Abstract
CRT is a cornerstone of therapy for patients with heart failure and reduced ejection fraction. By restoring left ventricular (LV) electrical and mechanical synchrony, CRT can reduce mortality, improve LV function and reduce heart failure symptoms. Since its introduction, many advances have been made that have improved the delivery of and enhanced the response to CRT. Improving CRT outcomes begins with proper patient selection so CRT is delivered to all populations that could benefit from it, and limiting the implantation of CRT in those with a small chance of response. In addition, advancements in LV leads and delivery technologies coupled with multimodality imaging and electrical mapping have enabled operators to place coronary sinus leads in locations that will optimise electrical and mechanical synchrony. Finally, new pacing strategies using LV endocardial pacing or His bundle pacing have allowed for CRT delivery and improved response in patients with poor coronary sinus anatomy or lack of response to traditional CRT.
Keywords: Heart failure, cardiac resynchronisation, biventricular pacemaker, biventricular defibrillator, His bundle pacing, leadless pacing
CRT is an essential treatment for patients with heart failure and reduced ejection fraction as it can restore left ventricular (LV) electrical and mechanical synchrony. It has been shown to increase quality of life, improve functional status, reduce hospitalisation, improve LV systolic function and reduce mortality in properly selected patients.[1,2] While CRT is an effective therapy, approximately 30% of patients treated with CRT do not benefit from it and some patients are negative responders. Improving outcomes with CRT begins with appropriate selection.
QRS Duration and Morphology
Since CRT targets electrical dyssynchrony, QRS duration and morphology have been used to determine which patients will receive maximum benefit from CRT. Based on subgroup analysis of large CRT trials, current guidelines consider CRT implants to be a Class I indication in patients with left bundle branch block (LBBB) and QRS >150 msec, with softer recommendations for QRS <150 msec and non-LBBB patients.[3]
An analysis of data from the Multicenter Automatic Defibrillator Implantation Trial-Cardiac Resynchronization Therapy (MADIT-CRT) trial demonstrated that only patients with LBBB had a reduction in heart failure events and that non-LBBB patients may have been harmed by CRT.[4] Similar data were published from the Resynchronization/Defibrillation for Ambulatory heart Failure Trial (RAFT).[5] No benefits were found to result from CRT in right bundle branch block (RBBB) patients in five randomised controlled clinical trials or in a subset of 1,233 patients with non-LBBB QRS morphology from four randomised trials.[6,7] However, data on QRS morphology are mixed and no large CRT trial has used QRS morphology as an enrolment criterion. There is no standardised definition of LBBB, especially with respect to predicting electrical dyssynchrony and response to CRT. In addition, some patients with RBBB and intraventricular conduction delay may have a LBBB-like activation pattern of the left ventricle and could respond to CRT.[8] To this end, the MADIT-CRT trial showed that, in patients with non-LBBB morphologies and PR intervals >230 msec, there was a 67% reduction in risk of the combined primary endpoint of heart failure and death and a 76% reduction in the risk of death in the CRT device (CRT-D) arm versus the ICD-only arm.[9] Data from the REsynchronization reVErses Remodeling in Systolic left vEntricular dysfunction (REVERSE) trial also showed benefit in patients with RBBB receiving CRT.[10]
QRS duration is a good predictor of CRT response. QRS duration is a criterion for every large clinical trial showing benefit in CRT. Trials randomising patients with narrow QRS (<120 msec or 130 msec) show no benefit or potential harm for patients receiving CRT, even in the presence of mechanical dyssynchrony.[11] An analysis of four trials by Cleland et al. showed that QRS duration was the best predicator of benefit from CRT placement, irrespective of QRS morphology, with response seen once QRS duration was >130 msec.[12] Subgroup analyses have shown that the greatest CRT benefit is derived in the cohort of patients with a QRS duration of 150 msec, as reflected in the guidelines for CRT placement.[3,10,12]
Since correcting dyssynchrony is the core benefit of CRT, imaging has been added to 12-lead ECG to expand and refine the population of patients that might benefit from this therapy. The presence of mechanical dyssynchrony on echocardiography or MRI has been shown to predict which patients perform best after CRT placement.[13,14] However, the Predictors of Response to CRT (PROSPECT) trial, which prospectively studied echocardiographic measures of dyssynchrony, found they had only modest sensitivity and specificity to predict CRT response with significant variability in the measurement of dyssychrony studied.[15] It was conjectured that this was due to the complexity of the dyssynchrony parameters and significant interobserver variability. Newer measures of mechanical dyssynchrony, as assessed by echocardiography and MRI, have shown promise.[13,14]
Apical rocking and septal flash are simple visual echocardiographic parameters of a LBBB-like contraction pattern that is characterised by late contraction of the lateral left ventricle. Apical rocking specifically refers to a short septal motion of the apex due to early contraction of the septum and late contraction of the lateral wall.[13] Septal flash is defined by early contraction of the septum causing a short rapid inward motion of the septum.[13]
The Relationship of Visually Assessed Apical Rocking and Septal Flash to Response and Long-term Survival Following CRT (PREDICT-CRT) trial assessed 1,060 patients for these parameters and found a 15% reduction in LV end-systolic volume in 77% of patients when both apical rocking and septal flash were present in contrast to 69% if only apical rocking was present and 56% in those with septal flash alone. These parameters were more predictive of echocardiographic response to CRT and long-term survival than QRS morphology, duration and other clinical variables.[13] Shoal et al. used cardiac MRI to assess the usefulness of the U-shaped contraction pattern, defined by a line of block in mechanical contraction between the septum and lateral left ventricle, consistent with a true LBBB contraction pattern, to predict CRT outcome. Patients who had a U-shaped propagation had an 80% response rate versus a 26% response rate in the group with homogenous propagation group (p<0.001).[16] Similarly, patients without dyssynchrony on MRI, based on a circumferential uniformity ratio estimate >0.70, had no clinical benefit with CRT and a 12-fold increased mortality rate.[14]
Invasive mapping studies have shown that QRS duration and morphology may not always be predictive of prolonged LV activation times that correlate with CRT response. Non-invasive ECG mapping techniques have been used to identify patients who may have abnormally late-activating regions of the left ventricle. In the Markers and Response to CRT (MARC) study of 240 patients who were followed prospectively, vectorcardiography-derived QRSarea was shown to be more predictive of echocardiographic response to CRT than QRS morphology and duration. The vectorcardiography was mathematically constructed from a standard digital 12-lead ECG and consists of three orthogonal leads X, Y, and Z that form a 3D vector loop.[17]
In addition, high-resolution non-invasive electrocardiographic imaging (ECGI) has shown promise in defining patients who are more likely to respond to CRT. Multiple studies have shown that ECGI measures of electrical dyssynchrony obtained using the CardioInsight™ system (Medtronic) better correlate with acute haemodynamic and long-term clinical response to CRT than the presence of LBBB.[18] Another smaller ECGI system with a 53-electrode ECG belt (Heartscape Technologies) has also shown the ability to predict echocardiographic response to CRT better than QRS duration or morphology.[19]
Lead Placement
Another strategy for improving CRT outcome is optimisation of lead placement. Since the lateral left ventricle is the latest-activating area in LBBB, the lateral or posterolateral left ventricle – in general – is the preferred target for LV lead placement, but the optimal place for such placement may vary for a given patient. Early data showed that the placement of anterolateral or posterolateral leads was superior to anterior lead placement.[20] However, analysis of the Comparison of Medical Therapy, Pacing and Defibrillation in Heart Failure (COMPANION) trial showed that no particular lead location was associated with an improved response.[21]
Other studies show that apical LV lead placement is associated with worse outcomes than non-apical leads.[22] The Targeted Left Ventricular Lead Placement to Guide CRT (TARGET) and Speckle Tracking Assisted Resynchronization Therapy for Electrode Region (STARTER) studies used speckle tracking echocardiography to determine the area of latest mechanical activation for placement of the LV lead. Patients randomised to the targeted LV lead placement group had lower rates of the combined endpoint of heart failure and death.[23,24] This approach is limited by the availability of specialised software and the need for optimal image quality.
LV leads should also be targeted to areas of viable myocardium. CRT patients with scar tissue in the posterolateral left ventricle assessed by cardiac MRI have minimal response to CRT.[25] Further data consistently show that LV leads placed in areas of viable myocardium – especially in viable segments with dyssynchrony – have high response rates to CRT versus LV leads placed in an area of scar and no dyssynchrony.[14]
Late-activating segments of the left ventricle can be targeted electrically. LV pacing from areas of late activation defined by either LV lead electrical delay (QLV is defined as the time between onset of the QRS on the surface ECG and the sensed signal on the LV lead) or interventricular delay (time of onset of large positive or negative peaks of the right ventricular to LV electrogram) have been shown to correlate with favourable acute haemodynamic and clinical responses.[26,27] Even patients with a lead placed in the LV apex had a good response to CRT if the lead showed late LV activation.[28] Leads placed in an electrically late-activating segment predicted by ECGI or vectorcardiography have also been shown to enhance acute response to CRT.[28–30] However, preliminary results from the CRT Implant Strategy Using the Longest Electrical Delay for Non-left Bundle Branch Block Patients (ENHANCE–CRT) pilot study, which randomised 248 patients to LV lead placement guided by QLV versus standard LV lead placement in non-LBBB subjects, showed no statistical difference.[31]
Applicability of CRT therapy can also be limited by the anatomic constraints of the coronary sinus (CS). In 5–10% of patients, LV lead placement is unsuccessful due to either CS inaccessibility, high LV pacing thresholds, or phrenic nerve stimulation.[1,2,6] In addition, >50% of patients have only one CS branch that is suitable for lead placement, making it difficult to target LV lead placement to areas of late activation or dyssynchrony in all subjects.[32]
New Catheter Approaches to LV Synchronisation
Endocardial LV pacing offers many potential benefits over epicardial LV pacing. LV endocardial leads can be targeted to any area of the left ventricle due to the lack of anatomic constraints from the CS. In addition, LV endocardial pacing thresholds are lower than epicardial leads and phrenic nerve stimulation can be more readily avoided. Animal and human studies have shown that the LV endocardium provides a favourable acute haemodynamic response to LV pacing. This is likely to due to more rapid LV endocardial impulse conduction leading to shorter LV activation times as compared with epicardial pacing.[33]
The most common technique for achieving LV endocardial pacing involves transseptal access across the intra-atrial septum to deliver a LV lead across the mitral annulus into the left ventricle. The largest trial of LV endocardial pacing with transseptal atrial placement, the ALternate Site Cardiac ResYNChronization (ALSYNC) trial, enrolled 138 patients and had an 89.4% procedural success rate in patients who were CRT non-responders or who had failed implants. The clinical response rate was 59%. However, despite anticoagulation there was a high rate of thromboembolic complications (stroke rate was 2.6 per 100 patient years and there were 14 transient ischaemic attacks in nine patients), but no cases of lead-related mitral regurgitation were seen.[34]
Another technique for LV endocardial pacing involves passing the lead through the intraventricular septum and into the lateral LV myocardium. In a preliminary study, all patients were successfully implanted with this technique and eight out of nine were considered responders. All patients were on anticoagulation and no cerebrovascular accidents or transient ischaemic attacks were reported during a mean follow-up of 8.7 months.[35] The larger Pilot Study of Interventricular Septal Puncture for Cardiac Resynchronization Therapy to Treat Heart Failure (LV-CONSEPT) trial (NCT01818765), designed to evaluate outcomes for this approach to LV lead placement, has completed enrolment.
Preliminary data from LV endocardial pacing via the LV septum has also been shown to improve haemodynamics versus right ventricular pacing and to have similar haemodynamic response to lateral LV endocardial biventricular pacing. This technique utilises a specialised pacing lead with a fixed 4 mm helix. The helix has a thin coating on the proximal portion, with only the distal 1.27 mm electrode exposed. The lead is screwed into the intraventricular septum until LV septal capture is confirmed. The advantage of this technique is that the lack of hardware in the left ventricle eliminates the need for anticoagulation.[36]
The final option for LV endocardial pacing is leadless pacing. The WiSE™ CRT System (EBR Systems) uses a leadless 9 mm pacing electrode directly implanted into the LV endocardium via retrograde or transseptal access. The electrode is powered by a generator implanted near the left ventricle in the intercostal space that delivers ultrasound to the electrode, which is then converted to electricity for pacing. The LV electrode will endothelialise, so long-term anticoagulation is not required. In the preliminary Safety and Performance of Electrodes Implanted in the Left Ventricle (SELECT-LV) study of 35 patients, there was a 97% success rate for implantation, with an 85% clinical response rate to CRT in patients that failed CRT implants or were non-responders. However, 23% of participants in this trial experienced significant adverse events.[37] The larger Simulation Of the Left Ventricular Endocardium for CRT (SOLVE-CRT) trial (NCT02922036) will look at 350 patients who are non-responders or who have failed CRT implants to assess the utility of this system.
Another option to improve CRT response is to pace the LV from two different sites. A study looking at 40 patients with permanent AF with a slow ventricular response randomised patients to conventional CRT versus CRT with two LV leads and showed higher LV ejection fraction (27% versus 35%) and smaller LV end-systolic volume (157 cm3 versus 134 cm3) with dual site LV pacing.[38] A second study compared 34 patients who had received CRT with two LV leads to a propensity score-matched population that had received conventional CRT.[39] The conventional CRT group had more frequent ventricular tachycardia and a higher all-cause mortality and heart transplant rate. Preliminary data from the 100-patient Triple-site versus Standard Cardiac Resynchronization Therapy (TRUST-CRT) study showed similar rates of adverse events and implantation success with triple-site versus standard CRT and an improved rate of clinical response, with only 12.5% of patients reporting New York Heart Association (NYHA) Class III symptoms versus 30% in the standard CRT group. However, triple-site CRT was associated with higher LV lead thresholds, lower pacing impedance and greater battery drain.[40] No further data have been published, although the trial completed enrolment in 2015. The recently published the triple-site CRT(V3) study showed that in 84 CRT non-responders randomised to a second LV lead versus control, there was no improvement in clinical response to CRT in the treatment group at 24 months, but there was a high rate (20%) of procedural complications.[41] Multiple randomised controlled trials of dual-site LV pacing have been completed but have not yet released their results.
Multisite LV pacing can also be delivered via a quadripolar LV lead. Multiple point pacing (MPP) is an option in most commercially available CRT systems and allows for dual-site LV pacing by using two separate bipoles from a quadripolar lead. By covering a larger area of LV myocardium, MPP can increase the speed of impulse propagation and reduce LV activation times. MPP has shown improvement over single-site LV pacing as assessed by pressure volume loops, LV dP/dtmax, global peak LV radial strain and LV outflow tract velocity time integral in selected patients.[42] In the MultiPoint Pacing IDE (MPP-IDE) study, MPP delivered from anatomically-separate bipoles resulted in an 87% clinical response rate and 100% response rate in patients who were non-responders at 3 months. MPP may be a good option to use in patients that do not initially respond to standard CRT therapy.[43] Figure 1 outlines a strategy for improving the clinical response of CRT non-responders.
Figure 1: Decision Tree for CRT Non-responders.
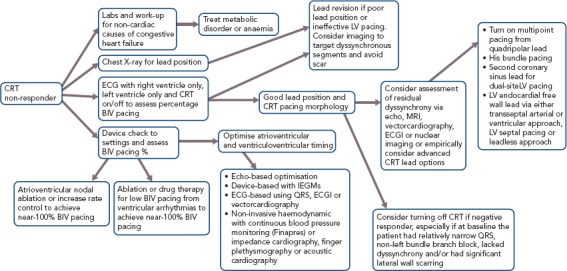
BIV = biventricular; ECGI = non-invasive high-resolution electrocardiographic mapping; IEGM = intracardiac electrogram; LV = left ventricular.
His bundle pacing is emerging as a first-line option for optimal CRT response in non-responders and in those with unfavourable CS anatomy. It is based on the premise that longitudinal dissociation exists in the proximal His bundle and disease within this bundle causes bundle branch block. Stimulation of the distal His bundle can narrow and potentially normalise a widened QRS. Animal and human studies from >40 years ago showed that temporary pacing of the distal His bundle resulted in QRS normalisation.[44,45] More recent work has shown that it is feasible to permanently pace the His with standard pacing leads and that His bundle pacing can result in narrowing of the QRS in 70–90% of patients.[46–48] In initial work, Lustagarten et al. noted similar outcomes between His bundle pacing and CS lead pacing for CRT, with significantly shorter procedure times with the former therapy.[49] A larger series reported by Sharma et al. showed a 90% success rate in narrowing the QRS with His bundle pacing.[50] The clinical response rate was 70% and mean ejection fraction increased from 30% to 44%. There was also a significant improvement in NYHA class, with participants’ symptoms improving by one NYHA class on average. Another recent series of 39 patients with RBBB from the same group showed that His bundle pacing was successful in 95% of patients and there was a favourable clinical response in 76% of patients.[50] His bundle pacing offers many potential advantages:
Faster impulse propagation due to endocardial and His–Purkinje system recruitment;[46,47]
Direct access to the anatomic area of interest;[46]
Lack of need for optimisation of CRT lead placement or ventriculoventricular (VV) timing, since recruitment of the distal His-Purkinje system and fascicles should result in normalisation of LV electrical activation;[49,50]
Endocardial pacing with QRS narrowing should avoid the negative CRT response that is sometimes associated with LV epicardial pacing due to ventricular proarrhythmia or unchanged dyssynchrony pattern.[51]
While His bundle pacing offers promise as a first-line therapy, current drawbacks include:
lack of randomised trials showing clinical improvement or mortality benefit;
high lead revision rates;
higher thresholds and current battery drain associated than for CRT; and
lack of significant long-term data of lead performance in the His position.
The on-going His Bundle Pacing Versus Coronary Sinus Pacing for CRT (His-SYNC) pilot trial (NCT02700425) will randomise 40 patients to CRT with His bundle pacing versus a LV lead. Further large clinical trials are required before this form of pacing can be considered a first-line method for CRT.
Narrow QRS
Narrow QRS patients are generally excluded from CRT due to the lack of benefit demonstrated in multiple large randomised controlled trials. However, selected patients with narrow QRS do benefit from CRT. Many studies have shown the CRT pacing coupled with atrioventricular (AV) node ablation, either with a LV lead or His bundle pacing, have reported improved heart failure symptoms and LV ejection fraction in patients with AF, LV dysfunction and congestive heart failure.[52,53] The recently published Abate and Place in AF plus CRT (APAF-CRT) trial randomised 109 patients with a narrow QRS and permanent AF to AV node ablation and CRT versus rate control. The CRT–AV node ablation group had a significant reduction in death from any cause or hospitalisation for heart failure (12% versus 33%) and a trend towards decreased mortality (4% versus 12%).[54]
While CRT for narrow QRS patients in sinus rhythm is currently contraindicated, some studies have suggested that in selected patients the majority of acute haemodynamic improvement from CRT is due to AV optimisation, thus improving LV preload rather than correcting dyssynchrony.[55] The His Optimized Pacing Evaluated for Heart Failure (HOPE-HF) trial (NCT02671903) will randomise 160 patients with a LV ejection fraction <40% with narrow QRS or RBBB and PR interval >200 msec to His bundle pacing with AV optimisation versus standard congestive heart failure therapy, testing the hypothesis that AV optimisation improves outcome in this group of patients.[56]
Atrioventricular and Ventriculoventricular Timing
Two initial large trials evaluated the use of AV and VV timing optimisation to improve CRT outcomes – SmartDelay determined AV Optimization: A Comparison of AV Optimization Methods Used in CRT (SMART-AV) and Frequent Optimization Study Using the QuickOpt Method (FREEDOM), but these showed no additional benefit over nominal settings.[57] As a consequence, guidelines currently only recommend AV and VV timing optimisation for CRT non-responders. Dynamic algorithms that change programmed settings based on frequent automatic assessments, thereby optimising AV and VV timing, have performed better. Adaptive CRT (aCRT) uses intrinsic conduction to determine timings and results in LV-only pacing with a sensed AV delay <220 msec and biventricular pacing otherwise. The aCRT algorithm was associated with a reduced 30-day readmission rate of 14.8% versus 24.8% in controls. Figure 2 shows optimisation of CRT with echocardiographic strain imaging using aCRT. Patients in the aCRT group with >50% LV-only pacing had an 82% clinical response rate versus 68% in the optimised biventricular pacing group.[58]
Figure 2: Optimisation of CRT with Adaptive CRT and Speckle Tracking Strain Imaging in Non-responders.
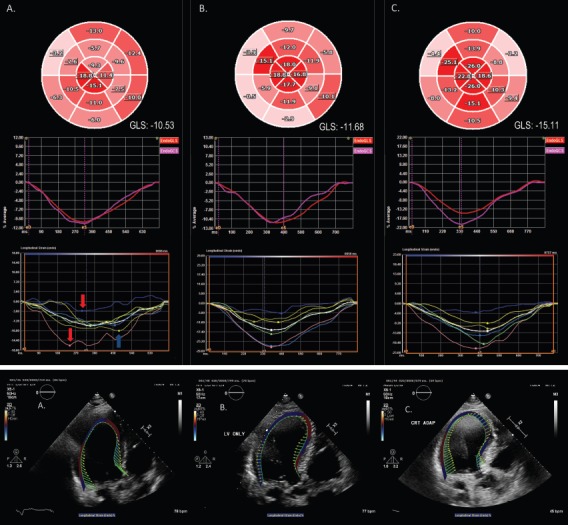
A 75-year old male CRT non-responder with an ejection fraction of 30%, left bundle branch block with QRS duration of 160 msec, and New York Heart Association Class III heart failure symptoms due to cardiac amyloidosis. (A) RV–LV=0 msec. Bull’s eye plot and endocardial GLS graph demonstrate impaired GLS (-10.53%) in an apical sparing pattern typical of cardiac amyloidosis. Segmental peak systolic strain curves illustrate a wide range in the timing of peak systolic strain, with early systolic shortening of the septum (red arrows) and late peak contraction of the lateral wall (blue arrow). (B) LV only. Note the similarly impaired GLS (-11.68%), though with time to peak strain occurring over a narrower range. (C) RV–LV=40 msec. Peak systolic deformation is markedly improved, particularly in the septal and inferior walls, with a significant increment in GLS (-15.11%). Note the narrow range of peak strain values. (D) Velocity vector tracking demonstrates severe septal–lateral wall dyssynchrony and severely impaired longitudinal strain with RV–LV=0 msec. (E) Some improvement with LV-only pacing. (F) Near-restoration of the synchrony of velocity, with convergence toward the centre of the LV, with RV–LV=40 msec. GLS = global longitudinal strain; LV = left ventricle; RV = right ventricle.
The Clinical Trial of the SonRtip Lead and Automatic AV–VV Optimization Algorithm in the PARADYM RF SonR CRT-D (RESPOND-CRT) trial assessed the clinical utility of the SonR™ (LinaNova) contractility sensor in CRT optimisation. The SonR sensor measures mechanical vibrations, which correlate with LV dP/dTmax. The study randomised 998 patients in a 2:1 fashion to receive weekly automatic CRT optimisation with SonR versus echocardiographic optimisation.[59] There was a 75% response rate in the SonR group versus 70% in the control group, with a 35% relative reduction in the risk of hospitalisation for heart failure. The SyncAV™ algorithm (Abbott) regularly calculates the PR interval from device electrograms and automatically adjusts the AV delay to allow for intrinsic septal activation before biventricular pacing. This algorithm shortens QRS duration during biventricular pacing to a greater extent than statically optimised AV and VV delays and LV-only pacing.[60] A recent randomised study showed that programming AV delays and VV timing to promote fusion with intrinsic septal activation and targeting the shortest QRS duration with CRT pacing resulted in a higher rate of reverse remodelling than nominal settings (74% versus 53%).[61] Table 1 summarises the various methods of AV and VV timing optimisation.
Table 1: Options for Optimisation of Atrioventricular Delays and Ventriculoventricular Timing in CRT Pacing.
| Echocardiographic Methods | |
| Ritter: pulsed wave Doppler of mitral inflow AV optimisation only | Doppler echocardiographic measurement of the time of MVC. AV delay [QRSonset-MVCSAVD–QRSonse-MVCLAVD]+ SAVD, where SAVD and LAVD are short (50–60 msec) and long AV delays (160–200 msec), respectively, and -MVC is the time interval between QRS onset (QRSonset) and MVC at short and long AV delay |
| Iterative: pulsed wave Doppler of mitral inflow AV optimisation only | AV delay is programmed by assessing mitral inflow pattern to allow for biventricular capture and separation of E and A waves without A wave truncation |
| Simplified (Meluzin): pulsed wave mitral inflow AV optimisation only | Longest AV delay with full biventricular capture – (5–10 msec) – (the time from the end of the A wave to onset of systolic MR) |
| Diastolic MR method (Ishikawa) AV optimisation only | Long AV delay is set to observe diastolic MR, and the LAVD – duration of diastolic MR is the optimal AV delay |
| Aortic or LVOT VTI: continuous wave Doppler of aortic flow AV and VV optimisation | AV delay and VV timing are serially programmed to achieve maximum aortic or LVOT VTI |
| Mitral VTI AV and VV optimisation | AV delay and VV timing are serially programmed to maximise diastolic mitral inflow of both E and A wave |
| MR jet AV and VV optimisation | The slope of continuous wave Doppler of the MR jet is measured as a marker of LV contractility. The AV and VV delays are serially programmed to maximise dP/dt |
| Tissue Doppler imaging AV and VV optimisation | VV timings are optimised to the maximum tissue Doppler velocity sum of all 16 segments of the LV |
| Speckle tracking strain imaging VV optimisation only | VV timings are optimised to peak global longitudinal strain of the LV |
| Device-based Methods | |
| SmartDelay™ (Boston Scientific) AV optimisation only | IEGM-based method that uses sensed atrial and paced atrial AV intervals and intrinsic RV to LV conduction time to calculate AV delay to allow for fusion between native septal activation and biventricular pacing |
| QuickOpt™ (Abbott) AV and VV optimisation | IEGM-based method that calculates AV interval based on length of RA lead IEGM duration to allow for ventricular pacing to occur after atrial depolarisation is complete. VV interval is calculated by comparing instrinic conduction between the RV and LV IEGMs and conduction time between RV and LV during RV and LV pacing |
| AdaptiveCRT™ (Medtronic) AV and VV optimisation | IEGM-based method that dynamically calculates AV delay every minute. LV-only pacing is delivered for native AV interval <220 msec and AV delay is time from RA sense or RA pace to RV sense – 40 msec. If instrinic AV interval >220 msec, then biventricular pacing is delivered after the end of the atrial IEGM and >50 msec before RV sense. VV interval is based on AV interval and time between RV sense and end of the ventricular IEGM on the far field signal |
| SyncAV™ (Abbott) AV optimisation only | IEGM-based method that calculates and dynamically sets AV delay by assessing instrinic AV delay every 256 beats and subtracting a programmed offset (50 msec nominally, but can be set to 10–60 msec) |
| SonR™ (LivaNova) AV and VV optimisation | Using a lead-based micro-accelerometer to detect mechanical vibrations (endocardial acceleration signal), AV and VV delays are dynamically optimised weekly during rest and exercise to maximise the peak endocardial acceleration signal, which is a surrogate for LV contractility |
| CRT AutoAdapt™ (Biotronic) AV and VV optimisation | IEGM-based method similar to AdaptiveCRT. AV interval to RV and LV is measured based on sensed and paced atrial beats. LV-only pacing is delivered if A-paced AV interval is <250 msec and A to LV interval is longer than A to RV interval, otherwise biventricular pacing is delivered. AV delay is dynamically set at 70% of AV interval or AV interval – 40 msec, depending on which is shorter |
| Other Methods | |
| Invasive haemodynamic AV and VV optimisation | An open-lumen micromanometer catheter or pressure wire directly placed in the LV is used to target maximum rate of increase of LV pressure (dP/dtmax) to optimise AV and VV timing |
| Impedance cardiography (Task Force® Monitor Systems, CNSystems) AV and VV optimisation | Multiple electrodes placed on the chest, neck, and abdomen measure transthoracic impedance. Increased aortic blood flow and cardiac output are associated with lower transthoracic impedance. AV and VV timings are optimised to target the lowest impedance value, which corresponds to maximum cardiac output |
| Acoustic cardiography (Audicor™, Inovise Medical) AV and VV optimisation | Using an ECG electrodes in V3 and V4 positions to detect the first, second and third heart sounds and the QRS, time from onset of the Q wave to the mitral component of S1 is measured (electromechanical activation time) and the strength of S3 is assessed. AV and VV timings are optimised for the shortest electromechanical activation time and strongest S3 |
| Finger plethysmography AV and VV optimisation | AV and VV delays are optimised using finger oximetry to target the maximum pulse amplitude of the finger plethysmogram wave form |
| Noninvasive blood pressure measurement AV and VV optimisation | AV and VV delays optimised with serial blood pressure measurements targeting the peak mean systolic blood pressure over multiple measurements |
Atrioventricular = AV; IEGM = intracardiac electrogram; LAVD = long atrioventricular delay; LVOT = left ventricular outflow tract; MR = mitral regurgitation; MVC = mitral valve closure; RA = right atrial; SAVD = short atrioventricular delay; VTI = velocity time integral; VV = ventriculoventricular. Adapted from Rowe and Kaye 2018[70] and Gorcsan et al. 2008.[71]
CRT with an ICD
There has been significant debate as to whether the addition of ICD therapy to CRT improves outcomes. The majority of patients who meet the CRT criteria are also suitable for an ICD for the primary prevention of sudden cardiac death. Furthermore, most patients enrolled in the pivotal trials demonstrating CRT benefit also received an ICD.[62]
Multiple retrospective studies have shown better survival in patients who received a CRT-D rather than a CRT pacemaker (CRT-P).[63,64] Additionally, in patients with ischaemic cardiomyopathy, CRT-D implantation was associated with improved mortality over CRT-P.[63,64] This may be because the patients who received CRT-P were generally older, more likely female and more often had non-ischaemic cardiomyopathy as well as a greater number of comorbidities, which likely contributed to their lower survival.[63,64] In its favour, CRT-P is costs less and has been associated with fewer complications than CRT-D.[65] It should be noted that large cohort studies have shown that in patients with non-ischaemic cardiomyopathy, the addition of ICD therapy to CRT is not associated with improved outcomes;[66] however, in non-ischaemic cardiomyopathy with scarring, specifically LV mid-wall fibrosis seen with cardiac MRI, CRT-D improves mortality and reduces major adverse cardiac events.[67]
The Prospective Observational Study of the ICD in Sudden Cardiac Death Prevention (PROSe-ICD) study developed a clinical decision tool using biomarkers and clinical variables to help guide those who would benefit from ICD therapy in addition to CRT.[68] The CeRtiTuDe study showed that while patients receiving CRT-P had a higher mortality, 95% of this excess mortality was not due to sudden cardiac death.[69] The guidelines currently recommend CRT-P for NYHA Class IV heart failure and favour CRT-D for younger patients with milder heart failure, with ischaemic heart disease and life expectancy >1 year.[65] CRT-P is recommended for older patients with more advanced congestive heart failure, severe renal dysfunction, frailty and lower life expectancy.[65]
Conclusion
CRT is a potent therapy for improving outcomes and reducing mortality in heart failure. Despite being an established first-line therapy, significant issues remain in patient selection and the proper delivery of CRT. On-going research into new tools and methods to improve CRT therapy will allow for further improvements in outcomes in what has already proven an innovative therapy for the treatment of symptomatic LV systolic dysfunction.
Clinical Perspective
To improve CRT, more accurate patient selection is needed to include new populations that will respond, and limit implantation in patient groups with poor outcomes.
Better coronary sinus lead placement location and device programming is needed to improve CRT outcomes with existing technology.
New pacing approaches are required to improve left ventricular synchronisation.
References
- 1.Bristow MR, Saxon LA, Boehmer J et al. Cardiac resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;350:2140–250. doi: 10.1056/NEJMoa032423. [DOI] [PubMed] [Google Scholar]
- 2.Cleland JG, Daubert JC, Erdmann E et al. Cardiac Resynchronization-Heart Failure (CARE-HF) Study Investigators. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352:1539–49. doi: 10.1056/NEJMoa050496. [DOI] [PubMed] [Google Scholar]
- 3.Daubert JC, Saxon L, Adamson PB et al. 2012 EHRA/HRS expert consensus statement on cardiac resynchronization therapy in heart failure: implant and follow-up recommendations and management. Europace. 2012;14:1236–86. doi: 10.1093/europace/eus222. [DOI] [PubMed] [Google Scholar]
- 4.Zareba W, Klein H, Cygankiewicz I et al. Effectiveness of cardiac resynchronization therapy by QRS morphology in the Multicenter Automatic Defibrillator Implantation Trial – Cardiac Resynchronization Therapy (MADIT-CRT) Circulation. 2011;123:1061–72. doi: 10.1161/CIRCULATIONAHA.110.960898. [DOI] [PubMed] [Google Scholar]
- 5.Birnie DH, Ha A, Higginson L et al. Impact of QRS morphology and duration on outcomes after cardiac resynchronization therapy: results from the Resynchronization-Defibrillation for Ambulatory Heart Failure Trial (RAFT) Circ Heart Fail. 2013;6:1190–98. doi: 10.1161/CIRCHEARTFAILURE.113.000380. [DOI] [PubMed] [Google Scholar]
- 6.Nery PB, Ha AC, Keren A et al. Cardiac resynchronization therapy in patients with left ventricular systolic dysfunction and right bundle branch block: A systematic review. Heart Rhythm. 2011;8:1083–7. doi: 10.1016/j.hrthm.2011.01.041. [DOI] [PubMed] [Google Scholar]
- 7.Kandala J, Upadhyay GA, Altman RK et al. QRS morphology, left ventricular lead location, and clinical outcome in patients receiving cardiac resynchronization therapy. Eur Heart J. 2013;34:2252–62. doi: 10.1093/eurheartj/eht123. [DOI] [PubMed] [Google Scholar]
- 8.Varma N. Left ventricular conduction delays and relation to QRS configuration in patients with left ventricular dysfunction. Am J Cardiol. 2009;103:1578–85. doi: 10.1016/j.amjcard.2009.01.379. [DOI] [PubMed] [Google Scholar]
- 9.Kutyifa V, Stockburger M, Daubert JP et al. PR interval identifies clinical response in patients with non-left bundle branch block: a Multicenter Automatic Defibrillator Implantation Trial-Cardiac Resynchronization Therapy substudy. Circ Arrhythm Electrophysiol. 2014;7:645–51. doi: 10.1161/CIRCEP.113.001299. [DOI] [PubMed] [Google Scholar]
- 10.Daubert C, Gold MR, Abraham WT et al. Prevention of disease progression by cardiac resynchronization therapy in patients with asymptomatic or mildly symptomatic left ventricular dysfunction: insights from the European cohort of the REVERSE (Resynchronization Reverses Remodeling in Systolic Left Ventricular Dysfunction) trial. J Am Coll Cardiol. 2009;54:1837–46. doi: 10.1016/j.jacc.2019.08.011. [DOI] [PubMed] [Google Scholar]
- 11.Ruschitza F, Abraham WT, Singh JP et al. Cardiac-resynchronization therapy in heart failure with a narrow QRS complex. N Engl J Med. 2013;369:1395–405. doi: 10.1056/NEJMoa1306687. [DOI] [PubMed] [Google Scholar]
- 12.Cleland JG, Abraham WT, Linde C et al. An individual patient meta-analysis of five randomized trials assessing the effects of cardiac resynchronization therapy on morbidity and mortality in patients with symptomatic heart failure. Eur Heart J. 2013;34:3547–56. doi: 10.1093/eurheartj/eht290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stankovic I, Prinz C, Ciarka A et al. Relationship of visually assessed apical rocking and septal flash to response and long-term survival following cardiac resynchronization therapy (PREDICT-CRT) Eur Heart J Cardiovasc Imaging. 2016;17:262–9. doi: 10.1093/ehjci/jev288. [DOI] [PubMed] [Google Scholar]
- 14.Bilchick K, Kuruvilla S, Hamirani Y et al. Impact of mechanical activation, scar, and electrical timing on cardiac resynchronization therapy response and clinical outcomes. J Am Coll Cardiol. 2014;63:1657–66. doi: 10.1016/j.jacc.2014.02.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chung ES, Leon AR, Tavazzi L et al. Results of the Predictors of Response to CRT (PROSPECT) trial. Circulation. 2008;117:2608–16. doi: 10.1161/CIRCULATIONAHA.107.743120. [DOI] [PubMed] [Google Scholar]
- 16.Sohal M, Shetty A, Duckett S et al. Noninvasive assessment of LV contraction patterns using CMR to identify responders to CRT. JACC Cardiovasc Imaging. 2013;6:864–73. doi: 10.1016/j.jcmg.2012.11.019. [DOI] [PubMed] [Google Scholar]
- 17.Maass AH, Vernooy K, Wijers SC et al. Refining success of cardiac resynchronization therapy using a simple score predicting the amount of reverse ventricular remodelling: results from the Markers and Response to CRT (MARC) study. Europace. 2018;20:e1–10. doi: 10.1093/europace/eux169. [DOI] [PubMed] [Google Scholar]
- 18.Ploux S, Lumens J, Whinnett Z et al. Noninvasive electrocardiographic mapping to improve patient selection for cardiac resynchronization therapy: Beyond QRS duration and left bundle branch block morphology. J Am Coll Cardiol. 2013;61:2435–43. doi: 10.1016/j.jacc.2013.01.093. [DOI] [PubMed] [Google Scholar]
- 19.Johnson WB, Vatterott PJ, Peterson MA et al. Body surface mapping using an ECG belt to characterize electrical heterogeneity for different left ventricular pacing sites during cardiac resynchronization: Relationship with acute hemodynamic improvement Heart Rhythm. 2017;14:385–91. doi: 10.1016/j.hrthm.2016.11.017. [DOI] [PubMed] [Google Scholar]
- 20.Dong YX, Powell BD, Asirvatham SJ et al. Left ventricular lead position for cardiac resynchronization: a comprehensive cinegraphic, echocardiographic, clinical, and survival analysis. Europace. 2012;14:1139–47. doi: 10.1093/europace/eus045. [DOI] [PubMed] [Google Scholar]
- 21.Saxon LA, Olshansky B, Volosin K et al. Influence of left ventricular lead location on outcomes in the COMPANION study. J Cardiovasc Electrophysiol. 2009;20:764–8. doi: 10.1111/j.1540-8167.2009.01444.x. [DOI] [PubMed] [Google Scholar]
- 22.Singh JP, Klein HU, Huang DT et al. Left ventricular lead position and clinical outcome in the multicenter automatic defibrillator implantation trial-cardiac resynchronization therapy (MADIT-CRT) trial. Circulation. 2011;123:1159–66. doi: 10.1161/CIRCULATIONAHA.110.000646. [DOI] [PubMed] [Google Scholar]
- 23.Khan FZ, Virdee MS, Palmer CR et al. Targeted left ventricular lead placement to guide cardiac resynchronization therapy: the TARGET study: a randomized, controlled trial. J Am Coll Cardiol. 2012;59:1509–18. doi: 10.1016/j.jacc.2011.12.030. [DOI] [PubMed] [Google Scholar]
- 24.Saba S, Marek J, Schwartzman D et al. Echocardiography-guided left ventricular lead placement for cardiac resynchronization therapy: results of the Speckle Tracking Assisted Resynchronization Therapy for Electrode Region trial. Circ Heart Fail. 2013;6:427–34. doi: 10.1161/CIRCHEARTFAILURE.112.000078. [DOI] [PubMed] [Google Scholar]
- 25.Bleeker GB, Kaandorp TA, Lamb HJ et al. Effect of posterolateral scar tissue on clinical and echocardiographic improvement after cardiac resynchronization therapy. Circulation. 2006;113:969–76. doi: 10.1161/CIRCULATIONAHA.105.543678. [DOI] [PubMed] [Google Scholar]
- 26.Singh JP, Fan D, Heist EK et al. Left ventricular lead electrical delay predicts response to cardiac resynchronization therapy. Heart Rhythm. 2006;3:1285–92. doi: 10.1016/j.hrthm.2006.07.034. [DOI] [PubMed] [Google Scholar]
- 27.Gold MR, Singh JP, Ellenbogen KA et al. Interventricular electrical delay Is predictive of response to cardiac resynchronization therapy. J Am Coll Cardiol Electrophysiol. 2016;2:438–47. doi: 10.1016/j.jacep.2016.02.018. [DOI] [PubMed] [Google Scholar]
- 28.Kandala J, Upadhyay GA, Altman RK et al. Electrical delay in apically positioned left ventricular leads and clinical outcome after cardiac resynchronization therapy. J Cardiovasc Electrophysiol. 2013;24:182–7. doi: 10.1111/j.1540-8167.2012.02428.x. [DOI] [PubMed] [Google Scholar]
- 29.Silva JN, S. Ghosh S, Bowman TM et al. Cardiac resynchronization therapy in pediatric congenital heart disease: Insights from noninvasive electrocardiographic imaging. Heart Rhythm. 2009;6:1178–85. doi: 10.1016/j.hrthm.2009.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Engels EB, Strik M, van Middendorp LB et al. Prediction of optimal cardiac resynchronization by vectors extracted from electrograms in dyssynchronous canine hearts. J Cardiovasc Electrophysiol. 2017;28:944–51. doi: 10.1111/jce.13241. [DOI] [PubMed] [Google Scholar]
- 31.Singh JP, Berger RD, Doshi RN B-LBCT01-03 – targeted left ventricular lead implantation in non-left bundle branch block patients: primary results of the enhance CRT pilot study. Heart Rhythm Society Meeting, May 2018
- 32.Khan FZ, Virdee MS, Gopalan D et al. Characterization of the suitability of coronary venous anatomy for targeting left ventricular lead placement in patients undergoing cardiac resynchronization therapy. Europace. 2009;11:1491–5. doi: 10.1093/europace/eup292. [DOI] [PubMed] [Google Scholar]
- 33.Hyde ER, Behar JM, Claridge S et al. Beneficial effect on cardiac resynchronization from left ventricular endocardial pacing Is mediated by early access to high conduction velocity tissue: Electrophysiological Simulation Study. Circ Arrhythm Electrophysiol. 2015;8:1164–72. doi: 10.1161/CIRCEP.115.002677. [DOI] [PubMed] [Google Scholar]
- 34.Morgan JM, Biffi M, Geller L et al. ALternate Site Cardiac ResYNChronization (ALSYNC): A prospective and multicentre study of left ventricular endocardial pacing for cardiac resynchronization therapy. Eur Heart J. 2016;37:2118–27. doi: 10.1093/eurheartj/ehv723. [DOI] [PubMed] [Google Scholar]
- 35.Betts TR, Gamble JH, Khiani R et al. Development of a technique for left ventricular endocardial pacing via puncture of the interventricular septum. Circ Arrhythm Electrophysiol. 2014;7:17–22. doi: 10.1161/CIRCEP.113.001110. [DOI] [PubMed] [Google Scholar]
- 36.Rademakers LM, van Hunnik A, Kuiper M et al. A possible role for pacing the LV septum in cardiac resynchronization therapy. J Am Coll Cardiol Electrophysiol. 2016;2:413–22. doi: 10.1016/j.jacep.2016.01.010. [DOI] [PubMed] [Google Scholar]
- 37.Reddy VY, Miller MA, Neuzil R et al. Cardiac Resynchronization Therapy with Wireless Left Ventricular Endocardial Pacing: The SELECT-LV Study. J Am Coll Cardiol. 2017;69:2119–29. doi: 10.1016/j.jacc.2017.02.059. [DOI] [PubMed] [Google Scholar]
- 38.Leclercq C, Gadler F, Kranig W et al. A randomized comparison of triple-site versus dual-site ventricular stimulation in patients with congestive heart failure. J Am Coll Cardiol. 2008;51:1455–62. doi: 10.1016/j.jacc.2007.11.074. [DOI] [PubMed] [Google Scholar]
- 39.Providencia R, Rogers D, Papageorgiou N et al. Long-term results of tri-ventricular versus bi-ventricular pacing in heart failure: a propensity-matched comparison. J Am Coll Cardiol Electrophysiol. 2016;2:825–35. doi: 10.1016/j.jacep.2016.05.015. [DOI] [PubMed] [Google Scholar]
- 40.Enarczyk R, Kowalski O, Sredniawa D et al. Implantation feasibility, procedure-related adverse events and lead performance during 1-year follow-up in patients undergoing triple-site cardiac resynchronization therapy: A substudy of TRUST CRT Randomized Trial. J Cardiovasc Electrophysiol. 2012;23:1228–36. doi: 10.1111/j.1540-8167.2012.02375.x. [DOI] [PubMed] [Google Scholar]
- 41.Bordachar P, Gras D, Clementy N et al. Clinical impact of an additional left ventricular lead in cardiac resynchronization therapy nonresponders: The V3 trial. Heart Rhythm. 2018;6:870–6. doi: 10.1016/j.hrthm.2017.12.028. [DOI] [PubMed] [Google Scholar]
- 42.Zanon F, Baracca E, Pastore G et al. Multipoint pacing by a left ventricular quadripolar lead improves the acute hemodynamic response to CRT compared with conventional biventricular pacing at any site. Heart Rhythm. 2015;12:975–81. doi: 10.1016/j.hrthm.2015.01.034. [DOI] [PubMed] [Google Scholar]
- 43.Niazi I, Baker J, Corbisiero R et al. Safety and efficacy of multipoint pacing in cardiac resynchronization therapy: The MultiPoint Pacing Trial. J Am Coll Cardiol Electrophysiol. 2017;3:1510–8. doi: 10.1016/j.jacep.2017.06.022. [DOI] [PubMed] [Google Scholar]
- 44.Narula OS. Longitudinal dissociation in the His bundle. Bundle branch block due to asynchronous conduction within the His bundle in man. Circulation. 1977;56:996–1006. doi: 10.1161/01.CIR.56.6.996. [DOI] [PubMed] [Google Scholar]
- 45.El-Sherif N, Amay YLF, Schonfield C et al. Normalization of bundle branch block patterns by distal His bundle pacing. Clinical and experimental evidence of longitudinal dissociation in the pathologic his bundle. Circulation. 1978;57:473–83. doi: 10.1161/01.CIR.57.3.473. [DOI] [PubMed] [Google Scholar]
- 46.Deshmukh P, Casavant DA, Romanyshyn M et al. Permanent, direct His-bundle pacing: a novel approach to cardiac pacing in patients with normal His-Purkinje activation. Circulation. 2000;101:869–77. doi: 10.1161/01.CIR.101.8.869. [DOI] [PubMed] [Google Scholar]
- 47.Lustgarten DL, Crespo EM, Arkhipova-Jenkins I et al. His-bundle pacing versus biventricular pacing in cardiac resynchronization therapy patients: a crossover design comparison. Heart Rhythm. 2015;12:1548–57. doi: 10.1016/j.hrthm.2015.03.048. [DOI] [PubMed] [Google Scholar]
- 48.Sharma PS, Dandamudi G, Herweg B et al. Permanent His bundle pacing as an alternative to biventricular pacing for cardiac resynchronization therapy: a multicenter experience. Heart Rhythm. 2018;15:413–20. doi: 10.1016/j.hrthm.2017.10.014. [DOI] [PubMed] [Google Scholar]
- 49.Lustgarten DL, Calame S, Crespo EM et al. Electrical resynchronization induced by direct His-bundle pacing. Heart Rhythm. 2010;7:15–21. doi: 10.1016/j.hrthm.2009.09.066. [DOI] [PubMed] [Google Scholar]
- 50.Sharma PS, Naperkowski A, Bauch TD et al. Permanent His bundle pacing for cardiac resynchronization therapy in patients with heart failure and right bundle Branch block. Circ Arrhythm Electrophysiol. 2018;11:e006613. doi: 10.1161/CIRCEP.118.006613. [DOI] [PubMed] [Google Scholar]
- 51.Scott PA, Yue AM, Watts E et al. Transseptal left ventricular endocardial pacing reduces dispersion of ventricular repolarization. Pacing Clin Electrophysiol. 2011;34:1258–66. doi: 10.1111/j.1540-8159.2011.03138.x. [DOI] [PubMed] [Google Scholar]
- 52.Doshi RN, Daoud EG, Fellows C et al. Left ventricular-based cardiac stimulation post AV nodal ablation evaluation (the PAVE study) J Cardiovasc Electrophysiol. 2005;16:1160–5. doi: 10.1111/j.1540-8167.2005.50062.x. [DOI] [PubMed] [Google Scholar]
- 53.Deshmukh PM, Romanyshyn M. Direct His-bundle pacing: present and future. Pacing Clin Electrophysiol. 2004;27:862–70. doi: 10.1111/j.1540-8159.2004.00548.x. [DOI] [PubMed] [Google Scholar]
- 54.Brignole M, Pokushalov E, Pentimalli F et al. APAF-CRT Investigators. A randomized controlled trial of atrioventricular junction ablation and cardiac resynchronization therapy in patients with permanent atrial fibrillation and narrow QRS. Eur Heart J. 2018;39:3999–4008. doi: 10.1093/eurheartj/ehy555. [DOI] [PubMed] [Google Scholar]
- 55.Jones S, Lumens J, Sohaib SM et al. Cardiac resynchronization therapy: mechanisms of action and scope for further improvement in cardiac function. Europace. 2017;19:1178–86. doi: 10.1093/europace/euw136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Keene D, Arnold A, Shun-Shin M et al. Rationale and design of the randomized multicenter His Optimized Pacing Evaluated for Heart Failure (HOPE-HF) trial. ESC Heart Failure. 2018;5:966–77. doi: 10.1002/ehf2.12315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ellenbogen KA, Gold MR, Meyer TE et al. Primary results from the SmartDelay determined AV optimization: a comparison to other AV delay methods used in cardiac resynchronization therapy (SMART-AV) trial: A randomized trial comparing empirical, echocardiography-guided, and algorithmic atrioventricular delay programming in cardiac resynchronization therapy. Circulation. 2010;122:2660–8. doi: 10.1161/CIRCULATIONAHA.110.992552. [DOI] [PubMed] [Google Scholar]
- 58.Starling RC, Krum H, Bril S et al. Impact of a novel adaptive optimization algorithm on 30-day readmissions: Evidence from the Adaptive CRT Trial. JACC Heart Fail. 2015;3:565–72. doi: 10.1016/j.jchf.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 59.Brugada J, Delnoy PP, Brachmann J et al. Contractility sensor guided optimization of cardiac resynchronization therapy: results from the RESPOND-CRT trial. Eur Heart J. 2017;38:730–8. doi: 10.1093/eurheartj/ehw526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Varma N, O’Donnell D, Bassiouny M et al. Programming cardiac resynchronization therapy for electrical synchrony: Reaching beyond left bundle branch block and left ventricular activation delay. J Am Heart Assoc. 2018;7:e007489. doi: 10.1161/JAHA.117.007489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Trucco E, Tolosana JM, Arbelo E et al. Improvement of reverse remodeling using electrocardiogram fusion-optimized intervals in cardiac resynchronization therapy: A randomized study. J Am Coll Cardiol Electrophysiol. 2018;4:181–9. doi: 10.1016/j.jacep.2017.11.020. [DOI] [PubMed] [Google Scholar]
- 62.Katritsis DG, Auricchio A. Do we need an implantable cardioverter-defibrillator for primary prevention in cardiac resynchronisation therapy patients? Arrhythm Electrophysiol Rev. 2018;7:157–8. doi: 10.15420/aer.2018.7.3.EO1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bogale N, Priori S, Cleland JGF et al. The European CRT Survey: 1 year (9–15 months) follow-up results. Eur J Heart Fail. 2012;14:61–73. doi: 10.1093/eurjhf/hfr158. [DOI] [PubMed] [Google Scholar]
- 64.Barra S, Providência R, Tang A et al. Importance of implantable cardioverter-defibrillator back-up in cardiac resynchronization therapy recipients: A systematic review and meta-analysis. J Am Heart Assoc. 2015;4:e002539. doi: 10.1161/JAHA.115.002539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brignole M, Auricchio A, Baron-Esquivias G et al. 2013 guidelines on cardiac pacing and cardiac resynchronization therapy. Eur Heart J. 2013;34:2281–329. doi: 10.1093/eurheartj/eht150. [DOI] [PubMed] [Google Scholar]
- 66.Barra S, Boveda S, Providencia R et al. Adding defibrillation therapy to cardiac resynchronization on the basis of the myocardial substrate. J Am Coll Cardiol. 2017;69:1669–78. doi: 10.1016/j.jacc.2017.01.042. [DOI] [PubMed] [Google Scholar]
- 67.Leyva F, Zegard A, Acquaye E et al. Outcomes of cardiac resynchronization therapy with or without defibrillation in patients with nonischemic cardiomyopathy. J Am Coll Cardiol. 2017;70:1216–27. doi: 10.1016/j.jacc.2017.07.712. [DOI] [PubMed] [Google Scholar]
- 68.Nauffal V, Zhang Y, Tanawuttiwat T et al. Clinical decision tool for CRT-P vs. CRT-D implantation: Findings from PROSE-ICD. PLoS ONE. 2017;12:e0175205. doi: 10.1371/journal.pone.0175205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Marijon E, Leclercq C, Narayanan K et al. Causes-of-death analysis of patients with cardiac resynchronization therapy: an analysis of the CeRtiTuDe cohort study. Eur Heart J. 2015;36:2767–76. doi: 10.1093/eurheartj/ehv455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rowe MK, Kaye GC. Advances in atrioventricular and interventricular optimization of cardiac resynchronization therapy – what’s the gold standard? Expert Rev Cardiovasc Ther. 2018;16:183–96. doi: 10.1080/14779072.2018.1427582. [DOI] [PubMed] [Google Scholar]
- 71.Gorcsan J 3rd, Abraham T, Agler DA et al. Echocardiography for cardiac resynchronization therapy: recommendations for performance and reporting – a report from the American Society of Echocardiography Dyssynchrony Writing Group endorsed by the Heart Rhythm Society. J Am Soc Echocardiogr. 2008;21:191–213. doi: 10.1016/j.echo.2008.01.003. [DOI] [PubMed] [Google Scholar]


