Abstract
Rationale
Neointimal hyperplasia is characterized by excessive accumulation of vascular smooth muscle cells (SMCs) leading to occlusive disorders such as atherosclerosis and stenosis. Blood vessel injury increases growth factor secretion and matrix synthesis, which promotes SMC proliferation and neointimal hyperplasia via focal adhesion kinase (FAK).
Objective
To understand the mechanism of FAK action in SMC proliferation and neointimal hyperplasia.
Methods and Results
Using combined pharmacological FAK catalytic inhibition (VS-4718) and SMC-specific FAK kinase-dead (KD, Myh11-Cre-ERT2) mouse models, we report that FAK regulates SMC proliferation and neointimal hyperplasia in part by governing GATA4-cyclin D1 signaling. Inhibition of FAK catalytic activity facilitates FAK nuclear localization, which is required for proteasome-mediated GATA4 degradation in the cytoplasm. Chromatin immunoprecipitation identified GATA4 binding to the mouse cyclin D1 promoter and loss of GATA4-mediated cyclin D1 transcription diminished SMC proliferation. Stimulation with platelet-derived growth factor or serum activated FAK and redistributed FAK from the nucleus to cytoplasm, leading to concomitant increase in GATA4 protein and cyclin D1 expression. In a femoral artery wire injury model, increased neointimal hyperplasia was observed in parallel with elevated FAK activity, GATA4 and cyclin D1 expression following injury in control, but not in VS-4718-treated and SMC-specific FAK-KD mice. Finally, lentiviral shGATA4 knockdown in the wire injury significantly reduced cyclin D1 expression, SMC proliferation, and neointimal hyperplasia compared to control mice.
Conclusions
Nuclear enrichment of FAK by inhibition of FAK catalytic activity during vessel injury blocks SMC proliferation and neointimal hyperplasia through regulation of GATA4-mediated cyclin D1 transcription.
Keywords: FAK, SMC, neointimal hyperplasia, GATA4, Cyclin D1, focal adhesion kinase, smooth muscle cell, proliferation, Atherosclerosis, Restenosis, Smooth Muscle Proliferation and Differentiation, Stenosis
INTRODUCTION
Excessive proliferation and migration of vascular smooth muscle cells (SMCs) contribute to occlusive cardiovascular diseases such as atherosclerosis.1 Although SMCs in mature vessels have low proliferative and migratory activity, physical and inflammatory stimuli following vessel injury increases growth factor secretion and matrix synthesis, which promotes SMC proliferation and migration into the intima leading to arterial stenosis.2–4, Current treatment strategies for stenotic atherosclerotic lesions include procedures such as balloon angioplasty, stenting, or bypass vein graft surgery that restore blood flow through the atherosclerotic vessel.5, 6 Although these procedures are effective, they damage the artery and can lead to restenosis or re-occlusion due to induction of SMC proliferation and migration. Drug-eluting stents and drug-coated balloons have been used to reduce restenosis,7, 8 but better treatment options are needed as size of the affected artery is still a major problem for intervention and prevention of restenosis.9–11
Focal adhesion kinase (FAK) is a protein tyrosine kinase that mediates integrin and growth factor signaling pathways associated with cell proliferation and migration.12, 13 Increased growth factor synthesis and matrix deposition are hallmarks of vessel injury,14, 15 which lead to aberrant FAK activation.16, 17 Platelet-derived growth factor (PDGF), a key factor in vascular remodeling, promotes FAK-mediated SMC migration and proliferation.17–21 Increased matrix stiffness also activates integrin downstream signaling through FAK, resulting in expression of cyclin D1.20, 22 FAK activity promotes SMC proliferation through increased protein stability of S-phase kinase-associated protein 2 (Skp-2), a ubiquitin ligase subunit that promotes degradation of cell cycle inhibitors p27 and p21.23–25 SMC-specific FAK knockout studies in SMCs revealed that FAK expression is important in promoting SMC hyperplasia upon vascular injury.21 FAK can function as either a kinase or a kinase-independent scaffold, but it is unknown how FAK regulates these cell cycle proteins and whether FAK-mediated SMC proliferation is dependent on its kinase activity or not.
FAK consists of N-terminal FERM (4.1, ezrin, radixin, and moesin homology), central kinase, and C-terminal focal adhesion targeting (FAT) domains.12 FAK localizes to focal adhesions via the FAT domain, where integrin and growth factor signaling lead to FAK tyrosine 397 (Y397) autophosphorylation, an indicator of FAK activation.26 FAK also shuttles between the cytoplasm and nucleus via nuclear localization and export signal within the FERM and kinase domains, respectively.27, 28 Nuclear FAK interacts with nuclear factors such as tumor suppressor p53, GATA4, MBD2, TAF9, MEF2, Runx1, NANOG, and RNA polymerase II.27, 29–35 These findings suggested a new paradigm for FAK in which nuclear FAK regulation of transcription factors may alter gene transcription.
Vessel injury alters expression of key transcription factors, such as serum response factor (SRF), myocardin, and Krüppel-like factor 4, which regulate expression of SMC contractile genes, SMC phenotype.36, 37 The GATA family of transcription factors, including GATA4, GATA5, and GATA6, are expressed in differentiated cardiac cells and SMCs.38 GATA6, the most commonly studied GATA transcription factor in SMCs, promotes SMC differentiation through cell cycle suppression and induction of SMC-specific genes through association with SRF and myocardin.39,40 Rapamycin, used on drug-eluting stents,41 promotes SMC differentiation via increased GATA6.42 Although there are some reports supporting GATA4 involvement in SMC-specific gene expression,43, 44 GATA4 expression levels are low in differentiated SMCs,38 and it is unknown how vascular injury regulates GATA factors in SMCs.
In this study, we observed a new link between FAK and GATA4 expression in SMC proliferation using in vitro and in vivo models. We report that (1) FAK catalytic inhibition-induced nuclear FAK reduces GATA4 protein levels via a proteasome-mediated pathway in SMCs, (2) mitogen stimuli, such as PDGF and serum, increase GATA4 protein stability through decreased nuclear FAK and increased FAK localization in the cytoplasm, (3) GATA4 promotes SMC proliferation through upregulation of cyclin D1 transcription, and (4) upregulation of GATA4 upon vascular injury is important for neointimal hyperplasia. These findings suggest that nuclear FAK-mediated GATA4 expression contributes to SMC proliferation and neointimal hyperplasia via regulation of cyclin D1 expression.
METHODS
The authors declare that all supporting data are available within the article and its Online Data Supplement. Detailed descriptions of experimental materials and methods used in this study are provided in the Online Data Supplement.
Generation of smooth muscle cell (SMC)-specific FAK-WT and -KD mice
SMC-specific Cre mice (Myh11-Cre-ERT2) were purchased from Jackson Laboratory. As Myh11-Cre-ERT2 was inserted into Y chromosome, only male mice were used.45 By crossing male Myh11-Cre-ERT2 mice with female FAK flox/flox (FAKFL/FL) mice, we generated FAKFL/FL;Myh11-Cre-ERT2 mice. Male FAKFL/FL;Myh11-Cre-ERT2 mice were crossed with female FAK wild-type (WT)/kinase-dead (KD) (FAKWT/KD) mice to generate FAKFL/WT;Myh11-Cre-ERT2 and FAKFL/KD;Myh11-Cre-ERT2 mice (Online Figure I). The flox FAK allele was excised by treating male mice (6-weeks-old) with tamoxifen (75 mg/kg, intraperitoneal, every other day) for 2 weeks, generating FAK−/WT;Myh11-Cre-ERT2 (FAK-WT) and FAK−/KD;Myh11-Cre-ERT2 (FAK-KD) mice.46
Femoral arterial injury
All animal procedures were performed under protocols approved by the Institutional Animal Care and Use Committee at the University of South Alabama. We performed femoral artery wire injury as described previously with minor modification.47 For FAK inhibitor experiments, mice were treated twice daily with vehicle (10 mM citrate, pH 6.0) or the FAK inhibitor VS-4718 (50 mg/kg, twice daily) by oral gavage. For SMC-specific FAK-WT and FAK-KD mice experiments, wire injury was performed after 2 weeks of tamoxifen injections. For GATA4 short hairpin RNA (shRNA) lentiviral infection of the femoral artery, concentrated lentivirus (107 transducing units) was mixed with nonionic surfactant (Pluronic F-127, Sigma-Aldrich) in phosphate-buffered saline and applied to artery as described previously.48
Cell culture
Primary mouse aortic SMCs were isolated from C57BL/6, FAKFL/FL, FAKFL/WT, and FAKFL/KD mice as previously reported with some modifications.49 Isolated FAKFL/WT and FAKFL/KD SMCs were treated with Cre adenovirus (50 MOI) to generate FAK-WT and FAK-KD SMCs. Isolated FAKFL/FL cells were transduced with FLAG-FAK-WT or nonnuclear localizing mutant (FLAG-FAK-NLM; R177A/R178A) lentivirus (20 MOI) and treated with Cre adenovirus to generate FLAG-FAK-WT and FLAG-FAK-NLM SMCs. Successful deletion of FAK flox allele was confirmed by immunoblotting.
Immunostaining
Paraffin-embedded sections were deparaffinized with Histo-Clear (National Diagnostics). Paraffin-embedded and frozen sections were fixed with cold acetone for 15 min. Cells grown on glass coverslips were fixed with 4% paraformaldehyde for 10 min. Samples were permeabilized with 0.1% Triton X-100 for 10 min, washed with PBS, and incubated with blocking solution containing 3% bovine serum albumin (BSA) and 1% goat serum for 1 h at RT. Samples were incubated with indicated primary antibodies overnight at 4°C. Samples were washed with PBS and incubated with conjugated goat anti-rabbit or anti-mouse secondary antibodies (1:1000) (Alexa Fluor 594 or 488, Thermo Fisher) for 1 h at RT. Species specific IgG or secondary antibodies were used as negative control. Nuclei were stained with DAPI (Sigma). Slides were mounted (Fluoromount-G, SouthernBiotech, Birmingham, AL), and images were acquired with a confocal microscope at 60-fold magnification (Nikon A1R, Nikon). Images were processed using Photoshop CS5. Quantification of all images were analyzed with ImageJ. At least 3 section images were acquired from each sample.
Statistical analysis
Data sets underwent Shapiro-Wilk test for normality, and statistical significance between experimental groups was determined with student t-test or two-way analysis of variance (2-way ANOVA) with Sidak multiple comparisons test (Prism software, v7.0d; GraphPad Software, La Jolla, CA). Power analyses were performed to determine sample size for 2-way ANOVA. Blinding procedures were not employed in this study due to the following reasons: high reproducibility, noticeable differences between specimen, and data acquisition by quantification.
RESULTS
Inhibition of FAK catalytic activity reduces SMC neointimal hyperplasia and proliferation
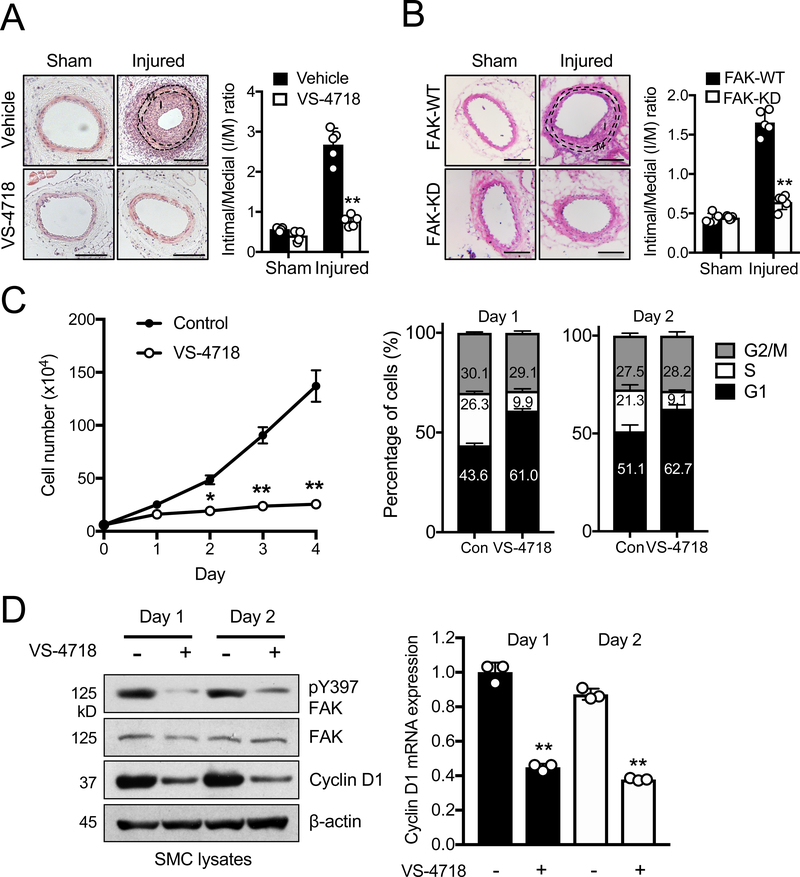
We evaluated the effect of FAK catalytic inhibition on SMC hyperplasia using femoral arterial wire injury model.47 Four weeks postinjury, arteries were analyzed by hematoxylin & eosin (H&E) staining to measure neointimal hyperplasia (Figure 1A). While injured arteries of vehicle-treated mice showed a significant increase in the intima/media (I/M) ratio, mice treated with FAK inhibitor VS-4718 had a lower I/M ratio similar to sham controls (Figure 1A). In order to further assess SMC-specific FAK catalytic activity in vivo, we used a Cre/loxP strategy to create new conditional SMC-specific FAK kinase-dead (FAK-KD) mice. As FAKKD/KD mouse is embryonic lethal,29, 50 we crossed FAKFL/FL;Myh11-Cre-ERT2 and FAKWild-type (WT)/KD mice to generate tamoxifen-inducible FAKFL/WT;Myh11-Cre-ERT2 and FAKFL/KD;Myh11-Cre-ERT2 mice45 (Online Figure I). Tamoxifen treatment led to deletion of FAK flox allele in SMCs, producing FAK−/WT;Myh11-Cre-ERT2 (FAK-WT) and FAK−/KD;Myh11-Cre-ERT2 (FAK-KD) SMCs in vivo. Four weeks postinjury, arteries were collected and neointimal hyperplasia was determined via H&E staining (Figure 1B). FAK-KD had a lower I/M ratio compared to FAK-WT mice (Figure 1B). Notably, FAK-WT mice showed less neointimal formation compared to vehicle-treated mice (Figures 1A and 1B), suggesting that single copy of FAK is less robust at promoting injury-induced neointimal formation. The results indicated that both pharmacological and genetic SMC-specific FAK catalytic inhibition significantly reduced SMC neointimal hyperplasia.
Figure 1. FAK catalytic inhibition attenuates SMC hyperplasia and proliferation through G1 cell cycle arrest.
(A and B) Representative H&E staining of femoral artery cross sections 4 weeks after wire injury for (A) vehicle or VS-4718 (50 mg/kg, twice daily) treated, and (B) genetic FAK-WT or FAK-KD mice. Scale bar, 50 μm. Intima/media (I/M) ratios were quantified (±SD; A, n=4; B, n=5). **P<0.005
(C) SMC proliferation assay and percentages of SMCs in each phase of the cell cycle were determined with or without VS-4718 (2.5 μM) in the presence of 10% serum (± SD; n=3). *P<0.01, **P<0.005
(D) Immunoblots of SMCs treated with or without VS-4718 (2.5 μM) in the presence of 10% serum for pY397 FAK, FAK, cyclin D1, and β-actin as loading control. Relative cyclin D1 mRNA levels were measured using RT-qPCR (±SEM; n=3). **P<0.005
Since SMC proliferation is critical in promoting neointimal hyperplasia, we tested the relation between FAK catalytic activity and SMC proliferation in vitro. A single treatment with VS-4718 reduced pY397 FAK (an active marker) in mouse aortic SMCs for 4 days (Online Figure IIA) and significantly reduced SMC proliferation (Figure 1C). VS-4718 also lowered expression of proliferating-cell nuclear antigen (PCNA), further supporting that FAK catalytic inhibition blocks cellular proliferation (Online Figure IIA).
FAK catalytic inhibition causes SMC cell cycle arrest at G1 in part by reducing cyclin D1 expression
Annexin V staining showed that 24 h FAK catalytic inhibition did not induce SMC apoptosis (Online Figure IIB), and that reduction in cell number was due to decreased cell proliferation (Figure 1C). We hypothesized that SMC cell cycling may be impaired upon FAK inhibition. Cell cycle analyses revealed that VS-4718 increased G1 and decreased S phase cell populations compared to control (Figure 1C), suggesting that FAK catalytic inhibition results in G1 arrest. It is known that cyclin D1 plays a critical role in G1/S phase transition by forming complexes with cyclin-dependent kinases 4/6 to phosphorylate retinoblastoma protein, resulting in cell cycle progression by phosphorylating E2F transcription factor 1.51 Therefore, we tested whether FAK activity could regulate cyclin D1 expression. VS-4718 treatment for 2 days reduced cyclin D1 protein expression (Figure 1D). As cyclin D1 expression is tightly regulated at a transcriptional level, we further tested whether VS-4718 reduced cyclin D1 transcription by using RT-qPCR. SMCs treated with VS-4718 had significantly less cyclin D1 mRNA when compared to control SMCs (Figure 1D). To ensure that these observations were not limited to mouse SMCs, we tested FAK catalytic inhibition in human coronary artery SMCs (hCASMCs). VS-4718 treatment reduced hCASMC proliferation, caused G1 arrest, and reduced cyclin D1 protein and mRNA expression (Online Figure IIIA–D). Using a human cyclin D1 promoter luciferase construct, we also observed that VS-4718 impaired cyclin D1 promoter activity in hCASMCs (Online Figure IIIE). Therefore, FAK catalytic inhibition prevented G1 to S cell cycle transition by potentially downregulating cyclin D1 transcription in mouse and human SMCs.
Nuclear FAK regulation of GATA4 and cyclin D1 expression in SMCs
Next, we investigated how FAK regulates cyclin D1 expression at a transcriptional level. Since our previous study demonstrated that stability of GATA4 transcription factor is regulated by nuclear FAK induced via inhibition of FAK catalytic activity,29 and others found that several cell cycle genes including cyclin D1 were downregulated in GATA4 knockout hearts,52 we chose GATA4 as a strong candidate for regulating FAK-mediated cyclin D1 transcription. Although GATA4 levels are lower in SMCs compared to GATA6,38 it is known that GATA4 also regulates SMC-specific gene expression through SRF and myocardin.44 First, we tested whether FAK catalytic inhibition altered GATA4 expression in SMCs. VS-4718 treatment significantly reduced GATA4 protein but had no effect on GATA6 in both mouse and human SMCs (Figure 2A and Online Figure IIIC). However, there was no change in GATA4 or GATA6 mRNA levels, suggesting that FAK catalytic inhibition does not regulate GATA4 protein levels on a transcriptional level (Figure 2A). As it is known that nuclear FAK regulates GATA4 protein stability in mouse fibroblasts,29 we used a proteasome inhibitor, MG132, to evaluate GATA4 stability in mouse SMCs. VS-4718 treatment significantly reduced GATA4 levels at 6 h, but MG132 restored GATA4 protein suggesting that FAK regulates GATA4 stability (Figure 2B). To determine if GATA4 degradation occurred in the cytoplasm or nucleus, SMCs were treated with Leptomycin B, a nuclear export blocker. Leptomycin B treatment blocked GATA4 degradation upon FAK catalytic inhibition (Online Figure IV), suggesting GATA4 degradation takes place in the cytoplasm. VS-4718 treatment for 12 h in both mouse and human SMCs increased nuclear FAK, which was correlated with reduced GATA4 and cyclin D1 staining (Figure 2C and Online Figure IIIF). Biochemical separation of SMCs into cytosolic and nuclear fractions also revealed that elevated levels of nuclear FAK upon VS-4718 treatment was associated with decreased GATA4 levels (Figure 2D). To verify if nuclear FAK is required for reducing GATA4 stability, we generated FLAG-tagged FAK-WT (FLAG-FAK-WT) and FAK nonnuclear localizing mutant (FLAG-FAK-NLM) in FAK−/− SMCs (Figure 2E). VS-4718 increased FLAG-FAK-WT nuclear localization and reduced GATA4 expression. We confirmed that FLAG-FAK-NLM still remained active in cytoplasm similar to FLAG-FAK-WT in control. Although VS-4718 reduced FAK catalytic activity in both FLAG-FAK-WT and FLAG-FAK-NLM SMCs as monitored by FAK pY397 staining (Figure 2E), VS-4718 did not induce FLAG-FAK-NLM nuclear localization or loss of GATA4 (Figure 2E). Biochemical analyses also supported that FLAG-FAK-NLM did not reduce GATA4 levels in the nucleus (Online Figure V). Therefore, nuclear FAK regulates GATA4 stability, but not GATA6, in SMCs.
Figure 2. Pharmacological FAK catalytic inhibition decreases GATA4 and cyclin D1 protein expression in SMCs.
All experiments were performed in the presence of 10% serum. (A) Immunoblots of SMCs treated with or without VS-4718 (2.5 μM) for pY397 FAK, FAK, GATA4, GATA6, and β-actin as loading control. Relative GATA4 and GATA6 mRNA levels were measured using RT-qPCR following treatment with VS-4718 (2.5 μM) for 2 days (±SEM; n=3). (B) Immunoblots of SMCs treated with VS-4718 (2.5 μM) only or VS-4718 together with MG132 (20 μM) for pY397 FAK, FAK, GATA4, GATA6, and β-actin as loading control. (C) Treatment with VS-4718 (2.5 μM) for 12 h increased nuclear FAK and reduced GATA4 and cyclin D1 expression as visualized by immunofluorescence staining of FAK, GATA4, cyclin D1, and GATA6 in SMCs. Scale bar 20 μm. (D) Immunoblots of SMC nuclear (N) and cytosolic (C) fractionated lysates with or without VS-4718 (2.5 μM) for FAK, GATA4, and GATA6. CREB and GAPDH as nuclear and cytosolic markers, respectively. (E) Comparison of FLAG-FAK-WT or FLAG-FAK-NLM nuclear localization and GATA4 levels in FAK−/− SMCs following treatment with VS-4718 (2.5 μM) for 12 h. Immunofluorescence staining of FLAG, FAK, pY397 FAK, GATA4, and GATA6 in SMCs. Scale bar, 20 mm. Immunoblots of SMCs for pY397 FAK, FAK, GATA4, GATA6, and β-actin as loading control.
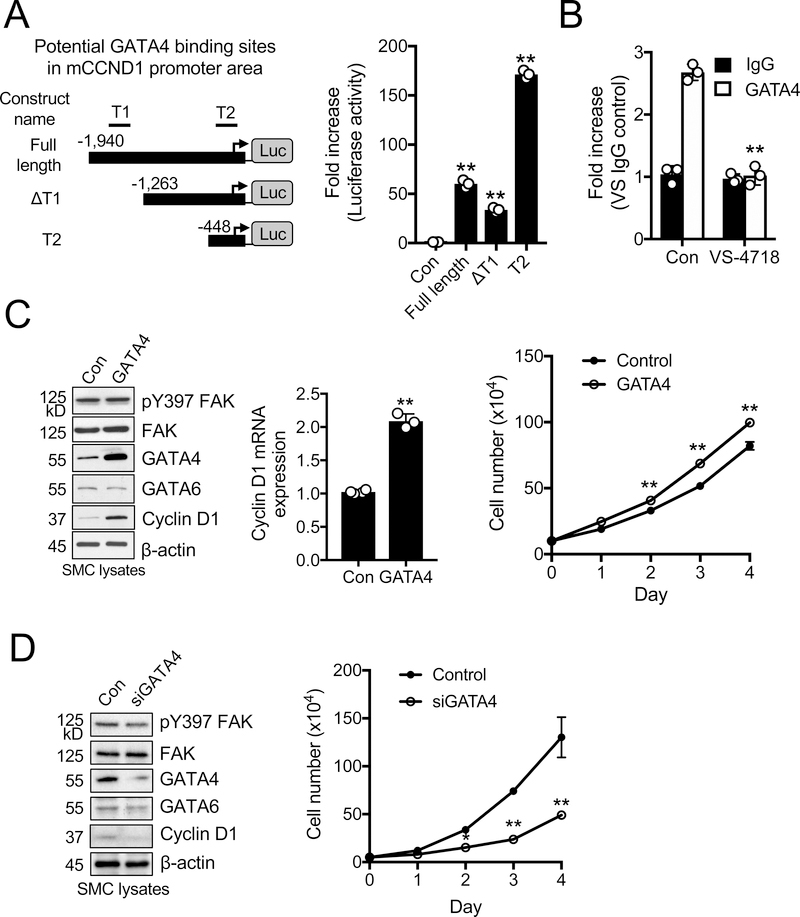
GATA4 regulation of cyclin D1 transcription in SMCs
As GATA4 regulation of cyclin D1 is not known, we looked into published GATA4 chromatin immunoprecipitation (ChIP) database using an interactive genome viewer to find potential GATA4-binding domains in the mouse cyclin D1 (mCCND1) promoter (Online Figure VI). We identified 2 potential GATA4 binding regions overlapping with active histone marks such as H3K4me3, named T1 (−1621 to −1400) and T2 (−238 to −178). Using these predicted sites, we generated mCCND1 promoter luciferase constructs containing either full-length, T1 deleted (ΔT1), or T2 only regions (Figure 3A). The T2 construct, containing the region closest to the transcription start site, showed the highest levels of luciferase activity (Figure 3B). As both the full-length and ΔT1 constructs had lower luciferase activity than T2, the presence of suppressive elements was implicated upstream of the T2 GATA4 binding site (Figure 3B). To determine whether GATA4 binds to the cyclin D1 promoter, we performed GATA4 ChIP in mouse SMCs. GATA4 pulldown enriched the T2 region compared to control IgG, suggesting that GATA4 directly mediates cyclin D1 transcription via the T2 site (Figure 3B). To further test whether GATA4 levels affect cyclin D1 expression, we performed a gain-of-function study by overexpressing GATA4 in SMCs. Stable GATA4 overexpression in SMCs increased cyclin D1 mRNA and protein expression, and increased SMC proliferation compared to control cells (Figure 3C). Additionally, GATA4 overexpression increased pre-spliced cyclin D1 mRNA in the nucleus (Online Figure VII), further supporting that GATA4 directly promotes cyclin D1 transcription. In contrast, GATA4 knockdown by using short interference RNA (siGATA4) reduced cyclin D1 protein expression in both mouse and human SMCs (Figure 3D and Online Figure VIII). siGATA4 also decreased SMC proliferation compared to scramble control (Figure 3D). In addition, cyclin D1 knockdown in control and GATA4 overexpressing SMCs reduced cell proliferation, indicating that cyclin D1 expression is indeed required for SMC proliferation (Online Figure IX). These results suggest that GATA4 promotes SMC proliferation through direct upregulation of cyclin D1 transcription.
Figure 3. GATA4 is a direct transcription factor for cyclin D1 and promotes SMC proliferation.
All experiments were performed in the presence of 10% serum. (A) Schematic of mouse cyclin D1 (mCCND1) promoter luciferase constructs with 2 putative GATA4 binding sites (T1 and T2) indicated. Arrow represents transcription start site at +1. Luciferase activity in SMCs was measured 24 h post-transfection (±SEM; n=3). **P<0.005 (B) ChIP analysis of GATA4 binding to the mCCND1 promoter in SMCs with or without VS-4718 (2.5 μM). Enrichment of potential site T2 over IgG control was quantified by RT-qPCR (±SEM; n=3). **P<0.005 (C) GATA4 overexpression increases SMC proliferation and cyclin D1 expression. Immunoblots of SMCs overexpressing GATA4 for pY397 FAK, FAK, GATA4, GATA6, cyclin D1, and β-actin as loading control. Relative cyclin D1 mRNA levels were measured using RT-qPCR (±SEM; n=3). Proliferation assay of GATA4-overexpressing SMCs (±SD; n=3). **P<0.005 (D) GATA4 knockdown decreases SMC proliferation and cyclin D1 expression. Immunoblots of SMCs transfected with either control or GATA4 siRNA (200 pmole for 48 h) for pY397 FAK, FAK, GATA4, GATA6, cyclin D1, and β-actin as loading control. Proliferation assay of SMCs following GATA4 siRNA knockdown (±SD; n=3). *P<0.05, **P<0.005
PDGF-induced cyclin D1 is dependent on GATA4 expression
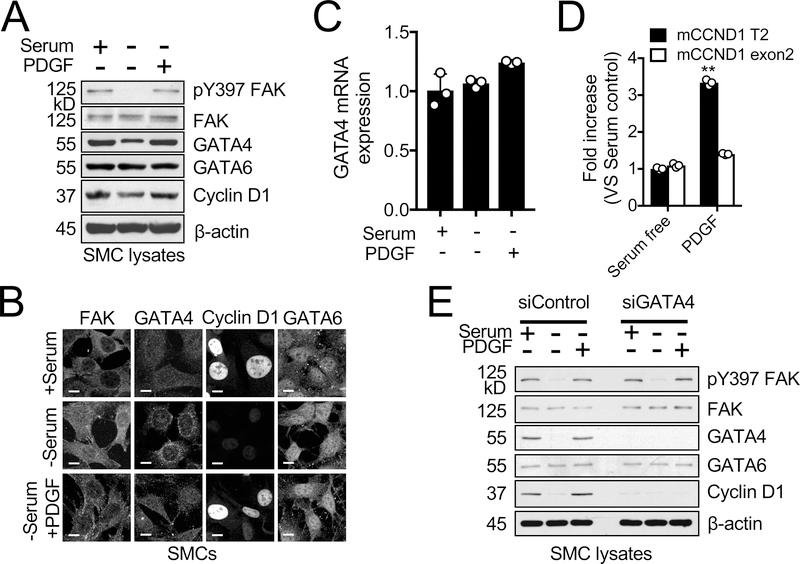
As GATA4 protein levels are low in SMCs in vivo,38 it is possible that proliferative SMCs located within the neointima or SMCs exposed to serum in vitro could have increased expression of GATA4. It is also well known that platelet-derived growth factor (PDGF) is increased in the neointima and is a strong promoter for SMC proliferation.53, 54 We tested the effect of serum or PDGF stimulation in mouse SMCs on GATA4 expression. Overnight serum-starved mouse SMCs exhibited decreased pY397 FAK levels (Figure 4A), but increased levels of nuclear FAK (Figure 4B). This was associated with decreased levels of GATA4 and cyclin D1 (Figure 4A and B). In addition to increased FAK cytoplasmic localization and pY397 FAK levels, stimulation with serum or PDGF also increased GATA4 and cyclin D1 expression (Figure 4A and B). Under all conditions, GATA6 levels did not change (Figure 4A and B). Neither serum nor PDGF stimulation changed GATA4 mRNA levels (Figure 4C), further supporting the idea that nuclear FAK regulates GATA4 protein stability independent of GATA4 transcription in mouse SMCs. In addition to regulating GATA4 stability, we observed FAK activity is also important for PDGF-mediated phosphorylation of GATA4 at serine 105, which promotes GATA4 transcriptional activity55 (Online Figure X). GATA4 ChIP assay revealed that PDGF stimulation increased GATA4 binding to the T2 region of the mCCND1 promoter, likely due to increased levels of GATA4 (Figure 4D). siGATA4 blocked serum- and PDGF-induced cyclin D1 expression in mouse SMCs (Figure 4E), further demonstrating that GATA4 expression is critical to promote mitogen-induced cyclin D1 expression in mouse SMCs. To test whether GATA4 expression is increased in proliferating SMCs, we used multipotent 10T½ cells that can be induced to exhibit a contractile SMC phenotype upon transforming growth factor β (TGFβ) or proliferative SMC phenotype upon PDGF treatment.56 While serum starvation and TGFβ treatment decreased pY397 FAK levels and increased FAK nuclear localization, serum and PDGF activated FAK and promoted FAK cytoplasmic redistribution (Online Figure XI). Serum or PDGF increased GATA4 and cyclin D1 (Online Figure XI). In contrast, TGFβ stimulation decreased both GATA4 and cyclin D1 expression in 10T½ cells (Online Figure XI). These data further support that FAK nuclear accumulation upon inhibition of FAK catalytic activity controls GATA4 protein stability and cyclin D1 expression in SMCs.
Figure 4. Mitogen-induced cyclin D1 expression is dependent on GATA4 in SMCs.
SMCs were serum-starved overnight, then treated with either serum (10% FBS) or PDGF (20 ng/ml) for 12 h. (A) Correlation between pY397 FAK, GATA4, and cyclin D1 levels following serum or PDGF stimulation of serum-starved SMCs as observed from immunoblots for pY397 FAK, FAK, GATA4, GATA6, cyclin D1, and β–actin as loading control. (B) Serum and PDGF redistribute FAK to cytoplasm and increase GATA4 and cyclin D1 as visualized by immunofluorescence staining of SMCs for FAK, GATA4, GATA6, and cyclin D1. Scale bar, 10 μm. (C) Relative GATA4 mRNA levels were measured using RT-qPCR (±SEM; n=3). (D) PDGF stimulation increased GATA4 binding to T2 site of mCCND1 promoter as seen by ChIP analysis (±SEM; n=3). mCCND1 Exon 2 primers used as negative control. **P<0.005
(E) Immunoblots of SMCs transfected with either siControl or siGATA4 (200 pmole for 48 h) for pY397 FAK, FAK, GATA4, GATA6, cyclin D1, and β-actin as loading control.
Kinase-dead FAK decreases GATA4 stability
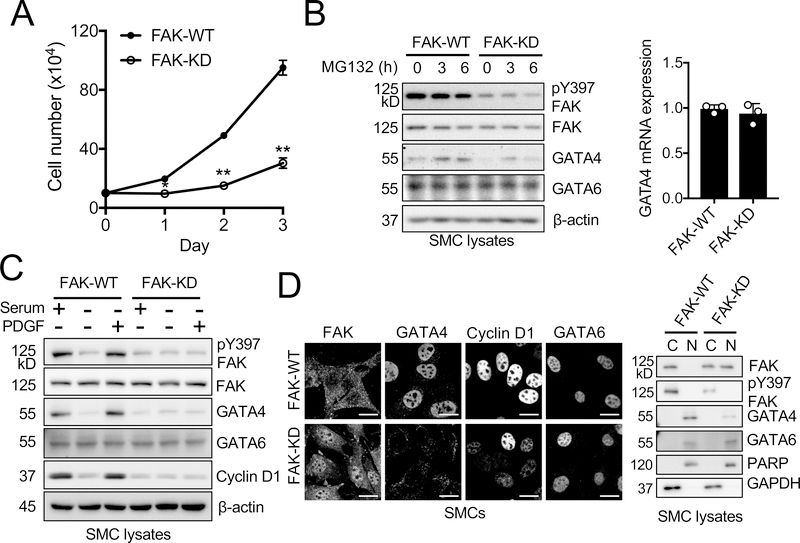
Although VS-4718 is a selective FAK inhibitor that is being tested for clinical use,57 it was necessary to verify the specific effect of FAK catalytic activity in SMC proliferation. We isolated FAK-WT and FAK-KD aortic SMCs from FAKFL/FL and FAKWT/KD mouse crossings (Online Figure I). FAK-KD SMCs proliferated at a slower rate compared to FAK-WT SMCs (Figure 5A). FAK-KD SMCs exhibited reduced pY397 FAK phosphorylation and significantly reduced GATA4 and cyclin D1 levels compared to FAK-WT SMCs (Figure 5B). GATA4 mRNA levels were not significantly different between FAK-WT and FAK-KD SMCs (Figure 5B). MG132 treatment restored GATA4 expression in FAK-WT and to a lesser extent in FAK-KD SMCs (Figure 5B). Serum or PDGF stimulation failed to increase GATA4 and cyclin D1 levels in FAK-KD, but not FAK-WT, SMCs (Figure 5C). Immunostaining revealed that FAK-KD SMCs showed much higher nuclear FAK and reduced GATA4 and cyclin D1 levels compared to FAK-WT SMCs (Figure 5D). Biochemical fractionation also confirmed that nuclear FAK was abundant and GATA4 levels were lower in FAK-KD SMCs (Figure 5D). There was no difference in GATA6 expression between FAK-KD and FAK-WT SMCs in all conditions (Figure 5). Next, we tested if cyclin D1 expression by itself is sufficient to promote cell proliferation in FAK-KD SMCs. However, cyclin D1 overexpression did not rescue proliferation defects of FAK-KD SMCs (Online Figure XII). Additionally, cyclin D1 overexpression had no effect on cell cycle inhibitors, such as p27 and p21, suggesting that cyclin D1 is necessary but not sufficient for SMC cell cyclin progression. These data further support the concept that nuclear FAK reduces GATA4 and cyclin D1 expression in SMCs.
Figure 5. FAK-KD SMCs exhibit prominent nuclear FAK localization, lower GATA4 protein expression, and slower proliferation.
Experiments were performed in the presence of 10% serum, unless indicated otherwise. (A) Proliferation assay was performed with FAK-WT and FAK-KD SMCs (±SD; n=3). *P<0.05, **P<0.005 (B) GATA4 is turned over faster in FAK-KD SMCs as seen by immunoblots of MG132 (20 μM, 3 or 6 h) treated FAK-WT and FAK-KD SMCs for pY397 FAK, FAK, GATA4, GATA6, and β–actin as loading control. Relative level of GATA4 mRNA in FAK-WT and FAK–KD SMCs was measured using RT-qPCR. (±SEM; n=3). (C) SMCs were serum-starved overnight and treated with either serum (10% FBS) or PDGF (20 ng/ml) for 12 h. Immunoblots of FAK-WT and FAK-KD SMCs for pY397 FAK, FAK, GATA4, GATA6, cyclin D1, and β-actin as loading control. (D) FAK-KD is enriched in the nucleus compared to FAK-WT. Representative immunofluorescence staining of FAK-WT and FAK-KD SMCs for FAK, GATA4, cyclin D1, and GATA6. Scale bars, 20 μm. Immunoblots of FAK-WT and FAK-KD SMC nuclear (N) and cytosolic (C) lysate for FAK, pY397 FAK, GATA4, and GATA6. PARP and GAPDH as nuclear and cytosolic markers, respectively.
Pharmacological FAK catalytic inhibition blocks FAK activation and GATA4 and cyclin D1 expression induced by wire injury
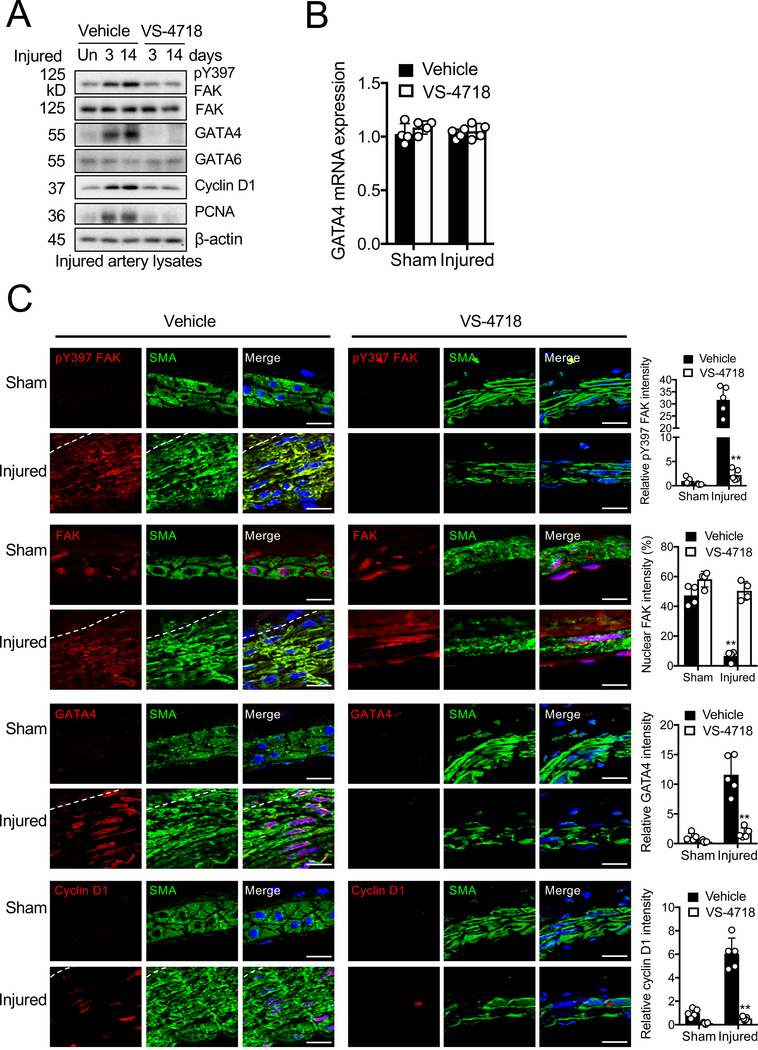
To gain further insights into the function of FAK in vascular remodeling in vivo, we evaluated the effect of FAK catalytic inhibition on SMCs in the femoral arterial wire injury model.47 As changes in gene and protein expression may occur rapidly after injury,58 we evaluated whether changes occurred in FAK, GATA4, GATA6, cyclin D1, and PCNA expression soon after vascular injury. Immunoblotting with artery lysates showed that wire injury increased the levels of pY397 FAK, GATA4, and cyclin D1 in femoral arteries within 3 days following injury compared to uninjured control (Figure 6A). However, VS-4718 treatment reduced wire injury-induced pY397 FAK, GATA4, cyclin D1, and PCNA levels compared to the vehicle-treated group (Figure 6A). Although GATA4 protein was increased in control after vascular injury and reduced upon FAK catalytic inhibition, there was no change in GATA4 mRNA expression in artery samples compared to uninjured control (Figure 6B). Immunostaining also showed increased pY397 FAK levels in the injured artery, and that GATA4 and cyclin D1 were also increased in the neointima 4 weeks following injury (Figure 6C and Online Figure XIIIA). These increases were blocked in mice treated with VS-4718 (Figure 6C and Online Figure XIIIB). We also observed increased nuclear FAK accumulation in VS-4718 treated artery samples (Figure 6C and Online Figure XIIIB). Relative fluorescence signals for nuclear FAK were significantly increased in vehicle- compared to VS-4718 treated mice (Figure 6C). Interestingly, we observed abundant nuclear FAK in uninjured control, suggesting that FAK in a healthy artery may remain less active. There were no differences in GATA6 levels between vehicle- and VS-4718-treated mice (Figure 6 and Online Figure XIII). These results indicate that VS-4718-induced loss of FAK activity and increased nuclear FAK blocks neointimal hyperplasia potentially through reduced expression of GATA4 and cyclin D1.
Figure 6. GATA4 is increased after wire injury and pharmacological FAK catalytic inhibition significantly blocks GATA4, cyclin D1 expression, and neointimal hyperplasia.
Mice were treated with vehicle or VS-4718 (50 mg/kg) twice daily following wire injury. (A) Immunoblots of femoral arteries 3 and 14 days postinjury for pY397 FAK, FAK, GATA4, GATA6, cyclin D1, PCNA, and β-actin as loading control. Un: uninjured artery. (B) Relative GATA4 mRNA expression in femoral arteries harvested 3 days postinjury were measured by RT-qPCR (±SEM; n=4 biological samples). (C) Immunofluorescence staining of femoral arteries 4 weeks postinjury for pY397 FAK, FAK, GATA4, cyclin D1, and α-SMA. Red, green (α-SMA), and blue (DAPI) were merged. Relative fluorescence intensity of nuclear FAK and indicated targets in α-SMA positive cells were quantified (±SD; n=5). Scale bar, 10 μm. Dashed lines, boundary between media and intima. **P<0.005
SMC-specific FAK catalytic inhibition prevents SMC hyperplasia in part via FAK-GATA4-cyclin D1 signaling
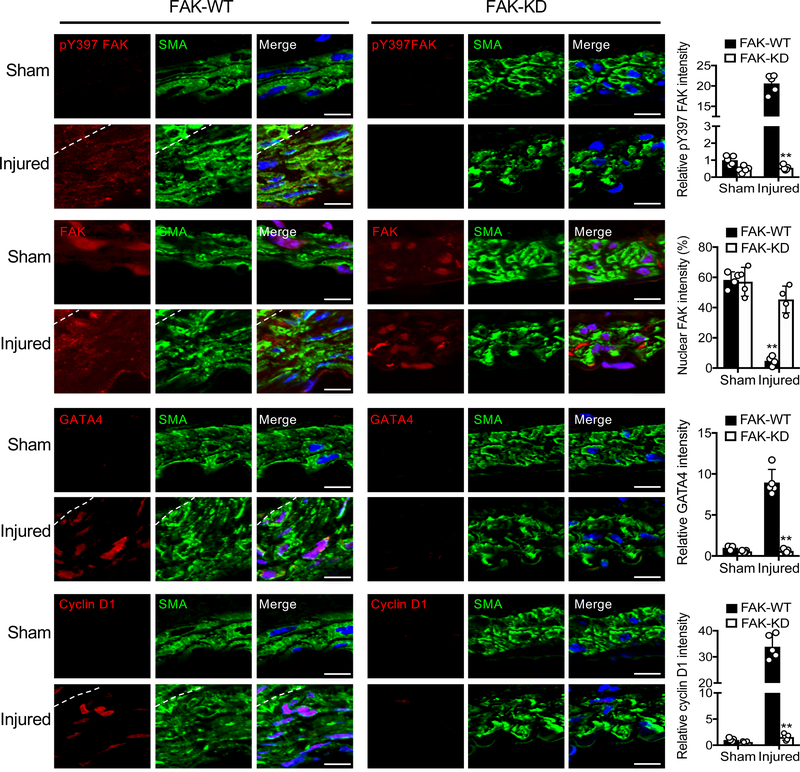
To evaluate SMC-specific FAK activity in wire injury model, we used our new FAK-KD model (Online Figure I). As SMC-specific FAK-KD mice showed significantly reduced SMC hyperplasia (Figure 1B), we further investigated changes in FAK activity, nuclear FAK, and expression of GATA4 and cyclin D1 following injury. Wire injury increased expression of GATA4 and cyclin D1 in FAK-WT mice, but not in FAK-KD mice (Figure 7 and Online Figure XIV). FAK activity (pY397 FAK) was significantly decreased in FAK-KD mice, verifying deletion of FAK flox allele after tamoxifen treatment compared to FAK-WT (Figure 7). While wire injury redistributed FAK from the nucleus to cytoplasm in FAK-WT mice, FAK-KD mice showed strong FAK nuclear localization in control and injured arteries (Figure 7). Relative fluorescence signals for nuclear FAK were also significantly increased in FAK-KD compared to FAK-WT mice (Figure 7). These results demonstrate that SMC-specific FAK activity contributes to SMC proliferation via upregulation of GATA4 and cyclin D1 in the neointimal regions (Figure 7).
Figure 7. SMC-specific FAK-KD mice develop reduced hyperplasia and exhibit less GATA4 and cyclin D1 expression after wire injury.
Immunofluorescence staining of FAK-WT and FAK-KD femoral arteries 4 weeks postinjury for pY397 FAK, FAK, GATA4, cyclin D1, and a-SMA. Red, green (α-SMA), and blue (DAPI) were merged. Relative fluorescence intensity of nuclear FAK and indicated targets in a-SMA positive cells were quantified (±SD; n=5). Scale bars, 10 μm. Dashed lines, boundary between media and intima. **P<0.005
shRNA knockdown of GATA4 abolished wire injury-induced neointimal hyperplasia
As wire injury-induced GATA4 upregulation was reduced by FAK catalytic inhibition (Figures 6 and 7), we evaluated whether GATA4 expression, per se, was important for neointimal formation following wire injury. We used either scramble or GATA4 shRNA (shGATA4) lentivirus co-expressing mCherry. Knockdown efficiency of 2 different GATA4 shRNAs were tested in mouse SMCs, and reduction of GATA4 and SMC proliferation were verified (Online Figure XV). One shGATA4 lentivirus was applied to the outside of the femoral artery following injury. shGATA4 completely abolished neointimal formation in injured arteries compared to the control lentivirus group (Figure 8A). Lentiviral infection was verified by monitoring mCherry expression, and we observed that shGATA4 lentivirus reduced wire injury-induced expression of GATA4 and cyclin D1 (Figure 8B and Online Figure XVI). Wire injury increased pY397 FAK levels in both scramble and shGATA4 arteries (Figure 8B and Online Figures XVI). Injured arteries of scramble and shGATA4 showed a significant decrease in FAK nuclear localization (Figure 8C and Online Figure XVII), suggesting that decreasing GATA4 levels may be enough to reduce SMC hyperplasia. Taken together, these data reveal that injury-induced upregulation of GATA4 contributes in neointimal hyperplasia.
Figure 8. Knockdown of GATA4 ameliorates wire injury-induced neointimal hyperplasia.
Femoral arteries were coated with either scramble or shGATA4 lentivirus immediately following wire injury. (A) Representative H&E staining of femoral artery cross sections 4 weeks after wire injury. Intima/media (I/M) ratios were quantified (± SD; n=5). Scale bar, 50 μm. **P<0.005 (B) Immunofluorescence staining 4 weeks postinjury for pY397 FAK, GATA4, cyclin D1, and α-SMA. Green and blue (DAPI) were merged. mCherry was used to verify successful viral infection. Relative fluorescence intensity of indicated targets in α-SMA positive cells and α-SMA thickness were (± SD; n=5). Scale bars, 10 μm. Dashed lines, boundary between media and intima. **P<0.005 (C) Fluorescence intensity of FAK in nucleus was quantified by ImageJ (±SD; n=4). **P<0.005
DISCUSSION
In this study, we discovered that inhibition of FAK catalytic activity blocked SMC proliferation by inducing G1 arrest in mouse and human SMCs (Figure 1 and Online Figure III). G1 arrest was a result of FAK catalytic inhibition-induced FAK nuclear localization, resulting in decreased stability of GATA4 protein (Figure 2) and reduced expression of cyclin D1 (Figure 1). Serum starvation of SMCs led to decreased FAK activity and increased FAK nuclear localization (Figure 4), and stimulation with serum or PDGF led to increased FAK activity and cytoplasmic localization (Figure 4). In the multipotent 10T½ cells, stimulation with TGFβ, which promotes a non-proliferative SMC phenotype, increased FAK nuclear localization and GATA4 protein levels (Online Figure XI); however, treatment with PDGF increased FAK cytoplasmic localization, GATA4, and cyclin D1 expression (Online Figure XI). As FAK cytoplasmic localization was positively correlated with GATA4 and cyclin D1 expression, we concluded that a FAK-GATA4-cyclin D1 axis may be a general mechanism to regulate SMC proliferation in mouse and human SMCs. In the wire injury model, we showed that injury-induced FAK activation and cytoplasmic redistribution could be reversed by pharmacological FAK catalytic inhibition, resulting in significantly deceased neointimal formation in part via nuclear FAK-GATA4-cyclin D1 axis (Figure 6).
Homozygous FAK kinase-dead (FAKKD/KD) knock-in mouse is embryonic lethal50 and FAK knockout models induce compensatory Pyk2 expression hindering the analyses of FAK-specific function.46 In order to study the role of FAK catalytic activity in SMCs, we developed a new tamoxifen-inducible SMC-specific (Myh11-Cre-ERT2) FAK-KD mouse (Online Figure I). SMCs isolated from FAK-KD mice proliferated slower and showed increased FAK nuclear localization compared to FAK-WT SMCs (Figure 5.) FAK-KD SMCs also had decreased GATA4 and cyclin D1 expression (Figure 5). In the femoral artery wire injury model, FAK-KD mice showed a significant reduction in SMC intimal thickening compared to FAK-WT mice (Figure 1B). FAK-KD mice also showed increased nuclear FAK and decreased GATA4 and cyclin D1 following wire injury compared to FAK-WT mice (Figure 7). The SMC-specific FAK-KD mouse model verified FAK-mediated GATA4-cyclin D1 connection in vitro and in vivo.
Our current animal models using pharmacological and genetic FAK catalytic inhibition reduced FAK cytoplasmic signaling and increased FAK nuclear localization, thus making it difficult to separate cytoplasmic and nuclear functions of FAK in the regulation of SMC proliferation. To help address this, we generated a nonnuclear localizing FAK mutant (FLAG-FAK-NLM) in FAK−/− mouse SMCs. Although Flag-FAK-NLM normally localizes to adhesions and shows pY397 autophosphorylation, VS-4718 treatment failed to induce FLAG-FAK-NLM nuclear localization and loss of GATA4 expression (Figure 2E and Online Figure V), suggesting that FAK nuclear localization is important for FAK-mediated GATA4 degradation. However, new animal models to better evaluate cytoplasmic versus nuclear FAK in neointimal formation are needed.
It is unknown what causes kinase-inhibited FAK to preferentially localize to the nucleus. This may be due to a conformational change that hides the nuclear export sequence and prevents it from leaving after it shuttles to the nucleus. Notably, we observed that nuclear FAK is abundant in healthy arteries and vessel injury redistributed FAK from the nucleus to cytoplasm, suggesting that FAK in a healthy condition is less active compared to injured artery (Figure 6, 7, and 8). It is also unknown what keeps FAK inside the nucleus or triggers its cytoplasmic localization following stimulation with serum or PDGF. Following vascular injury, SMCs express an endogenous inhibitor of FAK, termed FAK-related nonkinase (FRNK), that inhibits SMC proliferation to some extent.18, 59 Currently small molecule FAK inhibitors are mostly used as a cancer therapy,57 but several studies have investigated the efficacy of these inhibitors in the treatment of other diseases such as lung and liver fibrosis.60–62 In the present study, we observed that the FAK inhibitor VS-4718, currently in clinical development as a cancer therapy,63 significantly reduced SMC hyperplasia, suggesting that anticancer FAK inhibitors could also be evaluated as a potential therapy for vessel wall narrowing diseases.
GATA4 levels may be low in SMCs of mature vessels,38 but we observed that GATA4 mRNA was constantly expressed in SMCs in vitro and in vivo (Figure 2 and 6) and that wire injury increased GATA4 protein expression in vivo (Figure 6). Knocking down GATA4 using shRNA significantly prevented wire injury-induced neointimal formation (Figure 8), suggesting that GATA4 upregulation following wire injury is important for SMC proliferation and migration in vivo. Although GATA4 shRNA reduced cyclin D1 levels, it did not reduce pY397 FAK staining following wire injury, suggesting that targeting GATA4 in neointimal hyperplasia may be beneficial (Figure 8). As there currently are no GATA4-specific inhibitors available, further investigation into the regulation of GATA4 in SMCs in vivo is required.
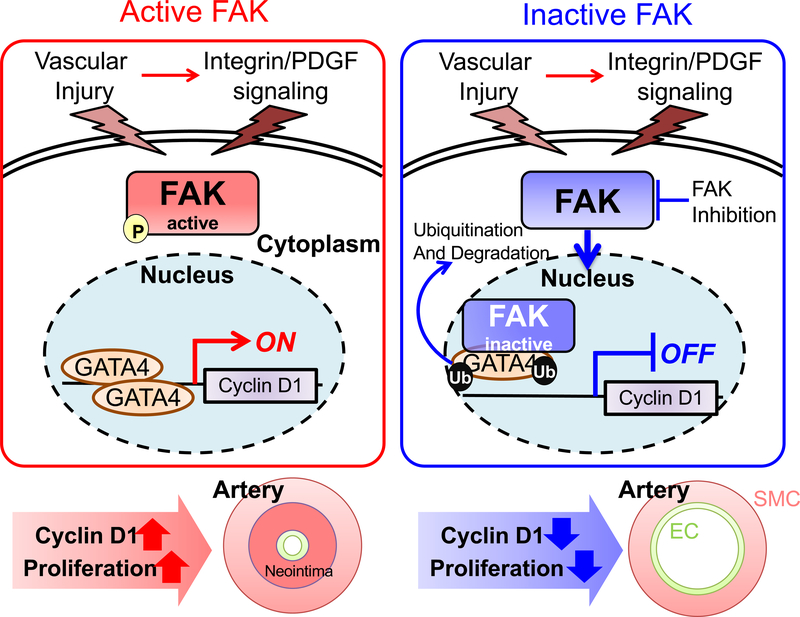
In summary, our new observations from this study is that healthy vessels have low FAK activity and abundant nuclear FAK (Figure 9). However, vascular injury promoted FAK activation and redistribution from the nucleus to cytoplasm (Figures 6–8 and Online Figures XIII, XIV, and XVI). We have demonstrated that regulation of FAK activity and cytoplasmic localization in SMCs is critical to promoting SMC proliferation in part via increased GATA4 stability and cyclin D1 expression. Targeting FAK catalytic activity or GATA4 levels may have potential in the treatment of vessel narrowing diseases.
Figure 9. Proposed model of FAK-GATA4-cyclin D1 regulation in SMC neointimal hyperplasia.
Upon vessel injury, growth factor secretion augments FAK activity and increases GATA4 stability and cyclin D1 expression leading to SMC proliferation and neointimal hyperplasia. Pharmacological or genetic FAK catalytic inhibition reduces SMC proliferation through increased nuclear FAK, which promotes GATA4 proteasomal degradation. Reduced GATA4 levels decreases cyclin D1 transcription and SMC proliferation. PDGF: platelet-derived growth factor, P: pY397 FAK, Ub: ubiquitin, EC: endothelial cells, SMC: smooth muscle cells.
Supplementary Material
NOVELTY AND SIGNIFICANCE.
What Is Known?
Vessel injury induces proliferation of vascular smooth muscle cells into the intima, leading to narrowing of the blood vessel.
Expression of focal adhesion kinase, a protein tyrosine kinase, is important in promoting smooth muscle cell proliferation.
What New Information Does This Article Contribute?
Focal adhesion kinase in healthy arteries is predominantly localized within nuclei of smooth muscle cells but is activated and redistributes to the cytoplasm following injury.
In injured arteries, focal adhesion kinase cytoplasmic localization increases GATA4 protein stability in the nucleus, which increases GATA4-mediated cyclin D1 transcription to promote SMC proliferation and hyperplasia.
Catalytic inhibition of focal adhesion kinase results in focal adhesion kinase retention in the nucleus and reduction in GATA4 protein expression in injured arteries, thus blocking smooth muscle cell proliferation and hyperplasia.
Current interventions to inhibit smooth muscle cell (SMC) proliferation, such as drug coated balloons and drug-eluting stents, are limited to vessel size and location. Therefore, a better understanding of SMC proliferation during vessel injury could lead to new therapeutic treatments. We are the first to report the molecular mechanism of how focal adhesion kinase (FAK) controls SMC proliferation and hyperplasia. We observed that FAK is predominantly located within the nucleus of healthy arteries, keeping GATA4 protein levels low by promoting proteasome-mediated degradation. However, vessel injury induces FAK activation resulting in FAK cytoplasmic localization, which increases expression of GATA4 protein, but not mRNA. This is also the first study to show that GATA4 plays a key role in SMC proliferation and that knockdown of GATA4 prevents injury-induced SMC hyperplasia. During vessel injury, forced nuclear localization of FAK through pharmacological or genetic inhibition of FAK catalytic activity reduced GATA4 protein and SMC proliferation. These findings highlight the importance of FAK subcellular localization in regulating SMC proliferation, and suggest that targeting GATA4 protein stability through forced nuclear localization of FAK could be used as a treatment to inhibit vessel narrowing.
ACKNOWLEDGEMENTS
We thank Drs. Elly Trepman and Mary Townsley for critical reading, and Nikon A1 Confocal microscope supported by National Institutes of Health S10RR027535 (to University of South Alabama).
SOURCE OF FUNDING
This work was supported by American Heart Association 12SDG10970000 (to S Lim), 16GRNT30960007 (to S Lim), National Institutes of Health R01 CA190688 (to E Ahn), R01 CA102310 (to D Schlaepfer) and R01 HL136432 (to S Lim).
Nonstandard Abbreviations and Acronyms
- ChIP
chromatin immunoprecipitation
- FAK
focal adhesion kinase
- FAT
focal adhesion targeting
- GATA4
GATA binding protein 4
- GATA6
GATA binding protein 6
- hCASMC
human coronary artery smooth muscle cell
- H&E
hematoxylin & eosin
- I/M ratio
intima/media ratio
- KD
kinase-dead
- mCCND1
mouse cyclin D1
- MOI
multiplicity of infection
- Myh11
myosin heavy chain 11
- NLM
nonnuclear localizing mutant
- PCNA
proliferating cell nuclear antigen
- RT-qPCR
real-time quantitative polymerized chain reaction
- Skp-2
S-phase kinase-associated protein 2
- SMC
smooth muscle cell
- TGFβ
transforming growth factor β
- PDGF
platelet-derived growth factor
- pY397
autophosphorylation at tyrosine 397
- shRNA
short hairpin RNA
- siRNA
short interference RNA
- α-SMA
α-smooth muscle actin
- SRF
serum response factor
- WT
wild-type
Footnotes
DISCLOSURES
There are no conflicts of interests.
REFERENCES
- 1.Bennett MR, Sinha S, Owens GK. Vascular Smooth Muscle Cells in Atherosclerosis. Circ Res. 2016;118:692–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Newby AC and Zaltsman AB. Molecular mechanisms in intimal hyperplasia. J Pathol. 2000;190:300–9. [DOI] [PubMed] [Google Scholar]
- 3.Louis SF and Zahradka P. Vascular smooth muscle cell motility: From migration to invasion. Exp Clin Cardiol. 2010;15:e75–85. [PMC free article] [PubMed] [Google Scholar]
- 4.Gomez D and Owens GK. Smooth muscle cell phenotypic switching in atherosclerosis. Cardiovasc Res. 2012;95:156–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collins MJ, Li X, Lv W, Yang C, Protack CD, Muto A, Jadlowiec CC, Shu C, Dardik A. Therapeutic strategies to combat neointimal hyperplasia in vascular grafts. Expert Rev Cardiovasc Ther. 2012;10:635–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mitchell RN and Libby P. Vascular remodeling in transplant vasculopathy. Circulation research. 2007;100:967–78. [DOI] [PubMed] [Google Scholar]
- 7.Ang H, Lin J, Huang YY, Chong TT, Cassese S, Joner M, Foin N. Drug-Coated Balloons: Technologies and Clinical Applications. Curr Pharm Des. 2018;24:381–396. [DOI] [PubMed] [Google Scholar]
- 8.Chaabane C, Otsuka F, Virmani R, Bochaton-Piallat ML. Biological responses in stented arteries. Cardiovasc Res. 2013;99:353–63. [DOI] [PubMed] [Google Scholar]
- 9.Masiero G, Mojoli M, Ueshima D, Tarantini G. Current concepts on coronary revascularization using BRS in patients with diabetes and small vessels disease. J Thorac Dis. 2017;9:S940–S949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alraies MC, Darmoch F, Tummala R, Waksman R. Diagnosis and management challenges of in-stent restenosis in coronary arteries. World J Cardiol. 2017;9:640–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Price MJ, Saito S, Shlofmitz RA, Spriggs DJ, Attubato M, McLaurin B, Popma Almonacid A, Brar S, Liu M, Moe E, Mehran R. First Report of the Resolute Onyx 2.0-mm Zotarolimus-Eluting Stent for the Treatment of Coronary Lesions With Very Small Reference Vessel Diameter. JACC Cardiovasc Interv. 2017;10:1381–1388. [DOI] [PubMed] [Google Scholar]
- 12.Mitra SK, Hanson DA, Schlaepfer DD. Focal adhesion kinase: in command and control of cell motility. Nat Rev Mol Cell Biol. 2005;6:56–68. [DOI] [PubMed] [Google Scholar]
- 13.Mitra SK and Schlaepfer DD. Integrin-regulated FAK-Src signaling in normal and cancer cells. Curr Opin Cell Biol. 2006;18:516–23. [DOI] [PubMed] [Google Scholar]
- 14.MacLeod DC, Strauss BH, de Jong M, Escaned J, Umans VA, van Suylen RJ, Verkerk A, de Feyter PJ, Serruys PW. Proliferation and extracellular matrix synthesis of smooth muscle cells cultured from human coronary atherosclerotic and restenotic lesions. J Am Coll Cardiol. 1994;23:59–65. [DOI] [PubMed] [Google Scholar]
- 15.Xu J and Shi GP. Vascular wall extracellular matrix proteins and vascular diseases. Biochim Biophys Acta. 2014;1842:2106–2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Han M, Wen JK, Zheng B, Liu Z, Chen Y. Blockade of integrin beta3-FAK signaling pathway activated by osteopontin inhibits neointimal formation after balloon injury. Cardiovasc Pathol. 2007;16:283–90. [DOI] [PubMed] [Google Scholar]
- 17.Brown XQ, Bartolak-Suki E, Williams C, Walker ML, Weaver VM, Wong JY. Effect of substrate stiffness and PDGF on the behavior of vascular smooth muscle cells: implications for atherosclerosis. J Cell Physiol. 2010;225:115–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taylor JM, Mack CP, Nolan K, Regan CP, Owens GK, Parsons JT. Selective expression of an endogenous inhibitor of FAK regulates proliferation and migration of vascular smooth muscle cells. Mol Cell Biol. 2001;21:1565–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sayers RL, Sundberg-Smith LJ, Rojas M, Hayasaka H, Parsons JT, Mack CP, Taylor JM. FRNK expression promotes smooth muscle cell maturation during vascular development and after vascular injury. Arterioscler Thromb Vasc Biol. 2008;28:2115–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bae YH, Mui KL, Hsu BY, Liu SL, Cretu A, Razinia Z, Xu T, Pure E, Assoian RK. A FAK-Cas-Rac-lamellipodin signaling module transduces extracellular matrix stiffness into mechanosensitive cell cycling. Sci Signal. 2014;7:ra57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mui KL, Bae YH, Gao L, Liu SL, Xu T, Radice GL, Chen CS, Assoian RK. N-Cadherin Induction by ECM Stiffness and FAK Overrides the Spreading Requirement for Proliferation of Vascular Smooth Muscle Cells. Cell Rep. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klein EA, Yin L, Kothapalli D, Castagnino P, Byfield FJ, Xu T, Levental I, Hawthorne E, Janmey PA, Assoian RK. Cell-cycle control by physiological matrix elasticity and in vivo tissue stiffening. Current biology : CB. 2009;19:1511–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bond M, Sala-Newby GB, Newby AC. Focal adhesion kinase (FAK)-dependent regulation of S-phase kinase-associated protein-2 (Skp-2) stability. A novel mechanism regulating smooth muscle cell proliferation. The Journal of biological chemistry. 2004;279:37304–10. [DOI] [PubMed] [Google Scholar]
- 24.Bond M, Sala-Newby GB, Wu YJ, Newby AC. Biphasic effect of p21Cip1 on smooth muscle cell proliferation: role of PI 3-kinase and Skp2-mediated degradation. Cardiovasc Res. 2006;69:198–206. [DOI] [PubMed] [Google Scholar]
- 25.Tanner FC, Yang ZY, Duckers E, Gordon D, Nabel GJ, Nabel EG. Expression of cyclin-dependent kinase inhibitors in vascular disease. Circ Res. 1998;82:396–403. [DOI] [PubMed] [Google Scholar]
- 26.Calalb MB, Polte TR, Hanks SK. Tyrosine phosphorylation of focal adhesion kinase at sites in the catalytic domain regulates kinase activity: a role for Src family kinases. Mol Cell Biol. 1995;15:954–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lim ST, Chen XL, Lim Y, Hanson DA, Vo TT, Howerton K, Larocque N, Fisher SJ, Schlaepfer DD, Ilic D. Nuclear FAK promotes cell proliferation and survival through FERM-enhanced p53 degradation. Mol Cell. 2008;29:9–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ossovskaya V, Lim ST, Ota N, Schlaepfer DD, Ilic D. FAK nuclear export signal sequences. FEBS Lett. 2008;582:2402–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lim ST, Miller NL, Chen XL, Tancioni I, Walsh CT, Lawson C, Uryu S, Weis SM, Cheresh DA, Schlaepfer DD. Nuclear-localized focal adhesion kinase regulates inflammatory VCAM-1 expression. The Journal of cell biology. 2012;197:907–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Luo SW, Zhang C, Zhang B, Kim CH, Qiu YZ, Du QS, Mei L, Xiong WC. Regulation of heterochromatin remodelling and myogenin expression during muscle differentiation by FAK interaction with MBD2. EMBO J. 2009;28:2568–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cardoso AC, Pereira AHM, Ambrosio ALB, Consonni SR, Rocha de Oliveira R, Bajgelman MC, Dias SMG, Franchini KG. FAK Forms a Complex with MEF2 to Couple Biomechanical Signaling to Transcription in Cardiomyocytes. Structure. 2016;24:1301–1310. [DOI] [PubMed] [Google Scholar]
- 32.Canel M, Byron A, Sims AH, Cartier J, Patel H, Frame MC, Brunton VG, Serrels B, Serrels A. Nuclear FAK and Runx1 Cooperate to Regulate IGFBP3, Cell-Cycle Progression, and Tumor Growth. Cancer Res. 2017;77:5301–5312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Serrels A, Lund T, Serrels B, et al. Nuclear FAK controls chemokine transcription, Tregs, and evasion of anti-tumor immunity. Cell. 2015;163:160–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sun S, Wu HJ, Guan JL. Nuclear FAK and its kinase activity regulate VEGFR2 transcription in angiogenesis of adult mice. Sci Rep. 2018;8:2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thiagarajan PS, Sinyuk M, Turaga SM, et al. Cx26 drives self-renewal in triple-negative breast cancer via interaction with NANOG and focal adhesion kinase. Nat Commun. 2018;9:578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev. 2004;84:767–801. [DOI] [PubMed] [Google Scholar]
- 37.Mack CP. Signaling mechanisms that regulate smooth muscle cell differentiation. Arterioscler Thromb Vasc Biol. 2011;31:1495–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Molkentin JD. The zinc finger-containing transcription factors GATA-4, −5, and −6. Ubiquitously expressed regulators of tissue-specific gene expression. J Biol Chem. 2000;275:38949–52. [DOI] [PubMed] [Google Scholar]
- 39.Perlman H, Suzuki E, Simonson M, Smith RC, Walsh K. GATA-6 induces p21(Cip1) expression and G1 cell cycle arrest. J Biol Chem. 1998;273:13713–8. [DOI] [PubMed] [Google Scholar]
- 40.Yin F and Herring BP. GATA-6 can act as a positive or negative regulator of smooth muscle-specific gene expression. J Biol Chem. 2005;280:4745–52. [DOI] [PubMed] [Google Scholar]
- 41.Abizaid A Sirolimus-eluting coronary stents: a review. Vasc Health Risk Manag. 2007;3:191–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xie Y, Jin Y, Merenick BL, et al. Phosphorylation of GATA-6 is required for vascular smooth muscle cell differentiation after mTORC1 inhibition. Sci Signal. 2015;8:ra44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Belaguli NS, Sepulveda JL, Nigam V, Charron F, Nemer M, Schwartz RJ. Cardiac tissue enriched factors serum response factor and GATA-4 are mutual coregulators. Molecular and cellular biology. 2000;20:7550–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chang DF, Belaguli NS, Iyer D, Roberts WB, Wu SP, Dong XR, Marx JG, Moore MS, Beckerle MC, Majesky MW, Schwartz RJ. Cysteine-rich LIM-only proteins CRP1 and CRP2 are potent smooth muscle differentiation cofactors. Developmental cell. 2003;4:107–18. [DOI] [PubMed] [Google Scholar]
- 45.Wirth A, Benyo Z, Lukasova M, Leutgeb B, Wettschureck N, Gorbey S, Orsy P, Horvath B, Maser-Gluth C, Greiner E, Lemmer B, Schutz G, Gutkind JS, Offermanns S. G12-G13-LARG-mediated signaling in vascular smooth muscle is required for salt-induced hypertension. Nat Med. 2008;14:64–8. [DOI] [PubMed] [Google Scholar]
- 46.Weis SM, Lim ST, Lutu-Fuga KM, Barnes LA, Chen XL, Gothert JR, Shen TL, Guan JL, Schlaepfer DD, Cheresh DA. Compensatory role for Pyk2 during angiogenesis in adult mice lacking endothelial cell FAK. J Cell Biol. 2008;181:43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sata M, Maejima Y, Adachi F, Fukino K, Saiura A, Sugiura S, Aoyagi T, Imai Y, Kurihara H, Kimura K, Omata M, Makuuchi M, Hirata Y, Nagai R. A mouse model of vascular injury that induces rapid onset of medial cell apoptosis followed by reproducible neointimal hyperplasia. J Mol Cell Cardiol. 2000;32:2097–104. [DOI] [PubMed] [Google Scholar]
- 48.Liu R, Jin Y, Tang WH, Qin L, Zhang X, Tellides G, Hwa J, Yu J, Martin KA. Ten-eleven translocation-2 (TET2) is a master regulator of smooth muscle cell plasticity. Circulation. 2013;128:2047–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kobayashi M, Inoue K, Warabi E, Minami T, Kodama T. A simple method of isolating mouse aortic endothelial cells. J Atheroscler Thromb. 2005;12:138–42. [DOI] [PubMed] [Google Scholar]
- 50.Lim ST, Chen XL, Tomar A, Miller NL, Yoo J, Schlaepfer DD. Knock-in mutation reveals an essential role for focal adhesion kinase activity in blood vessel morphogenesis and cell motility-polarity but not cell proliferation. J Biol Chem. 2010;285:21526–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bertoli C, Skotheim JM, de Bruin RA. Control of cell cycle transcription during G1 and S phases. Nat Rev Mol Cell Biol. 2013;14:518–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rojas A, Kong SW, Agarwal P, Gilliss B, Pu WT, Black BL. GATA4 is a direct transcriptional activator of cyclin D2 and Cdk4 and is required for cardiomyocyte proliferation in anterior heart field-derived myocardium. Mol Cell Biol. 2008;28:5420–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kaiser M, Weyand CM, Bjornsson J, Goronzy JJ. Platelet-derived growth factor, intimal hyperplasia, and ischemic complications in giant cell arteritis. Arthritis Rheum. 1998;41:623–33. [DOI] [PubMed] [Google Scholar]
- 54.Sirois MG, Simons M, Edelman ER. Antisense oligonucleotide inhibition of PDGFR-beta receptor subunit expression directs suppression of intimal thickening. Circulation. 1997;95:669–76. [DOI] [PubMed] [Google Scholar]
- 55.van Berlo JH, Elrod JW, Aronow BJ, Pu WT, Molkentin JD. Serine 105 phosphorylation of transcription factor GATA4 is necessary for stress-induced cardiac hypertrophy in vivo. Proc Natl Acad Sci U S A. 2011;108:12331–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hirschi KK, Rohovsky SA, D’Amore PA. PDGF, TGF-beta, and heterotypic cell-cell interactions mediate endothelial cell-induced recruitment of 10T½ cells and their differentiation to a smooth muscle fate. J Cell Biol. 1998;141:805–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shapiro IM, Kolev VN, Vidal CM, Kadariya Y, Ring JE, Wright Q, Weaver DT, Menges C, Padval M, McClatchey AI, Xu Q, Testa JR, Pachter JA. Merlin deficiency predicts FAK inhibitor sensitivity: a synthetic lethal relationship. Sci Transl Med. 2014;6:237ra68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mano T, Luo Z, Malendowicz SL, Evans T, Walsh K. Reversal of GATA-6 downregulation promotes smooth muscle differentiation and inhibits intimal hyperplasia in balloon-injured rat carotid artery. Circ Res. 1999;84:647–54. [DOI] [PubMed] [Google Scholar]
- 59.Walker HA, Whitelock JM, Garl PJ, Nemenoff RA, Stenmark KR, Weiser-Evans MC. Perlecan up-regulation of FRNK suppresses smooth muscle cell proliferation via inhibition of FAK signaling. Mol Biol Cell. 2003;14:1941–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lagares D, Busnadiego O, Garcia-Fernandez RA, et al. Inhibition of focal adhesion kinase prevents experimental lung fibrosis and myofibroblast formation. Arthritis Rheum. 2012;64:1653–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kinoshita K, Aono Y, Azuma M, Kishi J, Takezaki A, Kishi M, Makino H, Okazaki H, Uehara H, Izumi K, Sone S, Nishioka Y. Antifibrotic effects of focal adhesion kinase inhibitor in bleomycin-induced pulmonary fibrosis in mice. Am J Respir Cell Mol Biol. 2013;49:536–43. [DOI] [PubMed] [Google Scholar]
- 62.Zhao XK, Yu L, Cheng ML, et al. Focal Adhesion Kinase Regulates Hepatic Stellate Cell Activation and Liver Fibrosis. Sci Rep. 2017;7:4032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tanjoni I, Walsh C, Uryu S, et al. PND-1186 FAK inhibitor selectively promotes tumor cell apoptosis in three-dimensional environments. Cancer Biology & Therapy. 2010;9:762–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.