Abstract
Despite intense research efforts, maintenance of sinus rhythm in patients with non-paroxysmal AF remains challenging with suboptimal outcomes. A major limitation to the success of current ablation-based treatments is that our understanding of AF pathophysiology is incomplete. Advances in imaging and mapping tools have been reported to improve ablation outcomes. However, the role of these new approaches on the clinical care of patients with AF remains to be validated and better understood before wide adoption can occur. This article reviews the current techniques of imaging and mapping that can be applied in the management of patients with non-paroxysmal AF with a focus on their relevance to catheter ablation. Future applications and opportunities for new knowledge are also discussed.
Keywords: AF, catheter ablation, atrial mapping, atrial imaging, computed tomography, cardiac MRI
Maintenance of sinus rhythm in patients with non-paroxysmal AF is often challenging and complex. Catheter ablation is usually superior to anti-arrhythmic drug therapy alone. However, recurrence rates are high and have remained suboptimal. Although pulmonary vein isolation (PVI) is usually effective in treating paroxysmal AF, it is not sufficient for many patients with non-paroxysmal AF, particularly those with long-standing persistent AF. A major limitation is that our understanding of mechanisms of AF is incomplete. Electroanatomical remodelling adds substantially to the complexity of AF. Recent advances in imaging and mapping technology may facilitate better understanding and mapping of AF and subsequently improve outcomes of therapy.
The purpose of this article is to review current techniques of imaging and mapping that can be applied in the management of patients with non-paroxysmal AF with a focus on their relevance to catheter ablation. Future applications and opportunities for new knowledge will also be discussed.
Mechanisms of AF
Descriptions of AF wavefront properties are influenced by the study tools (optical mapping versus electroanatomical contact or noncontact mapping) and can explain some of the discrepancies in experimental studies and clinical observations.[1,2] Imaging studies have shown a close association with abnormal atrial architecture and clinical AF, but a unified model relating ultrastructural remodelling to arrhythmia maintenance is less than complete. Multidisciplinary approaches incorporating imaging, basic science, computer modelling, and clinical observations are vital for addressing these knowledge gaps.
Current models of non-paroxysmal AF often focus on both triggers and an atrial substrate able to perpetuate the arrhythmia. For patients with non-paroxysmal AF, ectopic triggers can include pulmonary vein and non-pulmonary vein triggers such as the superior vena cava, coronary sinus or crista terminalis.[3] Electroanatomical remodelling facilitates trigger formation, shortening of atrial refractory periods and the promotion of fibrosis.[4–6] Elimination of focal triggers in addition to PVI improves ablation outcomes.[7]
Two competing theories on AF maintenance have been proposed and have formed the basis for various ablation techniques. The first is the multiple wavelet theory, which describes AF as self-perpetuating rhythm independent of focal discharges but rather supported by a critical mass of myocardium allowing constant formation and dispersal of wavelets.[8,9] The second is a localised source model in which focal areas of re-entry or discharges sustain AF. These organised regions result in disorganised fibrillation due to wave-break as the impulses encounter tissue with anisotropy and conduction heterogeneity.[10] These competing mechanisms may not be mutually exclusive, particularly in patients with non-paroxysmal AF.[11]
Contemporary ablation strategies for non-paroxysmal AF are based on elimination of prevalent triggers (including pulmonary vein and other thoracic arrhythmogenic sites), dynamic real-time mapping of drivers of AF such as rotors and focal discharges or modification of the atrial substrate using a combination of anatomical lesion sets.
These approaches have been used alone or in combination with variable clinical outcomes. Hybrid techniques such as combined endocardial and epicardial ablation have been proposed to target the posterior wall and epicardial sources of AF.[12] Modulation of autonomic inputs through ablation of ganglionated plexi have also been considered as an adjunctive ablation strategy.[13]
Cardiac and Thoracic Imaging
Multimodality imaging can improve management of patients with non-paroxysmal AF and assist with ablation strategies (Figure 1). Traditional measurements of atrial size and morphology have been supplemented with functional and structural assessments through techniques such as strain imaging and late-gadolinium enhanced cardiac magnetic resonance imaging (LGE-CMR). Nuclear imaging such as PET can measure atrial inflammation and metabolism as it pertains to AF.[14–16] In a recent pilot study, left atrial uptake of [18]F-fluorodeoxyglucose was assessed through PET-CT scans.[16] Left atrial metabolism and scarring were found to be increased in patients with greater AF burdens. Further validation of these novel techniques is needed. Intraprocedural imaging, including intracardiac echocardiography and 3D rotational angiography, can be integrated with fluoroscopy and electroanatomical mapping to provide real-time information of challenging anatomy.[17] Current clinical applications of imaging include procedural planning, patient selection, prognostication of arrhythmia recurrence and evaluation of postoperative complications, such as pulmonary vein stenosis.
Figure 1: Left Atrial Imaging.
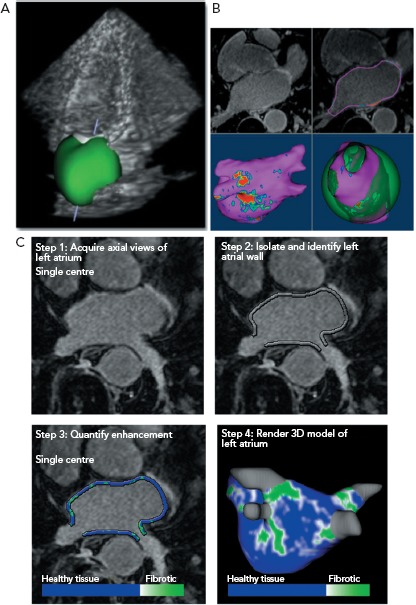
A: The use of 3D ultrasound to evaluate left atrial volume and functional parameters. An automated 3D reconstruction is superimposed on a greyscale echocardiography dataset. B: Cardiac MRI reconstruction of left atrial volume and morphology. Reconstruction of the 3D anatomic shell can be compared to a theoretical sphere shaped atrium to calculate the sphericity, which may hold prognostic significance. C: Late gadolinium enhanced cardiac MRI can be used to measure location of atrial fibrosis. The extent of fibrosis can be used to categorise patients into a novel scoring system, which has prognostic importance for arrhythmia recurrence after ablation procedures. Sources: Mor-Avi et al.;[21] Siebermai et al.[58] Reproduced with permission from Elsevier. den Uijl et al.[24] Reproduced with permission from John Wiley and Sons.
Atrial Size and Morphology
Atrial volume can be measured through a variety of non-invasive modalities including echocardiography, CT or CMR imaging with good agreement among the studies.[18–21] 3D echocardiography is more accurate and reproducible than 2D echocardiography and is preferred when available (Figure 1).[22] Increased atrial size is linked to poorer outcomes after ablation particularly in patients with non-paroxysmal AF.[23] Abnormal atrial morphology (increased sphericity) is a marker of advanced remodelling and also a predictor of poor outcomes including stroke (Figure 1B).[24,25] These phenotypic changes identify patients who may require more aggressive management to maintain sinus rhythm and prevent thromboembolic complications.
Anatomic variation in pulmonary vein anatomy is observed in 20–30% of patients undergoing catheter ablation and may impact long-term ablation outcomes.[26–28] Pre-procedural imaging of the pulmonary veins can be helpful in selecting an ablation strategy although the impact on outcomes, procedural safety, fluoroscopic exposure and long-term costs are less established.[29–31] Cross-sectional imaging can also identify the course of thoracic structures such as phrenic vein and coronary arteries. Understanding the proximity of these structures to the atrium can be critical when ablating near the left atrial appendage (LAA) or left lateral ridge,both of which are commonly targeted in patients with non-paroxysmal AF.[32] The left main coronary artery and left circumflex artery may course near the LAA ostia and cases of ablation-related coronary vasospasm have been reported.[32] Integrating electroanatomical data, intracardiac ultrasound and cross-sectional imaging is often used to avoid these complications. The oesophagus and its relationship to the posterior atrial wall can be visualised on cross-sectional imaging and superimposed into electroanatomical mapping systems, which may avoid ablation related oesophageal injury. Due to lateral oesophageal motility that occurs during ablation procedures,real-time monitoring of the oesophageal location and luminal temperature is likely superior to a static pre-procedural assessment.[33]
Atrial Wall Thickness
The atrium is a thin-walled, pliable structure whose size and geometry are dependent upon volume status and loading conditions. There is regional and inter-patient variability in wall thickness; pathologic and in vivo studies generally agree that the average left atrial wall thickness is between 1–4 mm and can range between 0.5–12 mm.[22,34] There is an age-related increase in left atrial wall thickness and men generally have thicker atrial walls than women.[35,36] While atrial volumes and structure are impacted by AF, a direct relationship between atrial wall thickness and AF burden is less clearly demonstrated.[37–39] Given its high spatial resolution, CT can most accurately measure atrial wall thickness in vivo although this is not without limitations. CT-based measurements are generally reported to be lower than ex vivo tissue samples, which may relate to the importance of loading conditions, the effect of pathological sample preparation or an inherent limitation to CT scanning.[11]
Assessment of left atrial wall thickness may have implications for patients with non-paroxysmal AF undergoing ablation procedures. Takahashi et al. performed high-resolution CT scans on 50 patients with AF and compared this to 25 control patients without AF. There was an increase in the thickness of the pulmonary vein–left atrial wall junction that was dependent on AF burden. Patients with thicker walls had higher incidences of ATP-provoked dormant conduction.[38] Suenari et al. demonstrated that regional atrial thickness at the left lateral ridge was an independent predictor or arrhythmia recurrence after PVI.[40] In a retrospective study of patients with non-paroxysmal AF undergoing LAA isolation, ostial wall thickness measured on pre-procedural CT scan was found to correlate with electrical reconnection. A retrospective evaluation of patients undergoing PVI reported that the ratio of ablation lesion force-time-integral to the underlying atrial wall thickness could accurately predict conduction gaps and dormant conduction.[41]
Wall thickness information could facilitate a strategy of tailored ablation energy delivery. Ablation energy delivery is generally titrated both by electrogram parameters (impedance changes and electrogram attenuation) as well as empirically based on anatomic location, with lower power delivery along the posterior wall and higher power delivery along thicker structures such as the left atrial ridge. This paradigm does not account for the wide range of wall thickness seen through all regions of the atrium. In a study of 60 patients with persistent AF undergoing catheter ablation, there was a similar range of wall thickness in areas normally found to be thinner such as the posterior wall (0.7–3.1 mm), as there were in thicker regions such as the left lateral ridge (0.5–3.5 mm) and mitral isthmus origin (0.9–2.8 mm). Whether a wall-thickness-guided ablation strategy would improve lesion durability is unclear. Opportunities for study also exist with other forms of titratable ablation energy such as the visually-guided laser balloon ablation system.[42] Investigational ablation technologies using low-intensity collimated ultrasound can directly measure left atrial wall thickness which may in the future be incorporated into dosing strategies.[43]
Atrial Strain
Pathological atrial remodelling can lead to chamber dilation, geometric distortion and fibrosis, which may lead to a substrate for arrhythmias including AF. These structural alterations also affect wall compliance and myocyte contractility with subsequent impairment of the normal atrial function as a conduit, reservoir and a contractile chamber. Real-time imaging with echocardiography and CMR can quantify atrial function through atrial strain assessment, which has been correlated with adverse cardiovascular outcomes include atrial arrhythmias.[44–46] Abnormal atrial strain is associated with an increased incidence of AF as well as higher post-ablation recurrence rates in patients with paroxysmal and non-paroxysmal AF.[47–49] In a study of 65 patients with paroxysmal and persistent AF who underwent multi-modality imaging, abnormal atrial strain parameters correlated with the extent of LGE-CMR derived atrial fibrosis independent of left atrial volumes.[50] Atrial strain and the extent of fibrosis were more severe in patients with persistent rather than paroxysmal AF. These observations highlight the overlapping relationship between structural, mechanical, and electrical atrial remodelling.
Unlike traditional measures of left atrial compliance and function that rely on pulmonary vein and transmitral flow, atrial strain can be assessed while in sinus rhythm or AF.[36] Wall strain may also be a useful surrogate for assessing wall fibrosis as measured on LGE-MRI[51] and correlates with ablation outcomes.
Atrial Fibrosis
Advances in spatial and temporal resolution of CMR imaging allow for accurate imaging of the thin-walled atrial structures (Figure 1C). Multiple parameters including atrial wall thickness, functional left atrial parameters and fibrosiscan be obtained simultaneously through CMR.[44,52,53] Assessment of atrial fibrosis through LGE-CMR has been performed for over a decade and has been shown to correlate spatially with both histopathological samples and endocardial voltage mapping.[54–56] However, there can be significant technical challenges that limit its broad applicability. Patients can be imaged while in AF although both irregular and rapid heart rates can limit image quality or render fibrosis assessment impossible.[57–59] Rhythm and rate control prior to imaging can be challenging in patients with non-paroxysmal AF. Cardiac devices may be sources of significant artifact.[60] Beyond image acquisition challenges, interpretation of LGE-CMR images is variable among centres and the optimal processing technique remains to be better described. Many protocols require expert operator input to select appropriate detection thresholds,limiting the external validation of these studies.[61] Despite these current challenges, inter- and intra-observer agreement in high-volume centres remains high.[62]
The efficacy of DE-MRI-guided ablation vs. Conventional catheter Ablation of Atrial Fibrillation (DECAAF) study was a large multicentre observational study of patients with AF undergoing catheter based ablation who had LGE-CMR performed prior to their procedures.[63] Of the 260 patients included in the final analysis, 75 (28.8%) had persistent AF and 17 (6.5%) permanent AF. The majority of patients had PVI only (68.1%). Arrhythmia recurrence post-ablation was related to the extent of global atrial fibrosis (unadjusted HR 1.06; 95% CI [1.03–10.8]; p<0.001) and was independent of covariates including age, sex, hypertension, congestive heart failure, left ventricular ejection fraction, left atrial volume or AF subtype among others. Similar relationships between the degree of left atrial fibrosis and outcomes after ablation have been shown in single centre studies.[64,65] LGE-MRI has also been used to evaluate left atrial substrate post-catheter ablation. Applications such as assessment of lesion quality, identifying conduction gaps, or quantifying residual fibrosis post-ablation have also been reported.[60,65–70] However, the clinical significance of these approaches remains to be determined.[71]
Given the correlation of atrial fibrosis and ablation outcomes in observational studies, LGE-CMR could be used in selection of ablation candidates. In the DECAAF study, the addition of fibrosis data to traditional clinical risk factors to recurrence prediction models resulted in a small but significant increase in prognostic accuracy for the recurrence of AF (risk difference 0.05; 95% CI [0.01–0.09]). The clinical utility of such strategies has not been prospectively investigated.
For patients with non-paroxysmal AF, LGE-CMR defined fibrosis may be used to guide the ablation strategy by providing a personalised ablation approach. A sub-study of the DECAAF trial examined 177 patients with follow up LGE-CMR performed 90 days after their initial ablation procedure. The amount of residual fibrosis (pre-ablation fibrosis that was not targeted through ablation lesions) was associated with arrhythmia occurrence.[72] Targeting LGE-CMR defined fibrosis may improve ablation outcomes and is the focus of the on-going DECAAF-2 trial.[73] Novel strategies incorporating LGE-MRI defined substrate into in silico atrial models to predict optimal ablation sites are also under investigation.[74]
Given the challenges in technique and reproducibility and lack of prospective studies, the current role of LGE-CMR for management of patients with non-paroxysmal AF remains limited. LGE-CMR defined fibrosis may harbour critical drivers of persistent AF.[75] Substrate-based ablation strategies (targeting fibrosis and channels based on atrial voltage mapping) have shown some efficacy but in a randomised clinical trial did not add benefit compared to traditional step-wise ablation strategies.[76–78] In lieu of specific targets, quantifying fibrosis may guide ablation by further refining classification of AF beyond the current paradigm (paroxysmal, persistent, long-standing persistent).[79] The degree of remodelling and fibrosis does not necessarily correlate directly with AF subtype,[63,76] which may explain in part why some patients with paroxysmal AF require more extensive ablation for clinical success, while others with non-paroxysmal AF have success with PVI alone.[78,80,81]
Mapping Atrial Electrical Activity
AF can be triggered or sustained by focal drivers that cluster in specific regions of the left and right atrium.[82] These regions are more prevalent in patients with non-paroxysmal than paroxysmal AF likely due to advanced remodelling associated with greater AF burden. Early strategies aimed at ablating these critical regions were based on analysis of local bipolar electrograms assessed on a point-by-point basis during AF. The various electrogram morphologies were thought to represent evidence of electrophysiological phenomenon associated with AF drivers such as high-frequency discharges, shortened refractory periods, micro-re-entry, or local conduction block.[10,83] Such approaches include complex fractionated electrograms (CFAE),dominant frequency analysis, high Shannon entropy, or sites with activity spanning large portions of the AF cycle length.[84–86] These approaches have been the subject of multiple clinical trials with equivocal results regarding their net clinical benefit, partially due to the subjective nature of identifying such sites and the low-specificity of these sites in locating critical drivers.[87]
CFAE ablation has been one of the more commonly performed mapping approaches, with various mapping systems incorporating automated detection algorithms.[83] Specific criteria for CFAEs have been proposed but most often operators will assess points manually, reducing the reproducibility of these techniques.
CFAE-based ablation strategies in addition to PVI in patients with persistent AF were the subject of the multicentre Substrate and Trigger Ablation for Reduction of Atrial Fibrillation II (STAR AF II) trial.[88] In this multicentre, randomised controlled trial, 589 patients were assigned to undergo PVI alone, or PVI with the addition of linear lesions, or the addition of left atrial CFAE ablation. The addition of CFAE ablation was associated with longer procedural and fluoroscopic times without improved long-term freedom from AF (18 month freedom from AF 59% versus 49%; p=0.15 for between-group differences). However, in another study CFAE ablation as a component of a stepwise ablation strategy aimed at restoration of sinus rhythm via ablation improved outcomes in patients with persistent and long-standing persistent AF.[89]
Non-invasive Mapping of AF
As opposed to point-by-point electrogram analysis, newer techniques can provide real-time mapping of electrical activity simultaneously across the atrium (Figure 2). These wide-field mapping techniques allows for direct visualisation of driver activity and assessment of their temporal stability. These techniques have provided key insights into the mechanism of AF and have corroborated with some observations made using optical and in silico mapping techniques.[90–92]
Figure 2: Mapping of Atrial Electrical Activity.
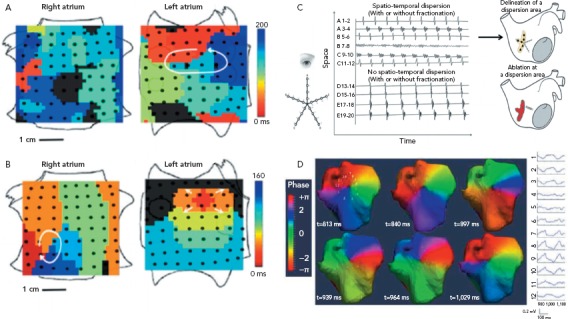
Invasive and non-invasive mapping techniques integrate multiple local and far-field electrocardiogram signals to represent wavefront propagation during AF. Analysis of these visualisations can help localise focal drivers of AF or the substrate necessary to sustain the arrhythmia. Panels A and B demonstrate simultaneous, bi-atrial activation maps created with a novel mapping system (Abbott). A: Left atrial rotor with counterclockwise activation (red to blue) with passive activation of the right atrium. B: Two focal sources present simultaneously, a counterclockwise right atrial rotor as well as a focal left atrial source showing centrifugal activation. C: Dispersion mapping using a PENTARAY™ (Biosense Webster) with both spatial and temporal dispersion indicating sites of interest. D: Epicardial phase mapping during AF generated by a noninvasive body mapping system (ECVUE, CardioInsight Technologies). Rotational activation is demonstrated in the posterior right upper atrium. Sources: Narayan et al.;[102] Seitz et al.[105] Reproduced with permission from Elsevier. Haissaguerre et al.[94] Reproduced with permission from Wolters Kluwer Health.
Body surface potentials generated from atrial electrical activity can be used to create real-time epicardial activation maps (Figure 2D). This approach was first used to non-invasively map AF by Guillem and colleagues using phase maps generated by 56 electrodes placed on the chest and back.[92] Additional techniques using 256-electrode vests integrated with CT imaging have been developed to provide high resolution (6 mm) 3D electroanatomical mapping.[93] Similar electrocardiographic imaging (ECGI) systems are available commercially and have been integrating into AF ablation strategies. Haissaguerre et al. used an ECGI system in 103 patients with persistent AF undergoing a driver-based ablation strategy and compared this with a historical control group who had undergone a step-wise ablation approach.[94] Patients underwent ablation starting in regions with the highest density of drivers with additional linear lesions made if AF failed to terminate. There was a median of four driver regions per patient, and the number of drivers increased with AF burden. As compared with the control group, patients undergoing the targeted ablation approach had similar clinical results (1 year freedom from AF 85% versus 87% in control group; p=non-significant) with less radiofrequency energy delivery (35±21 versus 65±33 minutes; p<0.0001). Similar results from multicentre studies using non-contact mapping of persistent AF have been reported[95] with favourable long-term outcomes (77% 1-year freedom from AF).
There are several limitations to non-invasive mapping that may limit its clinical applicability. Electrical activity generated by the atrium is generally low amplitude when measured on body surface electrodes, which may degrade signal quality. These systems reconstruct signals from epicardial structures only while there may be differential endocardial-epicardial activation during AF.[96] Sites identified as focal drivers could simply represent a wavefront breakthrough site from a passive endocardial structure not critical to AF propagation. Importantly, far-field signal contamination remains as a major challenge. Finally, the inter-atrial septum and LAA, which may harbour sites critical for AF, are poorly visualised with this technique.[93,97,98]
Invasive Mapping of AF
In a prior study, a multielectrode array catheter (EnSite™, Abbott) was used to reconstruct virtual unipolar electrograms from 64 non-contact electrodes to display voltage and activation maps on a 3D anatomical map during AF.[99] Noncontact mapping (NCM) has been used to map AF activation patterns,[99,100] although some studies did not find that rotors or focal sources were prevalent or necessary for AF propagation. NCM has been used successfully to identify conduction gaps in linear lesions sets, localise premature atrial contractures and target atrial tachycardias.[101] However, the use of NCM to ablate AF sources has not been described.
Focal impulse and rotor modulation (FIRM) mapping uses bi-atrial contact basket catheters along with a novel mapping system (Topera, Abbott; Figures 2A and 2B). This approach was the focus of the CONventional ablation for atrial fibrillation with or without Focal Impulse and Rotor Modulation (CONFIRM) trial, a single centre prospective randomised controlled trial of 92 patients undergoing ablation of AF (72% with persistent AF).[102] All patients underwent FIRM mapping and were randomised in 1:2 fashion to undergo FIRM directed ablation followed by conventional ablation versus a conventional ablation approach alone. In this series, rotors and focal impulses were seen in 97% of patients, demonstrated temporal stability of at least 10 minutes and were more numerous in patients with persistent versus paroxysmal AF. The FIRM-guided strategy resulted in higher rates of AF termination or slowing ablation and improved rates of single-procedure freedom from AF (82.4% versus 44.9% after median follow up of 273 days; p<0.001). Similar findings on the spatial and temporal stability of rotors and focal sources have been demonstrated in multicentre registries using the FIRM mapping approach.[82]
Regional atrial mapping with the use of multipole catheters (as opposed to point-by-point mapping or panoramic approaches) has been applied successfully to AF mapping and ablation (Figure 2C). Contact mapping avoids reliance on far-field signal interpretation inherent to non-invasive mapping while the use of multiple simultaneous points provides better analysis of the spatiotemporal components of electrogram surrogates of AF drivers, which may improve the specificity of ablation targets.[103,104] Seitz et al. performed a prospective study of 105 patients with AF, including 80 with non-paroxysmal AF, who underwent AF ablation guided by spatiotemporal dispersion observed with the use of a 20-electrode multispline catheter (PentaRay®, Biosense Webster).[105] These patients were compared with a historical cohort who underwent a conventional ablation approach (PVI followed by stepwise approach for patient with persistent AF). Patients who underwent the dispersion-guided ablation approach had lower rates of AF/AT recurrence after single or multiple procedures (45% versus 64%; log-rank p=0.026 and 15% versus 41%; log-rank p<0.001, respectively). In separate experiments the authors used optical mapping and numerical simulations to recreate their clinical findings of increased dispersion near the vicinity of active drivers. However, a limitation of this approach is that in the absence of a panoramic map of both atria it can be difficult to identify the primary drivers and true activation patterns. These combined experimental and clinical reports suggest that regional contact mapping may be useful tool for mapping and ablating non-paroxysmal AF. However, prospective studies are needed for further validation.
Invasive mapping of AF triggers can be performed with the use of traditional multipolar catheters strategically placed to maximise diagnostic yield.[106] Catheters can be simultaneously placed in regions commonly harbouring arrhythmogenic triggers such as the superior vena cava, cristae terminals, LAA and coronary sinus. AF can then be induced with high-dose isoproterenol infusion and sites of triggers can be targeted for ablation. These triggers may be more prevalent in patients with long-standing persistent AF compared to those with persistent AF and paroxysmal AF.[107] Trigger mapping has been shown in retrospective series to be a useful strategy in patients with prior failed ablations and long-standing persistent AF.[108,109]
Challenges and Future Direction
Real-time mapping to study AF mechanisms and/or guide ablation of non-paroxysmal AF has been implemented in various forms with promising, albeit mixed, results. Such discrepancies are likely because of the heterogeneous nature of non-paroxysmal AF as well as the relative merits and limitations of the mapping and ablation approaches. Acute termination of AF during targeted ablation is often demonstrated supporting the mechanistic validity of these approaches. However, it is unclear if AF termination is a clinically meaningful procedural endpoint.[10] Focal drivers may cluster in regions (such as the antrum of the pulmonary veins or posterior wall) often targeted in conventional ablation approaches, which may further limit the incremental benefit of additional ablation. Overall, superiority to anatomic or stepwise ablation approaches has not been convincingly demonstrated. For example, in the multicentre experience reported by Knecht et al. on the use of non-contact guided mapping of AF, radiofrequency ablation time and long-term clinical success were similar to traditional ablation approaches for persistent AF.[95] Multicentre reports and meta-analyses on the use of FIRM guided ablation have not consistently shown therapeutic benefit compared to traditional ablation strategies.[110–113]
Conclusion
Imaging and mapping technology continue to evolve providing a better understanding of anatomy, arrhythmic substrate and patterns of AF activation. These tools have been successfully implemented into ablation planning and execution at some centres. Future advances in imaging/mapping fidelity and automation could improve ease of use and facilitate real-world implementation.
The outcomes and clinical utility of ablation of predetermined targets based on anatomical landmarks with/without additional ablation of triggers, or tailored ablation of specific targets based on real-time dynamic mapping of AF mechanisms incorporating advanced imaging/mapping systems remain to be determined and will largely depend on further advances in these technologies.[114,115]
Clinical Perspective
Advanced mapping and imaging tools are critical components of investigation and clinical management of patients with non-paroxysmal AF.
Used together, these tools may help facilitate tailored ablation approaches although the prospective use of these techniques compared with existing strategies must be further investigated.
Improvements in mapping and imaging fidelity, automation and reproducibility would help increase the widespread adoption of these techniques.
References
- 1.Zaman JA, Baykaner T, Schricker AA Mechanistic targets for the ablation of atrial fibrillation. Glob Cardiol Sci Pract. 2017. [DOI] [PMC free article] [PubMed]
- 2.Zaman J, Peters NS. The rotor revolution. Circ Arrhythm Electrophysiol. 2014;7:1230–6. doi: 10.1161/CIRCEP.114.002201. [DOI] [PubMed] [Google Scholar]
- 3.Elayi CS, Biase LD, Bai R et al. Administration of isoproterenol and adenosine to guide supplemental ablation after pulmonary vein antrum isolation. J Cardiovasc Electrophysiol. 2013;24:1199–206. doi: 10.1111/jce.12252. [DOI] [PubMed] [Google Scholar]
- 4.Patterson E, Lazzara R, Szabo B et al. Sodium-calcium exchange initiated by the Ca2+ transient: an arrhythmia trigger within pulmonary veins. J Am Coll Cardiol. 2006;47:1196–206. doi: 10.1016/j.jacc.2005.12.023. [DOI] [PubMed] [Google Scholar]
- 5.Goette A, Honeycutt C, Langberg JJ. Electrical remodeling in atrial fibrillation. Circulation. 1996;94:2968–74. doi: 10.1161/01.CIR.94.11.2968. [DOI] [PubMed] [Google Scholar]
- 6.Schricker AA, Lalani GG, Krummen DE et al. Human atrial fibrillation initiates via organized rather than disorganized mechanisms. Circ Arrhythm Electrophysiol. 2014;7:816–24. doi: 10.1161/CIRCEP.113.001289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mohanty S, Mohanty P, Di Biase L et al. Long-term follow-up of patients with paroxysmal atrial fibrillation and severe left atrial scarring: comparison between pulmonary vein antrum isolation only or pulmonary vein isolation combined with either scar homogenization or trigger ablation. Europace. 2017;19:1790–7. doi: 10.1093/europace/euw338. [DOI] [PubMed] [Google Scholar]
- 8.Moe GK, Abildskov JA. Atrial fibrillation as a self-sustaining arrhythmia independent of focal discharge. Am Heart J. 1959;58:59–70. doi: 10.1016/0002-8703(59)90274-1. [DOI] [PubMed] [Google Scholar]
- 9.Haïssaguerre M, Sanders P, Hocini M et al. Catheter ablation of long-lasting persistent atrial fibrillation: critical structures for termination. J Cardiovasc Electrophysiol. 2005;16:1125–37. doi: 10.1111/j.1540-8167.2005.00307.x. [DOI] [PubMed] [Google Scholar]
- 10.Calkins H, Hindricks G, Cappato R et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Europace. 2018;20:e1–160. doi: 10.1093/europace/eux274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Konings KT, Kirchhof CJ, Smeets JR et al. High-density mapping of electrically induced atrial fibrillation in humans. Circulation. 1994;89:1665–80. doi: 10.1161/01.CIR.89.4.1665. [DOI] [PubMed] [Google Scholar]
- 12.Thosani AJ, Gerczuk P, Liu E et al. Closed chest convergent epicardial–endocardial ablation of non-paroxysmal atrial fibrillation – a case series and literature review. Arrhythmia Electrophysiol Rev. 2013;2:65–8. doi: 10.15420/aer.2013.2.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kampaktsis PN, Oikonomou EKY, Choi D et al. Efficacy of ganglionated plexi ablation in addition to pulmonary vein isolation for paroxysmal versus persistent atrial fibrillation: a meta-analysis of randomized controlled clinical trials. J Interv Card Electrophysiol. 2017;50:253–60. doi: 10.1007/s10840-017-0285-z. [DOI] [PubMed] [Google Scholar]
- 14.Dong A, Zhao T, Gong J et al. Diffuse FDG uptake of the bilateral atrial walls in a patient with atrial fibrillation. Clin Nucl Med. 2014;39:167–9. doi: 10.1097/RLU.0b013e318270894c. [DOI] [PubMed] [Google Scholar]
- 15.Kiuchi K, Fukuzawa K, Mori S et al. Feasibility of imaging inflammation in the left atrium post af ablation using pet technology. JACC Clin Electrophysiol. 2017;3:1466–7. doi: 10.1016/j.jacep.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 16.Ghannam M, Yun HJ, Ficaro EP Multiparametric assessment of left atrial remodeling using 18F-FDG PET/CT cardiac imaging: A pilot study. J Nucl Cardiol. 2018. epub ahead of press. [DOI] [PMC free article] [PubMed]
- 17.Potter TJD, Bardhaj G, Viggiano A et al. Three-dimensional rotational angiography as a periprocedural imaging tool in atrial fibrillation ablation. Arrhythmia Electrophysiol Rev. 2014;3:173–6. doi: 10.15420/aer.2014.3.3.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seemann F, Baldassarre LA, Llanos-Chea F et al. Assessment of diastolic function and atrial remodeling by MRI - validation and correlation with echocardiography and filling pressure. Physiol Rep. 2018;6:e13828. doi: 10.14814/phy2.13828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van den Berg NWE. Chan Pin Yin DRPP, Berger WR, et al. Comparison of non-triggered magnetic resonance imaging and echocardiography for the assessment of left atrial volume and morphology. Cardiovasc Ultrasound. 2018;16:17. doi: 10.1186/s12947-018-0134-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kühl JT, Lønborg J, Fuchs A et al. Assessment of left atrial volume and function: a comparative study between echocardiography, magnetic resonance imaging and multi slice computed tomography. Int J Cardiovasc Imaging. 2012;28:1061–71. doi: 10.1007/s10554-011-9930-2. [DOI] [PubMed] [Google Scholar]
- 21.Mor-Avi V, Yodwut C, Jenkins C et al. Real-Time 3D echocardiographic quantification of left atrial volume. JACC Cardiovasc Imaging. 2012;5:769–77. doi: 10.1016/j.jcmg.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 22.Walters TE, Ellims AH, Kalman JM. The role of left atrial imaging in the management of atrial fibrillation. Prog Cardiovasc Dis. 2015;58:136–51. doi: 10.1016/j.pcad.2015.07.010. [DOI] [PubMed] [Google Scholar]
- 23.von Bary C, Dornia C, Eissnert C et al. Predictive value of left atrial volume measured by non-invasive cardiac imaging in the treatment of paroxysmal atrial fibrillation. J Interv Card Electrophysiol. 2012;34:181–8. doi: 10.1007/s10840-011-9641-6. [DOI] [PubMed] [Google Scholar]
- 24.den Uijl DW, Cabanelas N, Benito EM et al. Impact of left atrial volume, sphericity, and fibrosis on the outcome of catheter ablation for atrial fibrillation. J Cardiovasc Electrophysiol. 2018;29:740–6. doi: 10.1111/jce.13482. [DOI] [PubMed] [Google Scholar]
- 25.Bisbal F, Guiu E, Calvo N et al. Left atrial sphericity: a new method to assess atrial remodeling. Impact on the outcome of atrial fibrillation ablation. J Cardiovasc Electrophysiol. 2013;24:752–9. doi: 10.1111/jce.12116. [DOI] [PubMed] [Google Scholar]
- 26.Kanaji Y, Miyazaki S, Iwasawa J et al. Pre-procedural evaluation of the left atrial anatomy in patients referred for catheter ablation of atrial fibrillation. J Cardiol. 2016;67:115–21. doi: 10.1016/j.jjcc.2015.02.016. [DOI] [PubMed] [Google Scholar]
- 27.Güler E, Güler GB, Demir GG et al. Effect of pulmonary vein anatomy and pulmonary vein diameters on outcome of cryoballoon catheter ablation for atrial fibrillation. Pacing Clin Electrophysiol. 2015;38:989–96. doi: 10.1111/pace.12660. [DOI] [PubMed] [Google Scholar]
- 28.Wei H-Q, Guo X-G, Zhou G-B et al. Procedural findings and clinical outcome of second-generation cryoballoon ablation in patients with variant pulmonary vein anatomy. J Cardiovasc Electrophysiol. 2019;30:32–8. doi: 10.1111/jce.13768. [DOI] [PubMed] [Google Scholar]
- 29.Thai W, Wai B, Truong QA. Preprocedural imaging for patients with atrial fibrillation and heart failure. Curr Cardiol Rep. 2012;14:584–92. doi: 10.1007/s11886-012-0293-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chyou JY, Biviano A, Magno P et al. Applications of computed tomography and magnetic resonance imaging in percutaneous ablation therapy for atrial fibrillation. J Interv Card Electrophysiol. 2009;26:47–57. doi: 10.1007/s10840-009-9404-9. [DOI] [PubMed] [Google Scholar]
- 31.Martinek M, Nesser H-J, Aichinger J et al. Impact of integration of multislice computed tomography imaging into three-dimensional electroanatomic mapping on clinical outcomes, safety, and efficacy using radiofrequency ablation for atrial fibrillation. Pacing Clin Electrophysiol. 2007;30:1215–23. doi: 10.1111/j.1540-8159.2007.00843.x. [DOI] [PubMed] [Google Scholar]
- 32.Romero J, Natale A, Di Biase L. How to perform left atrial appendage electrical isolation using radiofrequency ablation. Heart Rhythm. 2018;15:1577–82. doi: 10.1016/j.hrthm.2018.05.020. [DOI] [PubMed] [Google Scholar]
- 33.Good E, Oral H, Lemola K et al. Movement of the esophagus during left atrial catheter ablation for atrial fibrillation. J Am Coll Cardiol. 2005;46:2107–10. doi: 10.1016/j.jacc.2005.08.042. [DOI] [PubMed] [Google Scholar]
- 34.Cabrera JA, Ho SY, Climent V. Sánchez-Quintana D. The architecture of the left lateral atrial wall: a particular anatomic region with implications for ablation of atrial fibrillation. Eur Heart J. 2008;29:356–62. doi: 10.1093/eurheartj/ehm606. [DOI] [PubMed] [Google Scholar]
- 35.Pan N-H, Tsao H-M, Chang N-C et al. Aging dilates atrium and pulmonary veins: implications for the genesis of atrial fibrillation. Chest. 2008;133:190–6. doi: 10.1378/chest.07-1769. [DOI] [PubMed] [Google Scholar]
- 36.Hall B, Jeevanantham V, Simon R et al. Variation in left atrial transmural wall thickness at sites commonly targeted for ablation of atrial fibrillation. J Interv Card Electrophysiol. 2006;17:127–32. doi: 10.1007/s10840-006-9052-2. [DOI] [PubMed] [Google Scholar]
- 37.Imada M, Funabashi N, Asano M et al. Anatomical remodeling of left atria in subjects with chronic and paroxysmal atrial fibrillation evaluated by multislice computed tomography. Int J Cardiol. 2007;119:384–8. doi: 10.1016/j.ijcard.2006.07.162. [DOI] [PubMed] [Google Scholar]
- 38.Takahashi K, Okumura Y, Watanabe I et al. Relation between left atrial wall thickness in patients with atrial fibrillation and intracardiac electrogram characteristics and ATP-provoked dormant pulmonary vein conduction. J Cardiovasc Electrophysiol. 2015;26:597–605. doi: 10.1111/jce.12660. [DOI] [PubMed] [Google Scholar]
- 39.Beinart R, Abbara S, Blum A et al. Left atrial wall thickness variability measured by ct scans in patients undergoing pulmonary vein isolation. J Cardiovasc Electrophysiol. 2011;22:1232–6. doi: 10.1111/j.1540-8167.2011.02100.x. [DOI] [PubMed] [Google Scholar]
- 40.Suenari K, Nakano Y, Hirai Y et al. Left atrial thickness under the catheter ablation lines in patients with paroxysmal atrial fibrillation: insights from 64-slice multidetector computed tomography. Heart Vessels. 2013;28:360–8. doi: 10.1007/s00380-012-0253-6. [DOI] [PubMed] [Google Scholar]
- 41.Chikata A, Kato T, Sakagami S et al. Optimal force-time integral for pulmonary vein isolation according to anatomical wall thickness under the ablation line. J Am Heart Assoc. 2016;5:e003155. doi: 10.1161/JAHA.115.003155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bordignon S, Chun K-RJ, Gunawardene M et al. Energy titration strategies with the endoscopic ablation system: lessons from the high-dose vs. low-dose laser ablation study. Europace. 2013;15:685–9. doi: 10.1093/europace/eus352. [DOI] [PubMed] [Google Scholar]
- 43.Koruth JS, Schneider C, Avitall B et al. Pre-clinical investigation of a low-intensity collimated ultrasound system for pulmonary vein isolation in a porcine model. JACC Clin Electrophysiol. 2015;1:306–14. doi: 10.1016/j.jacep.2015.04.011. [DOI] [PubMed] [Google Scholar]
- 44.Olsen FJ, Bertelsen L, de Knegt MC Multimodality cardiac imaging for the assessment of left atrial function and the association with atrial arrhythmias. Circ Cardiovasc Imaging. 2016. p. 9. [DOI] [PubMed]
- 45.Gupta DK, Shah AM, Giugliano RP et al. Left atrial structure and function in atrial fibrillation: ENGAGE AF-TIMI 48. Eur Heart J. 2014;35:1457–65. doi: 10.1093/eurheartj/eht500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pagola J, González-Alujas T, Flores A et al. Left atria strain is a surrogate marker for detection of atrial fibrillation in cryptogenic strokes. Stroke. 2014;45:e164–6. doi: 10.1161/STROKEAHA.114.005540. [DOI] [PubMed] [Google Scholar]
- 47.Yasuda R, Murata M, Roberts R et al. Left atrial strain is a powerful predictor of atrial fibrillation recurrence after catheter ablation: study of a heterogeneous population with sinus rhythm or atrial fibrillation. Eur Heart J Cardiovasc Imaging. 2015;16:1008–14. doi: 10.1093/ehjci/jev028. [DOI] [PubMed] [Google Scholar]
- 48.Hammerstingl C, Schwekendiek M, Momcilovic D et al. Left atrial deformation imaging with ultrasound based two-dimensional speckle-tracking predicts the rate of recurrence of paroxysmal and persistent atrial fibrillation after successful ablation procedures. J Cardiovasc Electrophysiol. 2012;23:247–55. doi: 10.1111/j.1540-8167.2011.02177.x. [DOI] [PubMed] [Google Scholar]
- 49.Hwang HJ, Choi EY, Rhee SJ et al. Left atrial strain as predictor of successful outcomes in catheter ablation for atrial fibrillation: a two-dimensional myocardial imaging study. J Interv Card Electrophysiol. 2009;26:127–32. doi: 10.1007/s10840-009-9410-y. [DOI] [PubMed] [Google Scholar]
- 50.Kuppahally SS, Akoum N, Burgon NS et al. Left atrial strain and strain rate in patients with paroxysmal and persistent atrial fibrillation: relationship to left atrial structural remodeling detected by delayed-enhancement MRI. Circ Cardiovasc Imaging. 2010;3:231–9. doi: 10.1161/CIRCIMAGING.109.865683. [DOI] [PubMed] [Google Scholar]
- 51.Donal E, Lip GYH, Galderisi M et al. EACVI/EHRA Expert Consensus Document on the role of multi-modality imaging for the evaluation of patients with atrial fibrillation. Eur Heart J Cardiovasc Imaging. 2016;17:355–83. doi: 10.1093/ehjci/jev354. [DOI] [PubMed] [Google Scholar]
- 52.Varela M, Morgan R, Theron A et al. Novel MRI technique enables non-invasive measurement of atrial wall thickness. IEEE Trans Med Imaging. 2017;36:1607–14. doi: 10.1109/TMI.2017.2671839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Evin M, Cluzel P, Lamy J et al. Assessment of left atrial function by MRI myocardial feature tracking. J Magn Reson Imaging. 2015;42:379–89. doi: 10.1002/jmri.24851. [DOI] [PubMed] [Google Scholar]
- 54.Oakes RS, Badger TJ, Kholmovski EG et al. Detection and quantification of left atrial structural remodeling with delayed-enhancement magnetic resonance imaging in patients with atrial fibrillation. Circulation. 2009;119:1758–67. doi: 10.1161/CIRCULATIONAHA.108.811877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Harrison JL, Jensen HK, Peel SA et al. Cardiac magnetic resonance and electroanatomical mapping of acute and chronic atrial ablation injury: a histological validation study. Eur Heart J. 2014;35:1486–95. doi: 10.1093/eurheartj/eht560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Malcolme-Lawes LC, Juli C, Karim R et al. Automated analysis of atrial late gadolinium enhancement imaging that correlates with endocardial voltage and clinical outcomes: A 2-center study. Heart Rhythm. 2013;10:1184–91. doi: 10.1016/j.hrthm.2013.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Therkelsen SK, Groenning BA, Svendsen JH, Jensen GB. Atrial and ventricular volume and function in persistent and permanent atrial fibrillation, a magnetic resonance imaging study. J Cardiovasc Magn Reson. 2005;7:465–73. doi: 10.1081/jcmr-200053618. [DOI] [PubMed] [Google Scholar]
- 58.Siebermair J, Kholmovski EG, Marrouche N. Assessment of left atrial fibrosis by late gadolinium enhancement magnetic resonance imaging. JACC Clin Electrophysiol. 2017;3:791–802. doi: 10.1016/j.jacep.2017.07.004. [DOI] [PubMed] [Google Scholar]
- 59.Vijayakumar S, Kholmovski E, Haslam M et al. Dependence of image quality of late gadolinium enhancement MRI of left atrium on number of patients imaged: Results of multi-center trial DECAAF. J Cardiovasc Magn Reson. 2014;16 (Suppl 1):146. doi: 10.1186/1532-429X-16-S1-P146. [DOI] [Google Scholar]
- 60.Zghaib T, Nazarian S. New insights into the use of cardiac magnetic resonance imaging to guide decision making in atrial fibrillation management. Can J Cardiol. 2018;34:1461–70. doi: 10.1016/j.cjca.2018.07.007. [DOI] [PubMed] [Google Scholar]
- 61.Shah D. The role of MRI-detected left atrial delayed enhancement in selecting the right patient and choosing the optimal strategy for catheter ablation of atrial fibrillation. J Cardiovasc Electrophysiol. 2011;22:23–4. doi: 10.1111/j.1540-8167.2010.01904.x. [DOI] [PubMed] [Google Scholar]
- 62.Cochet H, Mouries A, Nivet H et al. Age, atrial fibrillation, and structural heart disease are the main determinants of left atrial fibrosis detected by delayed-enhanced magnetic resonance imaging in a general cardiology population. J Cardiovasc Electrophysiol. 2015;26:484–92. doi: 10.1111/jce.12651. [DOI] [PubMed] [Google Scholar]
- 63.Marrouche NF, Wilber D, Hindricks G et al. Association of atrial tissue fibrosis identified by delayed enhancement mri and atrial fibrillation catheter ablation: The DECAAF Study. JAMA. 2014;311:498–506. doi: 10.1001/jama.2014.3. [DOI] [PubMed] [Google Scholar]
- 64.McGann C, Akoum N. Patel, et al. Atrial fibrillation ablation outcome is predicted by left atrial remodeling on MRI. Circ Arrhythm Electrophysiol. 2014;7:23–30. doi: 10.1161/CIRCEP.113.000689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Peters DC, Wylie JV, Hauser TH et al. Recurrence of atrial fibrillation correlates with the extent of post-procedural late gadolinium enhancement. A Pilot Study. JACC Cardiovasc Imaging. 2009;2:308–16. doi: 10.1016/j.jcmg.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Peters DC, Wylie JV, Hauser TH et al. Detection of pulmonary vein and left atrial scar after catheter ablation with three-dimensional navigator-gated delayed enhancement MR imaging: initial experience. Radiology. 2007;243:690–5. doi: 10.1148/radiol.2433060417. [DOI] [PubMed] [Google Scholar]
- 67.Hunter RJ, Jones DA, Boubertakh R et al. Diagnostic accuracy of cardiac magnetic resonance imaging in the detection and characterization of left atrial catheter ablation lesions: a multicenter experience. J Cardiovasc Electrophysiol. 2013;24:396–403. doi: 10.1111/jce.12063. [DOI] [PubMed] [Google Scholar]
- 68.Knowles BR, Caulfield D, Cooklin M et al. 3-D visualization of acute RF ablation lesions using MRI for the simultaneous determination of the patterns of necrosis and edema. IEEE Trans Biomed Eng. 2010;57:1467–75. doi: 10.1109/TBME.2009.2038791. [DOI] [PubMed] [Google Scholar]
- 69.McGann CJ, Kholmovski EG, Oakes RS et al. New magnetic resonance imaging-based method for defining the extent of left atrial wall injury after the ablation of atrial fibrillation. J Am Coll Cardiol. 2008;52:1263–71. doi: 10.1016/j.jacc.2008.05.062. [DOI] [PubMed] [Google Scholar]
- 70.Spragg DD, Khurram I, Zimmerman SL et al. Initial experience with magnetic resonance imaging of atrial scar and co-registration with electroanatomic voltage mapping during atrial fibrillation: success and limitations. Heart Rhythm. 2012;9:2003–9. doi: 10.1016/j.hrthm.2012.08.039. [DOI] [PubMed] [Google Scholar]
- 71.Pontecorboli G, Figueras i, Ventura RM, Carlosena A et al. Use of delayed-enhancement magnetic resonance imaging for fibrosis detection in the atria: a review. Europace. 2016;19:180–189. doi: 10.1093/europace/euw053. [DOI] [PubMed] [Google Scholar]
- 72.Akoum N, Wilber D, Hindricks G et al. MRI assessment of ablation-induced scarring in atrial fibrillation: Analysis from the DECAAF Study. J Cardiovasc Electrophysiol. 2015;26:473–80. doi: 10.1111/jce.12650. [DOI] [PubMed] [Google Scholar]
- 73.Efficacy of delayed enhancement MRI-guided ablation vs conventional catheter ablation of atrial fibrillation. https://clinicaltrials.gov/ct2/show/NCT02529319 Available at. (accessed 29 April 2019)
- 74.Zahid S, Cochet H, Boyle PM et al. Patient-derived models link re-entrant driver localization in atrial fibrillation to fibrosis spatial pattern. Cardiovasc Res. 2016;110:443–54. doi: 10.1093/cvr/cvw073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Haissaguerre M, Shah AJ, Cochet H et al. Intermittent drivers anchoring to structural heterogeneities as a major pathophysiological mechanism of human persistent atrial fibrillation. J Physiol. 2016;594:2387–98. doi: 10.1113/JP270617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yang B, Jiang C, Lin Y et al. STABLE-SR (Electrophysiological Substrate Ablation in the Left Atrium During Sinus Rhythm) for the treatment of nonparoxysmal atrial fibrillation. Circ Arrhythm Electrophysiol. 2017;10:e005405. doi: 10.1161/CIRCEP.117.005405. [DOI] [PubMed] [Google Scholar]
- 77.Jadidi AS, Lehrmann H, Keyl C et al. Ablation of persistent atrial fibrillation targeting low-voltage areas with selective activation characteristics. Circ Arrhythm Electrophysiol. 2016;9:e002962. doi: 10.1161/CIRCEP.115.002962. [DOI] [PubMed] [Google Scholar]
- 78.Kottkamp H, Berg J, Bender R et al. Box isolation of fibrotic areas (BIFA): A patient-tailored substrate modification approach for ablation of atrial fibrillation. J Cardiovasc Electrophysiol. 2016;27:22–30. doi: 10.1111/jce.12870. [DOI] [PubMed] [Google Scholar]
- 79.January CT, Wann LS, Alpert JS et al. 2014 AHA/ACC/HRS Guideline for the management of patients with atrial fibrillation. J Am Coll Cardiol. 2014;64:e1–76. doi: 10.1016/j.jacc.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 80.Boveda S, Metzner A, Nguyen DQ et al. Single-procedure outcomes and quality-of-life improvement 12 months post-cryoballoon ablation in persistent atrial fibrillation: results from the multicenter CRYO4PERSISTENT AF Trial. JACC Clin Electrophysiol. 2018;4:1440–7. doi: 10.1016/j.jacep.2018.07.007. [DOI] [PubMed] [Google Scholar]
- 81.Omran H, Gutleben K-J, Molatta S et al. Second generation cryoballoon ablation for persistent atrial fibrillation: an updated meta-analysis. Clin Res Cardiol. 2018;107:182–92. doi: 10.1007/s00392-017-1171-5. [DOI] [PubMed] [Google Scholar]
- 82.Swarup V, Baykaner T, Rostamian A et al. Stability of rotors and focal sources for human atrial fibrillation: focal impulse and rotor mapping (FIRM) of AF sources and fibrillatory conduction. J Cardiovasc Electrophysiol. 2014;25:1284–92. doi: 10.1111/jce.12559. [DOI] [PubMed] [Google Scholar]
- 83.Deisenhofer I. Mapping of atrial fibrillation: strategies to understand an enigmatic arrhythmia. Herzschrittmachertherapie Elektrophysiologie. 2018;29:307–14. doi: 10.1007/s00399-018-0586-7. [DOI] [PubMed] [Google Scholar]
- 84.Nademanee K, McKenzie J, Kosar E et al. A new approach for catheter ablation of atrial fibrillation: mapping of the electrophysiologic substrate. J Am Coll Cardiol. 2004;43:2044–53. doi: 10.1016/j.jacc.2003.12.054. [DOI] [PubMed] [Google Scholar]
- 85.Haïssaguerre M, Hocini M, Sanders P et al. Localized sources maintaining atrial fibrillation organized by prior ablation. Circulation. 2006;113:616–25. doi: 10.1161/CIRCULATIONAHA.105.546648. [DOI] [PubMed] [Google Scholar]
- 86.Ganesan AN, Kuklik P, Lau DH et al. Bipolar electrogram shannon entropy at sites of rotational activation. Circ Arrhythm Electrophysiol. 2013;6:48–57. doi: 10.1161/CIRCEP.112.976654. [DOI] [PubMed] [Google Scholar]
- 87.Narayan SM, Shivkumar K, Krummen DE et al. Panoramic electrophysiological mapping but not electrogram morphology identifies stable sources for human atrial fibrillation. Circ Arrhythm Electrophysiol. 2013;6:58–67. doi: 10.1161/CIRCEP.111.977264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Verma A, Jiang C, Betts TR et al. Approaches to catheter ablation for persistent atrial fibrillation. N Engl J Med. 2015;372:1812–22. doi: 10.1056/NEJMoa1408288. [DOI] [PubMed] [Google Scholar]
- 89.Ammar S, Hessling G, Reents T et al. Importance of sinus rhythm as endpoint of persistent atrial fibrillation ablation. J Cardiovasc Electrophysiol. 2013;2:388–95. doi: 10.1111/jce.12045. [DOI] [PubMed] [Google Scholar]
- 90.Pandit SV, Jalife J. Rotors and the dynamics of cardiac fibrillation. Circ Res. 2013;112:849–62. doi: 10.1161/CIRCRESAHA.111.300158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Guillem MS, Climent AM, Rodrigo M et al. Presence and stability of rotors in atrial fibrillation: evidence and therapeutic implications. Cardiovasc Res. 2016;109:480–92. doi: 10.1093/cvr/cvw011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Guillem MS, Climent AM, Castells F et al. Noninvasive mapping of human atrial fibrillation. J Cardiovasc Electrophysiol. 2009;20:507–13. doi: 10.1111/j.1540-8167.2008.01356.x. [DOI] [PubMed] [Google Scholar]
- 93.Cuculich PS, Wang Y, Lindsay BD et al. Noninvasive characterization of epicardial activation in humans with diverse atrial fibrillation patterns. Circulation. 2010;122:1364–72. doi: 10.1161/CIRCULATIONAHA.110.945709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Haissaguerre M, Hocini M, Denis A et al. Driver domains in persistent atrial fibrillation. Circulation. 2014;130:530–8. doi: 10.1161/CIRCULATIONAHA.113.005421. [DOI] [PubMed] [Google Scholar]
- 95.Knecht S, Sohal M, Deisenhofer I et al. Multicentre evaluation of non-invasive biatrial mapping for persistent atrial fibrillation ablation: the AFACART study. Europace. 2017;19:1302–9. doi: 10.1093/europace/euw168. [DOI] [PubMed] [Google Scholar]
- 96.Verheule S, Eckstein J, Linz D et al. Role of endo-epicardial dissociation of electrical activity and transmural conduction in the development of persistent atrial fibrillation. Prog Biophys Mol Biol. 2014;115:173–85. doi: 10.1016/j.pbiomolbio.2014.07.007. [DOI] [PubMed] [Google Scholar]
- 97.Santangeli P, Marchlinski FE. Techniques for the provocation, localization, and ablation of non–pulmonary vein triggers for atrial fibrillation. Heart Rhythm. 2017;14:1087–96. doi: 10.1016/j.hrthm.2017.02.030. [DOI] [PubMed] [Google Scholar]
- 98.Romero J, Gianni C, Di Biase L, Natale A. Catheter ablation for long-standing persistent atrial fibrillation. Methodist DeBakey Cardiovasc J. 2015;11:87–93. doi: 10.14797/mdcj-11-2-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lee G, McLellan AJA, Hunter RJ et al. Panoramic characterization of endocardial left atrial activation during human persistent AF: Insights from non-contact mapping. Int J Cardiol. 2017;228:406–11. doi: 10.1016/j.ijcard.2016.11.085. [DOI] [PubMed] [Google Scholar]
- 100.Yamabe H, Kanazawa H, Ito M et al. Prevalence and mechanism of rotor activation identified during atrial fibrillation by noncontact mapping: Lack of evidence for a role in the maintenance of atrial fibrillation. Heart Rhythm. 2016;13:2323–30. doi: 10.1016/j.hrthm.2016.07.030. [DOI] [PubMed] [Google Scholar]
- 101.Kumagai K, Nakashima H. Noncontact mapping-guided catheter ablation of atrial fibrillation. Circ J. 2009;73:233–41. doi: 10.1253/circj.CJ-08-0700. [DOI] [PubMed] [Google Scholar]
- 102.Narayan SM, Krummen DE, Shivkumar K et al. Treatment of atrial fibrillation by the ablation of localized sources: CONFIRM (Conventional Ablation for Atrial Fibrillation With or Without Focal Impulse and Rotor Modulation) trial. J Am Coll Cardiol. 2012;60:628–36. doi: 10.1016/j.jacc.2012.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rostock T, Rotter M, Sanders P et al. High-density activation mapping of fractionated electrograms in the atria of patients with paroxysmal atrial fibrillation. Heart Rhythm. 2006;3:27–34. doi: 10.1016/j.hrthm.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 104.Narayan SM, Wright M, Derval N et al. Classifying fractionated electrograms in human atrial fibrillation using monophasic action potentials and activation mapping: Evidence for localized drivers, rate acceleration, and nonlocal signal etiologies. Heart Rhythm. 2011;8:244–53. doi: 10.1016/j.hrthm.2010.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Seitz J, Bars C. Théodore G, et al. AF ablation guided by spatiotemporal electrogram dispersion without pulmonary vein isolation. J Am Coll Cardiol. 2017;69:303–21. doi: 10.1016/j.jacc.2016.10.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Della Rocca DG, Mohanty S, Trivedi C et al. Percutaneous treatment of non-paroxysmal atrial fibrillation: A paradigm shift from pulmonary vein to non-pulmonary vein trigger ablation? Arrhythmia Electrophysiol Rev. 2018;7:256–60. doi: 10.15420/aer.2018.56.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hung Y, Lo L-W, Lin Y-J et al. Characteristics and long-term catheter ablation outcome in long-standing persistent atrial fibrillation patients with non-pulmonary vein triggers. Int J Cardiol. 2017;241:205–11. doi: 10.1016/j.ijcard.2017.04.050. [DOI] [PubMed] [Google Scholar]
- 108.Lin D, Frankel DS, Zado ES et al. Pulmonary vein antral isolation and nonpulmonary vein trigger ablation without additional substrate modification for treating longstanding persistent atrial fibrillation. J Cardiovasc Electrophysiol. 2012;23:806–13. doi: 10.1111/j.1540-8167.2012.02307.x. [DOI] [PubMed] [Google Scholar]
- 109.Mohanty S, Trivedi C, Gianni C et al. Procedural findings and ablation outcome in patients with atrial fibrillation referred after two or more failed catheter ablations. J Cardiovasc Electrophysiol. 2017;28:1379–86. doi: 10.1111/jce.13329. [DOI] [PubMed] [Google Scholar]
- 110.Buch E, Share M, Tung R et al. Long-term clinical outcomes of focal impulse and rotor modulation for treatment of atrial fibrillation: A multicenter experience. Heart Rhythm. 2016;13:636–41. doi: 10.1016/j.hrthm.2015.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mohanty S, Mohanty P, Trivedi C et al. Long-term outcome of pulmonary vein isolation with and without focal impulse and rotor modulation mapping. Circ Arrhythm Electrophysiol. 2018;11:e005789. doi: 10.1161/CIRCEP.117.005789. [DOI] [PubMed] [Google Scholar]
- 112.Ramirez FD, Birnie DH, Nair GM et al. Efficacy and safety of driver-guided catheter ablation for atrial fibrillation: A systematic review and meta-analysis. J Cardiovasc Electrophysiol. 2017;28:1371–8. doi: 10.1111/jce.13313. [DOI] [PubMed] [Google Scholar]
- 113.Baykaner T, Rogers AJ, Meckler GL et al. Clinical implications of ablation of drivers for atrial fibrillation: a systematic review and meta-analysis. Circ Arrhythm Electrophysiol. 2018;11:e006119. doi: 10.1161/CIRCEP.117.006119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Romero J, Michaud GF, Avendano R et al. Benefit of left atrial appendage electrical isolation for persistent and long-standing persistent atrial fibrillation: a systematic review and meta-analysis. Europace. 2018;20:1268–78. doi: 10.1093/europace/eux372. [DOI] [PubMed] [Google Scholar]
- 115.Vein of Marshall ethanol infusion for persistent atrial fibrillation. https://clinicaltrials.gov/ct2/show/NCT01898221 Available at. (accessed 29 April 2019)


