Abstract
Capillary electrochromatography (CEC) is a micro-scale separation technique which is a hybrid between capillary electrophoresis (CE) and liquid chromatography (LC). CEC can be performed in packed, monolithic and open-tubular columns. In recent three years (from 2016 to 2018), enormous attention for CEC has been the development of novel stationary phases. This review mainly covers the development of novel stationary phases for open-tubular and monolithic columns. In particular, some biomaterials attracted increasing interest. There are no significant breakthroughs in technology and principles in CEC. The typical CEC applications, especially chiral separations are described.
Keywords: Capillary electrochromatography, Open-tubular column, Monolithic column, Novel stationary phases, Separation modes
1. Introduction
Capillary electrochromatography (CEC) is the dynamic integration of high efficiency of capillary electrophoresis (CE) and high selectivity of high performance liquid chromatography (HPLC) [1,2]. The diversity of the stationary phases makes CEC more effective separation selectivity [[3], [4], [5], [6], [7]] and the inhibition of the flat flow profile generated in CEC for the peak broadening is beneficial for the improvement of resolution [8]. In the past decades, CEC has been generally recognized as a powerful electrokinetic separation technique due to its superiority. The core of CEC is the development of column technology which comprises packed, monolithic and open-tubular columns. Compare to packed columns, monolithic and open-tubular columns are always prevalent in CEC owing to the inherent advantages of simple preparation and fritless design. Packed column is decreasingly reported in recent years.
Open-tubular columns are extremely suitable for the introduction of innovative materials as the stationary phases [7,9]. The novel materials are grouped as microporous materials, nanoparticles and biomaterials. The acceptable chromatographic performance deriving from these materials makes open-tubular columns more promising. However, the shortcomings of low phase ratios and sample capacity of open-tubular columns are the limiting factors for some applications [5,6]. Monolithic columns overcome the shortcomings of open-tubular columns and possess higher efficiency and resolution. The monolithic stationary phases have become one of major formats of CEC with the advantages of simplicity of preparation and well controlled porous properties and surface chemistries [3,4]. Advances in column technology propel the development of CEC.
In CEC, the uncharged and charged substances can be separated based on the interactions between solutes and the stationary phases or a combination of such interactions and inherent electrophoretic mobility of the solutes [10,11]. The separation modes have been mainly classified into reversed-phase, ion-exchange, hydrophilic partitioning, chiral recognition and ligand affinity behavior. CEC has been applied for the analysis of biomolecules like proteins, peptides, amino acids, chiral separations and the analysis of pharmaceutical and their metabolites in a series of matrices. The abundant applications are the fundament of attracting increasing attention for CEC.
This review presents an overview of the development in CEC during the period from 2016 to 2018, according to 54 primary research papers. Open-tubular columns are the main trend in CEC. The introduction of novel materials as stationary phases propels the development of open-tubular and monolithic columns. However, there is no progress in the separation modes. The extensive applications indicate that the intrinsic advantages of CEC are widely recognized.
2. Open-tubular column
Open-tubular columns provide a very feasible pattern for novel materials to be used as stationary phases in CEC. The successful employment of various stationary phases possessing acceptable chromatographic selectivity continues to be the driving motivation in the development of open-tubular columns. In recent three years, numerous materials have been applied in open-tubular capillary electrochromatography (OT-CEC). These materials are classified as microporous materials, nanoparticles, polymer-based materials, silica-based materials and biomaterials.
2.1. Microporous materials
Microporous materials reported in this review include metal organic frameworks (MOFs), covalent organic frameworks (COFs), porous organic cages (POCs) and knitted aromatic polymers (KAPs), which are prevalent in OT-CEC due to their abundant porous structure and favourable chromatographic performance.
2.1.1. Metal organic frameworks (MOFs)
MOFs, a class of coordination polymers, consist of metal ions and organic units. MOFs have been employed as stationary phases in chromatography due to their distinctive advantages, such as outstanding porosity, large surface area and controllable surface chemical properties. By changing the building blocks, thousands of MOFs are synthesized for different applications. MOF-5, MOF-180, HKUST-1 (Hong Kong University of Science and Technology-1), UiO-66-NH2 (University of Oslo-66-NH2), ZIF-8 (zeolite imidazolate framework-8) and chiral MOFs draw continuous attention and have been widely applied in CEC.
Chen’s group developed MOF-5 [12], MOF-180 [13], UiO-66-NH2 [14] and ZIF-8 [15] as stationary phases in OT-CEC for the separation of small organic molecules. These MOFs were immobilized on the inner wall of COOH-terminated or polydopamine-coated capillary by liquid-phase epitaxy growth method. MOF-5 is made of Zn2+ and organic ligands 1,4-benzenedicarboxylic acid. The procedure of the immobilization of MOF-5 on the inner wall of the capillary is shown in Fig. 1. The framework of MOF-5 possesses large amount of the structures of octahedral Zn–O–C clusters linking with dicarboxylate benzene struts, which is good for the separation of the substances with benzene structures. MOF-180 is built by the octahedral Zn4O(CO2)6 and 4,4′,4″-[benzene-1,3,5-triyl-tris(ethyne-2,1-diyl)] tribenzoate with cage size 15 × 23 Å. The size of the pore structures was proved to be beneficial for the separation of analytes according to molecular size. UiO-66-NH2, a kind of Zr(IV)-based MOFs, is composed of ZrCl4 and 2-amino-1,4-benzenedicarboxylic acid. The abundant tetrahedral and octahedral cages and excellent pH stability make UiO-66-NH2 material very promising. The resultant open-tubular column with UiO-66-NH2 as stationary phases shows good separation performance for chlorobenzenes, phenoxyacids and phenols. Zeolite imidazolate frameworks (ZIFs) are a subclass of MOFs. ZIF-8 is synthesized with ZnCl2 and 2-methylimidazole by Zn-Im-Zn links. The similarity of the structure of ZIF-8 with zeolites makes ZIF-8 have high chemical stability. Polydopamine is a good support for the growth of ZIF-8 on the inner wall of capillary. The successful separation of phenols demonstrates the powerful chromatography performance of ZIF-8. Compared with MOF-5 and MOF-180, UiO-66-NH2 and ZIF-8 show more outstanding pH stability. The separation performance of these materials is powerful. Various analytes are separated successfully based on the reserved-phase mechanism. In particular, size exclusion of MOF-180 contributes to the chromatographic separations. These materials are promising materials as stationary phase in CEC separations.
Fig. 1.
Schematic of the growth of MOF-5 onto inner surface of fused-silica capillary.
Reprinted from [12], with permission from Elsevier [OR APPLICABLE SOCIETY COPYRIGHT OWNER]. License Number 4542281434189.
Pan et al. [16] also developed a one-dimensional open-tubular column with ZIF-8 as stationary phases for simultaneous separation of neutral and cationic analytes. Qu and co-workers [17] synthesized a ZIF-8 modified open-tubular column by a layer-by-layer approach in order to improve the phase-ratio. The size of the ZIF-8 crystals became larger with the increase of growth cycles, which was beneficial for enhancing the phase-ratio. This ZIF-8 modified open-tubular column achieved the separation of the phenolic isomers. The HKUST-1 materials were used by Xu and co-workers [18] as stationary phases through a layer-by-layer self-assembly strategy for OT-CEC. Compared to ZIF-8 crystals grafted on the inner wall of open-tubular column by a layer-by-layer method, the size of the HKUST-1 crystals was not obviously influenced with the increase of growth cycles but the quantity of HKUST-1 crystals increased. Layer-by-layer strategy is beneficial for the increase of the amount of the MOF stationary phases, which can improve the phase-ratio and column capacity.
The chiral MOF [Cu(mal)(bpy)]·H2O was synthesized with Cu2+ and organic linkers malic acid (mal) and 4,4-bipyridyl (byp). It was used as chiral stationary phases for the enantioseparation of chiral drugs including diastereoisomers ephedrine hydrochloride and pseudoephedrine hydrochloride, as well as enantiomers d-penicillamine and l-penicillamine, d-phenylalanine and l-phenylalanine by Ma and co-workers [19]. This MOF material was first modified with silane reagent 3-aminopropyltriethoxysilane (APTES), then covalent bonded on the inner wall of capillary. Moreover, it has been applied in the chiral separation of ephedrine and pseudoephedrine in the extractive of traditional Chinese medicine ephedra. Wang and co-workers [20] also employed the chiral MOF [Cu(mal)(bpy)]·H2O as stationary phases for the capillary electrochromatographic determination of cephalosporin antibiotics cefapirin, ceftiofur and cefixime. The same material, MOF [Cu(mal)(bpy)]·H2O, can be used as a chiral stationary phase and an achiral stationary phase, which indicates the wide application of chiral MOFs.
JLU-Liu23 is a homochiral zeolite-like metal-organic framework with unique DNA like double-helicity structure and permanent chiral porosity [21]. Pan et al. [22] were the first to introduce this chiral material as stationary phases in OT-CEC for the enantioseparation of chiral monoamine neurotransmitters and analogues. As shown in Fig. 2, excellent chiral separations were obtained. JLU-Liu23 was formed with CuI, 1,3-bis(2-benzimidazol)benzene and 1,4-diazabicyclo[2.2.2]-octane, possessing two interpenetrated chiral structures which are chiral three-periodic zeolitic framework and chiral DNA-like double-helical chain, respectively. The specific chiral structures made great contribution to the enantiomer separation. Pan et al. [23] also developed an open-tubular column with homochiral MOF [Zn(s-nip)2]n as chiral stationary phases which was immobilized on the inner wall of capillary by in situ growth method using ZnO as nucleating agents. The homochiral MOF [Zn(s-nip)2]n could perfectly separate the model analytes such as the enantiomers of three monoamine neurotransmitters, the diastereoisomers of ephedrine and pseudoephedrine, the isomers of nitrophenols and analogues of bisphenols. Using ZnO as nucleating agents provides a new strategy for the growth of MOFs on the inner wall of capillary. The chiral separation ability of JLU-Liu23 and [Zn(s-nip)2]n for chiral monoamine neurotransmitters is similar, and the separation mechanism is mainly based on chiral microenvironment. In particular, [Zn(s-nip)2]n is also suitable for the separation of positional isomers.
Fig. 2.
SEM image of the homochiral MOF JLU-Liu23 coated capillary column (A). The electropherograms of the model chiral analytes under optimum conditions (B). Separation conditions: 15% (v/v) methanol, 10 mM borate; injection: 0.5 psi, 3 s; applied voltage: 15 kV; detection wavelength: 214 nm; capillary: 75 μm i.d., 50.2 cm total length (40 cm effective length); capillary temperature: 20 °C .
Reprinted from [21], with permission from Journal of the American Chemical Society. Copyright (2016) American Chemical Society.
2.1.2. Covalent organic frameworks (COFs)
COFs are a kind of covalent porous polymers with precisely integrated and formed sequence. The structure of COFs is mainly built by covalent bond including B–O–B, B–C–O, C N and N N. These materials have been widely used in chromatography field because of the advantages of high surface area, large amount of cavities and unique chemistry property.
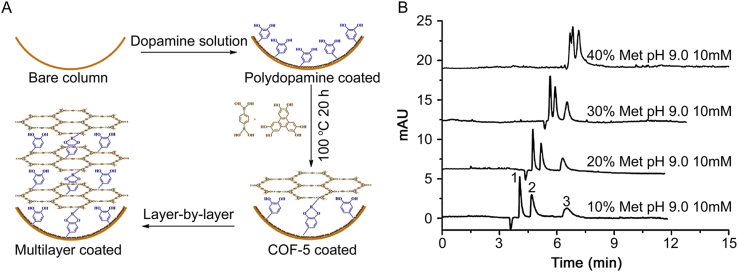
COF-5 is synthesized with 1,4-diboronic acid (BDBA) and 2,3,6,7,10,11-hexahydroxytriphenylene (HHTP) through the dehydration reaction between BDBA and HHTP [24]. It belongs to boron-based COFs. Chen’s group [25] were the first to use COF-5 as novel stationary phases for the separation of neutral, acidic and basic analytes (Fig. 3). Through a polydopamine-assisted layer-by-layer method, the prepared open-tubular column modified with COF-5 exhibited more powerful separation selectivity. In the work, COF-5 is firstly used as CEC stationary phases and COF-5 modified column exhibits good reproducibility. The COF-5 modified column is very promising in CEC separations.
Fig. 3.
Schematic of the growth of multilayer COF-5 on the inner wall of polydopamine-coated capillary (A). The separation of alkylbenzenes (B); voltage, 11 kV; injection, 10 mbar × 5 s; UV detection at 210 nm; temperature, 25 °C; peak identities: 1, ethylbenzene; 2, n-propylbenzene; 3, n-butylbenzene. Capillary column: 31.0 cm total length; 22.5 cm effective length, 50 μm i.d. × 360 μm o.d.
Reprinted from [25], with permission from Elsevier [OR APPLICABLE SOCIETY COPYRIGHT OWNER]. License Number 4542300144191.
Covalent organic framework LZU1 (COF-LZU1, LZU1 is for Lanzhou University-1) is an imine-based COF with excellent properties of structural regularity, unusual stability and good porosity, which is formed from p-phenylenediamine and 1,3,5-benzenetricarboxaldehyde [26]. Chen’s group [27] introduced COF-LZU1 into OT-CEC as stationary phases by in situ growth method based on Schiff reaction to separate neutral compounds, amino acids and non-steroidal anti-inflammatory drugs. Niu et al. [28] prepared the COF-LZU1-modified open-tubular column by the ring-opening reaction between the amino group and the epoxy group from the as-synthesized COF-LZU1 and glycidoxypropyltrimethoxysilane (GLYMO) modified on the inner wall of the capillary, respectively. The obtained column achieved the excellent separation of neutral substances including alkylbenzenes and polyaromatic hydrocarbons (PAHs). The in situ growth method is very simple for the COF-LZU1-modified open-tubular column. These two researches complement each other and the chromatographic performance of COF-LZU1 is fully developed.
Schiff base network-1 (SNW-1) is made of 1,4-benzenedialdehyde and melamine through a Schiff base approach. Ye et al. [29] synthesized APTES-modified SNW-1, and then the material was attached onto the inner wall of capillary by covalent bonding method. Due to the π-π and hydrogen-bond interactions provided by SNW-1 crystals, the separations of sulfonamides, cephalosporins, amino acids and parabens were achieved perfectly. The fabrication method of SNW-1-modified column is very universal and good separations are achieved with the better resolution and higher column efficiency than those of the common column.
COFs are a class of promising stationary phases for CEC. The successful applications of various COFs in open tubular column offer more options for the development of CEC. However, for the real sample analysis using these columns, the research is not enough.
2.1.3. Porous organic cages (POCs)
POCs are a class of shape-persistent, three-dimensional organic molecules with permanent cavities. In particular, some cage molecules such as CC2, CC3, CC5, CC9 and CC10 can self-assemble into crystalline materials with permanent porosity through weak intermolecular forces. POCs have good solubility in organic solvents. Among POCs, some are chiral POCs. As new type of microporous materials, they have attracted increasing attention.
Zhang et al. [30] were the first to employ a homochiral POC (CC3-R) as stationary phases in OT-CEC by a static coating method. The prepared column was suitable for the separation of chiral compounds and positional isomers comprising furoin, benzoin and alprenlol. The chiral recognition might be attributed to host-guest inclusion interactions, chiral steric fit of the analytes in the cage cavities, π-π interactions, dipole-dipole interactions and van der Waals forces. The mechanism is complex and cannot be elucidated explicitly. Compared with chiral MOFs, POCs are soluble in common organic solvents. Therefore, POCs can be coated onto substrates to form membranes by a static method. The POCs materials broaden the sources of chiral stationary phases.
2.1.4. Knitted aromatic polymers (KAPs)
KAPs are constructed with aromatic compounds like benzene or biphenyl which were connected with rigid methylene bridges from the cross-linker formaldehyde dimethyl acetal (FDA) through the Friedel-Crafts reaction [31]. The method is like a “knitting” process without requirement for monomers with specific functionalities. KAPs are potential reverse-phase materials attracting increasing interests.
Chen’s group [32] developed a new strategy for in situ immobilization of the KAP which is made by the monomers benzenes on the inner wall of capillary. The silylating reagent phenyltrimethoxysilane containing phenyl was taken as the monomer. The monomer could covalent bond on the inner wall of capillary. Then the phenyls were knitted together to form the KAP. The method is simple and convenient. The separation of neutral compounds and small biomolecules was achieved based on the powerful hydrophobicity of the KAP.
2.2. Nanoparticles
Nanoparticles, in the size range of 1–100 nm, have attracted continuous attention in OT-CEC due to their superiorities of enlarged phase patio, good chemical stability and controllable functionality. Fang and co-workers [33] developed the thiols β-cyclodextrins (SH-β-CDs) modified gold nanoparticles (GNPs) as the stationary phases for chiral separations. The GNPs were immobilized on the 3-mercaptopropyl-trimethoxysilane (MPTMS)-modified capillary through a layer-by-layer self-assembly strategy. SH-β-CDs were attached on the GNPs layer by S–Au covalent bonding. Compared to the monolayer GNPs film column, the three-layer GNPs film column showed good enantioseparation performance. Zhou and co-workers [34] employed the SH-β-CDs conjugated polydopamine-gold nanoparticles (PDA–AuNPs) as chiral stationary phases for the separation of seven chiral drugs comprising tryptophan, phenylalanine, histidine, fexofenadine, promethazine, tropicamide and terbutaline. The results were acceptable. Compared to the former work, PDA was introduced into the layer-by-layer self-assembly strategy of GNPs to make the multiplayer GNPs film more firming. Al-Hossaini et al. [35] prepared an open-tubular column coated with polydiallyldimethylammonium chloride-stabilized gold nanoparticles (pDDA-GNPs) as stationary phases for the separation of dynorphin metabolites. Through electrostatic adsorption, the pDDA-GNPs were immobilized on the inner wall of the capillary. The positively charged stationary phases inhibited the adsorption of the positively charged peptides.
Zhang and co-workers [36] immobilized the titanium dioxide (TiO2) nanoparticles on the inner wall of capillary as stationary phases by a PDA technology for the separation of proteins. The hydroxyls from PDA layer could chelate with Ti4+ as the nucleation sites for the graft of TiO2 nanoparticles (Fig. 4). Successful separations of variants of β-lactoglobulin and glycoisoforms of ovalbumin were obtained, which demonstrated the powerful selectivity of TiO2 nanoparticles for the separation of proteins.
Fig. 4.
Schematic illustration of the formation of PDA (A) and TiO2 NPs deposited on the PDA coated OT column (B). Electrochromatograms of separation of proteins in different columns (C): (a) bare capillary; (b) PDA coated OT column; (c) TiO2 coated OT column (LPD 1 h); (d) TiO2@PDA coated OT column (LPD 1 h). Experimental conditions: mobile phase, 40 mM phosphate (pH 9.0); separation voltage, 15 kV; injection, 3.45 × 103 Pa for 5 s; detection wavelength, 214 nm; temperature, 25 °C. Peak identification: (1) ConA, (2) a-Lac, (3) b-Lg, and (4) BSA.
Reprinted from [36], with permission from Elsevier [OR APPLICABLE SOCIETY COPYRIGHT OWNER]. License Number 4542330330355.
Liu and co-workers [37] used fibrous mesoporous silica nanoparticles (fSiO2) modified with polymer (Ploy (2-(dimethylamino) ethyl methacrylate)) (PDMAEMA) as stationary phases for the separation of polycyclic aromatic hydrocarbons (PAHs) and proteins. Qu and co-workers [38] synthesized the graphene oxide-SiO2 hybrid nanoparticles as stationary phases in OT-CEC.
The remarkable feature of nanoparticles is the large surface-to-volume ratio. In OT-CEC, the gold nanoparticles and silica nanoparticles are traditional materials which are beneficial to improving the phase-ratio and column capacity. The successful application of TiO2 nanoparticles broadens the sources of nanoparticle materials.
2.3. Polymer-based materials
Polymer-based stationary phases have been widely applied in OT-CEC due to their advantages of diverse functionalities which can be achieved by changing the monomers. The separation performance of polymer-based stationary phases is convincing. Compared with silica-based materials, the pH stability of polymer-based materials is better.
Sepehrifar et al. [39] prepared a novel stimuli-responsive polymeric (SRP) stationary phase for the separation of acidic and basic compounds in OT-CEC. The stationary phase was a Y-shaped block copolymer with two dissimilar chain compositions (poly(2-dimethylaminoethylmethacrylate)-block-poly(acrylic acid), PDMAEMA-b-PAA). The direction of electroosmotic flow (EOF) could be controlled by changing the pH of the mobile phase.
Polyhedral oligomeric silsesquioxane (POSS) was firstly used by Zhao et al. [40] to prepare new molecularly imprinted polymers (MIPs) for enantiomer separation. The imprinted monolithic stationary phase was synthesized with a mixture of PSS-(1-propylmethacrylate)-heptaisobutyl substituted (MA0702), S-amlodipine (template), methacrylic acid (functional monomer), and 2-methacrylamidopropylmethacrylate (crosslinker) in a binary porogenic system of toluene and isooctane. Higher resolution of the enantiomers including amlodipine, naproxen and zopiclone was achieved using the POSS-based MIPs open-tubular column.
Qi’s group [41] developed a chiral ligand exchange stationary phase poly(maleic anhydride-styrene-methacryloyl-l-arginine methyl ester) for the enantioseparation of d,l-amino acids. The chiral stationary phase showed excellent enantioseparation performance.
Xiao et al. [42] prepared a novel poly(norepinephrine)-modified open-tubular column through the self-polymerization strategy for the separation of basic and acidic proteins including two variants of bovine serum albumin and two variants of β-lactoglobulin. The separations were acceptable and the column showed good repeatability with the run-to-run, day-to-day, and column-to-column relative standard deviations of migration times of proteins less than 3.40%.
2.4. Silica-based materials
Silica-based materials exhibit excellent advantages of good chemical stability and ease of chemical modification, which make these materials prevalent in OT-CEC. However, the scarcity of silylating reagents limits the development of silica-based materials.
Liu et al. [43] developed a highly uniform porous layer open tubular (PLOT) column using a single-step in-situ biphasic reaction which could efficiently achieve the control of the stationary phase properties such as thickness, morphology and distribution of pores. The stationary phase, a porous-layer with ∼240 nm thickness, was modified on the inner wall of the capillary. For the mixture of naphthalene and biphenyl, perfect base-line separation was achieved with resolution > 3.0 and theoretical plate numbers over 6 × 104 m−1. Furthermore, the PLOT column was used for the separation of peptides and PAHs and the results were acceptable.
Svobodová et al. [44] synthesized a silica sol-gel containing porphyrin-brucine conjugate as stationary phases for the analysis of biologically compounds including oligopeptides, nucleobases, nucleosides and nucleotides. Compared to the sol–gel stationary phases just possessing Si–O–Si structure [43], the introduction of porphyrin-brucine conjugate obviously enhances the separation performance. The incorporation of an appropriate modifier into sol–gel stationary phases provides a new idea for the development of silica-based materials.
2.5. Biomaterials
Biomaterials are a class of promising stationary phase materials in OT-CEC owing to their unique structure and properties. The unique interactions between stationary phases and analytes, such as chiral or affinity selectivity, are mainly based on the distinct microenvironments from the biomaterials. The abundant biomaterials promote the development of OT-CEC.
Bovine serum albumin (BSA), a traditional biomaterial, was introduced into open-tubular column as stationary phases by Jia group [45] through an electrostatic self-assembly strategy for the separation of the monoclonal antibodies (mAbs). The same group [46] also employed the fibrin as stationary phase in OT-CEC for the separation of the mAbs. The prepared columns showed powerful separation performance.
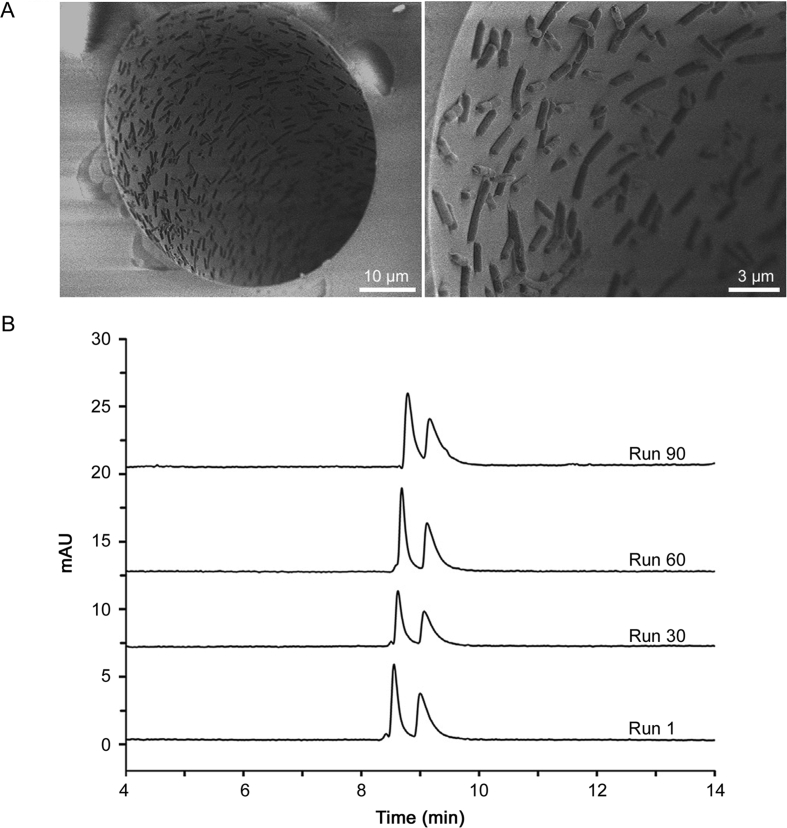
Fu and co-workers [47] were the first to use bacteria (Escherichia coli) as innovative chiral stationary phases in OT-CEC for enantiomer separation (Fig. 5). The bacteria were adhered onto the inner wall of positively charged polyethyleneimine (PEI)-modified capillaries. Because of the intrinsic chirality and chirality-triggered biological processes and behaviors of bacteria, the enantioseparation of fluoroquinolone enantiomers was successfully achieved. The resultant columns were used at least 90 consecutive runs. Some enantiomers are separated, but the peak shape and the resolution are unsatisfying. The results demonstrate the chiral separation selectivity of the Escherichia coli is not powerful. However, this work is very interesting and creative. It seems to be a promising direction for chiral separation in CEC.
Fig. 5.
SEM images of the inner wall of freshly prepared E. coli@capillary (A) (the cross section and the inner wall). Electropherograms of ofloxacin enantiomers of different runs on E. coli@capillary (B). Experimental conditions: back-ground electrolyte, 50 mM phosphate buffer, pH 6.0; applied voltage, 15 kV; UV detection at 280 nm; temperature, 20 °C; capillary column, 48.5 cm (40 cm effective length) × 50 mm i.d.
Reprinted from [47], with permission from Elsevier [OR APPLICABLE SOCIETY COPYRIGHT OWNER]. License Number 4542301230546.
3. Monolithic column
Monolithic columns are a convenient and efficient column type in CEC with large column capacity, high selectivity and high efficiency. Various monolithic columns have been developed by changing the functional monomers and cross-linkers. Taking account of unsystematic diversity of the monomers and cross-linkers, it is suitable to classify the monolithic columns into two groups, i.e. direct copolymerization and incorporation strategy according to the fabrication method of monolithic stationary phases.
3.1. Direct copolymerization
Deep eutectic solvents (DESs), analogues of ionic liquids, are novel green solvents synthesized with quaternary ammonium salts and hydrogen-bond donors. Chen’s group [48] used a DES which consisted of chlorocholine chloride and itaconic acid as functional monomer to prepare a new DES-based monolithic column for CEC (Fig. 6). The obtained monolithic column showed good separation performance for the neutral compounds, phenols, toluidines, nucleosides, nucleotide bases and alkaloids based on the synergistic effect of hydrophobic interaction, hydrogen-bond interaction and electronic interaction. The relative standard deviations (RSDs) of migration time of chlorobenzenes for intra-day, inter-day, column-to-column and batch-to-batch were all less than 3.08%.
Fig. 6.
Schematic illustration for fabrication of DES-based monolithic column by in situ polymerization.
Reprinted from [48], with permission from Elsevier [OR APPLICABLE SOCIETY COPYRIGHT OWNER]. License Number 4542310894790.
The quinine derivative, N-benzylquininium chloride, was firstly combined with acrylamide as monomers by Chen’s group [49] to prepare a novel anion-exchange monolithic column for the electrochromatographic separation of acidic compounds including 2-chlorobenzoic acid, mandelic acid, 4-hydroxybenzoic acid, indole-3-acetic acid, 2-aminoterephthalic acid, 3,5-pyridinedicarboxylic acid, benzoic acid and 4-aminobenzoic acid. Excellent baseline separation of eight acid compounds was achieved with high resolution, high column efficiency and good reproducibility based on the electrophoretic mobility with anion-exchange, π-π stacking and hydrogen bonding interactions.
Chen’s group [50] also prepared a new monolithic column with ionic liquid (1-allyl-methylimidazolium chloride, AlMeIm+Cl−) and styrene as monomers, ethylene dimethacrylate (EDMA) as the cross-linker for CEC. The column efficiency for toluene was 2.70 × 105 m−1. The resultant monolithic column was suitable for the separation of neutral compounds, acidic analytes and phenols. Compared with the DES-based monolithic column, this monolithic column has higher column efficiency and better resolution.
Dixit et al. [51] developed a zirconia hybrid monolithic column modified with carbamoylated azithromycin by a single-step sol-gel approach for enantioseparation of acidic chiral drugs using non-aqueous capillary electrochromatography (NACEC). The obtained monolithic column was applied for the chiral separation of six acidic drugs and the results were acceptable.
Zhang et al. [52] synthesized a novel hybrid monolithic column with the ionic liquid (1-butyl-3-vinylimidazolium-bis[(trifluoromethyl)sulfonyl]imide), VBIMNTF2) as the monomer and a methacryl substituted polyhedral oligomeric silsesquioxane (POSS-MA) as the cross-linker by photoinitiated free-radical polymerization. The fabrication process can be finished within 7 min. Successful electrochromatographic separations of alkylbenzenes, phenols, anilines and PAHs were achieved based on the powerful selectivity from the combination of hydrophobic interactions, π-π stacking, electrostatic interaction and electrophoretic mobility.
Wang et al. [53] fabricated an ionic liquid (IL)-bonded multifunctional monolithic column through the in-situ polycondensation of urea-formaldehyde (UF) and a lab-made acylamino-functionalized IL (1-acetylamino-propyl-3-methylimidazolium bromide, [AAPMIm]Br). The monoliths were rapidly synthesized within 10 min, possessing satisfactory permeability and mechanical stability. Phenols, benzoic acid and its homologues, and enkephalins were perfectly separated because of the presence of multiple retention mechanisms including hydrophilic interaction, hydrogen bond, anion-exchange and cation-exclude interactions.
Vergara-Barberán et al. [54] synthesized a series of monolithic columns using poly(ethylene glycol) diacrylates (PEGDA, Mn 250, 575 and 700) as single monomers in a binary porogen solvent of methanol and ethyl ether. By comparing the chromatographic performance of these columns, the PEGDA 700 monolithic column exhibited the best separation performance for alkylbenzenes, organophosphorous pesticides (OPPs), benzoic acid derivatives and sulfonamides (Fig. 7). For sulfonamides, the highest column efficiency was up to 144000 m−1.
Fig. 7.
SEM micrograph of the PEGDA 700 based monolithic column (A); CEC separation of OPPs on a PEGDA 700 monolithic column (B). CEC conditions: mobile phase, 50:50 (v/v) ACN/water containing 5 mM acetic/acetate buffer at pH 3.0; electrokinetic injection, 10 kV × 7 s; voltage, − 20 kV. Peak identification: 1, uracil; 2, fensulfothion; 3, malathion; 4, prophenofos; 5, dialifos; 6, chlorpyrifos; and 7, sulprofos.
Reprinted from [54], with permission from John Wiley and Sons [OR APPLICABLE SOCIETY COPYRIGHT OWNER]. License Number 4542340477967.
Murauer et al. [55] prepared a monolithic column with the monomer 1-vinylimidazole and the dicationic crosslinker 3,3’-(hexane-1,6-diyl)bis(1-vinylimidazolium) bromide, in a ternary porogen system of 1-propanol, 1-decanol and water for the pressured electrochromatographic separation of methylxanthines. Murauer et al. [56] also developed a novel zwitterionic monolithic column for the separation of phenolic acids in coffee bean extracts in CEC. Qiu et al. [57] developed a phosphorylcholine-based hydrophilic monolithic column to determine the impurities of a positively charged drug pramipexole.
3.2. Incorporation strategy
Incorporation strategy is very convenient for the introduction of novel materials without double bond such as MOFs, nanoparticles and carbon nanotubes as stationary phases in monolithic columns. It promotes the application of novel materials in monolithic column for CEC.
Ganewatta and co-workers [58] synthesized two types of monolithic stationary phases including poly(glycidyl methacrylate-co-ethylene glycol dimethacrylate) monolith (poly(GMA-co-EDMA)) and a diol derivative of the poly(GMA-co-EDMA) monolith incorporated with hydroxyl functionalized multiwalled carbon nanotubes (OH-MWCNTs), respectively. The OH-MWCNTs were covalently and/or physically entrapped into these two kinds of poly(GMA-co-EDMA) monolithic columns. Successful separations of alkylbenzenes, toluene derivatives, aniline compounds, phenols and polyaromatic hydrocarbons were achieved. Ganewatta and co-workers [59] also prepared a monolithic column poly(glyceryl monomethacrylate-co-ethylene glycol dimethacrylate) (poly(GMM-co-EDMA)) incorporated with the gluconamide-modified fumed silica nanoparticles (FSNPs) for the hydrophilic electrochromatographic separation of small polar solutes, phenols and nucleobases. The FSNPs were modified with N-(3-triethoxysilylpropyl)gluconamide through pre-column or on-column functionalization reactions which were corresponded to p-gluconamide-FSNPs or o-gluconamide-FSNPs, respectively. The monolithic column incorporated with o-gluconamide-FSNPs showed more powerful selectivity than those columns incorporated with p-gluconamide-FSNPs.
Mesoporous silica nanoparticles modified with amino groups (NH2-MSN) were used by Xu and co-workers [60] to prepare a poly(GMA-EDMA-NH2-MSN) monolith (Fig. 8). The surface of the monolithic stationary phases was functionalized with pepsin as chiral selector. The resultant monolithic column was applied for the enantioseparation of fifteen basic chiral drugs where nine drugs were baseline resolved and six drugs were partly separated. Furthermore, compared with the monolithic column without NH2-MSN, the resultant monolithic column with NH2-MSN significantly improved the chiral separation selectivity for all analytes. Miao and co-workers [61] immobilized pepsin on the surface of the poly(GMA-co-EDMA) monolith incorporated with carboxylated single-walled carbon nanotubes (c-SWNTs) for the enantioseparation of ten basic chiral drugs.
Fig. 8.
Scheme for the preparation of poly(GMA-EDMA-NH2-MSN) monolithic column (A) and pepsin immobilization (B).
Reprinted from [60], with permission from Elsevier [OR APPLICABLE SOCIETY COPYRIGHT OWNER]. License Number 4542341348775.
Chen’s group [62] developed an ionic liquid (AlMeIm+Cl−) polymer monolithic column incorporated with ZIF-8 as stationary phases for enhancing reversed electrochromatographic separation of neutral compounds, anilines and phenols. The successful separations were attributed to powerful synergistic effect derived from the same imidazole ring structure of ionic liquid and organic ligands of ZIF-8. Carrasco-Correa and co-workers [63] synthesized a methacrylate-based monolith incorporated with ZIF-8-derived nanoporous carbons for the separations of PAHs and non-steroidal anti-inflammatory drugs in CEC.
Lanthanide metal-organic frameworks NKU-1, [Eu2(ABTC)1.5(H2O)3(DMA)] (NKU-1), synthesized with Eu(III) ions and 3,3′,5,5′-azo benzene tetracarboxylic acid ligands was introduced into poly(butyl methacrylate-co-ethylene glycol dimethacrylate) (poly(BMA-co-EDMA)) monolith for the separations of alkylbenzenes, PAHs, aniline series and naphthyl substitutes in CEC [64]. The highest column efficiency of the Ln-MOFs-incorporated monolithic column was 2.1 × 105 m−1.
Zhao et al. [65] used vinylized graphene oxide (GO) and styrene as the monomers and divinylbenzene as the cross-linker to prepare GO-bonded monolithic stationary phases for the separation of neutral and polar compounds. Li and co-workers [66] synthesized the 3-(trimethoxysilyl) propylmethacrylate modified graphene oxide (GO-MPS) and used it as the monomer to prepare a monolithic column in green porogen system of DESs and ILs.
Direct copolymerization is simple and time-saving. Compared with incorporation strategy, the pore structure and surface chemical property of the stationary phases are controllable. Incorporation strategy provides more options in terms of the stationary phases for the preparation of monolithic column. Various stationary phase materials propel the development of CEC.
4. Separation modes
In the development of CEC, the separation modes mainly include reversed-phase, hydrophilic interaction, ion-exchange, mixed-mode and chiral separations. There are no innovative separation modes.
4.1. Reversed-phase separations
Reversed-phase separations are universal in CEC. The hydrophobicity of the stationary phases is the foundation of reversed-phase separations for neutral compounds. In particular, for the separations of the analytes with aromatic groups, π-π stacking interactions also play an important role. In this review, numerous reversed-phase electrochromatographic columns developed for the separation of the compounds with different polarity are summarized.
The organic ligands of the MOFs (MOF-5, MOF-180, UiO-66-NH2, HKUST-1, NKU-1) and COFs (COF-5, COF-LZU1, SNW-1) are the aromatic compounds such as 1,4-benzenedicarboxylic acid [12], 1,3,5-benzenetricarboxlic acid [13,18], 3,3′,5,5′-azo benzene tetracarboxylic acid [64], 2-amino-1,4-benzenedicarboxylic acid [14], 1,4-benzenediboronic acid and 2,3,6,7,10,11-hexahydroxytriphenylene [25], 1,3,5-triformylbenzene and 1,4-diaminobenzene [27,28] as well as 1,4-benzenedialdehyde and melamine [29]. In particular, the imidazole, 2-methylimidazole [[15], [16], [17],62,63] is used as the organic ligand for the synthesis of ZIF-8. Because of the abundant benzene structure or imidazolium rings from these organic ligands, the MOFs and COFs possess powerful hydrophobic and π-π stacking interactions which demonstrate these materials are suitable to be used as reversed-phase stationary phases.
Ganewatta and co-workers [58] used MWCNTs-based stationary phases for the reversed-phase separations of alkylbenzenes, toluene derivatives, anilines, phenols and PAHs, while Li and co-workers [66] applied the GO-functionalized monoliths to separate alkylphenones, alkylbenzenes and the tryptic digests from bovine serum albumin. The highly conjugated π-electron system of MWCNTs and GO provides powerful hydrophobic and π-π stacking interactions with aromatic compounds. Zhao et al. [65] also employed a GO-bonded monolithic stationary phases for the separation of neutral and polar compounds.
Chen’s group [48] used a DES-based monolithic column for the separations of the neutral compounds, phenols, toluidines, nucleosides, nucleotide bases and alkaloids based on the hydrophobic interactions. Murauer et al. [55] prepared vinylimidazole-based monolithic stationary phases for the pressured electrochromatographic separation of the methylxanthines according to their hydrophobicity.
4.2. Hydrophilic interaction separations
Hydrophilic interaction separations are beneficial for the separation of highly polar analytes. Murauer et al. [56] synthesized novel zwitterionic monolithic stationary phases for the separation of phenolic acids. Qiu et al. [57] separated the impurities of a positively charged drug pramipexole using a phosphorylcholine-based hydrophilic monolithic column.
4.3. Ion-exchange separations
Ion-exchange separations, especially anion-exchange separations, have attracted considerable attention in CEC. The anion-exchange separations are an effective approach for the separation of acid compounds, which can overcome the shortcomings of long analysis time or strong retention of solutes. The quinine derivative-based monolithic column was used by Chen’s group [49] to separate eight acidic compounds. The successful separation was attributed to the anion-exchange interaction.
4.4. Mixed-mode separations
Mixed-mode separations are achieved depending on at least two different interactions between the stationary phases and the solutes. It is preferential than single-mode separations due to the strong multiple interactions from the mixed-mode stationary phases. Wang et al. [53] prepared an IL-bonded multifunctional monolithic column with anion-exchange/hydrophilic interaction mixed-mode for the separation of the benzoic acid and its homologues. Chen’s group [50] synthesized a novel monolithic column with IL and styrene bifunctional stationary phases for the separations of neutral compounds, amino acids and phenols under reversed-phase/anion-exchange mixed-mode.
4.5. Chiral separations
In recent three years, the traditional chiral stationary phases, cyclodextrin-based materials [33,34,67], pepsin [60,61], azithromycin [51], molecularly imprinted polymers [40] and ligand exchanger [41] are still employed for the chiral separation in CEC. The chiral selectivity is satisfying. The introduction of novel chiral stationary materials including chiral MOFs [22], POCs [30] and bacteria [47] vastly promoted the development of chiral separations. JLU-Liu23, a chiral MOF, was used to separate chiral monoamine neurotransmitters [22], while the chiral POC (CC3-R) was applied for the enantiomer separations of furoin, benzoin, and alprenlol [30]. In particular, it should be pointed out that Escherichia coli was firstly employed as chiral stationary phases for the separations of ofloxacin and lomefloxacin enantiomers [47].
5. Applications
CEC is a simple and practical technique for drug analysis, environmental analysis, biological sample analysis and chiral separation. The analytes including small organic, biological and chiral molecules have been separated successfully in CEC. The wide applications indicate CEC is very promising.
The separations of small organic molecules consisting of alkylbenzenes [12,13,25,27,28,32,48,50,52,54,58,64,66], PAHs [18,28,37,43,48,52,[63], [64], [65]], chlorobenzenes [13,14,32,50], aromatic acids [13,39,49,53,54], phenols [14,15,17,18,48,50,52,53,58,59,62,68], anilines [12,25,28,39,48,52,62,64], parabens [29] and organophosphorus pesticides [54] have been investigated, while the separations of drugs such as cephalosporin antibiotics [20,29], impurities of pramipexole [57], sulfonamides [29,32,54] and non-steroidal anti-inflammatory drugs [27,32,63], especially the plant natural ingredients including purine alkaloids [55], flavonoids [16] and phenoxyacids [14,56], have been achieved. Biological molecules, amino acids [27,32,50], peptides [32,35,44,53], nucleotides [57], nucleosides [32,44,57], nucleobases [44,59], monoamine neurotransmitters [16] and proteins [[36], [37], [38],42,45,46,69] have also been separated successfully. For the real samples, protein digests [43,66], CEC showed strong separation selectivity. Chiral separation, the research focus in CEC, has been attracting interests of separation scientists. In recent three years, the enantiomer separation of amino acids [19,34,41,69], chiral drugs [23,33,34,47,51,61,67] and monoamine neurotransmitters [19,22,23] have been investigated.
6. Conclusions
In CEC, the development of novel stationary phases has always been the research focus. With the development of materials science, there are amounts of innovative materials, such as MOFs, COFs, POCs, KAPs and DESs. Open-tubular columns and monolithic columns are the excellent and accessible supporter for the evaluation of the chromatographic performance of novel stationary phases, especially open-tubular columns. Furthermore, the introduction of biomaterials, e.g. bacteria, as stationary phases also provides a unique strategy for the development of CEC. The wide applications demonstrate that the employment of new materials as stationary phases is promising. As an advanced analytical technique, CEC has huge development potential.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant Nos. 81872828 and 81573384).
Footnotes
Peer review under responsibility of Xi'an Jiaotong University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jpha.2019.05.002.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Cikalo M.G., Bartle K.D., Robson M.M., et al. Capillary electrochromatography-tutorial review. Analyst. 1998;123:87R–102R. [Google Scholar]
- 2.Colón L.A., Guo Y., Fermier A. Peer reviewed: capillary electrochromatography. Anal. Chem. 1997;69:461A–467A. [Google Scholar]
- 3.Svec F. Recent developments in the field of monolithic stationary phases for capillary electrochromatography. J. Sep. Sci. 2005;28:729–745. doi: 10.1002/jssc.200400086. [DOI] [PubMed] [Google Scholar]
- 4.Svec F., Peters E.C., Sýkora D., et al. Design of the monolithic polymers used in capillary electrochromatography columns. J. Chromatogr. A. 2000;887:3–29. doi: 10.1016/s0021-9673(99)01232-7. [DOI] [PubMed] [Google Scholar]
- 5.Eeltink S., Rozing G.P., Kok W.Th. Recent applications in capillary electrochromatography. Electrophoresis. 2003;24:3935–3961. doi: 10.1002/elps.200305638. [DOI] [PubMed] [Google Scholar]
- 6.Kapnissi-Christodoulou C.P., Zhu X., Warner I.M. Analytical separations in open-tubular capillary electrochromatography. Electrophoresis. 2003;24:3917–3934. doi: 10.1002/elps.200305640. [DOI] [PubMed] [Google Scholar]
- 7.Tarongoy F.M., Jr., Haddad P.R., Boysen R.I., et al. Open tubular-capillary electrochromatography: developments and applications from 2013 to 2015. Electrophoresis. 2016;37:66–85. doi: 10.1002/elps.201500339. [DOI] [PubMed] [Google Scholar]
- 8.Robson M.M., Cikalo M.,G., Myers P., et al. Capillary electrochromatography: a review. J. Microcolumn Sep. 1997;9:357–372. [Google Scholar]
- 9.Tarongoy F.M., Jr., Haddad P.R., Quirino J.P. Recent developments in open tubular capillary electrochromatography from 2016 to 2017. Electrophoresis. 2018;39:34–52. doi: 10.1002/elps.201700280. [DOI] [PubMed] [Google Scholar]
- 10.Altria K.D. Overview of capillary electrophoresis and capillary electrochromatography. J. Chromatogr. A. 1999;856:443–463. doi: 10.1016/s0021-9673(99)00830-4. [DOI] [PubMed] [Google Scholar]
- 11.Bartle K.D., Myers P. Theory of capillary electrochromatography. J. Chromatogr. A. 2001;916:3–23. doi: 10.1016/s0021-9673(01)00709-9. [DOI] [PubMed] [Google Scholar]
- 12.Bao T., Tang P., Mao Z., et al. An immobilized carboxyl containing metal-organic framework-5 stationary phase for open-tubular capillary electrochromatography. Talanta. 2016;154:360–366. doi: 10.1016/j.talanta.2016.03.089. [DOI] [PubMed] [Google Scholar]
- 13.Tang P., Bao T., Chen Z. Novel Zn-based MOFs stationary phase with large pores for capillary electrochromatography. Electrophoresis. 2016;37:2181–2189. doi: 10.1002/elps.201600067. [DOI] [PubMed] [Google Scholar]
- 14.Tang P., Wang R., Chen Z. In situ growth of Zr-based metal-organic framework UiO-66-NH2 for open-tubular capillary electrochromatography. Electrophoresis. 2018;39:2619–2625. doi: 10.1002/elps.201800057. [DOI] [PubMed] [Google Scholar]
- 15.Li Y., Bao T., Chen Z. Polydopamine-assisted immobilization of zeolitic imidazolate framework-8 for open-tubular capillary electrochromatography. J. Sep. Sci. 2017;40:954–961. doi: 10.1002/jssc.201601152. [DOI] [PubMed] [Google Scholar]
- 16.Pan C., Lv W., Wang G., et al. Simultaneous separation of neutral and cationic analytes by one dimensional open tubular capillary electrochromatography using zeolitic imidazolate framework-8 as stationary phase. J. Chromatogr. A. 2017;1484:98–106. doi: 10.1016/j.chroma.2017.01.017. [DOI] [PubMed] [Google Scholar]
- 17.Qu Q., Xuan H., Zhang K., et al. Layer-by-layer assembly of zeolite imidazolate framework-8 as coating material for capillary electrochromatography. Electrophoresis. 2016;37:2175–2180. doi: 10.1002/elps.201600121. [DOI] [PubMed] [Google Scholar]
- 18.Xu Y.-Y., Lv W.-J., Ren C.L., et al. In situ preparation of multilayer coated capillary column with HKUST-1 for separation of neutral small organic molecules by open tubular capillary electrochromatography. J. Chromatogr. A. 2018;1532:223–231. doi: 10.1016/j.chroma.2017.11.064. [DOI] [PubMed] [Google Scholar]
- 19.Ma J., Ye N., Li J. Covalent bonding of homochiral metal-organic framework in capillaries for stereoisomer separation by capillary electrochromatography. Electrophoresis. 2016;37:601–608. doi: 10.1002/elps.201500342. [DOI] [PubMed] [Google Scholar]
- 20.Wang X., An J., Li J., et al. A capillary coated with a metal-organic framework for the capillary electrochromatographic determination of cephalosporins. Microchim. Acta. 2017;184:1345–1351. [Google Scholar]
- 21.Luo X., Cao Y., Wang T., et al. Host-guest chirality interplay: a mutually induced formation of a chiral ZMOF and its double-helix polymer guests. J. Am. Chem. Soc. 2016;138:786–789. doi: 10.1021/jacs.5b12516. [DOI] [PubMed] [Google Scholar]
- 22.Pan C., Lv W., Niu X., et al. Homochiral zeolite-like metal-organic framework with DNA like double-helicity structure as stationary phase for capillary electrochromatography enantioseparation. J. Chromatogr. A. 2018;1541:31–38. doi: 10.1016/j.chroma.2018.02.015. [DOI] [PubMed] [Google Scholar]
- 23.Pan C., Wang W., Chen X. In situ rapid preparation of homochiral metal-organic framework coated column for open tubular capillary electrochromatography. J. Chromatogr. A. 2016;1427:125–133. doi: 10.1016/j.chroma.2015.12.020. [DOI] [PubMed] [Google Scholar]
- 24.Cote A.P., Benin A.I., Ockwig N.W., et al. Porous, crystalline, covalent organic frameworks. Science. 2005;310:1166–1170. doi: 10.1126/science.1120411. [DOI] [PubMed] [Google Scholar]
- 25.Bao T., Tang P., Kong D., et al. Polydopamine-supported immobilization of covalent-organic framework-5 in capillary as stationary phase for electrochromatographic separation. J. Chromatogr. A. 2016;1445:140–148. doi: 10.1016/j.chroma.2016.03.085. [DOI] [PubMed] [Google Scholar]
- 26.Ding S.Y., Gao J., Wang Q., et al. Construction of covalent organic framework for catalysis: Pd/COF-LZU1 in Suzuki-Miyaura coupling reaction. J. Am. Chem. Soc. 2011;133:19816–19822. doi: 10.1021/ja206846p. [DOI] [PubMed] [Google Scholar]
- 27.Kong D., Bao T., Chen Z. In situ synthesis of the imine-based covalent organic framework LZU1 on the inner walls of capillaries for electrochromatographic separation of nonsteroidal drugs and amino acids. Microchim. Acta. 2017;184:1169–1176. [Google Scholar]
- 28.Niu X., Ding S., Wang W., et al. Separation of small organic molecules using covalent organic frameworks-LZU1 as stationary phase by open-tubular capillary electrochromatography. J. Chromatogr. A. 2016;1436:109–117. doi: 10.1016/j.chroma.2016.01.066. [DOI] [PubMed] [Google Scholar]
- 29.Ye N., Wang X., Liu Q., et al. Covalent bonding of Schiff base network-1 as a stationary phase for capillary electrochromatography. Anal. Chim. Acta. 2018;1028:113–120. doi: 10.1016/j.aca.2018.04.037. [DOI] [PubMed] [Google Scholar]
- 30.Zhang J.-H., Zhu P.-J., Xie S.-M., et al. Homochiral porous organic cage used as stationary phase for open tubular capillary electrochromatography. Anal. Chim. Acta. 2018;999:169–175. doi: 10.1016/j.aca.2017.11.021. [DOI] [PubMed] [Google Scholar]
- 31.Li B., Gong R., Wang W., et al. A new strategy to microporous polymers: knitting rigid aromatic building blocks by external cross-linker. Macromolecules. 2011;44:2410–2414. [Google Scholar]
- 32.Tang P., Chen Z. Capillary electrochromatography using knitted aromatic polymer as the stationary phase for the separation of small biomolecules and drugs. Talanta. 2018;178:650–655. doi: 10.1016/j.talanta.2017.10.004. [DOI] [PubMed] [Google Scholar]
- 33.Fang L.-L., Wang P., Wen X.L., et al. Layer-by-layer self-assembly of gold nanoparticles/thiols beta-cyclodextrin coating as the stationary phase for enhanced chiral differentiation in open tubular capillary electrochromatography. Talanta. 2017;167:158–165. doi: 10.1016/j.talanta.2017.01.082. [DOI] [PubMed] [Google Scholar]
- 34.Zhou L., Zhang B., Li S., et al. Enantioselective open-tubular capillary electrochromatography using a β-cyclodextrin–gold nanoparticles–polydopamine coating as a stationary phase. New J. Chem. 2018;42:17250–17258. [Google Scholar]
- 35.Al-Hossaini A.M., Suntornsuk L., Lunte S.M. Separation of dynorphin peptides by capillary electrochromatography using a polydiallyldimethylammonium chloride gold nanoparticle-modified capillary. Electrophoresis. 2016;37:2297–2304. doi: 10.1002/elps.201600006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang Y., Wang W., Ma X., et al. Polydopamine assisted fabrication of titanium oxide nanoparticles modified column for proteins separation by capillary electrochromatography. Anal. Biochem. 2016;512:103–109. doi: 10.1016/j.ab.2016.08.015. [DOI] [PubMed] [Google Scholar]
- 37.Liu Y., Liu Q., Yu H., et al. Polymer-modified fibrous mesoporous silica nanoparticles as coating material for open-tubular capillary electrochromatography. J. Chromatogr. A. 2017;1499:196–202. doi: 10.1016/j.chroma.2017.03.074. [DOI] [PubMed] [Google Scholar]
- 38.Qu Q., Xuan H., Zhang K., et al. Graphene oxide-SiO2 hybrid nanostructure as coating material for capillary electrochromatography. Electrophoresis. 2016;37:1367–1375. doi: 10.1002/elps.201500548. [DOI] [PubMed] [Google Scholar]
- 39.Sepehrifar R., Boysen R.I., Danylec B., et al. Application of pH-responsive poly(2-dimethyl-aminoethylmethacrylate)-block-poly(acrylic acid) coatings for the open-tubular capillary electrochromatographic analysis of acidic and basic compounds. Anal. Chim. Acta. 2016;917:117–125. doi: 10.1016/j.aca.2016.02.045. [DOI] [PubMed] [Google Scholar]
- 40.Zhao Q.-L., Zhou J., Zhang L.-S., et al. Coatings of molecularly imprinted polymers based on polyhedral oligomeric silsesquioxane for open tubular capillary electrochromatography. Talanta. 2016;152:277–282. doi: 10.1016/j.talanta.2016.02.019. [DOI] [PubMed] [Google Scholar]
- 41.Zhao L., Qiao J., Zhang K., et al. Construction of chiral ligand exchange capillary electrochromatography for D,L-amino acids enantioseparation and its application in glutaminase kinetics study. J. Chromatogr. A. 2018;1548:104–110. doi: 10.1016/j.chroma.2018.03.031. [DOI] [PubMed] [Google Scholar]
- 42.Xiao X., Zhang Y., Wu J., et al. Poly(norepinephrine)-coated open tubular column for the separation of proteins and recombination human erythropoietin by capillary electrochromatography. J. Sep. Sci. 2017;40:4636–4644. doi: 10.1002/jssc.201700720. [DOI] [PubMed] [Google Scholar]
- 43.Liu X., Sun S., Nie R., et al. Highly uniform porous silica layer open-tubular capillary columns produced via in-situ biphasic sol-gel processing for open-tubular capillary electrochromatography. J. Chromatogr. A. 2018;1538:86–93. doi: 10.1016/j.chroma.2018.01.024. [DOI] [PubMed] [Google Scholar]
- 44.Svobodova J., Kofronova O., Benada O., et al. Separation of oligopeptides, nucleobases, nucleosides and nucleotides using capillary electrophoresis/electrochromatography with sol-gel modified inner capillary wall. J. Chromatogr. A. 2017;1517:185–194. doi: 10.1016/j.chroma.2017.08.014. [DOI] [PubMed] [Google Scholar]
- 45.Zhang Y., Wang W., Xiao X., et al. Separation of monoclonal antibody charge state variants by open tubular capillary electrochromatography with immobilised protein as stationary phase. J. Chromatogr. A. 2016;1466:180–188. doi: 10.1016/j.chroma.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 46.Xiao X., Wang W., Zhang Y., et al. Facile preparation of fibrin coated open tubular column for characterization of monoclonal antibody variants by capillary electrochromatography. J. Pharm. Biomed. Anal. 2017;140:377–383. doi: 10.1016/j.jpba.2017.03.014. [DOI] [PubMed] [Google Scholar]
- 47.Fu Q., Zhang K., Gao D., et al. Escherichia coli adhesive coating as a chiral stationary phase for open tubular capillary electrochromatography enantioseparation. Anal. Chim. Acta. 2017;969:63–71. doi: 10.1016/j.aca.2017.03.036. [DOI] [PubMed] [Google Scholar]
- 48.Wang R., Mao Z., Chen Z. Monolithic column with polymeric deep eutectic solvent as stationary phase for capillary electrochromatography. J. Chromatogr. A. 2018;1577:66–71. doi: 10.1016/j.chroma.2018.09.046. [DOI] [PubMed] [Google Scholar]
- 49.Mao Z., Qin X., Chen Z. Monolithic column functionalized with quinine derivative for anion-exchange capillary electrochromatography. Electrophoresis. 2018;39:3006–3012. doi: 10.1002/elps.201800253. [DOI] [PubMed] [Google Scholar]
- 50.Mao Z., Chen Z. Monolithic column modified with bifunctional ionic liquid and styrene stationary phases for capillary electrochromatography. J. Chromatogr. A. 2017;1480:99–105. doi: 10.1016/j.chroma.2016.12.030. [DOI] [PubMed] [Google Scholar]
- 51.Dixit S., Lee I.S., Park J.H. Carbamoylated azithromycin incorporated zirconia hybrid monolith for enantioseparation of acidic chiral drugs using non-aqueous capillary electrochromatography. J. Chromatogr. A. 2017;1507:132–140. doi: 10.1016/j.chroma.2017.05.046. [DOI] [PubMed] [Google Scholar]
- 52.Zhang B., Lei X., Deng L., et al. Ultrafast preparation of a polyhedral oligomeric silsesquioxane-based ionic liquid hybrid monolith via photoinitiated polymerization, and its application to capillary electrochromatography of aromatic compounds. Microchim. Acta. 2018;185:318. doi: 10.1007/s00604-018-2847-x. [DOI] [PubMed] [Google Scholar]
- 53.Wang J., Wu F., Xia R., et al. Rapid fabrication of ionic liquid-functionalized monolithic column via in-situ urea-formaldehyde polycondensation for pressurized capillary electrochromatography. J. Chromatogr. A. 2016;1449:100–108. doi: 10.1016/j.chroma.2016.04.069. [DOI] [PubMed] [Google Scholar]
- 54.Vergara-Barberan M., Mompo-Rosello O., Herrero-Martinez J.M., et al. Poly(ethylene glycol) diacrylate based monolithic capillary columns for the analysis of polar small solutes by capillary electrochromatography. J. Sep. Sci. 2018;41:2632–2639. doi: 10.1002/jssc.201800184. [DOI] [PubMed] [Google Scholar]
- 55.Murauer A., Bakry R., Partl G., et al. Optimization of an innovative vinylimidazole-based monolithic stationary phase and its use for pressured capillary electrochromatography. J. Pharm. Biomed. Anal. 2019;162:117–123. doi: 10.1016/j.jpba.2018.08.054. [DOI] [PubMed] [Google Scholar]
- 56.Murauer A., Bakry R., Schottenberger H., et al. An innovative monolithic zwitterionic stationary phase for the separation of phenolic acids in coffee bean extracts by capillary electrochromatography. Anal. Chim. Acta. 2017;963:136–142. doi: 10.1016/j.aca.2017.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Qiu D., Li F., Zhang M., et al. Preparation of phosphorylcholine-based hydrophilic monolithic column and application for analysis of drug-related impurities with capillary electrochromatography. Electrophoresis. 2016;37:1725–1732. doi: 10.1002/elps.201600066. [DOI] [PubMed] [Google Scholar]
- 58.Ganewatta N., El Rassi Z. Monolithic capillary columns consisting of poly(glycidyl methacrylate-co-ethylene glycol dimethacrylate) and their diol derivatives with incorporated hydroxyl functionalized multiwalled carbon nanotubes for reversed-phase capillary electrochromatography. Analyst. 2018;143:270–279. doi: 10.1039/c7an01426k. [DOI] [PubMed] [Google Scholar]
- 59.Ganewatta N., El Rassi Z. Poly(glyceryl monomethacrylate-co-ethylene glycol dimethacrylate) monolithic columns with incorporated bare and surface modified gluconamide fumed silica nanoparticles for hydrophilic interaction capillary electrochromatography. Talanta. 2018;179:632–640. doi: 10.1016/j.talanta.2017.11.062. [DOI] [PubMed] [Google Scholar]
- 60.Xu S., Mo R., Jin C., et al. Mesoporous silica nanoparticles incorporated hybrid monolithic stationary phase immobilized with pepsin for enantioseparation by capillary electrochromatography. J. Pharm. Biomed. Anal. 2017;140:190–198. doi: 10.1016/j.jpba.2017.03.013. [DOI] [PubMed] [Google Scholar]
- 61.Miao C., Bai R., Xu S., et al. Carboxylated single-walled carbon nanotube-functionalized chiral polymer monoliths for affinity capillary electrochromatography. J. Chromatogr. A. 2017;1487:227–234. doi: 10.1016/j.chroma.2017.01.025. [DOI] [PubMed] [Google Scholar]
- 62.Mao Z., Bao T., Li Z., et al. Ionic liquid-copolymerized monolith incorporated with zeolitic imidazolate framework-8 as stationary phases for enhancing reversed phase selectivity in capillary electrochromatography. J. Chromatogr. A. 2018;1578:99–105. doi: 10.1016/j.chroma.2018.10.008. [DOI] [PubMed] [Google Scholar]
- 63.Carrasco-Correa E.J., Martinez-Vilata A., Herrero-Martinez J.M., et al. Incorporation of zeolitic imidazolate framework (ZIF-8)-derived nanoporous carbons in methacrylate polymeric monoliths for capillary electrochromatography. Talanta. 2017;164:348–354. doi: 10.1016/j.talanta.2016.11.027. [DOI] [PubMed] [Google Scholar]
- 64.Zhang L.-S., Du P.-Y., Gu W., et al. Monolithic column incorporated with lanthanide metal-organic framework for capillary electrochromatography. J. Chromatogr. A. 2016;1461:171–178. doi: 10.1016/j.chroma.2016.07.015. [DOI] [PubMed] [Google Scholar]
- 65.Zhao H., Wang Y., Cheng H., et al. Graphene oxide decorated monolithic column as stationary phase for capillary electrochromatography. J. Chromatogr. A. 2016;1452:27–35. doi: 10.1016/j.chroma.2016.05.001. [DOI] [PubMed] [Google Scholar]
- 66.Li X.-X., Zhang L.-S., Wang C., et al. Green synthesis of monolithic column incorporated with graphene oxide using room temperature ionic liquid and eutectic solvents for capillary electrochromatography. Talanta. 2018;178:763–771. doi: 10.1016/j.talanta.2017.10.014. [DOI] [PubMed] [Google Scholar]
- 67.Liu Z., Du Y., Feng Z. Enantioseparation of drugs by capillary electrochromatography using a stationary phase covalently modified with graphene oxide. Microchim. Acta. 2017;184:583–593. [Google Scholar]
- 68.Yu X., Zhou W., Chen Z. In situ immobilization of layered double hydroxides as stationary phase for capillary electrochromatography. J. Chromatogr. A. 2017;1530:219–225. doi: 10.1016/j.chroma.2017.11.021. [DOI] [PubMed] [Google Scholar]
- 69.Geng Z., Song Q., Yu B., et al. Using ZIF-8 as stationary phase for capillary electrophoresis separation of proteins. Talanta. 2018;188:493–498. doi: 10.1016/j.talanta.2018.06.027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.