessed for both IOH and IOV. LOD and LLOQ were automatically computed by the software. LOD concentration level was established according to the following ratio, LOD=LOQ/3.3. LLOQ was established at 1 μg/mL representing the first level of the dosing range. Three calibration series prepared in three different days were achieved. Each series comprised seven calibration levels, each calibration level being extracted twice. Next, two injections per extraction and per calibration level were performed. Concentration response relationship was evaluated for concentration levels comprised between 1 μg/mL and 500 μg/mL. In order to assess the trueness, precision and overall accuracy, three independent series were prepared in three different days. Each series comprised four concentration levels, from 1 μg/mL to 250 μg/mL, with five replicates per concentration level. Each replicate was further injected twice. The overall accuracy was evaluated by taking into consideration the total error meaning the sum of all systematic and random errors.
Autosampler analyte stability was assessed at three different concentration levels (1, 25, and 500 μg/mL, respectively). For each concentration level two extractions were performed and each extraction was injected twice. Samples were stored at 4 °C in the autosampler and were injected immediately after sample preparation ended, this time moment being set as T0. Afterwards, samples were re-injected after 24 h (T24) and 48 h (T48), respectively. Stability of the samples was calculated relative to the analytical signals obtained for the T0 injected samples.
3. Results and discussion
3.1. LC-MS/MS analysis method
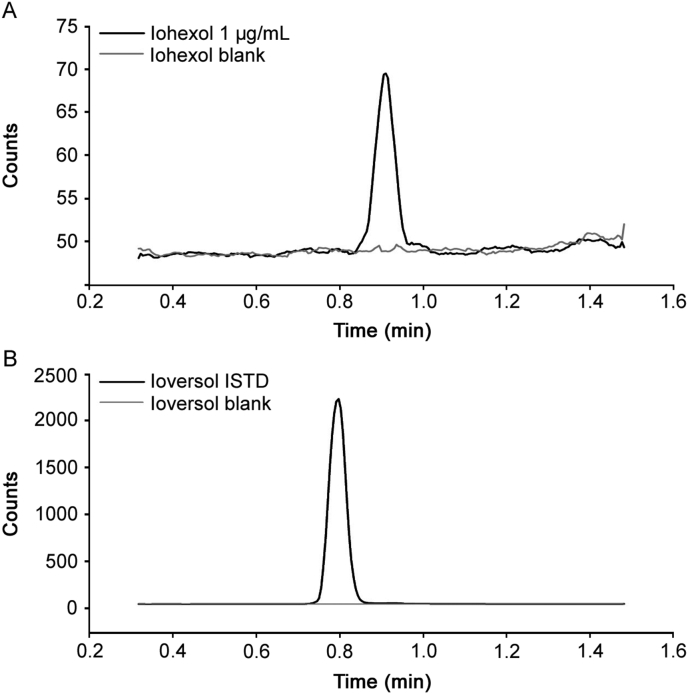
The nature of the solution used to solubilize IOH after sample preparation is of high importance since it may affect analyte retention and peak shape. When using a solution rich in organic modifier (80 % ACN or 80 % MeOH), IOH was not retained on the stationary phase. In order to provide analyte retention, a solution composed of 80 % water and 20 % MeOH with 0.1 % FA was used to retain both IOH and IOV on the stationary phase. As shown in Fig. 1, the optimized mobile phase gradient was able to separate IOH and IOV in less than 1 min.
Fig. 1.
Iohexol (IOH) and ioversol (IOV) representative chromatograms: (A) chromatogram of IOH spiked blood at 1 μg/mL, and blank blood; (B) chromatogram of IOV ISTD spiked in the extraction solvent, and blank blood.
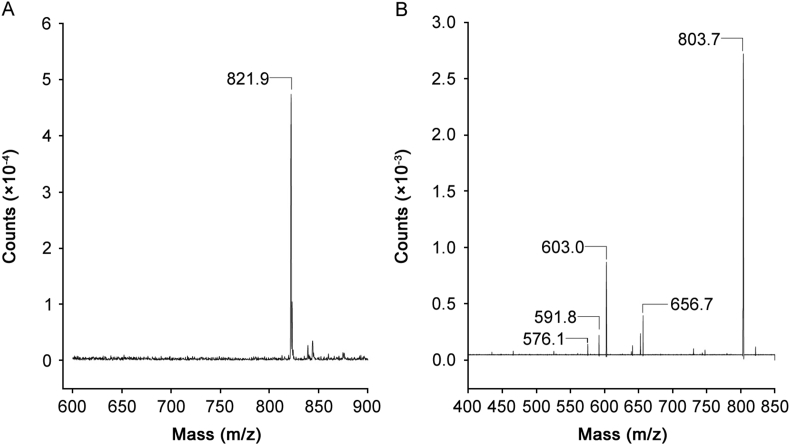
Two MRM transitions were chosen for IOH according to the fragmentation pattern, one being used as quantifier transition and the other as qualifier transition. As shown in Fig. 2, the two most intense fragments of the parent ion IOH 821.9 m/z were obtained after the following transitions, IOH 821.9 (+) m/z→803.7 (+) m/z; 821.9 (+) m/z→603.0 (+) m/z. The most intense transition corresponds to the loss of a water molecule.
Fig. 2.
IOH mass spectra: (A) IOH parent ion 821.9m/z; (B) product ions of IOH 821.9m/z positive ionization mode.
3.2. VAMS extraction optimisation
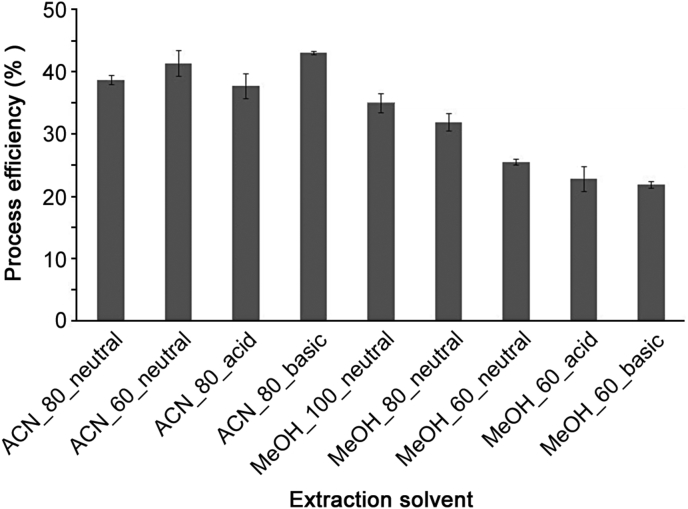
The extraction solution has to be carefully chosen, as it is responsible for analyte extraction from the VAMS sample. A total of 18 different extraction solutions were tested by assessing the process efficiency of the analyte extraction (Table 1). Out of 18 solvents only 12 extraction mixtures were injected into the LC-MS/MS system. Indeed, as can be seen in Table 1, six gave colored extracts or extracts with residues. Using 100 % ACN (neutral, acid or basic) no chromatographic peak was detected, suggesting a total protein precipitation that might have trapped the analytes inside the VAMS. For nine extraction solutions, process efficiency (%) was computed (Fig. 3). Even though the basic solution containing 80 % ACN +20 % water was found to yield the highest process efficiency, it was not chosen since Ostro plates usually do not stand pH above 10. Finally the neutral solution containing 60 % ACN was chosen as suitable extraction medium for VAMS.
Table 1.
VAMS extraction solvent screening.
| Solvent type | Coloration | Residue presence | LC-MS/MS injection |
|---|---|---|---|
| ACN 100 % Neutral | Colorless-Transparent | No | Yes |
| ACN 80 % + 20 % H2O Neutral | Colorless-Transparent | No | Yes |
| ACN 60 % + 40 % H2O Neutral | Colorless-Transparent | No | Yes |
| ACN 100 % + FA 0.1 % | Colorless-Transparent | No | Yes |
| ACN 80 % + 20 % H2O + FA 0.1 % | Colorless-Transparent | No | Yes |
| ACN 60 % + 40 % H2O + FA 0.1 % | Yellow-Red | No | No |
| ACN 100 % + NH3 0.1 % | Colorless-Transparent | No | Yes |
| ACN 80 % + 20 % H2O + NH3 0.1 % | Colorless-Transparent | No | Yes |
| ACN 60 % + 40 % H2O + NH3 0.1 % |
Yellow-Red |
Yes |
No |
| MeOH 100 % Neutral | Colorless-Transparent | No | Yes |
| MeOH 80 % + 20 % H2O Neutral | Colorless-Transparent | No | Yes |
| MeOH 60 % + 40 % H2O Neutral | Colorless-Transparent | No | Yes |
| MeOH 100 % + FA 0.1 % | Yellow-Red | Yes | No |
| MeOH 80 % + 20 % H2O + FA 0.1 % | Yellow-Red | Yes | No |
| MeOH 60 % + 40 % H2O + FA 0.1 % | Colorless-Transparent | No | Yes |
| MeOH 100 % + NH3 0.1 % | Yellow-Red | Yes | No |
| MeOH 80 % + 20 % H2O + NH3 0.1 % | Yellow-Red | Yes | No |
| MeOH 60 % + 40 % H2O + NH3 0.1 % | Colorless-Transparent | No | Yes |
Fig. 3.
Process efficiency rates (%) ± SD for IOH using different extraction solvents - solvent screening (k=2 replicates for each experiment/solvent, n=2 injections for each replicate).
3.3. Short- to medium-term stability
IOH was proved to be stable in dried blood VAMS matrix for at least 22 h. After 22 h and up until 12 days, the degradation of IOH in the dried blood VAMS occurred but was minimum, i.e. only 9 % decrease in recovery rate. Those results were similar for both concentration levels tested (low level 1 μg/mL and high level 50 μg/mL). This short- to medium-term stability study confirms the applicability of the VAMS devices in the context of GFR or pharmacokinetic profile assessment of IOH. Usually, GFR evaluation protocol using IOH clearance rates involves repeated venous blood collection from the patient treated a priori with an in bolus dose of IOH. Samples are thus collected each hour with a recurrence of up to 8 h. VAMS samples can either be stored in some conditions until analysis (darkness, room temperature, static air current), immediately extracted and analyzed using LC-MS/MS or extracted while dried extracts are kept at –80 °C and further analyzed. It is thus important to assess the stability of the dried extracts in the freezer at –80 °C.
The second part of the stability study consisted in evaluating the stability of IOH in dried extracts obtained after the VAMS processing while the dried extracts being stored for 12 days at –80 °C in the freezer. The recovery rates were computed as relative ratios (%) between the IOH concentrations determined in dried extracts (kept for 12 days at -80 °C) and the IOH concentrations determined in freshly obtained VAMS, dried for 2 h which were immediately processed and injected (used as reference). IOH was stable in dried extracts stored at –80 °C for up to 12 days with an overall recovery rate of 96.5 % ± 11.6 % for low level and 91.6 % ± 14.0 % for high level with no statistical difference relative to reference 2 h concentration level. Thus it is possible to gather a higher amount of samples, process them up to dried extracts level and further store them in the freezer until LC-MS/MS analysis.
3.4. Matrix effects
Whole blood is a very complex matrix composed of different types of compounds such as low molecular weight molecules, sugars, amino acids, lipids; high molecular weight molecules, proteins, and cells being able to interfere with the LC-MS/MS analysis. Given the complexity of the matrix, many compounds may interfere with the ionization process, inducing a high degree of variability and affecting detection and quantitation of the analyte. Matrix effect was tested at four different concentration levels (Table 2). For all tested levels a positive matrix effect was observed, i.e. an ion enhancement occurred.
Table 2.
Iohexol matrix effects, extraction recoveries and process efficiencies (mean ± SD, n=2, k=2, m=4).
| Concentration (μg/mL) | Matrix effects (%) | Extraction recovery (%) | Process efficiency (%) |
|---|---|---|---|
| 1 | 119.8 ± 4.5 | 59.7 ± 0.8 | 71.5 ± 1.2 |
| 5 | 130.7 ± 3.7 | 63.2 ± 0.6 | 82.6 ± 0.4 |
| 100 | 127.5 ± 1.1 | 53.6 ± 0.1 | 68.4 ± 0.2 |
| 250 | 155.1 ± 1.2 | 65.7 ± 0.1 | 107 ± 1.4 |
ER defines the extraction efficiency of the IOH from the dried blood VAMS samples under established conditions. Slight differences in extraction recovery rates for different concentration levels can be observed. This difference may be explained by the fact that the internal standard is not added to the spiked blood but in the extraction solution. Thus, the internal standard is not able to compensate for the extraction rate inconsistencies over different concentration levels. PE is a combination of ME and ER. As shown in Table 2, PE rates were higher than ER for all concentration levels since a positive ME (ionization enhancement) occurred.
3.5. Method validation
3.5.1. Selectivity
In order to evaluate the method selectivity, six different sources of human blood samples were tested. A representative chromatogram for IOH spiked at 1 μg/mL in whole human blood is displayed in Fig. 1 A. There was no interference peak at the retention time of IOH using the chosen MRM transitions. Method selectivity for IOV was also tested. There was no interference peak at the retention time of IOV for the blank (Fig. 1 B).
3.5.2. Calibration curve
Considering that the dosing range was comprised between 1 and 500 μg/mL IOH in whole blood, different calibration models were assessed. A quadratic regression model using a weighting factor of 1/x2 was chosen as it gave the best results in terms of trueness for all calibration points. The equations and correlation coefficients are displayed in Table 3.
Table 3.
Regression & calibration parameters – calibration range 1-500 μg/mL using VAMS devices (n=2, k=2, m=7)
| Series | Regression model - weighting | Slope | Intercept | Quadratic term | r |
|---|---|---|---|---|---|
| 1 | Quadratic 1/X² | 0.01216 | 0.001966 | –0.00000358 | 0.9975 |
| 2 | Quadratic 1/X² | 0.01249 | –0.001099 | –0.00000490 | 0.9956 |
| 3 | Quadratic 1/X² | 0.01280 | 0.0007651 | –0.00000699 | 0.9920 |
3.5.3. Trueness and precision
Trueness and precision results are presented in Table 4. As can be seen in Table 4, relative bias between the theoretical concentrations and the experimental ones never exceeds 7 % for all validation levels. In bioanalysis the maximum acceptable threshold is ±15 %. Precision also complies with the imposed threshold of ±15 % for both intra-day (repeatability) and inter-day precision (intermediate precision). The highest RSD value was 13.67 % for inter-day precision.
Table 4.
Trueness, intra and inter-day precision and accuracy for the quantitation of iohexol using VAMS devices (n=2, k=5, m=4).
| Concentration (μg/mL) | Trueness (%) | Trueness relative bias (%) | Precision (% RSD) |
Accuracy (%) β-expectation tolerance limits | |
|---|---|---|---|---|---|
| Intra-day (k=5) | Inter-day (k=20) | ||||
| 1 | 100.1 | 0.1 | 7.42 | 10.27 | –16.27, 16.47 |
| 5 | 99.54 | –0.46 | 9.77 | 9.78 | –14.06, 13.14 |
| 100 | 97.55 | –2.45 | 11.38 | 13.67 | –22.91, 18.01 |
| 250 | 93.06 | –6.94 | 8.55 | 8.55 | –18.83, 4.949 |
3.5.4. Accuracy and linearity of the results
Accuracy takes into account overall experimental errors composed of systematic and random errors. Thus, accuracy is a combination of trueness and precision. As can be seen in Table 4, beta-expectations tolerance limits never exceed 23 %. The linear regression model that fitted the back-calculated concentrations as a function of the introduced concentrations gave rise to the following equation: Y=1.186 + 0.9310 X. The coefficient of correlation was r=0.9916.
3.5.5. LLOQ, LOD and carryover
LLOQ was set at 1 μg/mL IOH in whole blood since this was the first and lowest concentration level for which adequate accuracy was encountered. LOD was computed by the validation software at a level of 0.303 μg/mL. Indeed, at this concentration level, the signal-to-noise ratio calculated using peak areas was around 3. Carryover was evaluated by injecting processed VAMS samples coming from whole blood spiked with IOH at high concentration levels, namely 250 and 500 μg/mL. Further on, blank samples were injected in order to assess the presence of any chromatographic peak. Due to the exterior and interior rinsing of the needle with the two different solvents, no chromatographic peaks have been detected either for IOH or IOV. In marginal cases small chromatographic peaks could have been detected for IOH but they never exceeded 7.5 % of LLOQ peak intensity.
3.5.6. Autosampler analyte stability
IOH proved to be stable when stored in the autosampler at 4 °C for all three concentration levels tested for at least 48 h. Recovery rates of IOH varied between 94.47 % ± 1.10 % and 107.66 % ± 1.28 % relative to T0.
3.6. HemaPEN studies
The sample preparation protocol for VAMS was used to test HemaPEN devices. A linear regression model (no weighting) was the best to describe the concentration-response relationship over the concentration domain of interest (Table 5). Intra-device repeatability RSDs (%) calculated using relative analytical responses at six different concentration levels, taking into account four replicates (4 filters for each HemaPEN) per concentration level were all bellow 10 %. Those results were considered as very promising. Furthermore, HemaPEN filters can be an example for “less is more” since only 2.74 μL of whole blood per each filter was enough to analyze IOH with extraction recovery rates of more than 80 % for each concentration level, using the same protocol applied to VAMS devices (Table 6).
Table 5.
Regression analysis, repeatability (n=2, k=4, m=6) for HemaPENs.
| Concentration (μg/mL) | Regression model | Equation | R2 | CV (%) |
|---|---|---|---|---|
| 1 | Linear | y=0.007116x + 0.003641 | 0.9989 | 8.09 |
| 5 | 6.93 | |||
| 7.5 | 2.33 | |||
| 20 | 3.88 | |||
| 100 | 3.20 | |||
| 250 | 0.75 |
Table 6.
Extraction recovery rates (%) (n=2, k=4, m=3) for HemaPENs.
| Concentration (μg/mL) | Extraction recovery rates (%) | SD (%) |
|---|---|---|
| 1 | 82.27 | 8.82 |
| 10 | 87.01 | 3.95 |
| 100 | 83.83 | 10.40 |
4. Conclusions
A fully validated analytical method for IOH dosage from VAMS samples was developed. The innovative sample preparation protocol, high throughput and reduced analysis time make this method potentially of interest for IOH analysis from whole blood samples. Medium-term stability of IOH in dried whole blood samples allows remote collection, transportation and delayed analysis, which confirms and highlights the advantages of this kind of sample collection systems. By collecting only 10 μL of whole blood, VAMS devices reduce the invasiveness of the sample collection procedure avoiding recurrent venipuncture. The new HemaPEN device was also tested and seems to be another interesting alternative to VAMS.
However, further investigations on large cohorts using multiple patients and healthy human subjects have to be made in order to establish whether the GFR calculated with the help of IOH dosage from whole blood VAMS are well correlated with the GFR calculated based on IOH dosage from plasma samples. Moreover, this study has to be well harmonized using the same calibration standards and to assess and validate the results in intra- and inter-laboratory studies.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Footnotes
Peer review under responsibility of Xi'an Jiaotong University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jpha.2019.06.003.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Brändström E., Grzegorczyk A., Jacobsson L. GFR measurement with iohexol and 51Cr-EDTA. A comparison of the two favoured GFR markers in Europe. Nephrol. Dial. Transplant. 1998;13:1176–1182. doi: 10.1093/ndt/13.5.1176. [DOI] [PubMed] [Google Scholar]
- 2.Delanaye P., Ebert N., Melsom T. Iohexol plasma clearance for measuring glomerular filtration rate in clinical practice and research: a review. Part 1: how to measure glomerular filtration rate with iohexol? Clin. Kidney J. 2016;9:682–699. doi: 10.1093/ckj/sfw070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Delanaye P., Melsom T., Ebert N. Iohexol plasma clearance for measuring glomerular filtration rate in clinical practice and research: a review. Part 2: why to measure glomerular filtration rate with iohexol? Clin. Kidney J. 2016;9:700–704. doi: 10.1093/ckj/sfw071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luis-Lima S., Gaspari F., Negrín-Mena N. Iohexol plasma clearance simplified by dried blood spot testing. Nephrol. Dial. Transplant. 2018;33:1597–1603. doi: 10.1093/ndt/gfx323. [DOI] [PubMed] [Google Scholar]
- 5.Staples A., Wong C., Schwartz G.J. Iohexol-measured glomerular filtration rate in children and adolescents with chronic kidney disease: a pilot study comparing venous and finger stick methods. Pediatr. Nephrol. 2019;34:459–464. doi: 10.1007/s00467-018-4110-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Castagnet S., Blasco H., Vourc’h P. Routine determination of GFR in renal transplant recipients by HPLC quantification of plasma iohexol Concentrations and comparison with estimated GFR. J. Clin. Lab. Anal. 2012;26:376–383. doi: 10.1002/jcla.21537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Baere S., Smets P., Finch N. Quantitative determination of exo- and endo-iohexol in canine and feline samples using high performance liquid chromatography with ultraviolet detection. J. Pharm. Biomed. Anal. 2012;61:50–56. doi: 10.1016/j.jpba.2011.11.022. [DOI] [PubMed] [Google Scholar]
- 8.Nyssen L., Delanaye P., Le Goff C. A simple LC-MS method for the determination of iohexol and iothalamate in serum, using ioversol as an internal standard. Clin. Chim. Acta. 2016;463:96–102. doi: 10.1016/j.cca.2016.10.021. [DOI] [PubMed] [Google Scholar]
- 9.Van Houcke S.K., Seaux L., Cavalier E. Determination of iohexol and iothalamate in serum and urine by capillary electrophoresis. Electrophoresis. 2016;37:2363–2367. doi: 10.1002/elps.201600084. [DOI] [PubMed] [Google Scholar]
- 10.Brown S.C.W., O’Reilly P.H. Iohexol clearance for the determination of glomerular filtration rate in clinical practice: evidence for a new gold standard. J. Urol. 1991;146:675–679. doi: 10.1016/s0022-5347(17)37891-6. [DOI] [PubMed] [Google Scholar]
- 11.Kok M.G.M., Fillet M. Volumetric absorptive microsampling: current advances and applications. J. Pharm. Biomed. Anal. 2018;147:288–296. doi: 10.1016/j.jpba.2017.07.029. [DOI] [PubMed] [Google Scholar]
- 12.Bjornstad P., Karger A.B., Maahs D.M. Measured GFR in routine clinical practice—the promise of dried blood spots. Adv. Chron. Kidney Dis. 2018;25:76–83. doi: 10.1053/j.ackd.2017.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salvador C., Tøndel C., Mørkrid L. Glomerular filtration rate measured by iohexol clearance: a comparison of venous samples and capillary blood spots. Scand. J. Clin. Lab. Invest. 2015;75:710–716. doi: 10.3109/00365513.2015.1091091. [DOI] [PubMed] [Google Scholar]
- 14.Maahs D.M., Bushman L., Kerr B. A practical method to measure GFR in people with type 1 diabetes. J. Diabet. Complicat. 2014;28:667–673. doi: 10.1016/j.jdiacomp.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 15.Barco S., Castagnola E., Moscatelli A. Volumetric adsorptive microsampling-liquid chromatography tandem mass spectrometry assay for the simultaneous quantification of four antibiotics in human blood: method development, validation and comparison with dried blood spot. J. Pharm. Biomed. Anal. 2017;145:704–710. doi: 10.1016/j.jpba.2017.07.033. [DOI] [PubMed] [Google Scholar]
- 16.Thiry J., Evrard B., Nys G. Sampling only ten microliters of whole blood for the quantification of poorly soluble drugs: itraconazole as case study. J. Chromatogr., A. 2017;1479:161–168. doi: 10.1016/j.chroma.2016.12.009. [DOI] [PubMed] [Google Scholar]
- 17.Protti M., Mandrioli R., Mercolini L. Tutorial: volumetric absorptive microsampling (VAMS) Anal. Chim. Acta. 2019;1046:32–47. doi: 10.1016/j.aca.2018.09.004. [DOI] [PubMed] [Google Scholar]
- 18.Nys G., Gallez A., Kok M.G.M. Whole blood microsampling for the quantitation of estetrol without derivatization by liquid chromatography-tandem mass spectrometry. J. Pharm. Biomed. Anal. 2017;140:258–265. doi: 10.1016/j.jpba.2017.02.060. [DOI] [PubMed] [Google Scholar]
- 19.Thiry J., Kok M.G.M., Collard L. Bioavailability enhancement of itraconazole-based solid dispersions produced by hot melt extrusion in the framework of the Three Rs rule. Eur. J. Pharm. Sci. 2017;99:1–8. doi: 10.1016/j.ejps.2016.12.001. [DOI] [PubMed] [Google Scholar]
- 20.Velghe S., Stove C.P. Volumetric absorptive microsampling as an alternative tool for therapeutic drug monitoring of first-generation anti-epileptic drugs. Anal. Bioanal. Chem. 2018;410:2331–2341. doi: 10.1007/s00216-018-0866-4. [DOI] [PubMed] [Google Scholar]
- 21.Qu Y., Brady K., Apilado R. Capillary blood collected on volumetric absorptive microsampling (VAMS) device for monitoring hydroxychloroquine in rheumatoid arthritis patients. J. Pharm. Biomed. Anal. 2017;140:334–341. doi: 10.1016/j.jpba.2017.03.047. [DOI] [PubMed] [Google Scholar]
- 22.Protti M., Vignali A., Sanchez Blanco T. Enantioseparation and determination of asenapine in biological fluid micromatrices by HPLC with diode array detection. J. Sep. Sci. 2018;41:1257–1265. doi: 10.1002/jssc.201701315. [DOI] [PubMed] [Google Scholar]
- 23.Trajan scientific and medical. 2019. https://www.trajanscimed.com/pages/microsampling
- 24.Enderle Y., Foerster K., Burhenne J. Clinical feasibility of dried blood spots: analytics, validation, and applications. J. Pharm. Biomed. Anal. 2016;130:231–243. doi: 10.1016/j.jpba.2016.06.026. [DOI] [PubMed] [Google Scholar]
- 25.Matuszewski B.K., Constanzer M.L., Chavez-Eng C.M. Strategies for the assessment of matrix effect in quantitative bioanalytical methods based on HPLC – MS/MS. Anal. Chem. 2003;75:3019–3030. doi: 10.1021/ac020361s. Anal. Chem. 75 (2003) 3019–3030. [DOI] [PubMed] [Google Scholar]
- 26.Bioanalytical Method Validation Guidance for Industry, FDA. <https://www.fda.gov/downloads/drugs/guidances/ucm070107.pdf>.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.